Extract
We extend our appreciation for the remarkable work from Scaravilli et al. [1] and their systematic scoping review regarding potential early plasmatic biomarkers for the diagnosis of primary graft dysfunction (PGD) after lung transplantation (LTx). The authors shed light on the promise of biomarkers for time-dependent PGD phenotyping and identified important knowledge gaps in the field.
Shareable abstract
This editorial highlights the importance of research towards PGD-specific biomarkers, suggesting strategic sample collection and advanced analysis techniques to expand our knowledge of PGD mechanisms and potential phenotyping https://bit.ly/3UUElw0
We extend our appreciation for the remarkable work from Scaravilli et al. [1] and their systematic scoping review regarding potential early plasmatic biomarkers for the diagnosis of primary graft dysfunction (PGD) after lung transplantation (LTx). The authors shed light on the promise of biomarkers for time-dependent PGD phenotyping and identified important knowledge gaps in the field.
PGD is an important cause of early morbidity and mortality after LTx. In fact, PGD is an umbrella term encompassing the ongoing pathobiological mechanisms occurring in the grafts within the first 72 postoperative hours. The PGD definition is based on the level of hypoxaemia measured by the pressure of arterial oxygen over the fraction of inspired oxygen and the presence of bilateral alveolar infiltrates on chest radiography [2]. Based on these parameters, PGD is graded to identify the recipients' severe postoperative acute lung injury and potentially poor outcomes. However, this grading algorithm suffers from the subjective interpretation of the chest radiograph, illustrating the need for implementation of more objectively quantifiable biomarkers for the diagnosis of true PGD. Most studies included in the review reported PGD grading based on the earlier 2005 definition [3]. Furthermore, PGD parameters are affected and altered by a wide range of other post-LTx complications, including cardiogenic oedema, pneumonia, aspiration pneumonitis, rejection and obstruction of vascular or airway anastomoses [4]. These confounding factors lead to a heterogeneous presentation, all under the PGD umbrella. Differentiation of PGD phenotypes could help clinicians to recognise the underlying causes of lung oedema and injury, each requiring a well-defined treatment strategy. Therefore, the findings of Scaravilli et al. [1] are an important step forward. Their review underscores the pressing need for further international prospective studies using updated diagnostic criteria and advanced methodologies. These could build a more detailed view of PGD grading and the underlying mechanisms of the PGD phenotypes that are yet to be defined.
More extensive insight into PGD pathobiology is an important step toward improved post-LTx outcomes. Characterisation of molecular signatures allow PGD phenotyping and the clinical integration of PGD-specific biomarkers is essential to achieve this goal. Such biomarkers will not only allow early detection and monitoring of PGD progression but will also enable more objective assessment of future therapeutic interventions. To date, there is no consensus on the clinical implementation of early biomarkers for PGD. However, PGD biomarkers should ideally meet the following criteria: 1) detectability in readily available samples like blood; 2) high specificity and sensitivity for the overall PGD presentation and different phenotypes; 3) availability of robust and cost-effective detection methods; and 4) pathobiological and mechanistic relevance.
The work of Scaravilli et al. [1] identified five candidate biomarkers – sRAGE (soluble receptor for advanced glycation end products), ICAM (intercellular adhesion molecule), PAI-1 (plasminogen activator inhibitor-1), SP-D (surfactant protein-D) and FSTL-1 (follistatin-related protein 1) – which were validated in predicting PGD. Other candidate biomarkers discussed in this review might be epiphenomena not mechanistically related to PGD. The findings in the review have profound significance and identified biomarkers with the potential to differentiate PGD phenotypes. However, before these biomarkers can be clinically implemented, they require further validation through global multicentre and prospective studies. Surprisingly, endothelin (ET)-1 was absent from their search results, although multiple studies have indicated that elevated levels of ET-1 correlate with donor lung assessment, and that high levels in both donor and recipient contribute to the development of PGD [5]. High levels of ET-1 are associated with increased expression of vascular endothelial growth factor and increased vascular permeability, leading to pulmonary oedema.
Strategic sample collection for PGD research
The scoping review only evaluated studies on early plasma biomarkers and their correlation with distinct PGD grades at specific time points [2]. Plasma samples are readily available but only characterise PGD from a systemic perspective. Therefore, plasma analyses are potentially confounded by nonspecific post-LTx changes. This necessitates linking potential biomarkers with pathobiological and mechanistic PGD pathways.
To obtain a more local and lung graft-oriented perspective on PGD, alternative sources of samples and methods for biomarkers research should be incorporated. 1) Bronchoalveolar lavage (BAL) fluid is a valuable option but it is mildly invasive and is typically reserved to rule out and detect causes of post-LTx infections. Routine BAL within the first 72 h poses logistical challenges and does not allow sampling at multiple time-points. Additionally, laboratory equipment and techniques to analyse BAL biomarkers are often unavailable. 2) Endobronchial lung tissue biopsies offer another source for biomarker analysis. Endoscopy for BAL and biopsy is more invasive and challenging to implement in daily practice but will be key to gain insight into the molecular pathways and identification of potential biomarkers. Compared to plasma samples, analysis of BAL or tissue biopsies require more time, are more labour-intensive and more costly. Furthermore, localisation of BAL or biopsies may potentially induce a sample bias [6]. Endobronchial biopsies are routinely used in clinical practice to rule out or diagnose allograft rejection but could also be used for biomarker analysis and PGD phenotyping, offering a molecular-level insight into disease status. Actual wedge biopsies of lung parenchyma taken during the transplant are very valuable for the early phase but limited to specific research purposes on identifying key mechanisms of PGD.
An unexplored resource for local sample collection is the pleural space, which is easily accessible when a chest drain is in place during the first days to weeks after LTx. Pleural fluid potentially offers a unique view of PGD pathobiology because lung graft lymphatics, which are not anastomosed, drain directly in the recipient chest cavity. Pleural fluid consists of cells including leukocytes, proteins, metabolites and debris containing donor lung RNA and DNA [7]. Analysis of pleural fluid offers a novel approach for identifying mechanistic mediators and biomarkers. These mediators might reflect molecular PGD pathways including endothelial and epithelial damage, oxidative stress, and donor and recipient leukocyte migration.
Precision molecular analysis of collected samples
Expanding the range and type of analyses in the rapidly growing landscape of biomolecular techniques will be crucial to identify the most relevant PGD biomarkers. Scaravilli et al. [1] noted that previous studies have primarily used ELISA or multiplex bead array assays. These methods only allow detection of a limited number of biomarkers, not allowing a comprehensive and multiomics approach. Furthermore, the low number of biomarkers that were analysed impairs the integration of findings across different study cohorts.
To advance our understanding of the multifactorial biological processes involved in PGD, it will be essential to integrate transcriptome, metabolome and proteome profiling. Analysing gene expression patterns from different cell types, including endothelial cells, epithelial cells and fibroblasts, could reveal distinct molecular responses. Transcriptomics can distinguish more favourable genetic graft profiles from those grafts developing PGD. Furthermore, employing single-cell sequencing analyses allows for detailed exploration of molecular characteristics at the cellular level but requires immediate tissue processing. Proteomics not only facilitates detailed pathway analysis but can also shed more light on cell-to-cell communication in PGD pathobiology. Understanding these protein-mediated pathways is crucial for gaining further insights into the processes driving PGD. There is an urgent need to identify unique protein patterns in recipients developing PGD at different time-points and different severities. Lastly, spatial omics recently emerged as a new technique to histologically visualise RNA, protein and metabolome changes in the lung tissue. The ultimate goal is be to develop a predefined biomarker panel enabling rapid and cost-effective sample analysis. While current multiplex assays can monitor up to 30 markers, a comprehensive biomarker survey targeting a protein signature of early PGD remains absent [8].
Establishing such a PGD biomarker panel could significantly improve early detection and prediction of clinical outcomes. These panels would also allow therapeutic efficacy monitoring and potentially define different PGD phenotypes to reach individualised treatment strategies.
Complex multimodal data generated by various techniques that are employed to identify biomarkers now bring the challenge of integrating large volumes of information at different 'omics levels (protein, RNA, etc.). This challenge can be overcome with artificial intelligence (AI) and, in particular, machine learning (ML) pipelines, where large amounts of data could be used to train AI/ML models to classify patients into different PGD phenotypes. Furthermore, these models could use the multiomics data to identify biomarkers that are specific to certain PGD phenotypes. Finally, clinical parameters including donor/recipient characteristics and surgical variables could also be incorporated into the models together with the biomolecular results, in order to accurately determine a comprehensive PGD phenotype for each individual LTx recipient.
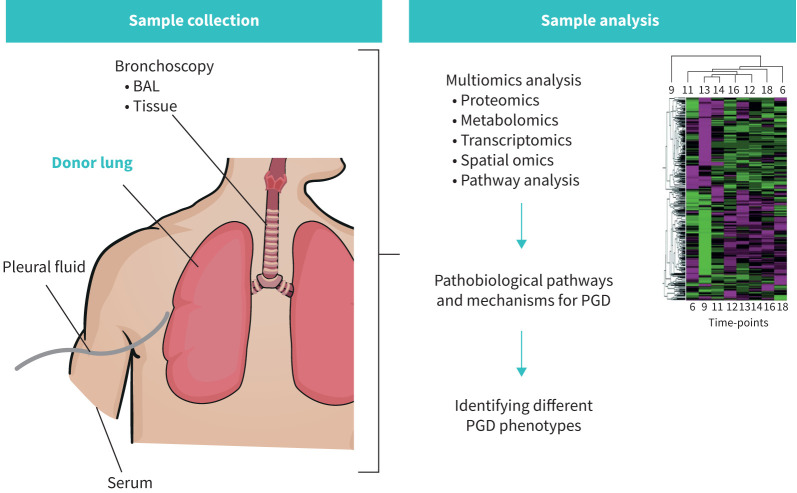
Combining recent transcriptomic and proteomic methodologies with well-chosen graft-specific sample collection has strong potential to obtain essential insights into PGD phenotype development through biomarker research (figure 1).
FIGURE 1.
The potential sources of lung graft-specific sample collection and integrated omics analyses to advance research on biomarkers, phenotypes and underlying mechanisms of primary graft dysfunction (PGD) after lung transplantation. BAL: bronchoalveolar lavage.
In conclusion, we would encourage collaborative research initiatives among LTx centres, clinicians and scientists to invest in PGD biomarker research and studies on pathobiological mechanisms of different PGD phenotypes. Bringing the PGD definition to a more comprehensive and higher level will advance the field of LTx and will contribute to improved patient outcome.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Scaravilli V, Turconi G, Colombo SM, et al. Early serum biomarkers to characterise different phenotypes of primary graft dysfunction after lung transplantation: a systematic scoping review. ERJ Open Res 2024; 10: 00121-2024. doi: 10.1183/23120541.00121-202438226065 [DOI] [Google Scholar]
- 2.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading – a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017; 36: 1097–1103. doi: 10.1016/j.healun.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Carby M, Bag R, et al. Report of the ISHLT working group on primary lung graft dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005; 24: 1454–1459. doi: 10.1016/j.healun.2004.11.049 [DOI] [PubMed] [Google Scholar]
- 4.Van Slambrouck J, Van Raemdonck D, Vos R, et al. A focused review on primary graft dysfunction after clinical lung transplantation: a multilevel syndrome. Cells 2022; 11: 745. doi: 10.3390/cells11040745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salama M, Andrukhova O, Hoda MA, et al. Concomitant endothelin-1 overexpression in lung transplant donors and recipients predicts primary graft dysfunction. Am J Transplant 2010; 10: 628–636. doi: 10.1111/j.1600-6143.2009.02957.x [DOI] [PubMed] [Google Scholar]
- 6.Chao BT, Sage AT, Yeung JC, et al. Identification of regional variation in gene expression and inflammatory proteins in donor lung tissue and ex vivo lung perfusate. J Thorac Cardiovasc Surg 2023; 166: 1520–1528. doi: 10.1016/j.jtcvs.2023.07.013 [DOI] [PubMed] [Google Scholar]
- 7.Haddad O, Thomas M. Postoperative management and acute complications after lung transplantation. Curr Chall Thorac Surg 2023; 5: 16–16. doi: 10.21037/ccts-20-182 [DOI] [Google Scholar]
- 8.Van Gool A, Corrales F, Čolović M, et al. Analytical techniques for multiplex analysis of protein biomarkers. Expert Rev Proteomics 2020; 17: 257–273. doi: 10.1080/14789450.2020.1763174 [DOI] [PubMed] [Google Scholar]