Abstract
Introduction
A subset of people with Parkinson's disease (PD) develop dementia faster than others. We aimed to profile PD cognitive subtypes at risk of dementia based on their rate of cognitive decline.
Method
Latent class mixed models stratified subtypes in Parkinson's Progression Markers Initiative (PPMI) (N = 770) and ICICLE‐PD (N = 212) datasets based on their decline in the Montreal Cognitive Assessment over at least 4 years. Baseline demographic and cognitive data at diagnosis were compared between subtypes to determine their clinical profile.
Results
Four subtypes were identified: two with stable cognition, one with steady decline, and one with rapid decline. Performance on Judgement of Line Orientation, but not category fluency, was associated with a steady decline in the PPMI dataset, and deficits in category fluency, but not visuospatial function, were associated with a steady decline in the ICICLE‐PD dataset.
Discussion
People with PD susceptible to cognitive decline demonstrate unique clinical profiles at diagnosis, although this differed between cohorts.
Highlights
Four cognitive subtypes were revealed in two Parkinson's disease samples.
Unique profiles of cognitive impairment were related to cognitive decline.
Judgement of Line Orientation/category fluency predictive of steady decline.
Global deficits related to rapid cognitive decline and increased dementia risk.
Keywords: clinical neuropsychology, cognitive impairment, dementia, latent class mixed model, Parkinson's disease
1. BACKGROUND
The cognitive implications of Parkinson's disease (PD) are heterogenous and complex. For almost 80% of people with PD, cognitive decline eventually culminates in dementia. 1 Yet it is not fully understood why people with PD experience such a vast array of symptoms spanning multiple cognitive domains, nor why some progress faster than others. 2 To better explore the pathology, prognosis, and treatment of cognitive symptoms in PD, efforts have been made to develop clinically relevant subtypes using data‐driven techniques.
In our systematic review, all but one study exploring data‐driven cognitive subtypes in PD used machine learning or structural equation modeling methods on baseline data, with only a few evaluating their progression over time. 3 Andersson et al. (2021) instead used latent class mixed modeling to stratify cognitive subtypes based on their rate of cognitive decline, 4 exploring executive, memory, and visuospatial composite scores as outcome variables to reveal two subtypes: one cognitively stable group and another, much smaller, group with rapid decline. However, due to the use of composite scores, it was unclear if subtypes could be defined by impairments on specific cognitive measures at baseline. The outcome measures also lacked clinical relevance, being impractical in a busy clinic setting to administer specialized neuropsychological assessments.
The present study therefore applied the Montreal Cognitive Assessment (MoCA), a common clinical tool that is sensitive to cognitive decline in PD, 5 as the outcome measure in latent class mixed models to identify PD subtypes based on the rate of cognitive decline in two large, longitudinal cohorts. Cognitive profiles for each subtype were developed by comparing groups on baseline neuropsychological measures to explore predictors of cognitive decline. Based on the dual syndrome hypothesis, 6 , 7 it was hypothesized that baseline memory and visuospatial function would be associated with cognitive decline.
2. METHOD
2.1. Participants
2.1.1. PPMI
Parkinson's Progression Markers Initiative (PPMI) data were used, 8 accessed on November 15, 2022. PPMI is an international, observational, cohort study with almost 50 sites across 13 countries. 8 The project was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice (GCP) guidelines. Local ethics committees of the participating sites provided relevant approvals and informed consent was obtained from each participant. Further details are available at https://www.ppmi‐info.org/data. Eligibility was contingent on a diagnosis of PD no longer than 2 years prior to enrolment and drug naïve status. Participants with young‐onset PD (age < 50) and dementia were excluded. Data were available annually from baseline up to year 7. Participants with two or more data points were included, resulting in N = 770 baseline participants.
2.1.2. ICICLE‐PD
Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation‐Parkinson's Disease (ICICLE‐PD) data were used, 9 accessed on October 10, 2022. ICICLE‐PD was conducted in accordance with the Declaration of Helsinki and GCP guidelines. 9 , 10 Ethical approval was provided by the Newcastle and North Tyneside Research Ethics Committee, and written, informed consent was obtained from all participants. Recruitment for ICICLE‐PD took place from the community in Cambridgeshire and Newcastle upon Tyne/Gateshead, United Kingdom. All participants were recently diagnosed people with PD meeting Queen's Square Brain Bank Criteria, with working knowledge of English and no significant baseline cognitive impairment (Mini‐Mental State Examination [MMSE] < 24 or Diagnostic and Statistical Manual of Mental Disorders Revision IV [DSM‐IV] diagnosis of dementia). Data were available every 18‐months from baseline to year 4.5. Participants with two or more data points were included, resulting in N = 212 baseline participants.
RESEARCH IN CONTEXT
Systematic Review: People with Parkinson's disease (PD) experience cognitive decline at different rates. The dual syndrome hypothesis predicts that memory/visuospatial impairments (posterior‐cortical subtype) predict a rapid decline. Our previous systematic review demonstrated the clinical significance of data‐driven PD cognitive subtypes, however most studies did not explore predictors of longitudinal cognitive decline.
Interpretation: Data‐driven PD cognitive subtypes with different rates of decline were revealed, confirming the heterogeneity of cognitive prognoses. Participants with rapid decline showed widespread baseline cognitive deficits, while people with steady decline were older and showed deficits in category fluency in one dataset and visuospatial function in another. As category fluency and visuospatial function are both posterior‐cortical tasks, these findings somewhat align with the dual syndrome hypothesis.
Future Directions: Prospective studies should characterize and explore the cognitive prognosis of posterior‐cortical impaired PD. Developing a PD subtype at risk of dementia will contribute to targeted clinical trials for early intervention and person‐centered care.
2.2. Measures
Both ICICLE‐PD and PPMI datasets included core demographic variables (age, education, sex, disease duration) and the MoCA as a measure of global cognition. 5 The Movements Disorders Society Unified Parkinson's Disease Rating Scale Part III (MDS‐UPDRS III) and Hoehn and Yahr stage measured PD motor severity, 11 and the Geriatric Depression Scale short form (GDS‐15) measured depression. 12 Only ICICLE‐PD included Levodopa Equivalent Daily Dose (LEDD) as PPMI participants were drug naïve at enrolment. PPMI included cerebrospinal fluid (CSF; alpha‐synuclein, beta‐amyloid, pTau, tTau) and genetic (apolipoprotein E4 [APOE4]) biomarkers, which were unavailable for analysis from ICICLE‐PD. Medical notes of all participants, including those who withdrew, were reviewed to capture any diagnosis of dementia (PDD) in the ICICLE‐PD study only, as described previously. 10
2.2.1. PPMI: Cognitive measures
Five neuropsychological tests had baseline data available for analysis in the PPMI dataset: the Symbol Digit Modality Test (SDMT) measured attention, 13 the Letter Number Sequencing (LNS) task measured attention and working memory, 13 the delayed scale from the Hopkins Verbal Learning Test‐Revised (HVLT) measured verbal episodic memory, 14 category fluency (animals in 60 s) measured semantic memory, 15 and Benton's Judgement of Line Orientation (JLO) measured visuospatial function. 16
2.2.2. ICICLE‐PD: Cognitive measures
Eight baseline neuropsychological tests from the ICICLE‐PD study were available for analysis. 9 , 10 Several measures were derived from the Cambridge Neuropsychological Test Automated Battery (CANTAB), 17 including Pattern Recognition Memory (PRM) and Paired Associates Learning (PAL), and Spatial Recognition Memory (SRM) measures of visual memory, and the One Touch Stockings of Cambridge (OTS) measure of executive function. The Simple Reaction Time (SRT) and Choice Reaction Time (CRT) scales from the Cognitive Drug Research test battery measured attention/processing speed. 18 Category fluency (animals in 90 s) measured semantic memory, 15 and pentagon copying from the MMSE measured visuospatial function. 6
2.3. Statistics
2.3.1. Latent class mixed model
Analysis and visualization were performed using R version 3.6.3. For each dataset, latent class mixed models were created to identify subgroups with similar longitudinal cognitive trajectories, using iterative maximum likelihood methods to estimate linear mixed models with latent classes within the data. 19 Total MoCA score was the outcome variable, time (assessment number) was a fixed effect, and a random intercept term was included in each model. Due to the nongaussian nature of the outcome variable, models were fitted to the data using the lcmm package, 19 which includes parameterized nonlinear transformations of the data.
PPMI was used as an exploratory dataset to determine the optimal number of latent classes. Models with two, three, and four latent classes using both the beta cumulative distribution function and quadratic I‐splines transformations were compared on the Bayesian Information Criterion (BIC) to select the model of best fit. 20 Once the optimal number of classes was determined, a confirmatory analysis was performed using the ICICLE‐PD data, applying the optimal number of classes and using the BIC to determine the nonlinear transformation of best fit. Posterior probabilities were used to assess model quality, with higher values reflecting better fit. Final models were visualized using dplyr and ggplot2 packages. 21 , 22
2.3.2. Post‐hoc comparisons
Post‐hoc tests compared classes on demographic and cognitive variables. Analysis of variance (ANOVA), Kruskal–Wallis tests, or chi‐squared test, as appropriate, with Bonferroni‐adjusted post‐hoc tests were performed (adjusted significance threshold of p < 0.001). Significant results for cognitive variables were confirmed with analysis of covariance (ANCOVA) including age and education as covariates and Class as the dependent variable. Survival and cumulative survival to dementia were calculated for each subtype in the ICICLE‐PD dataset using Kaplan–Meier plots. Statistical comparisons were performed using SPSS 27.0.1.0.
3. RESULTS
3.1. Participants
The baseline demographic and clinical characteristics of each dataset were compared (Table 1). Compared to the PPMI dataset, the ICICLE‐PD dataset comprised older, less educated participants with shorter disease duration, greater motor severity, and slightly more severe cognitive impairment (p < 0.001 for all).
TABLE 1.
Comparison of baseline demographic and clinical variables across datasets.
| Dataset | ||||||
|---|---|---|---|---|---|---|
| PPMI (N = 770) | ICICLE‐PD (N = 212) | |||||
| Measure | Mean/N | SD | Mean/N | SD | Test statistic | P‐value |
| Age (year) | 62.28 | 9.65 | 65.96 | 9.73 | t = 4.917 | <0.001 |
| Education (year) | 15.94 | 3.69 | 12.80 | 3.69 | U = 120987 | <0.001 |
| Sex | V = 0.018 | 0.564 | ||||
| Male | 476 | 135 | ||||
| Female | 297 | 77 | ||||
| Dis. duration (month) | 17.81 | 21.93 | 5.49 | 5.05 | U = 117575 | <0.001 |
| MDS‐UPDRS‐III | 20.47 | 9.68 | 27.57 | 11.68 | U = 51464 | <0.001 |
| H&Y | V = 0.253 | <0.001 | ||||
| 1 | 254 | 54 | ||||
| 2 | 444 | 122 | ||||
| 3 | 21 | 35 | ||||
| 4 | 0 | 1 | ||||
| MoCA | 26.71 | 2.70 | 25.30 | 3.44 | U = 91442 | <0.001 |
| GDS | 2.46 | 2.74 | 2.87 | 2.64 | U = 69480 | 0.003 |
Note: Significant p‐values (<0.001) are bolded.
Abbreviations: Dis., disease; GDS, Geriatric Depression Scale; MDS‐UPDRS‐III, Movement Disorder Society Unified Parkinson's Disease Rating Scale III; MoCA, Montreal Cognitive Assessment.
3.1.1. Missing data
Little's MCAR test performed on both datasets found data was not Missing Completely at Random, 23 PPMI: = 200.464, p < 0.001, ICICLE‐PD: = 81.731, p < 0.001. As more severely cognitively impaired participants were more likely to drop out, perhaps due to their greater impairment, the data were assumed to be Missing at Random. Missing data were thus handled using Available Case Analysis and the implications of this are explored in the discussion. 24 Notably, 55% of the biospecimen data from the PPMI dataset were missing, with Class 4 being excluded from biomarker analyses due to a lack of available data.
3.2. PPMI
3.2.1. Latent class mixed model
Full R output including assessment of assumptions, comparisons between alternative models, and final model parameters are provided in Supplementary material 1 (Table S1, Figures S1–S3). BIC values tended to decrease with each additional class, with the lowest BIC value attributed to the beta cumulative distribution function transformed 4‐Class model (Figure 1). Class 1 (N = 31) and Class 2 (N = 608) reflected cognitively intact groups with stable cognition over time. Class 3 (N = 111) comprised cognitively intact participants with a steady decline over time, whereas Class 4 (N = 10) comprised severely impaired participants with poor cognition and rapid decline. Good separation of classes was indicated by posterior probabilities that were roughly above 0.7 for each class (Table S2).
FIGURE 1.
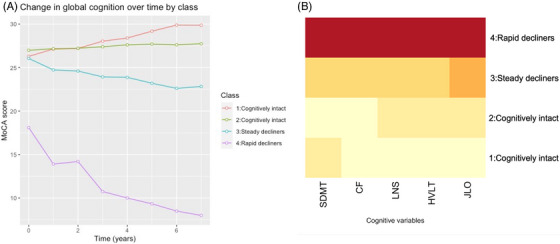
(A) Mean MoCA score at each timepoint by class (PPMI). (B) Heatmap of relative cognitive scores per class within PPMI dataset (darker colors reflecting poorer scores). Note: As each test is scored on a different scale, the colors of the heatmap do not reflect specific values. CF, category fluency; HVLT, Hopkin's Verbal Learning Test; JLO, Judgement of Line Orientation; LNS, Letter Number Sequencing; MoCA, Montreal Cognitive Assessment; PPMI, Parkinson's Progression Markers Initiative; SDMT, Symbol Digit Modalities Test.
3.2.2. Attrition
Only Class 1 maintained most participants from baseline until year 7 (84%). The majority of participants in Class 2, Class 3, and Class 4 had dropped out by year 7 (75%, 63%, and 90% attrition, respectively). Notably, 50% of participants in Class 4 had dropped out by the second year and only one participant remained in the final year (Table S3).
3.2.3. Comparison of baseline clinical and cognitive profiles
Table 2 summarizes the differences in baseline clinical and cognitive measures across classes. Class 4 had significantly higher depression scores, longer disease duration, and more severe motor disease than the cognitively intact groups (Class 1 and Class 2). Class 3 was significantly older, scored significantly higher on the depression scale, and had poorer global cognition than Class 2, yet demonstrated better global cognition and significantly less severe motor severity than Class 4. There were no significant differences in education or sex among subtypes. Of the biomarkers, only Class 1–3 differed with respect to beta‐amyloid levels, F(2) = 3.24, p = 0.040, although this did not withstand adjustment for age or multiple comparisons. No other significant differences were revealed. In terms of cognitive measures, Class 1 and Class 2 demonstrated the best performance across all measures and Class 4 performed most poorly across all measures. Class 3 outperformed Class 4 on all measures except for the JLO, and only scored significantly lower than Class 1 on the JLO. A heatmap is provided in Figure 1 for a visual depiction of cognitive performance by class, with darker colors reflecting poorer performance on a given cognitive measure.
TABLE 2.
Baseline PPMI clinical and cognitive variables (mean and standard deviation or N) by class.
| Measures |
Class 1: Cognitively intact N = 31 (5%) |
Class 2: Cognitively intact N = 608 (79%) |
Class 3: Steady decliners N = 111 (14%) |
Class 4: Rapid decliners N = 10 (1%) |
Significant differences * | ||||
|---|---|---|---|---|---|---|---|---|---|
| M/N | SD | M/N | SD | M/N | SD | M/N | SD | ||
| Clinical measures | |||||||||
| Age | 60.55 | 8.26 | 61.75 | 9.77 | 64.69 | 8.68 | 69.43 | 9.86 | 2 < 3 |
| Education | 16.29 | 3.13 | 16.15 | 3.58 | 14.96 | 3.80 | 11.00 | 5.48 | |
| Sex | |||||||||
| Male | 22 | 376 | 62 | 7 | |||||
| Female | 9 | 233 | 48 | 3 | |||||
| Dx duration (month) | 10.79 | 14.71 | 16.85 | 20.65 | 23.11 | 27.25 | 49.22 | 27.01 | 1,2 < 4 |
| MDS‐UPDRS‐III | 18.84 | 7.25 | 20.48 | 9.58 | 19.33 | 9.15 | 30.20 | 16.42 | 1,2,3 < 4 |
| H&Y | |||||||||
| 1 | 17 | 200 | 32 | 1 | |||||
| 2 | 14 | 354 | 61 | 3 | |||||
| 3 | 0 | 15 | 4 | 2 | |||||
| 4 | 0 | 0 | 0 | 0 | |||||
| MoCA | 26.29 | 1.69 | 26.99 | 2.39 | 26.05 | 3.02 | 18.10 | 3.72 | 1,2,3 > 4; 2 > 3 |
| GDS | 2.06 | 2.62 | 2.31 | 2.65 | 3.09 | 2.81 | 6.30 | 3.30 | 1,2 < 4; 2 < 3 |
| Biomarkers | |||||||||
| CSF Alpha‐synuclein | 1328 | 527 | 1481 | 641 | 1561 | 582 | – | – | |
| CSF Beta‐amyloid | 784 | 285 | 902 | 335 | 836 | 354 | – | – | |
| CSF pTau | 12.8 | 4.46 | 14.3 | 5.12 | 15.3 | 5.49 | – | – | |
| CSF tTau | 151 | 48.3 | 165 | 59.5 | 176 | 61.3 | – | – | |
| APOE4 | |||||||||
| 0 | 25 | 359 | 90 | 6 | |||||
| 1 | 6 | 98 | 21 | 4 | |||||
| 2 | 0 | 9 | 0 | 0 | |||||
| Cognitive measures | |||||||||
| SDMT | 41.84 | 6.69 | 43.31 | 10.25 | 35.02 | 10.69 | 12.00 | 9.51 | 1,2,3 > 4; 2 > 3 |
| CF | 21.97 | 4.40 | 21.66 | 5.54 | 18.67 | 5.19 | 11.00 | 3.40 | 1,2,3 > 4; 2 > 3 |
| LNS | 11.19 | 2.12 | 10.63 | 2.72 | 8.92 | 2.70 | 3.80 | 2.35 | 1,2,3 > 4; 2 > 3 |
| HVLT | 8.84 | 1.86 | 8.57 | 2.66 | 7.25 | 2.85 | 2.30 | 2.06 | 1,2,3 > 4 |
| JLO | 13.45 | 1.65 | 12.78 | 2.31 | 11.31 | 2.92 | 9.22 | 2.63 | 1,2 > 3 |
Abbreviations: CF, category fluency; CSF, cerebrospinal fluid; Dx, disease; GDS, Geriatric Depression Scale; HVLT, Hopkin's Verbal Learning Test; H&Y, Hoehn & Yahr; JLO, Judgement of Line Orientation; LNS, Letter Number Sequencing; MDS‐UPDRS‐III, Movement Disorder Society Unified Parkinson's Disease Rating Scale III; MoCA, Montreal Cognitive Assessment; SDMT, Symbol Digit Modalities Test.
p < 0.001.
3.3. ICICLE‐PD
3.3.1. Latent class mixed model
Given that the exploratory analysis identified four latent classes, only four class models were investigated using the ICICLE‐PD dataset. Supplementary material 1 (Tables S4–S5, Figures S4–S6) details the model selection process and parameters for each model transformation. The final model was created using the I‐Splines transformation (Figure 2). Class 1 (N = 59) and Class 2 (N = 83) reflected cognitively intact groups with relatively stable cognition over time. Class 3 (N = 57) comprised mildly impaired participants with steady decline, whereas Class 4 (N = 13) comprised severely impaired participants with rapid decline. Good separation of classes was indicated by posterior probabilities that were above 0.7 (Table S6).
FIGURE 2.
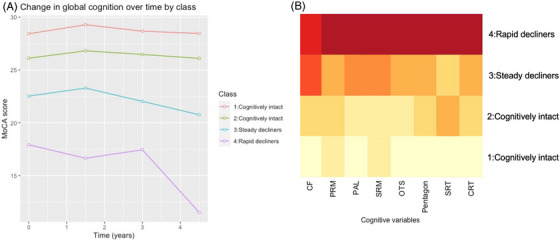
(A) Mean MoCA score at each timepoint by class (ICICLE‐PD). (B) Heatmap of cognitive scores per class within ICICLE‐PD dataset (darker colors reflecting poorer scores). Note: As each test is scored on a different scale, the colors of the heatmap do not reflect specific values. CF, category fluency; CRT, Choice Reaction Time; MoCA, Montreal Cognitive Assessment; OTS, One Touch Stockings; PAL, Paired Associate Learning; PRM, Paired Recognition Memory; SRM, Spatial Recognition Memory; SRT, Simple Reaction Time.
3.3.2. Attrition
Class 4 exhibited the greatest attrition, with the majority of participants having dropped out by the final assessment (69%). Class 3 similarly lost the majority of participants by the final assessment (56%). Class 1 and Class 2 had lower attrition rates, with only 20% and 41% of participants dropping out by the final assessment, respectively (Table S7).
3.3.3. Time to dementia
Forty‐six participants developed PDD between baseline and 72‐month follow‐up. Cumulative survival was significantly different between groups (= 120.8, p < 0.001 Figure S7). Cumulative dementia probability was higher for Class 4 (84.6%) and Class 3 (56.2%) compared to Class 1 (21.8%) and for Class 2 (26.3%). Mean estimated time to dementia diagnosis was also fastest in Class 4 (2.85 years, 95% confidence interval [CI]: 1.71–3.99, n = 11) followed by Class 3 (5.99 years, 95% CI: 5.34–6.65, n = 25) compared to Class 1 (7.66 years, 95% CI: 7.49–7.82) and Class 2 (8.09 years, 95% CI: 7.75–8.42), where only a small number of participants developed PDD over 6 years (N = 2 and N = 8, respectively).
3.3.4. Comparison of baseline measures
At baseline, Class 1 participants were significantly younger and more educated than Class 3 and Class 4 and in addition had significantly less severe motor problems than Class 4 (Table 3). Class 3 was also less educated than Class 2. While Class 3 and Class 4 both reflected cognitively impaired groups, baseline MoCA scores indicated milder cognitive impairment in Class 3 compared to Class 4. Similarly, although Class 2 remained cognitively intact over time, their mean baseline MoCA score of 26.12 ± 1.86 was just above the threshold for normal cognition (MoCA > 26). 25 Notably, there were no differences among classes in terms of PD duration, sex, or depression. In terms of baseline cognitive profiles, Class 1 demonstrated the highest performance across all measures and Class 4 performed most poorly, scoring significantly lower than Class 1 on all measures. While Class 3 performed significantly more poorly than Class 1 on all but the SRT and CRT measures, the greatest difference in performance was on category fluency. A heatmap is again provided in Figure 2 for a visual depiction of cognitive performance by class, with darker colors reflecting poorer performance on a given cognitive measure.
TABLE 3.
Baseline ICICLE‐PD clinical and cognitive variables (mean and standard deviation or N) by class.
| Measures |
Class 1: Cognitively intact N = 59 (28%) |
Class 2: Cognitively intact N = 83 (39%) |
Class 3: Steady decliners N = 57 (27%) |
Class 4: Rapid decliners N = 13 (6%) |
Significant differences * | ||||
|---|---|---|---|---|---|---|---|---|---|
| M/N | SD | M/N | SD | M/N | SD | M/N | SD | ||
| Clinical measures | |||||||||
| Age | 60.56 | 8.85 | 65.04 | 9.20 | 71.01 | 8.37 | 74.23 | 5.92 | 1,2 < 3,4; 1 < 2 |
| Education | 14.64 | 3.48 | 12.95 | 3.40 | 11.05 | 3.43 | 11.15 | 3.60 | 1 > 3,4; 2 > 3 |
| Sex | |||||||||
| Male | 33 | 53 | 41 | 8 | |||||
| Female | 26 | 30 | 16 | 5 | |||||
| Dx duration (month) | 5.36 | 4.91 | 5.17 | 5.00 | 6.36 | 5.62 | 4.34 | 2.79 | |
| MDS‐UPDRS‐III | 24.22 | 11.48 | 27.84 | 11.49 | 29.12 | 11.72 | 34.23 | 10.22 | 1 < 4 |
| H&Y | |||||||||
| 1 | 19 | 23 | 11 | 1 | |||||
| 2 | 35 | 46 | 34 | 7 | |||||
| 3 | 5 | 13 | 12 | 5 | |||||
| 4 | 0 | 1 | 0 | 0 | |||||
| MoCA | 28.43 | 1.29 | 26.12 | 1.86 | 22.53 | 2.35 | 17.91 | 2.39 | 2,3,4 < 1; 3,4 < 2 |
| LEDD | 129.03 | 95.98 | 187.30 | 177.41 | 195.50 | 154.52 | 210.77 | 119.75 | |
| GDS | 2.14 | 2.04 | 3.17 | 2.96 | 3.13 | 2.46 | 3.15 | 3.29 | |
| Cognitive measures | |||||||||
| CF | 25.05 | 6.04 | 22.10 | 5.39 | 17.16 | 5.81 | 16.38 | 8.10 | 3,4 < 1; 3 < 2 |
| PRM | 21.26 | 1.96 | 19.92 | 3.00 | 18.67 | 2.28 | 14.36 | 5.30 | 3,4 < 1; 4 < 3,2 |
| PAL a | 1.69 | 0.44 | 1.82 | 0.59 | 2.38 | 0.86 | 2.89 | 0.98 | 1,2 < 3,4 |
| SRM | 16.14 | 1.99 | 15.79 | 1.90 | 14.25 | 2.11 | 12.36 | 1.80 | 3,4 < 2,1 |
| OTS | 16.54 | 2.64 | 15.10 | 2.75 | 12.78 | 4.43 | 9.55 | 6.36 | 3,4 < 1 |
| Pentagon | 3,4 < 2,1 | ||||||||
| 0 | 0 | 2 | 0 | 2 | |||||
| 1 | 0 | 7 | 13 | 5 | |||||
| 2 | 59 | 74 | 44 | 6 | |||||
| SRT a | 329.90 | 96.08 | 371.84 | 165.61 | 366.24 | 69.43 | 415.12 | 80.74 | 1 < 4 |
| CRT a | 502.22 | 85.07 | 537.13 | 88.28 | 566.62 | 86.70 | 637.99 | 114.72 | 1 < 4 |
Abbreviations: CF, category fluency; CRT, Choice Reaction Time; Dx, disease; GDS, Geriatric Depression Scale; H&Y, Hoehn & Yahr; LEDD, Levodopa Equivalent Daily Dose; MDS‐UPDRS‐III, Movement Disorder Society Unified Parkinson's Disease Rating Scale III; MoCA, Montreal Cognitive Assessment; OTS, One Touch Stockings; PAL, Paired Associate Learning; PRM, Paired Recognition Memory; SRM, Spatial Recognition Memory; SRT, Simple Reaction Time.
Higher scores indicate poorer performance.
p < 0.001.
4. DISCUSSION
The present paper identified and profiled subtypes of cognitive impairment in PD defined by their rate of longitudinal global cognitive decline. Four subtypes were identified: two cognitively intact subtypes with stable cognition, a severely impaired subtype with rapid decline and high attrition, and a subtype with a unique cognitive profile and steady decline.
4.1. Summary of subtype profiles
In both cohorts, most participants belonged to cognitively intact subtypes with stable cognition, and only a minority of participants demonstrated rapid cognitive decline. Rapid decliners generally tended to be older and less educated, with greater motor disease severity than the cognitively intact subtypes. They also demonstrated the poorest performance across all cognitive measures and the highest attrition rates. As both PPMI and ICICLE‐PD protocols rigorously evaluated and excluded those with dementia, and ICICLE‐PD data revealed that rapid decliners developed dementia faster than others, the profile of this subtype may reflect people at the latest stages of prodromal PDD.
The groups with steady cognitive decline differed most across cohorts. This subtype had abnormal global cognition at baseline in the ICICLE‐PD dataset (mean MoCA < 26) and was on the threshold of normal cognition in the PPMI dataset (mean MoCA = 26). Compared to the cognitively intact subtype, this subtype was older in both datasets and less educated in the ICICLE‐PD dataset. A higher proportion of this subtype was identified in the ICICLE‐PD dataset (PPMI: 14%; ICICLE‐PD: 27%). Differences in the cohorts that should be considered include increased age, lower education, and greater motor severity of the ICICLE‐PD sample compared to the PPMI. ICICLE‐PD also recruited a more representative community‐based sample compared to the PPMI study. Nevertheless, this subtype demonstrated a faster rate and increased risk of developing dementia compared to cognitively intact groups in the ICICLE‐PD survival analysis.
4.2. Profiling risk of cognitive decline
In terms of cognitive performance, subtypes with steady cognitive decline performed most poorly compared to cognitively intact subtypes on the JLO task in the PPMI dataset and category fluency in the ICICLE‐PD dataset. In line with the hypothesis, category fluency and visuospatial deficits appeared to be related to steady decline. This aligns with findings from Williams‐Gray et al. (2009) who revealed that category fluency and visuospatial (pentagon copying) impairments were predictive of both cognitive decline (MMSE) and dementia within 5 years of diagnosis. 26 However, a single syndrome with both category fluency and visuospatial deficits was not identified in the present study.
This discrepancy may be partly attributed to differences in mean age (PPMI: 62 years; ICICLE‐PD: 71 years) and education (PPMI: 16 years; ICICLE‐PD: 11 years) between the two cohorts. Consequently, mild deficits in JLO may be uniquely associated with rapid cognitive decline in those with younger age of PD onset and higher education. Importantly, ICICLE‐PD did not include the JLO task and instead measured visuospatial function using pentagon copying from the MMSE. The discrepancy in results may therefore be due to differences in the sensitivity of visuospatial measures employed, suggesting that JLO may be more sensitive to cognitive decline in PD than pentagon copying. Only one previous study (N = 42) found the JLO to be predictive of PDD at a 7.5‐year follow‐up, 27 warranting further investigations to confirm its predictive utility in specific samples.
Conversely, results from the ICICLE‐PD cohort suggest that mild deficits in category fluency may be associated with steady cognitive decline in those with older age of PD onset and lower education. This is consistent with previous studies, which have found category fluency to be a strong predictor for cognitive decline in PD. 6 , 28 , 29 Interestingly, most samples were on average 70 years old with disease duration <5 years, suggesting that category fluency may be a useful screening tool for cognitive decline in PD with older age of onset. This is also supported by the present findings, where category fluency performance was related to a steady decline in an older, less educated de novo sample (ICICLE‐PD) but not related to a steady decline in a younger, highly educated de novo (PPMI) sample. However, differences in the timing of the test (PPMI: 60 s; ICICLE‐PD: 90 s) may also explain this discrepancy, suggesting a 90 s category fluency test may be more sensitive to cognitive decline in PD than a 60 s test.
4.3. Clinical implications
The present results confirm the prognostic value of cognitive subtyping in PD and work toward profiling people at increased risk of cognitive decline, which is critical to facilitate person‐centered care and early intervention, and inform selection criteria for clinical trials. 30 Cognitive subtyping will also facilitate the identification of biomarkers for dementia risk in PD, for instance through multimodal studies using MRI and PET data from PPMI and ICICLE‐PD datasets. Although the present results from the PPMI biomarker analysis were nonsignificant, likely due to substantial missing data, future research should consider prospectively evaluating biomarkers for PD cognitive subtypes to minimize data loss and maximize statistical power. Finally, understanding prominent cognitive subtypes will help to inform neuropsychological test selection in PD, which currently varies significantly across clinical and research settings. 31 , 32
4.4. Strengths and limitations
A major strength of the present study was the use of the MoCA to stratify subtypes. As it is commonly used to screen for cognitive impairment in PD, using the MoCA allowed for an accurate representation of the prognosis as it would present in the clinic. The use of two large, well‐defined, newly diagnosed PD cohorts was also a strength, and the exploratory‐confirmatory study design facilitated the comparison of subtypes across independent datasets. While different cognitive measures used across the two datasets made comparison of cognitive profiles difficult, subtypes with comparable longitudinal cognitive decline were revealed, providing important insight into the prognosis of cognitive subtypes in PD.
There was also insufficient biospecimen data available for the ICICLE‐PD dataset, preventing biomarker analyses for this cohort. Analyses were also restricted to the 4.5–7 years of follow‐up for which there was data, which limited interpretation of the findings to only the early stages of the disease given the average PD disease duration of 15 years. 33 Regardless, these results are useful for identifying those who are at risk of early cognitive decline and imminent dementia. However, future studies may benefit from the application of random forests or gradient boosting machines (GBMs) to mitigate limited follow‐up duration.
Additionally, small sample sizes of the more impaired subtypes likely affected statistical comparisons across groups, potentially masking significant differences on clinical and cognitive variables. Generative Adversarial Networks (GANs) may be employed in future studies to increase sample size by generating synthetic data. Unavoidable bias arising from selective attrition must also be acknowledged. Greater attrition in people with cognitive decline is likely, as impaired cognition is a barrier to participation in studies. 34 Data from those who dropped out were not Missing Completely at Random, 23 and measures of cognition at timepoints with attrition were therefore likely systematically overestimated (i.e., higher than if there were no attrition), with the rate of decline disproportionately underestimated for rapid decliners due to greater attrition rates. We attempted to mitigate this by looking at survival data in the ICICLE‐PD analysis, while acknowledging that PPMI and ICICLE‐PD attrition rates fared well in comparison to analogous studies (e.g., 41% attrition at 5 years 35 ; 64% attrition at 6 years 28 ).
5. CONCLUSIONS
The present study identified subtypes of PD with discrete rates of global cognitive decline and delineated their baseline clinical and cognitive profiles. These profiles help to inform the clinical picture of people with PD who are susceptible to cognitive decline, which is not only beneficial to determine prognostic factors but also for selection into clinical trials and patient‐centered care. Ultimately, the results of the present study highlight clinical profiles susceptible to cognitive decline in PD and encourage future research into the predictive relevance of cognitive subtypes in PD.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the Supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We acknowledge Professor David Burn for his significant role in the ICICLE‐PD study. This research was supported by the National Institute for Health and Care Research (NIHR) Newcastle Biomedical Research Centre (BRC). The views expressed are not necessarily those of the NIHR or the Department of Health and Social Care. ICICLE‐PD was funded by Parkinson's UK (J‐0802, G‐1301, G‐1507). The research was supported by the Lockhart Parkinson's Disease Research Fund, National Institute for Health Research (NIHR), Newcastle Biomedical Research Unit and Centre based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University, and the NIHR Cambridge Biomedical Research Centre (NIHR203312). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. R.A.L. is supported by a Janet Owens Parkinson's UK Senior Research Fellowship (F‐1801). C.H.W.G. is supported by an RCUK/UKRI Fellowship awarded by the MRC (MR/W029235/1). A.J.Y. is supported by the Newcastle NIHR Biomedical Research Centre, in addition to grants from EU Innovative Medicines Initiative (IMI), Michael J. Fox Foundation, Parkinson's UK, Lewy Body Society, NIHR Heath Technology Assessment (HTA), Dunhill medical Trust, Weston Brain Institute, and Intercept pharmaceuticals. This work was supported by the NHMRC Fellowship to N.D. (APP1137339), as well as the Australian Government Research Training Program scholarship awarded to D.P. PPMI – a public‐private partnership – is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including 4D Pharma, Abbvie, AcureX, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson's, AskBio, Avid Radiopharmaceuticals, BIAL, Biogen, Biohaven, BioLegend, BlueRock Therapeutics, Bristol‐Myers Squibb, Calico Labs, Celgene, Cerevel Therapeutics, Coave Therapeutics, DaCapo Brainscience, Denali, Edmond J. Safra Foundation, Eli Lilly, Gain Therapeutics, GE HealthCare, Genentech, GSK, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lundbeck, Merck, Meso Scale Discovery, Mission Therapeutics, Neurocrine Biosciences, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi, Servier, Sun Pharma Advanced Research Company, Takeda, Teva, UCB, Vanqua Bio, Verily, Voyager Therapeutics, the Weston Family Foundation, and Yumanity Therapeutics.
Pourzinal D, Lawson RA, Yarnall AJ, et al. Profiling people with Parkinson's disease at risk of cognitive decline: Insights from PPMI and ICICLE‐PD data. Alzheimer's Dement. 2024;16:e12625. 10.1002/dad2.12625
DATA AVAILABILITY STATEMENT
Data used in the preparation of this article were obtained in July, 2023 from the Parkinson's Progression Markers Initiative (PPMI) database (https://www.ppmi‐info.org/access‐data‐specimens/download‐data), RRID:SCR_006431. For up‐to‐date information on the study, visit https://www.ppmi‐info.org. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
REFERENCES
- 1. Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837‐844. doi: 10.1002/mds.21956 [DOI] [PubMed] [Google Scholar]
- 2. Goldman JG, Vernaleo BA, Camicioli R, et al. Cognitive impairment in Parkinson's disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Parkinsons Dis. 2018;4(1):19. doi: 10.1038/s41531-018-0055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pourzinal D, Yang J, Lawson RA, Mcmahon KL, Byrne GJ, Dissanayaka NN. Systematic review of data‐driven cognitive subtypes in Parkinson disease. Eur J Neurol. 2022;29(11):3395‐3417. doi: 10.1111/ene.15481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson S, Josefsson M, Stiernman LJ, Rieckmann A. Cognitive decline in Parkinson's disease: a subgroup of extreme decliners revealed by a data‐driven analysis of longitudinal progression. Front Psychol. 2021;12:729755. doi: 10.3389/fpsyg.2021.729755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biundo R, Weis L, Bostantjopoulou S, et al. MMSE and MoCA in Parkinson's disease and dementia with Lewy bodies: a multicenter 1‐year follow‐up study. J Neural Transm. 2016;123(4):431‐438. doi: 10.1007/s00702-016-1517-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams‐Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(7):1787‐1798. doi: 10.1093/brain/awm111 [DOI] [PubMed] [Google Scholar]
- 7. Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener Dis. 2013;11(2):79‐92. doi: 10.1159/000341998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Parkinson Progression Marker Initiative . The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629‐635. doi: 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE‐PD study. Neurology. 2014;82(4):308‐316. doi: 10.1212/wnl.0000000000000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawson RA, Williams‐Gray CH, Camacho M, et al. Which neuropsychological tests? Predicting cognitive decline and dementia in Parkinson's disease in the ICICLE‐PD Cohort. J Parkinsons Dis. 2021;11(3):1297‐1308. doi: 10.3233/jpd-212581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MD‐UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41‐47. doi: 10.1002/mds.21198 [DOI] [PubMed] [Google Scholar]
- 12. Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165‐173. doi: 10.1300/J018v05n01_09 [DOI] [Google Scholar]
- 13. Lezak MD. Neuropsychological Assessment. 5th ed. Oxford University Press; 2012. [Google Scholar]
- 14. Benedict RHB, Brandt J. Hopkin's Verbal Learning Test‐Revised/Brief Visuospatial Memory Test‐Revised: Professional Manual Supplement. PAR; 2007. [Google Scholar]
- 15. Goodglass H. The Assessment of Aphasia and Related Disorders. Lea & Febiger; 1972. [Google Scholar]
- 16. Calamia M, Markon K, Denburg NL, Tranel D. Developing a short form of Benton's Judgment of Line Orientation Test: an item response theory approach. Clin Neuropsychol. 2011;25(4):670‐684. doi: 10.1080/13854046.2011.564209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CANTAB® . 2019: Cambridge Cognition. https://www.cantab.com
- 18. Simpson PM, Surmon DJ, Wesnes KA, Wilcock GK. The cognitive drug research computerized assessment system for demented patients: a validation study. Int J Geriatr Psychiatry. 1991;6(2):95‐102. doi: 10.1002/gps.930060208 [DOI] [Google Scholar]
- 19. Proust‐Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R Package lcmm. J Stat Softw. 2017;78(2):1‐56. 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 20. Kuha J. AIC and BIC: comparisons of assumptions and performance. Sociol Methods Res. 2004;33(2):188‐229. doi: 10.1177/0049124103262065 [DOI] [Google Scholar]
- 21. Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer‐Verlag; 2016. [Google Scholar]
- 22. Wickham H, François R. dplyr: A Grammar of Data Manipulation. 2023. [Google Scholar]
- 23. Little RJ, Rubin DB. Statistical Analysis with Missing Data. John Wiley & Sons; 2019. [Google Scholar]
- 24. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a Brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 26. Williams‐Gray CH, Evans JR, Goris An, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow‐up of the CamPaIGN cohort. Brain. 2009;132(11):2958‐2969. doi: 10.1093/brain/awp245 [DOI] [PubMed] [Google Scholar]
- 27. Galtier I, Nieto A, Mata M, Lorenzo JN, Barroso J. Analyses of visuospatial and visuoperceptual errors as predictors of dementia in Parkinson's disease patients with subjective cognitive decline and mild cognitive impairment. J Int Neuropsychol Soc. 2021;27(7):722‐732. doi: 10.1017/S1355617720001216 [DOI] [PubMed] [Google Scholar]
- 28. Hobson P, Meara J. Mild cognitive impairment in Parkinson's disease and its progression onto dementia: a 16‐year outcome evaluation of the Denbighshire cohort. Int J Geriatr Psychiatry. 2015;30(10):1048‐1055. doi: 10.1002/gps.4261 [DOI] [PubMed] [Google Scholar]
- 29. Domellöf ME, Ekman U, Forsgren L, Elgh E. Cognitive function in the early phase of Parkinson's disease, a five‐year follow‐up. Acta Neurol Scand. 2015;132(2):79‐88. doi: 10.1111/ane.12375 [DOI] [PubMed] [Google Scholar]
- 30. Greenland JC, Williams‐Gray CH, Barker RA. The clinical heterogeneity of Parkinson's disease and its therapeutic implications. Eur J Neurosci. 2019;49(3):328‐338. doi: 10.1111/ejn.14094 [DOI] [PubMed] [Google Scholar]
- 31. Hoogland J, Van Wanrooij LL, Boel JA, et al. Detecting mild cognitive deficits in Parkinson's disease: comparison of neuropsychological tests. Mov Disord. 2018;33(11):1750‐1759. doi: 10.1002/mds.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wood K‐L, Myall DJ, Livingston L, et al. Different PD‐MCI criteria and risk of dementia in Parkinson's disease: 4‐year longitudinal study. NPJ Parkinsons Dis. 2016;2(1):15027. doi: 10.1038/npjparkd.2015.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poortvliet PC, Gluch A, Silburn PA, Mellick GD. The Queensland Parkinson's Project: an overview of 20 years of mortality from Parkinson's disease. J Mov Disord. 2021;14(1):34‐41. 10.14802/jmd.20034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burke SL, Hu T, Naseh M, et al. Factors influencing attrition in 35 Alzheimer's Disease Centers across the USA: a longitudinal examination of the National Alzheimer's Coordinating Center's Uniform Data Set. Aging Clin Exp Res. 2019;31(9):1283‐1297. doi: 10.1007/s40520-018-1087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Broeders M, De Bie RMA, Velseboer DC, Speelman JD, Muslimovic D, Schmand B. Evolution of mild cognitive impairment in Parkinson disease. Neurology. 2013;81(4):346‐352. doi: 10.1212/WNL.0b013e31829c5c86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
Data used in the preparation of this article were obtained in July, 2023 from the Parkinson's Progression Markers Initiative (PPMI) database (https://www.ppmi‐info.org/access‐data‐specimens/download‐data), RRID:SCR_006431. For up‐to‐date information on the study, visit https://www.ppmi‐info.org. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.


