Abstract
Introduction
Type 2 (T2) asthma is often associated with chronic rhinosinusitis with nasal polyposis (CRSwNP). Additionally, nonsteroidal anti-inflammatory drug (NSAID) intolerance leads to NSAID-exacerbated respiratory disease (N-ERD). Previous transcriptomic data in non-CRSwNP T2 asthma patients showed differentially expressed genes. We focused on ALOX15, CLC, CYSLTR2, HRH4 and SMPD3 to investigate their role in T2 asthma.
Methods
The study included 100 healthy controls and 103 T2 asthma patients, divided into patients with asthma (n=54), patients with asthma and CRSwNP (n=29) and patients with N-ERD (n=20). Quantitative PCR analysis was performed on blood-derived RNA samples first to validate the five differentially expressed genes. The data were further analysed to find potential associations and biomarkers.
Results
Patients, regardless of stratification, exhibited significantly higher gene expression than healthy controls. The patterns of association revealed that ALOX15 was exclusively present in the non-comorbidity group, SMPD3 and CLC in the comorbidity groups, and HRH4 in all patient groups. ALOX15, CYSLTR2 and SMPD3 expression showed potential as biomarkers to confirm the diagnosis of T2 asthma using peripheral blood eosinophils as the initial criterion. Peripheral blood eosinophils combined with gene expression, especially SMPD3, may improve the diagnosis. CLC and CYSLTR2 expression play a specific role in discriminating N-ERD.
Discussion
We validated the transcriptomic data of five differentially expressed genes in T2 asthma. Different patterns of association were identified in patient stratification, suggesting that different molecular mechanisms underlie the spectrum of T2 asthma. Potential biomarkers were also found and used to design an algorithm with practical diagnostic utility for T2 asthma, including risk stratification for N-ERD.
Shareable abstract
Analysis of ALOX15, CLC, CYSLTR2, HRH4 and SMPD3 gene expression in different T2 asthma patient groups reveals possible associations in disease mechanisms and biomarkers, and a valuable algorithm for T2 asthma diagnosis and N-ERD risk assessment https://bit.ly/3vu6PTv
Introduction
Asthma is a major global health burden affecting 1–18% of the population [1]. It is a heterogeneous disease characterised by chronic airway inflammation resulting from complex immunological processes. According to inflammation, asthma is classified as type 2 (T2) and non-T2 [2, 3]. Typically, T2 asthma is characterised by increased levels of type 2 cytokines and eosinophils in the blood and/or airways. In addition, in the case of allergic asthma, sensitisation to aeroallergens will be present [2, 4].
In addition to the inflammatory type, the complexity of asthma is increased by comorbidities. Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a comorbidity with a 7% prevalence in asthma patients, increasing up to 40% in nonsteroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease (N-ERD) [5, 6]. This specific clinical group is associated with the highest rates of polyp recurrence, even with systemic corticosteroid treatment and endoscopic sinus surgery [1, 7].
Recently, a significant effort has been made to find specific asthma biomarkers to aid in more accurate diagnosis and disease management [4]. In this sense, precision medicine is becoming more commonly applied. Nevertheless, for asthma to be approached in this way, its heterogeneity must be considered an essential variable in scientific studies. In a previous transcriptomic study on a cohort of patients with allergic asthma (T2 asthma phenotype), we described several genes that were highly differentially expressed in patients compared to healthy subjects. Among the most differentially expressed genes (DEGs) (|fold-change|≥1.5), only IL5RA and PTGDR2 were validated and postulated as potential biomarkers [8, 9]. PTGDR2 also discriminated between asthma groups [9]. Based on these findings and transcriptome scoring [9], we focused on other DEGs with similar fold-change and p-adjusted values, selecting ALOX15, CLC, CYSLTR2, HRH4 and SMPD3. We aimed to study their expression to confirm this differential upregulation in asthma patients and to determine their potential as possible biomarkers, individually or as a set, and their role in the molecular mechanisms of T2 asthma, considering the heterogeneity of disease in asthma patients in terms of CRSwNP and N-ERD.
Methods
Further details are given in the supplementary material.
Study population
Individuals were recruited from the Allergy Department of Salamanca University Hospital. The local Clinical Research Ethics Committee approved the study (PI 2020-02-433) and all subjects provided written informed consent.
Gene expression analysis
A transcriptomic RNA-sequencing (RNA-seq) analysis was performed as previously reported (repository NCBI-PRJNA686899) [8, 9]. A heatmap was generated from the data using Morpheus (https://software.broadinstitute.org/morpheus). The quantitative PCR (qPCR) expression validation analysis of our selected genes was performed on RNA samples from the peripheral blood of the subjects, as previously described [9].
Statistical analysis
Data were analysed using SPSS software, version 26 (IBM, Armonk, NY, USA).
Results
Study population
The study population comprised 203 subjects (table 1); 100 were healthy controls (HCs) and 103 were patients diagnosed with T2 asthma. The asthma patient population was stratified into three mutually exclusive groups: patients with asthma and without CRSwNP or NSAID intolerance (asthma group, n=54); patients with asthma and CRSwNP and without NSAID intolerance (asthma+CRSwNP group, n=29); and patients with asthma, CRSwNP and NSAID intolerance (N-ERD group, n=20). Both peripheral blood eosinophil (PBE) levels and total IgE levels were significantly higher (p<0.05) in the total asthma population compared to HCs. When stratifying the patients, we found a significant increase in atopy in the asthma group compared to the other groups. However, we did not observe significant differences in total IgE levels among asthma groups. PBE levels were significantly higher in all patient groups compared to HCs; in particular, the asthma+CRSwNP group had significantly higher PBE levels than the asthma group (table 1).
TABLE 1.
Clinical and phenotypic characteristics of the study population
| HCs | Patients | ||||
|---|---|---|---|---|---|
| Total | Asthma | Asthma+CRSwNP | N-ERD | ||
| Subjects (n) | 100 | 103 | 54 | 29 | 20 |
| Age (years) | 59.1 ±18.0 | 44.2±19.7* | 36.9±18.7* | 49.5±18.9*,# | 56.6±14.9# |
| Female sex | 65.7 | 59.2 | 57.4 | 58.6 | 65.0 |
| Atopy | 0 | 70.9* | 87.0* | 58.6*,# | 45.0*,# |
| PBE (cells·µL−1) | 113.0±78.47 | 486.5±335.4* | 395.2±254.3* | 626.2±429.2*,# | 530.6±310.6* |
| Total IgE (kU·L−1) | 67.7±103.5 | 420.7±591.7* | 500.1±684.3* | 338.7±504.8* | 309.7±363.3* |
Values are expressed as mean±sd or %, unless otherwise indicated. Only statistically significant differences are indicated. HC: healthy control; CRSwNP: chronic rhinosinusitis with nasal polyposis; N-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; PBE: peripheral blood eosinophil. *: p<0.05 compared to HCs; #: p<0.05 compared to the asthma group.
Gene expression of ALOX15, CLC, CYSLTR2, HRH4 and SMPD3
The heatmap generated from previous RNA-seq [8, 9] showed ALOX15, CLC, CYSLTR2, HRH4 and SMPD3 as the most upregulated genes (supplementary figure S1). The relative gene expression of these five DEGs, determined by qPCR analysis in our validation study, is shown in table 2. All patient groups showed significantly higher gene expression levels than HCs. This finding is consistent with our previous transcriptomic study, which showed upregulation of these genes in the asthma population compared to controls [8, 9]. Comparing the patient groups, we found that expression levels of CLC in the N-ERD group and HRH4 in the asthma+CRSwNP group were significantly higher than in the asthma group.
TABLE 2.
Gene expression analysis of ALOX15, CLC, CYSLTR2, HRH4 and SMPD3
| Patients | |||||
|---|---|---|---|---|---|
| HCs | Total | Asthma | Asthma +CRSwNP | N-ERD | |
| ALOX15 | 1.88±1.51 | 10.95±9.04* | 10.35±8.63* | 13.22±10.60* | 9.28±7.29* |
| CLC | 2.21±1.43 | 10.87±12.06* | 8.49±11.72* | 11.13±10.86* | 16.92±13.02*,# |
| CYSLTR2 | 2.83±1.13 | 8.05±4.44* | 7.59±4.58* | 8.41±4.28* | 8.75±4.33* |
| HRH4 | 1.78±0.98 | 4.43±2.63* | 3.83±2.68* | 5.28±2.33*,# | 4.81±2.61* |
| SMPD3 | 2.44±1.34 | 11.99±13.58* | 12.38±16.83* | 11.63±7.70* | 11.44±10.59* |
Gene expression values (mean±sd) determined by quantitative PCR (2-ΔΔCt). Data were analysed by ANOVA and post hoc test or Kruskal–Wallis analysis. Only statistically significant differences are indicated after adjustment by sex, age and atopy. HC: healthy control; CRSwNP: chronic rhinosinusitis with nasal polyposis; N-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease. *: p<0.05 compared to HCs; #: p<0.05 compared to the asthma group.
Correlations among ALOX15, CLC, CYSLTR2, HRH4 and SMPD3
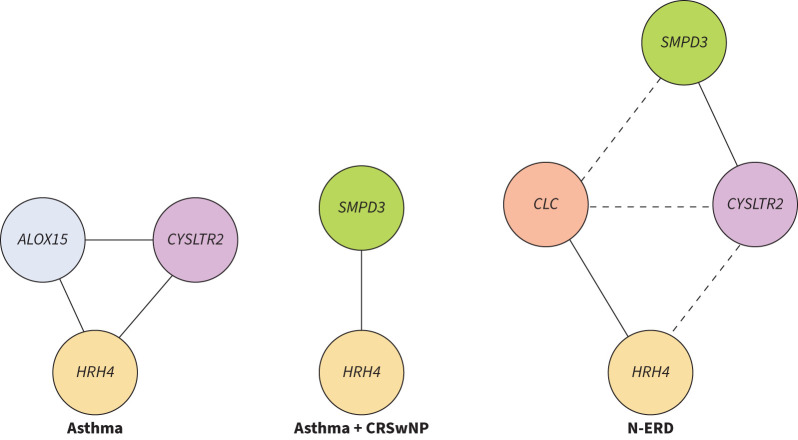
We analysed gene expression for possible associations using correlation analysis (table 3) and created a graph to visualise the correlations with r>0.7 (i.e. highly correlated) (figure 1). HRH4 was highly correlated with different genes in different groups, i.e. ALOX15 and CYSLTR2 in the asthma group, SMPD3 in the asthma+CRSwNP group and CLC in the N-ERD group. ALOX15 had highly significant correlations with several genes in the asthma group. By contrast, SMPD3 showed poorer correlation with the other genes in the asthma group but was highly correlated with HRH4 in the asthma+CRSwNP group and with CYSLTR2 in the N-ERD group. CYSLTR2 showed high correlations, especially with ALOX15 and HRH4 in the asthma group and with SMPD3 in the N-ERD group. Finally, CLC only showed high correlations with other genes in the N-ERD group. Overall, our data showed various association patterns among the patient groups. Although we cannot be sure of the nature of these associations, they suggest that our five genes may be involved in different molecular mechanisms of T2 asthma, depending on the type of patient.
TABLE 3.
Gene expression correlation matrix
| ALOX15 | CLC | CYSLTR2 | HRH4 | SMPD3 | |
|---|---|---|---|---|---|
| Healthy controls | |||||
| ALOX15 | 1 | 0.190 | 0.388* | 0.556* | 0.214 |
| CLC | 0.190 | 1 | 0.090 | 0.340* | 0.245* |
| CYSLTR2 | 0.388* | 0.090 | 1 | 0.438* | 0.342* |
| HRH4 | 0.556* | 0.340* | 0.438* | 1 | 0.091 |
| SMPD3 | 0.214 | 0.245* | 0.342* | 0.091 | 1 |
| Asthma | |||||
| ALOX15 | 1 | 0.635* | 0.755* | 0.859* | 0.423* |
| CLC | 0.635* | 1 | 0.602* | 0.585* | 0.199 |
| CYSLTR2 | 0.755* | 0.602* | 1 | 0.748* | 0.415* |
| HRH4 | 0.859* | 0.585* | 0.748* | 1 | 0.355* |
| SMPD3 | 0.423* | 0.199 | 0.415* | 0.355* | 1 |
| Asthma+CRSwNP | |||||
| ALOX15 | 1 | −0.071 | 0.468* | 0.491* | 0.368* |
| CLC | −0.071 | 1 | 0.339 | 0.611* | 0.571* |
| CYSLTR2 | 0.468* | 0.339 | 1 | 0.640* | 0.633* |
| HRH4 | 0.491* | 0.611* | 0.640* | 1 | 0.780* |
| SMPD3 | 0.368* | 0.571* | 0.633* | 0.780* | 1 |
| N-ERD | |||||
| ALOX15 | 1 | 0.209 | 0.459* | 0.242 | 0.393 |
| CLC | 0.209 | 1 | 0.611* | 0.717* | 0.458* |
| CYSLTR2 | 0.459* | 0.611* | 1 | 0.663* | 0.730* |
| HRH4 | 0.242 | 0.717* | 0.663* | 1 | 0.314 |
| SMPD3 | 0.393 | 0.458* | 0.730* | 0.314 | 1 |
Data presented as Pearson's correlation coefficients. Bolding indicates statistically significant values higher than 0.7. Grey shaded boxes are repeated values in the bivariate matrix. CRSwNP: chronic rhinosinusitis with nasal polyposis; N-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease. *: p<0.05.
FIGURE 1.
Graphical representation of gene expression associations in T2 asthma patient groups. The solid lines in the graph indicate significant correlations (p<0.05) between gene expressions with a Pearson's correlation coefficient higher than 0.7 (r>0.7). The line length is inversely proportional to the correlation coefficient, i.e. shorter lines in the graph indicate a higher correlation between the two gene expressions. The dashed lines represent moderate-low significant correlations (p<0.05) between two separate high correlations in the nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (N-ERD) group. CRSwNP: chronic rhinosinusitis with nasal polyposis.
ALOX15, CLC, CYSLTR2, HRH4 and SMPD3 as potential biomarkers
The differential gene expression and correlation analyses showed that ALOX15, CLC, CYSLTR2, HRH4 and SMPD3 could be potential biomarkers for identifying T2 asthma. Furthermore, they could help discriminate between different asthma populations. To further explore the potential of these genes as biomarkers, we performed a receiver operating characteristic (ROC) curve analysis to determine the discriminatory capacity of these genes (table 4). PBE, a clinically accepted biomarker for T2 asthma [4], was also included in the analysis for comparison with gene expressions. We evaluated the ability of the potential biomarkers to discriminate between HCs and the general population of T2 asthma patients or between the patient groups (asthma, asthma+CRSwNP and N-ERD) and HCs (reference group). We also evaluated whether the potential biomarkers could differentiate patient groups, using the asthma group or the asthma+CRSwNP group as a reference to compare with asthma+CRSwNP and N-ERD patients, respectively. Our results showed that PBE levels had the highest area under the curve (AUC) for differentiating T2 asthma patients from HCs in all comparisons (table 4).
TABLE 4.
Receiver operating characteristic curve analysis of PBE, ALOX15, CLC, CYSLTR2, HRH4 and SMPD3
| Healthy controls versus |
Asthma versus |
Asthma +CRSwNP versus |
||||
|---|---|---|---|---|---|---|
| Total | Asthma | Asthma +CRSwNP | N-ERD | Asthma +CRSwNP | N-ERD | |
| PBE | 0.95 (0.92–0.98) | 0.94 (0.90–0.98) | 0.97 (0.94–1.00) | 0.97 (0.94–1.00) | 0.68 (0.57–0.81) | 0.45* (0.29–0.62) |
| ALOX15 | 0.93 (0.90–0.97) | 0.93 (0.89–0.98) | 0.96 (0.92–1.00) | 0.91 (0.83–0.98) | 0.57* (0.45–0.71) | 0.38* (0.22–0.54) |
| CLC | 0.86* (0.80–0.91) | 0.79* (0.70–0.87) | 0.93 (0.88–0.99) | 0.93 (0.86–1.00) | 0.66 (0.55–0.78) | 0.63 (0.46–0.81) |
| CYSLTR2 | 0.92 (0.88–0.96) | 0.91 (0.85–0.96) | 0.93 (0.87–0.99) | 0.93 (0.86–1.00) | 0.57* (0.44–0.70) | 0.53 (0.36–0.70) |
| HRH4 | 0.85* (0.79–0.91) | 0.78* (0.70–0.87) | 0.95 (0.90–1.00) | 0.88* (0.79–0.97) | 0.72 (0.61–0.83) | 0.44* (0.26–0.61) |
| SMPD3 | 0.91 (0.87–0.95) | 0.88 (0.82–0.94) | 0.95 (0.91–1.00) | 0.91 (0.84–0.98) | 0.62 (0.50–0.75) | 0.42* (0.25–0.60) |
Data are presented as the area under the receiver operating characteristic curve (AUC) (95% confidence interval). For each patient group (columns), yellow shading indicates the highest absolute value of the AUC; grey shaded cells indicate the AUC is significantly lower than the highest AUC value; in all other cells, the AUC values are statistically equivalent (p>0.05) to the highest AUC value; CRSwNP: chronic rhinosinusitis with nasal polyposis; N-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; PBE: peripheral blood eosinophils. *: p<0.05.
Nevertheless, ALOX15, CYSLTR2 and SMPD3 expression also had AUCs comparable to the PBE value in all groups (p>0.05), suggesting their potential use as biomarkers for distinguishing T2 asthma patients from HCs. When analysing the discriminatory capacity of the potential biomarkers in differentiating between patient groups (table 4), PBE, CLC and SMPD3 had comparable AUCs to the higher value (HRH4) for differentiating asthma+CRSwNP patients from the asthma group. For discriminating N-ERD patients from the asthma+CRSwNP group, the AUCs of CLC and CYSLTR2 were the best. Interestingly, the AUC of ALOX15 expression (one of the best for discriminating HCs from patients) was consistently lower (p<0.05) than the best ones in all comparisons between patient groups.
Diagnostic value of ALOX15, CLC, CYSLTR2, HRH4 and SMPD3
Because ROC analyses showed potential candidates for discriminating patients from HCs or between patient groups, we next used logistic regression analysis to determine which might be good predictors of a specific condition (table 5). We determined their diagnostic value in each condition using cut-offs calculated from the ROCs (table 6). Because PBE, ALOX15, CYSLTR2 and SMPD3 exhibited comparable AUCs in all patient groups compared to HCs (table 4), they were analysed to differentiate patients from HCs. In this case, they all proved good predictors for distinguishing T2 asthma (table 5). Among them, PBE had the highest diagnostic sensitivity (92.2%) (table 6, upper panels). However, the analysed gene expressions had higher specificities than PBE.
TABLE 5.
Regression analysis of potential biomarkers
| Healthy controls versus patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SLR | Biomarker | PBE | ALOX15 | CYSLTR2 | SMPD3 | |||||
| OR (95% CI) | 1.023 (1.013–1.034) | 2.625 (1.724–3.996) | 3.628 (1.893–6.952) | 2.438 (1.651–3.600) | ||||||
| p-value | <0.05 | <0.05 | <0.05 | <0.05 | ||||||
| MLR | Biomarker | PBE | + | ALOX15 | PBE | + | CYSLTR2 | PBE | + | SMPD3 |
| OR (95% CI) | 1.018 (1.005–1.031) | 2.206 (1.285–3.788) | 1.021 (1.008–1.034) | 3.124 (1.379–7.074) | 1.020 (1.009–1.032) | 1.985 (1.169–3.371) | ||||
| p-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | ||||
| Patients | |||||||
|---|---|---|---|---|---|---|---|
| Asthma versus Asthma+CRSwNP | Asthma+CRSwNP versus N-ERD# | ||||||
| SLR | Biomarker | PBE | HRH4 | CLC | SMPD3 | CLC | CYSLTR2 |
| OR (95% CI) | 1.002 (1.001–1.004) | 1.258 (1.043–1.517) | 1.020 (0.980–1.061) | 1.008 (0.973–1.044) | 1.106 (1.022–1.196) | 1.072 (0.921–1.248) | |
| p-value | <0.05 | <0.05 | 0.342 | 0.651 | <0.05 | 0.368 | |
| MLR | Biomarker | PBE | + | HRH4 | CLC | ||
| OR (95% CI) | 1.002 (1.001–1.004) | 1.077 (0.832–1.393) | 1.106 (1.022–1.196) | ||||
| p-value | <0.05 | 0.574 | <0.05 | ||||
Potential biomarkers with significantly higher area under the receiver operating characteristic curve values (no shading, table 3) were tested individually by simple logistic regression (SLR). Only significant biomarkers were further tested by multiple logistic regression (MLR). Bolding indicates statistically significant values. Values were adjusted for sex, age and atopy. PBE: peripheral blood eosinophils; CRSwNP: chronic rhinosinusitis with nasal polyposis; N-ERD: nonsteroidal inti-inflammatory drug-exacerbated respiratory disease. #: PBE was also used as a confounding variable.
TABLE 6.
Diagnostic values of potential biomarkers
| Healthy controls versus patients | |||||||
|---|---|---|---|---|---|---|---|
| Biomarkers (cut-off) | Combinations (cut-off) | ||||||
| PBE (≥195) | ALOX15 (≥4.07) | CYSLTR2 (≥4.12) | SMPD3 (≥3.75) | PBE (≥195) and ALOX15 (≥4.07) | PBE (≥195) and CYSLTR2 (≥4.12) | PBE (≥195) and SMPD3 (≥3.75) | |
| S (%) | 92.20 | 81.60 | 83.50 | 81.60 | 75.70 | 81.60 | 78.60 |
| SP (%) | 84.00 | 94.00 | 90.00 | 89.00 | 96.00 | 97.00 | 99.00 |
| LR (+) | 5.76 | 13.60 | 8.35 | 7.42 | 18.93 | 27.20 | 78.60 |
| LR (−) | 0.09 | 0.20 | 0.18 | 0.21 | 0.25 | 0.19 | 0.22 |
| AUC (95% CI) | 0.95 (0.92–0.98) | 0.93 (0.90–0.97) | 0.92 (0.88–0.96) | 0.91 (0.87–0.95) | 0.99 (0.99–1.000) | 0.99 (0.99–1.000) | 0.99 (0.98–1.000) |
| Patients | ||||
|---|---|---|---|---|
| Asthma versus Asthma+CRSwNP | Asthma+CRSwNP versus N-ERD | |||
| Biomarkers (cut-off) | Biomarkers (cut-off) | Combination (cut-off) | ||
| PBE (≥455) | CLC (≥14.30) | CYSLTR2 (≥11.58) | CLC (≥14.30) & CYSLTR2 (≥11.58) | |
| S (%) | 65.52 | 60.00 | 35.00 | 30.00 |
| SP (%) | 74.10 | 79.31 | 82.76 | 96.55 |
| LR (+) | 2.53 | 2.90 | 2.03 | 8.70 |
| LR (−) | 0.47 | 0.50 | 0.79 | 0.73 |
| AUC (95% CI) | 0.68 (0.57–0.81) | 0.63 (0.46–0.81) | 0.53 (0.36–0.70) | 0.75 (0.61–0.89) |
Cut-off values for each potential biomarker were calculated from receiver operating characteristic curve data by the Jouden index. PBE is cells·µL−1 and gene expression from quantitative PCR as 2-ΔΔCt. PBE: peripheral blood eosinophils; S: sensitivity; SP: specificity; LR: likelihood ratio; AUC: area under the receiver operating characteristic curve; CRSwNP: chronic rhinosinusitis with nasal polyposis; N-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease.
Likelihood ratios (LRs) above 10 (LR+) or below 0.1 (LR−) are considered to provide strong evidence to rule in or rule out a diagnosis, respectively [10]. Under this premise, PBE is a good biomarker to rule out disease (LR−=0.09). In contrast, gene expressions performed better to confirm the diagnosis, especially ALOX15 (LR+=13.60). Combining PBE with gene expression resulted in higher LR+ values (and AUCs) than gene expression alone, especially the combination of PBE with SMPD3 (LR+=78.60), but also CYSLTR2 combined with PBE, given that the OR value of CYSLTR2 (OR=3.12; table 5, upper-panel) was higher than for the other genes.
When discriminating between patient groups (tables 5 and 6, lower panels), the evaluated potential biomarkers were less effective than those analysed in differentiating patients from HCs. Only PBE could be considered to discriminate asthma+CRSwNP patients from the asthma group with moderate LR+ (2.53, table 6). CLC and CYSLTR2 expression deserve special attention in differentiating N-ERD patients from asthma patients with CRSwNP and NSAID-tolerance. They had the highest AUC values (table 4), but only CLC had a significant weight in the regression analysis (table 5, lower panels). Given the aetiopathogenic role of CYSLTR2 in N-ERD [11], we also decided to analyse the diagnostic value of CYSLTR2. The results showed that CLC moderately differentiated N-ERD patients (LR+=2.90; AUC=0.63). However, we found that combining CLC with CYSLTR2 discriminated N-ERD patients more accurately from the asthma+CRSwNP group (LR+=8.70; AUC=0.75).
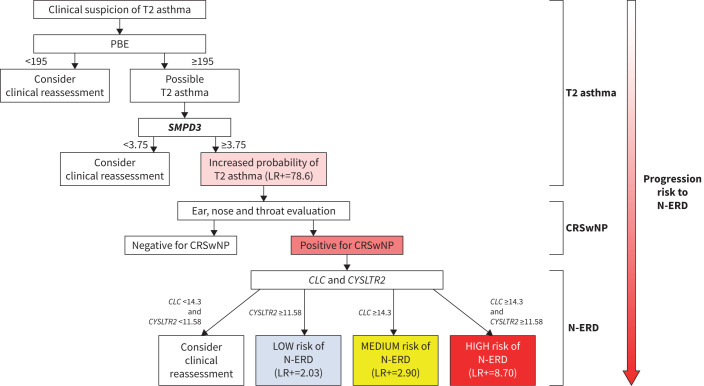
Based on all the diagnostic value data, we propose an algorithm using PBE as a starting point to diagnose T2 asthma and N-ERD (figure 2).
FIGURE 2.
Algorithm to assist in the diagnosis of T2 asthma and nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (N-ERD). The proposed algorithm starts with the peripheral blood eosinophil (PBE) counts as part of the T2 asthma diagnostic criteria. Our PBE cut-off (<195 cells·µL−1) can exclude the disease (likelihood ratio (LR)−=0.09). However, as other cut-offs exist (Global Initiative for Asthma guidelines [1]), negative results are left to the clinician's discretion (clinical reassessment is suggested). Also, obtaining airway samples for these patients would be advisable. If positive (≥195 cells·µL−1), the test should be confirmed. SMPD3 expression (cut-off >3.75 as per 2-ΔΔCt) in combination with PBE can accurately confirm the positive result (LR+=78.60), thus diagnosing T2 asthma. If the SMPD3 cut-off is <3.75, a negative result cannot be accurately given and a clinical reassessment should be considered. The diagnosis of chronic rhinosinusitis with nasal polyposis (CRSwNP) is assumed to be made by physicians (ear, nose and throat evaluation). If positive, the CLC and/or CYSLTR2 expression (considering the indicated cut-off points) can either indicate a low, medium or high risk for N-ERD. If both CLC and CYSLTR2 expression cut-offs are below their thresholds, a negative result cannot be accurately given and clinical reassessment should be undertaken. The boxes displaying a gradient of colour from light red (T2 asthma) to dark red (N-ERD) correspond to a possible disease progression. See text and table 6 for further details of cut-off points and LRs.
Discussion
This study aimed to validate the differential expression of ALOX15, CLC, CYSLTR2, HRH4 and SPMD3, previously identified in a transcriptomic analysis [8, 9], in a similar independent T2 asthma population and to extend this validation to two other T2 asthma plus comorbidity populations (asthma+CRSwNP and N-ERD).
Our results showed upregulation of the five genes in all groups compared to HCs and some significant differences in the expression among the patient groups. Additionally, we found several patterns of potential associations between gene expressions that depended on patient stratification.
CYSLTR2, SMPD3 and especially ALOX15 expression showed a good discriminatory capacity, statistically comparable to PBE, in confirming T2 asthma, the phenotype common to all patients, and could be proposed as disease-specific biomarkers. Furthermore, combining them with PBE improved the confirmatory diagnosis. In this case, PBE with SMPD3 expression was the best confirmatory combination for diagnosing T2 asthma and was included in our diagnostic algorithm (figure 2). Indeed, Global Initiative for Asthma guidelines indicate a cut-off of ≥150 cells·µL−1 to suspect T2 asthma [1]. However, this level is also associated with the lower level of response to biologicals in clinical trials [12] and is intended to avoid misclassifying patients as having non-T2 asthma [1]. So, our threshold could have a better predictive value for T2 asthma, particularly with a concomitant SMPD3 value >3.75 (as per 2-ΔΔCt). This demonstrates that combining biomarkers enhances diagnostics over individual biomarkers, as previously suggested [4].
ALOX15 encodes the arachidonate 15-lipoxygenase protein (15-LOX), which is involved in several inflammatory diseases, including asthma [13]. In addition to its diagnostic value, ALOX15 showed major changes in the correlation analysis in the asthma group compared to HCs. Therefore, our results align with those highlighting the role of ALOX15 in asthma [13, 14]. However, ALOX15 expression was not a suitable biomarker for differentiating among the patient groups. These findings may be in contrast to a previous study describing significantly increased expression of ALOX15 in nasal polyp cells from N-ERD patients which concluded that the dysregulation of arachidonic acid metabolism via the 15-LOX pathway contributes to increased inflammation in N-ERD disease [15]. We found no significant differences in ALOX15 expression among patient groups (table 2). However, the data showed a decrease in gene expression in the N-ERD group compared to the asthma+CRSwNP group. Although the differences between our results and those mentioned above could be due to using different cell types (nasal polyp cells versus peripheral blood cells), other hypotheses could be raised. Because 15-LOX has pro- and anti-inflammatory properties [13, 16], non-increased or even reduced ALOX15 expression, as we observed, could also be associated with the increased severity of N-ERD disease. How the balance between pro- and anti-inflammatory properties of 15-LOX, mediated by ALOX15 expression, is regulated or represented by specific cell types could be a topic for further research.
Similar to ALOX15, PBE levels were irrelevant in discriminating N-ERD patients. However, CLC expression could be helpful for diagnosing N-ERD, a result that agrees with previous reports [17]. CLC encodes Charcot–Leyden crystal protein. First described in the late 1800s [18, 19], it has been proposed as a valuable biomarker of eosinophilic T2 inflammation in several diseases [20–22]. CLC expression was significantly higher in the N-ERD group than the asthma group (table 2), and its AUC value was the highest for differentiating N-ERD from asthma+CRSwNP patients (table 3). Given the low LR+ values (table 6), CLC expression alone may not be sufficient to differentiate N-ERD patients. Nevertheless, combining CLC and CYSLTR2 expression discriminated N-ERD disease more accurately. Although the results of these genes are not entirely conclusive for N-ERD and further research is needed, the combination of CLC and CYSLTR2 expression could be a valuable novel tool in diagnosing NSAID hypersensitivity, where the gold standard is currently the aspirin (acetylsalicylic acid) or other NSAID challenge [23], with the potential risk of severe reactions [1]. Therefore, this combination was proposed for the diagnosis and follow-up of N-ERD in our algorithm (figure 2). This result could be useful before considering an oral challenge with acetylsalicylic acid, because it allows for risk stratification.
CYSLTR2 encodes the G protein-coupled receptor (GPCR) cysteinyl leukotriene receptor 2, which binds the cysteinyl leukotrienes C4 and D4, potent lipid inflammatory mediators in asthma [24]. CYSLTR2 is expressed in various cell types, including eosinophils [24, 25]. Several genetic studies have linked this receptor to asthma [26–28], and a role in the immunopathology of N-ERD disease has been reported previously [11]. We also found that CYSLTR2 expression highly correlated with other genes in the N-ERD group (figure 1), so it may be reasonable to consider this gene a critical player in the specific molecular mechanisms driving N-ERD disease. Moreover, the usefulness of CYSLTR2 expression as a biomarker to confirm T2 asthma diagnosis when combined with PBE levels suggests that this gene may also play a role in molecular mechanisms common to all patients. We found a high correlation between CYSLTR2 and ALOX15 in the asthma group, indicating some association. That is consistent with evidence that in asthma, CYSLTR2 and 15-LOX are involved in signalling pathways starting from a common precursor, arachidonic acid [13, 24].
HRH4 encodes the GPCR protein histamine receptor H4 [29], which is linked to several inflammatory processes [30]. In allergic asthma, histamine signalling via HRH4 is implicated in the immune-inflammatory response [31, 32]. Although HRH4 expression was significantly higher in asthma patients with CRSwNP (table 2), our subsequent analyses suggest that its expression may not be as effective a biomarker to differentiate that patient group as PBE. Nonetheless, we observed that HRH4 expression highly correlated with other gene expressions in all patient groups (figure 1), suggesting a transversal role in the molecular mechanisms underlying T2 asthma. That is a likely hypothesis, given the several lines of evidence linking the receptor to this disease [30–32].
Our results on SMPD3 are exciting because there is less evidence for its association with asthma than for the other genes. SMPD3 encodes neutral sphingomyelinase II, which is involved in sphingomyelin metabolism [33]. This protein has been proposed as a novel target in COPD [34]. In a recent study, SMPD3 was significantly associated with atopy and/or atopic asthma in children and adolescents [35]. Although our results on SMPD3 are based on an adult population, they are consistent with the findings of the latter study. In the asthma group (mostly allergic), SMPD3 expression was significantly higher than in HCs. Furthermore, its AUC was among the best for discriminating this patient group (versus HCs; table 4). However, the importance of SMPD3 could be extended to all T2 asthma patients, given that the combination of PBE with this gene was the best to confirm a T2 asthma diagnosis (figure 2), suggesting that future research needs to focus on SMPD3 and its possible functional role in the underlying mechanisms of this asthma phenotype. The role of SMPD3 in T2 asthma may be part of the lipid-mediated signalling mechanisms that are common in asthma. Our results support this hypothesis because we found that SMPD3 was highly correlated with CYSLTR2 (N-ERD group), which is involved in the signalling pathway mediated by the lipid mediator leukotrienes [24]. We also found that SMPD3 expression was highly correlated with HRH4 in the asthma+CRSwNP group. Interestingly, the correlation between SMPD3 and genes encoding GPCR proteins (HRH4 and CYSLTR2) in the CRSwNP patient groups suggests possible SMPD3-GPCR-mediated signalling mechanisms specific to this comorbidity.
Our study's strengths include the identification of potential biomarkers among the DEGs and using them to design an algorithm with practical diagnostic utility for T2 asthma and N-ERD. As shown in figure 2, our algorithm proposes PBE as a starting point because it is the most sensitive biomarker. For the subsequent confirmatory step, we opted for SMPD3, because PBE and SMPD3 were the best combination of biomarkers for diagnosing T2 asthma. CLC and CYSLTR2 were suggested as valuable tools for N-ERD diagnosis. Furthermore, considering the potential progression from T2 asthma to N-ERD [7], PBE, CLC and CYSLTR2 could be considered to monitor this transition. In particular, combining CLC and CYSLTR2 could signal a potential risk gradient (low-medium-high) for N-ERD.
In addition, identifying patterns of association between gene expressions provided data to better understand the molecular mechanisms underlying the spectrum of T2 asthma.
One study limitation is the possible differences between peripheral blood gene expression and that of the airways. Future research could include airway samples to improve the findings. Additionally, validation of N-ERD risk stratification results will require a larger sample size for broader generalisation. Finally, results cannot be extrapolated to non-T2 asthma.
In conclusion, a variety of possible relevant roles emerged for the five DEGs in our study, which could be seen as a reflection of the heterogeneity of this disease and highlights the importance of focusing research on the type of asthma patient beyond the common phenotype, bringing us closer to the concept of personalised medicine.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00909-2023.SUPPLEMENT (197.5KB, pdf)
Figure S1 00909-2023.SUPPLEMENTARYFIGURE (409.8KB, tif)
Acknowledgments
The authors would like to express their gratitude to the staff of the Allergy Department at Salamanca University Hospital, particularly Milagros González Prieto, Cristina Regalado González and Rosa Aguadero Martín, for their valuable collaboration in providing samples and retrieving clinical information. The authors also thank Fundación del Instituto de Estudios de Ciencias de la Salud de Castilla y León and the Institute of Biomedical Research of Salamanca.
Provenance: Submitted article, peer reviewed.
Ethics statement: The local Clinical Research Ethics Committee approved the study (PI 2020-02-433) and all subjects gave informed written consent.
Conflict of interest: A. García-Sánchez has received payment for lectures from Leti.
Conflict of interest: M. Estravís has received payment for lectures from Sanofi in the last 3 years.
Conflict of interest: J. Ramos-González has received payments for lectures from AstraZeneca, Chiesi, GSK, Novartis, Sanofi, Menarini and Boehringer; and for consultancy from AstraZeneca, GSK, Novartis and Sanofi.
Conflict of interest: M. Gil-Melcón has received payment for lectures from AstraZeneca, GSK and Sanofi.
Conflict of interest: I. Dávila has received payment for lectures, including service on speaker's bureaus, from Allergy Therapeutics, AstraZeneca, Chiesi, Diater, GSK, Leti, Novartis and Sanofi; for consultancy from Allergy Therapeutics, ALK-Abello, AstraZeneca, GSK, Merck, MSD, Novartis and Sanofi; and grants from Thermofisher Diagnostics.
Conflict of interest: The rest of the authors declare no conflict of interest. The funders had no role in the study's design, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Support statement: This study has been funded by the projects PI20/00268, funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union (EU); RD21/0002/0054 and PMP22/00124, funded by ISCIII and NextGeneration EU, Mecanismo para la Recuperación y la Resiliencia; IMP/00009, funded by ISCIII (CIBERCV) and European Regional Development Fund (ERDF) “A way to make Europe”; by grant PID2021-125117OB-I00 funded by MCIN/AEI/10.13039/501100011033 “A way to make Europe”; and by grant IES161P20, funded by Junta de Castilla y León and by ERDF “A way to make Europe”. E. Moreno-Jiménez is recipient of a predoctoral grant PFIS, funded by ISCIII through the project “FI-21/00048” and co-funded by the EU. N. Morgado's contract is financed by Programa Investigo (NextGenerationEU funds of the Recovery and Resilience Facility), fellowship numbers 2022 C23.I01.P03.S0020–0000205. M. Gómez-García is recipient of a Sara Borrell postdoctoral fellowship from the ISCIII (CD23/00185), co-funded by the European Social Fund. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma . Difficult-to-treat and Severe Asthma in Adolescent and Adult Patients: Diagnosis and Management. 2022. Available from: http://ginasthma.org
- 2.Hammad H, Lambrecht BN. The basic immunology of asthma. Cell 2021; 184: 1469–1485. doi: 10.1016/j.cell.2021.02.016 [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Melén E, Menzies-Gow AN. Holy grail: the journey towards disease modification in asthma. Eur Respir Rev 2022; 31: 210183. doi: 10.1183/16000617.0183-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamant Z, Vijverberg S, Alving K, et al. Toward clinically applicable biomarkers for asthma: an EAACI position paper. Allergy 2019; 74: 1835–1851. doi: 10.1111/all.13806 [DOI] [PubMed] [Google Scholar]
- 5.Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID-exacerbated respiratory disease (N-ERD) – a EAACI position paper. Allergy 2019; 74: 28–39. doi: 10.1111/all.13599 [DOI] [PubMed] [Google Scholar]
- 6.Langdon C, Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy 2016; 9: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczeklik A, Niżankowska E, Duplaga M. Natural history of aspirin-induced asthma. Eur Respir J 2000; 16: 432–436. doi: 10.1034/j.1399-3003.2000.016003432.x [DOI] [PubMed] [Google Scholar]
- 8.Elena-Pérez S, Heredero-Jung DH, García-Sánchez A, et al. Molecular analysis of IL-5 receptor subunit ɑ as a possible pharmacogenetic biomarker in asthma. Front Med 2021; 7: 624576. doi: 10.3389/fmed.2020.624576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Sánchez A, Estravís M, Martin MJ, et al. PTGDR2 expression in peripheral blood as a potential biomarker in adult patients with asthma. J Pers Med 2021; 11: 827. doi: 10.3390/jpm11090827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden SR, Brown MD. Likelihood ratio: a powerful tool for incorporating the results of a diagnostic test into clinical decisionmaking. Ann Emerg Med 1999; 33: 575–580. doi: 10.1016/S0196-0644(99)70346-X [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Barrett NA, Kanaoka Y, et al. Type 2 cysteinyl leukotriene receptors drive IL-33-dependent type 2 immunopathology and aspirin sensitivity. J Immunol 2018; 200: 915–927. doi: 10.4049/jimmunol.1700603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGregor MC, Krings JG, Nair P, et al. Role of biologics in asthma. Am J Respir Crit Care Med 2019; 199: 433–445. doi: 10.1164/rccm.201810-1944CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh NK, Rao GN. Emerging role of 12/15-lipoxygenase (ALOX15) in human pathologies. Prog Lipid Res 2019; 73: 28–45. doi: 10.1016/j.plipres.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Li J, Zhang Y, et al. Arachidonic acid 15-lipoxygenase: effects of its expression, metabolites, and genetic and epigenetic variations on airway inflammation. Allergy Asthma Immunol Res 2021; 13: 684–696. doi: 10.4168/aair.2021.13.5.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens WW, Staudacher AG, Hulse KE, et al. Activation of the 15-lipoxygenase pathway in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2021; 147: 600–612. doi: 10.1016/j.jaci.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snodgrass RG, Brüne B. Regulation and functions of 15-lipoxygenases in human macrophages. Front Pharmacol 2019; 10: 1–12. doi: 10.3389/fphar.2019.00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devouassoux G, Pachot A, Laforest L, et al. Galectin-10 mRNA is overexpressed in peripheral blood of aspirin-induced asthma. Allergy Eur J Allergy Clin Immunol 2008; 63: 125–131. doi: 10.1111/j.1398-9995.2007.01558.x [DOI] [PubMed] [Google Scholar]
- 18.Charcot JM, Robin C. Observation de leucocythemie [Observation of leukocythaemia]. Mem Soc Biol 1853; 5: 44–50. [Google Scholar]
- 19.Leyden E. Zur Kenntniss des Bronchial-Asthma [On the knowledge of bronchial asthma]. Virchows Arch Pathol Anat Physiol Klin Med 1872; 54: 324–344. [Google Scholar]
- 20.Tomizawa H, Yamada Y, Arima M, et al. Galectin-10 as a potential biomarker for eosinophilic diseases. Biomolecules 2022; 12: 1–16. doi: 10.3390/biom12101385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aegerter H, Smole U, Heyndrickx I, et al. Charcot–Leyden crystals and other protein crystals driving type 2 immunity and allergy. Curr Opin Immunol 2021; 72: 72–78. doi: 10.1016/j.coi.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weller PF, Wang H, Melo RCN. The Charcot–Leyden crystal protein revisited – A lysopalmitoylphospholipase and more. J Leukoc Biol 2020; 108: 105–112. doi: 10.1002/JLB.3MR0320-319RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nizankowska E, Bestyńska-Krypel A, Ćmiel A, et al. Oral and bronchial provocation tests with aspirin for diagnosis of aspirin-induced asthma. Eur Respir J 2000; 15: 863–869. doi: 10.1034/j.1399-3003.2000.15e09.x [DOI] [PubMed] [Google Scholar]
- 24.Kanaoka Y, Austen KF. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv Immunol; 2019; 142: 65–84. doi: 10.1016/bs.ai.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Mita H, Hasegawa M, Saito H, et al. Levels of cysteinyl leukotriene receptor mRNA in human peripheral leucocytes: significantly higher expression of cysteinyl leukotriene receptor 2 mRNA in eosinophils. Clin Exp Allergy 2001; 31: 1714–1723. doi: 10.1046/j.1365-2222.2001.01184.x [DOI] [PubMed] [Google Scholar]
- 26.Thompson MD, Capra V, Clunes MT, et al. Cysteinyl leukotrienes pathway genes, atopic asthma and drug response: from population isolates to large genome-wide association studies. Front Pharmacol 2016; 7: 299. doi: 10.3389/fphar.2016.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.dos S Jesus T, dos S Costa R, Alcântara-Neves NM, et al. Variants in the CYSLTR2 are associated with asthma, atopy markers and helminths infections in the Brazilian population. Prostaglandins Leukot Essent Fat Acids 2019; 145: 15–22. doi: 10.1016/j.plefa.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 28.Pillai SG, Cousens DJ, Barnes AA, et al. A coding polymorphism in the CYSLT2 receptor with reduced affinity to LTD4 is associated with asthma. Pharmacogenetics 2004; 14: 627–633. doi: 10.1097/00008571-200409000-00007 [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Itadani H, Hidaka Y, et al. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun 2000; 279: 615–620. doi: 10.1006/bbrc.2000.4008 [DOI] [PubMed] [Google Scholar]
- 30.Mehta P, Miszta P, Rzodkiewicz P, et al. Enigmatic histamine receptor H4 for potential treatment of multiple inflammatory, autoimmune, and related diseases. Life (Basel) 2020; 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann D. Role of the histamine H4-receptor in bronchial asthma. Handb Exp Pharmacol 2016; 347–359. doi: 10.1007/164_2016_11 [DOI] [PubMed] [Google Scholar]
- 32.Salcedo C, Pontes C, Merlos M. Is the H4 receptor a new drug target for allergies and asthma? Front Biosci (Elite Ed) 2013; 5: 178–187. doi: 10.2741/E606 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Cao Y. The sphingomyelin synthase family: proteins, diseases, and inhibitors. Biol Chem 2017; 398: 1319–1325. doi: 10.1515/hsz-2017-0148 [DOI] [PubMed] [Google Scholar]
- 34.Filosto S, Castillo S, Danielson A, et al. Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. Am J Respir Cell Mol Biol 2011; 44: 350–360. doi: 10.1165/rcmb.2009-0422OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y, Gruzieva O, Wang T, et al. Transcriptomics of atopy and atopic asthma in white blood cells from children and adolescents. Eur Respir J 2019; 53: 1900102. doi: 10.1183/13993003.00102-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00909-2023.SUPPLEMENT (197.5KB, pdf)
Figure S1 00909-2023.SUPPLEMENTARYFIGURE (409.8KB, tif)