Abstract
Necrotic enteritis (NE) is a disease of worldwide distribution, which affects young broilers and causes economic losses on a scale of 6 billion dollars per year. For decades, NE was controlled in poultry flocks by dietary administration of low doses of antimicrobial growth promoters (AGPs). However, an increase in NE incidence was noted after the AGP ban. This study aimed to compare the effect of an antibiotic (Enramycin) diet to a combination of sodium butyrate, hydrolyzed yeast, and zinc proteinate (ViligenTM) on broiler diets regarding performance, blood parameters, intestinal permeability, morphology and lesions, and carcass yield of broilers challenged with Eimeria spp. and Clostridium perfringens to simulate subclinical necrotic enteritis. A total of 1,150 one-day-old male broiler chickens with an initial average weight of 43.9 ± 0.65 g were allocated to 50 experimental pens. Animals were divided into 5 groups: Negative control (NC) without additives; Positive control (PC) with 0.12 g/ton of Enramycin (8%); V500, V1000, and V1500 with the addition of 500, 1.000, and 1.500 g/ton of Viligen, respectively. All animals were challenged by Eimeria spp. at 7 d of age and by C. perfringens at 17, 18, and 19 d for induction of subclinical NE. The broilers fed with all concentrations of Viligen showed similar performance, blood parameters, intestinal permeability, and carcass yield compared to PC broilers. However, NC broilers showed higher FCR compared to PC broilers from 1 to 33 d (1.42 vs. 1.39) (P = 0.048) and from 1 to 42 d (1.51 vs. 1.49) (P < 0.001). V1500 broilers had fewer intestinal lesions at 28 d when compared to the PC treatment (P < 0.05) and showed that higher Viligen inclusion resulted in lower intestinal damage. At 21 d, the V500 group showed higher intestinal morphology characteristics (VH:VD 4.9 vs. 3.5) compared to the PC treatment (P < 0.001). Thus, in this study, the dietary addition of Viligen to broilers challenged by an experimental model of subclinical NE resulted in lower intestinal damage and similar performance to that obtained by the addition of Enramycin.
Key words: antibiotic-free, broiler, gut health, necrotic enteritis, nutrition
INTRODUCTION
The dietary addition of antimicrobial growth promoters (AGPs) has been employed for a long time in animal production to modulate the microbiota and thus enhance productive performance (Shah et al., 2023). However, due to escalating antimicrobial resistance and the presence of residues in meat, the European Union banned antibiotics as growth promoters in animal diets in 2006 (European Union, 2003). Countries such as Brazil, a major broiler meat exporter, are also transitioning towards banning AGPs (MAPA, 2020), which has contributed to the increased prevalence of microbial enteric diseases in broilers, such as necrotic enteritis (NE) (Fathima et al., 2022).
Necrotic enteritis is a multifactorial gastroenteritis, with Clostridium perfringens (C. perfringens) being the principal etiological agent (Fasina et al., 2016), affecting various species including humans (Gautam et al., 2024). C. perfringens is a gram-positive, spore-forming, anaerobic bacillus (Adhikari et al., 2020), constituting part of the soil and native microbiota of broilers (Fasina and Lillehoj, 2019). It exhibits pathogenicity in favorable growth environments, necessitating the presence of predisposing factors, particularly injuries caused by Eimeria spp. (Moore, 2016). The clinical form of NE is characterized by intestinal lesions, high morbidity and mortality rates in broilers. In contrast, the subclinical form is more common, presenting with reduced performance, diarrhea, and apathy (Timbermont et al., 2011), leading to increased condemnations in production plants (Fathima et al., 2022). The estimated global cost of NE ranges between 2 to 6 billion dollars annually (Wade et al., 2016).
The industry has been developing vaccines and nutraceuticals with mitigation effects to manage bird health and performance when using diets without AGPs. However, even after years of research, subclinical NE remains one of the greatest challenges for the poultry industry (Emami et al., 2019). Butyrate, a short-chain fatty acid produced through microbial fermentation, holds potential as a mitigant for subclinical NE (Bansal et al., 2021). Butyrate can modulate the immune response (Bortoluzzi et al., 2017), provide carbon as an energy source for enterocytes (Liu et al., 2019), and control the pathogenic microbial population (Timbermont et al., 2009). These properties could enhance the immune response (Kim et al., 2024), alleviating necrotic enteritis lesions, as demonstrated in studies by Song et al. (2017), where the addition of microencapsulated sodium butyrate to the diet of broilers contaminated with Eimeria spp. and C. perfringens increased weight gain (WG) and feed conversion (FC) compared to broilers fed unsupplemented diets.
Yeast cell wall (YCW) is another feed additive that has been incorporated into animal diets to enhance productive performance and modulate immunological responses and the intestinal microbiome (Ahiwe et al., 2019). Yeast cell wall products generally consist of polysaccharides not digestible by monogastric animals, which selectively stimulate the growth and/or activity of specific bacteria in the gut (Gibson and Roberfroid, 1995) The microbiota stimulated by prebiotics may result in competitive exclusion of pathogens by producing antimicrobial metabolites, competing for limited nutrients, or attaching to receptor sites that pathogens otherwise occupy (Vandeplas et al., 2010). Alqhtani et al. (2024) demonstrated that dietary supplementation of Saccharomyces cerevisiae cell wall for broilers challenged with Clostridium perfringens restored growth performance.
Zinc is an essential trace element (Yan et al., 2016) involved in antioxidant and immunomodulatory functions (Naz et al., 2016), intestinal development, and regeneration after an enteric challenge (MacDonald, 2000). Organic sources of this mineral, such as zinc proteinate, reduce the formation of complexes between zinc and other minerals, thereby increasing its bioavailability (Lebel et al., 2014). Bortoluzzi et al. (2019) added ZnSO4 and zinc proteinate to the diet of broilers challenged with Eimeria maxima and C. perfringens and observed that the organic source reduced the expression of pro-inflammatory cytokines and the inflammatory response.
The subclinical form is the most recurrent presentation of the disease in the field (Gautam et al., 2024). The difficulty in diagnosis is caused by the absence of clinical signs and mortality, indicating a reduction in performance (Khalique et al., 2020). According to Van der Klein et al. (2023), the combination of variables such as feed conversion, weight gain, feed consumption, associated with scores of intestinal lesions and mortality, can more clearly reveal the intensity of necrotic enteritis in field broilers. Moreover, depending on the degree of intestinal injury caused by toxins, intestinal permeability is impaired, potentially causing bacterial translocation and colangiohepatitis (Nicholds et al., 2021). Therefore, the analysis of intestinal permeability, assessment of blood parameters, and yield can provide further insight into the severity of the disease.
To date, the potential synergy of sodium butyrate, hydrolyzed yeast, and zinc proteinate in the diet of broilers has not been explored. The present study hypothesizes that the combination of such compounds benefits gut health and animal performance, mitigating the adverse effects of subclinical NE. Therefore, this study aimed to evaluate the effect of dietary addition of sodium butyrate, hydrolyzed yeast, and zinc proteinate in synergy, comparing with Enramycin, on performance, carcass yield, blood parameters, intestinal permeability, intestinal lesions, and intestinal morphology of broiler chickens challenged with an experimental model of subclinical necrotic enteritis.
MATERIALS AND METHODS
Experimental Broiler and Design
This study was conducted at the Poultry Research Center of Western Paraná State University, Unioeste (Marechal Cândido Rondon, PR, Brazil). All procedures, including the use of broilers, their management, and care, were in accordance with the National Council for Control and Animal Experimentation and approved by the Animal Use Ethics Committee of the university (34/2019).
A total of 1,150 one-day-old male broilers (Cobb 500, Cobb Vantress Ltd., Cascavel, PR, BR), with an initial average weight of 43.9 ± 0.65 g, were allocated to 50 experimental pens (1.76 m²) containing new pine shavings as litter with 10cm of depth. The broilers were assigned to 5 treatment groups, each with 10 pens (replications) and 23 broilers per pen in a completely randomized design.
Experimental broilers were fed ad libitum with the dietary treatments from 1 to 42 d of age, as follows: negative control (NC), a basal diet without additives; positive control (PC), a basal diet with 0.12 g/ton of Enramycin (8%), being removed for the final stage; (V500), a basal diet with 500 g/ton of Viligen; (V1000), a basal diet with 1,000 g/ton of Viligen; and (V1500), a basal diet with 1,500 g/ton of Viligen. All diets were formulated based on corn and soybean meal (isoproteic and isocaloric diets), according to feed composition and nutritional requirements proposed by Rostagno et al. (2017). Diets were divided into pre-initial (1–10 d), initial (11–21 d), growth (22–33 d), and final (34–42-days-old) phases shown in Table 1. Salinomycin (12%) was added to all treatments to avoid clinical disease and was removed at the final stage.
Table 1.
Ingredient and calculated chemical composition of experimental diets.
| Ingredients% | Pre initial | Initial | Growth | Final |
|---|---|---|---|---|
| Corn (7,88%) | 54.62 | 56.91 | 64.55 | 68.83 |
| Soybean meal (46%) | 35.69 | 31.65 | 23.53 | 19.51 |
| Meat and bone meal | 3.00 | 4.00 | 3.00 | - |
| Poultry offal meal | 3.00 | 4.00 | 3.00 | 2.50 |
| Feather meal | - | - | 1.50 | 4.00 |
| Degomade soybean oil | 1.13 | 1.60 | 2.09 | 2.55 |
| Salt (NaCl) | 0.45 | 0.41 | 0.41 | 0.43 |
| Dicalcium phosphate | 0.68 | 0.05 | 0.24 | 0.69 |
| Limestone | 0.29 | 0.28 | 0.25 | 0.46 |
| DL-methionine (98%) | 0.33 | 0.31 | 0.27 | 0.20 |
| L-Lysine (54%) | 0.26 | 0.27 | 0.44 | 0.48 |
| L-threonine (99%) | 0.05 | 0.05 | 0.06 | 0.02 |
| L-valine (98%) | 0.007 | 0.004 | 0.23 | - |
| 1Vitamin supplement | 0.13 | 0.13 | 0.10 | 0.10 |
| 2Mineral Supplement | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline Chloride (60%) | 0.050 | 0.050 | 0.050 | - |
| 3Antioxidant | 0.01 | 0.01 | 0.01 | 0.01 |
| 4Enramycin | 0.012 | 0.012 | 0.012 | - |
| 5Salinomycin | 0.05 | 0.05 | 0.03 | - |
| 6Inerte | 0.16 | 0.16 | 0.16 | 0.16 |
| Chemical composition (%) | ||||
| Metabolizable energy (kcal/kg) | 2.975 | 3.020 | 3.150 | 3.200 |
| Crude protein (%) | 24.20 | 23.50 | 20.60 | 19.00 |
| Calcium (%) | 0.97 | 0.88 | 0.76 | 0.63 |
| Total phosphorus (%) | 0.71 | 0.66 | 0.59 | 0.49 |
| Available phosphorus (%) | 0.46 | 0.42 | 0.37 | 0.29 |
| Na (%) | 0.22 | 0.22 | 0.21 | 0.2 |
| K (%) | 0.86 | 0.80 | 0.67 | 0.59 |
| Lysine (%) | 1.31 | 1.25 | 1.12 | 1.01 |
| Methionine (%) | 0.72 | 0.71 | 0.62 | 0.48 |
| Met. + cystine (%) | 0.97 | 0.93 | 0.83 | 0.75 |
| Isoleucine (%) | 0.91 | 0.87 | 0.74 | 0.70 |
| Valine (%) | 1.01 | 0.97 | 1.07 | 0.83 |
| Threonine (%) | 0.86 | 0.83 | 0.74 | 0.67 |
| Tryptophan (%) | 0.26 | 0.24 | 0.20 | 0.18 |
Vitamin supplement. composition per kg of diet: Vitamin A (min) 14.3 I.U.; Vitamin D3 (min) 5.2 I.U.; Vitamin E (min) 71.5 I.U.; Vitamin K3 (min) 3,9 mg; Vitamin B1 (min) 2,3 mg; Vitamin B2 (min) 9.1 mg; Pantothenic acid (min) 0.01 g; Vitamin B6 (min) 5.2 mg; Vitamin B12 (min) 32.5 mcg; Nicotinic Acid (min) 0.08 g; Folic acid (min) 2,6 mg; Biotin (min) 0,3 mg; Selenium (min) 0,4 mg.
Mineral supplement. composition per kg of diet: Iron (min) 0.05 g; Copper (min) 0.01 g; Manganese (min) 0.06 g; Zinc (min) 0.06 g; Iodine (min) 1 mg.
BHT, butylated hydroxytoluene,
Enramycin-based performance enhancer (8%) added in pre-initial, initial, and growth phases of positive control (PC) treatment.
Anticoccidian.
Washed sand or Viligen.
Viligen (Alltech, Inc; Nicholasville, KY), comprising sodium butyrate, dehydrated hydrolyzed yeast, and zinc proteinate, was added to the formula to replace inert material on a weight basis.
NE Infection
All groups were challenged, and the infection was divided into two phases. The first phase involved the administration of a solution containing Eimeria spp. oocysts, while the second phase involved the inoculation of Clostridium perfringens via gavage. The aim was to simulate a subclinical challenge, adapting the methodologies of Santiani et al., 2015) and Bortoluzzi et al. (2019), respectively. To challenge the animals with Eimeria oocysts, samples of infected litter were collected from the field, and the orthopantomogram (OPG) test was performed to search for Eimeria spp. oocysts, following the methodology described by Benedet (2019). One hundred and fifty oocysts were found per gram of collected sample. Subsequently, the litter was placed in buckets and covered with water supersaturated with sodium chloride to float the oocysts. The supernatant containing the oocysts was then collected using the methodology described by Santiani et al. (2015). On d 7, the water supply to all broilers via nipple drinkers was suspended for 6 h and replaced by infant drinkers containing the supernatant. At 17, 18, and 19 d of age, all broilers individually received 0.5 mL of broth containing 108 colony-forming units (CFU) per mL of C. perfringens strains into the crop using a Pasteur pipette via the esophagus, following the protocol of Bortoluzzi et al. (2019). The strain used was provided by the Mercolab Laboratory and was obtained from field samples to simulate a subclinical field challenge.
Growth Performance
During the experiment, body weight and feed consumed were recorded on d 1, 10, 21, 33, and 42. The feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR; feed intake/weight gain) were calculated by adjusting for mortality, which was recorded daily for FI and FCR corrections, following the method described by Sakomura and Rostagno (2016).
Blood Analysis
On d 21 and 40, 2 broilers were randomly selected per pen for blood metabolite evaluation. The methodology followed was of Tesser et al (2023). These broilers were fasted for 6 h following blood collection by ulnar vein puncture, using vacuum tubes without anticoagulant. The blood samples were allowed to settle for 15 min for coagulation, and then centrifuged at 1,050 g for 10 min (Kasvi K14–4000. Kasvi. São José dos Pinhais. PR. BR) at room temperature to collect the serum. Serum samples were stored at -20°C for further analyses. Serum concentrations of cholesterol (CHOL), triglycerides (TG), glucose (GLU), total proteins (TP), albumin (ALB), creatinine (CRE), uric acid (UA), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), aspartate aminotransferase (AST), and urea (URE) were analyzed using a high-performance automatic spectrophotometer (Flexor EL200; ELITech Latin America, Brazil) with automatic calibration using commercial kits (Elitech, Clinical Systems, Elitech Group, Paris, FR).
Gut Permeability
On d 21, one broiler per pen was randomly selected to evaluate gut permeability, and received 0.5 mL of Fluorescein Isothiocyanate Dextran (FITC-d) (100 mg, MW 4,000; Sigma-Aldrich, Canada) (2.2 mg/bird) using a Pasteur pipette via the esophagus to the crop. FITC-d is a large molecule that, under normal conditions, cannot cross the epithelial barrier. However, the tight junctions are disrupted during intestinal inflammation, allowing FITC-d to enter circulation. Thus, the level of gut permeability is directly proportional to the blood level of FITC-d. Two hours after the oral gavage, blood was collected from the ulnar vein to determine the FITC-d concentration per mL of serum and plasma, using a microplate reader at a wavelength of 485 nm and an emission wavelength of 528 nm. The FITC-d concentration per mL of serum was calculated based on a standard curve. The higher the gut permeability, the higher the blood level of FITC-d." this methodology was adapted from Bortoluzzi et al. (2019).
Gut Morphometry
On d 21 and 42, one broiler from each of 5 pens per treatment (n = 25) was randomly selected, individually weighed, euthanized, and sacrificed in accordance with the guidelines of the National Council for Control of Animal Experimentation (Conselho Nacional de Controle de Experimentação Animal – CONCEA), as outlined in Normative Resolution No. 37, dated 15 February 2018. Following euthanasia, the broilers underwent plucking and evisceration. A section of the jejunum was collected at the midpoint between the bile duct entry and Meckel's diverticulum. The tissue was then fixed in 10% buffered formalin and routinely processed. Subsequently, all samples were dehydrated, infiltrated, and embedded in paraffin following standard histological procedures, and stained with hematoxylin and eosin, as adapted by Belote et al. (2018). For intestinal morphometry, two slides per animal were prepared, and 5 intact intestinal villi and crypts per slide were observed to determine villus height, crypt depth, and villus-to-crypt ratio.
Intestinal Macroscopic Lesions
On d 28, 2 broilers per pen were randomly selected, sacrificed, weighed, and examined for the presence of macroscopic findings compatible with necrotic enteritis (NE), such as liver lesions, Peyer's patch lesions, Eimeria maxima lesions, Eimeria tenella lesions, suffusion, friable intestine, necrotic foci, balloon-like lesions in the intestine, and twisted towel. Each variable was individually evaluated for severity, adapted from Mesa et al. (2014), wherein a degree of severity (DS) was assigned between 0 and 3, where 0 indicated no change, grade 1 indicated mild changes, grade 2 indicated moderate alterations, and grade 3 indicated severe alterations.
For each bird, the mean severity score for each injury was calculated as follows: score = (∑ degrees of severity) / number of evaluations. The mean score (MS) represents the average score of lesions found in twenty broilers per treatment."
Carcass Yield, Liver, and Fat Weight
On d 42, 2 broilers per pen were weighed and euthanized to determine carcass yield. The weight of the eviscerated carcass (excluding the head, neck, feet, and abdominal fat) was calculated relative to the live weight of the broiler. For the yields of breast, leg, and wing cuts, the weight of the water-chilled eviscerated carcass was considered. Additionally, the percentage of abdominal fat (deposited fat) and the liver (without the gall bladder) were evaluated relative to the live weight.
Statistical Analysis
The data were subjected to homogeneity (Levene) and normality tests (Shapiro–Wilk). Analysis of variance was conducted using General Linear Models in SPSS version 25. Dunnett's test was employed to compare the treatments PC vs. NC, V500, V1000, and V1500, with the PC treatment considered as the control. Additionally, regression analysis was performed using the NC (0), V500, V1000, and V1500 treatments. All statistical procedures were conducted at a significance level of 5% (P < 0.05).
RESULTS
Growth Performance
Animals from the NC group exhibited a higher feed conversion ratio (FCR) compared to the positive control treatment (PC) during the periods of 1 to 33 d (1.42 vs. 1.39) (P = 0.048) and 1 to 42 d (1.51 vs. 1.49) (P < 0.001). However, there were no significant differences (P > 0.05) in performance between the Viligen treatment groups and the PC group throughout the experimental period (Table 2).
Table 2.
Effect of dietary treatments on growth performance of broilers.
| Treatments1 |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | V500 | V1,000 | V1,500 | SEM5 | ANOVA | Linear | Quadratic |
| 1-10 d | |||||||||
| FI2 g | 258 | 259 | 258 | 261 | 263 | 2.43 | 0.977 | 0.088 | 0.271 |
| BWG3, g | 211 | 214 | 213 | 215 | 214 | 1.86 | 0.990 | 0.174 | 0.323 |
| FCR4 | 1.22 | 1.21 | 1.21 | 1.22 | 1.23 | 0.01 | 0.311 | 0.425 | 0.506 |
| 1-21 d | |||||||||
| FI, g | 1,205 | 1,188 | 1,201 | 1,199 | 1,192 | 5.28 | 0.854 | 0.130 | 0.154 |
| BWG, g | 911 | 910 | 918 | 923 | 911 | 3.66 | 0.759 | 0.885 | 0.322 |
| FCR | 1.32 | 1.31 | 1.31 | 1.29 | 1.31 | 0.01 | 0.294 | 0.264 | 0.181 |
| 1-33 d | |||||||||
| FI, g | 3,119 | 3,059 | 3,079 | 3,084 | 3,076 | 10.98 | 0.536 | 0.194 | 0.372 |
| BWG, g | 2,190 | 2,199 | 2,195 | 2,197 | 2,197 | 8.04 | 0.997 | 0.080 | 0.238 |
| FCR | 1.42a | 1.39 | 1.40 | 1.40 | 1.40 | 0.01 | 0.048 | 0.140 | 0.340 |
| 1-42 d | |||||||||
| FI, g | 4,734 | 4,701 | 4,750 | 4,733 | 4,721 | 17.17 | 0.922 | 0.392 | 0.406 |
| BWG, g | 3,128 | 3,164 | 3,187 | 3,181 | 3,168 | 11.89 | 0.558 | 0.452 | 0.277 |
| FCR | 1.51a | 1.49 | 1.49 | 1.49 | 1.49 | 0.01 | <0.001 | 0.220 | 0.149 |
NC – Control diet, without antimicrobials; PC – Control diet, added 0.12 g/ton of Enramycin (8%); V500 – Control diet, added 500 g/ton of Viligen; V1,000 – Control diet, added 1,000 g/ton of Viligen; V1,500 – Control diet, added 1,500 g/ton of Viligen.
FI = Feed intake.
BWG = Body weight gain.
FCR = Feed conversion ratio.
SEM = Standard error of the mean.
Differs from the positive control (PC) treatment by Dunnett's test (P < 0.05).
Blood Analyses
At 21 d, the results of blood metabolite concentrations showed no significant difference between the PC and the other treatments (P > 0.05). However, a significant linear effect was observed for ALT (P = 0.020) and AST (P = 0.040), with increasing concentrations as the Viligen dose increased (Table 3).
Table 3.
Effect of dietary treatments on blood metabolites of 21-day-old broilers.
| Treatments1 |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | V500 | V1,000 | V1,500 | SEM11 | ANOVA | Linear | Quadratic | |
| UA2 (mg/DL) | 7.21 | 6.61 | 5.79 | 8.28 | 8.22 | 0.35 | 0.137 | 0.233 | 0.617 |
| Alt3,12 (IU/L) | 6.06 | 5.84 | 6.09 | 6.90 | 7.28 | 0.26 | 0.391 | 0.020 | 0.193 |
| Albumin (g/L) | 16.33 | 16.15 | 15.24 | 16.63 | 16.89 | 0.23 | 0.209 | 0.361 | 0.612 |
| Ast4,13 (IU/L) | 189.13 | 177.47 | 178.76 | 189.23 | 193.25 | 7.23 | 0.943 | 0.040 | 0.260 |
| Cho5 (mg/DL) | 116.70 | 115.59 | 105.63 | 119.11 | 113.75 | 2.30 | 0.407 | 0.820 | 0.956 |
| Crea6 (mg/DL) | 0.20 | 0.20 | 0.19 | 0.20 | 0.19 | 0.01 | 0.919 | 0.753 | 0.904 |
| Ggt7 (IU/L) | 10.68 | 10.89 | 10.74 | 9.24 | 9.07 | 0.36 | 0.341 | 0.068 | 0.362 |
| Glu8 (mg/DL) | 258.73 | 266.61 | 246.82 | 259.72 | 266.62 | 3.49 | 0.371 | 0.821 | 0.535 |
| TP9 (g/L) | 26.89 | 26.80 | 24.98 | 26.96 | 26.36 | 0.43 | 0.553 | 0.906 | 0.915 |
| Trig10(mg/DL) | 58.69 | 63.96 | 47.25 | 69.20 | 62.09 | 3.55 | 0.379 | 0.776 | 0.929 |
| Urea (mg/DL) | 3.24 | 4.14 | 3.75 | 4.72 | 3.99 | 0.20 | 0.216 | 0.844 | 0.959 |
NC – Control diet, without growth promoter antimicrobials; PC – Control diet, added 0.12g/ton of Enramycin (8%); 500 - Control diet, added 500 g/ton of Viligen; 1,000 - Control diet, added 1,000 g/ton of Viligen; 1,500 - Control diet, added 1,500 g/ton of Viligen.
Uric Acid.
Alanine Amino transferase.
Aspartate Amino Transferase.
Cholesterol.
Creatinine.
Gama GT.
Glucose.
Total protein.
Triglycerides.
SEM = Standard error of the mean.
Linear effect (P < 0.05) of the treatments on the ALT (y=1.0251x + 5.7578 R² = 0.9595) 13Linear effect (P< 0.05) of the treatments on the AST (y=11.562x + 176 R² = 0.9224).
At 42 d of age, there was no significant difference between the PC and the other treatments in terms of blood metabolite concentrations (P > 0.05). Nonetheless, a significant quadratic effect was observed for the variables GGT (P = 0.011), with a minimum point (12.9 IU/L) observed at the estimated Viligen level of 375 g/ton, and urea (P = 0.011), with a maximum point (3.92 mg/dL) observed at the estimated Viligen level of 375 g/ton (Table 4).
Table 4.
Effect of dietary treatments on blood metabolites of 42-day-old broilers.
| Treatments¹ |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | NC | PC | 500 | 1,000 | 1,500 | SEM11 | ANOVA | Linear | Quadratic |
| UA2 (mg/DL) | 3.64 | 3.53 | 3.40 | 3.16 | 3.23 | 0.79 | 0.137 | 0.122 | 0.335 |
| Alt3 (IU/L) | 9.81 | 11.69 | 9.62 | 10.11 | 9.92 | 0.43 | 0.648 | 0.330 | 0.454 |
| Albumin (g/L) | 16.42 | 16.75 | 16.13 | 16.81 | 16.44 | 0.21 | 0.839 | 0.901 | 0.969 |
| Ast4 (IU/L) | 463.68 | 498.23 | 458.97 | 499.51 | 442.86 | 15.63 | 0.723 | 0.430 | 0.803 |
| Cho5 (mg/DL) | 119.71 | 123.72 | 123.47 | 127.34 | 125.90 | 2.28 | 0.863 | 0.271 | 0.659 |
| Crea6 (m/ DL) | 0.13 | 0.13 | 0.13 | 0.13 | 0.12 | 0.01 | 0.936 | 0.721 | 0.200 |
| Ggt7,12 (IU/L) | 13.68 | 13.08 | 12.92 | 13.39 | 14.45 | 0.54 | 0.910 | 0.142 | 0.011 |
| Glu8 (mg/DL) | 260.64 | 254.19 | 264.36 | 250.52 | 260.83 | 2.42 | 0.375 | 0.875 | 0.992 |
| TP9 (g/L) | 29.48 | 29.41 | 29.51 | 30.25 | 29.39 | 0.35 | 0.932 | 0.786 | 0.705 |
| Trig10(m/DL) | 70.51 | 74.37 | 70.09 | 60.96 | 64.43 | 2.93 | 0.617 | 0.154 | 0.379 |
| Urea13 (mg/DL) | 3.32 | 3.86 | 3.91 | 3.75 | 3.37 | 0.16 | 0.669 | 0.141 | 0.011 |
NC – Control diet, without growth promoter antimicrobials; PC – Control diet, added 0.12g/ton of Enramycin (8%); 500 - Control diet, added 500 g/ton of Viligen; 1,000 - Control diet, added 1,000 g/ton of Viligen; 1,500 - Control diet, added 1,500 g/ton of Viligen.
Uric Acid.
Alanine Amino transferase.
Aspartate Amino Transferase.
Cholesterol.
Creatinine.
Gama GT
Glucose.
Total protein.
Triglycerides.
SEM = Standard error of the mean.
Quadractic effect (P < 0.05) of the treatments on the GGT (y = 1.223x2 – 0.9187x + 13.079 R² = 0.9999)
Quadractic effect (P < 0.05) of the treatments on the UREA (y = -0.4341x2 + 0.3252x + 3.8579
R² = 0.9999).
Gut Permeability and Morphometry
At 21 d, there was no significant difference (P > 0.05) between PC and all other treatments for serum concentrations of FITC-d. Additionally, at 21 d, the V500 treatment increased the height of villi and the villus:crypt ratio compared to the positive control. However, there were no differences in gut morphometry at 42 d (Table 5).
Table 5.
Effect of dietary treatments on concentration of FITC-d on serum and gut morphometry.
| Treatments¹ |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PC | V500 | V1,000 | V1,500 | SEM4 | ANOVA | Linear | Quadratic | |
| Conc. of FITC-d at 21 d of age | |||||||||
| 3Serum (ng/mL) | 242 | 229 | 244 | 199 | 208 | 0.01 | 0.632 | 0.317 | 0.725 |
| Morphometry | |||||||||
| 21 d of age | |||||||||
| Villus height (µm) | 780 | 762 | 1,067a | 887 | 806 | 19.40 | <0.001 | 0.953 | 0.560 |
| Crypt depth (µm) | 228 | 238 | 229 | 219 | 259 | 5.97 | 0.271 | 0.606 | 0.374 |
| Villus height: crypt depth | 3.5 | 3.5 | 4.9a | 4.2 | 3.4 | 0.12 | <0.001 | 0.841 | 0.372 |
| 42 d of age | |||||||||
| Villus height (µm) | 1,169 | 1,24 | 1,034 | 1,232 | 1,209 | 27.07 | 0.109 | 0.860 | 0.827 |
| Crypt depth (µm) | 223 | 192 | 201 | 186 | 230 | 5.62 | 0.054 | 0.352 | 0.548 |
| Villus height: crypt depth | 5.7 | 7.0 | 5.7 | 6.5 | 5.7 | 0.19 | 0.121 | 0.399 | 0.763 |
NC – Control diet, without growth promoter antimicrobials; PC – Control diet, added 0.12g/ton of Enramycin (8%); 500 - Control diet, added 500 g/tonof Viligen; 1,000 - Control diet, added 1,000 g/ton of Viligen; 1,500 - Control diet, added 1,500 g/ton of Viligen.
2Concentration of FITC-d on Serum.
SEM = Standard error of the mean.
Differs from the positive control treatment by Dunnett's test (P < 0.05).
Intestinal Lesions Score
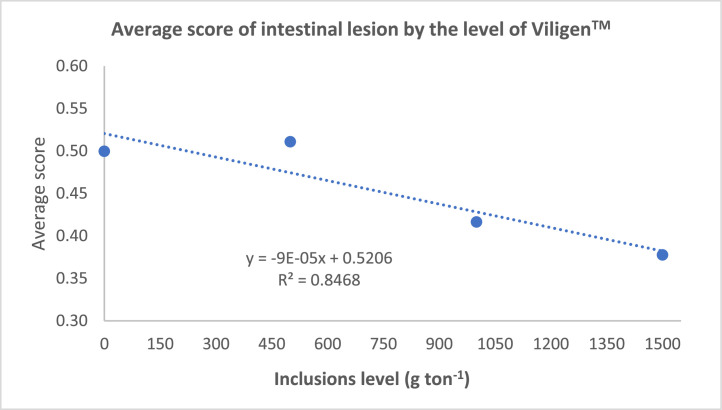
It was observed that the animals that received V1500 presented lower scores of intestinal injuries than the animals that received the PC treatment (Table 6). Additionally, a significant linear effect was observed as a function of Viligen dose (Figure 1). As the Viligen supplementation level increased, the scores of intestinal injuries decreased.
Table 6.
Effect of dietary treatments on mean score for degree of severity of intestinal lesions.
| Treatments¹ |
P- value |
||||||
|---|---|---|---|---|---|---|---|
| NC | PC | 500 | 1,000 | 1,500 | 3SEM | ANOVA | |
| Mean Score² | 0.50 | 0.45 | 0.51 | 0.42 | 0.38a | 0.21 | 0.038 |
NC – Control diet, without growth promoter antimicrobials; PC – Control diet, added 0.12g/ton of Enramycin (8%); 500 - Control diet, added 500 g/ton of Viligen; 1,000 - Control diet, added 1,000 g/ton of Viligen; 1,500 - Control diet, added 1,500 g/ton of Viligen.
Mean score: mean of the score lesion find on treatment.
SEM = Standard error of the mean;
Differs from the positive control treatment by Dunnett's test (P<0.05)
Figure 1.
Linear effect of dietary treatments on mean score for degree of severity of intestinal lesions of 28-day-old broilers challenged with Eimeria spp. and C. perfringens.
Carcass Yield, Liver, and Fat Weight
There was no significant difference between the PC and other treatments on carcass characteristics of broilers slaughtered at 42 d (P > 0.05) (Table 7).
Table 7.
Effect of dietary treatments on carcass characteristics, liver and fat weight.
| Treatments¹ |
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item (%) | NC | PC | V500 | V1,000 | V1,500 | SEM4 | ANOVA | Linear | Quadratic |
| HCY² | 69.71 | 69.87 | 69.64 | 70.47 | 69.22 | 0.13 | 0.061 | 0.722 | 0.779 |
| CCY³ | 70.72 | 70.80 | 70.69 | 71.40 | 70.43 | 0.14 | 0.278 | 0.875 | 0.792 |
| Breast | 29.43 | 28.66 | 29.00 | 29.11 | 29.05 | 0.11 | 0.324 | 0.177 | 0.129 |
| Thighs | 31.86 | 31.77 | 31.74 | 31.63 | 32.11 | 0.09 | 0.552 | 0.436 | 0.421 |
| Wing | 10.34 | 10.32 | 10.39 | 10.07 | 10.18 | 0.05 | 0.310 | 0.345 | 0.753 |
| Tender lones | 6.41 | 6.22 | 6.29 | 6.16 | 6.19 | 0.04 | 0.418 | 0.537 | 0.866 |
| Liver | 1.80 | 1.86 | 1.86 | 1.84 | 1.83 | 0.02 | 0.865 | 0.104 | 0.336 |
| Abdominal Fat | 1.41 | 1.59 | 1.36 | 1.34 | 1.46 | 0.03 | 0.110 | 0.528 | 0.076 |
NC – Control diet, without growth promoter antimicrobials; PC – Control diet, added 0.12g/ton of Enramycin (8%); 500 - Control diet, added 500 g/ton of Viligen; 1,000 - Control diet, added 1,000 g/ton of Viligen; 1,500 - Control diet, added 1,500 g/ton of Viligen.
HCY= Hot Carcass Yield.
CCY = Cold Carcass Yield.
SEM = Standard error of the mean
DISCUSSION
Growth Performance
NE was induced using a method aimed at closely simulate a subclinical challenge in the field. When evaluating performance parameters, we found that the severity degree of the disease was mild, which is consistent with the objective of the challenge. In the present study, animals in the NC group showed a higher FCR than animals in the PC group. This finding is consistent with the results of Sun et al. (2023), who reported that subclinical NE often results in higher FCR and poor growth performance due to intestinal damage, toxin actions, and consequent lower nutrient absorption. There was no significant difference in the performance parameters between the PC and Viligen treatments, confirming the functionality of the synergy of Viligen compounds in the performance of broilers under sanitary challenge. This has been separately evidenced in previous studies through intestinal analyses (Bortoluzzi et al., 2019; Yang et al., 2023; Alqhtani et al., 2024).
Blood Analyses
The effect of NE on blood metabolites of broilers is still poorly understood and varies according to the severity of the disease. In the present study, there was no significant difference between the treatments when compared to the PC for all variables analyzed at both ages. However, at 21 d of age, the enzymes ALT and AST showed a linear response, increasing as the concentration of Viligen in the diet increased. Such a response was not observed at 42 d of age, which may be associated with the recent infection of the broilers (d 18) and the metabolism of the compounds after the challenge. Serum levels outside the standard range of AST and ALT enzymes are associated with hepatic cell damage (Zhu et al., 2022), which was not observed in the study. Although at 42 d, a significant quadratic effect was observed for the variables GGT (P = 0.011), and Urea (P = 0.011). Thus, it is concluded that Viligen supplementation had not negatively affected the serum biochemical indicators of broiler chickens.
Gut Permeability and Intestinal Morphometry
In this trial, dietary treatments did not influence the FITC-d values in serum. NE is characterized by increased intestinal permeability (Latorre et al., 2018) caused by enzymes and toxins produced by C. perfringens. C. perfringens enterotoxin (CPE) is one of the toxins produced, which binds to proteins that form tight junctions, called claudins (Benz & Popoff, 2018), forming a pore in the plasma membrane, consequently inducing cell apoptosis. Higher doses of CPE lead to several pore formations in the cell membrane, leading to a massive cell death process called apoptosis (Freedman et al., 2016). This process leads to an increase in intestinal permeability that can result in the passage of microorganisms and toxins into the bloodstream (Emami et al., 2019) and can be measured through the administration of FITC-d, a compound with a molecular weight of 3 to 5 kDa that, under normal conditions, does not cross the epithelial barrier. However, during intestinal inflammation, FITC-d can be found in the bloodstream (Baxter et al., 2017). In our study, neither the performance-enhancing antibiotic (Enramycin 8%) nor Viligen administration prevented the passage of FITC-d into the bloodstream. Moreover, there was no statistical difference between NC and PC, which may be associated with subclinical NE, causing less damage to the intestinal epithelium.
At 21 d, the broilers from the V500 group had greater villus height and villus:crypt ratio compared to the PC group. Sikandar et al. (2017) added sodium butyrate to broiler diets and found improved morphology of lymphoid organs and gut mucosa in the broiler, which is similar to our findings. Butyrate is considered an important energy source for enterocytes, allowing greater cell mitosis in the crypt, villus height, and consequent villus/crypt relationship (Ahsan et al., 2016). Butyrate, associated with the hydrolyzed yeast and zinc properties, may have allowed the superior gut morphology observed in the V500 treatment group.
Intestinal Lesions Score
The evaluation of intestinal lesions is commonly used to assess the severity of an experimental NE model (Cooper and Songer, 2009). The infection model proposed in this study is consistent with the lesions observed in subclinical NE, as described by Paiva & McElroy (2014) and Timbermont et al. (2011). Analysis of the lesions revealed that the V1500 group had lower intestinal injury scores compared to the PC treatment. The Viligen dosage showed an inverse relationship with the intestinal injury score, suggesting that higher doses of Viligen support intestinal health under subclinical NE challenge.
Bortoluzzi et al. (2019) demonstrated that organic (proteinate) and inorganic (sulfate) zinc supplementation in the diet of broilers infected with Eimeria maxima and C. perfringens exerted an anti-inflammatory effect. This effect was associated with crypt cell proliferation, which enhances cell turnover, thereby maintaining the intestinal barrier's structure and function (Hu et al., 2013 ; Mocchegiani et al., 2013). Dietary supplementation with yeast-derived products reduces the presence of intestinal lesions consistent with NE (Tian et al., 2016; Ahiwe et al., 2019; Liu et al., 2019). Yeast cell wall compounds bind to the cell walls of pathogenic microorganisms and the host epithelium, decreasing the adherence of pathogenic microorganisms (Liu et al., 2017) and stimulating the release of macrophages, monocytes, and cytokines to enhance the host immune response (Tian et al., 2016).
Carcass Yield, and Liver and Fat Weight
In the present study, dietary treatments did not show a significant difference in the carcass yield variables being in accordance with the performance parameters, in which there was no difference between the treatments. These results agree with Pascual et al. (2020) and Kyoung et al. (2023), that included YCW in the diet of broilers, and showed that there was a significant improvement in performance, but found no difference in the carcass yield of the animals analyzed. This condition may be directly linked to the concurrent subclinical enteritis challenge at the intestinal level, without major systemic changes that could cause a reduction in muscle position.
CONCLUSIONS
The addition of varying levels of Viligen to the diet of broilers challenged with an experimental model of subclinical necrotic enteritis resulted in performance comparable to that of animals receiving Enramycin. However, a lower intestinal lesion score was observed with higher concentrations of Viligen. This study demonstrates that Enramycin and Viligen offers benefits to intestinal health and helps alleviate the adverse effects of subclinical NE in broilers challenged with Eimeria spp. and C. perfringens.
DISCLOSURES
The authors declare no conflicts of interest.
Acknowledgments
The authors thank the Alltech for financing and Universidade do Oeste do Paraná (UNIOESTE) for supporting this study.
REFERENCES
- Adhikari P., Kiess A., Adhikari R., Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020;29:515–534. [Google Scholar]
- Ahiwe E.U., Chang'a E.P., Abdallh M.E., Al-Qahtani M., Kheravii S.K., Wu S., Graham H., Iji P.A. Dietary hydrolysed yeast cell wall extract is comparable to antibiotics in the control of subclinical necrotic enteritis in broiler chickens. Br. Poult. Sci. 2019;60:757–765. doi: 10.1080/00071668.2019.1664727. [DOI] [PubMed] [Google Scholar]
- Ahsan U., Cengiz Ö., Raza I., Kuter E., Chacher M.F., Iqbal Z., Umar S., Çakir S. Sodium butyrate in chicken nutrition: the dynamics of performance, gut microbiota, gut morphology, and immunity. World's Poult. Sci. J. 2016;72:265–275. [Google Scholar]
- Alqhtani A.H., Al Sulaiman A.R., Alharthia A.S., Abudabos A.E. Dietary supplementation of prebiotic yeast Saccharomyces cerevisiae cell wall promotes growth performance and intestinal health in broiler chickens challenged with Clostridium perfringens. British Poultry Sci. 2024;2:129–136. doi: 10.1080/00071668.2023.2296938. [DOI] [PubMed] [Google Scholar]
- Bansal M., Alenezi T., Fu Y., Almansour A., Wang H., Gupta A., Liyanage R., Graham D.B., Hargis B.M., Sun X. Specific Secondary Bile Acids Control Chicken Necrotic Enteritis. Pathogens. 2021;10(8):1041. doi: 10.3390/pathogens10081041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M.F.A., Merino-Guzman R., Latorre J.D., Mahaffey B.D., Yang Y., Teague K.D., Graham L.E., Wolfenden A.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017;4:1–6. doi: 10.3389/fvets.2017.00056. http://www.frontiersin.org/Journal/10.3389/fvets.2017.00056/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belote B.L., Tujimoto-Silva A., Hummelgen P.H., Sanches A.W.D., Wammes J.C.S., Hayashi R.M., Santin E. Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poult. Sci. 2018;97:2287–2294. doi: 10.3382/ps/pey064. [DOI] [PubMed] [Google Scholar]
- Benedet, J. P., 2019. Avaliação de tratamentos de camas de frangos contra Clostridium perfringens, enterobactérias e oocistos de Eimeria spp. em aviários dark house e convencional de frango de corte. Dissertação de mestrado – IFC. Concórdia. 1–47. (Brazilian Portuguese, abstract in English).
- Benz R., Popoff M.R. Clostridium perfringens enterotoxin: the toxin forms highly cation-selective channels in lipid bilayers. Toxins. 2018;10:341–354. doi: 10.3390/toxins10090341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Lumpkins B., Mathis G.F., França M., King W.D., Graugnard D.E., Dawson K.A., Applegate T.J. Zinc source modulates intestinal inflammation and intestinal integrity of broiler chickens challenged with coccidia and Clostridium perfringens. Poult. Sci. 2019;98:2211–2219. doi: 10.3382/ps/pey587. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Pedroso A.A., Mallo J.J., Puyalto M., Kim W.K., Applegate T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017;96:3981–3993. doi: 10.3382/ps/pex218. [DOI] [PubMed] [Google Scholar]
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA) Instrução Normativa No 01, de 13 de Janeiro de 2020. https://www.in.gov.br/en/web/dou/-/instrucao-normativa-n-1-de-13-de-janeiro-de-2020-239402385 (accessed 30 October 2021) (Brazilian Portuguese).
- Cooper K.K., Songer J.G. Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2009;15:55–60. doi: 10.1016/j.anaerobe.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Calik A., White M.B., Young M., Dalloul R.A. Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019;7:231. doi: 10.3390/microorganisms7080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Union Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union. 2003;50:29. [Google Scholar]
- Fasina Y.O., Newman M.M., Stough J.M., Liles M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016;95:247–260. doi: 10.3382/ps/pev329. [DOI] [PubMed] [Google Scholar]
- Fasina Y.O., Lillehoj H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathima S., Al Hakeem W.G., Shanmugasundaram R., Selvaraj R.K. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms. 2022;10:1958. doi: 10.3390/microorganisms10101958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J.C., Shrestha A., McClane B.A. Clostridium perfringens enterotoxin: action, genetics, and translational applications. Toxins. 2016;8:73–89. doi: 10.3390/toxins8030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam H., Ayalew E.L., Shaik N.A., Subhasinghe I., Popowich S., Chow‑Lockerbie B., Dixon A., Ahmed K.A., Tikoo S.K., Gomis S. Exploring the predictive power of jejunal microbiome composition in clinical and subclinical necrotic enteritis caused by Clostridium perfringens: insights from a broiler chicken model. J. Transl. Med. 2024;22:80. doi: 10.1186/s12967-023-04728-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Hu C.H., Qian Z.C., Song J., Luan Z.S., Zuo A.Y. Effects of zinc oxide-montmorillonite hybrid on growth performance, intestinal structure, and function of broiler chicken. Poult Sci. 2013;92:143–150. doi: 10.3382/ps.2012-02250. [DOI] [PubMed] [Google Scholar]
- Khalique A., Zeng D., Shoaib M., Wang H., Qing X., Rajput D.S., Pan K., Ni X. Probiotics mitigating subclinical necrotic enteritis (SNE) as potential alternatives to antibiotics in poultry. AMB Express. 2020;10:50. doi: 10.1186/s13568-020-00989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.M., Liu J., Whitmore M.A., Tobin I., Zhao Z., Zhang G. Two intestinal microbiota-derived metabolites, deoxycholic acid and butyrate, synergize to enhance host defense peptide synthesis and alleviate necrotic enteritis. J. Anim. Scien. Biotech. 2024;15:29. doi: 10.1186/s40104-024-00995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoung H., Kim E., Cho J.H., Lee H., Kim Y., Park K.I., Kim H.B., Song M. Dietary yeast cell wall enhanced intestinal health of broiler chickens by modulating intestinal integrity, immune responses, and microbiota. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F.A., Hernandez-Velasco X., Kwon Y.M., Ricke S.C., Bielke L.R., Hargis B.M., Tellez G. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;5:199. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel A., Matte J.J., Guay F. Effect of mineral source and mannan oligosaccharide supplements on zinc and copper digestibility in growing pigs. Arch. Anim. Nutr. 2014;68:370–384. doi: 10.1080/1745039X.2014.954357. [DOI] [PubMed] [Google Scholar]
- Liu J.D., Bayir H.O., Cosby D.E., Cox N.A., Williams S.M., Fowler J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers. Poult. Sci. 2017;96:3638–3644. doi: 10.3382/ps/pex174. [DOI] [PubMed] [Google Scholar]
- Liu J.D., Lumpkins B., Mathis G., Williams S.M., Fowler J. Evaluation of encapsulated sodium butyrate with varying releasing times on growth performance and necrotic enteritis mitigation in broilers. Poult. Sci. 2019;98:3240–3245. doi: 10.3382/ps/pez049. [DOI] [PubMed] [Google Scholar]
- MacDonald R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- Mesa D., Lourenço M.C., Westphal P., Kraieski A., Santin E. Modelo de protocolo experimental para induzir, classificar e avaliar as enterites inespecíficas em frangos de corte. Pesq. Vet. Bras. 2014;34:929–936. (Brazilian Portuguese, abstract in English) [Google Scholar]
- Mocchegiani E., Romeo J., Malavolta M., Costarelli L., Giacconi R., Diaz L.E., Marcos A. Zinc: dietary intake and impact of supplementation on immune function in elderly. Age. 2013;35:839–860. doi: 10.1007/s11357-011-9377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian. Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- Naz S., Idris M., Khalique M.A., Zia-Ur-rahman A.I.A., Abdelrahman M.M., Khan R.U., Chand N., Farooq U., Ahmad S. The activity and use of zinc in poultry diets. Worlds Poult. Sci. J. 2016;72:159–167. [Google Scholar]
- Nicholds J.F., McQuain C., Hofacre C.L., Mathis G.F., Fuller A.L., Telg B.E., Montoya A.F., Williams S.M., Berghaus R.D., Jones M.K. The effect of different species of eimeria with Clostridium perfringens on performance parameters and induction of clinical necrotic enteritis in broiler chickens. Avian Dis. 2021;65:132–137. doi: 10.1637/aviandiseases-D-20-00106. 2021. [DOI] [PubMed] [Google Scholar]
- Paiva D., McElroy A. Necrotic enteritis: Applications for the poultry industry. J. Appl. Poult. Res. 2014;23:557–566. [Google Scholar]
- Pascual A., Pauletto M., Giantin M., Radaelli G., Ballarin C., Birolo M., Zomeño C., Dacasto M., Bortoletti M., Vascellari M., Xiccato G., Trocino A. Effect of dietary supplementation with yeast cell wall extracts on performance and gut response in broiler chickens. J. Anim. Sci. Biotechnol. 2020;11:40. doi: 10.1186/s40104-020-00448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno H.S., Albino L.F.T., Hannas M.I. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 4 ed. Viçosa; UFV: 2017. p. 488. [Google Scholar]
- Santiani F., Gomes D.J., Piva M.M., Carneiro D.C.S., Mingotti T.R., Prior K.C., Weber C.L., Silva T.M. Comparativo de prevalência de Eimeria spp. em cama fermentada e não fermentada de frangos de corte e lesões encontradas no intestino das aves, na região do meio oeste catarinense. Brazilian J. Poult. Sci. 2015;21:201–208. (Brazilian Portuguese, abstract in English) [Google Scholar]
- Shah B.R., Hakeem W.A., Shanmugasundaram R., Selvaraj R.K. Effect of symbiotic supplementation on production performance and severity of necrotic enteritis in broilers during an experimental necrotic enteritis challenge. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102959. https://www.sciencedirect.com/science/article/pii/S0032579123004789 Accessed April 15, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar A., Zaneb H., Younus M., Masood S., Aslam A., Khattak F., Ashraf S., Yousaf M.S., Rehman H. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas J Anim. Sci. 2017;30:690–699. doi: 10.5713/ajas.16.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Li H., Wu Y., Zhen W., Wang Z., Xia Z., Guo Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim. Feed Sci. Technol. 2017;232:6–15. [Google Scholar]
- Sakomura N.K., Rostagno H.S. 2 ed. FUNEP; Jaboticabal: 2016. Métodos de pesquisa em nutrição de monogástricos; p. 262. [Google Scholar]
- Sun, N., Xue, Y., Wei, S., Wu, B., Wang, H., Zeng, D., Zhao, Y., Khalique, A., Pan, K., Zeng, Y., Shu, G., Jing, B., Xueqin, N. Compound probiotics improve body growth performance by enhancing intestinal development of broilers with subclinical necrotic enteritis. Probiotics Antimicrob. Proteins, 15:558–572. [DOI] [PubMed]
- Tesser G.L.S., Junior N.R., Campos F.P., Costa A.P.G.C., Sartor H., Kaufmann C., Junior J.V.G., Eyng C., Nunes R.V. Efects of feeding diets with zinc‑l‑selenomethionine on growth performance of broilers subjected to cyclic heat stress. Trop. Anim. Health Prod. 2023;55:384. doi: 10.1007/s11250-023-03779-x. [DOI] [PubMed] [Google Scholar]
- Tian X., Shao Y., Wang Z., Guo Y. Effects of dietary yeast β-glucans supplementation on growth performance, gut morphology, intestinal Clostridium perfringens population and immune response of broiler chickens challenged with necrotic enteritis. Anim. Feed Sci. Technol. 2016;215:144–155. [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., Ducatelle R., Van Immerseel F. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2009;39:117–121. doi: 10.1080/03079451003610586. [DOI] [PubMed] [Google Scholar]
- Van der Klein S.A.S., Evans C., Marchal J.L.M., Gibbs K. Elucidating the varying impact of necrotic enteritis using performance and health indicators in broiler infection models. Avian Dis. 2023;67:326–339. doi: 10.1637/aviandiseases-D-23-00048. [DOI] [PubMed] [Google Scholar]
- Vandeplas S., Dubois Dauphin R., Beckers Y., Thonart P., Théwis A. Salmonella in chicken: current and developing strategies to reduce contamination at farm level. J Food Prot. 2010;73:774–785. doi: 10.4315/0362-028x-73.4.774. [DOI] [PubMed] [Google Scholar]
- Yan R., Zhang L., Yang X., Wen C., Zhou Y.M. Bioavailability evaluation of zinc-bearing palygorskite as a zinc source for broiler chickens. Appl Clay Sci. 2016;119:155–160. [Google Scholar]
- Yang T., Sun Y., Dai Z., Liu J., Xiao S., Liu Y., Wang X., Yang S., Sun Y., Zhenglie D., Liu J., Xiao S., Liu Y., Wang X., Yang S., Zhang R., Yang C., Dai B. Microencapsulated sodium butyrate alleviates immune injury and intestinal problems caused by Clostridium Perfringens through gut microbiota. Animals. 2023;13:3784. doi: 10.3390/ani13243784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B., Keyburn A.L., Haring V., Ford M., Rood J.I., Moore R.J. The adherent abilities of Clostridium perfringens strains are critical for the pathogenesis of avian necrotic enteritis. Vet. Microbiol. 2016;197:53–61. doi: 10.1016/j.vetmic.2016.10.028. [DOI] [PubMed] [Google Scholar]
- Zhu X., Zhang Y., Zhao Y., Tao L., Liu H., Dong W., Yang D., Li L. Effects of dietary supplementation with itaconic acid on the growth performance, nutrient digestibility, slaughter variables, blood biochemical parameters, and intestinal morphology of broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101732. [DOI] [PMC free article] [PubMed] [Google Scholar]