Abstract
Egg production is an important economic trait in layer ducks and understanding the genetics basis is important for their breeding. In this study, a genome-wide association study (GWAS) for egg production traits in 303 female Longyan Shan-ma ducks was performed based on a genotyping-by-sequencing strategy. Sixty-two single nucleotide polymorphisms (SNPs) associated with egg weight traits were identified (P < 9.48 × 10-5), including 8 SNPs at 5% linkage disequilibrium (LD)-based Bonferroni-corrected genome-wide significance level (P < 4.74 × 10-6). One hundred and nineteen SNPs were associated with egg number traits (P < 9.48 × 10-5), including 13 SNPs with 5% LD-based Bonferroni-corrected genome-wide significance (P < 4.74 × 10-6). These SNPs annotated 146 target genes which contained known candidate genes for egg production traits, such as prolactin and prolactin releasing hormone receptor. This study identified that these associated genes were significantly enriched in egg production-related pathways (P < 0.05), such as the oxytocin signaling, MAPK signaling, and calcium signaling pathways. It was notable that 18 genes were differentially expressed in ovarian tissues between higher and lower egg production in Shan-ma ducks. The identified potential candidate genes and pathways provide insight into the genetic basis underlying the egg production trait of layer ducks.
Key words: duck, GWAS, egg weight, egg number, candidate gene
INTRODUCTION
Ducks are the second most popular poultry after chickens. In layer ducks, egg production is an important economic trait because it determines the economic income of poultry farmers (Bello et al., 2022). Egg production traits including the age at first egg (AFE), egg weight (EW), number of eggs laid (EN) and egg production rate (EPR) are influenced by genetic and other factors (Xu et al., 2022). For traits that are genetically determined, heritability of EW and EN traits ranged from medium to high, and AFE had low heritability (Lin et al., 2016; Xu et al., 2022). In poultry, associated genetic markers can be used to dissect and quantify genetic variations in egg production traits (Yuan et al., 2015). Over the past 20 years, genome‐wide association studies (GWAS) have been effective and widely used methods for dissecting the genomic variants associated with traits (Tan et al., 2023) have revealed the genetic basis for egg production traits in poultry, including chickens (Liu et al., 2011; Wolc et al., 2012; Wolc et al., 2014; Yi et al., 2015; Yuan et al., 2015; Zhang et al., 2015b; Psifidi et al., 2016; Fan et al., 2017; Pértille et al., 2017; Azmal et al., 2019; Kudinov et al., 2019; Liu et al., 2019; Azmal et al., 2020; KARACAÖREN, 2020; Khaltabadi Farahani et al., 2020; Lien et al., 2020; Tarsani et al., 2020; Tarsani et al., 2021; Gao et al., 2022; Khaltabadi Farahani et al., 2022; Wang et al., 2022; Fu et al., 2023; Chen et al., 2024; Ma et al., 2024; Yang et al., 2024), goose (Zhao et al., 2020; Gao et al., 2021) and ducks (Liu et al., 2021; Xu et al., 2022). It can be seen that the number of studies of GWAS in chicken egg production traits is the largest, where more than 500 genes for chicken egg production traits have been identified in the above-mentioned GWAS studies. Through GWAS, different potential candidate genes for egg production traits were identified in different chicken populations, hens, broilers, meat-and-egg or crossbred chicken, such as PRKAR2B, HMGA2, LEMD3, GRIP1, EHBP1, MAP3K7, MYH (Khaltabadi Farahani et al., 2022), AR, YIPF6, and STARD8 for EW (Ma et al., 2024), GDF15, BHLHE40, JUND, GDF3, COMP, ITPR1, ELF3, ELL, CRLF1 and IFI30 (Tarsani et al., 2020), and NEO1, ADPGK, and CYP11A1 (Fu et al., 2023) for EN. Up to now, more than 580 quantitative trait loci (QTLs) for the EN, EW, AFE and EPR traits in chicken have been deposited in the Animal QTL database (Hu et al., 2022).
One study in ducks showed that there were only 4 single nucleotide polymorphisms (SNPs) on chromosome 3 significantly associated with EW were identified using 330 F2 ducks produced by reciprocal crosses of mallards and Peking ducks with 9,584,532 SNPs (Liu et al., 2021). In another GWAS for egg production traits in laying ducks, 12 SNPs for AFE and 17 SNPs for EW43 on chromosome 25, and 9 SNPs on chromosome 2 and 3 SNPs on chromosome 29 for EW66 were found using 166 Shaoxing ducks with 6,746,746 SNPs (Xu et al., 2022). Some genes for these 3 traits were identified, including GRIK4, ARHGEF12, ACAD8, THYN1, CA2, and GAMT. Research using GWAS for egg production in ducks was relatively scarce, confirming a need to elucidate the candidate genes and genetic mechanisms.
The Longyan Shan-ma duck is the most popular breed of laying ducks in China, with over 300 million of these ducks are raised in southern China (Xia et al., 2015; Xia et al., 2022). Based on the information from the Ministry of Agriculture and Rural Affairs of the People's Republic of China selected and released 10 excellent agricultural germplasm resources for 2022 were selected and released (https://www.moa.gov.cn/xw/zwdt/202305/t20230522_6428124.htm).The breed is a local breed with one of the highest egg productions in the world. The average laying EN more than was 330 at 72 weeks in Longyan Shan-ma duck of the high yield line (Sun et al., 2020a). This study used a genotyping by sequencing (GBS)-based GWAS assay to identify candidate genes and signal pathways for egg production traits (EN and EW) in the Longyan Shan-ma duck, which will provide an understanding of the potential genetic basis for egg production traits in layer ducks.
MATERIALS AND METHODS
Ducks and Phenotypes
This study was approved by the Longyan University Ethics Committee. The animal trial was carried in according to the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China).
Longyan Shan-ma female ducks of the fourth-generation high yield line were raised at the Longyan Shan-ma Duck Original Breeding Farm, as described in previous studies (Sun et al., 2023a,b). Birds were incubated at the same time, the starting and growing periods of 20 individuals raised in a large cage and underwent the laying period in individual cages. A total of 288 individuals with complete egg production records were used in this study. From 19 weeks to 44 weeks, the egg number of each individual was recorded as EN19 to EN44. Egg numbers in different stages were calculated as EN20 to EN24, EN25 to EN29, EN30 to EN34, EN35 to EN39, and EN40 to EN44. The egg weight at 23, 29, 33, 37, 40, and 43 weeks of age was based on the weight of the eggs collected in 5 days. Average egg weight (EWA) and total egg weight (EWT) were based on above-mentioned egg number and egg weight traits.
Genomic Sequencing, Quality Control, and Imputation
Genomic sequencing, quality control, and imputation have been described elsewhere (Sun et al., 2023a, 2023b). Briefly, the blood of each duck was sampled from the brachial vein at the wing using citrate-based anticoagulant syringes. The concentration and quality of each individual genomic DNA (gDNA) was extracted by the traditional phenol-chloroform method and was used for the GBS-based library construction. The DNA Libraries were sequenced as 150 bp and paired-end on an Illumina Novaseq platform (Illumina, Inc., CA). After removing adapters, poly-N reads and low-quality reads from raw data, clean data were obtained and aligned to the duck reference genome ZJU1.0 (https://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/015/476/345/GCF_015476345.1_-ZJU1.0/GCF_015476345.1_ZJU1.0_genomic.fna.gz) using Burrows-Wheeler Aligner software (Version bwa-0.7.17) (Li and Durbin, 2009). With the parameters as “-q 1 -C 50 -t AD, DP -m 2 -F 0.002,” the raw SNP sets were called by SAMtools software (Version 1.10) (Li et al., 2009), and SNPs further annotated using ANNOVAR tool (Wang et al., 2010).
Quality control of samples and SNPs were performed using Plink (version 1.9) (Renteria et al., 2013) through excluding them for failing to meet one or more of the following conditions including: sample call rate less than 80%, SNP call rate less than 90%, minor allele frequency (MAF) less than 5%, Hardy-Weinberg equilibrium test P < 10-6, and SNPs on the Z chromosome. Missing genotypes of SNPs were then imputed using Beagle software (version 5.4: 22Jul22.46e) (Browning et al., 2018) with the default parameter.
Genetic Parameter Estimation
Based on the genetic relationship matrix between pairs of samples, genetic parameters of egg production traits, SNP-based heritability (h2) and genetic correlation (rg), were estimated with restricted maximum likelihood and bivariate genome-based restricted maximum likelihood analysis methods using GCTA software (Version gcta-1.94.1-linux-kernel-3-x86_64) (Yang et al., 2013). The phenotypic correlations (rp) were calculated using IBM SPSS Statistics 19.0 software (IBM Corp., Armonk, NY). Plots of phenotypic and genetic correlation were drawn with by “corrplot” package in R software (v4.2.3).
Association Analysis
SNPs-trait association analysis was performed using an univariate linear mixed model (LMM) based on all SNPs in autosomes using the GEMMA software, v0.98.4 (Zhou and Stephens, 2012). The same software was used to calculate the centered kinship matrix (K) based on all SNPs in autosomes using GEMMA v0.98.4 software. The statistical model was calculated as:
where was phenotypic values, was the common mean, was the kinship matrix, was the effect of the SNP and was the random residual. The trait values were treated as numerical values. The significance of the associations was determined with a Wald test. Based on the previous principal components (PCs) analysis for the population of ducks (Sun et al., 2023b), the corresponding number of PCs were not included in the model. Circle Manhattan plots and Quantile-Quantile (QQ) plots for the GWAS results were generated with the CMplot package (https://cran.r-project.org/web/packages/CMplot/index.html) using R software version 4.2.3.
Function Enrichment Analyses of Identified Associated Genes
Gene Ontology (GO) term and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses for our GWAS identified egg production trait associated genes were performed using the KOBAS web-based software (Version 3.0) (Bu et al., 2021).
Expression Levels of Identified Associated Genes in Ovarian Tissue Between Higher and Lower Laying Ducks
Expression levels of egg production traits-associated genes in ovarian tissue between higher and lower laying ducks were in Longyan Shan-ma ducks (Sun et al., 2020c) and Shan-ma ducks (Chang et al., 2024). Longyan Shan-ma ducks were obtained from the Longyan Shan-ma Duck Original Breeding Farm (Longyan, China), and Shan-ma ducks were raised in Zhoukou Guiliu Breeding Duck Breeding Co., Ltd (Zhoukou, China). The egg productions of higher and lower laying ducks had significant differences in these ducks (Longyan Shan-ma ducks, 360 ± 13 VS 300 ± 13, from first laying to 497 days; Shan-ma ducks, 215 ± 8 VS 159 ± 21, from 120 days to 350 days). The detailed information for egg productions, RNA extraction and sequencing were descripted in previous studies (Sun et al., 2020c; Chang et al., 2024). The RNA-seq raw data were obtained from National Center for Biotechnology Information (NCBI, accession number: PRJNA601181) and China National Center for Bioinformation (CNCB, accession number: PRJCA016268). Clean Data were acquired from raw data after removing adapter and low-quality reads using Fast QC software (Version 0.12.0, website: https://github.com/s-andrews/FastQC). Then clean data were mapped to the duck reference genome (ZJU1.0) using HISAT software (Version 2.2.1) (Kim et al., 2015; Zhang et al., 2021). Expression level of each gene in each sample were obtained using StringTie software (Version 2.2.0) (Pertea et al., 2015). Differentially expressed genes (DEGs) were determined using DESeq2 package (Love et al., 2014). Significant DEGs were obtained with the genes fold change |log2fold change | equal to or exceeding 1, and P < 0.05.
RESULTS
Phenotype and Genotype Data
Total 40 EW and EN traits were recorded and calculated, and the descriptive statistics are shown in Table 1. The EWA and EN44 of Longyan Shan-ma duck was 65.59 g and 155.18, respectively. Relatively large variations were found in EWT and EN traits, whit coefficient of variation (C.V.) values ranging from 13.75 to 31.80.
Table 1.
Descriptive statistics and heritability of egg weight and egg number traits.
| Trait | N | Mean | SD | Min | Max | C.V. | h2 |
|---|---|---|---|---|---|---|---|
| EW20 (g) | 282 | 56.84 | 4.74 | 42.72 | 71.10 | 8.34 | 0.18 ± 0.11 |
| EW23 (g) | 285 | 60.38 | 4.62 | 42.20 | 74.62 | 7.65 | 0.09 ± 0.07 |
| EW29 (g) | 283 | 65.79 | 4.81 | 50.68 | 80.80 | 7.31 | 0.15 ± 0.10 |
| EW33 (g) | 281 | 68.20 | 4.94 | 48.65 | 86.00 | 7.25 | 0.07 ± 0.08 |
| EW37 (g) | 286 | 68.98 | 4.55 | 52.46 | 81.06 | 6.59 | 0.31 ± 0.11 |
| EW40 (g) | 277 | 69.24 | 4.84 | 51.36 | 81.68 | 6.99 | 0.19 ± 0.10 |
| EW43 (g) | 274 | 70.12 | 4.68 | 56.12 | 83.60 | 6.68 | 0.23 ± 0.12 |
| EWA (g) | 288 | 65.59 | 3.94 | 53.03 | 78.53 | 6.01 | 0.21 ± 0.11 |
| EWT (kg) | 288 | 10.20 | 1.65 | 4.92 | 13.66 | 16.19 | 0.10 ± 0.07 |
| EN19 | 288 | 4.07 | 1.29 | 0 | 5 | 31.8 | 0.03 ± 0.06 |
| EN20 | 288 | 9.95 | 2.78 | 0 | 12 | 27.96 | 0.02 ± 0.06 |
| EN21 | 288 | 16.10 | 3.92 | 0 | 19 | 24.32 | 0.08 ± 0.08 |
| EN22 | 287 | 22.40 | 4.76 | 2 | 26 | 21.25 | 0.08 ± 0.08 |
| EN23 | 287 | 28.55 | 5.55 | 4 | 33 | 19.42 | 0.08 ± 0.08 |
| EN24 | 287 | 34.61 | 6.38 | 5 | 40 | 18.44 | 0.11 ± 0.09 |
| EN25 | 287 | 40.77 | 7.08 | 7 | 47 | 17.37 | 0.13 ± 0.09 |
| EN26 | 287 | 46.85 | 7.82 | 14 | 54 | 16.68 | 0.13 ± 0.09 |
| EN27 | 287 | 52.70 | 8.70 | 19 | 61 | 16.51 | 0.14 ± 0.10 |
| EN28 | 288 | 58.54 | 9.66 | 23 | 68 | 16.50 | 0.13 ± 0.10 |
| EN29 | 287 | 64.60 | 10.25 | 26 | 75 | 15.86 | 0.08 ± 0.08 |
| EN30 | 286 | 70.84 | 10.75 | 32 | 82 | 15.17 | 0.04 ± 0.07 |
| EN31 | 287 | 76.77 | 11.81 | 29 | 89 | 15.38 | 0.02 ± 0.06 |
| EN32 | 287 | 82.81 | 12.53 | 32 | 96 | 15.14 | 0.00 ± 0.05 |
| EN33 | 287 | 88.76 | 13.30 | 37 | 103 | 14.98 | 0.00 ± 0.05 |
| EN34 | 287 | 94.74 | 13.98 | 41 | 110 | 14.75 | 0.00 ± 0.06 |
| EN35 | 287 | 100.64 | 14.52 | 48 | 117 | 14.43 | 0.00 ± 0.06 |
| EN36 | 287 | 106.63 | 15.15 | 55 | 124 | 14.21 | 0.00 ± 0.06 |
| EN37 | 287 | 112.74 | 15.73 | 62 | 131 | 13.95 | 0.00 ± 0.06 |
| EN38 | 287 | 118.99 | 16.37 | 65 | 138 | 13.75 | 0.00 ± 0.06 |
| EN39 | 288 | 125.07 | 17.38 | 61 | 145 | 13.89 | 0.00 ± 0.05 |
| EN40 | 288 | 131.20 | 18.11 | 66 | 152 | 13.81 | 0.00 ± 0.05 |
| EN41 | 288 | 137.31 | 18.98 | 72 | 159 | 13.82 | 0.00 ± 0.05 |
| EN42 | 288 | 143.42 | 19.95 | 76 | 166 | 13.91 | 0.00 ± 0.06 |
| EN43 | 288 | 149.17 | 20.95 | 81 | 173 | 14.04 | 0.00 ± 0.06 |
| EN44 | 288 | 155.18 | 21.89 | 81 | 180 | 14.11 | 0.00 ± 0.06 |
| EN20_24 | 287 | 30.53 | 5.78 | 1 | 35 | 18.93 | 0.09 ± 0.08 |
| EN25_29 | 287 | 30.06 | 6.13 | 6 | 35 | 20.38 | 0.03 ± 0.06 |
| EN30_34 | 287 | 30.16 | 6.59 | 3 | 35 | 21.86 | 0.00 ± 0.05 |
| EN35_39 | 287 | 30.66 | 6.32 | 5 | 35 | 20.61 | 0.00 ± 0.06 |
| EN40_44 | 287 | 30.22 | 7.47 | 0 | 35 | 24.73 | 0.00 ± 0.06 |
For the genotype sequencing quality of gDNA samples, read quality scores of the Q20 (1 error in 100) were more than or equal to 93.89%, and Q30 (1 error in 1000) ranged from 84.38% to 91.39% in Table S1. The mapping rate of clear data are not less than 96.88% in Table S2, showing that the sequencing quality and mapping rate of genotype data could meet the subsequent research needs.
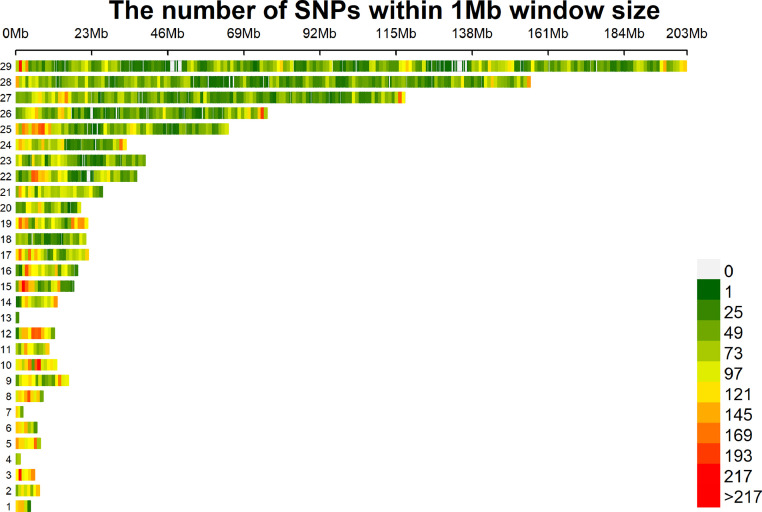
After quality control of samples and SNPs, 62,266 SNPs were distributed on 1 to 29 chromosomes in an SNP density plot (Figure 1), and 303 female ducks were used for further study. A genome-wide significance and suggestive significance threshold of 4.79 × 10-6 (0.05/10,428) and 9.59 × 10-5 (1/10,428) respectively, were determined using 10,428 independent SNPs based Bonferroni correction to reduce false positive probability, with detail described in the previous studies (Sun et al., 2013; Sun et al., 2023a).
Figure 1.
SNP density plot.
The heritability of egg weight and egg number traits varied from 0.00 ± 0.05 to 0.31 ± 0.11 (Table 1). Positive phenotypic correlations were found amongst EW traits and EN traits (Figure S1). Strong positive genetic correlations were identified amongst most egg weight traits, and egg number traits, such as EW20 and EW23, EN23 and EN29 (Figure S2).
GWAS Results
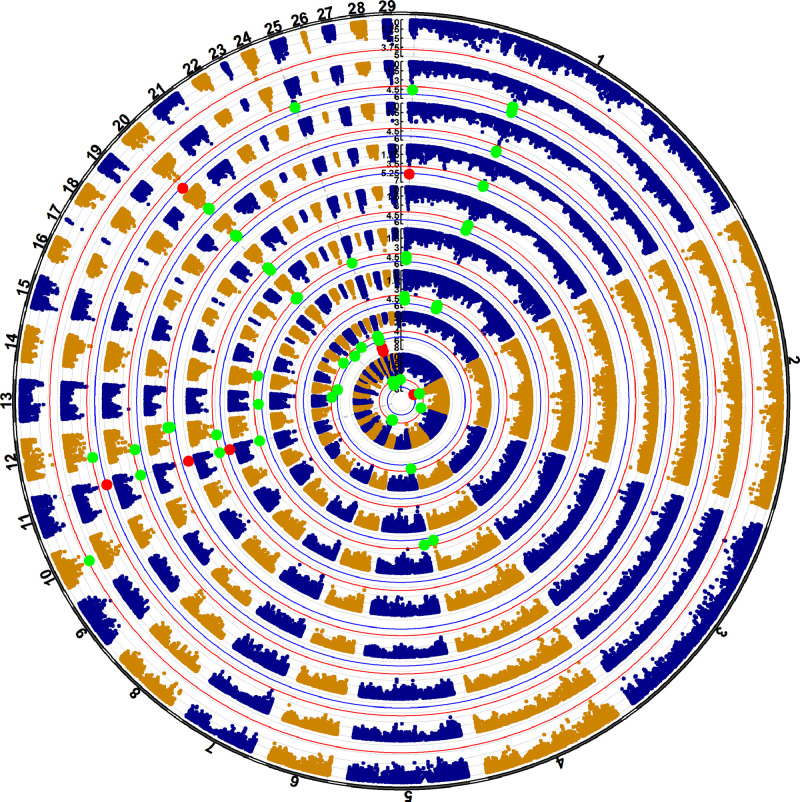
QQ and Manhattan plots were used to visualize the GWAS results. It can be seen from the QQ plots that there were no obvious statistic deviates of the observed distribution from the expected distribution of all traits GWAS results (Figure S3). One hundred and 2 associations were identified for egg weight traits, distributed on chromosomes 1–5, 7, 10–14, 18, 20, 21, 24, 25, 29, seen in Figure 2 and Table S3. These associations contained 10 with Bonferroni-corrected genome-wide significance, and 92 with genome-wide suggestive significance. Ten genome-wide significance associations were involved with 8 SNPs distributed on chromosomes 1, 2, 11, 20, and 25, including chr1:204589614:A>C, chr1:204589589:A>G, chr2:158970332:G>C, chr11:12633344:A>G, chr20:1616158:C>T, chr25:568645:C>T, chr25:604153:C>T, and chr25:634286:G>A. There were 92 genome-wide suggestive significance associations involved with 59 SNPs and their 44 genes (Table S3). Previous studies have shown that some gene functions were related to follicular and ovarian development, such as HYDIN axonemal central pair apparatus protein (HYDIN) (Chen et al., 2023), collagen type I alpha 2 chain (COL1A2) (Zhou et al., 2020), FTO alpha-ketoglutarate dependent dioxygenase (FTO) (Yang et al., 2012; Zang et al., 2023), and forkhead box L1 (FOXL1) (Uhlenhaut and Treier, 2011).
Figure 2.
Circular-Manhattan plot for egg weight traits. From inner circle to outer circle, EW20, EW23, EW29, EW33, EW37, EW40, EW43, EWA, and EWT.
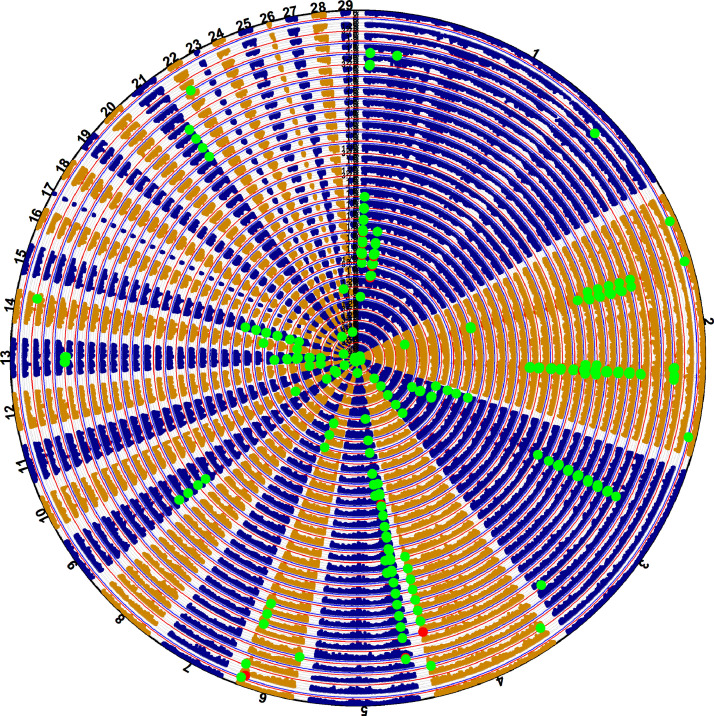
Forty hundred and fourteen associations were associated with egg number traits (Figure 3), including 17 Bonferroni-corrected genome-wide significance associations, and 397 genome-wide suggestive significance associations, distributed on chromosomes 1–7, 9, 10, 12–15, 21, 22, 25, 27 (Table S4). These genome-wide significance and suggestive associations were involved in 119 SNPs and their 98 nearby genes. For these genes, some of them were the known candidate genes for egg production traits, such as prolactin (PRL) (Wang et al., 2011; Zhang et al., 2015a; Chuekwon and Boonlum, 2017; Bai et al., 2019) and prolactin releasing hormone receptor (PRLHR) (Liu et al., 2019) and some had functions are related to ovarian and follicular, such as forkhead box O1 (FOXO1), plakophilin 4 (PKP4), and protein kinase cGMP-dependent 1 (PRKG1) (Shi and LaPolt, 2003; Struk et al., 2019; Gad et al., 2020).
Figure 3.
Circular-Manhattan plot for egg number traits. From inner circle to outer circle, EN 19 to EN44, EN20 to EN24, EN25 to EN29, EN30 to EN34, EN35 to EN39, and EN40 to EN44.
Function Enrichment Analyses of Identified Associated Genes
The EW and EN traits associated genes identified by the GWAS were significantly enriched in 41 GO terms (corrected P-value < 0.05) (Table S5). In cellular component ontology, 43 genes were mainly enriched in cytoplasm, 43 genes in plasma membrane, 29 genes in nucleoplasm, 28 genes in integral component of membrane. A total of 49 genes were enriched in biological process ontology, such as 13 genes in positive regulation of transcription by RNA polymerase II, 9 in the Regulation of transcription by RNA polymerase II, and 9 genes in the positive regulation of transcription, DNA-templated. In molecular function ontology, 95 genes were enriched in protein binding.
Twenty-eight egg weight and egg number traits associated genes identified by this GWAS were significantly enriched in 32 pathways (P-value < 0.05) (Table S6). Fourteen genes including arginine vasopressin receptor 1B (AVPR1B), calcium/calmodulin dependent protein kinase IG (CAMK1G), COL1A2, FOXO1, glycine receptor alpha 1 (GLRA1), potassium inwardly rectifying channel subfamily J member 3 (KCNJ3), MYC proto-oncogene, bHLH transcription factor (MYC), nuclear factor of activated T cells 1 (NFATC1), neuromedin U receptor 2 (NMUR2), PRL, PRLHR, tachykinin receptor 2 (TACR2), transforming growth factor beta receptor 2 (TGFBR2), and vascular endothelial growth factor A (VEGFA) enriched in 8 pathways have been verified to play an important role in egg production in ducks, including AGE RAGE signaling pathway in diabetic complications (Yan et al., 2022), neuroactive ligand-receptor interaction (Yan et al., 2022; He et al., 2023; Chang et al., 2024), relaxin signaling pathway (Yan et al., 2022), oxytocin signaling pathway (Qiu et al., 2020; Lu et al., 2021), MAPK signaling pathway (Bao et al., 2020; Zou et al., 2020; Bello et al., 2021; Bhavana et al., 2022; Yan et al., 2022; He et al., 2023; Zhang et al., 2023; Xin et al., 2024), calcium signaling pathway (Tao et al., 2017; Bao et al., 2020; Sun et al., 2020b; Bello et al., 2021; He et al., 2023; Xin et al., 2024), PI3K-Akt signaling pathway (Bao et al., 2020; Ma et al., 2022; Yan et al., 2022; Zhang et al., 2023), and TGF-beta signaling pathway (Zhu et al., 2017; Zhang et al., 2023).
Egg Production Traits Associated Genes Ovarian Tissue Expression
Based on the regulatory role of the ovarian tissue in egg production in duck (Tao et al., 2017; Bao et al., 2020; Sun et al., 2020c; Bhavana et al., 2022; Chang et al., 2024), 18 egg production traits-associated genes obtained by this GWAS were differentially expressed between Shan-ma ducks with higher and lower egg production (Table 2). Seven up-regulated expressed genes included NMUR2, transmembrane protein 272 (TMEM272), GLRA1, N-deacetylase and N-sulfotransferase 4 (NDST4), PRL, anoctamin 4 (ANO4), and leucine rich repeat and fibronectin type III domain containing 5 (LRFN5). Eleve down-regulated expressed genes contained synaptotagmin 16 (SYT16), inositol polyphosphate-5-phosphatase A (INPP5A), coiled-coil domain containing 148 (CCDC148), LOC101796494, VEGFA, PRKG1, PKP4, Nik related kinase (NRK), TGFBR2, SPT3 homolog, SAGA and STAGA complex component (SUPT3H), and ADAM metallopeptidase with thrombospondin type 1 motif 2 (ADAMTS2).
Table 2.
Eighteen egg production traits associated genes differentially expressed in ovarian tissue between higher and lower Shan-ma ducks.
| Breed | Gene | Log2FoldChange | P value |
|---|---|---|---|
| Longyan Shan-ma | NMUR2 | 6.20 | 2.34 × 10-6 |
| Longyan Shan-ma | TMEM272 | 5.79 | 2.52 × 10-5 |
| Longyan Shan-ma | GLRA1 | 5.05 | 1.45 × 10-3 |
| Longyan Shan-ma | NDST4 | 1.75 | 4.40 × 10-4 |
| Shan-ma | PRL | 1.08 | 1.65 × 10-2 |
| Shan-ma | ANO4 | 1.06 | 6.97 × 10-3 |
| Shan-ma | LRFN5 | 1.01 | 5.98 × 10-3 |
| Shan-ma | SYT16 | −1.10 | 1.77 × 10-3 |
| Longyan Shan-ma | INPP5A | −1.10 | 4.30 × 10-2 |
| Longyan Shan-ma | CCDC148 | −1.10 | 6.68 × 10-3 |
| Longyan Shan-ma | LOC101796494 | −1.17 | 3.19 × 10-4 |
| Longyan Shan-ma | VEGFA | −1.17 | 3.40 × 10-2 |
| Longyan Shan-ma | PRKG1 | −1.22 | 1.12 × 10-2 |
| Longyan Shan-ma | PKP4 | −1.35 | 1.23 × 10-2 |
| Longyan Shan-ma | NRK | −1.42 | 3.09 × 10-2 |
| Longyan Shan-ma | TGFBR2 | −1.61 | 9.43 × 10-3 |
| Longyan Shan-ma | SUPT3H | −1.70 | 1.40 × 10-2 |
| Longyan Shan-ma | ADAMTS2 | −2.54 | 5.70 × 10-4 |
DISCUSSION
The EW and EN are significant production traits in ducks, and uncovering their genetic basis is crucial in selecting for breeding performance in layer duck production. In this study, 181 SNPs including 21 genome wide significant SNPs and 160 suggestive significant SNPs associated with egg weight and egg number traits in a Chinese famous layer duck population were identified using a univariate linear mixed model with GBS-based whole genome SNPs. A total of 146 associated genes of these 181 SNPs were identified. Some known candidate genes and pathways for egg weight and egg number traits were found, such as PRL and PRLHR and the oxytocin signaling, MAPK signaling, and calcium signaling pathways. Importantly, 30 associated genes revealed by this GWAS were differentially expressed in hypothalamic-pituitary-ovarian tissues between higher and lower egg production in laying ducks. These candidate genes and pathways provide insight into genetic basis underlying the egg production trait in ducks.
The SNP-based genetics parameters were estimated for egg weight and egg number traits in 303 layering ducks. The heritability of egg weight traits was low to high moderate at 0.70 to 0.31, and egg number traits have low heritability at 0.00 to 0.14. This study results were much lower than those reported for egg weight and egg number in Longyan Shan-ma ducks of previous studies (Lin et al., 2016; Sun et al., 2020a). The heritability estimation of the genetic parameters in this study used the SNPs-based pedigree, while the previous heritability estimation used the pedigree. This may be due to the fact that SNPs that are significantly associated with quantitative traits usually explain only a portion of the genetic variation, affecting heritability (Yang et al., 2017).
Potential candidate genes of egg weight and egg number traits were identified using transcriptome analysis, quantitative trait loci (QTL) mapping, and GWAS method (Bello et al., 2022), were used, with GWAS studies identifying fewer genes for these traits (Liu et al., 2021; Xu et al., 2022). For the egg weight traits, the functions of some genes identified by this GWAS were related to ovarian and follicular development. The gene HYDIN encoded the HYDIN axonemal central pair apparatus protein, whose function is related with duck follicular development, with upregulated expression between the small white follicle and the small yellow follicle (Chen et al., 2023). The gene COL1A2 encoded collagen type I alpha 2 chain. Comparing the 400-μm follicles with 800-μm follicles in chickens, COL1A2 was upregulated, influencing growing follicle development (Zhou et al., 2020). The FTO encoded a nuclear protein of the AlkB related nonhaem iron and 2-oxoglutarate-dependent oxygenase superfamily and variations of FTO were associated with body mass index in human (Yang et al., 2012) and carcass traits in ducks (Gan et al., 2015). The FTO-mediated m6A demethylation can regulates GnRH expression in the hypothalamus, affecting the function of the ovaries and follicles (Zang et al., 2023). The gene FOXL1 encodes a member of the forkhead/winged helix-box (FOX) family of transcription factors, which have key role in ovarian folliculogenesis (Uhlenhaut and Treier, 2011).
For the EN traits, some genes identified by this GWAS were known candidate genes for egg production traits, such as PRL and PRLHR, where PRL encoded prolactin that is mainly produced in the cephalic lobe of the anterior pituitary gland (Kansaku et al., 2005) and PRLHR encoded a prolactin releasing hormone receptor, also known as G-protein-coupled receptor 10, the receptor for prolactin releasing peptide (Su et al., 2015). Polymorphisms of the PRL and PRLHR genes associated with egg production traits have been investigated. One SNP 5961 C>T polymorphism of the PRL gene was significantly associated with egg production and egg weight in the F2 resource population of white Liancheng X white Kaiya (Wang et al., 2011). In a Muscovy duck population, SNPs T-884C and T-335C of PRL were significantly associated with the AFE, EN59 and egg number in ducks at age 300 d (Zhang et al., 2015a). In Khaki Campbell ducks, the 359 C>A polymorphism in intron1 of the PRL gene was associated with egg production at 300 days of age (Chuekwon and Boonlum, 2017). In 2 Chinese domestic laying duck populations (Jinding and Youxian), the 412A > G polymorphism of the PRL gene in intron 1 was associated with egg production and egg weight traits (Bai et al., 2019). In a hen population, 13 SNPs of the PRLHR gene located on chromosome 6 associated with AFE were detected (Liu et al., 2019).
Some genes associated with egg number traits identified by this GWAS, were related to ovarian and follicular. The gene FOXO1 belongs to the fork-head family of transcription factors, whose levels regulated expression in ovarian follicular development, atresia and luteinization physiological processes (Shi and LaPolt, 2003). The gene PKP4 encoded plakophilin 4, which may be a component of desmosomal plaques and other adhesion plaques. Compared to large oocytes, PKP4 was highly expressed in small oocytes in pigs (Gad et al., 2020) and PRKG1 encoded protein kinase cGMP-dependent 1, a self-regulation and the foraging gene (Struk et al., 2019). PRKG1 was expressed at an upregulated level in the ovarian tissue of Xinjiang Yili geese with high egg production (Wu et al., 2020).
Based on the above studies, many associated genes of egg weight and egg number traits identified by this GWAS have functions related to the development of the ovaries and oviducts. Some genes were enriched in egg production-related signaling pathways in ducks, such as FOXO1 and COL1A2 in AGE RAGE signaling pathway in diabetic complications, and COL1A2 and PRL in the PI3K-Akt signaling pathway. These genes can be used as important potential candidate genes for duck egg production traits, needing further study.
Egg production traits may have similar genetics basis between ducks and chickens. Previous GWAS studies identified associated genes for egg production traits in chickens were listed in Table S7. Of 316 genes, only 9 genes were common genes for egg production traits both in ducks of this study and in chickens of previous studies, including CCDC148, deleted in lymphocytic leukemia 7 (DLEU7) (Liu et al., 2018), family with sequence similarity 204 member A (FAM204A) (Liu et al., 2019), FOXO1 (Yi et al., 2015), glycerol-3-phosphate dehydrogenase 2 (GPD2) (Ma et al., 2024), HYDIN (Zhao et al., 2021), musashi RNA binding protein 2 (MSI2) (Ma et al., 2024), nemo like kinase (NLK) (Ma et al., 2024), neurensin 1 (NRSN1) (Gao et al., 2022), nuclear receptor subfamily 4 group A member 2 (NR4A2) (Ma et al., 2024), and PRLHR (Liu et al., 2019). Although there were fewer common potential candidate genes for egg production traits in ducks and chickens identified through GWAS studies, their potential candidate genes were enriched in 8 same pathways, containing neuroactive ligand-receptor interaction, calcium signaling, cellular senescence, MAPK signaling, TGF-beta signaling, phosphatidylinositol signaling system, vascular smooth muscle contraction, and adherens junction (Table S6 and S8). These results suggested that there are both similarities and differences in the genetic basis of egg production traits between ducks and chickens.
Because the cis-SNPs can regulate their target gene expression (Kim et al., 2012; Zeng et al., 2017), this study has been confirmed that the SNPs identified by the GWAS may affect the egg production traits by regulating the expression of their nearby genes. Seven up-regulated (NMUR2, TMEM272, GLRA1, NDST4, PRL, ANO4, LRFN5), and 11 down-regulated (SYT16, INPP5A, CCDC148, LOC101796494, VEGFA, PRKG1, PKP4, NRK, TGFBR2, SUPT3H, and ADAMTS2) expressed genes in ovarian tissue adjacent to the SNP associated with the egg production traits were identified between Shan-ma ducks with higher and lower egg production, such as SNP chr6:924673:T>G located at intron region of gene PRKG1 on chromosome 6, and which was more decreased expressed in ovarian tissue with the individuals having a high egg-laying number. This may be due to the cis-acting effect of this SNP to regulate gene PRKG1 expression, resulting in increased the egg production. These 18 DEGs in ovarian tissue of Shan-ma ducks may have key regulating role in egg production traits, especially functions of some genes were concerned with the development of the ovaries and follicles, such as PRL, PKP4, and PRKG1. These candidate genes associated with the egg production traits of ducks require further examination.
CONCLUSIONS
A GWAS based on GBS strategy were carried out to identify the associations between SNPs and egg production traits EW and EN in Longyan Shan-ma ducks. For EW traits, 8 genome-wide significant and 54 suggestive SNPs were detected. For EN traits, 119 SNPs including 13 genome-wide significant SNPs were found. These SNPs were harbored to 146 target genes that significantly enriched in egg production-related pathways, such as oxytocin signaling, MAPK signaling, and calcium signaling pathways. These associated genes contained known candidate genes for egg production traits, such as PRL and PRLHR. Eighteen genes were differentially expressed in the ovarian tissue between higher and lower egg production in Shan-ma ducks. This study will provide insight into the genetic basis underlying the egg production trait in ducks.
DISCLOSURES
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
This study is supported by the Foreign Cooperation Projects of Fujian Provincial Science and Technology Department (2021I0045), Key Research and Breeding Projects and Industrialization Foundations of Agricultural Improved Breeding in Breed Industry Innovation and Industrialization Project (2021–2025) of Fujian Province (zycxny20211014), and Science and Technology Department Leading Project of Fujian Province (2022N0027). We also thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.104032.
Appendix. Supplementary materials
References
- Azmal S.A., Bhuiyan A.A., Omar A.I., Ma S., Sun C., Han Z., Zhang M., Zhao S., Li S. Novel polymorphisms in RAPGEF6 gene associated with egg-laying rate in Chinese Jing Hong chicken using genome-wide SNP scan. Genes. 2019;10:384. doi: 10.3390/genes10050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmal S.A., Nan J., Bhuiyan A.A., Elokil A.A., Ali M.I., Adetula A.A., Ma S., Sun C., Han Z., Yuan J. A genome-wide single nucleotide polymorphism scan reveals genetic markers associated with fertility rate in Chinese Jing Hong chicken. Poult. Sci. 2020;99:2873–2887. doi: 10.1016/j.psj.2019.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D.-P., Hu Y.-Q., Li Y.-B., Huang Z.-B., Li A. Polymorphisms of the prolactin gene and their association with egg production traits in two Chinese domestic ducks. Br. Poult. Sci. 2019;60:125–129. doi: 10.1080/00071668.2019.1567909. [DOI] [PubMed] [Google Scholar]
- Bao X., Song Y., Li T., Zhang S., Huang L., Zhang S., Cao J., Liu X., Zhang J. Comparative transcriptome profiling of ovary tissue between black muscovy duck and white muscovy duck with high-and low-egg production. Genes. 2020;12:57. doi: 10.3390/genes12010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello S.F., Adeola A.C., Nie Q. The study of candidate genes in the improvement of egg production in ducks - a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello S.F., Xu H., Guo L., Li K., Zheng M., Xu Y., Zhang S., Bekele E.J., Bahareldin A.A., Zhu W., Zhang D., Zhang X., Ji C., Nie Q. Hypothalamic and ovarian transcriptome profiling reveals potential candidate genes in low and high egg production of white Muscovy ducks (Cairina moschata) Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavana K., Foote D.J., Srikanth K., Balakrishnan C.N., Prabhu V.R., Sankaralingam S., Singha H.S., Gopalakrishnan A., Nagarajan M. Comparative transcriptome analysis of Indian domestic duck reveals candidate genes associated with egg production. Sci. Rep. 2022;12:10943. doi: 10.1038/s41598-022-15099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning B.L., Zhou Y., Browning S.R. A one-penny imputed genome from next-generation reference panels. Am. J. Hum. Genet. 2018;103:338–348. doi: 10.1016/j.ajhg.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu D., Luo H., Huo P., Wang Z., Zhang S., He Z., Wu Y., Zhao L., Liu J., Guo J., Fang S., Cao W., Yi L., Zhao Y., Kong L. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49:W317–W325. doi: 10.1093/nar/gkab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Guo R., Zeng T., Sun H., Tian Y., Han X., Cao Y., Xu L., Duan M., Lu L. Analysis of transcriptomic differences in the ovaries of high-and low-laying ducks. Genes. 2024;15:181. doi: 10.3390/genes15020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wu Y., Wang J., Pi J. Transcription profiling analysis of follicles at different develop mental stages in duck ovaries. Res. Square. 2023 [Google Scholar]
- Chen X., Li X., Zhong C., Jiang X., Wu G., Li G., Yan Y., Yang N., Sun C. Genetic patterns and genome-wide association analysis of eggshell quality traits of egg-type chicken across an extended laying period. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuekwon K., Boonlum S. Association of prolactin gene with egg production in Khaki Campbell ducks. Walailak J. Sci. Tech. 2017;14:849–853. [Google Scholar]
- Fan Q., Wu P., Dai G., Zhang G., Zhang T., Xue Q., Shi H., Wang J. Identification of 19 loci for reproductive traits in a local Chinese chicken by genome-wide study. Genet. Mol. Res. 2017;16:1–8. doi: 10.4238/gmr16019431. [DOI] [PubMed] [Google Scholar]
- Fu M., Wu Y., Shen J., Pan A., Zhang H., Sun J., Liang Z., Huang T., Du J., Pi J. Genome-wide association study of egg production traits in shuanglian chickens using whole genome sequencing. Genes. 2023;14:2129. doi: 10.3390/genes14122129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad A., Nemcova L., Murin M., Kinterova V., Kanka J., Laurincik J., Benc M., Pendovski L., Prochazka R. Global transcriptome analysis of porcine oocytes in correlation with follicle size. Mol. Reprod. Dev. 2020;87:102–114. doi: 10.1002/mrd.23294. [DOI] [PubMed] [Google Scholar]
- Gan W., Song Q., Zhang N., Xiong X., Wang D., Li L. Association between FTO polymorphism in exon 3 with carcass and meat quality traits in crossbred ducks. Genet. Mol. Res. 2015;14:6699–6714. doi: 10.4238/2015.June.18.14. [DOI] [PubMed] [Google Scholar]
- Gao G., Gao D., Zhao X., Xu S., Zhang K., Wu R. Genome-wide association study-based identification of SNPs and haplotypes associated with goose reproductive performance and egg quality. Front Genet. 2021;12:360. doi: 10.3389/fgene.2021.602583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Xu W., Zeng T., Tian Y., Wu C., Liu S., Zhao Y., Zhou S., Lin X., Cao H. Genome-wide association study of egg-laying traits and egg quality in LingKun chickens. Front.Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.877739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Chen Q., Ouyang Q., Hu J., Shen Z., Hu B., Hu S., He H., Li L., Liu H. Transcriptomic analysis of the thyroid and ovarian stroma reveals key pathways and potential candidate genes associated with egg production in ducks. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z.-L., Park C.A., Reecy J.M. Bringing the animal QTLdb and CorrDB into the future: meeting new challenges and providing updated services. Nucleic Acids Res. 2022;50:D956–D961. doi: 10.1093/nar/gkab1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansaku N., Ohkubo T., Okabayashi H., Guémené D., Kuhnlein U., Zadworny D., Shimada K. Cloning of duck PRL cDNA and genomic DNA. Gen. Comp. Endocrinol. 2005;141:39–47. doi: 10.1016/j.ygcen.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Karacaören B. Functional and whole regression-based genome-wide association analyses for weightmeasurements of chicken eggs. Turk. J. Vet. Anim. Sci. 2020;44:9–16. [Google Scholar]
- Khaltabadi Farahani A., Mohammadi H., Moradi M., Ghasemi H. Identification of potential genomic regions for egg weight by a haplotype-based genome-wide association study using Bayesian methods. Br. Poult. Sci. 2020;61:251–257. doi: 10.1080/00071668.2020.1724879. [DOI] [PubMed] [Google Scholar]
- Khaltabadi Farahani A., Mohammadi H., Moradi M., Ghasemi H., Hajkhodadadi I. Genomic-wide association study for egg weight-related traits in Rhode Island Red breed using Bayesian methods. Ani. Prod. Res. 2022;11:41–53. [Google Scholar]
- Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Cho H., Lee D., Webster M.J. Association between SNPs and gene expression in multiple regions of the human brain. Transl. Psychiat. 2012;2:e113. doi: 10.1038/tp.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudinov A.A., Dementieva N.V., Mitrofanova O.V., Stanishevskaya O.I., Fedorova E.S., Larkina T.A., Mishina A.I., Plemyashov K.V., Griffin D.K., Romanov M.N. Genome-wide association studies targeting the yield of extraembryonic fluid and production traits in Russian White chickens. BMC Genom. 2019;20:1–12. doi: 10.1186/s12864-019-5605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., S. Genome Project Data Processing The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien C.-Y., Tixier-Boichard M., Wu S.-W., Chen C.-F. Identification of QTL and loci for egg production traits to tropical climate conditions in chickens. Livest. Sci. 2020;234 [Google Scholar]
- Lin R.L., Chen H.P., Rouvier R., Marie-Etancelin C. Genetic parameters of body weight, egg production, and shell quality traits in the Shan Ma laying duck (Anas platyrhynchos) Poult. Sci. 2016;95:2514–2519. doi: 10.3382/ps/pew222. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang L., Guo Z., Xu Q., Fan W., Xu Y., Hu J., Zhang Y., Tang J., Xie M. Genome-wide association and selective sweep analyses reveal genetic loci for FCR of egg production traits in ducks. Genet. Sel. Evol. 2021;53:1–16. doi: 10.1186/s12711-021-00684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li D., Liu J., Chen S., Qu L., Zheng J., Xu G., Yang N. A genome-wide SNP scan reveals novel loci for egg production and quality traits in white leghorn and brown-egg dwarf layers. PLoS One. 2011;6:e28600. doi: 10.1371/journal.pone.0028600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Sun C., Yan Y., Li G., Wu G., Liu A., Yang N. Genome-wide association analysis of age-dependent egg weights in chickens. Front. Genet. 2018;9:128. doi: 10.3389/fgene.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Yang N., Yan Y., Li G., Liu A., Wu G., Sun C. Genome-wide association analysis of egg production performance in chickens across the whole laying period. BMC Genet. 2019;20:1–9. doi: 10.1186/s12863-019-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M., Anders S., Huber W. Differential analysis of count data–the DESeq2 package. Genome Biol. 2014;15:10–1186. [Google Scholar]
- Lu, L., C. A. Asiamah, R. Ye, Y. Pan, P. Jiang, Y. Su, and Z. Zhao. 2021. Enrichment and verification of differentially expressed miRNAs in the ovarian tissue of Leizhou black ducks.
- Ma L., Yu X., Wang Z., Wu J., Li H., Huang L., Zhang J. Preliminary comparative transcriptome profiling of ovary tissue in white muscovy duck with high-and low-egg production. Genes. 2022;12:57. doi: 10.3390/genes12010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Ying F., Li Z., Bai L., Wang M., Zhu D., Liu D., Wen J., Zhao G., Liu R. New insights into the genetic loci related to egg weight and age at first egg traits in broiler breeder. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M., Pertea G.M., Antonescu C.M., Chang T.-C., Mendell J.T., Salzberg S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pértille F., Moreira G.C.M., Zanella R., Nunes J.d.R.d.S., Boschiero C., Rovadoscki G.A., Mourão G.B., Ledur M.C., Coutinho L.L. Genome-wide association study for performance traits in chickens using genotype by sequencing approach. Sci. Rep. 2017;7:41748. doi: 10.1038/srep41748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psifidi A., Banos G., Matika O., Desta T.T., Bettridge J., Hume D.A., Dessie T., Christley R., Wigley P., Hanotte O. Genome-wide association studies of immune, disease and production traits in indigenous chicken ecotypes. Genet. Sel. Evol. 2016;48:1–16. doi: 10.1186/s12711-016-0252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M., Zhang Z., Xiong X., Du H., Li Q., Yu C., Gan W., Liu H., Peng H., Xia B. High-throughput sequencing analysis identified microRNAs associated with egg production in ducks ovaries. PeerJ. 2020;8:e8440. doi: 10.7717/peerj.8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria M.E., Cortes A., Medland S.E. Using PLINK for Genome-Wide Association Studies (GWAS) and data analysis. Methods Mol. Biol. 2013;1019:193–213. doi: 10.1007/978-1-62703-447-0_8. [DOI] [PubMed] [Google Scholar]
- Shi F., LaPolt P. Relationship between FoxO1 protein levels and follicular development, atresia, and luteinization in the rat ovary. J. Endocrinology. 2003;179:195–203. doi: 10.1677/joe.0.1790195. [DOI] [PubMed] [Google Scholar]
- Struk A.A., Mugon J., Huston A., Scholer A.A., Stadler G., Higgins E.T., Sokolowski M.B., Danckert J. Self-regulation and the foraging gene (PRKG1) in humans. PNAS. 2019;116:4434–4439. doi: 10.1073/pnas.1809924116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q., Wang Y., Zhao J., Ma C., Wu T., Jin T., Xu J. Polymorphisms of PRLHR and HSPA12A and risk of gastric and colorectal cancer in the Chinese Han population. BMC Gastroenterol. 2015;15:1–5. doi: 10.1186/s12876-015-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Li Y., Lin R., Chen H., Wu Q., Li J., Chen Y., Lin Z. Estimation of genetic parameters for egg production and weight traits in Longyan Shan-Ma duck. Chinese J. Anim. Sci. 2020;56:51–55. [Google Scholar]
- Sun Y., Wu Q., Lin R., Chen H., Gan Q., Shen Y., Wang Y., Xu P., Chen F., Liu J. Genome-wide association study of egg quality traits in Longyan Shan-Ma duck. Scientia Agricult. Sinica. 2023;56:572–586. [Google Scholar]
- Sun Y., Wu Q., Lin R., Chen H., Zhang M., Jiang B., Wang Y., Xue P., Gan Q., Shen Y., Chen F., Liu J., Zhou C., Lan S., Pan H., Deng F., Yue W., Lu L., Jiang X., Li Y. Genome-wide association study for the primary feather color trait in a native Chinese duck. Front. Genet. 2023;14 doi: 10.3389/fgene.2023.1065033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wu Q., Pan J., Li T., Liu L., Chen D., Zhang X., Chen H., Li Y., Lin R. Identification of differentially expressed genes and signalling pathways in the ovary of higher and lower laying ducks. Br. Poult. Sci. 2020;61:609–614. doi: 10.1080/00071668.2020.1792834. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wu Q., Pan J., Li T., Liu L., Chen D., Zhang X., Chen H., Li Y., Lin R. Identification of differentially expressed genes and signalling pathways in the ovary of higher and lower laying ducks. Br. Poult. Sci. 2020;61:609–614. doi: 10.1080/00071668.2020.1792834. [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhao G., Liu R., Zheng M., Hu Y., Wu D., Zhang L., Li P., Wen J. The identification of 14 new genes for meat quality traits in chicken using a genome-wide association study. BMC Genom. 2013;14:458. doi: 10.1186/1471-2164-14-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., He Z., Fahey A.G., Zhao G., Liu R., Wen J. Research progress and applications of genome-wide association study in farm animals. AROH. 2023;1:56–77. [Google Scholar]
- Tao Z., Song W., Zhu C., Xu W., Liu H., Zhang S., Huifang L. Comparative transcriptomic analysis of high and low egg-producing duck ovaries. Poult. Sci. 2017;96:4378–4388. doi: 10.3382/ps/pex229. [DOI] [PubMed] [Google Scholar]
- Tarsani E., Kranis A., Maniatis G., Avendano S., Hager-Theodorides A.L., Kominakis A. Deciphering the mode of action and position of genetic variants impacting on egg number in broiler breeders. BMC Genom. 2020;21:1–12. doi: 10.1186/s12864-020-06915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsani E., Kranis A., Maniatis G., Hager-Theodorides A.L., Kominakis A. Detection of loci exhibiting pleiotropic effects on body weight and egg number in female broilers. Sci. Rep. 2021;11:7441. doi: 10.1038/s41598-021-86817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut N.H., Treier M. Forkhead transcription factors in ovarian function. Reproduction. 2011;142:489. doi: 10.1530/REP-11-0092. [DOI] [PubMed] [Google Scholar]
- Wang C., Liang Z., Yu W., Feng Y., Peng X., Gong Y., Li S. Polymorphism of the prolactin gene and its association with egg production traits in native Chinese ducks. S. Afr. J. Anim. Sci. 2011;41:63–69. [Google Scholar]
- Wang D.-d., Zhang Y.-y., Teng M.-l., Zhang W., Xu C.-l., Jiang K.-r., Zheng M., Li Z.-j., Tian Y.-d., Kang X.-t. Integrative analysis of hypothalamic transcriptome and genetic association study reveals key genes involved in the regulation of egg production in indigenous chickens. J. Integr. Agr. 2022;21:1457–1474. [Google Scholar]
- Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolc A., Arango J., Jankowski T., Dunn I., Settar P., Fulton J., O'sullivan N., Preisinger R., Fernando R., Garrick D. Genome-wide association study for egg production and quality in layer chickens. J. Anim. Breed. Genet. 2014;131:173–182. doi: 10.1111/jbg.12086. [DOI] [PubMed] [Google Scholar]
- Wolc A., Arango J., Settar P., Fulton J., O'sullivan N., Preisinger R., Habier D., Fernando R., Garrick D., Hill W. Genome-wide association analysis and genetic architecture of egg weight and egg uniformity in layer chickens. Anim. Genet. 2012;43:87–96. doi: 10.1111/j.1365-2052.2012.02381.x. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhao X., Chen L., Wang J., Duan Y., Li H., Lu L. Transcriptomic analyses of the hypothalamic-pituitary-gonadal axis identify candidate genes related to egg production in Xinjiang Yili geese. Animals. 2020;10:90. doi: 10.3390/ani10010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W.G., Abouelezz K.F.M., Makled M.N., Wang S., Chen W., Zhang Y.N., Elokil A.A., Wang S.L., Huang X.B., Li K.C., Zheng C.T. Effects of dietary substitution of peanut meal for soybean meal on egg production, egg quality, oxidative status, and yolk fatty acid profile in laying ducks. Animal. 2022;16 doi: 10.1016/j.animal.2022.100652. [DOI] [PubMed] [Google Scholar]
- Xia W.G., Zhang H.X., Lin Y.C., Zheng C.T. Evaluation of dietary calcium requirements for laying Longyan shelducks. Poult. Sci. 2015;94:2932–2937. doi: 10.3382/ps/pev281. [DOI] [PubMed] [Google Scholar]
- Xin Q., Li L., Zhao B., Shi W., Hao X., Zhang L., Miao Z., Zhu Z., Huang Q., Zheng N. The network regulation mechanism of the effects of heat stress on the production performance and egg quality of Jinding duck was analyzed by miRNA‒mRNA. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Wang Z., Qu Y., Li Q., Tian Y., Chen L., Tang J., Li C., Li G., Shen J., Tao Z., Cao Y., Zeng T., Lu L. Genome-wide association studies and haplotype-sharing analysis targeting the egg production traits in Shaoxing duck. Front. Gene.t. 2022;13 doi: 10.3389/fgene.2022.828884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Liu H., Hu J., Han X., Qi J., Ouyang Q., Hu B., He H., Li L., Wang J., Zeng X. Transcriptomic analyses of the HPG axis-related tissues reveals potential candidate genes and regulatory pathways associated with egg production in ducks. BMC Genom. 2022;23:281. doi: 10.1186/s12864-022-08483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Lee S.H., Goddard M.E., Visscher P.M. Humana Press; Totowa, NJ: 2013. Genome-wide complex trait analysis (GCTA): methods, data analyses, and interpretations. [DOI] [PubMed] [Google Scholar]
- Yang J., Loos R.J., Powell J.E., Medland S.E., Speliotes E.K., Chasman D.I., Rose L.M., Thorleifsson G., Steinthorsdottir V., Mägi R. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490:267–272. doi: 10.1038/nature11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zeng J., Goddard M.E., Wray N.R., Visscher P.M. Concepts, estimation and interpretation of SNP-based heritability. Nat. Genet. 2017;49:1304–1310. doi: 10.1038/ng.3941. [DOI] [PubMed] [Google Scholar]
- Yang S., Ning C., Yang C., Li W., Zhang Q., Wang D., Tang H. Identify candidate genes associated with the weight and egg quality traits in Wenshui Green Shell-laying chickens by the copy number variation-based genome-wide association study. Vet. Sci. 2024;11:76. doi: 10.3390/vetsci11020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G., Shen M., Yuan J., Sun C., Duan Z., Qu L., Dou T., Ma M., Lu J., Guo J. Genome-wide association study dissects genetic architecture underlying longitudinal egg weights in chickens. BMC Genom. 2015;16:1–14. doi: 10.1186/s12864-015-1945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Sun C., Dou T., Yi G., Qu L., Qu L., Wang K., Yang N. Identification of promising mutants associated with egg production traits revealed by genome-wide association study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang S., Yin X., Li P. FTO-mediated m6A demethylation regulates GnRH expression in the hypothalamus via the PLCβ3/Ca2+/CAMK signalling pathway. Commun. Biol. 2023;6:1297. doi: 10.1038/s42003-023-05677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng P., Zhou X., Huang S. Prediction of gene expression with cis-SNPs using mixed models and regularization methods. BMC Genom. 2017;18:1–11. doi: 10.1186/s12864-017-3759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Xu Z., He J., Ji C., Zhang Y., Zhang X. Polymorphisms in the 5′-flanking regions of the GH, PRL, and Pit-1 genes with Muscovy duck egg production. J. Anim. Sci. 2015;93:28–34. doi: 10.2527/jas.2014-8071. [DOI] [PubMed] [Google Scholar]
- Zhang G., Fan Q., Wang J., Zhang T., Xue Q., Shi H. Genome-wide association study on reproductive traits in Jinghai Yellow Chicken. Anim. Reprod. Sci. 2015;163:30–34. doi: 10.1016/j.anireprosci.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Zhang L., Xie J., Sun G., Ji R., Li X., Zhang X., Wang J. Identification of differentially expressed genes and signaling pathways in Gaoyou duck ovary at different physiological stages. Front. Vet Sci. 2023;10 doi: 10.3389/fvets.2023.1190998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Park C., Bennett C., Thornton M., Kim D. Rapid and accurate alignment of nucleotide conversion sequencing reads with HISAT-3N. Genome Res. 2021;31:1290–1295. doi: 10.1101/gr.275193.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Chen J., Lin W., Xie Q. Genome-wide association analysis reveals key genes responsible for egg production of lion head goose. Front. Genet. 2020;10 doi: 10.3389/fgene.2019.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Nie C., Zhang J., Li X., Zhu T., Guan Z., Chen Y., Wang L., Lv X.Z., Yang W. Identification of candidate genomic regions for chicken egg number traits based on genome-wide association study. BMC Genom. 2021;22:610. doi: 10.1186/s12864-021-07755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Ma Y., Zhao D., Mi Y., Zhang C. Transcriptome profiling analysis of underlying regulation of growing follicle development in the chicken. Poult. Sci. 2020;99:2861–2872. doi: 10.1016/j.psj.2019.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012;44:821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Miao Z., Chen H., Xin Q., Li L., Lin R., Huang Q., Zheng N. Ovarian transcriptomic analysis of Shan Ma ducks at peak and late stages of egg production. Asian. Austral. J. Anim. 2017;30:1215. doi: 10.5713/ajas.16.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Asiamah C.A., Lu L.-l., Liu Y., Pan Y., Chen T., Zhao Z., Su Y. Ovarian transcriptomic analysis and follicular development of Leizhou black duck. Poult. Sci. 2020;99:6173–6187. doi: 10.1016/j.psj.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.