Abstract
Postacute symptoms are not uncommon after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with pre-Omicron variants. How the Omicron variant and coronavirus disease 2019 (COVID-19) booster vaccination influence the risk of postacute symptoms is less clear. We analyzed data from a nationwide Danish questionnaire study, EFTER-COVID, comprising 36 109 individuals aged ≥15 years who were tested between July 2021 and January 2022, to evaluate the associations of the Omicron variant and COVID-19 booster vaccination with postacute symptoms and new-onset general health problems 4 months after infection with SARS-CoV-2. Risk differences (RDs) were estimated by comparing Omicron cases with controls, comparing Omicron cases with Delta cases, and comparing Omicron cases vaccinated with 3 doses with those vaccinated with 2 doses, adjusting for age, sex, body mass index, self-reported chronic diseases, Charlson comorbidity index, health-care occupation, and vaccination status. Four months after testing for SARS-CoV-2 during the Omicron period, cases experienced substantial postacute symptoms and new-onset health problems in comparison with controls; the largest RD was observed for memory issues (RD = 7.4%; 95% CI, 6.4-8.3). However, risks were generally lower than those in the Delta period, particularly for dysosmia (RD = –15.0%; 95% CI, −17.0 to −13.2) and dysgeusia (RD = –11.2%; 95% CI, −13.2 to −9.5). Booster vaccination was associated with fewer postacute symptoms and new-onset health problems 4 months after Omicron infection as compared with 2 doses of COVID-19 vaccine.
Keywords: booster vaccination, coronavirus disease 2019, COVID-19, long COVID, postacute symptomatology, SARS-CoV-2, SARS-CoV-2 Omicron variant, severe acute respiratory syndrome coronavirus 2
Introduction
A significant proportion of individuals who recovered from mild infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the earlier parts of the coronavirus disease 2019 (COVID-19) pandemic continued to report persistent symptoms and health conditions with new onset, several months after the acute phase of the disease.1,2 The degree to which the prevalence, duration, and severity of these symptoms differ for the more recent SARS-CoV-2 variants and by vaccination status is less clear. Of particular public health interest, given its massive spread, is the association between the Omicron variant and postacute symptoms.
Infection with SARS-CoV-2 during the period of Omicron predominance is seen to cause less severe acute illness than previous variants among vaccinated populations.3 Infection during the Omicron period has also been reported to lead to similar or milder postacute symptoms than the Delta variant 1‑4 months after a positive test.3,4 Furthermore, it is not clear to what degree, if any, vaccination protects against or reduces the severity of postacute symptoms following breakthrough infections, which have been particularly common for the Omicron variant.
Regarding variants arising prior to Omicron, vaccination with 1 or 2 doses of COVID-19 vaccine before infection with SARS-CoV-2 may have had a protective effect against long-term COVID-19,5 hereafter called “long COVID.” However, the impact of 3 doses of COVID-19 vaccine on postacute symptomatology is uncertain.
In this study, we used data from a large nationwide Danish questionnaire survey on long COVID to evaluate the risk of 24 postacute symptoms, including physical, cognitive, and fatigue-related symptoms, and 5 new-onset health conditions 4 months after infection with the SARS-CoV-2 Omicron variant. We compared Omicron cases to both test-negative controls from the same period in which Omicron was predominant and cases from the period of Delta predominance. In addition, we evaluated the effect of booster vaccination on postacute symptoms and new-onset health conditions 4 months after infection during the Omicron period, by comparing cases vaccinated with 3 doses of COVID-19 vaccine to cases who had received only 2 doses of vaccine.
Methods
Study design and population
We created a study cohort comprising respondents from a Danish nationwide questionnaire study on long COVID, EFTER-COVID (“after COVID”),6 which is described in detail elsewhere.2 Specifically, residents of Denmark aged 15 years or more who tested positive for SARS-CoV-2 for the first time during the periods July 15, 2021-November 15, 2021, and December 28, 2021-January 15, 2022, were included in the study along with test-negative individuals matched on date of testing at a 2:3 ratio. Results of all reverse-transcriptase polymerase chain reaction tests were obtained from the national COVID-19 surveillance system at the Statens Serum Institut.7 EFTER-COVID was designed to contain a baseline questionnaire and a number of follow-up questionnaires comprising different study tracks, each focusing on physical, cognitive, or fatigue-related symptoms. Notably, the design was organized so that each invited individual was randomly assigned to be followed up in only 1 of the 3 study tracks, in order to keep questionnaires brief and accessible, while still providing rich data on all tracks. In the current study, we included data from the baseline questionnaire and the follow-up questionnaire, completed 1 and 4 months after the date of testing positive or negative, respectively.
Infections with either the Delta variant or the Omicron variant were defined on the basis of periods of predominance (periods where it has been estimated that the variant accounted for more than 95% of cases based on national surveillance with whole genome sequencing or variant reverse-transcriptase polymerase chain reaction). The Delta period was defined as July 15, 2021-November 15, 2021, and the Omicron period was defined as December 28, 2021-January 15, 2022. Individuals who were tested during the intermediate transitional period were not included in the study.8
Exclusion criteria
We excluded the following participants: (1) cases who were reinfected during the Omicron period, (2) controls who reported having been found seropositive between the test date and the date of completing the 4-month follow-up questionnaire, and (3) individuals who received the first dose of vaccine within 14 days prior to testing for SARS-CoV-2—that is, without the full effect of the first vaccine dose on the testing date (see Figure S1, available at https://doi.org/10.1093/aje/kwad225).
Data sources
The baseline questionnaire contained questions on acute symptoms and on lifestyle, education, employment, physical condition, alcohol consumption, smoking, height, weight, and selected chronic diseases. The follow-up questionnaire contained questions on study-track–specific symptoms and new-onset general health problems after testing for SARS-CoV-2, referring to the time period comprising the 14 days prior to questionnaire completion, 4 months after the test. The questions on new-onset general health problems were asked of all participants, regardless of the study track. Several of the tracks were based on validated questionnaires. The fatigue-related track was based on the Fatigue Assessment Scale (FAS) (©FAS, ILD Care Foundation; www.ildcare.nl)9-11 together with postexertional malaise (PEM)-related questions from the DePaul Symptom Questionnaire,12 and the cognitive symptoms track was based on the Cognitive Complaints in Bipolar Disorder Rating Assessment (COBRA).13,14
For a response to be considered complete, it was obligatory to respond to all of the questions, except for the questions on height, weight, and alcohol consumption. The 4-month follow-up questionnaire is shown in Appendix S1.
All individuals residing in Denmark are assigned a unique personal identification number in the Danish Civil Registration System.15 We used this personal identifier to link information on relevant covariates obtained from national registers. This included data on age and sex from the Civil Registration System,15 comorbid conditions from the Danish National Patient Register,16 and health-care occupation from the national COVID-19 surveillance system.17,18 The information on comorbid conditions (including dates of hospitalizations16 and corresponding diagnoses19) was used to calculate the Charlson comorbidity index for every participant based on hospital contacts during the 5 years before the test date.
The COVID-19 vaccination status of participants was obtained from the Danish Vaccination Register,20 which contains individual-level and linkable information on all vaccines administered in Denmark, including the date of vaccination and the type of vaccine used. In Denmark, the SARS-CoV-2 vaccines predominantly used have been (1) the Pfizer/BioNTech vaccine (BNT162b2; Pfizer, Inc, and BioNTech SE), comprising 86.1% of all SARS-CoV-2 vaccines administered (based on second doses given before May 24, 2022); (2) the Moderna vaccine (mRNA-1273; Moderna, Inc), comprising 13.8% of all vaccines; (3) the Oxford-AstraZeneca vaccine (ChAdOx1-S; Oxford University and AstraZeneca AB), comprising 0.1%; and (4) the Janssen (Johnson & Johnson) vaccine (Ad26.COV2.S; Janssen Pharmaceutica), comprising less than 0.1%.21
Statistical analysis
Outcome prevalences between (1) Omicron cases and controls, (2) Omicron cases and Delta cases, and (3) Omicron cases vaccinated with 2 doses and cases vaccinated with 3 doses were compared using risk differences (RDs). RDs, along with 95% CIs, were estimated using parametric g-computation22-25 on logistic regression; the estimates were adjusted for age, sex, body mass index, self-reported chronic diseases from the baseline questionnaire, Charlson comorbidity index, health-care occupation, and vaccination status. When comparing cases vaccinated with 2 doses during the Omicron period with those vaccinated with 3 doses, estimates were additionally adjusted for the week of infection due to the possible differential impact of the BA.1 and BA.2 subvariants gradually developing at the beginning of the Omicron period. The 95% CIs were estimated using bootstrap resampling with replacement (1000 iterations). We considered the following outcomes: (1) postacute symptoms appearing within 14 days prior to filling out the 4-month follow-up questionnaire and (2) general health problems (difficulties concentrating, memory issues, mental exhaustion, physical exhaustion, and sleep problems) arising within 14 days prior to filling out the 4-month follow-up questionnaire, given that there was no experience of each of the latter health problems in the 6-month period leading up to the test date (new onset).
Charlson comorbidity index was included in the analyses as a categorical variable with 4 levels: scores of 0, 1, 2, or ≥3. In the baseline questionnaire, participants were asked supplementary questions about relevant chronic diseases commonly treated in primary care (diabetes, asthma, high blood pressure, chronic obstructive pulmonary disease or other lung disease, headache or migraine, and other chronic disease). The presence of any of these chronic diseases was included in the analyses as a dichotomous variable (yes/no). Body mass index (weight (kg)/height (m)2) was included as a categorical variable with 3 levels: (1) obese (obesity was defined as a body mass index ≥30 for individuals aged 18 years or more, and for adolescents aged 15‑17 years international cutoff points for obesity by sex and age were used26); (2) nonobese; and (3) unknown (when height or weight information was missing from the baseline questionnaire). Vaccination status comprised a combination of number of vaccinations and timing of the most recent vaccination, defined as a categorical variable with 7 levels: (1) unvaccinated; (2) vaccinated with 1 dose within 3 months prior to testing; (3) vaccinated with 1 dose more than 3 months prior to testing; (4) vaccinated with 2 doses, with the second dose given within 3 months prior to testing; (5) vaccinated with 2 doses, with the second dose given more than 3 months prior to testing; (6) vaccinated with 3 doses, with the third dose given within 3 months prior to testing; and (7) vaccinated with 3 doses, with the third dose given more than 3 months prior to testing. When comparing cases vaccinated with 2 doses during the Omicron period to cases vaccinated with 3 doses, vaccination status was included as a dichotomous variable with 2 levels: (1) second dose of COVID-19 vaccine given within 3 months prior to testing and (2) second dose of COVID-19 vaccine given more than 3 months prior to testing.
In addition, FAS9-11 scores for fatigue were dichotomized into 2 groups: no fatigue (10‑21) and substantial fatigue (22‑50). PEM12 scores (DePaul Symptom Questionnaire) were dichotomized into “PEM present,” defined as frequency and severity scores of at least 2 (“about half of the time”) and 2 (“moderate”) on any question, respectively, and “PEM not present otherwise” (see Appendix S1, pp. 14-15). COBRA13,14 scores for cognitive complaints were dichotomized into normal (0-8.56) and caseness (8.57-48). Track-specific scores (except for the noncumulative PEM score) and the number of postacute physical symptoms 4 months after testing for SARS-CoV-2 were modeled as count outcomes using Poisson regression in order to obtain rate ratios (RRs).
Data management and statistical analyses were conducted using R software, version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).27 The R packages “riskCommunicator”28 and “forestploter” 29 were used for modeling and generating forest plots, respectively.
Results
Participants
Among EFTER-COVID participants tested during the study inclusion period (July 15, 2021-November 15, 2021 and December 28, 2021-January 15, 2022) who had completed the baseline questionnaire, 16 088 individuals with a positive test during the period of Delta predominance and 66 670 individuals with either a positive (n = 34 086) or a negative (n = 32 584) test during the period of Omicron predominance had received an invitation to complete the 4-month follow-up questionnaire by May 16, 2022. The 4-month follow-up questionnaire was completed by 13 577 (39.8%) of the cases and 15 809 (48.5%) of the controls in the Omicron period, respectively, and 8186 (50.9%) of the cases in the Delta period.
We excluded 291 Omicron-reinfected cases, 951 control participants who reported testing seropositive between the test date and the time of completion of the 4-month follow-up questionnaire, and 221 participants who completed a primary vaccination course within 14 days prior to testing for SARS-CoV-2—that is, without the full effect of the first vaccine dose on the testing date (Figure S1).
The final study cohort (n = 36 109) comprised 28 128 participants from the Omicron period (13 274 cases and 14 854 controls) and 7981 cases from the Delta period. With regard to sex and age, among those who were tested during the Omicron period, 17 046 (60.6%) were females and 11 082 (39.4%) were males, with median ages of 57 years (interquartile range (IQR), 46‑66) and 62 years (IQR, 52‑71), respectively. Correspondingly, among participants who tested positive during the Delta period, 4820 (60.4%) were females and 3161 (39.6%) were males, with median ages of 53 years (IQR, 40‑65) and 59 years (IQR, 46‑70), respectively. Among participants who were tested during the Omicron period, 11 426 (40.6%) reported at least 1 chronic disease, whereas the corresponding number and proportion for the Delta period were 3078 (38.6%). According to the EFTER-COVID study design, the distribution of participants within each study track during the Omicron period was as follows: physical track, 15 211 (54.1%); fatigue-related track, 6377 (22.7%); and cognitive track, 6540 (23.3%). During the Delta period, the distribution within each track was similar: physical track, 4231 (53%); fatigue-related track, 1831 (22.9%); and cognitive track, 1919 (24%). Across all study tracks during the Omicron and Delta periods and regardless of test result, study participants more often were middle-aged, were female, and had a low Charlson comorbidity index. High blood pressure was the most frequently self-reported chronic disease (Table S1, Table S2). Table S3 shows the characteristics of participants and nonparticipants at the 4-month follow-up questionnaire.
Risk of postacute symptoms 4 months after SARS-CoV-2 testing
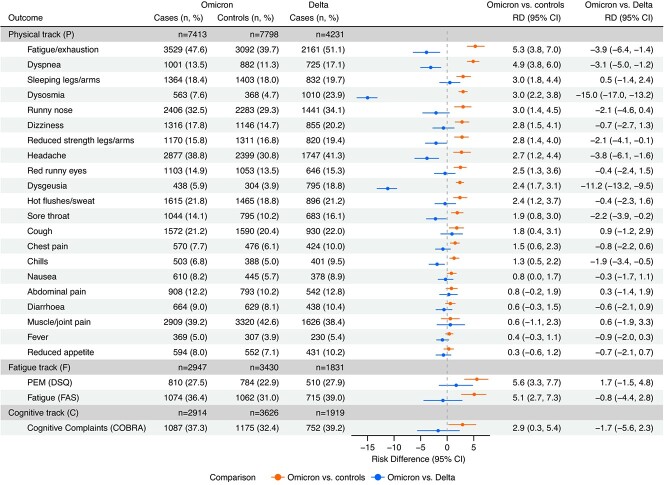
Among cases diagnosed during the Omicron period, the most prominent postacute symptoms 4 months after infection were fatigue/exhaustion (47.6%), muscle/joint pain (39.2%), headache (38.8%), and runny nose (32.5%) (Figure 1). Cases diagnosed during the Delta period reported similar prevalences of the following symptoms: fatigue/exhaustion (51.1%), muscle/joint pain (38.4%), headache (41.3%), and runny nose (34.1%). When comparing cases with controls during the Omicron period, RDs were elevated for 18 out of 24 postacute symptoms. We observed the largest RDs for PEM (RD = 5.6%; 95% CI, 3.3-7.7), fatigue/exhaustion (RD = 5.3%; 95% CI, 3.8-7.0), substantial fatigue (FAS; RD = 5.1%; 95% CI, 2.7-7.3), and dyspnea (RD = 4.9%; 95% CI, 3.8-6.0). In contrast, when comparing symptoms between Omicron and Delta cases, we observed significantly lower RDs for 8 out of the aforementioned 18 postacute symptoms (Figure 1). However, the most remarkable result was the large risk reductions for dysosmia (RD = –15.0%; 95% CI, –17.0 to −13.2) and dysgeusia (RD = –11.2%; 95% CI, –13.2 to −9.5) after infection during the Omicron period, as compared with the Delta period.
Figure 1.
Risk differences (RDs) for postacute coronavirus disease 2019 symptoms in a comparison of Omicron cases with Omicron controls and Omicron cases with Delta cases 4 months after reverse-transcriptase polymerase chain reaction testing for severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection (n = 36 109), Denmark, July 2021-January 2022. The period of SARS-CoV-2 Delta variant predominance was July 15, 2021-November 15, 2021; the period of SARS-CoV-2 Omicron variant predominance was December 28, 2021‑January 15, 2022. RDs were adjusted for age, sex, body mass index, self-reported chronic diseases, Charlson comorbidity index, health-care occupation, and vaccination status. “Omicron vs. controls” and “Omicron vs. Delta” refer to the comparison of Omicron cases with Omicron controls and the comparison of Omicron cases with Delta cases, respectively. Omicron controls and Delta cases were used as the reference groups, respectively. Sample sizes for each study track by variant and test result are included. Bars represent 95% confidence intervals (CIs). COBRA, Cognitive Complaints in Bipolar Disorder Rating Assessment; DSQ, DePaul Symptom Questionnaire; FAS, Fatigue Assessment Scale; PEM, postexertional malaise.
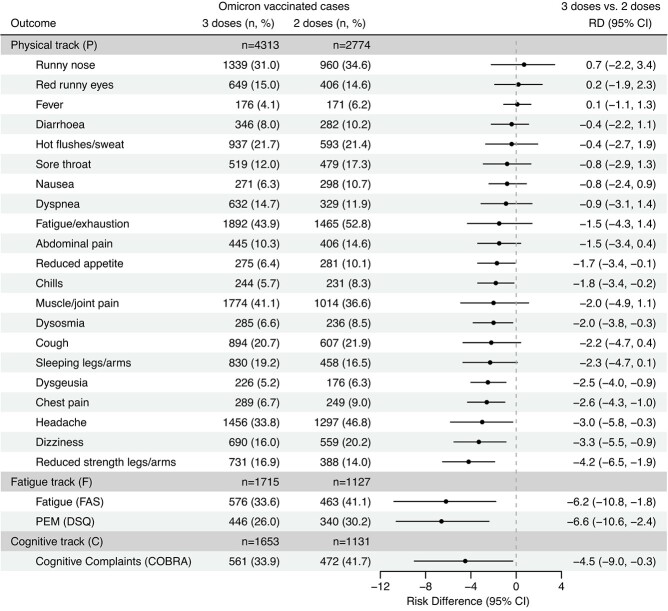
When comparing cases vaccinated with 3 doses to cases vaccinated with 2 doses during the Omicron period, we observed significantly decreased RDs for 11 out of 24 postacute symptoms and no significantly increased RDs (Figure 2). The largest RDs were observed for PEM (RD = –6.6%; 95% CI, –10.6 to −2.4), substantial fatigue (FAS; RD = –6.2%, 95% CI: –10.8 to −1.8), and reduced strength in the legs/arms (RD = –4.2%, 95% CI: –6.5 to −1.9).
Figure 2.
Risk differences (RDs) for postacute coronavirus disease 2019 (COVID-19) symptoms in a comparison of cases vaccinated with 3 doses of COVID-19 vaccine to cases vaccinated with 2 doses, 4 months after infection with severe acute respiratory syndrome coronavirus (SARS-CoV-2) during the period of SARS-CoV-2 Omicron variant predominance (n = 12 713), Denmark, December 2021-January 2022. The Omicron period was December 28, 2021-January 15, 2022. RDs were adjusted for age, sex, body mass index, self-reported chronic diseases, Charlson comorbidity index, health-care occupation, vaccination status (timing of vaccination with second dose), and week of infection during the Omicron period. Omicron cases vaccinated with 2 doses were used as the reference group. Sample sizes for each study track by vaccine dose are included. Bars represent 95% confidence intervals (CIs). COBRA, Cognitive Complaints in Bipolar Disorder Rating Assessment; DSQ, DePaul Symptom Questionnaire; FAS, Fatigue Assessment Scale; PEM, postexertional malaise.
Risk of general health problems with new onset 4 months after SARS-CoV-2 testing
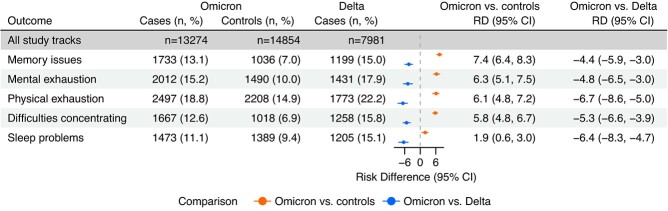
At least 1 postacute health problem with new onset 4 months posttest was reported by 4991 (37.6%) cases and 4437 (29.9%) controls during the Omicron period and 3370 (42.2%) cases during the Delta period. When comparing cases with controls during the Omicron period, RDs for all 5 postacute health problems with new onset were significantly increased. The largest RDs were observed for memory issues (RD = 7.4%; 95% CI, 6.4-8.3), followed by mental exhaustion (RD = 6.3%; 95% CI, 5.1-7.5), physical exhaustion (RD = 6.1%; 95% CI, 4.8-7.2), difficulties concentrating (RD = 5.8%; 95% CI, 4.8-6.7), and sleep problems (RD = 1.9%; 95% CI, 0.6-3.0) (Figure 3). When comparing cases diagnosed during the Omicron period with cases diagnosed during the Delta period, we observed significantly reduced RDs for all 5 new-onset health problems: memory issues (RD = –4.4%; 95% CI, –5.9 to −3.0), mental exhaustion (RD = –4.8%; 95% CI, –6.5 to −3.0), physical exhaustion (RD =–6.7%; 95% CI, –8.6 to −5.0), difficulties concentrating (RD = –5.3%; 95% CI, –6.6 to −3.9), and sleep problems (RD = –6.4%; 95% CI, –8.3 to −4.7) (Figure 3).
Figure 3.
Risk differences (RDs) for new-onset general health problems in a comparison of coronavirus disease 2019 Omicron cases with Omicron controls and Omicron cases with Delta cases 4 months after reverse-transcriptase polymerase chain reaction testing for severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection (n = 36 109), Denmark, July 2021-January 2022. The period of SARS-CoV-2 Delta variant predominance was July 15, 2021-November 15, 2021; the period of SARS-CoV-2 Omicron variant predominance was December 28, 2021-January 15, 2022. RDs were adjusted for age, sex, body mass index, self-reported chronic diseases, Charlson comorbidity index, health-care occupation, and vaccination status. “Omicron vs. controls” and “Omicron vs. Delta” refer to the comparison of Omicron cases with Omicron controls and the comparison of Omicron cases with Delta cases, respectively. Omicron controls and Delta cases were used as the reference groups, respectively. Sample sizes by variant and test result are included. Bars represent 95% confidence intervals (CIs).
We estimated RDs for postacute general health problems with new onset 4 months after testing positive for SARS-CoV-2 during the Omicron period, comparing participants vaccinated with 2 doses to those vaccinated with 3 doses. We observed that cases vaccinated with 3 doses less frequently reported new-onset mental exhaustion (RD = –4.2%; 95% CI, –6.6 to −1.8), memory issues (RD = –2.5%; 95% CI, –4.5 to −0.4), and difficulties concentrating (RD = –2.3%; 95% CI, –4.1 to −0.4) compared with those vaccinated with 2 doses, 4 months after infection during the Omicron period (Figure 4).
Figure 4.
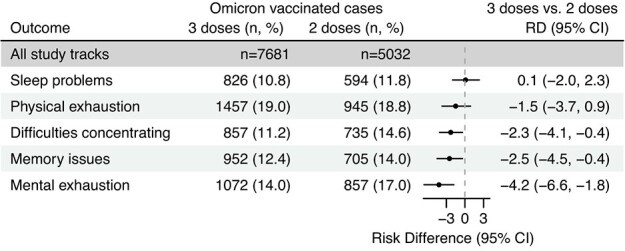
Risk differences (RDs) for new-onset general health problems in a comparison of coronavirus disease 2019 (COVID-19) cases vaccinated with 3 doses of COVID-19 vaccine to cases vaccinated with 2 doses, 4 months after infection with severe acute respiratory syndrome coronavirus (SARS-CoV-2), during the Omicron period (n = 12 713), Denmark, December 2021-January 2022. The period of SARS-CoV-2 Omicron variant predominance was December 28, 2021-January 15, 2022. RDs were adjusted for age, sex, body mass index, self-reported chronic diseases, Charlson comorbidity index, health-care occupation, vaccination status (timing of vaccination with second dose), and week of infection during the Omicron period. Omicron cases vaccinated with 2 doses were used as the reference group. Sample sizes by vaccine dose are included. Bars represent 95% confidence intervals (CIs).
Track-specific scores and number of postacute physical symptoms 4 months after SARS-CoV-2 testing
During the period of Omicron predominance, cases had 14% more postacute physical symptoms than controls (RR = 1.14; 95% CI, 1.12-1.16). Conversely, Omicron cases had 11% fewer postacute physical symptoms than Delta cases (RR = 0.89; 95% CI, 0.86-0.91) 4 months after the test date. Cases vaccinated with 3 doses before infection during the Omicron period had 8% fewer postacute physical symptoms than those vaccinated with 2 doses (RR = 0.92; 95% CI, 0.89-0.95) 4 months after a positive test. Results observed when modeling track-specific scores (Table S4, Figure S2) were consistent with the trends observed for the results obtained from the RD models (Figure 1, Figure 2).
Discussion
Key findings
Four months after testing positive for SARS-CoV-2 during the Omicron period, COVID-19 cases more frequently reported postacute symptoms and health problems with new onset than did controls in the same period and less frequently reported postacute symptoms and health problems with new onset than did cases during the period of Delta predominance. Moreover, among cases diagnosed during the Omicron period, participants who had received 3 doses of COVID-19 vaccine before infection reported fewer symptoms than participants who had received 2 doses of vaccine.
Other studies
Persistence of postacute symptoms after infection with SARS-CoV-2 has also been reported by other investigators,2,4,30-32 with regard to primarily pre-Omicron variants; however, differences in estimates of risk, time of measurement, and definitions of long COVID vary from study to study and should be taken into account in interpretation. In several studies, fatigue and dyspnea comprised the 2 main postacute symptoms up to 4 months after a positive test during both the Delta and Omicron periods, in line with the findings of the present study.3,4,33 Interestingly, in a Norwegian register-based study,4 post–COVID-19 complaints in general practice persisted to a similar extent after both Omicron and Delta infections, in contrast to our observations that Omicron is associated with lower risk of numerous postacute symptoms and new-onset general health problems, when compared with Delta. In a UK study on self-reported symptom data, Antonelli et al3 recently reported that experiencing long COVID was less common after infection during the Omicron period compared with the Delta period, with reported prevalences of 4.5% and 10.8%, respectively.
Postacute symptoms arising within 2-12 months of infection with pre-Omicron variants were more frequently reported in individuals hospitalized during the acute phase of infection than in those with mild infection.2,30,34-36 In a UK study on COVID-19 risk factors, vaccination was associated with reduced odds of hospitalization.37
The latter study was also included in a recent meta-analysis5 in which vaccination with 1 or 2 doses before pre-Omicron infections protected against long COVID in some studies, but not all (odds ratios ranged between 0.22 and 1.93). Our results suggest that vaccination with a third dose provides some protection against postacute symptoms and new-onset general health conditions after infection during Omicron, compared with being vaccinated with 2 doses. Our finding is reassuring, since even though vaccination reduces the severity of COVID-19, its impact on preventing or treating long COVID has been unclear.5 Hence, reliance on vaccination as a sole mitigation strategy may not optimally reduce the societal risk of long COVID5—for example, due to low vaccine uptake and no evidence of a strong preventive effect. Therefore, adequate follow-up in future vaccine trials would be beneficial in order to define and evaluate long COVID as an outcome.5
In Denmark, the number of monthly referrals to specialized long COVID clinics in major hospitals has decreased remarkably during the Omicron period as compared with the period before the emergence of the Omicron variant,36 consistent with the findings of the present study observing fewer postacute sequelae 4 months after infection.
Strengths and limitations
The key strengths of the present study were its remarkable study population size and the inclusion of date-matched controls, which allowed us to take the background prevalence of symptoms and general health conditions into account. The questionnaires were designed to minimize potential recall bias; in the 4-month follow-up questionnaire, all questions on postacute symptoms and new-onset general health problems referred to the past 14 days. Furthermore, in contrast to many previous studies which evaluated primarily unvaccinated individuals, the vast majority of the study population were fully vaccinated with 2 or 3 doses and nonhospitalized, thus enabling a unique long COVID study focused primarily on persons who experienced mild disease during the acute phase of infection with SARS-CoV-2.
The main limitations of the present study were its self-reporting nature, potential participation bias, and the lack of direct testing for variants. Regarding persons who underwent testing for SARS-CoV-2 during the Omicron period, higher response rates were reported among controls than among cases, which reduces concern about selection bias. Regarding test-positives, we excluded persons whose positive test referred to reinfection with SARS-CoV-2, in order to minimize the impact of postacute symptoms potentially caused by an earlier infection; thus, our results are generalizable only to first-time infections. The lack of direct variant verification at the individual level is unlikely to have had an impact on our results given that during the periods studied, the dominating variant accounted for more than 95% of all cases. Furthermore, any misclassification is likely to have been nondifferential and therefore unlikely to have influenced our findings.
Perspectives
Postacute symptoms after infection with SARS-CoV-2 during the Omicron period, along with the potential severity and duration of SARS-CoV-2 infection during this period, encompass a notable concern given the number of infections that have occurred globally. By utilizing self-reported information on health outcomes combined with registry data, the present study provides much needed information on the most recent and most common SARS-CoV-2 variant causing infections during the pandemic. This can help public health authorities better evaluate the full impact of different pandemic strategies and help patients better understand a condition about which we still have much to learn. More research on long COVID is urgently needed, particularly on severity and duration, as well as studies attempting to identify long COVID phenotypes consisting of multiple symptoms and health problems.
Conclusion
In the present nationwide questionnaire study, we found that infection with SARS-CoV-2 during the period of Omicron predominance was associated with postacute symptoms and general health problems with new onset, 4 months after a positive test; however, compared with infections diagnosed during the Delta period, symptoms and health problems were reassuringly less common. In comparison with persons with 2 doses of vaccine, vaccination with a third dose before infection with SARS-CoV-2 during the Omicron period was associated with fewer postacute symptoms and general health problems with new onset 4 months after a positive test.
Supplementary Material
Acknowledgments
We thank all EFTER-COVID participants for filling in the questionnaire. We also thank the members of the EFTER-COVID stakeholder group, consisting of representatives from research institutions, hospital departments, and health institutions, for their comments on the questionnaires.
Contributor Information
Lampros Spiliopoulos, Department of Epidemiology Research, Statens Serum Institut, 2300 Copenhagen, Denmark.
Anna Irene Vedel Sørensen, Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, 2300 Copenhagen, Denmark.
Peter Bager, Department of Epidemiology Research, Statens Serum Institut, 2300 Copenhagen, Denmark; Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, 2300 Copenhagen, Denmark.
Nete Munk Nielsen, Department of Epidemiology Research, Statens Serum Institut, 2300 Copenhagen, Denmark; Focused Research Unit in Neurology, Department of Neurology, Hospital of Southern Jutland, University of Southern Denmark, 6200 Aabenraa, Denmark.
Jørgen Vinsløv Hansen, Department of Epidemiology Research, Statens Serum Institut, 2300 Copenhagen, Denmark.
Anders Koch, Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, 2300 Copenhagen, Denmark; Department of Infectious Diseases, Rigshospitalet University Hospital, 2100 Copenhagen, Denmark; Global Health Section, Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, 1014 Copenhagen, Denmark.
Inger Kristine Meder, Department of Epidemiology Research, Statens Serum Institut, 2300 Copenhagen, Denmark.
Poul Videbech, Centre for Neuropsychiatric Depression Research, Mental Health Centre Glostrup, 2600 Glostrup, Denmark; Institute for Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Steen Ethelberg, Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, 2300 Copenhagen, Denmark; Global Health Section, Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, 1014 Copenhagen, Denmark.
Anders Hviid, Department of Epidemiology Research, Statens Serum Institut, 2300 Copenhagen, Denmark; Pharmacovigilance Research Centre, Department of Drug Design and Pharmacology, University of Copenhagen, 2100 Copenhagen, Denmark.
Funding
This study was conducted as part of the Statens Serum Institut’s advisory tasks for the Danish Ministry of Health.
Data availability
The data used in this study comprised sensitive individual-level information from completed questionnaires and national registry data. According to Danish data protection legislation, the authors are not allowed to share these sensitive data directly upon request. However, the data are available for research upon reasonable request to the Danish Health Data Authority (registry data; e-mail: kontakt@sundhedsdata.dk) and Statens Serum Institut (questionnaire data; e-mail: aii@ssi.dk) within the framework of the Danish data protection legislation and any required permission from authorities.
Conflict of interest
The authors received no support from any organization for this work. N.M.N. reports receiving grants from the A.P. Møller Foundation, Lilly and Herbert Hansen’s Fund, and the Greenland Research Council, all unrelated to the present study. A.K. is the President of the Danish Greenlandic Society for Circumpolar Health and the Past President of the International Union for Circumpolar Health, unrelated to this study. A.H. is a Scientific Board Member of the Vaccine Monitoring Collaboration for Europe (VAC4EU) and reports receiving grants from the Novo Nordisk Foundation, the Danish Medical Research Council, the European Medicines Agency, the Lundbeck Foundation, and the Global Vaccine Data Network, all unrelated to the present study. The authors report no other relationships or activities that could appear to have influenced this work.
References
- 1. World Health Organization . A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. Published October 6, 2021. Accessed October 4, 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- 2. Sørensen AIV, Spiliopoulos L, Bager P, et al. A nationwide questionnaire study of post-acute symptoms and health problems after SARS-CoV-2 infection in Denmark. Nat Commun. 2022;13(1):4213–4218. 10.1038/s41467-022-31897-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antonelli M, Pujol JC, Spector TD, et al. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–2264. 10.1016/S0140-6736(22)00941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magnusson K, Kristoffersen DT, Dell’Isola A, et al. Post-covid medical complaints after SARS-CoV-2 Omicron vs Delta variants—a prospective cohort study. medRxiv. 10.1101/2022.05.23.22275445, May 25, 2022, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Byambasuren O, Stehlik P, Clark J, et al. Impact of COVID-19 vaccination on long COVID: a systematic review and meta-analysis. medRxiv. 10.1101/2022.06.20.22276621, June 22, 2022, preprint: not peer reviewed. [DOI] [Google Scholar]
- 6. Statens Serum Institut . EFTER-COVID [in Danish]. Updated December 11, 2023. Accessed August 29, 2022. https://covid19.ssi.dk/overvagningsdata/undersoegelser/efter-covid
- 7. Schønning K, Dessau RB, Jensen TG, et al. Electronic reporting of diagnostic laboratory test results from all healthcare sectors is a cornerstone of national preparedness and control of COVID-19 in Denmark. APMIS. 2021;129(7):438–451. 10.1111/apm.13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michlmayr D, Hansen CH, Gubbels SM, et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: a Danish cohort study using two years of nationwide PCR-test data. SSRN. 10.2139/ssrn.4054807, March 10, 2022, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J. 2012;40(1):255–263. 10.1183/09031936.00002512 [DOI] [PubMed] [Google Scholar]
- 10. De Kleijn WPE, De Vries J, Wijnen PAHM, et al. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med. 2011;105(9):1388–1395. 10.1016/j.rmed.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 11. De Vries J, Michielsen H, Van Heck GL, et al. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS). Br J Health Psychol. 2004;9(3):279–291. 10.1348/1359107041557048 [DOI] [PubMed] [Google Scholar]
- 12. Cotler J, Holtzman C, Dudun C, et al. A brief questionnaire to assess post-exertional malaise. Diagnostics. 2018;8(3):66. 10.3390/diagnostics8030066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen JH, Støttrup MM, Nayberg E, et al. Optimising screening for cognitive dysfunction in bipolar disorder: validation and evaluation of objective and subjective tools. J Affect Disord. 2015;187:10–19. 10.1016/j.jad.2015.07.039 [DOI] [PubMed] [Google Scholar]
- 14. Rosa AR, Mercadé C, Sánchez-Moreno J, et al. Validity and reliability of a rating scale on subjective cognitive deficits in bipolar disorder (COBRA). J Affect Disord. 2013;150:29–36. 10.1016/j.jad.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CB, Gøtzsche H, Møller JØ, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. https://content.ugeskriftet.dk/sites/default/files/scientific_article_files/2018-10/dmb3816.pdf [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7):30–33. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 17. Danish Patient Safety Authority . Autorisationsregistret [in Danish]. Accessed October 23, 2023. https://stps.dk/sundhedsfaglig/autorisation/autorisationsregistret
- 18. Statistics Denmark. Dansk Branchekode DB07, v3:2014- [in Danish]. Accessed October 4, 2022. https://www.dst.dk/da/Statistik/dokumentation/nomenklaturer/db07
- 19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 20. Grove Krause T, Jakobsen S, Haarh M, et al. The Danish Vaccination Register. Euro Surveill. 2012;17(17):2. 10.2807/ese.17.17.20155-en [DOI] [PubMed] [Google Scholar]
- 21. Statens Serum Institut . Covid-19 Vaccine Dashboard (regional). [in Danish]. 2022. Accessed October 4, 2022. https://experience.arcgis.com/experience/9824b03b114244348ef0b10f69f490b4
- 22. Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol. 2011;173(7):731–738. 10.1093/aje/kwq472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Westreich D, Cole SR, Young JG, et al. The parametric g-formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat Med. 2012;31(18):2000–2009. 10.1002/sim.5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: a step-by-step illustration of causal inference methods. Am J Epidemiol. 2009;169(9):1140–1147. 10.1093/aje/kwp015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model. 1986;7(9-12):1393–1512. 10.1016/0270-0255(86)90088-6 [DOI] [Google Scholar]
- 26. Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J. 2000;320(7244):1240–1243. 10.1136/bmj.320.7244.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2022. Accessed October 4, 2022. http://www.R-project.org/ [Google Scholar]
- 28. Grembi JA, Rogawski McQuade ET. Introducing riskCommunicator: an R package to obtain interpretable effect estimates for public health. PloS One. 2022;17(7):e0265368. 10.1371/journal.pone.0265368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dayimu A. forestploter: Create Flexible Forest Plot. Version 1.1.1. Published March 21, 2022. Accessed January 4, 2024. https://cran.rstudio.com/web/packages/forestploter/index.html
- 30. Yoo SM, Liu TC, Motwani Y, et al. Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med. 2022;37(8):1988–1995. 10.1007/s11606-022-07523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021;21(10):1373–1382. 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 7 July 2022. Published July 7, 2022. Accessed July 7, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongo-ingsymptomsfollowingcoronaviruscovid19infectionintheuk/7july2022
- 34. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571–6512. 10.1038/s41467-021-26513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28(7):1461–1467. 10.1038/s41591-022-01840-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Danish Broadcasting Corporation . Klinniker oplever markant fald i antallet af patienter, der bliver henvist med coronasenfølger [in Danish]. Published June 26, 2022. Accessed October 4, 2022. https://www.dr.dk/nyheder/indland/klinikker-oplever-markant-fald-i-antallet-af-patienter-der-bliver-henvist-med
- 37. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22(1):43–55. 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study comprised sensitive individual-level information from completed questionnaires and national registry data. According to Danish data protection legislation, the authors are not allowed to share these sensitive data directly upon request. However, the data are available for research upon reasonable request to the Danish Health Data Authority (registry data; e-mail: kontakt@sundhedsdata.dk) and Statens Serum Institut (questionnaire data; e-mail: aii@ssi.dk) within the framework of the Danish data protection legislation and any required permission from authorities.