Abstract
Objective.
We assessed the association of APOE genotype with cerebrovascular disease (CVD) in a large neuropathological database maintained by the National Alzheimer’s Coordinating Center (NACC). Such a comprehensive investigation of APOE and CVD pathology has not heretofore been conducted. We focused on APOE e2, an established neuroprotective genetic variant against Alzheimer’s disease.
Methods.
To implement these objectives APOE associations in the NACC database of 1275 brains with 11 CVD pathologies, including old and recent infarcts, hemorrhages, cerebral amyloid angiopathy, and arteriosclerosis, were examined. These pathologies were uniformly and semi-quantitatively measured across 39 ADC sites. We utilized chi square statistics and ordinal regression to assess the significance of associations, and Bonferroni corrected for multiple comparisons.
Results.
Of the cases, 98 were e2/e3 or e2/e2 genotypes (“e2” carriers), 621 were e3 homozygotes (“e3” group), and 556 were e4/e3 (442) or e4/e4 (114) genotypes (“e4” group). Results indicated that the APOE e4 allele significantly increased risk for cerebral amyloid angiopathy (CAA). After stratification by CAA presence/absence, we found that in those cases in which CAA was present, APOE e2 significantly increased risk for gross hemorrhage. All other associations were negative. I
Conclusions.
In this, the largest study of APOE e2 effects on pathologically verified CVD, e2 was not protective against any CVD pathology compared to e3 homozygotes, including CAA. Regarding the latter pathology, e4 was associated with increases in its severity. Furthermore, and perhaps unexpectedly, e2 significantly increased risk of acute/subacute gross hemorrhage in the presence of CAA. Thus, there were limits to e2 neuroprotection against amyloidosis, despite its known and large protective effects against diffuse and neuritic amyloid plaques compared to e3/e3 and e4 carriers in this very collection.
Introduction
Cerebrovascular disease (CVD) is a frequent co-morbidity of Alzheimer’s disease (AD). In post-mortem studies, CVD may be present in more than 90% of AD cases, and cases of mixed dementia (AD + CVD) outnumber cases of “pure” AD. In a large post-mortem sample, 27% of cases had AD and CVD pathology, while only 3% had AD pathology only.(1) With respect to genetic risk for AD and CVD, apolipoprotein E (APOE) e4 associations with multiple brain vascular pathologies (e.g., infarcts, hemorrhages, cerebral amyloid angiopathy CAA) have been studied, but e2, the APOE allele associated with AD neuroprotection(2, 3) has not been examined systematically in relation to vascular pathologies. Here, we examined whether e2 has protective effects against common types of cerebrovascular disease in a large and well-characterized post-mortem series collected in the National Alzheimer’s Coordinating Center (NACC) v. 10 database. Importantly, our results are derived directly from post-mortem examination of brain tissue rather than inferentially from clinical and neuroimaging data.
The association of APOE e2 with cerebral amyloid angiopathy (CAA) and a major sequela of CAA, lobar intracerebral hemorrhage (ICH), has been inconsistent in post mortem studies. In a small meta-analysis of neuropathologic cases found that e4 but not e2 was associated with CAA,(4) though more recent neuropathology studies have found that both e2 and e4 were associated with CAA.(5, 6) With respect to ICH, Biffi et al (N>6000 cases and controls) found that both e2 and e4 increased risk of lobar ICH based on results from brain imaging data.(7) An earlier meta-analysis based on imaging and clinical findings found that e2, but not e4, increased this risk.(8)
We interrogated the NACC post-mortem human brain v10 database in particular because it used up-to-date pathological criteria, advanced immunohistochemical techniques, and had a large number of cases. Critically, we elected not to utilize clinical diagnoses, AD related or otherwise for AD, for our analytic strategy. Our rationale was to make as few assumptions as possible about clinical phenotypic validity or reliability or about formal diagnostic neuropathologic criteria. Rather, we sought to understand the impact of APOE genotype on a frequent co-morbidity of AD, which is CVD. We believe that this approach not only is novel but more importantly unbiased and powerful as we were able to include all cases in the collection with CVD data (>1200). Furthermore, it minimizes errors in clinical diagnosis or inferences from neuroimaging diagnoses. These diagnostic approaches may have varying degrees of reliability and/or be inconsistent with neuropathology, which remains the gold standard for the diagnoses of CVD and AD.(9, 10)
Methods
We accessed the NACC Neuropathology Data Set v. 10 (December 2016) to conduct this study. It is the most recent version of the NACC neuropathology database with increased granularity for CVD variables. It assembles neuropathological data collected in a uniform manner from 39 Alzheimer’s Disease Center (ADC) sites in the United States. Semi-quantitative ratings were made by immunohistochemistry, histochemistry, microscopic visualization, or visual inspection and appropriate regional examinations. It includes 1275 cases with CVD data and is derived from the updated 2014 neuropathology forms.(11, 12) Severity ratings for each pathology are described in detail in the Codebook, as well as criteria for the identification of pathology. In all cases 0 indicates absence of pathology and higher numbers indicate more severe pathology. Several variables were rated dichotomously for presence or absence of CVD pathology (e.g., gross hemorrhage). With respect to gross hemorrhage, NACC does not have a not have a specific measure of lobar intracerebral hemorrhage, but we assume that gross hemorrhage captures the large majority of cases of lobar hemorrhage in this sample We utilized all brains in the v. 10 collection of NACC with neuropathological CVD measures, irrespective of clinical diagnosis.
Approximately 44% of the sample were e4 carriers and 8% were e2/e2 or e2/e3 carriers, which were combined to classify e2-positive cases. E3 homozygotes comprised 48% of the sample. Approximately 51% of the sample had high probabilities of AD neuropathological change as based on ABC score. Table 1 lists the APOE genotypic Ns, demographics, and the proportion of cases for each genotype that meet ABC criteria for “high” AD neuropathology change score. Additionally, over 95% of the sample had at least one CVD pathology. In the demographic table the percentage of cases demonstrating CVD pathology by APOE genotype is also displayed.
Table 1.
Demographics of the NACC v. 10 CVD Sample
| Genotype | N | Age | Sex (%female) | AD ABC Neuropath Change: % med and high prob | Any vascular pathology % |
|---|---|---|---|---|---|
| e2/e2 & e2/e3 | 98 | 80.37+12.66 | 56.1 | 29.80 | 97.96 |
| e3/e3 | 621 | 82.50+11.25 | 53.6 | 61.03 | 97.10 |
| e3/e4 | 442 | 80.24+10.09 | 51.1 | 84.62 | 99.10 |
| e4/e4 | 114 | 75.43+8.91 | 56.1 | 86.49 | 100.0 |
Nearly all (95%) of the sample was self-identified as Caucasian. Generalizability to other ethnic groups may be limited.
Statistical Approach.
Our statistical plan follows. All analyses were conducted in SAS 9.4.
We first conducted a series of chi square analyses in order to determine if there were disproportionate frequencies of one or another APOE genotype namely “e2” (comprised of e2/e2s and e2/e3 cases), “e3” (e3 homozygotes) and “e3/e4” or e4/e4” (comprised of e3/e4 and e4/e4 cases) associated with neuropathological staging or presence/absence of pathology. Importantly the e2/e4 genotype (N=37 cases) was not included in these analyses. It was examined in a separate series of analyses.
If findings were positive, we refined our analysis by conducting two planned contrasts in regression models in which e2 was contrasted with e3, and e2 was contrasted with e4, as predictors. In these regressions we adjusted for sex and age at death. If the pathological outcome was binary, we utilized logistic regression. If the outcome was ordinal, we utilized ordinal regression.
Given the number of chi-square analyses that we conducted, we used a Bonferroni correction to reduce the probability of type I error. Thus, for 11 chi square analyses, we set significance at p<.0045. We considered .004<p<.01 trend level significance. For planned contrasts using ORs, we considered p<.05 significant. P values for ORs were derived from maximum likelihood estimate Wald chi squares. For chi square e3/e4 and e4/e4 were separated. For the regressions they were combined.
We elected to examine APOE genotype associations with the following comprehensive vascular pathologies obtained in the NACC post-mortem brain series: parenchymal pathologies including old and recent infarcts and microinfarcts and old and recent hemorrhages and microhemorrhages, We also examined vessel pathologies, including arteriosclerosis and CAA (13). Last, hippocampal sclerosis was examined, as it has been associated in some cohorts with CVD (14). The diagnosis of hippocampal sclerosis did not include the presence of TDP-43 pathology as a criterion.
Results
CAA.
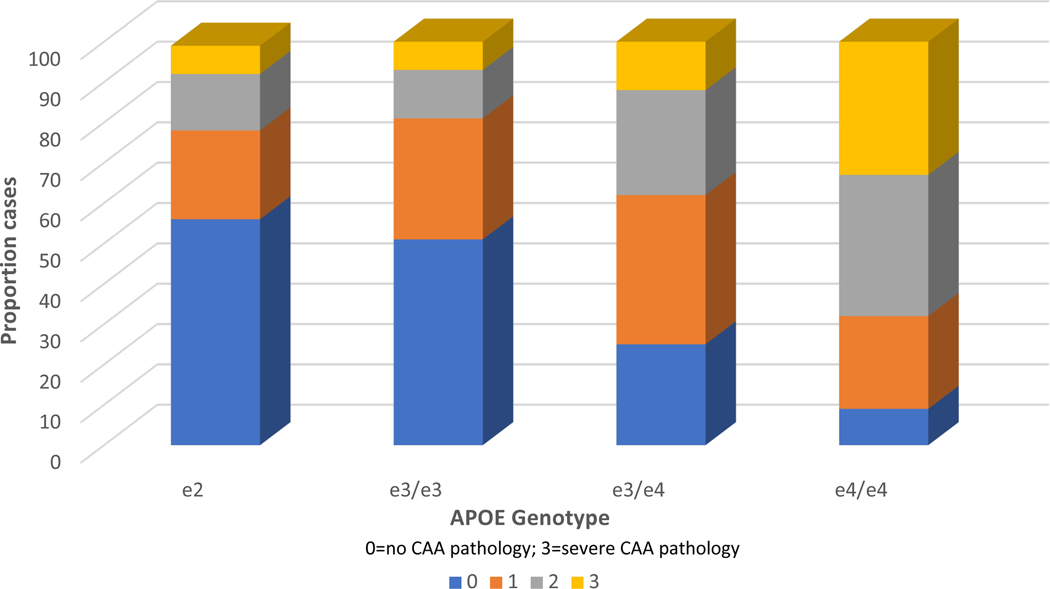
CAA was observed in 61% of all cases in the database. APOE was significantly associated with CAA (X2=188.47, p<.0001) and as can be seen in be seen in both Figure 1 and Table 2, e4 was associated with a disproportionate number of CAA cases. APOE e2 and e3 did not differ in ordinal regression (p>.10). E2 was, however, significantly associated with reduced severity of CAA when contrasted with e4 in ordinal regressions (OR=.24, 95% CI .16-.34, p<.0001), as was e3 (data not shown).
Figure 1.
APOE genotype frequencies associated with CAA severity levels. Within each genotype column, coloured rows represent the relative proportion of cases in each severity stage. These proportions are expressed as percentages and add to 100. Note especially the increased proportion of the most severe CAA pathology in E4 cases. APOE, apolipoprotein E; CAA, cerebral amyloid angiopathy.
Table 2.
APOE Genotype by CAA Severity
| APOE | 0 | 1 | 2 | 3 | Total |
|---|---|---|---|---|---|
| E2 | 55 | 22 | 14 | 7 | 98 |
| E3/E3 | 314 | 188 | 74 | 45 | 621 |
| E3/E4 | 112 | 162 | 114 | 54 | 442 |
| E4/E4 | 10 | 27 | 39 | 38 | 114 |
| TOTAL CASES | 491 | 399 | 241 | 144 | 1275 |
For the e2/e4 genotype group that thus includes both the protective and risk variant, we sought to determine if the protective variant could to some degree moderate the effects of e4 on CAA. The e2/e4 genotype was associated with a significantly higher proportion of CAA cases than e3/e3 (49% CAA present) and the other remaining e2 cases (44% CAA present), and was similar to other e3/e4 carrier genotypes in terms of frequency of CAA present cases (76% of e2/e4 cases and 75% of e3/e4 cases).
Gross hemorrhages.
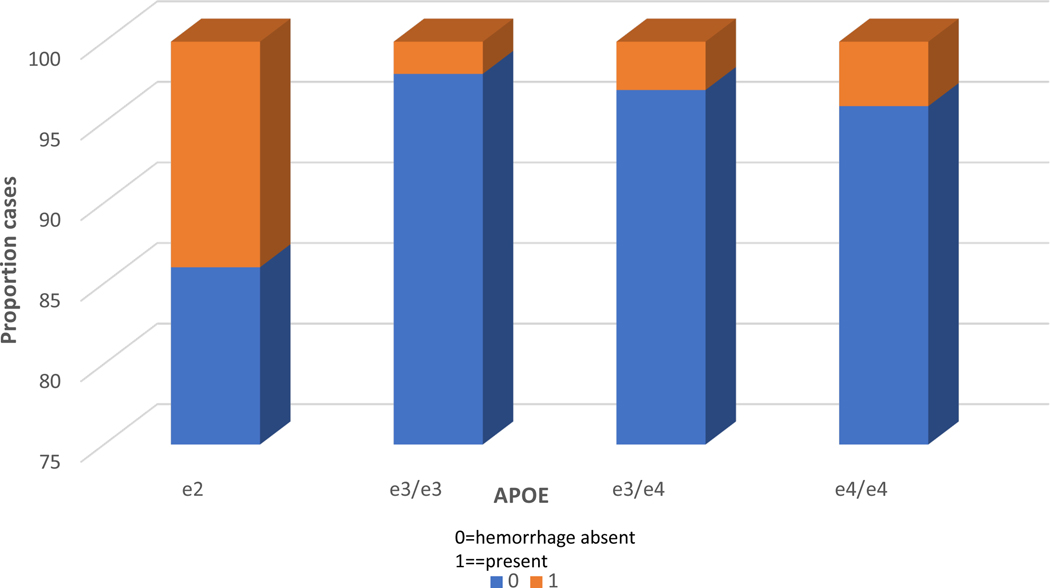
Acute/subacute gross hemorrhages were associated with APOE at the trend level (X2=10.45, p=.01). E3/e3 cases had the lowest frequency of CVD pathology and e2 cases the highest frequency with an OR=3.31, 95% CI 1.29–8.54, p=.01, when contrasted. We then conducted a stratified analysis in which we assessed APOE associations only within the CAA-present group i.e., CAA severity score of 1 or greater. Results were highly significant despite the decrease in sample size (N=623) with X2 increasing to 18.04, p= .0001. The distribution of genotypes can be seen in Figure 2. E2 was associated with the greatest proportion of acute gross hemorrhages (OR=20.693, 95% CI=4.675– 91.594, p<.0001) contrasted with e3, and OR=6.03, 95% CI=2.02–17.981, p=.001) contrasted with e4. In the CAA absent group, APOE associations were not significant (X2=3.73, p=.29).
Figure 2.
APOE genotype frequencies associated with acute gross haemorrhage in cases with cerebral amyloid angiopathy. Within each genotype column, coloured rows represent the relative proportion of cases in each severity stage. These proportions are expressed as percentages and add to 100. Note the increased proportion of acute gross haemorrhage in E2 cases. APOE, apolipoprotein E.
The finding was also assessed in an interaction model (APOE genotype x CAA present/absent) in which results were adjusted for sex and age at death. Results were significant at the trend level for the interaction term in this model (OR=.40, CI .13–1.18, Wald=2.75, p=.09), as well as the CAA term (OR=5.49, CI .99–30.44, Wald=3.80, p=.05). Thus, these terms modified risk of hemorrhage. The APOE main effect term was not significant (p=.18).
Other CVD pathologies.
For all other CVD related pathologies, APOE genotypic associations were non-significant, as shown in chi-square analyses in Table 3. These include old and recent gross infarcts and microinfarcts and old hemorrhages, recent microhemorrhages, and arteriosclerosis. Also, APOE genotype was not associated with hippocampal sclerosis.
Table 3.
Chi- Square Associations of APOE Genotypes with CVD Variables (N=1275)
| Variable | Severity Range (for all variables 0=absent) | % cases pathology present | X2 Value | Probability |
|---|---|---|---|---|
| Parenchymal pathology | ||||
| Old gross infarct | 0–1 | 1.29 | 0.77 | |
| Old gross hemorrhage | 0–1 | 1.95 | 0.58 | |
| Old microinfarct | 0–1 | 2.67 | 0.45 | |
| Acute gross infarct | 0–1 | 0.21 | 0.98 | |
| Acute microinfarct | 0–1 | 2.25 | 0.52 | |
| Acute gross hemorrhage | 0–1 | 10.45 | 0.01 | |
| Acute microhemorrhage. | 0–1 | 1.97 | 0.58 | |
| Old microbleed | 0–1 | 3.05 | 0.39 | |
| Hippocampal sclerosis | 0–3 | 11.90 | 0.22 | |
| Vessel pathology | ||||
| Arteriosclerosis | 0–3 | 13.91 | 0.13 |
Discussion
Study Design.
The study design has several strengths. We used a direct and unbiased approach that made no a priori assumptions about pathologies and so included all brains with rated CVD in the v. 10 collection. It is thus transdiagnostic; we did not impose any filter or a priori bias on our analyses. It is the largest single study of e2 effects on multiple post-mortem human cerebrovascular pathologies. Another strength of the study is that we do not have to rely on measures of CVD risk such as the Hachinski Scale. Rather we have direct measures of CVD pathology. Last, NACC data were collected prospectively and without regard for the specific hypotheses that we tested.
CAA.
This study of APOE alleles and CVD in the NACC v10 database identified several significant associations. First, we found a highly significant association of e4 with CAA in keeping with the literature and the known effect of e4 in increasing Abeta plaque pathology. In an earlier version of the NACC database, CAA co-occurred with AD neuropathology, was associated both with infarcts and hemorrhages, and was significantly more frequent in e4 genotype carriers; e2 was not examined.(15) In our study, e2 was not associated with reductions in CAA in contrast to e3 homozygotes. It was only associated with reductions in pathology in comparison to e4 carriers, with or without adjustments for AD histopathology. This was perhaps unexpected as we previously observed that e2 was associated with robust reductions in diffuse and neuritic plaques in this NACC sample compared to e3 homozygotes and e4 carriers.(16) Also, with respect to CAA, we found that the e2/e4 genotype was similar to other e4 carrier genotypic cases in that it increased the risk for CAA.
e2 Association with Gross Hemorrhage.
A second major positive finding derives from the established relationship between CAA and ICH. Thus, we also sought to determine if APOE was associated with gross hemorrhage (insofar as gross hemorrhage reflects ICH in the NACC database) as modulated by CAA. Here we found that e2 was associated with increased risk of acute/subacute gross hemorrhage, but only in those cases in which CAA was present after the sample was stratified. Lobar hemorrhage is a frequent co-morbidity of CAA and prior findings suggest that e2 is associated with this potentially severe outcome of CAA (with ORs of 2.20 and 1.32).(7, 8) We appreciate that the NACC data set does not have a unitary measure of lobar ICH, but gross hemorrhage likely captures the large majority of cases of lobar hemorrhage in this sample. However NACC neuropathology does not subdivide “gross hemorrhage” and includes parenchymal, subdural, and subarachnoid hemorrhage. Our assumption that acute/subacute gross hemorrhage captures lobar hemorrhage is based on the fact that no other NACC CVD variable could reasonably capture it (the NACC v 10 neuropath data dictionary available on line). NACC did not otherwise subdivide this diagnosis Further, it may at first seem surprising that e2 was not associated with old gross hemorrhages. This may be due to the relatively small number of such cases and the likelihood that ICH was a proximal cause of death in this sample given the high mortality associated with this cerebrovascular accident. Finally, we cannot rule out the possibility that CAA is not a mediator of the gross hemorrhage observed in these APOE ε2 cases bearing CAA, but rather an underlying condition (e.g., diabetes, hypertension, arteriosclerosis). The mechanism of e2 risk effects on hemorrhage or ICH is unclear and perhaps complex. While earlier research suggested that e2 might have compromising effects on the vasculature,(17) other recent experimental work has suggested that e2 may promote clearance of Abeta via the blood-brain barrier.(18)
Limits to e2 Neuroprotection.
We observed obvious limits to the neuroprotection properties of e2. Not only was it not associated with reductions in CVD pathologies such as CAA (discussed above), infarcts, or microhemorrhages, but it promoted gross hemorrhage in the presence of CAA. It also did not reduce the association of the e2/e4 genotype with CAA. This is highly consistent with our prior work on neurodegenerative pathologies in this database (i.e., diffuse plaques, neuritic plaques, tau Braak stage) in that e2/e4 cases were not neuroprotective, but rather “behaved” like other e4 genotypes.
Non-significant Associations.
APOE e4 associations with all other CVD related pathologies were non-significant. It is perhaps unexpected that APOE e4 was not associated with CVD given the established disadvantageous role of the allele in cardiovascular disease. Thus, APOE e4 has been significantly associated with increased risk of coronary disease (myocardial infarction or coronary artery stenosis).(19, 20) Nevertheless, it has been difficult to observe a consistent e4 related increase in risk for ischemic stroke.(21, 22) Even in large meta analyses using imaging and clinical data and comprising more than 20000 cases and controls, e4 was identified as a risk factor only inconsistently.(8, 23) Our study refines and extends this work in a carefully and uniformly characterized post-mortem series that measured both micro and gross infarcts that are considered the gold standard in the field. In parallel, we found neither an increased risk nor protective effects of e2 on infarcts (large or small), though e2 has been associated with decreased risk of coronary disease.(19)
Study Limitations.
There are several limitations to this study. The case series has an ascertainment bias in that approximately 51% of cases met pathological criteria for high AD neuropathological change scores by ABC system. However, that would not necessarily preclude other pathologies of interest and might even promote them on the basis of being intrinsic to AD. While a history of stroke is usually an exclusion criterion for ADCs, the overall frequency of cerebrovascular disease was nevertheless high in this NACC sample and it would not preclude individuals from incident stroke with being followed in the ADC programs. As noted, our study is limited by the absence of a unique ICH pathology; NACC used instead gross hemorrhage. We also did not detail APOE associations with number and locations of infarcts and microinfarcts, given our overall negative findings. Also, lacunes and infarcts were not distinguished in the NACC database.
Conclusion
This study is fully trans-diagnostic and treats de novo the impact of APOE genotype on CVD pathologies with no a priori biases or filters. We demonstrated that e4 has robust effects on CAA pathology, while e2 was associated with recent gross hemorrhagic pathology, especially in the presence of CAA. These neuropathological findings obtained in patients presenting to ADCs emphasize that the neuroprotective effect of APOE e2 may differ based on the type of neuropathology, but need to be confirmed in general population autopsy samples that have been examined systematically and in the same manner.
Acknowledgments
Funding Statement
This study is funded by the National Institute on Aging grant award R01AG051346 (to TG) and Columbia University Irving Medical Center.
Footnotes
Contributorship
TEG designed the study, conducted statistical analyses, and wrote the initial draft. EDH and DPD revised the study and did scientific editing.
Competing Interests
None declared.
Ethics Approval
Ethics approval by an ethics committee or institutional review board was not required for this study as it used a public data base.
References
- 1.Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275(3):251–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC Jr., et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–4. [DOI] [PubMed] [Google Scholar]
- 3.Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF, et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83(5):623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rannikmae K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N, et al. APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(3):300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L, Boyle PA, Nag S, Leurgans S, Buchman AS, Wilson RS, et al. APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol Aging. 2015;36(11):2946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PT, Pious NM, Jicha GA, Wilcock DM, Fardo DW, Estus S, et al. APOE-epsilon2 and APOE-epsilon4 correlate with increased amyloid accumulation in cerebral vasculature. J Neuropathol Exp Neurol. 2013;72(7):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68(6):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudlow C, Martinez Gonzalez NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analyses of 31 studies among 5961 cases and 17,965 controls. Stroke. 2006;37(2):364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71(11):804–11. [DOI] [PubMed] [Google Scholar]
- 10.McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, et al. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. 2016;14(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besser LM, Kukull WA, Teylan MA, Bigio EH, Cairns NJ, Kofler JK, et al. The Revised National Alzheimer’s Coordinating Center’s Neuropathology Form-Available Data and New Analyses. J Neuropathol Exp Neurol. 2018;77(8):717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Center NAsC. Neuropathology Data Set Coding Guidebook. 2014(Version 10). [Google Scholar]
- 13.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30(5):637–49. [DOI] [PubMed] [Google Scholar]
- 14.Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016;131(5):659–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71(4):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg TE, Huey ED, Devanand DP. Association of APOE e2 Genotype with Alzheimer’s and Non-Alzheimer’s Neurodegenerative Pathologies. Nat Commun. 2020;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70(6):871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298(11):1300–11. [DOI] [PubMed] [Google Scholar]
- 20.Haan MN, Mayeda ER. Apolipoprotein E Genotype and Cardiovascular Diseases in the Elderly. Curr Cardiovasc Risk Rep. 2010;4(5):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan TA, Shah T, Prieto D, Zhang W, Price J, Fowkes GR, et al. Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int J Epidemiol. 2013;42(2):475–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturgeon JD, Folsom AR, Bray MS, Boerwinkle E, Ballantyne CM, Atherosclerosis Risk in Communities Study I. Apolipoprotein E genotype and incident ischemic stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2005;36(11):2484–6. [DOI] [PubMed] [Google Scholar]
- 23.Wei LK, Au A, Menon S, Griffiths LR, Kooi CW, Irene L, et al. Polymorphisms of MTHFR, eNOS, ACE, AGT, ApoE, PON1, PDE4D, and Ischemic Stroke: MetaAnalysis. J Stroke Cerebrovasc Dis. 2017;26(11):2482–93. [DOI] [PubMed] [Google Scholar]