Abstract
Background
Amitriptyline is a tricyclic antidepressant that was synthesised in 1960 and introduced as early as 1961 in the USA, but is still regularly used. It has also been frequently used as an active comparator in trials on newer antidepressants and can therefore be called a 'benchmark' antidepressant. However, its efficacy and safety compared to placebo in the treatment of major depression has not been assessed in a systematic review and meta‐analysis.
Objectives
To assess the effects of amitriptyline compared to placebo or no treatment for major depressive disorder in adults.
Search methods
We searched the Cochrane Depression, Anxiety and Neurosis Group's Specialised Register (CCDANCTR‐Studies and CCDANCTR‐References) to August 2012. This register contains relevant randomised controlled trials from: The Cochrane Library (all years), EMBASE (1974 to date), MEDLINE (1950 to date) and PsycINFO (1967 to date). The reference lists of reports of all included studies were screened and manufacturers of amitriptyline contacted for details of additional studies.
Selection criteria
All randomised controlled trials (RCTs) comparing amitriptyline with placebo or no treatment in patients with major depressive disorder as diagnosed by operationalised criteria.
Data collection and analysis
Two review authors independently extracted data. For dichotomous data, we calculated the odds ratio (OR) with 95% confidence intervals (CI). We analysed continuous data using standardised mean differences (with 95% CI). We used a random‐effects model throughout.
Main results
The review includes 39 trials with a total of 3509 participants. Study duration ranged between three and 12 weeks. Amitriptyline was significantly more effective than placebo in achieving acute response (18 RCTs, n = 1987, OR 2.67, 95% CI 2.21 to 3.23). Significantly fewer participants allocated to amitriptyline than to placebo withdrew from trials due to inefficacy of treatment (19 RCTs, n = 2017, OR 0.20, 95% CI 0.14 to 0.28), but more amitriptyline‐treated participants withdrew due to side effects (19 RCTs, n = 2174, OR 4.15, 95% CI 2.71 to 6.35). Amitriptyline also caused more anticholinergic side effects, tachycardia, dizziness, nervousness, sedation, tremor, dyspepsia, sedation, sexual dysfunction and weight gain. In subgroup and meta‐regression analyses the results of the primary outcome were robust towards publication year (1971 to 1997), mean participant age at baseline, mean amitriptyline dose, study duration in weeks, pharmaceutical sponsor, inpatient versus outpatient setting and two‐arm versus three‐arm design. However, higher severity at baseline was associated with higher superiority of amitriptyline (P = 0.02), while higher responder rates in the placebo groups were associated with lower superiority of amitriptyline (P = 0.05). The results of the primary outcome were rather homogeneous, reflecting comparability of the trials. However, methods of randomisation, allocation concealment and blinding were usually poorly reported. Not all studies used intention‐to‐treat analyses and in many of them standard deviations were not reported and often had to be imputed. Funnel plots suggested a possible publication bias, but the trim and fill method did not change the overall effect size much (seven adjusted studies, OR 2.64, 95% CI 2.24 to 3.10).
Authors' conclusions
Amitriptyline is an efficacious antidepressant drug. It is, however, also associated with a number of side effects. Degree of placebo response and severity of depression at baseline may moderate drug‐placebo efficacy differences.
Keywords: Adult; Humans; Amitriptyline; Amitriptyline/therapeutic use; Antidepressive Agents, Tricyclic; Antidepressive Agents, Tricyclic/therapeutic use; Depressive Disorder, Major; Depressive Disorder, Major/drug therapy; Placebo Effect; Randomized Controlled Trials as Topic
Plain language summary
Amitriptyline for the treatment of depression
Amitriptyline is a tricyclic antidepressant drug that has been used for decades in the treatment of depression. The current review includes 39 trials with a total of 3509 participants and confirms its efficacy compared to placebo or no treatment. This finding is important, because the efficacy of antidepressants has recently been questioned. However, the review also demonstrated that amitriptyline produces a number of side effects such as vision problems, constipation and sedation. It is a limitation of this review that many studies have been poorly reported, which might have led to bias.
Summary of findings
Summary of findings for the main comparison. Amitriptyline versus placebo for major depressive disorder.
| Amitriptyline versus placebo for major depressive disorder | ||||||
| Patient or population: adults with major depressive disorder Settings: inpatients and outpatients Intervention: amitriptyline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amitriptyline | |||||
| Response to treatment At least 50% reduction of a depression scale (mainly Hamilton Depression Rating Scale) Follow‐up: 3 to 12 weeks | 313 per 1000 | 546 per 1000 (509 to 582) | OR 2.64 (2.28 to 3.06) | 3228 (31 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Death due to suicide | See comment | See comment | Not estimable3 | ‐ | See comment | No study reported on this outcome |
| Quality of life | See comment | See comment | Not estimable3 | ‐ | See comment | No study reported on this outcome |
| Acceptability of treatment Drop‐out for any reason Follow‐up: 3 to 12 weeks | 403 per 1000 | 324 per 1000 (271 to 386) | OR 0.71 (0.55 to 0.93) | 2400 (24 studies) | ⊕⊝⊝⊝ very low1,4,5 | |
| Overall tolerability Drop‐out due to adverse events Follow‐up: 3 to 12 weeks | 45 per 1000 | 165 per 1000 (114 to 232) | OR 4.15 (2.71 to 6.35) | 2174 (19 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The randomisation and allocation methods were usually unclear. Drop‐outs were often not clearly described. 2 There was a possibility of publication bias, but according to the trim and fill method the suspected missing studies would not have changed the overall effect size much. 3 Not a single study reported on this outcome. 4 There was moderate heterogeneity (I² = 48%). Some studies showed superiority of amitriptyline and others of placebo. 5 Acceptability of treatment was measured indirectly by the number of participants leaving the studies prematurely.
Background
Description of the condition
Major depressive disorder is a common condition with a lifetime prevalence of 15% to 18% (Berger 2004). Its main symptoms are a depressed mood and lack of interest or pleasure in activities. These are often accompanied by a range of other problems including fatigue, loss of appetite and weight, poor concentration, decreased libido, sleep problems, inappropriate guilt feelings and suicidal ideation. The World Health Organization (WHO) estimates that depression affects about 121 million people in the world (WHO 2005). Some authors describe a lower prevalence of major depression, according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders by the American Psychiatric Association (DSM‐IV), in Japan (lifetime prevalence 3% to 7%) compared to Western countries, suggesting that the prevalence of major depression might be lower in Asian countries (Kawakami 2007), but this is controversial. However, by the year 2020 depression could become the most common disease after cardiovascular diseases worldwide (Gayetot 2007). The degree of disability and suffering of those with depression can be dramatic. For example, in the year 2005, people with unipolar depressive disorders were placed second in terms of disability adjusted life years (DALY) in Germany (WHO 2005). Suicide rates are clearly higher in those with major depression than in the general population (Berger 2004).
Description of the intervention
Various psychotherapeutic and psychopharmacological interventions are available for the treatment of major depressive disorder. Among the psychological therapies, the efficacy of cognitive behavioural interventions is probably the best examined (Cuijpers 2010; Gloaguen 1998). The mainstay of pharmacological treatment are the various classes of antidepressants.
The first tricyclic antidepressant (TCA) was imipramine, introduced in 1955. Various other TCAs and monoamine oxidase inhibitors (MAOIs) followed soon after. Amitriptyline, the antidepressant examined in this review, is a TCA that was already in use in 1961 and is still frequently used nowadays. As an example, with 94 million defined daily doses (DDD) it was still the third most frequently prescribed antidepressant in Germany after citalopram (209 DDD) and mirtazapine (107 DDD) in 2008 (Lohse 2009). Amitriptyline also offers a large variability in dosing, often ranging between 25 mg and 150 mg, but sometimes even less or more. A typical adult dose for inpatients is 150 mg daily. The drug is associated with a number of side effects such as blurred vision, constipation, urination problems, dry mouth, delirium, vertigo and sedation. Overdoses can be life‐threatening due to cardiac arrhythmias and other factors. Indeed, fatal toxicities have been shown to be more frequent under tricyclic antidepressants than under newer antidepressants such as selective serotonin reuptake inhibitors (SSRIs) (Henry 1995). Apart from the treatment of major depressive disorder, amitriptyline is also used in the treatment of other forms of depression, chronic pain, migraine and anxiety disorders, although for many of the latter it does not have an official indication.
In the last three decades the TCAs have been partly replaced by newer agents, especially selective serotonin reuptake inhibitors (SSRIs) but also selective noradrenaline reuptake inhibitors or selective serotonin and noradrenaline reuptake inhibitors. The main advantage of the SSRIs is their better overall tolerability compared to TCAs (Barbui 2000). However, there is debate as to whether TCAs such as amitriptyline may be more efficacious than SSRIs, in particular in more severely ill inpatients (Guaiana 2007).
How the intervention might work
One of the hypotheses for the aetiology of depression is dysfunction of the monoamine system, including the neurotransmitters serotonin and norepinephrine. Amitriptyline increases the concentrations of these neurotransmitters in the synaptic cleft by inhibiting their reuptake into the presynaptic neuron. The reuptake inhibition is achieved by blocking the noradrenaline and serotonin transporters. Amitriptyline has a relatively similar affinity for these two receptors whereas clomipramine, for example, has a far greater affinity for the serotonin relative to the noradrenaline transporter or desipramine and nortriptyline for which the reverse applies.
Amitriptyline, however, also functions as an antagonist at various other neuroreceptors such as histamine H1 receptors, muscarinic cholinoreceptors, alpha 1 adrenoreceptors and 5‐HT2a receptors, which may serve as links to its putative side effects.
Why it is important to do this review
In many countries amitriptyline is still a frequently used antidepressant. For example, in 2008 it was the third most frequently prescribed antidepressant in Germany (94 million DDDs) (Lohse 2009). In the UK, approximately 13 people per 1000 were prescribed amitriptyline for depression in 2010 according to a large primary care‐based prescription database (GPRD 2011) ) (please note this is based on an estimate only as the GPRD does not record the indication for which the drug was prescribed). Therefore it is important to define its efficacy and safety compared to placebo. Furthermore, recent reviews have found only small differences between new antidepressants and placebo, putting into question the efficacy of antidepressants in general. For example, Barbui 2008 compared the selective serotonin reuptake inhibitor paroxetine with placebo and the absolute difference in responder rates was only 10% (53% responded to drug versus 42% to placebo, N = 22 trials, n = 5222 participants; risk difference 10%, 95% confidence interval (CI) 7% to 13%; response ratio 1.2, 95% CI 1.2 to 1.3) and the effect size was only 0.31 (95% CI 0.22 to 0.40). Turner 2008 showed that the effect sizes of new antidepressants are smaller if unpublished trials are included. Kirsch 2008 concluded from their systematic review that new antidepressants should only be used in the most severely ill patients and not in mild forms of depression for which they are frequently prescribed, although the methodology of the review has been criticised by other researchers (McAllister‐Williams 2008). However, all these analyses were derived from studies on newer antidepressants. To the best of our knowledge a methodologically sound systematic review comparing the effects of the classical antidepressant amitriptyline with placebo is not available. The results of this review can be an important contribution to the present polarised debate. This review also adds to the portfolio of Cochrane reviews on antidepressants for depression. In particular, it augments the information available on amitriptyline, for which a systematic review comparing it with other antidepressants is already available (Guaiana 2007). The results will also be used in a network meta‐analysis on antidepressants currently being conducted by members of the Cochrane Depression, Anxiety and Neurosis Group.
Objectives
To assess the effects of amitriptyline compared to placebo or no treatment for major depressive disorder in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials. We only included studies with adequate randomisation (for example computer‐generated randomisation lists) and allocation (for example allocation by an independent person in the hospital pharmacy) procedures, or if the details of randomisation and allocation were unclear, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We excluded quasi‐randomised studies such as those using allocation by day of the week, date of birth or alternate allocation due to the evidence of a strong relationship between allocation concealment and direction of effect (Schulz 1995).
There was no minimum duration of the included studies, and there was no upper limit of the duration as long as the participants were initially acutely ill.
There was no language restriction, in order to avoid the problem of ‘language bias’ (Egger 1997).
Only the first phases of cross‐over studies were used, to avoid carry‐over effects.
Types of participants
Adults aged 18 years or older with acute unipolar major depressive disorder according to any standardised diagnostic criteria such as the DSM‐IV, DSM‐III‐R, DSM‐III diagnostic codes 296.2 or 296.3 (APA 1980; APA 1987; APA 1994), WHO International Classification of Diseases (ICD) ICD‐10 (F32 or F33) (WHO 1992), ICD‐9 (WHO 1978), Research Diagnostic Criteria (Spitzer 1978) or Feighner criteria (Feighner 1972) were included.
There were no limits in terms of setting, gender or ethnicity and there was no upper age limit.
We included studies in which less than 20% of the participants were suffering from bipolar depression, dysthymia or neurotic depression. We also included participants with a concurrent secondary diagnosis of another psychiatric disorder. We included participants treated in primary care and specialty behavioural health or psychiatry as well as participants treated in inpatient and outpatient settings. We excluded studies in participants with no or only subclinical symptoms at baseline, which are usually conducted to address the relapse preventing effects of antidepressants. We excluded participants with a concurrent primary diagnosis of Axis I or II disorders and participants with a serious concomitant medical illness.
Types of interventions
The experimental treatment was amitriptyline: any dose, any oral mode of administration (tablets, capsules or liquid form).
The comparator substance was placebo, either active (an inert substance that mimics the side effects of amitriptyline) or inactive.
Treatment must have been given as monotherapy.
Types of outcome measures
Primary outcomes
The primary outcome was the number of patients who responded to treatment, defined as a reduction of at least 50% on the Hamilton Depression Rating Scale (HAM‐D) (Hamilton 1960), the Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979) or any other depression scale, or 'much or very much improved' (score 1 or 2) on the Clinical Global Impression (CGI) Improvement Scale (Guy 1976). All response rates were calculated from the total number of randomised patients. Where more than one criterion was provided, we used the HAM‐D for judging the response and then followed the sequence described above. Despite the problems surrounding scale‐derived response cutoffs (Leucht 2007), dichotomous outcomes can be understood more intuitively by clinicians than the mean values of rating scales and are therefore preferred.
When studies reported response rates at various time points of the trial, we had decided a priori to subdivide the treatment indices as follows.
Early response, between one and five weeks; the time point closest to two weeks was given preference.
Acute phase treatment response, between six and 12 weeks; the time point given in the original study as the study endpoint was given preference.
Follow‐up response, between four and six months; the time point closest to 24 weeks was given preference.
The acute phase treatment response, that is between six and 12 weeks, was our primary outcome of interest.
Secondary outcomes
The number of participants in remission, as defined by either: (a) a score of 7 or less on the 17‐item HAM‐D and 8 or less for all the other longer versions of HAM‐D; (b) a score of 10 or less on the MADRS (Zimmerman 2004); (c) 'not ill or borderline mentally ill' (score 1 or 2) on the CGI‐Severity (Guy 1976); or (d) other criteria as defined by the trial authors. All remission rates were calculated out of the total number of randomised patients. Where two or more scales are provided, we preferred the first criteria for judging remission. ‘Remission’ is a state of relative absence of symptoms. This outcome added to the primary outcome ‘response’ to treatment. The disadvantage of 'remission' is that its frequency depends on the initial severity of the participants. If they were only relatively mildly ill, many will be classified as in remission while only few will be in the case of high average severity at baseline. Therefore, studies and meta‐analyses usually apply response and not remission as the primary outcome.
Change scores from baseline or endpoint score at the time point in question (early response, acute phase response or follow‐up response as defined above) on the HAM‐D or MADRS, or any other validated depression scale. The results of mean values of depression rating scales can be more sensitive than dichotomous response data. Therefore, they should also be presented even though their interpretation is less intuitive than with dichotomous response data. Change data were preferred to endpoint data but both had to be presented separately because we used the standardised mean difference as an effect size measure for which pooling of endpoint and change data is not appropriate (Higgins 2008, page 269). We preferred change scores to endpoint scores because they, to a certain extent, take into account small baseline imbalances.
Social adjustment, social functioning including the Global Assessment of Function scores (Luborsky 1962).
Health‐related quality of life as measured by validated disease‐specific and generic scales such as the Short Form (SF)‐36 (Ware 1993) or the Health of the Nation Outcome Scales (HoNOS) (Wing 1994).
-
Various reasons for dropping out of the studies:
due to any reason, as a measure of the overall acceptability of treatment
due to inefficacy of treatment, as a global efficacy measure
due to adverse events, as a global measure of tolerability
-
Death:
natural causes
suicide
suicide attempts
-
Side effects:
number of participants experiencing at least one side effect
agitation or anxiety
blurred vision
constipation
urination problems
delirium
diarrhoea
dry mouth
fits
insomnia
hypotension
nausea
sedation or somnolence
vomiting
vertigo
We anticipated including the following main outcomes in a 'Summary of findings' table using GRADEpro (Brozek 2008): response to treatment, acceptability of treatment (drop‐out due to any reason), quality of life, death due to suicide and overall tolerability (drop‐out due to adverse events).
Search methods for identification of studies
CCDAN's Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintain two clinical trials registers at their editorial base in Bristol, UK, a references register and a studies‐based register. The CCDANCTR‐References Register contains over 30,000 reports of randomised controlled trials in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual. Please contact the CCDAN Trials Search Co‐ordinator for further details. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 ‐), EMBASE (1974 ‐) and PsycINFO (1967 ‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organization’s trials portal (ICTRP), drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCDAN's generic search strategies can be found on the Group‘s website.
Electronic searches
With the assistance of the Cochrane Collaboration Depression, Anxiety and Neurosis Group (CCDAN) Trials Search Co‐ordinator (TSC), we searched the Group's controlled trials registers (CCDANCTR‐References and CCDANCTR‐Studies) up to 30 August 2012 using the following terms:
((Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder*" or "Affective Symptoms") and amitriptylin* and placebo*)
We also searched the clinical trial databases ClinicalTrials.gov, the International Standard Randomised Controlled Trial Number Register (ISRCTN) and the WHO Trials portal (ICTRP) with the term amitriptylin* (to 30 August 2012).
Searching other resources
Reference searching
We inspected the references of all identified studies for more trials.
Personal contact
We contacted the first author of each included study for any missing information on the included studies.
Drug companies
We contacted the major manufacturer of amitriptyline to ask about further relevant studies and for missing information on identified studies.
Handsearching
Appropriate journals and conference proceedings relating to amitriptyline treatment for depression have been handsearched and incorporated into the CCDAN databases.
Data collection and analysis
Selection of studies
Two review authors independently inspected all titles and abstracts identified by the searches. Disagreement was resolved by discussion and if necessary a third review author was involved. Where doubt still remained, we acquired the full article for further inspection. Once the full articles were obtained, at least two review authors independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, and a third review author, we sought further information from the study authors.
Data extraction and management
Two review authors independently extracted data from all selected trials. When there was disagreement it was resolved by discussion with a third review author. When possible, we contacted the study authors to resolve any dilemma. We extracted data on standard, simple forms that were piloted using a random sample of 10 studies.
Assessment of risk of bias in included studies
Two authors independently assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome assessment, the completeness of outcome data, selective reporting and other biases. If the raters disagreed the final rating was made by consensus and with the involvement (if necessary) of a third member of the review group. We categorised each domain as high risk of bias, low risk of bias or unclear risk of bias.
Measures of treatment effect
1. Continuous data
As we expected that the studies would frequently use different scales to measure the same concept (for example either the HAM‐D or the MADRS to evaluate the overall degree of depression) the standardised mean difference (SMD) was the effect measure for continuous outcomes.
2.1 Change versus endpoint data
We used endpoint data only when change data were not available.
2.2 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion.
(a) We entered data from studies of, for example, at least 200 participants in the analysis irrespective of the following rules, because skewed data pose less of a problem in large studies.
(b) Endpoint data: when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean and divide this by the standard deviation. If this value is lower than one, it strongly suggests a skew and the study was excluded. If this ratio is higher than one but below two, there is suggestion of a skew. We entered the study and tested whether its inclusion or exclusion substantially changed the results. If the ratio was larger than two the study was included because skew is less likely (Altman 1996; Higgins 2008).
(c) When continuous data are presented on a scale which includes the possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We planned to enter such studies because change data tend to be less skewed and because excluding studies would also lead to bias, because not all the available information would be used.
3. Binary data
We calculated the odds ratio (OR) and its 95% confidence interval (CI).
Unit of analysis issues
Cross‐over trials
For trials which had a cross‐over design we only considered results from the first randomisation period to avoid carry‐over effects (Elbourne 2002).
Cluster‐randomised trials
If we encountered cluster‐randomised trials we included them following the rules presented in the Cochrane Handbook (Higgins 2008).
Trials with multiple dose groups
Some studies might address the effects of different doses of amitriptyline compared to placebo. In the case of dichotomous outcomes we summed the sample sizes and the number of people with events across both groups. For continuous outcomes we combined means and standard deviations using the methods described in chapter 7 (section 7.7.3.8) of the Cochrane Handbook (Higgins 2008).
Dealing with missing data
1. Missing participants
Dichotomous data
We analysed all data on the basis of the intention‐to‐treat (ITT) principle: drop‐outs were always included in this analysis. Where participants were withdrawn from the trial before the endpoint, it was assumed that their condition remained unchanged if they had stayed in the trial. This is conservative for outcomes related to response to treatment (because these participants will be considered to have not responded to treatment). It is not conservative for adverse events but we think that for the adverse events of interest in our review (see outcomes) a worst‐case scenario is clinically unlikely. When there were missing data and the method of 'last observation carried forward' (LOCF) had been used to do an ITT analysis, then we used the LOCF data with due consideration of the potential bias and uncertainty introduced.
Continuous data
The Cochrane Handbook recommends avoiding imputations of continuous data and suggests rather that the data must be used in the form presented by the original authors. Whenever ITT data were presented by the authors they were preferred to ‘per protocol or completer’ data sets.
2. Missing data
We contacted the original study authors for missing data.
3. Missing statistics
When only the standard error (SE) or P values were reported, we calculated standard deviations (SDs) according to Altman (Altman 1996). In the absence of supplemental data after requests to the authors, we estimated the SDs from CI, t values or P values as described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008); or imputed them according to a validated method (Furukawa 2006). We examined the validity of these imputations in a sensitivity analysis.
Assessment of heterogeneity
We started to assess heterogeneity by visual inspection of the forest plots. We also calculated I2 statistics and analysed them on the basis of the Cochrane Handbook recommendations (I2 values of 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity). In addition to the I 2 statistic (Higgins 2003) we presented the Chi2 and its P value and considered the direction and magnitude of the treatment effects. As the Chi2 test is underpowered to detect heterogeneity in meta‐analyses with few studies, should it exist, we used a P value of 0.10 as a threshold of statistical significance.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These biases are described in section 10.1 of the Cochrane Handbook (Higgins 2008). We investigated reporting bias by constructing funnel plots. We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size.
Data synthesis
We employed the random‐effects model for all analyses (Der‐Simonian 1986). We understand that there is no closed argument for preference of either the fixed‐effect or random‐effects model. The random‐effects method incorporates an assumption that the different studies are estimating different yet related intervention effects. This does seem true for us as we a priori expected some clinical heterogeneity between the patients in the different trials. We examined, however, whether use of a fixed‐effect model led to a substantial difference in the primary outcome.
Subgroup analysis and investigation of heterogeneity
We explored potential causes of heterogeneity by performing subgroup analysis and random‐effects restricted maximum‐likelihood meta‐regression. We are aware that subgroup analyses are observational by nature and therefore considered the results to be exploratory and not explanatory. Nevertheless, we addressed the following a priori defined potential effect modifiers of the primary outcome.
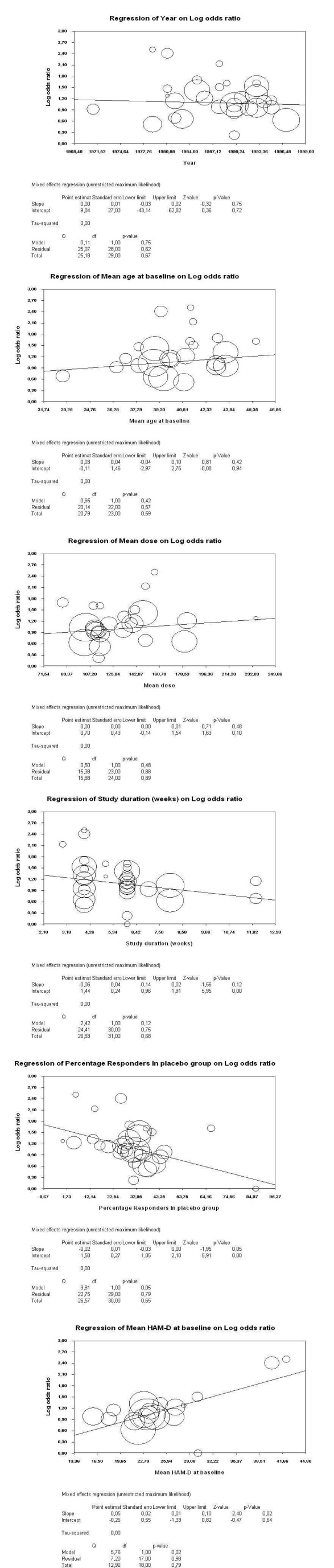
Depression severity at baseline using the mean HAM‐D score at baseline as a moderator in a meta‐regression: because it is known that antidepressants are more efficacious in more severely ill patients (Kirsch 2008).
Mean age at baseline: the rationale was that drug pharmacokinetics and metabolism change with age.
Mean amitriptyline dose: in a secondary analysis of their work, Furukawa 2003 found that higher doses of tricyclic antidepressants might be somewhat more efficacious than lower doses.
Study duration, in weeks: to find out whether longer duration studies showed greater drug to placebo differences than shorter trials.
Percentage number of participants who responded to placebo: studies on selective serotonin reuptake inhibitors have shown that the degree of placebo response has increased in recent years and that this can limit drug to placebo differences (Walsh 2002). We explored whether this is also the case in the amitriptyline studies.
Publication year: old meta‐analyses (e.g. Davis 1993) found much bigger differences between antidepressants and placebo than recent systematic reviews (e.g. Barbui 2008). We explored whether this impression can be confirmed by statistical analysis.
Diagnostic system (subgroup analysis): we compared studies that used operationalised diagnostic criteria (DSM‐IV, DSM‐III‐R, DSM‐III, ICD‐10, Research Diagnostic Criteria, Feighner criteria) with studies using the non‐operationalised criteria ICD‐9. As participants diagnosed by the latter criteria might be quite different from those applying operationalised criteria, we planned to investigate this in a subgroup analysis.
Pharmaceutical sponsor (yes or no): pharmaceutical companies have an inevitable conflict of interest. Therefore, we compared the results of industry sponsored and non‐industry sponsored trials. As long as only medication was provided by a pharmaceutical company, such studies were not classified as primarily industry sponsored.
Two‐arm versus three‐arm studies (e.g. amitriptyline versus SSRI versus placebo): we carried out this subgroup analysis because early work suggested that the antidepressant‐placebo difference is smaller in three‐arm than in two‐arm studies (Greenberg 1992).
Inpatient versus outpatient studies: Barbui 2004 found that amitriptyline might be more efficacious than SSRIs in inpatients, while there was no difference in outpatients. This subgroup analysis therefore explored whether this was also the case when amitriptyline was compared with placebo.
Sensitivity analysis
The following sensitivity analyses of the primary outcome were planned.
Exclusion of non‐double‐blind studies.
Fixed‐effect instead of random‐effects model.
Exclusion of studies using imputed statistics.
Exclusion of cluster‐randomised trials.
Exclusion of cross‐over trials.
Exclusion of studies that used ICD‐9 for the diagnostic criteria.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
A PRISMA diagram is presented in Figure 1. The electronic search in September 2010 and the update search in August 2012 yielded 466 potentially relevant references; 27 were identified in clinicaltrials.gov or ISRCTN, one by cross‐referencing and 10 records were sent by two pharmaceutical companies (five by each company). In total we screened 504 reports; we excluded 360 reports on the basis of the abstract. We inspected in detail but finally excluded 82 full reports on 81 studies (for reasons see below). Sixty reports on 39 RCTs with a total of 3509 participants met the inclusion criteria and 36 RCTs provided data for at least one outcome. One study is awaiting assessment.
1.
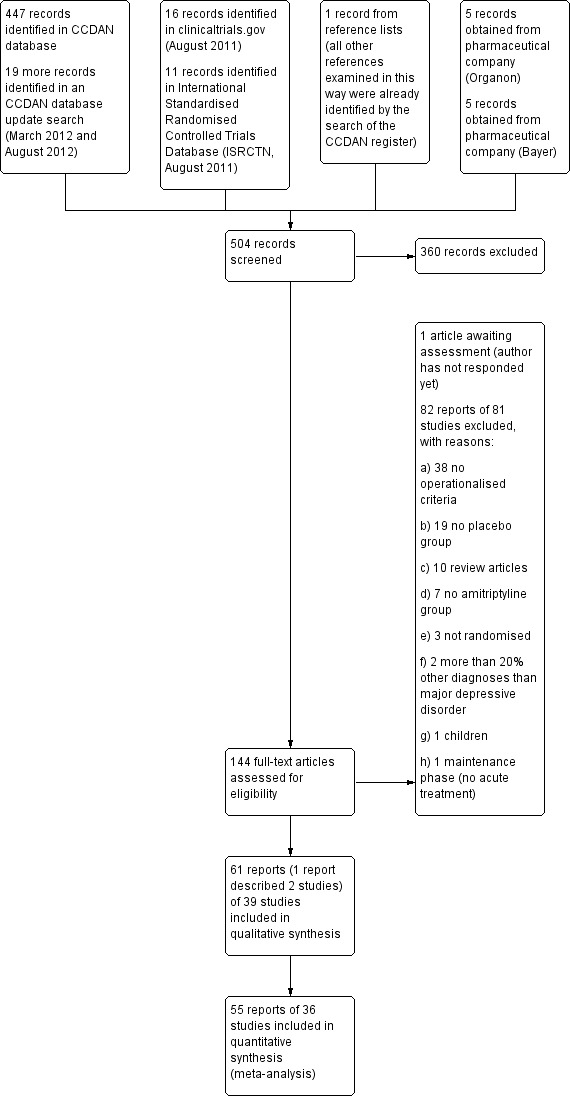
Study flow diagram.
We contacted all first authors and seven authors replied; two of them provided us with additional information. We also contacted pharmaceutical companies manufacturing amitriptyline: two companies replied, one company (Organon) provided us with additional information of already published studies as well as data from two unpublished trials.
Included studies
Design
Length of treatment
In 17 studies the randomised phase was six weeks (Bhatia 1991; Bremner 1995; Carman 1991; Gelenberg 1990; Hicks 1988; Jacobson 1990; Kusalic 1993; Organon 3‐020 unpublished; Organon 84062 unpublished; Paykel 1988a; Preskorn 1983; Rickels 1982; Rickels 1985; Rowan 1980; Smith 1990; Stratas 1984; Wilcox 1994) and in 13 studies the randomised phase lasted four weeks (Amsterdam 1986; Blashki 1971; Claghorn 1983; Feighner 1979; Georgotas 1982; Hormazabal 1985; Katz 1993; Katz 1993a; Kupfer 1979; Langlois 1985; Shipley 1981; Roffman 1982; van de Merwe 1984a). There were respectively two studies with a duration of three weeks (Klieser 1988; McNair 1984a), five weeks (Hoschl 1989; Raft 1981), eight weeks (Lydiard 1997; Reimherr 1990) and 12 weeks (Mynors‐Wallis 1995; Thomson 1982). One study lasted seven weeks (Bakish 1992).
Sample size
The mean number of participants per study was 92.2 (SD 76.5), with a minimum sample size of 12 (van de Merwe 1984a) and a maximum of 299 (Reimherr 1990). In one study the number of participants was not reported (Preskorn 1983).
Cluster/cross‐over design
There was only one study with a cross‐over design (McNair 1984a), but the description of the first treatment phase did not provide any usable data. There was no cluster‐randomised trial.
Participants
Age
Overall the mean age was 40.06 years (SD 2.96); the mean age was provided in 27 studies. Of these 27 trials, six trials provided information only for the mean age of all arms. Regarding the other 12 trials, two provided only the age range which was 17 to 73 and 21 to 65, respectively, and 10 trials did not provide any relevant information. In six studies patients over 65 years could have been included (Amsterdam 1986; Blashki 1971; Bremner 1995; Katz 1993a; Preskorn 1983; Roffman 1982).
Diagnosis
All studies enrolled patients suffering from major depression, 20 according to DSM‐III criteria (Bhatia 1991; Bremner 1995; Carman 1991; Gelenberg 1990; Hicks 1988; Hoschl 1989; Jacobson 1990; Katz 1993; Klieser 1988; Langlois 1985; Organon 3‐020 unpublished; Organon 84062 unpublished; Preskorn 1983; Reimherr 1990; Rickels 1982; Rickels 1985; Smith 1990; Roffman 1982; Wilcox 1994), 12 according to RDC (Amsterdam 1986;Claghorn 1983; Georgotas 1982; Kupfer 1979; McNair 1984a; Mynors‐Wallis 1995; Paykel 1988a; Rowan 1980; Shipley 1981; Stratas 1984; Thomson 1982; van de Merwe 1984a), four according to DSM‐III‐R criteria (Bakish 1992; Katz 1993a; Kusalic 1993; Lydiard 1997), one according to its own operationalised criteria (Blashki 1971) and two according to Feighner criteria (Feighner 1979; Raft 1981).
Intervention
All studies compared amitriptyline with placebo, three of them only in a two‐arm comparison (Kupfer 1979; Paykel 1988a; Shipley 1981), whereas 36 used a three or more arms design. All studies had a placebo arm; there was no trial with 'no treatment' in the comparator group.
Setting
In 25 studies the participants were outpatients (Amsterdam 1986; Bakish 1992; Blashki 1971; Bremner 1995; Carman 1991; Claghorn 1983; Feighner 1979; Gelenberg 1990; Jacobson 1990; Kusalic 1993; Langlois 1985; Lydiard 1997; McNair 1984a; Mynors‐Wallis 1995; Organon 3‐020 unpublished; Organon 84062 unpublished; Paykel 1988a; Reimherr 1990; Rickels 1982; Rickels 1985; Rowan 1980; Smith 1990; Stratas 1984; Thomson 1982; Wilcox 1994). In eight studies the participants were inpatients (Bhatia 1991; Hicks 1988; Hoschl 1989; Klieser 1988; Kupfer 1979; Preskorn 1983; Raft 1981; Shipley 1981), whereas in two studies outpatients and inpatients were included (Hormazabal 1985; van de Merwe 1984a). In four studies the setting remained unclear (Georgotas 1982; Katz 1993; Katz 1993a; Roffman 1982).
Dosage of study drug
The mean dosage of amitriptyline was 139.6 mg/day (SD 40.4); nine trials did not specify the mean dosage (Carman 1991; Georgotas 1982; Katz 1993; Katz 1993a; Organon 84062 unpublished; Preskorn 1983; Rowan 1980; Roffman 1982; Shipley 1981). In 30 studies the dosage of amitriptyline was within the therapeutic dosage range (25 to 300 mg/day, 25 mg was only a starting dose in some studies which could be increased); in two studies only the maximum dosage was reported (Amsterdam 1986; Mynors‐Wallis 1995). Only one study did not provide any information about the mean dose or the dosage range (Preskorn 1983).
Twenty‐nine trials used a flexible and eight trials a fixed‐dosage regimen (Blashki 1971; Klieser 1988; Kupfer 1979; Langlois 1985; Mynors‐Wallis 1995; Shipley 1981; Roffman 1982; Thomson 1982). The dosage regimen remained unclear in two studies (Kusalic 1993; Preskorn 1983).
Primary outcome
The primary outcome used in the great majority of studies was change from baseline on the HAM‐D. Specifically, 24 studies used the scale HAM‐D‐17 and in one study the HAM‐D‐17 was only used as threshold for inclusion, but there were no response and remission data anyway (van de Merwe 1984a). In nine studies the HAM‐D‐21 was used (Amsterdam 1986; Claghorn 1983; Gelenberg 1990; Georgotas 1982; Hormazabal 1985; Rickels 1982; Rickels 1985; Roffman 1982; Stratas 1984) and in one study the HAM‐D‐24 (Feighner 1979). One study used the first 18 items of the 21‐item HAM‐D (Thomson 1982) and one study the first 16 items (Hoschl 1989). Two studies did not provide any information (Klieser 1988; Preskorn 1983).
Response definitions
In 13 studies response was defined as showing at least a 50% reduction in the HAM‐D (Amsterdam 1986; Bakish 1992; Bremner 1995; Claghorn 1983; Feighner 1979; Gelenberg 1990; Jacobson 1990; Kusalic 1993; Organon 3‐020 unpublished; Organon 84062 unpublished; Rickels 1985; Smith 1990; Wilcox 1994), whereas there was no definition of response in 15 studies (Carman 1991; Georgotas 1982; Hormazabal 1985; Katz 1993;Katz 1993a; Klieser 1988; Langlois 1985; McNair 1984a; Mynors‐Wallis 1995; Preskorn 1983; Raft 1981; Rowan 1980; Shipley 1981; Stratas 1984; van de Merwe 1984a). In one study response was defined as a 50% reduction in HAM‐D‐17 baseline score without subsequent deterioration beyond 20% of achieved HAM‐D score (Roffman 1982) and in one study as an improvement with HAM‐D < 10 (Hoschl 1989). In one study a HAM‐D score of 12 was used as the cutoff score for responders (Kupfer 1979), in one study response was defined as a CGI ≤ 2 (Lydiard 1997) and in one study response was defined as a moderate or marked global improvement (Rickels 1982).
Remission definitions
In one study remission was defined as recovery (the criteria were a HAM‐D score ≤ 7 and Beck Depression Inventory (BDI) ≤ 8 (Mynors‐Wallis 1995)) and in one study remission was defined as a fall to 4 points or less on the total HAM‐D score (Thomson 1982), whereas all other studies did not provide a definition of remission.
Sponsorship
Twenty‐six studies were sponsored by a drug company (Amsterdam 1986; Bakish 1992; Bhatia 1991; Bremner 1995; Carman 1991; Claghorn 1983; Gelenberg 1990; Georgotas 1982; Hicks 1988; Hormazabal 1985; Jacobson 1990; Katz 1993; Katz 1993a; Langlois 1985; Lydiard 1997; McNair 1984a; Organon 3‐020 unpublished; Organon 84062 unpublished; Rickels 1982; Rickels 1985; Rowan 1980; Smith 1990; Roffman 1982; Thomson 1982; van de Merwe 1984a; Wilcox 1994). In two studies the sponsorship was unclear (Klieser 1988; Reimherr 1990) whereas 11 studies were not sponsored (Blashki 1971; Feighner 1979; Hoschl 1989; Kupfer 1979; Kusalic 1993; Mynors‐Wallis 1995; Paykel 1988a; Preskorn 1983; Raft 1981; Shipley 1981; Stratas 1984). It should be noted that we did not classify a study as industry‐sponsored when only the medication was provided. Moreover, in none of the industry‐sponsored studies was the focus on amitriptyline. Either the sponsor was the manufacturer of another antidepressant or the sponsor produced both amitriptyline and another, newer antidepressant, but the focus was on the new antidepressant. Amitriptyline was rather an active comparator in addition to placebo versus the new antidepressant in these trials.
Excluded studies
Eighty‐two abstracts on 81 studies for which we assessed the full publications were excluded because they did not use operationalised criteria (N = 38), did not have a placebo group (N = 19) or amitriptyline group (N = 7), were review articles (N=10), were not randomised (N = 3), had included more than 20% of participants with other diagnoses than major depressive disorder (N = 2), studied children (N = 1) or were conducted in stable participants (N = 1).
Studies awaiting classification
One study is currently awaiting assessment (Kahn 2008). The available report is just a follow‐up of a potentially eligible trial. Too little information on the relevant original study could be obtained from the authors, so we decided to classify this study as awaiting assessment.
Risk of bias in included studies
A summary of the 'Risk of bias' assessment is provided in Figure 2 and Figure 3.
2.
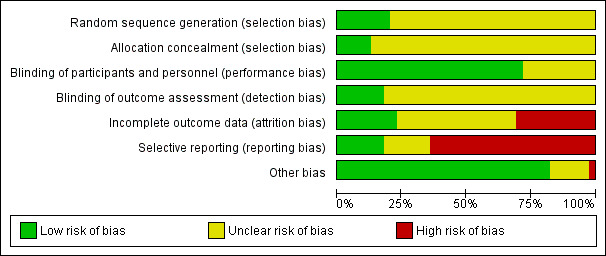
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
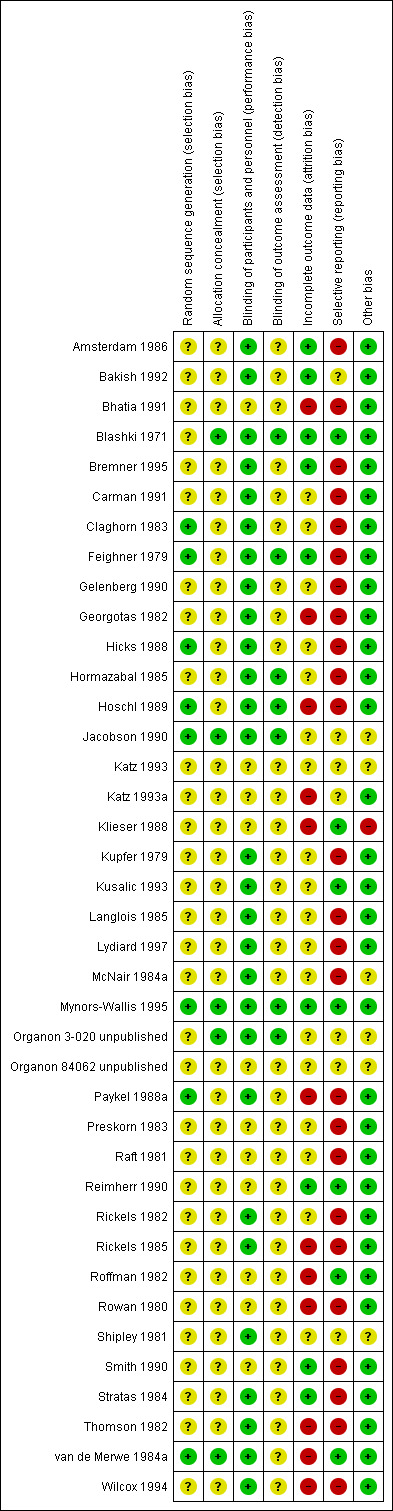
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The vast majority of the studies were just stated to be randomised without indicating details of how the sequence was generated. The same held true for allocation concealment. Thus, it is unclear whether participants were adequately randomised and allocated.
Blinding
All studies were described as double‐blind, although not all RCTs provided at least a few details (e.g. statements such as "identical capsules") as to how blinding was assured. Very few studies made a statement about the blinding of assessor (detection bias).
Incomplete outcome data
In approximately 30% of the studies we felt that there was a high risk of bias due to incomplete outcome data. The main reasons for this were that the study authors either did not report reasons for drop‐out clearly enough or presented only completer analyses. Moreover, frequently more participants in the placebo group clearly dropped out due to inefficacy of treatment and more participants in the drug group dropped out due to adverse events.
Selective reporting
A general problem was that standard deviations were often not reported so that in the vast majority of the trials we had to apply the mean SDs from the "meta‐analysis of new generation antidepressants" (MANGA) project (Cipriani 2009).
Other potential sources of bias
Klieser 1988 reported only interim results. It is unclear whether the study has been completed. There were no other clear sources of bias.
Effects of interventions
See: Table 1
Amitriptyline versus placebo
1. Primary outcome ‐ response to treatment
4.
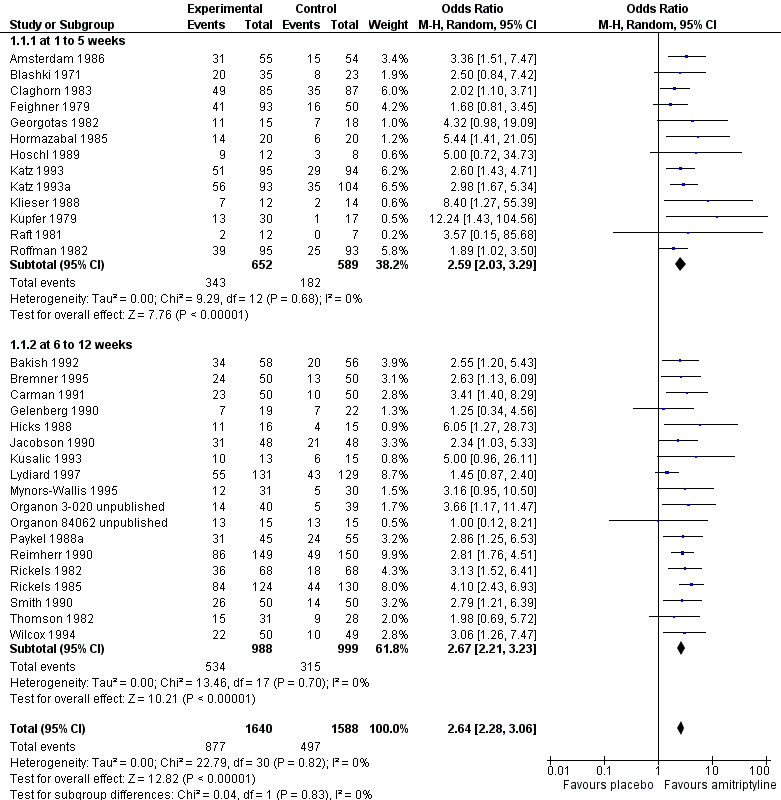
Forest plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Response.
1.1. Analysis.
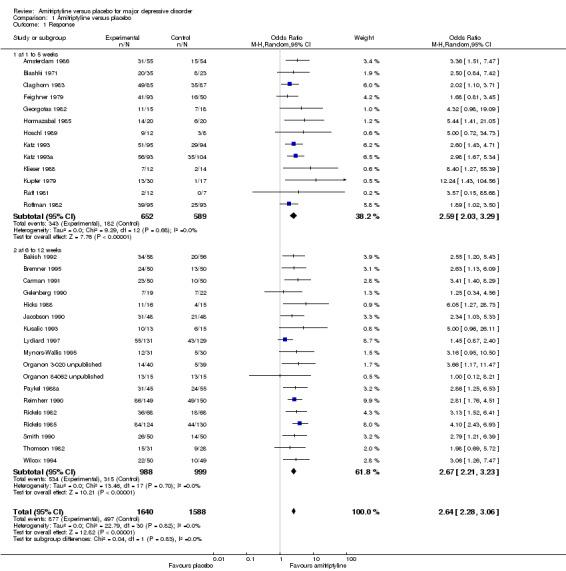
Comparison 1 Amitriptyline versus placebo, Outcome 1 Response.
a) Early response (one to five weeks)
Significantly more participants in the amitriptyline group than in the placebo group responded to treatment (odds ratio (OR) 2.59, 95% confidence interval (CI) 2.03 to 3.29, P < 0.00001, I² = 0%, 13 randomised controlled trials (RCTs), 1241 participants).
b) Acute‐phase response (6 to 12 weeks)
Significantly more participants in the amitriptyline group than in the placebo group responded to treatment (OR 2.67, 95% CI 2.21 to 3.23, P < 0.00001, I² = 0%, 18 RCTs, 1987 participants).
c) Overall results (1 to 12 weeks, i.e. combining early response and acute‐phase response)
Significantly more participants in the amitriptyline group than in the placebo group responded to treatment (OR 2.64, 95% CI 2.28 to 3.06, P < 0.00001, I² = 0%, 31 RCTs, 3228 participants).
2. Remission
1.2. Analysis.
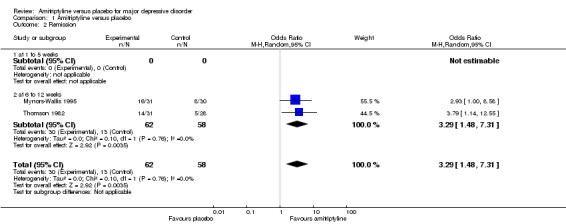
Comparison 1 Amitriptyline versus placebo, Outcome 2 Remission.
a) Early phase (one to five weeks)
No data available.
b) Acute phase (6 to 12 weeks)
Significantly more participants in the amitriptyline group than in the placebo group remitted (OR 3.29, 95% CI 1.48 to 7.31, P = 0.004, I² = 0%, two RCTs, 120 participants).
c) Overall results (1 to 12 weeks)
As there were only data for the acute phase (6 to 12 weeks), the overall results correspond to 2b).
3. Mean severity of depression reduction from baseline to endpoint
1.3. Analysis.
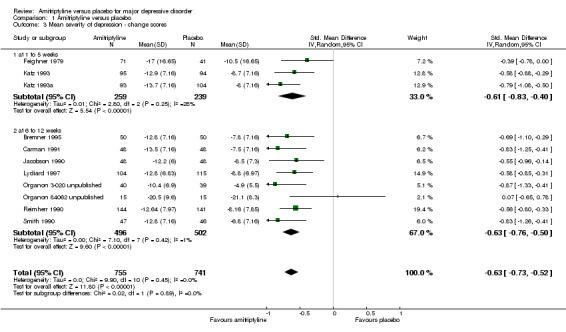
Comparison 1 Amitriptyline versus placebo, Outcome 3 Mean severity of depression ‐ change scores.
a) Early phase (one to five weeks)
The data suggest that amitriptyline was superior to placebo (standardised mean difference (SMD) ‐0.61, 95% CI ‐0.83 to ‐0.40, P < 0.00001, I² = 28%, three RCTs, 498 participants).
b) Acute phase (6 to 12 weeks)
The data suggest that amitriptyline was superior to placebo (SMD ‐0.63, 95% CI ‐0.76 to ‐0.50, P < 0.00001, I² = 1%, eight RCTs, 998 participants).
c) Overall results (1 to 12 weeks)
The data suggest that amitriptyline was superior to placebo (SMD ‐0.63, 95% CI ‐0.73 to ‐0.52, P < 0.00001, I² = 0%, 11 RCTs, 1496 participants).
4. Mean severity of depression at endpoint
1.4. Analysis.
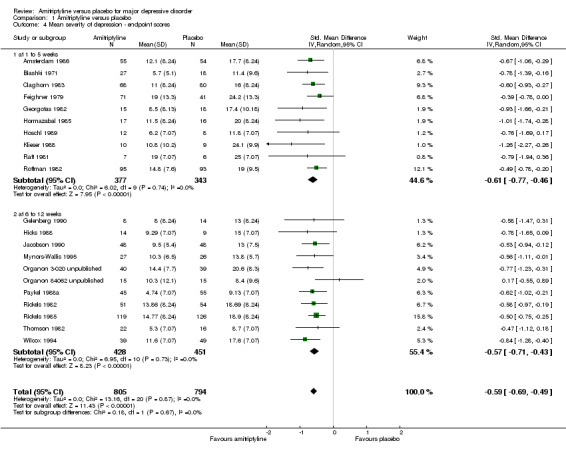
Comparison 1 Amitriptyline versus placebo, Outcome 4 Mean severity of depression ‐ endpoint scores.
a) Early phase (one to five weeks)
The data suggest that amitriptyline was superior to placebo (SMD ‐0.61, 95% CI ‐0.77 to ‐0.46, P < 0.00001, I² = 0%, 10 RCTs, 720 participants).
b) Acute phase (6 to 12 weeks)
The data suggest that amitriptyline was superior to placebo (SMD ‐0.57, 95% CI ‐0.71 to ‐0.43, P < 0.00001, I² = 0%, 11 RCTs, 879 participants).
c) Overall results (1 to 12 weeks)
The data suggest that amitriptyline was superior to placebo (SMD ‐0.59, 95% CI ‐0.69 to ‐0.49, P < 0.00001, I² = 0%, 21 RCTs, 1599 participants).
5. Drop‐out due to any reason
1.5. Analysis.
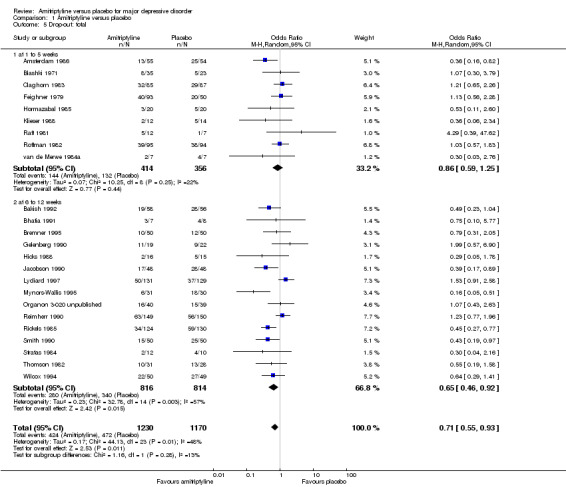
Comparison 1 Amitriptyline versus placebo, Outcome 5 Drop‐out: total.
a) Early phase (one to five weeks)
The drop‐out rates due to any reason showed no statistically significant superiority of amitriptyline compared to placebo (OR 0.86, 95% CI 0.59 to 1.25, P = 0.44, nine RCTs, 770 participants).
b) Acute phase (6 to 12 weeks)
The drop‐out rates due to any reason in the acute phase revealed a non‐significant trend in favour of amitriptyline (OR 0.65, 95% CI 0.46 to 0.92, P = 0.02, 15 RCTs, 1630 participants). There was moderate heterogeneity (Tau² = 0.23; Chi² = 32.78, df = 14 (P = 0.003); I² = 57%).
c) Overall results (1 to 12 weeks)
The overall drop‐out rates revealed a significant superiority of amitriptyline (OR 0.71, 95% CI 0.55 to 0.93, P = 0.01, 24 RCTs, 2400 participants). The results were moderately heterogeneous (Tau² = 0.17; Chi² = 44.13, df = 23 (P = 0.005); I² = 48%) with some studies favouring amitriptyline and others placebo. Drop‐out due to any reason is not an operationalised outcome which may explain some of the heterogeneity.
6. Drop‐out due to inefficacy
1.6. Analysis.
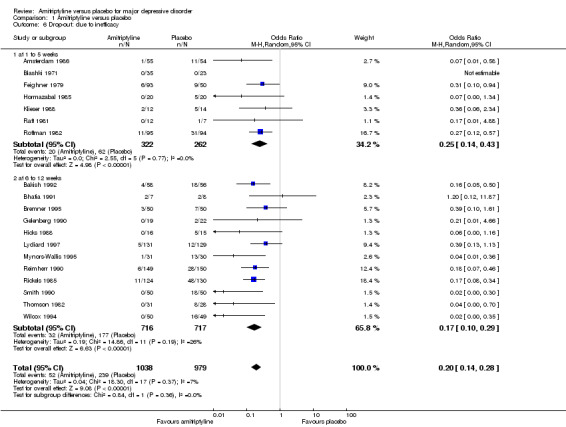
Comparison 1 Amitriptyline versus placebo, Outcome 6 Drop‐out: due to inefficacy.
a) Early phase (one to five weeks)
The drop‐out rates due to inefficacy suggest that amitriptyline is superior to placebo (OR 0.25, 95% CI 0.14 to 0.43, P < 0.00001, seven RCTs, 584 participants).
b) Acute phase (6 to 12 weeks)
The drop‐out rates due to inefficacy suggest that amitriptyline is superior to placebo (OR 0.17, 95% CI 0.10 to 0.29, P < 0.00001, 12 RCTs, 1433 participants).
c) Overall results (1 to 12 weeks)
The drop‐out rates due to inefficacy suggest that amitriptyline is superior to placebo (OR 0.20, 95% CI 0.14 to 0.28, P < 0.00001, 19 RCTs, 2017 participants).
7. Drop‐out due to adverse events
1.7. Analysis.
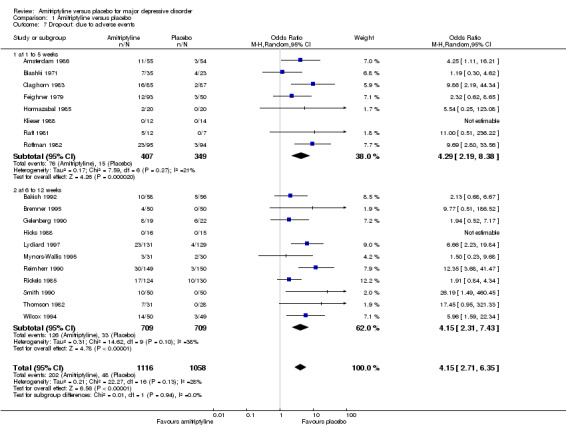
Comparison 1 Amitriptyline versus placebo, Outcome 7 Drop‐out: due to adverse events.
a) Early phase (one to five weeks)
The drop‐out rates due to adverse events suggest that amitriptyline is inferior to placebo (OR 4.29, 95% CI 2.19 to 8.38, P < 0.0001, eight RCTs, 756 participants).
b) Acute phase (6 to 12 weeks)
The drop‐out rates due to adverse events suggest that amitriptyline is inferior to placebo (OR 4.15, 95% CI 2.31 to 7.43, P < 0.00001, 11 RCTs, 1418 participants).
c) Overall results (1 to 12 weeks)
The drop‐out rates due to adverse events suggest that amitriptyline is inferior to placebo (OR 4.15, 95% CI 2.71 to 6.35, P < 0.00001, 19 RCTs, 2174 participants).
Side effects
8. Total number of participants experiencing at least one side effect
Overall significantly more participants in the amitriptyline group experienced at least one side effect (OR 4.64, 95% CI 2.45 to 8.78, P < 0.00001, seven RCTs, 802 participants). There was substantial heterogeneity (Tau² = 0.40; Chi² = 16.70, df = 6 (P = 0.01); I² = 64%), but with one exception (Raft 1981) all studies at least tended to favour placebo. Inspection of Raft 1981 revealed no obvious reason for the heterogeneity (Analysis 1.8).
1.8. Analysis.
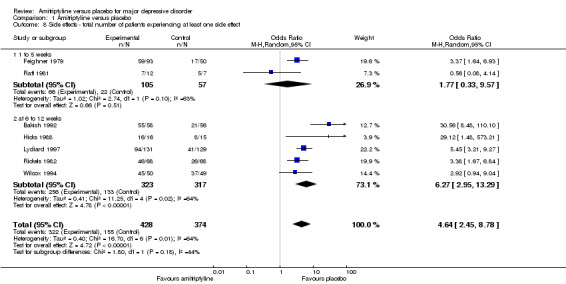
Comparison 1 Amitriptyline versus placebo, Outcome 8 Side effects ‐ total number of patients experiencing at least one side effect.
9. Anticholinergic: any anticholinergic effects (dry mouth, constipation, visual disturbances)
Overall significantly more participants in the amitriptyline group experienced any anticholinergic adverse effects (OR 6.33, 95% CI 3.44 to 11.65, P < 0.00001, two RCTs, 279 participants) (Analysis 1.9).
1.9. Analysis.
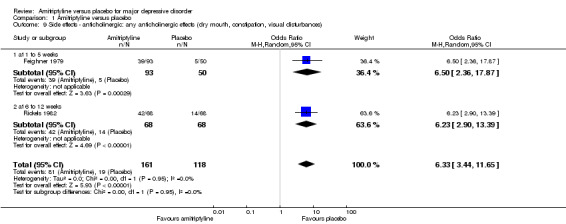
Comparison 1 Amitriptyline versus placebo, Outcome 9 Side effects ‐ anticholinergic: any anticholinergic effects (dry mouth, constipation, visual disturbances).
10. Anticholinergic: constipation
Overall significantly more participants in the amitriptyline group suffered from constipation (OR 3.39, 95% CI 2.36 to 4.88, P < 0.00001, nine RCTs, 1255 participants) (Analysis 1.10).
1.10. Analysis.
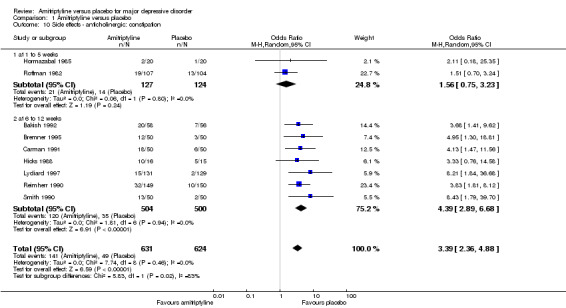
Comparison 1 Amitriptyline versus placebo, Outcome 10 Side effects ‐ anticholinergic: constipation.
11. Anticholinergic: dry mouth
Overall significantly more participants in the amitriptyline group suffered from dry mouth (OR 13.50, 95% CI 9.38 to 19.42, P < 0.00001, 11 RCTs, 1414 participants). There was moderate heterogeneity (Tau² = 0.16; Chi² = 12.28, df = 7 (P = 0.09); I² = 43%), but the effects of all studies were in favour of placebo (Analysis 1.11).
1.11. Analysis.
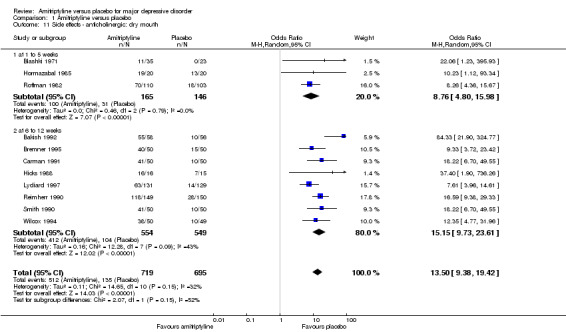
Comparison 1 Amitriptyline versus placebo, Outcome 11 Side effects ‐ anticholinergic: dry mouth.
12. Anticholinergic: nasal congestion
The adverse event nasal congestion was only recorded by Hormazabal 1985. There was no statistically significant difference between the amitriptyline and placebo group (OR 0.18, 95% CI 0.01 to 4.01, P = 0.28, one RCT, 40 participants) (Analysis 1.12).
1.12. Analysis.
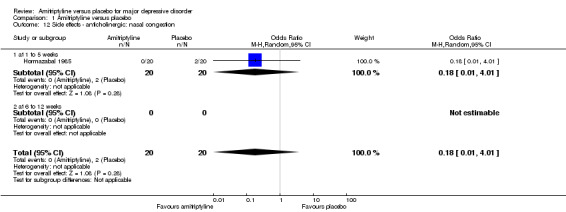
Comparison 1 Amitriptyline versus placebo, Outcome 12 Side effects ‐ anticholinergic: nasal congestion.
13. Anticholinergic: urination problems
Overall significantly more participants in the amitriptyline group experienced urination problems (OR 8.73, 95% CI 1.95 to 39.12, P = 0.005, three RCTs, 418 participants) (Analysis 1.13).
1.13. Analysis.
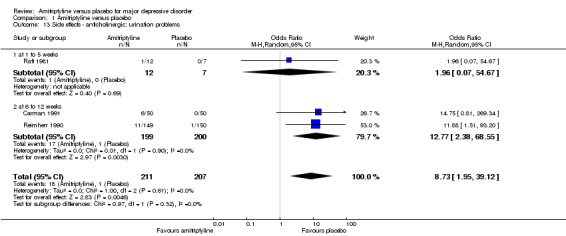
Comparison 1 Amitriptyline versus placebo, Outcome 13 Side effects ‐ anticholinergic: urination problems.
14. Anticholinergic: vision problems (amblyopia, blurred vision)
Overall significantly more participants in the amitriptyline group experienced vision problems (OR 3.73, 95% CI 2.39 to 5.82, P < 0.00001, 10 RCTs, 1055 participants) (Analysis 1.14).
1.14. Analysis.
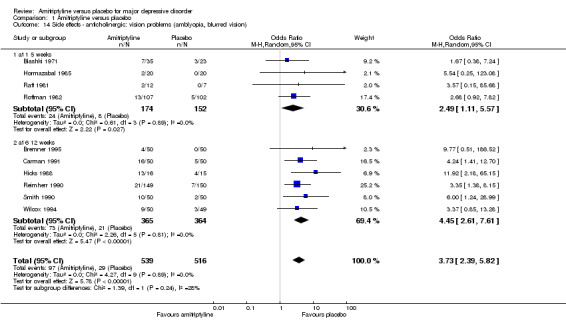
Comparison 1 Amitriptyline versus placebo, Outcome 14 Side effects ‐ anticholinergic: vision problems (amblyopia, blurred vision).
15. Cardiovascular: hypertension
The adverse event hypertension was only recorded by Smith 1990. There was no statistically significant difference between the amitriptyline and placebo group (OR 2.14, 95% CI 0.50 to 9.07, P = 0.30, one RCT, 100 participants) (Analysis 1.15).
1.15. Analysis.
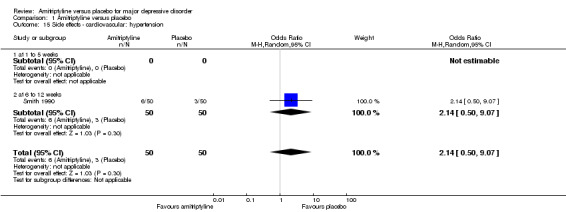
Comparison 1 Amitriptyline versus placebo, Outcome 15 Side effects ‐ cardiovascular: hypertension.
16. Cardiovascular: hypotension
The adverse event hypotension was only recorded by Smith 1990. There was no statistically significant difference between the amitriptyline and placebo group (OR 3.91, 95% CI 0.77 to 19.83, P = 0.10, one RCT, 100 participants) (Analysis 1.16).
1.16. Analysis.
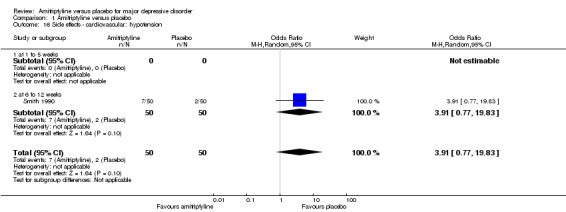
Comparison 1 Amitriptyline versus placebo, Outcome 16 Side effects ‐ cardiovascular: hypotension.
17. Cardiovascular: lightheadedness
The adverse event lightheadedness was only recorded by Hicks 1988. There was no statistically significant difference between the amitriptyline and placebo group (OR 3.79, 95% CI 0.75 to 19.04, P = 0.11, one RCT, 31 participants) (Analysis 1.17).
1.17. Analysis.
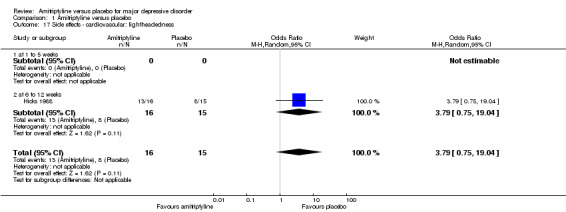
Comparison 1 Amitriptyline versus placebo, Outcome 17 Side effects ‐ cardiovascular: lightheadedness.
18. Cardiovascular: palpitations
The adverse event palpitations was only recorded by Reimherr 1990. There was no statistically significant difference between the amitriptyline and placebo group (OR 3.15, 95% CI 0.84 to 11.87, P = 0.09, one RCT, 299 participants) (Analysis 1.18).
1.18. Analysis.
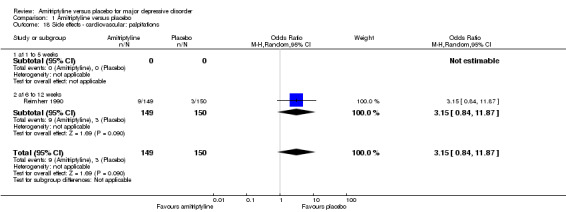
Comparison 1 Amitriptyline versus placebo, Outcome 18 Side effects ‐ cardiovascular: palpitations.
19. Cardiovascular: tachycardia
Overall significantly more participants in the amitriptyline group suffered from tachycardia (OR 3.88, 95% CI 1.71 to 8.80, P = 0.001, five RCTs, 384 participants) (Analysis 1.19).
1.19. Analysis.
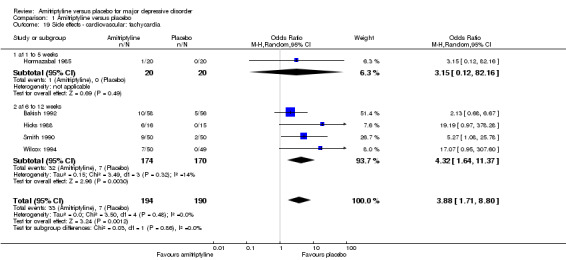
Comparison 1 Amitriptyline versus placebo, Outcome 19 Side effects ‐ cardiovascular: tachycardia.
20. Central nervous: agitation
There was no statistically significant difference between the amitriptyline and placebo group (OR 1.52, 95% CI 0.79 to 2.93, P = 0.21, two RCTs, 339 participants) (Analysis 1.20).
1.20. Analysis.
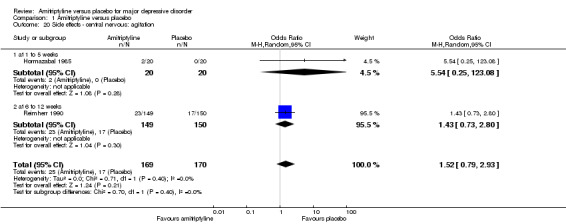
Comparison 1 Amitriptyline versus placebo, Outcome 20 Side effects ‐ central nervous: agitation.
21. Central nervous: amnesia
The adverse event amnesia was only recorded by Reimherr 1990. There was no statistically significant difference between the amitriptyline and placebo group (OR 13.63, 95% CI 0.76 to 244.23, P = 0.08, one RCT, 299 participants) (Analysis 1.21).
1.21. Analysis.
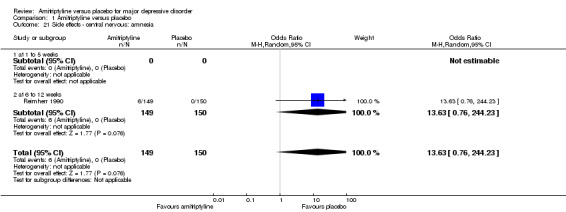
Comparison 1 Amitriptyline versus placebo, Outcome 21 Side effects ‐ central nervous: amnesia.
22. Central nervous: confusion
There was no statistically significant difference between the amitriptyline and placebo group (OR 2.76, 95% CI 0.50 to 15.33, P = 0.25, four RCTs, 228 participants). There was substantial heterogeneity (Tau² = 1.36; Chi² = 5.08, df = 2 (P = 0.08); I² = 61%) but as only three studies reported this outcome, two of which favoured placebo and one amitriptyline, this result is not robust in any case (Analysis 1.22).
1.22. Analysis.
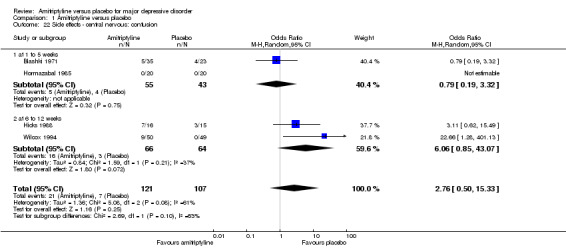
Comparison 1 Amitriptyline versus placebo, Outcome 22 Side effects ‐ central nervous: confusion.
23. Central nervous: disco‐ordination
The adverse event disco‐ordination was only recorded by Smith 1990. There was no statistically significant difference between the amitriptyline and placebo‐treated group (OR 6.68, 95% CI 0.77 to 57.70, P = 0.08, one RCT, 100 participants) (Analysis 1.23).
1.23. Analysis.
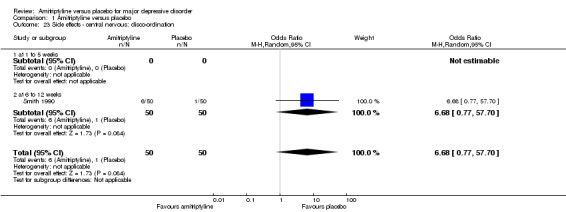
Comparison 1 Amitriptyline versus placebo, Outcome 23 Side effects ‐ central nervous: disco‐ordination.
24. Central nervous: dizziness
Overall significantly more participants in the amitriptyline group suffered from dizziness (OR 2.92, 95% CI 2.07 to 4.11, P < 0.00001, eight RCTs, 1246 participants) (Analysis 1.24).
1.24. Analysis.
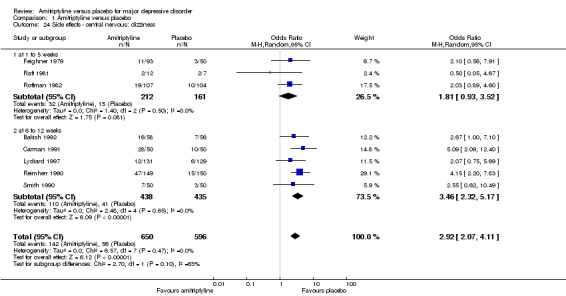
Comparison 1 Amitriptyline versus placebo, Outcome 24 Side effects ‐ central nervous: dizziness.
25. Central nervous: headache
There was no statistically significant difference between the amitriptyline and placebo group (OR 0.84, 95% CI 0.54 to 1.29, P = 0.42, nine RCTs, 1173 participants) (Analysis 1.25).
1.25. Analysis.
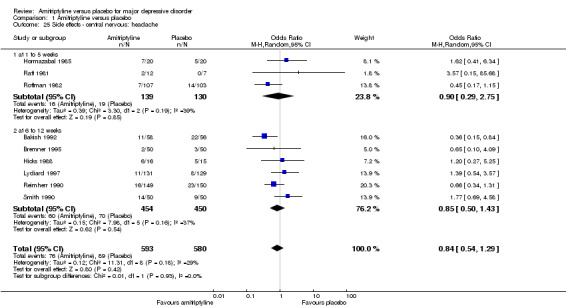
Comparison 1 Amitriptyline versus placebo, Outcome 25 Side effects ‐ central nervous: headache.
26. Central nervous: increased activity
The adverse event increased activity was only recorded by Hormazabal 1985. There was no statistically significant difference between the amitriptyline and placebo group (OR 3.15, 95% CI 0.12 to 82.16, P = 0.49, one RCT, 40 participants) (Analysis 1.26).
1.26. Analysis.
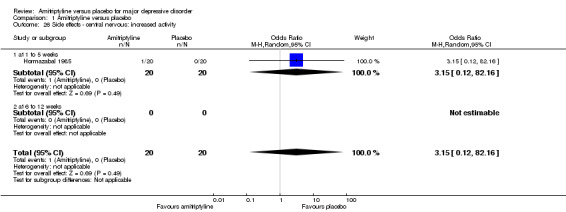
Comparison 1 Amitriptyline versus placebo, Outcome 26 Side effects ‐ central nervous: increased activity.
27. Central nervous: insomnia
There was no statistically significant difference between the amitriptyline and placebo group (OR 0.70, 95% CI 0.39 to 1.24, P = 0.22, five RCTs, 923 participants) (Analysis 1.27).
1.27. Analysis.
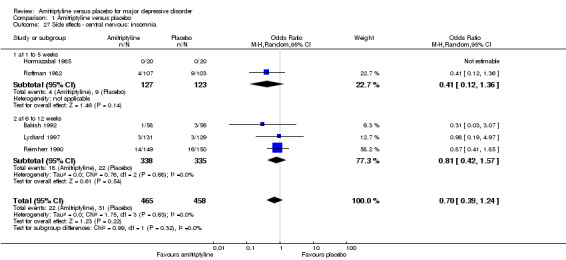
Comparison 1 Amitriptyline versus placebo, Outcome 27 Side effects ‐ central nervous: insomnia.
28. Central nervous: nervousness
There was no statistically significant difference between the amitriptyline and placebo group (OR 2.46, 95% CI 0.73 to 8.35, P = 0.001, four RCTs, 449 participants). There was moderate heterogeneity (Tau² = 0.79; Chi² = 6.16, df = 3 (P = 0.10); I² = 51%) among the three available studies. Obvious reasons explaining the heterogeneity could not be identified (Analysis 1.28).
1.28. Analysis.
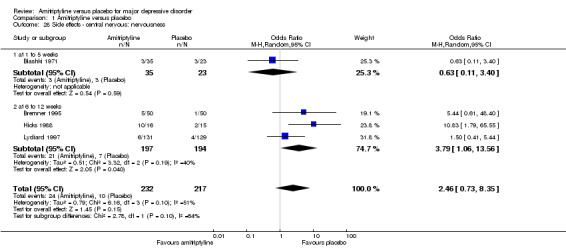
Comparison 1 Amitriptyline versus placebo, Outcome 28 Side effects ‐ central nervous: nervousness.
29. Central nervous: sedation/sleepiness/somnolence/drowsiness
Overall significantly more participants in the amitriptyline group suffered from sedation/sleepiness/somnolence/drowsiness (OR 5.50, 95% CI 3.69 to 8.20, P < 0.00001, 13 RCTs, 1690 participants). There was moderate heterogeneity (Tau² = 0.27; Chi² = 24.98, df = 12 (P = 0.01); I² = 52%), because a single outlier study showed an advantage of amitriptyline (Blashki 1971). Excluding this study reduced heterogeneity to an I2 value of 22% (Analysis 1.29).
1.29. Analysis.
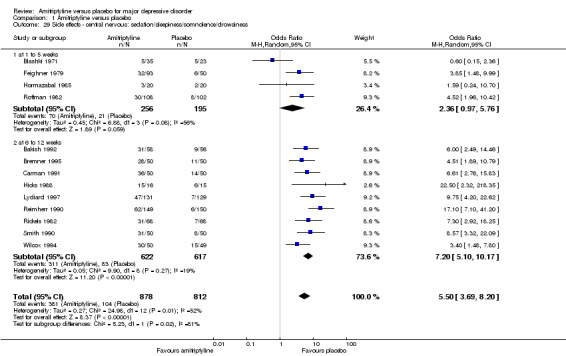
Comparison 1 Amitriptyline versus placebo, Outcome 29 Side effects ‐ central nervous: sedation/sleepiness/somnolence/drowsiness.
30. Central nervous: tremor
Overall significantly more participants in the amitriptyline group suffered from tremor (OR 5.68, 95% CI 3.19 to 10.10, P < 0.00001, 10 RCTs, 1230 participants) (Analysis 1.30).
1.30. Analysis.
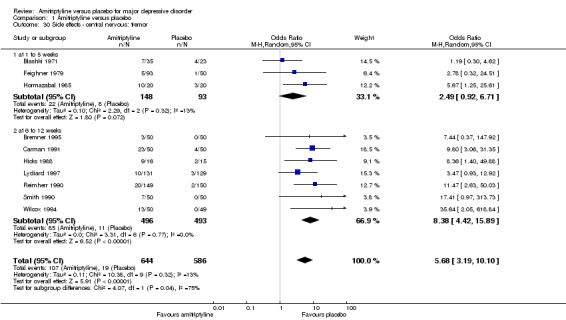
Comparison 1 Amitriptyline versus placebo, Outcome 30 Side effects ‐ central nervous: tremor.
31. Dermal: rash
There was no statistically significant difference between the amitriptyline and placebo group (OR 7.44, 95% CI 0.37 to 147.92, P = 0.19, two RCTs, 140 participants) (Analysis 1.31).
1.31. Analysis.
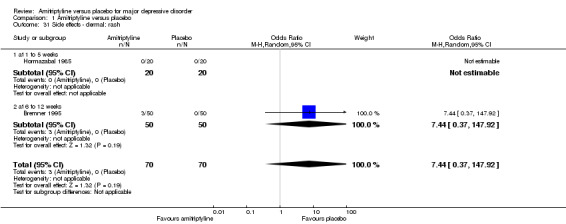
Comparison 1 Amitriptyline versus placebo, Outcome 31 Side effects ‐ dermal: rash.
32. Dermal: sweating
There was no statistically significant difference between the amitriptyline and placebo group (OR 1.82, 95% CI 0.28 to 12.00, P = 0.53, two RCTs, 339 participants) (Analysis 1.32).
1.32. Analysis.
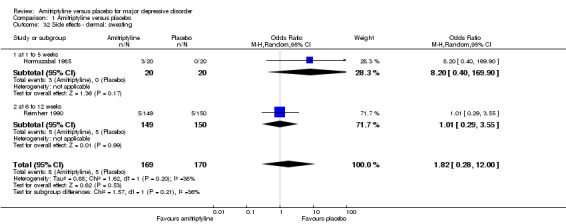
Comparison 1 Amitriptyline versus placebo, Outcome 32 Side effects ‐ dermal: sweating.
33. Gastrointestinal: anorexia
The adverse event anorexia was only recorded by Reimherr 1990. There was no statistically significant difference between the amitriptyline and placebo group (OR 0.20, 95% CI 0.02 to 1.70, P = 0.14, one RCT, 299 participants) (Analysis 1.33).
1.33. Analysis.
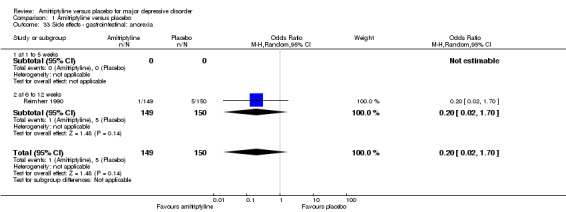
Comparison 1 Amitriptyline versus placebo, Outcome 33 Side effects ‐ gastrointestinal: anorexia.
34. Gastrointestinal: diarrhoea
There was no statistically significant difference between the amitriptyline and placebo group (OR 0.51, 95% CI 0.21 to 1.24, P = 0.14, two RCTs, 339 participants) (Analysis 1.34).
1.34. Analysis.
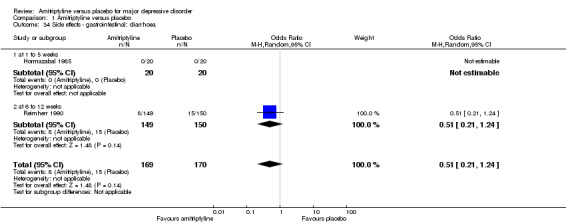
Comparison 1 Amitriptyline versus placebo, Outcome 34 Side effects ‐ gastrointestinal: diarrhoea.
35. Gastrointestinal: dyspepsia
Overall significantly more participants in the amitriptyline group suffered from gastralgia (OR 6.79, 95% CI 2.49 to 18.52, P = 0.0002, five RCTs, 859 participants) (Analysis 1.35).
1.35. Analysis.
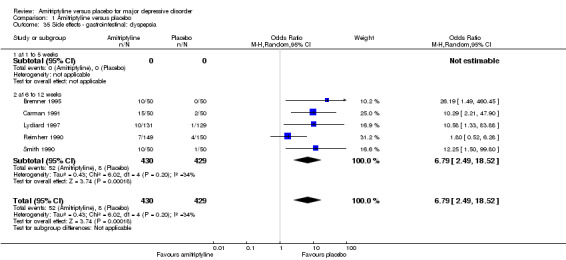
Comparison 1 Amitriptyline versus placebo, Outcome 35 Side effects ‐ gastrointestinal: dyspepsia.
36. Gastrointestinal: gastralgia
There was no statistically significant difference between the amitriptyline and placebo group (OR 1.89, 95% CI 0.82 to 4.35, P = 0.38, two RCTs, 172 participants) (Analysis 1.36).
1.36. Analysis.
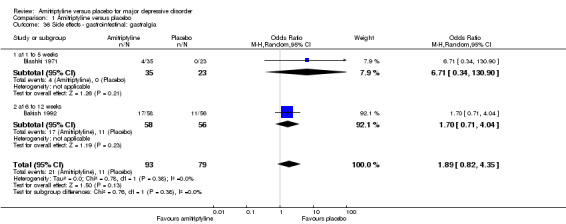
Comparison 1 Amitriptyline versus placebo, Outcome 36 Side effects ‐ gastrointestinal: gastralgia.
37. Gastrointestinal: increased appetite
Overall significantly more participants in the amitriptyline group suffered from increased appetite (OR 4.01, 95% CI 1.95 to 8.24, P = 0.0002, three RCTs, 460 participants) (Analysis 1.37).
1.37. Analysis.
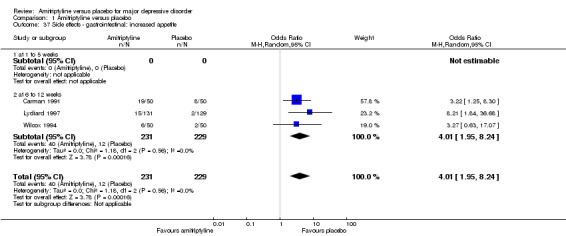
Comparison 1 Amitriptyline versus placebo, Outcome 37 Side effects ‐ gastrointestinal: increased appetite.
38. Gastrointestinal: nausea
There was no statistically significant difference between the amitriptyline and placebo group (OR 1.22, 95% CI 0.49 to 3.04, P = 0.68, six RCTs, 749 participants). There was moderate heterogeneity (Tau² = 0.47; Chi² = 7.52, df = 4 (P = 0.11); I² = 47%). Excluding the single outlier study (Lydiard 1997) that showed an advantage of amitriptyline reduced the I² value to 0%, but there was still no significant difference between groups (Analysis 1.38).
1.38. Analysis.
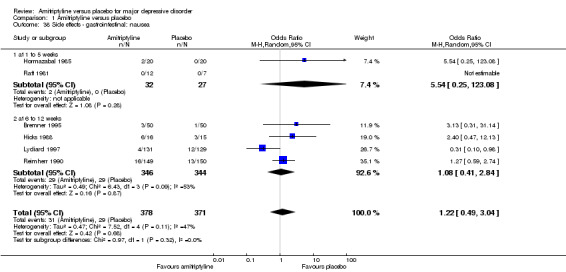
Comparison 1 Amitriptyline versus placebo, Outcome 38 Side effects ‐ gastrointestinal: nausea.
39. Gastrointestinal: vomiting
The adverse event vomiting was only recorded by Reimherr 1990. There was no statistically significant difference between the amitriptyline and placebo group (OR 1.01, 95% CI 0.14 to 7.24, P = 0.99, one RCTs, 299 participants) (Analysis 1.39).
1.39. Analysis.
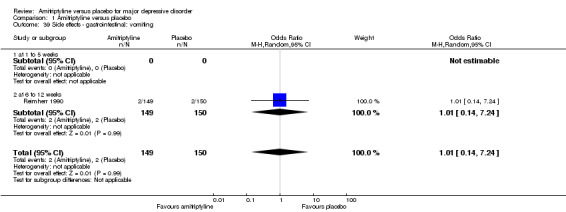
Comparison 1 Amitriptyline versus placebo, Outcome 39 Side effects ‐ gastrointestinal: vomiting.
40. Gastrointestinal: weight gain
The adverse event weight gain was only recorded by Smith 1990. Significantly more participants in the amitriptyline group gained weight (OR 12.25, 95% CI 1.50 to 99.80, P = 0.002, one RCT, 100 participants) (Analysis 1.40).
1.40. Analysis.
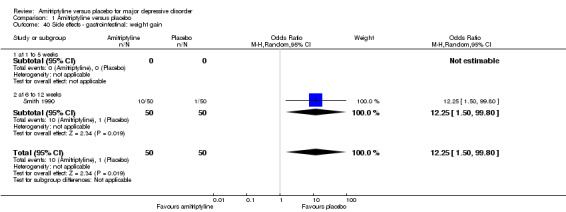
Comparison 1 Amitriptyline versus placebo, Outcome 40 Side effects ‐ gastrointestinal: weight gain.
41. General: fatigue/asthenia/slowed down
Overall significantly more participants in the amitriptyline group suffered from this adverse event (OR 2.44, 95% CI 1.52 to 3.91, P = 0.0002, six RCTs, 1051 participants) (Analysis 1.41).
1.41. Analysis.
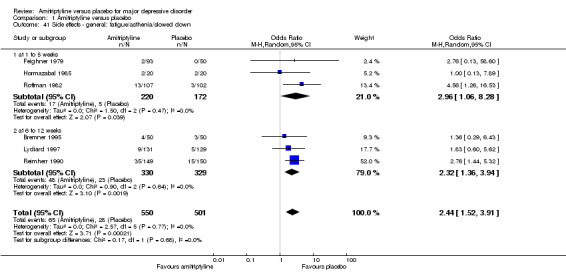
Comparison 1 Amitriptyline versus placebo, Outcome 41 Side effects ‐ general: fatigue/asthenia/slowed down.
42. Sexual: impotence
The adverse event impotence was only recorded by Bremner 1995. There was no statistically significant difference between the amitriptyline and placebo group (OR 9.77, 95% CI 0.51 to 186.52, P = 0.13, one RCT, 100 participants) (Analysis 1.42).
1.42. Analysis.
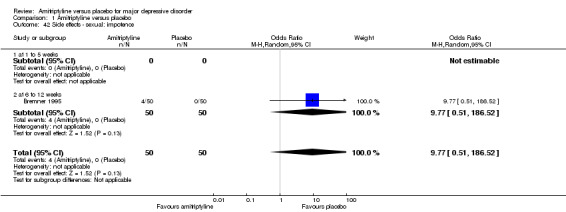
Comparison 1 Amitriptyline versus placebo, Outcome 42 Side effects ‐ sexual: impotence.
43. Sexual: any sexual dysfunction
Overall significantly more participants in the amitriptyline group suffered from sexual dysfunction (OR 16.59, 95% CI 4.54 to 60.64, P < 0.0001, two RCTs, 442 participants) (Analysis 1.43).
1.43. Analysis.
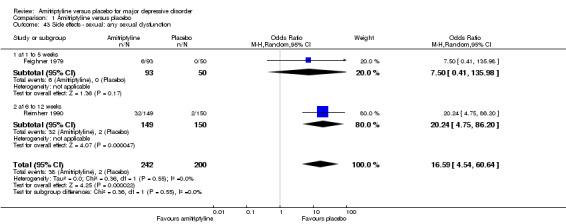
Comparison 1 Amitriptyline versus placebo, Outcome 43 Side effects ‐ sexual: any sexual dysfunction.
44. Missing outcomes
No data were available for the outcomes 'social adjustment', 'quality of life' and 'death'.
45. Subgroup analyses
There was no difference between industry‐sponsored and non‐industry‐sponsored trials (test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.97), I² = 0%), inpatient versus outpatient studies (test for subgroup differences: Chi² = 3.92, df = 3 (P = 0.27), I² = 23.4), two‐arm versus three‐arm trials (test for subgroup differences: Chi² = 0.48, df = 1 (P = 0.49), I² = 0%). There were no data for the subgroup analysis comparing studies using operationalised criteria versus studies using ICD‐9 (Analysis 1.44; Analysis 1.45; Analysis 1.46).
1.44. Analysis.
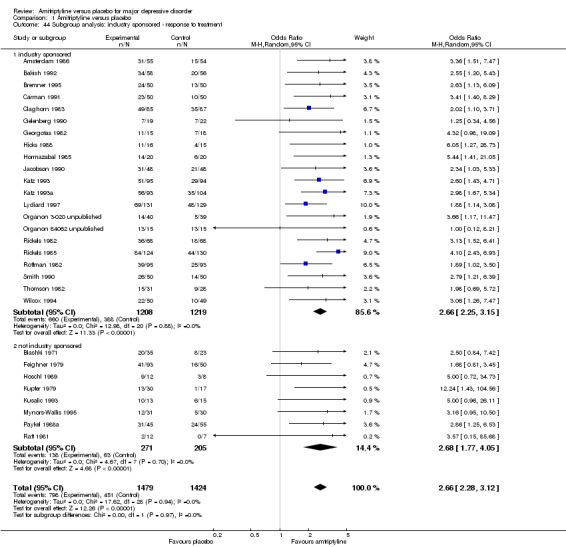
Comparison 1 Amitriptyline versus placebo, Outcome 44 Subgroup analysis: industry sponsored ‐ response to treatment.
1.45. Analysis.
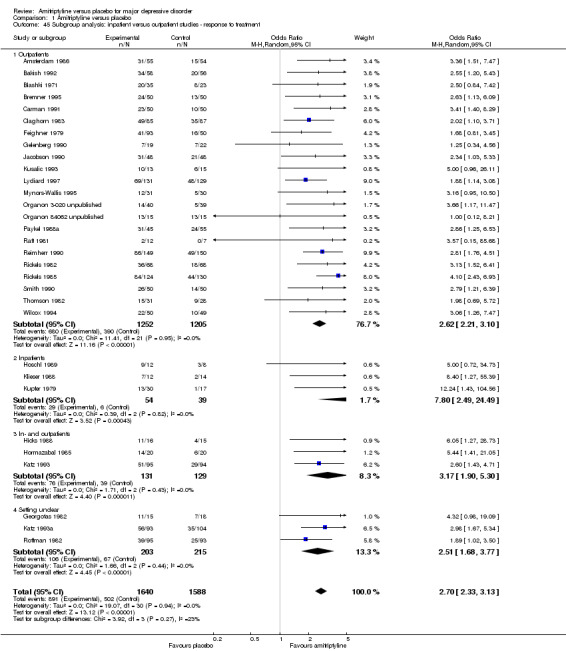
Comparison 1 Amitriptyline versus placebo, Outcome 45 Subgroup analysis: inpatient versus outpatient studies ‐ response to treatment.
1.46. Analysis.
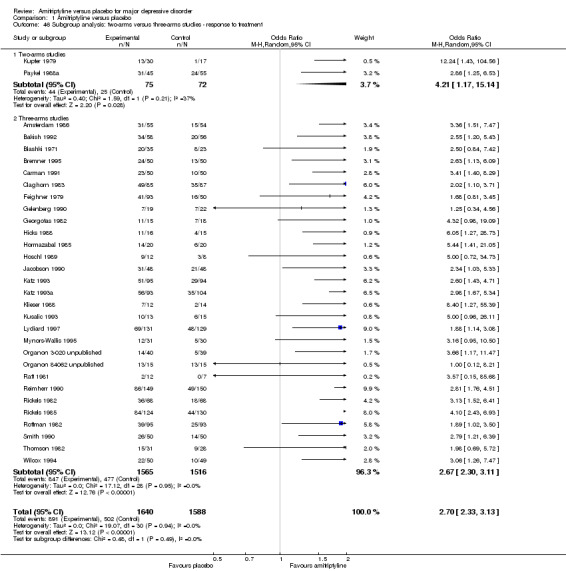
Comparison 1 Amitriptyline versus placebo, Outcome 46 Subgroup analysis: two‐arms versus three‐arms studies ‐ response to treatment.
46. Meta‐regressions
The following potential effect moderators had no statistically significant effects on the primary outcome (for details see Figure 5): publication year (slope 0.00, 95% CI ‐0.03 to 0.02, P = 0.75), mean age at baseline (slope 0.03, 95% CI ‐0.04 to 0.10, P = 0.42), mean amitriptyline dose (slope 0.00, 95% CI 0.00 to 0.01, P = 0.48), study duration (slope ‐0.06, 95% CI ‐0.14 to 0.02, P = 0.12). Only higher depression severity at baseline as measured by the Hamilton Depression Rating Scale (HAM‐D) was associated with significantly higher drug efficacy (slope 0.05, 95% CI 0.01 to 0.10, P = 0.02). Higher percentage responder rates in the placebo groups were associated with almost statistically significant lower drug‐placebo differences (slope ‐0.02, 95% CI 0.01 to ‐0.03, P = 0.05).
5.
47. Sensitivity analyses
Excluding studies for which standard deviations had to be imputed (all studies pooled: OR 2.55, 95% CI 1.93 to 3.36, P < 0.00001, nine RCTs, 936 participants) and applying a fixed‐effect model rather than a random‐effects model (OR 2.71, 95% CI 2.34 to 3.14, P < 0.00001, 31 RCTs, 3228 participants) did not lead to any important changes in the primary outcome. The other preplanned sensitivity analyses did not apply (Analysis 1.47; Analysis 1.48).
1.47. Analysis.
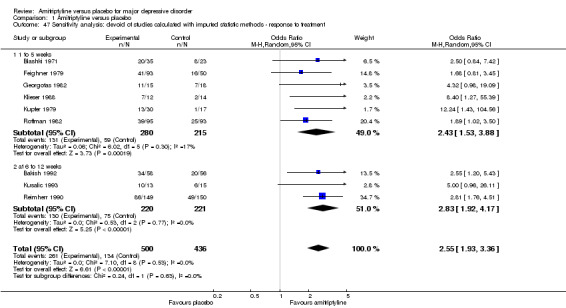
Comparison 1 Amitriptyline versus placebo, Outcome 47 Sensitivity analysis: devoid of studies calculated with imputed statistic methods ‐ response to treatment.
1.48. Analysis.
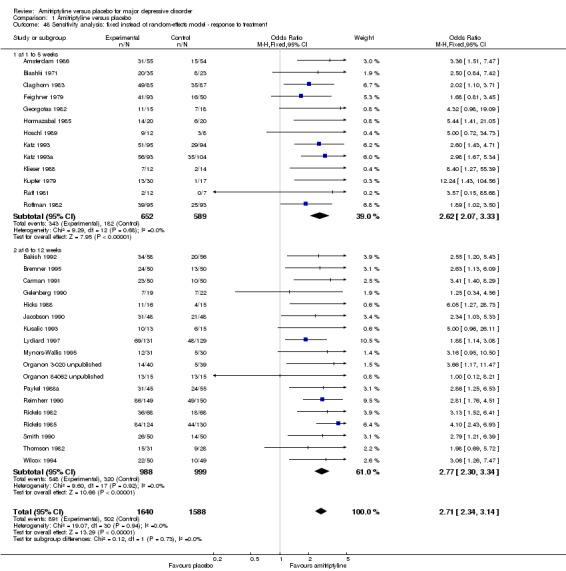
Comparison 1 Amitriptyline versus placebo, Outcome 48 Sensitivity analysis: fixed instead of random‐effects model ‐ response to treatment.
Publication bias
A funnel plot of the primary outcome (response to treatment) was asymmetrical (Egger's test was not significant, P = 0.19, but the trim and fill method (Duval 2000) suggested missing trials) suggesting that small studies may not have been published, especially in the one to five weeks category (Figure 6). When a trim and fill method was applied the adjusted relative risk (RR) did not, however, change much (RR 2.81, 95% CI 2.4 to 3.3; Figure 7).
6.
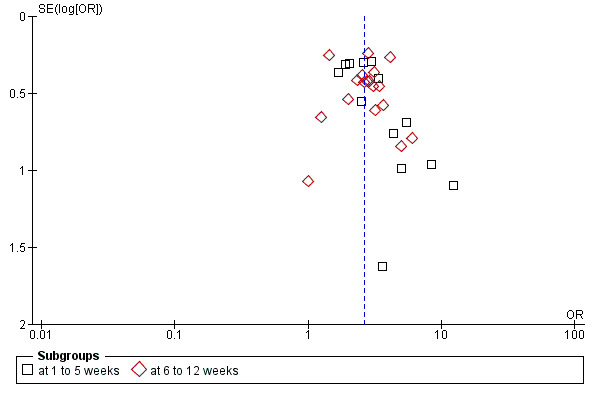
Funnel plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Response.
7.
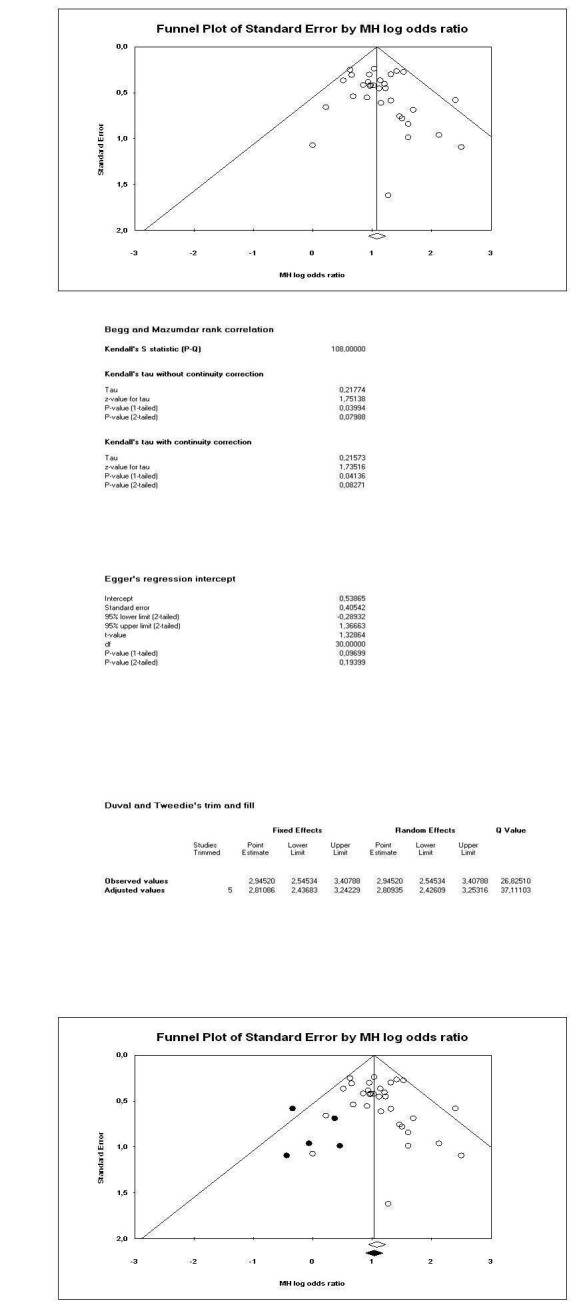
Summary of findings
We judged the quality of the outcomes 'response to treatment' and 'overall tolerability' to be moderate, and that of 'acceptability of treatment' to be very low. No data on the other two a priori defined outcomes for the 'Summary of findings' table, 'death due to suicide' and 'quality of life', were available. Therefore, the quality of any recommendations for these outcomes also has to be rated as very low. We implemented these judgements in our interpretation of the findings (see below).
Discussion
Summary of main results
Amitriptyline is a classical tricyclic antidepressant, but its effects compared to placebo had to our knowledge not been assessed by a systematic review. This report, based on 39 randomised controlled trials (RCTs) and 3509 participants, clearly demonstrated its efficacy for the acute treatment of major depressive disorder. The difference compared to placebo was considerable in the primary outcome, response to treatment (odds ratio (OR) 2.59 (95% confidence interval (CI) 2.03 to 3.29, I²= 0%) for early response, OR 2.67 (95% CI 2.21 to 3.23, I²= 0%) for acute‐phase response and OR 2.64 (95% CI 2.28 to 3.06, I²= 0%) when all studies were pooled). This means that 546 (95% CI 509 to 582) per 1000 amitriptyline‐treated participants would respond compared to 313 per 1000 placebo‐treated participants. This was corroborated by secondary outcomes such as number of participants in remission (OR 3.29, 95% CI 1.48 to 7.31, I²= 0%), mean reduction of depressive symptoms (standardised mean difference (SMD) ‐0.63, 95% CI ‐0.73 to ‐0.52, I²= 0%) or drop‐out due to inefficacy of treatment (OR 0.20, 95% CI 0.14 to 0.28, I²= 0%).
These results were robust to a number of effect moderators such as publication year (range 1971 to 1997), mean participant age at baseline, mean amitriptyline dose, study duration in weeks, pharmaceutical sponsor, inpatient versus outpatient setting and two‐arm versus three‐arm design. Concerning pharmaceutical sponsor it should be noted that in all studies the sponsor was either not the manufacturer of amitriptyline or if it was a third, newer antidepressant was the one of interest. In these cases amitriptyline was rather used as an active control in studies comparing a new compound with placebo. We nevertheless undertook this analysis, because even though the focus was the other antidepressant, the sponsors may have been biased to find superior outcomes for drugs compared to placebo. However, higher severity at baseline was associated with higher superiority of amitriptyline (P = 0.02), while higher responder rates in the placebo groups were associated with lower superiority of amitriptyline (just not meeting the conventional threshold of statistical significance, P = 0.05). As such our results confirm previous findings that antidepressants are more effective in more severely ill patients (e.g. Kirsch 2008). The result for placebo response is important because increasing placebo response has been identified as a major problem in recent antidepressant drugs trials (Walsh 2002). However, we highlight that meta‐regression is an observational (non‐randomised) method and that we undertook many meta‐regressions, raising the problem of multiple testing.
Results for acceptability of treatment as measured by dropping out of the studies for any reason suggested a superiority of amitriptyline (324 (95% CI 271 to 386) out of 1000 amitriptyline‐treated participants compared to 403 out of 1000 placebo‐treated participants would drop out), although there was heterogeneity and drop‐out due to any reason is also a very indirect measure of acceptability. Here, a superiority of amitriptyline in drop‐outs due to inefficacy appeared to have outweighed its inferiority in drop‐outs due to side effects.
The review also documented amitriptyline's well‐known side effects such as the various anticholinergic effects (constipation, dry mouth, nasal congestion, urination problems, vision problems), dizziness, sedation, tachycardia, sexual dysfunction and weight gain. The overall tolerability of amitriptyline was lower than that of placebo. According to the 'Summary of findings' table 165 out of 1000 amitriptyline‐treated participants compared to 45 out of 1000 placebo‐treated patients discontinued the studies due to adverse events.
As data on death were too rarely reported, this review could not clarify whether amitriptyline is associated with increased mortality due to side effects or whether it reduces mortality by preventing suicides. Moreover, there were virtually no data on outcomes that may be particularly important for patients such as quality of life and social functioning.
Overall completeness and applicability of evidence
The available studies have been published in a variety of settings such as in hospitals and in outpatient clinics or in primary and specialised care making the results generalisable. Moreover, the primary outcome was not changed by various potential effect modifiers. The number of included studies (39) and participants (3509) should make the results rather robust, at least concerning the primary outcome. Trikalinos 2004 have shown that as a rule of thumb once 1000 participants have been included in a meta‐analysis, further trials are unlikely to change the effect size much. As a limitation, the two longest studies (Mynors‐Wallis 1995; Thomson 1982) lasted 12 weeks. Thus data for the predefined category 'follow‐up response' were not available. Moreover, we emphasise that much less information is available for secondary efficacy (e.g. remission) and tolerability outcomes. Without having original protocols available it is impossible to tell whether these outcomes were not measured or simply not recorded.
A funnel plot suggested a potential for publication bias although we undertook a thorough search to retrieve all relevant RCTs. This is not surprising because amitriptyline is a very old compound and serious attempts to limit publication bias have only been made in the last two decades. Indeed, some data on three unpublished trials were provided by a pharmaceutical company (Organon, manufacturer of mirtazapine), but manufacturers of amitriptyline either did not respond or did let us know that data on amitriptyline are no longer available. It is unlikely that only three studies have not been published in the last 50 years. Nevertheless, when the relative risk of the primary outcome was adjusted by the trim and fill method (Duval 2000) it did not change to a considerable degree.
Quality of the evidence
As it is frequently the case in RCTs on psychotropic agents, the exact methods of randomisation and allocation concealment were often not reported so that it is unclear whether there was a bias. Although all studies were double‐blind, our results illustrate that amitriptyline is associated with a lot of adverse events that may have uncovered whether participants were on drug or on placebo. Indeed, Moncrieff 2004 found a standardised mean difference of 0.39 (95% CI 0.24 to 0.54) when they compared antidepressants with active placebos (treatments that are ineffective but mimic the side effects of antidepressants) which is lower than the SMD for depression at endpoint of 0.59 (95% CI 0.49 to 0.69) in the current review. A number of studies did not apply intention‐to‐treat analyses, but examined only the study completers which can lead to bias. Moreover, when last observation carried forward data were presented, the definitions for 'modified ITT' were often quite relaxed (e.g. participants had to be in a study for at least two weeks to be included in the analysis). As a consequence, according to our 'Summary of findings' table the evidence on 'response to treatment' and 'overall tolerability' was only moderate, that on 'acceptability of treatment' was very low and no data on the other two a priori defined outcomes for the 'Summary of findings' table, 'death due to suicide' and 'quality of life', were available.
Potential biases in the review process
We feel that the major potential bias in our review process was that we often had to employ the mean standard deviations of the studies in the MANGA project (Cipriani 2009) since standard deviations of depression scales were frequently not reported in the included studies. Moreover, these results frequently had to be used for the imputation of responder rates. While this procedure was planned a priori in the protocol, it might have led to bias. The more studies have to be imputed, the more imprecision may be introduced (Furukawa 2003). Nevertheless, a sensitivity analysis excluding imputed values yielded similar results. As a further limitation we want to mention that we made many statistical tests. This might have led to a type 1 error, that is, reporting a spurious association especially in some secondary outcomes with relatively high P values. Nevertheless, our results had very low P values that would have survived quite some adjustment for multiple testing.
Agreements and disagreements with other studies or reviews
We are not aware of any systematic review that exclusively compared amitriptyline with placebo. Storosum 2001 compared tricyclic antidepressants with placebo and found a SMD in terms of depression severity of only 0.33 (95% CI 0.27 to 0.39), while in contrast the SMD on depression severity at endpoint of the current review was 0.60 (95% CI 0.49 to 0.71). The main difference to the current review is that Storosum 2001 included only studies in which amitriptyline was used as a third arm in trials comparing newer antidepressants with placebo. As most of the studies in this review were three‐arm studies also, this design issue is not a likely explanation for the discrepancy. As Storosum 2001 included only studies on new antidepressants, cohort effects related to publication year are a more plausible explanation. Older studies have limitations, because methods in terms of blinding, rating scales, external auditing and statistics may have been less well developed (Leucht 2012). Moreover, as mentioned above, serious efforts to address publication bias have only been made in the last decade. Therefore, we may have missed old studies with negative results. However, modern studies also have serious problems which may reduce drug placebo differences. Severely ill, suicidal patients who might respond best to treatment are excluded by the protocols for ethical reasons; additionally, because so many antidepressants are available, the motivation for people to participate in a clinical trial may be small. For the same reason there are few treatment‐naive patients, and there is the phenomenon of so‐called 'professional patients' ‐ people who participate in clinical trials partly for financial benefits and enter one trial after the other (Leucht 2012). These and other factors may also in part explain why the current review finds a clearly higher superiority of amitriptyline compared to placebo than SSRIs compared to placebo (e.g. Barbui 2008; Turner 2008), while head‐to‐head comparisons of amitriptyline and SSRIs did not show a clear efficacy difference (Guaiana 2007).
Authors' conclusions
Implications for practice.
The review demonstrates that amitriptyline is an effective treatment for major depressive disorder which is associated with a number of adverse effects. As data on death were not reported, this review could not clarify whether amitriptyline increases mortality by its side effects or reduces it by preventing suicides. However, due to its relatively well‐documented efficacy together with its low cost (amitriptyline is available as a generic drug, and inexpensive in at least some countries) amitriptyline should not be forgotten as a treatment option, especially for those patients who have not responded to safer drugs.
Implications for research.
Reporting of quality indicators such as procedures for allocation concealment and reporting of outcomes remains insufficient in antidepressant drug trials. Strict adherence to the CONSORT statement (Moher 2001) would make such studies much more informative.
Due to the lack of financial interest in compounds that are no longer patent protected, it is unlikely that further randomised controlled trials (RCTs) will be conducted; but given the solid evidence for amitriptyline's effectiveness such RCTs would be warranted. Future trials should address other outcomes than solely efficacy and side effects. Most importantly they should address functional outcomes such as ability to work and quality of life, and examine the mortality associated with amitriptyline, because these outcomes may be especially informative for patients.
Acknowledgements
We thank the editorial team of CCDAN for its generous support. We thank Magdolna Tardy for her help in the final stages of this review. We also thank Drs. Claghorn, Kahn, Kupfer, Bakish, Klieser, Follingstad, Stassen and Höschl and the pharmaceutical companies Organon, Merck Sonoro, Hexal and Bayer for replying to our letters. Organon provided us with additional data from already published studies as well as with data from two unpublished trials.
Data and analyses
Comparison 1. Amitriptyline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response | 31 | 3228 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [2.28, 3.06] |
| 1.1 at 1 to 5 weeks | 13 | 1241 | Odds Ratio (M‐H, Random, 95% CI) | 2.59 [2.03, 3.29] |
| 1.2 at 6 to 12 weeks | 18 | 1987 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [2.21, 3.23] |
| 2 Remission | 2 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 3.29 [1.48, 7.31] |
| 2.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 at 6 to 12 weeks | 2 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 3.29 [1.48, 7.31] |
| 3 Mean severity of depression ‐ change scores | 11 | 1496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.73, ‐0.52] |
| 3.1 at 1 to 5 weeks | 3 | 498 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.83, ‐0.40] |
| 3.2 at 6 to 12 weeks | 8 | 998 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.76, ‐0.50] |
| 4 Mean severity of depression ‐ endpoint scores | 21 | 1599 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.69, ‐0.49] |
| 4.1 at 1 to 5 weeks | 10 | 720 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.77, ‐0.46] |
| 4.2 at 6 to 12 weeks | 11 | 879 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.71, ‐0.43] |
| 5 Drop‐out: total | 24 | 2400 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.55, 0.93] |
| 5.1 at 1 to 5 weeks | 9 | 770 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.25] |
| 5.2 at 6 to 12 weeks | 15 | 1630 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.92] |
| 6 Drop‐out: due to inefficacy | 19 | 2017 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.14, 0.28] |
| 6.1 at 1 to 5 weeks | 7 | 584 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.14, 0.43] |
| 6.2 at 6 to 12 weeks | 12 | 1433 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.10, 0.29] |
| 7 Drop‐out: due to adverse events | 19 | 2174 | Odds Ratio (M‐H, Random, 95% CI) | 4.15 [2.71, 6.35] |
| 7.1 at 1 to 5 weeks | 8 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 4.29 [2.19, 8.38] |
| 7.2 at 6 to 12 weeks | 11 | 1418 | Odds Ratio (M‐H, Random, 95% CI) | 4.15 [2.31, 7.43] |
| 8 Side effects ‐ total number of patients experiencing at least one side effect | 7 | 802 | Odds Ratio (M‐H, Random, 95% CI) | 4.64 [2.45, 8.78] |
| 8.1 1 to 5 weeks | 2 | 162 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [0.33, 9.57] |
| 8.2 at 6 to 12 weeks | 5 | 640 | Odds Ratio (M‐H, Random, 95% CI) | 6.27 [2.95, 13.29] |
| 9 Side effects ‐ anticholinergic: any anticholinergic effects (dry mouth, constipation, visual disturbances) | 2 | 279 | Odds Ratio (M‐H, Random, 95% CI) | 6.33 [3.44, 11.65] |
| 9.1 at 1 to 5 weeks | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 6.5 [2.36, 17.87] |
| 9.2 at 6 to 12 weeks | 1 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 6.23 [2.90, 13.39] |
| 10 Side effects ‐ anticholinergic: constipation | 9 | 1255 | Odds Ratio (M‐H, Random, 95% CI) | 3.39 [2.36, 4.88] |
| 10.1 at 1 to 5 weeks | 2 | 251 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.75, 3.23] |
| 10.2 at 6 to 12 weeks | 7 | 1004 | Odds Ratio (M‐H, Random, 95% CI) | 4.39 [2.89, 6.68] |
| 11 Side effects ‐ anticholinergic: dry mouth | 11 | 1414 | Odds Ratio (M‐H, Random, 95% CI) | 13.50 [9.38, 19.42] |
| 11.1 at 1 to 5 weeks | 3 | 311 | Odds Ratio (M‐H, Random, 95% CI) | 8.76 [4.80, 15.98] |
| 11.2 at 6 to 12 weeks | 8 | 1103 | Odds Ratio (M‐H, Random, 95% CI) | 15.15 [9.73, 23.61] |
| 12 Side effects ‐ anticholinergic: nasal congestion | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 12.1 at 1 to 5 weeks | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 12.2 at 6 to 12 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Side effects ‐ anticholinergic: urination problems | 3 | 418 | Odds Ratio (M‐H, Random, 95% CI) | 8.73 [1.95, 39.12] |
| 13.1 at 1 to 5 weeks | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 1.96 [0.07, 54.67] |
| 13.2 at 6 to 12 weeks | 2 | 399 | Odds Ratio (M‐H, Random, 95% CI) | 12.77 [2.38, 68.55] |
| 14 Side effects ‐ anticholinergic: vision problems (amblyopia, blurred vision) | 10 | 1055 | Odds Ratio (M‐H, Random, 95% CI) | 3.73 [2.39, 5.82] |
| 14.1 at 1 5 weeks | 4 | 326 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [1.11, 5.57] |
| 14.2 at 6 12 weeks | 6 | 729 | Odds Ratio (M‐H, Random, 95% CI) | 4.45 [2.61, 7.61] |
| 15 Side effects ‐ cardiovascular: hypertension | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [0.50, 9.07] |
| 15.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 at 6 to 12 weeks | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [0.50, 9.07] |
| 16 Side effects ‐ cardiovascular: hypotension | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 3.91 [0.77, 19.83] |
| 16.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 at 6 to 12 weeks | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 3.91 [0.77, 19.83] |
| 17 Side effects ‐ cardiovascular: lightheadedness | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 3.79 [0.75, 19.04] |
| 17.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 at 6 to 12 weeks | 1 | 31 | Odds Ratio (M‐H, Random, 95% CI) | 3.79 [0.75, 19.04] |
| 18 Side effects ‐ cardiovascular: palpitations | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.84, 11.87] |
| 18.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.84, 11.87] |
| 19 Side effects ‐ cardiovascular: tachycardia | 5 | 384 | Odds Ratio (M‐H, Random, 95% CI) | 3.88 [1.71, 8.80] |
| 19.1 at 1 to 5 weeks | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 19.2 at 6 to 12 weeks | 4 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 4.32 [1.64, 11.37] |
| 20 Side effects ‐ central nervous: agitation | 2 | 339 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.79, 2.93] |
| 20.1 at 1 to 5 weeks | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 5.54 [0.25, 123.08] |
| 20.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.73, 2.80] |
| 21 Side effects ‐ central nervous: amnesia | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 13.63 [0.76, 244.23] |
| 21.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 13.63 [0.76, 244.23] |
| 22 Side effects ‐ central nervous: confusion | 4 | 228 | Odds Ratio (M‐H, Random, 95% CI) | 2.76 [0.50, 15.33] |
| 22.1 at 1 to 5 weeks | 2 | 98 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.19, 3.32] |
| 22.2 at 6 to 12 weeks | 2 | 130 | Odds Ratio (M‐H, Random, 95% CI) | 6.06 [0.85, 43.07] |
| 23 Side effects ‐ central nervous: disco‐ordination | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 6.68 [0.77, 57.70] |
| 23.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 at 6 to 12 weeks | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 6.68 [0.77, 57.70] |
| 24 Side effects ‐ central nervous: dizziness | 8 | 1246 | Odds Ratio (M‐H, Random, 95% CI) | 2.92 [2.07, 4.11] |
| 24.1 at 1 to 5 weeks | 3 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [0.93, 3.52] |
| 24.2 at 6 to 12 weeks | 5 | 873 | Odds Ratio (M‐H, Random, 95% CI) | 3.46 [2.32, 5.17] |
| 25 Side effects ‐ central nervous: headache | 9 | 1173 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.29] |
| 25.1 at 1 to 5 weeks | 3 | 269 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.29, 2.75] |
| 25.2 at 6 to 12 weeks | 6 | 904 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.50, 1.43] |
| 26 Side effects ‐ central nervous: increased activity | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 26.1 at 1 to 5 weeks | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 26.2 at 6 to 12 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Side effects ‐ central nervous: insomnia | 5 | 923 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.39, 1.24] |
| 27.1 at 1 to 5 weeks | 2 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.36] |
| 27.2 at 6 to 12 weeks | 3 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.42, 1.57] |
| 28 Side effects ‐ central nervous: nervousness | 4 | 449 | Odds Ratio (M‐H, Random, 95% CI) | 2.46 [0.73, 8.35] |
| 28.1 at 1 to 5 weeks | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.11, 3.40] |
| 28.2 at 6 to 12 weeks | 3 | 391 | Odds Ratio (M‐H, Random, 95% CI) | 3.79 [1.06, 13.56] |
| 29 Side effects ‐ central nervous: sedation/sleepiness/somnolence/drowsiness | 13 | 1690 | Odds Ratio (M‐H, Random, 95% CI) | 5.50 [3.69, 8.20] |
| 29.1 at 1 to 5 weeks | 4 | 451 | Odds Ratio (M‐H, Random, 95% CI) | 2.36 [0.97, 5.76] |
| 29.2 at 6 to 12 weeks | 9 | 1239 | Odds Ratio (M‐H, Random, 95% CI) | 7.20 [5.10, 10.17] |
| 30 Side effects ‐ central nervous: tremor | 10 | 1230 | Odds Ratio (M‐H, Random, 95% CI) | 5.68 [3.19, 10.10] |
| 30.1 at 1 to 5 weeks | 3 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [0.92, 6.71] |
| 30.2 at 6 to 12 weeks | 7 | 989 | Odds Ratio (M‐H, Random, 95% CI) | 8.38 [4.42, 15.89] |
| 31 Side effects ‐ dermal: rash | 2 | 140 | Odds Ratio (M‐H, Random, 95% CI) | 7.44 [0.37, 147.92] |
| 31.1 at 1 to 5 weeks | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 at 6 to 12 weeks | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 7.44 [0.37, 147.92] |
| 32 Side effects ‐ dermal: sweating | 2 | 339 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.28, 12.00] |
| 32.1 at 1 to 5 weeks | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 8.2 [0.40, 169.90] |
| 32.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.29, 3.55] |
| 33 Side effects ‐ gastrointestinal: anorexia | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.70] |
| 33.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.70] |
| 34 Side effects ‐ gastrointestinal: diarrhoea | 2 | 339 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.24] |
| 34.1 at 1 to 5 weeks | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.24] |
| 35 Side effects ‐ gastrointestinal: dyspepsia | 5 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 6.79 [2.49, 18.52] |
| 35.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 at 6 to 12 weeks | 5 | 859 | Odds Ratio (M‐H, Random, 95% CI) | 6.79 [2.49, 18.52] |
| 36 Side effects ‐ gastrointestinal: gastralgia | 2 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 1.89 [0.82, 4.35] |
| 36.1 at 1 to 5 weeks | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 6.71 [0.34, 130.90] |
| 36.2 at 6 to 12 weeks | 1 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [0.71, 4.04] |
| 37 Side effects ‐ gastrointestinal: increased appetite | 3 | 460 | Odds Ratio (M‐H, Random, 95% CI) | 4.01 [1.95, 8.24] |
| 37.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 at 6 to 12 weeks | 3 | 460 | Odds Ratio (M‐H, Random, 95% CI) | 4.01 [1.95, 8.24] |
| 38 Side effects ‐ gastrointestinal: nausea | 6 | 749 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.49, 3.04] |
| 38.1 at 1 to 5 weeks | 2 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 5.54 [0.25, 123.08] |
| 38.2 at 6 to 12 weeks | 4 | 690 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.84] |
| 39 Side effects ‐ gastrointestinal: vomiting | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.24] |
| 39.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.24] |
| 40 Side effects ‐ gastrointestinal: weight gain | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 12.25 [1.50, 99.80] |
| 40.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 at 6 to 12 weeks | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 12.25 [1.50, 99.80] |
| 41 Side effects ‐ general: fatigue/asthenia/slowed down | 6 | 1051 | Odds Ratio (M‐H, Random, 95% CI) | 2.44 [1.52, 3.91] |
| 41.1 at 1 to 5 weeks | 3 | 392 | Odds Ratio (M‐H, Random, 95% CI) | 2.96 [1.06, 8.28] |
| 41.2 at 6 to 12 weeks | 3 | 659 | Odds Ratio (M‐H, Random, 95% CI) | 2.32 [1.36, 3.94] |
| 42 Side effects ‐ sexual: impotence | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 9.77 [0.51, 186.52] |
| 42.1 at 1 to 5 weeks | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.2 at 6 to 12 weeks | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 9.77 [0.51, 186.52] |
| 43 Side effects ‐ sexual: any sexual dysfunction | 2 | 442 | Odds Ratio (M‐H, Random, 95% CI) | 16.59 [4.54, 60.64] |
| 43.1 at 1 to 5 weeks | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 7.50 [0.41, 135.98] |
| 43.2 at 6 to 12 weeks | 1 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 20.24 [4.75, 86.20] |
| 44 Subgroup analysis: industry sponsored ‐ response to treatment | 29 | 2903 | Odds Ratio (M‐H, Random, 95% CI) | 2.66 [2.28, 3.12] |
| 44.1 industry sponsored | 21 | 2427 | Odds Ratio (M‐H, Random, 95% CI) | 2.66 [2.25, 3.15] |
| 44.2 not industry sponsored | 8 | 476 | Odds Ratio (M‐H, Random, 95% CI) | 2.68 [1.77, 4.05] |
| 45 Subgroup analysis: inpatient versus outpatient studies ‐ response to treatment | 31 | 3228 | Odds Ratio (M‐H, Random, 95% CI) | 2.70 [2.33, 3.13] |
| 45.1 Outpatients | 22 | 2457 | Odds Ratio (M‐H, Random, 95% CI) | 2.62 [2.21, 3.10] |
| 45.2 Inpatients | 3 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 7.80 [2.49, 24.49] |
| 45.3 In‐ and outpatients | 3 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 3.17 [1.90, 5.30] |
| 45.4 Setting unclear | 3 | 418 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [1.68, 3.77] |
| 46 Subgroup analysis: two‐arms versus three‐arms studies ‐ response to treatment | 31 | 3228 | Odds Ratio (M‐H, Random, 95% CI) | 2.70 [2.33, 3.13] |
| 46.1 Two‐arms studies | 2 | 147 | Odds Ratio (M‐H, Random, 95% CI) | 4.21 [1.17, 15.14] |
| 46.2 Three‐arms studies | 29 | 3081 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [2.30, 3.11] |
| 47 Sensitivity analysis: devoid of studies calculated with imputed statistic methods ‐ response to treatment | 9 | 936 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [1.93, 3.36] |
| 47.1 1 to 5 weeks | 6 | 495 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [1.53, 3.88] |
| 47.2 at 6 to 12 weeks | 3 | 441 | Odds Ratio (M‐H, Random, 95% CI) | 2.83 [1.92, 4.17] |
| 48 Sensitivity analysis: fixed instead of random‐effects model ‐ response to treatment | 31 | 3228 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.71 [2.34, 3.14] |
| 48.1 at 1 to 5 weeks | 13 | 1241 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [2.07, 3.33] |
| 48.2 at 6 to 12 weeks | 18 | 1987 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.77 [2.30, 3.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amsterdam 1986.
| Methods | 4‐week, randomised, double‐blind, single‐centre study, followed by a 1‐week placebo elimination period with exclusion of placebo‐responders | |
| Participants | Psychiatric outpatients with an RDC diagnosis of major depression and anxiety symptoms, with a minimum Hamilton Depression Rating score of at least 18 on a 21‐item scale, a minimum score of 9 on the Raskin Depression scale and an 8 on the Covi Anxiety scale after the placebo elimination period Age range: 21 to 67 years Gender: amitriptyline M38 F17; placebo M31 F19 Exclusion criteria: schizophrenia, acute mania or bipolar I disorder, dementia, mental retardation, substance abuse, significant medical illness contraindicative to TCA, significant hepatic, renal, endocrine or cardiovascular disorders |
|
| Interventions | Amitriptyline: 55 participants Placebo: 54 participants Amitriptyline dose range:maximum 300 mg, mean 182 mg, flexible dosing |
|
| Outcomes | Primary outcome: HAM‐D (21‐item score) Secondary outcome: Hamilton Anxiety Rating Scale, Clinical Global Impressions scale, treatment‐emergent symptoms scale for adverse experiences |
|
| Notes | Response defined as "showing at least a 50% reduction in the HDRS" Remission: no results 3‐arm study comparing zimeldine with amitriptyline and placebo The study was sponsored by a grant‐in‐aid from Astra‐Pharmaceuticals, Merck Sharpe and Dome and "The Mr.& Mrs. Jack Warsaw Fund for Research in Biological Psychiatry" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We performed a randomised, double‐blind clinical trial..." |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Medication was administered in identical looking capsules, each containing...." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons for drop‐out indicated, reasons for drop‐out unbalanced, an ITT analysis was performed including all participants |
| Selective reporting (reporting bias) | High risk | Results of HARS and CGI not for all patients reported, missing standard deviations |
| Other bias | Low risk | No clear evidence for other bias |
Bakish 1992.
| Methods | 7‐week, prospective, randomised, double‐blind, multi‐centre study, followed by a 1‐week placebo wash‐out period | |
| Participants | Psychiatric outpatients with a major depressive episode according to DSM‐III and scoring a minimum of 18 points on the 17‐item HAM‐D Age range (amitriptyline and placebo): 22 to 64 years Gender: amitriptyline M29 F28; placebo M35 F20 (sex and age of 2 patients, one in the AMI and one in the PBO group, are missing) Exclusion criteria: high suicidal risk, depression associated with mood incongruent psychotic features, manic or acute confusional states, significant organic disease, alcohol or drug abuse, recent treatment with MAO‐inhibitors within past 2 weeks, TCA within past week or ECT within past 6 months, women with childbearing potential, not using an effective form of contraception, pregnant and lactating women, concomitant use of antihypertensive, diuretic, anticholinergic or sympathomimetic agents was prohibited |
|
| Interventions | Amitriptyline: 58 participants Placebo: 56 participants Amitriptyline dose range: 50 mg to 150 mg, mean 112 mg, flexible dosing |
|
| Outcomes | Primary outcome: HAM‐D (17‐item score) Secondary outcome: physician's global assessment of efficacy, adverse events |
|
| Notes | Response defined as "50% reduction in the total HAM‐D score" Remission: no results 3‐arm study comparing moclobemide to amitriptyline and placebo No sponsorship mentioned, but one of the authors was a representative of Hoffmann‐LaRoche, Canada Answer of the author upon request: "sponsored by Hoffmann‐LaRoche" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated within each study centre" |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Capsules identical in appearance and taste", "to guarantee double‐blind conditions the active capsules were supplemented with placebo capsules in the blister packs" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Capsules identical in appearance and taste", "to guarantee double‐blind conditions the active capsules were supplemented with placebo capsules in the blister packs" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for all drop‐outs indicated, reasons for drop‐out unbalanced, ITT analysis including all but 4 patients without a post baseline assessment |
| Selective reporting (reporting bias) | Unclear risk | Only response rate based on HAM‐D score described (results in percent), no mean HAM‐D at endpoint or change from baseline to endpoint |
| Other bias | Low risk | No clear other bias |
Bhatia 1991.
| Methods | 6‐week, randomised, double‐blind, 2‐centre study, unclear placebo wash‐out, probably drug naive | |
| Participants | Psychiatric outpatients fulfilling SDSM‐II criteria for major depression with melancholia, having a minimum score of 26 on the HAM‐D (unclear how many items, HAM‐D 1967) Age: range of the total group 19 to 60, mean age amitriptyline group. 38.0 ± 15.1, placebo group 45.6 ± 10.2 Sex: in total M11, F10 Exclusion criteria: free of significant medical disorders, β‐HCG negative, need of any psychotropic medications, opiate analgesics, adrenergic agonists or antagonists, ECT or MAO‐inhibitors for 2 weeks, TCA within 3 days prior to investigation, drug or alcohol abuse |
|
| Interventions | Amitriptyline: 7 participants Placebo: 8 participants Amitriptyline dose range: 150 mg to 300 mg |
|
| Outcomes | Primary outcome: platelet α‐2 receptor activity Secondary outcome: HAM‐D (HAM‐D 1967, probably 17 items) No data or definition for response and remission |
|
| Notes | Sponsor: Upjohn Pharmaceutical Company 3‐arm study: amitriptyline, adinazolam and placebo with age‐ and sex‐matched healthy controls Patients acquired as outpatients, hospitalised for 1 week, further outpatients for 7 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind", no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind", no further details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for all drop‐outs addressed, drop‐out reasons were balanced between groups, but completer analysis on 8 out of originally 15 participants |
| Selective reporting (reporting bias) | High risk | Value for HAM‐D reduction only presented for the total group, missing standard deviations for HAM‐D |
| Other bias | Low risk | No other bias |
Blashki 1971.
| Methods | 4‐week, double‐blind, randomised, multi‐centre study, placebo wash‐out phase unclear, but probably drug naive patients | |
| Participants | General practice setting, patients diagnosed with depression and anxiety based on operationalised diagnostic criteria by the authors which are described in detail. Age: minimum 15 years, total mean 37.7 years Sex: only women Exclusion criteria: organic brain disorder, schizophrenia, epilepsy, alcoholism, mental retardation, ECT in previous 3 months, antidepressant medication in previous month |
|
| Interventions | Amitriptyline: 35 participants Placebo: 23 participants Amitriptyline dose: 2 separate groups with doses of either 75 mg or 150 mg |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcome: clinical rating, Zung scale, Taylor scale |
|
| Notes | Sponsor: Roche Products No definition or data for response and remission 3‐arm study: amitriptyline at 2 dosage levels (75 mg or 150 mg), amylobarbitone 150 mg or placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clearly stated, but randomisation very likely due to double‐blind and correct allocation. Quote: "Every general practitioner was given a number of coded bottles each containing a four‐week supply of capsules. When he admitted a patient to the trial he noted the patients name against the code on the label and instructed her to take one capsule three times a day. The code was kept separately, so that both the general practitioner and the psychiatrist were blind as regards the contents of the capsules received by any patient." |
| Allocation concealment (selection bias) | Low risk | Quote: "Every general practitioner was given a number of coded bottles each containing a four‐week supply of capsules. When he admitted a patient to the trial he noted the patients name against the code on the label and instructed her to take one capsule three times a day. The code was kept separately, so that both the general practitioner and the psychiatrist were blind as regards the contents of the capsules received by any patient." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, general practitioners and psychiatrist were kept blind throughout the study". Drugs and placebo were "all prepared in identical orange capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants, general practitioners and psychiatrist were kept blind throughout the study". Drugs and placebo were "all prepared in identical orange capsules". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons for drop‐out reported and relatively balanced across groups, therefore ‐ although only completer analysis ‐ no obvious bias |
| Selective reporting (reporting bias) | Low risk | All outcomes, described in the method section are published in the results section |
| Other bias | Low risk | No evident other bias |
Bremner 1995.
| Methods | 6‐week, double‐blind, randomised, single‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depressive episode, moderate to severe, having a minimum score of 18 on the 17‐item HAM‐D Age: range 18 to 93 years, mean age 38 years Sex: amitriptyline M13, F37; placebo M15, F35 Exclusion criteria: primary diagnosis of schizophrenia (atypical depressive type), bipolar disorder or adjustment disorder, anxiety as he primary disorder, known active suicidal tendencies, known cognitive deficiencies, known alcohol or drug abuse during the last 6 months, relevant renal, hepatic, respiratory, cardiovascular, cerebrovascular diseases, narrow‐angle glaucoma, clinically sign. Prostatic hypertrophy, seizure disorders, drug allergy or hypersensitivity reaction to TCA or related compounds, hyperthyroidism, abnormal EEG, pregnancy or unreliable contraception, nursing, concomitant medication, ECG 3 months prior to baseline, study medication within 3 months of baseline, MAO‐inhibitors within 14 days of baseline, psychotropic medication including antidepressives within 7 days of baseline, HAM‐D score reduction ≥ 20% in a 7‐day placebo wash‐out period |
|
| Interventions | Amitriptyline: 50 participants Placebo: 50 participants Amitriptyline dose: range 40 mg to 280 mg, overall mean dose 133 mg |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcomes: CGI, MADRS, SDS, Zung |
|
| Notes | Sponsor: Organon Inc., West Orange New Jersey Response‐definition: percentage of patients with ≥ 50% reduction from baseline in total HAM‐D scores No data on remission 3‐arm study, comparing mirtazapine with amitriptyline and placebo (total 150 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomly assigned to treatment" |
| Allocation concealment (selection bias) | Unclear risk | Not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treatment prepared as indistinguishable‐looking capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Treatment prepared as indistinguishable‐looking capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All drop‐outs and their reasons indicated, although difference in reasons between drug and placebo possibly not problematic due to acceptable rates, ITT analysis including all participants with at least 14 days of treatment |
| Selective reporting (reporting bias) | High risk | Missing standard deviations |
| Other bias | Low risk | No evident other bias |
Carman 1991.
| Methods | 6‐week, double‐blind, randomised, placebo wash‐out with elimination of placebo responders, number of participating centres unclear | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depression, moderately to severe, having a minimum score of 18 on the 17‐item HAM‐D Age: no data Sex: no data Exclusion criteria: fertile females without adequate contraception, major or unstable medical problems, other psychiatric diagnosis, age < 18 years |
|
| Interventions | Amitriptyline: 50 participants Placebo: 50 participants Amitriptyline dose: range 60 mg to 300 mg |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcomes: MADRS, CGI, SDS |
|
| Notes | Sponsor: no sponsor mentioned, but one of the authors is from Organon Inc. Response: no response data Remission: no remission data Only 17‐item HAM‐D depression change score 3‐arm study comparing mianserin, amitriptyline and placebo (total 150 participants) One author is also an co‐author of Wilcox 1994 and representative of Organon. Due to identical parameters like equal numbers of patients randomised, same study design and duration, equal dosing and almost identical response rates we had the suspicion, that both publications are describing the same trial, but there are also outcomes which are different like the mean baseline HAM‐D or the mean dose. So we finally decided that these trials were independent and included both. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised, double‐blind active‐ and placebo‐controlled" |
| Allocation concealment (selection bias) | Unclear risk | Not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Randomised, double‐blind active‐ and placebo‐controlled", "identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Randomised, double‐blind active‐ and placebo‐controlled", "identical capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It seems that not all drop‐outs have been reported. Modified ITT analysis (at least 14 days of treatment) and true ITT did not lead to different results |
| Selective reporting (reporting bias) | High risk | Missing standard deviations |
| Other bias | Low risk | No obvious other bias |
Claghorn 1983.
| Methods | 4‐week, double‐blind, randomised, multi‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting RDC‐criteria (Spitzer) for major depressive disorder, having a minimum score of 18 on the 21‐item HAM‐D Age: range AMI 20 to 65 years, PBO 20 to 64 years, mean AMI 39.0 ± 12.7 years, PBO 39.1 ± 12.5 years Sex: AMI M41, F44; PBO M36, F51 Exclusion criteria: females of childbearing potential with no effective contraception, somatic illness, pre‐existing psychiatric conditions (schizophrenia, schizoaffective disorders, epilepsy, alcohol or drug dependence, lactating or pregnant women) |
|
| Interventions | Amitriptyline: 85 participants Placebo: 87 participants Amitriptyline dose: range 75 to 300 mg, mean 180 mg |
|
| Outcomes | Primary outcome: 21‐item HAM‐D Secondary outcome: CGI |
|
| Notes | Sponsor: unclear, 1 author was a representative of Astra Pharmaceutic Products, Inc Response: at least a 50% reduction of the HAM‐D total score at endpoint Remission: no data 3‐arm study, comparing zimeldine to amitriptyline and placebo (total 263 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned sequentially to one of three treatment groups by means of a previously randomised medication code" |
| Allocation concealment (selection bias) | Unclear risk | Not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identically appearing opaque capsules, containing either zimeldine or amitriptyline or placebo, a separate supply was prepared for each patient" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Identically appearing opaque capsules, containing either zimeldine or amitriptyline or placebo, a separate supply was prepared for each patient". It appears that therapist was also the rater, but this is not certain |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomisation was before wash‐out which complicated the judgement as to whether there was a bias. All reasons for drop‐out listed. ITT of all participants with at least 14 days of treatment, visit‐wise analysis of completers of each visit |
| Selective reporting (reporting bias) | High risk | Missing standard deviations |
| Other bias | Low risk | No obvious other bias |
Feighner 1979.
| Methods | 4‐week, double‐blind, randomised, multi‐centre study, placebo wash‐out, exclusion of placebo responders not reported | |
| Participants | Patients meeting Feighner criteria for primary depression, having a minimum score of 20 on the 24‐item HAM‐D, setting: "the patients of four physicians were from their private practices, those of the other two were selected from university research clinics" Age: AMI mean 40.9 years; PBO mean 40.9 years Sex: AMI M40, F53; PBO M17, F33 Exclusion criteria: pre‐existing psychiatric conditions like schizophrenia, alcoholism, hysteria and antisocial personality, further patients with serious medical illness or suicidal risks, recent treatment with ECT or MAO‐inhibitor |
|
| Interventions | Amitriptyline: 93 participants Placebo: 50 participants Amitriptyline dose: range 100 mg to150 mg, mean 115 mg |
|
| Outcomes | Primary outcome: 24‐item HAM‐D, efficacy assessment | |
| Notes | Sponsor: unclear Response: reduction of the total HAM‐D score to half or less of the baseline score Remission: no definition 4‐arm study comparing limbitrol, amitriptyline, chlordiazepoxide and placebo (total 337 randomised participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned sequentially to one of four treatment groups by means of previously randomised medication code. Randomisation was in blocks of seven patients: limbitrol‐2, amitriptyline‐2, chlordiazepoxide‐2, placebo‐1" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double‐blind, quote: "all determinations were made “blind”, before the double‐blind code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated double‐blind, quote: "all determinations were made “blind”, before the double‐blind code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons for drop‐outs indicated, overall similar drop‐out rates in placebo and amitriptyline group. More amitriptyline patients dropped out due to adverse events, more placebo patients due to inefficacy of treatment, modified LOCF results (at least 1 post baseline visit) for 279 patients of 337 randomised patients and for 112 of 143 patients receiving AMI or PBO. Appears to be acceptable as no difference between excluded and included participants |
| Selective reporting (reporting bias) | High risk | Missing standard deviations |
| Other bias | Low risk | No obvious other bias |
Gelenberg 1990.
| Methods | 6‐week, double‐blind, randomised, single‐centre study, placebo wash‐out, exclusion of placebo responder not reported | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depression or Feighner criteria for primary depression, moderately ill Age: range 21 to 62 years (total) Sex: M19, F43 (total) Exclusion criteria: pregnancy or childbearing age, patients with other psychiatric or serious medical illness, patients with chemical dependencies |
|
| Interventions | Amitriptyline: 19 participants Placebo: 22 participants Amitriptyline dose: range 50 mg to 350 mg, mean 114 mg |
|
| Outcomes | Primary outcome: 21‐item HAM‐D Secondary outcome: CGI, salivary flow |
|
| Notes | Sponsor: Kali‐Duphar‐Laboratories (clovoxamine); The Arbour Research Foundation Response: 50% or greater improvement in the HAM‐D total score Remission: no definition, no data 3‐arm study comparing clovoxamine with amitriptyline and placebo (total 62 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "assigned at random", no further details |
| Allocation concealment (selection bias) | Unclear risk | No details presented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "conducted double‐blind", "identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "conducted double‐blind", "identical capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All reasons for drop‐outs indicated, considerable drop‐out rate (50%), but reasons equally distributed, only completer‐analysis, 1 participant does not add up |
| Selective reporting (reporting bias) | High risk | Results for all outcomes reported, but missing standard deviations |
| Other bias | Low risk | No other obvious risk of bias |
Georgotas 1982.
| Methods | 4‐week, double‐blind, randomised, single‐centre study, placebo wash‐out, exclusion of placebo responder unclear | |
| Participants | Setting unclear, patients meeting RDC‐criteria for major depressive disorder, having a minimum score of 18 on the HAM‐D (unclear which item HAM‐D was used) Concerning the number of participants, the authors only describe the completers sample of 52 patients throughout the results, although there are baseline data from 60 patients Age: AMI mean 36.1 ± 2.84 (SE), PBO mean 39.5 ± 3.11 Sex: AMI M12, F3; PBO M10, F8 Exclusion criteria: intercurrent medical illness, childbearing potential, need to take other medications |
|
| Interventions | Amitriptyline: unclear, at least 15 participants completed 4 weeks of treatment Placebo: unclear, at least 18 participants completed 4 weeks of treatment Amitriptyline dose: range 150 mg to 300 mg |
|
| Outcomes | Primary outcome: 21‐item HAM‐D Secondary outcome: CGI, BDI |
|
| Notes | Sponsor: unclear, but "The authors acknowledge the assistance of ..., Ass. Director, Clin Research Astra Pharmaceuticals " Response: no results for response Remission: no results for remission 3‐arm study comparing zimeldine to AMI and PBO (total of 52 participants completing the trial, number of patients with baseline data N = 60) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no further details |
| Allocation concealment (selection bias) | Unclear risk | No details presented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were given placebo tablets identical in appearance to zimeldine and amitriptyline ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients were given placebo tablets identical in appearance to zimeldine and amitriptyline ...", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 60 participants with at least 2 weeks of treatment, but only results of 52 completers were presented. It is unclear to which treatment groups the 8 less than 2‐week completers belonged and how many participants were randomised |
| Selective reporting (reporting bias) | High risk | Adverse events were not reported in a usable way for meta‐analysis (no numbers, only results of significant statistical tests) |
| Other bias | Low risk | No obvious other bias |
Hicks 1988.
| Methods | 6‐week, double‐blind, randomised, single‐centre study, placebo wash‐out, exclusion of placebo‐responder not reported | |
| Participants | Patients meeting DSM‐III criteria for major depression with melancholia, having a minimum score of 26 on the HAM‐D, patients were referred by physicians or newspaper advertisement, inpatients for 10 to 14 days, then outpatients until the end of the study Age: range 18 to 59 (total), AMI mean 42.2, PBO mean 40.8 Sex: AMI M5, F11; PBO M5, F10 Exclusion criteria: pregnancy, major medical illness, epilepsy, glaucoma, hypothyroidism, active alcohol or drug abuse, ECT, MAOI therapy or therapy with one of the investigational drugs in previous 2 weeks |
|
| Interventions | Amitriptyline: 16 participants Placebo: 15 participants Amitriptyline dose: mean 142 mg, range 25 mg to 300 mg |
|
| Outcomes | Primary outcome: HAM‐D (Hamilton 1960, probably 17 items) Secondary outcome: Carol Rating Scale for Depression, Core Symptom Checklist, Physicians and Patients Global Assessment Scale, Self‐Rating Symptoms Scale |
|
| Notes | Sponsor: Upjohn Company and a PHS Research Grant Response: no results for response Remission: no results for remission 3‐arm study comparing adinazolam with amitriptyline and placebo (48 participants in total) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assigned by blocked randomization on admission to the hospital" |
| Allocation concealment (selection bias) | Unclear risk | No details presented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical capsules", "double‐blind conditions were maintained throughout the evaluation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Identical capsules", "double‐blind conditions were maintained throughout the evaluation", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All drop‐outs and reasons indicated. Placebo drop‐outs mainly due to inefficacy, amitriptyline drop‐outs mainly due to improvement/loss to follow‐up, presents both completer and ITT analysis (although only the former could be used for our analysis) |
| Selective reporting (reporting bias) | High risk | Results for all outcomes mentioned in the methods section were presented, missing SD for HAM‐D score |
| Other bias | Low risk | No obvious other bias |
Hormazabal 1985.
| Methods | 4‐week, double‐blind, randomised study, number of study centres not reported, placebo wash‐out not reported | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depressive episode, severely depressed Age: range 20 to 93 years in total AMI mean 43.9 ± 15.3, PBO mean 42.3 ± 14.5 years Sex: AMI M3, F17; PBO M4, F16 Exclusion criteria: uncontrolled organic (cardiovascular, renal, hepatic) disease, pregnancy or puerperium |
|
| Interventions | Amitriptyline: 20 participants Placebo: 20 participants Amitriptyline dose: range 50 mg to 150 mg, mean 86.4 mg |
|
| Outcomes | Primary outcome: 21‐item HAM‐D Secondary outcome: global evaluation |
|
| Notes | Sponsor: not reported, but one author is a representative of Hoffmann La Roche Response: no definition, no data Remission: no definition, no data 3‐arm study comparing cianopramine with amitriptyline and placebo (total 60 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated...", no further details |
| Allocation concealment (selection bias) | Unclear risk | No details presented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Capsules of identical appearance containing placebo, 1mg cianopramine or 25mg amitriptyline were provided, together with their codes sealed in envelopes. These could be opened only in emergency, and were otherwise to be returned unopened at the end of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Capsules of identical appearance containing placebo, 1mg cianopramine or 25mg amitriptyline were provided, together with their codes sealed in envelopes. These could be opened only in emergency, and were otherwise to be returned unopened at the end of the study." "blind raters" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for all drop‐outs reported; reasons in placebo group inefficacy, in drug group adverse events, but overall degree acceptable (25%), for efficacy analysis on completer analysis presented |
| Selective reporting (reporting bias) | High risk | Missing standard deviations for HAM‐D results |
| Other bias | Low risk | No obvious other bias |
Hoschl 1989.
| Methods | 5‐week, double‐blind, randomised, single‐centre study, 3 days of placebo wash‐out, exclusion of placebo responder not reported | |
| Participants | Psychiatric inpatients meeting DSM‐III criteria for major depression, no threshold reported, baseline HAM‐D > 35 Age: AMI mean 44 ± 9; PBO mean 49 ± 7 Sex: only women Exclusion criteria: abnormal body temperature, ECG, blood pressure or basic biometrical screening |
|
| Interventions | Amitriptyline: 12 participants Placebo: 8 participants Amitriptyline dose: range 75 mg to 175 mg, mean 115 ± 35 |
|
| Outcomes | Primary outcome: HAM‐D, (probably 17‐item HAM‐D, only first 16 items were measured) Secondary outcome: Zung Self Rating Scale, Aitken Scale, General Clinical Impression of the Physician |
|
| Notes | Sponsor: Knoll Pharmaceutics Pharmacy Bohnice only provided medication Response: improvement with HAM‐D < 10 Remission: no definition, no data 4‐arm study comparing verapamil, AMI, PBO and state‐adjusted treatment in affective disorders. There is one group of 20 patients suffering from MD DSM‐III, for which separated results were available; this group was included in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Coin toss" (information received from author) |
| Allocation concealment (selection bias) | Unclear risk | No explanation presented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "capsules of similar appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "capsules of similar appearance", "blind rater" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐outs for subgroup of participants with major depressive disorder not indicated, unclear whether results are ITT or only completers |
| Selective reporting (reporting bias) | High risk | Missing standard deviations |
| Other bias | Low risk | No obvious other bias |
Jacobson 1990.
| Methods | 4‐week (according to abstract) or 6‐week (according to Organon and review by Bech 2001), double‐blind, randomised, flexible‐dose trial, number of study centres not reported, but mentioned that there was only 1 investigator, placebo wash‐out with exclusion of placebo responders | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for a major depressive episode (single or recurrent), baseline 17‐item HAM‐D ≥ 18 Age: no data Sex: no data Exclusion criteria: ≥ 25% decrease in total HAM‐D score during the placebo wash‐out period, history of schizophrenia or other psychoses, atypical depression, adjustment disorder, drug or alcohol abuse, drug overdose in the previous 4 months, active suicidal tendencies; patients with clinically relevant renal, cardiovascular, respiratory or cerebrovascular diseases, prostatic hypertrophy, narrow‐angle glaucoma, urinary retention, unstable diabetes, seizure disorder or clinically relevant EEG changes; no ECT in the previous 3 months, adequate dose of an antidepressant (≥ 150 mg amitriptyline or equivalent for at least 6 weeks) in the month preceding the trial; women of childbearing potential without adequate contraception, mothers either breastfeeding or 6 months post partum |
|
| Interventions | Amitriptyline: 48 participants Placebo: 48 participants Amitriptyline dose (mean dose last week of therapy): 115.1 mg/d |
|
| Outcomes | 17‐item HAM‐D, MADRS, CGI, ZDS, PDI, Drug account (primary outcome not defined) | |
| Notes | The information we used was from the abstract, the review by Bech 2001 and from data we received from Organon 3‐arm, dose finding study comparing mirtazapine with amitriptyline and placebo Sponsor: Organon, The Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "lists for randomisation were centrally prepared using random number tables, and the randomisation was blinded for the investigator" |
| Allocation concealment (selection bias) | Low risk | Quote "...capsules packed in coded packages." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: medication "prepared as indistinguishable capsules packed in coded packages" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: medication "prepared as indistinguishable capsules packed in coded packages", double‐blind trial, no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants might be slightly different from the number of patients randomised (the number of participants for amitriptyline is 48 in Bech 2001 and 47 in the data set provided by Organon) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Katz 1993.
| Methods | 4‐week, double‐blind, randomised study, number of study centres not reported, placebo wash‐out, exclusion of placebo‐responder not reported | |
| Participants | Patients meeting DSM‐III criteria for major depression, having a minimum score of 18 on the HAM‐D total score (17‐item HAM‐D), setting unclear Age: AMI mean 42.6 years, PBO mean 44.7 years Sex: AMI M53, F42; PBO M54, F40 Exclusion criteria: clinically significant hepatic, disease, glaucoma, epileptic seizures, hypertension, endocrine disorder, prostatic hypertrophy, renal disease, cerebral vascular disease (including significant EEG findings), clinical laboratory findings, bone marrow depression, blood dyscrasia, hypersensitivity to TCA or tetracyclic AD, women of childbearing potential, pregnant or nursing, patients at risk of suicide |
|
| Interventions | Amitriptyline: 95 participants Placebo: 94 participants Amitriptyline dose: range 50 to 150 mg |
|
| Outcomes | Primary outcome: HAM‐D total score Secondary outcome: HAM‐D items 1,7, 8 and 14 separately |
|
| Notes | Sponsor: Ciba Geigy Response: no definition, no data Remission: no definition, no data 2 treatment protocols were described (P1 and P3), Katz 1993 describes protocol 3, a 3‐arm study, comparing oxaprotiline with amitriptyline and placebo (total 278 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no detail |
| Allocation concealment (selection bias) | Unclear risk | No detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated double‐blind, no detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated double‐blind, no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Katz 1993a.
| Methods | 4‐week, double‐blind, randomised study, number of study centres not reported, placebo wash‐out, exclusion of placebo‐responder not reported | |
| Participants | Patients meeting DSM‐III‐R criteria for major depression, having a minimum score of 18 on the HAM‐D total score (17‐item HAM‐D), setting unclear Age: AMI mean 43.2 years, PBO mean 44.0 years Sex: AMI M45, F48; PBO M55, F49 Exclusion criteria: atypical and double depressions, clinically significant hepatic, disease, glaucoma, epileptic seizures, hypertension, endocrine disorder, prostatic hypertrophy, renal disease, cerebral vascular disease (including significant EEG findings), clinical laboratory findings, bone marrow depression, blood dyscrasia, hypersensitivity to TCA or tetracyclic AD, women of childbearing potential, pregnant or nursing, patients at risk of suicide |
|
| Interventions | Amitriptyline: 93 participants Placebo: 104 participants Amitriptyline dose: range 75 to 225 mg |
|
| Outcomes | Primary outcome: HAM‐D total score Secondary outcome: HAM‐D items 1,7, 8 and 14 separately |
|
| Notes | Sponsor: Ciba Geigy Response: no definition, no data Remission: no definition, no data 2 treatment protocols were described (P1 and P3), Katz 1993 a describes protocol 1, a 3‐arm study, comparing oxaprotiline with amitriptyline and placebo (total 278 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fully‐randomised, three‐compartment, multi‐centre trial" |
| Allocation concealment (selection bias) | Unclear risk | No details presented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind treatment", no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind treatment", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐outs not indicated, ITT (all participants who received at least 1 dose) |
| Selective reporting (reporting bias) | Unclear risk | Only efficacy results presented, unclear whether there were other outcomes |
| Other bias | Low risk | No obvious other bias |
Klieser 1988.
| Methods | 3‐week, double‐blind, randomised, single‐centre study, 3 days placebo wash‐out, exclusion of placebo responder not reported | |
| Participants | Psychiatric inpatients meeting DSM‐III criteria for major depressive disorder, being severely ill, closed ward Age: AMI mean 42 ± 4.9, PBO mean 41.1 ± 5.2 Sex: AMI M3, F9; PBO M5, F9 Exclusion criteria: not reported |
|
| Interventions | Amitriptyline: 12 participants Placebo: 14 participants Amitriptyline dose: 150 mg, fixed dosing schedule |
|
| Outcomes | Primary outcome: HAM‐D (number of items unclear) Secondary outcome: BPRS, HAMA, AMDP |
|
| Notes | Sponsor: unclear Response: no definition, no data Remission: no definition, no data 3‐arm study comparing trazodone with amitriptyline and placebo (total 26 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "on an random basis", no further details |
| Allocation concealment (selection bias) | Unclear risk | No details presented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind trial", no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind trial", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for drop‐outs not presented, mean HAM‐D only based on completers, responder rates (CGI) ITT |
| Selective reporting (reporting bias) | Low risk | No obvious selective reporting |
| Other bias | High risk | Only interim analysis presented, unclear whether study was finished |
Kupfer 1979.
| Methods | 4‐week, double‐blind, randomised, single‐centre study, 1 week drug‐free treatment preceded the 4 weeks treatment phase | |
| Participants | Psychiatric inpatients meeting RDC criteria for major depressive syndrome, having a minimum score of 30 on the 17‐item HAM‐D, Age: AMI mean 42.1 ± 2.5; PBO mean 40.0 ± 3.9 Sex: AMI M9, F21; PBO M6, F11 Exclusion criteria: not reported |
|
| Interventions | Amitriptyline: 30 participants Placebo: 17 participants Amitriptyline dose: fixed schedule, 4 capsules per day, increasing doses according to a time schedule, mean over 4 weeks 157 mg |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcome: weight gain |
|
| Notes | Sponsor: 2 NIMH grants Response: HAM‐D score of 12 was used as the cutoff score for responders Remission: no definition, no data 2‐arm study comparing amitriptyline with placebo (total 47 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were treated in a random design", no further details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Throughout the study patients received a constant number of capsules that appeared identical, so that both, the patients and the staff remained blind to the medication" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical study capsules", "both the patients and the staff remained "blind" to the medication" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Identical study capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of drop‐outs not mentioned, unclear whether there were any |
| Selective reporting (reporting bias) | High risk | Mean HAM‐D not mentioned, paper focuses on weight gain, unlikely that no other outcomes were measured |
| Other bias | Low risk | No obvious other bias |
Kusalic 1993.
| Methods | 6‐week, double‐blind study, number of study centres not reported, placebo lead‐in period of 1 week, exclusion of placebo responder not reported | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R criteria for major depressive episode, having a minimum score 18 of on the 17‐item HAM‐D Age: range (total) 22 to 61 years, mean (total) 41.3 ± 10.1 Sex: M24, F14 (total) Inclusion criteria: "Subjects were in good physical health according to physical examination, blood count, T3/4 laboratory and ECG" |
|
| Interventions | Amitriptyline: 13 participants Placebo: 15 participants Amitriptyline dose: mean 109.93 ± 5.11 |
|
| Outcomes | Primary outcome: thyroid assay Secondary outcome: response |
|
| Notes | Sponsor: unclear Response: "improvement: 50% decline in the HRSD score by the end of the treatment period" Remission: no definition, no data 3‐arm study comparing amitriptyline, moclobemide and placebo (total 39 patients) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details presented, assumed because double‐blind trial |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The medication was given in capsules that were identical in shape, size and color”, "double‐blind" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The medication was given in capsules that were identical in shape, size and color”, "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “The medication was given in capsules that were identical in shape, size and color”, "double‐blind", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs not presented, unclear whether there were any |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | There appear to be 2 typos about numbers, no obvious other bias |
Langlois 1985.
| Methods | 4‐week, double‐blind, randomised, single‐centre study, placebo wash‐out unclear | |
| Participants | Psychiatric outpatients meeting DSM‐III and RDC criteria for major depressive disorder, having a minimum score of 20 on the HAM‐D (HAM‐D 1960, probably 17‐item HAM‐D) Age: not reported Sex: not reported Exclusion criteria: antidepressive or antipsychotic treatment for at least 2 weeks prior to entering the study, not in good physical health or abnormal laboratory values |
|
| Interventions | Amitriptyline: 15 participants Placebo: 15 participants Amitriptyline dose:range 150 mg to 225 mg, fixed schedule with increasing doses over 2 weeks, mean over 4 weeks 206 mg |
|
| Outcomes | Primary outcome: plasma levels Secondary outcome: side effects to check toxic reactions |
|
| Notes | Sponsor: grant‐in‐aid from Astra Läkemedel AB Response: no definition, no data Remission: no definition, no data 3‐arm study comparing zimeldine to amitriptyline and placebo (total 45 patients) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not presented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind basis", "identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind basis", "identical capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of drop‐outs in the AMI and PBO group are not reported |
| Selective reporting (reporting bias) | High risk | Report focuses on side effects of zimeldine, no efficacy data, no side effects of AMI or PBO |
| Other bias | Low risk | No obvious other bias |
Lydiard 1997.
| Methods | 8‐week, double‐blind, randomised, multi‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R criteria for major depression, having a minimum score of 18 on the 17‐item HAM‐D Age: AMI mean 39.0 years; PBO mean 40.2 years Sex: AMI M41, F90; PBO M43, F86 Exclusion criteria: 17‐item HAM‐D < 18, improvement during placebo wash‐out, acute or chronic organic mental disorder, obsessive compulsive disorder, post‐traumatic stress disorder, schizophrenia, paranoid disorders, psychotic disorders not elsewhere classified, severe personality disorder, significant medical illness, recent history of substance abuse or dependence, current suicide risk, history of neurologic disease, narrow‐angle glaucoma, significant prostate syndromes, requirement of additional psychotropic drugs, received sertraline, recent participation in investigational drug study, no response to adequate trials of antidepressants, depot during past 6 months, fluoxetine within 1 month, daily psychotropic medication within 2 weeks, MAO‐I within 3 weeks, significant ECG or laboratory abnormalities, pregnancy or unreliable contraception |
|
| Interventions | Amitriptyline: 131 participants Placebo: 129 participants Amitriptyline dose: range 50 mg to 150 mg, mean final dose 103.1 mg |
|
| Outcomes | Primary outcome: 17‐item HAM‐D, CGI Secondary outcome: MADRS, CGI‐Improvment, CGI‐Severity, GAS, Quality of Life Enjoyment and Satisfaction Questionnaire, HRQOL‐II, POMS, BDI |
|
| Notes | Sponsor: Pfizer Inc. Response: CGI ≤ 2 Remission: no definition, no data 3‐arm study comparing sertraline to amitriptyline and placebo (total 392 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no further details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Medication provided as identical capsules in blister pack format and was administered orally" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Medication provided as identical capsules in blister pack format and was administered orally" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Medication provided as identical capsules in blister pack format and was administered orally", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All drop‐out reasons reported, different numbers for amitriptyline and placebo drop‐outs due to side effects (17.6% and 5.3%, respectively), ITT analysis (at least 1 dose and 1 post baseline rating), last observation carried forward |
| Selective reporting (reporting bias) | High risk | Results for MADRS, social adjustment and health‐related quality of life not reported (only P values) |
| Other bias | Low risk | No clear other bias |
McNair 1984a.
| Methods | 3‐week, double‐blind, randomised, single‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting RDC criteria for major depression, threshold not reported Age: AMI mean 32.9, PBO mean 34.7 Sex: AMI M6, F4; PBO M3, F7 Exclusion criteria: psychosis, sociopathy, alcoholism, drug addiction, CNS impairment, hypersensitivity to TCA |
|
| Interventions | Amitriptyline: 10 participants Placebo: 10 participants Amitriptyline dose: 25 mg to 150 mg, final mean dose 118 mg |
|
| Outcomes | Primary outcome: HSCL, TESS Secondary outcome: HAM‐D (Hamilton 1967, probably 17‐item Ham‐D), Raskin Depression Scale, POMS, CGI |
|
| Notes | Sponsor: Lederle Laboratories, a division of American Cyanamid Corporation Response: no definition, no data Remission: no definition, no data 3‐arm cross‐over study comparing amitriptyline and placebo or amoxapine and placebo (total N = 20) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind", "identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind", "identical capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons and numbers of drop‐outs in each group reported, relatively high overall drop‐out rate, unclear whether ITT |
| Selective reporting (reporting bias) | High risk | No data for HAM‐D reported, only the results of the statistical tests, no means, no SD, no responder rates |
| Other bias | Unclear risk | Cross‐over study, but we used only the first phase |
Mynors‐Wallis 1995.
| Methods | 12‐week, double‐blind, randomised, single‐centre study, placebo wash‐out unclear | |
| Participants | Patients meeting RDC criteria for major depression, having a minimum score of 13 on the 17‐item HAM‐D, focusing on general care setting Age: range AMI 18 to 58 years; PBO 21 to 60 years Sex: AMI M7, F24; PBO M9, F21 Exclusion criteria: another psychiatric disorder before onset of depression, receiving current psychological or antidepressant drug therapy, current psychotic symptoms, serious suicidal intent, history of schizophrenia, recent drug or alcohol misuse, physical problems contraindicating amitriptyline treatment |
|
| Interventions | Amitriptyline: 31 participants Placebo: 30 participants Amitriptyline dose: maximum 150 mg, mean dose 139 mg, fixed dosing schedule |
|
| Outcomes | Primary outcome: Present State Examination, 17‐item HAM‐D Secondary outcome: BDI, modified social adjustment scale |
|
| Notes | Sponsor: Wellcome Trust funded a training fellowship for 1 author, Warner‐Lambert provided amitriptyline and placebo Response: no definition, no data Remission: recovery criteria are HAM‐D score ≤ 7 and BDI ≤ 8 3‐arm study comparing problem‐solving treatment, amitriptyline plus standard clinical management and placebo plus standard clinical management (total 91 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised controlled trial,...", "randomly allocated", "stratified to make sure that the three groups contained patients with depressive symptoms of similar severity" |
| Allocation concealment (selection bias) | Low risk | Quote: "System of sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical capsules", "both patient and therapist were blind to the content of the capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Identical capsules", "double‐blind", "two experienced research interviewers who were blind to treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals reported, 82 (at least 4 therapy sessions) out of 91 patients included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | Data for all outcomes reported |
| Other bias | Low risk | No obvious other bias |
Organon 3‐020 unpublished.
| Methods | 6‐week, double‐blind, randomised study, number of centres not clear, placebo wash‐out (quote: "after a 3 to 7‐days placebo‐washout period eligible patients were randomised") | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for a major depressive episode (single or recurrent), baseline 17‐item HAM‐D ≥ 18 Age: no data Sex: no data Exclusion criteria: ≥ 25% decrease in total HAM‐D score during the placebo wash‐out period, history of schizophrenia or other psychoses, atypical depression, adjustment disorder, drug or alcohol abuse, drug overdose in the previous 4 months, active suicidal tendencies; patients with clinically relevant renal, cardiovascular, respiratory or cerebrovascular diseases, prostatic hypertrophy, narrow‐angle glaucoma, urinary retention, unstable diabetes, seizure disorder or clinically relevant EEG changes; no ECT in the previous 3 months, adequate dose of an antidepressant (≥ 150 mg amitriptyline or equivalent for at least 6 weeks) in the month preceding the trial; women of childbearing potential without adequate contraception, mothers either breastfeeding or 6 months post partum |
|
| Interventions | Amitriptyline: 40 participants Placebo: 49 participants Amitriptyline dose (mean dose in the last week of treatment): 133.7 mg/d |
|
| Outcomes | 17‐item HAM‐D, MADRS, CGI, ZDS, PDI, drug account (primary outcome not defined) | |
| Notes | The information we used was from the review by Bech 2001 and from data we received from Organon 3‐arm, dose finding study comparing mirtazapine with amitriptyline and placebo Sponsor: Organon, The Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "lists for randomisation were centrally prepared using random number tables, and the randomisation was blinded for the investigator" |
| Allocation concealment (selection bias) | Low risk | Quote "...capsules packed in coded packages." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: medication "prepared as indistinguishable capsules packed in coded packages" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: medication "prepared as indistinguishable capsules packed in coded packages", double‐blind trial, no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants might be slightly different from the number of patients randomised (the number of participants for amitriptyline is 40 in Bech 2001 and 38 in the data set provided by Organon, for placebo the numbers are 39 and 37) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Organon 84062 unpublished.
| Methods | 6‐week, double‐blind, randomised, single‐centre study, placebo wash‐out unclear | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for a major depressive episode (single or recurrent), baseline 17‐item HAM‐D ≥ 18 Age: no data Sex: no data Exclusion criteria: not reported |
|
| Interventions | Amitriptyline: 15 participants Placebo: 15 participants Amitriptyline dose: no information provided |
|
| Outcomes | 17‐item HAM‐D, MADRS, CGI, ZDS, PDI, drug account (primary outcome not defined) | |
| Notes | We received the data from Organon 3‐arm, dose finding study comparing mirtazapine with amitriptyline and placebo Sponsor: Organon, The Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | Not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Paykel 1988a.
| Methods | 6‐week, double‐blind, randomised study, 41 general practitioners participating, unclear placebo wash‐out | |
| Participants | Patients meeting RDC criteria for major depression, having a minimum score of 6 on the 17‐item HAM‐D, focusing on general care setting Age: no data for MD sample, overall range 18 to 64 Sex: no data for the MD sample Exclusion criteria: case of RDC minor or intermittent depression, previous history of schizophrenia, concurrent RDC diagnosis of phobic, generalised anxiety or obsessive disorder, history of drug dependence, recent history of habitual excessive alcohol intake, evidence of brain damage or mental retardation (estimated IQ below 70), language or other problems prevented adequate co‐operation in assessment procedures, evidence of physical disorder precluding antidepressants, suicidal risk of such degree as to contraindicate placebo, receiving an antidepressant or having seen a psychiatrist in the last 3 months |
|
| Interventions | Amitriptyline: 45 participants Placebo: 55 participants Amitriptyline dose: range 125 mg to 175 mg, flexible dosing schedule |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcome: Raskin B‐Area Score, 7‐point global rating of severity and change |
|
| Notes | Sponsor: Parke Davis and Co, Ltd. provided medication, project grant by Medical Research Council Response: no definition, no data Remission: no definition, no data 2‐arm study comparing amitriptyline and placebo (total of the MD sample 100 participants, total of the allocated sample: 141 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Double‐blind treatment according to previously prepared randomisation schedules" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind treatment with identical 25mg capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind treatment with identical 25mg capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 141 out of 178 completed at least 4 weeks and are included in this report, this is almost a completer analysis. Reasons for drop‐outs in each group not presented. |
| Selective reporting (reporting bias) | High risk | No SDs |
| Other bias | Low risk | No obvious other bias |
Preskorn 1983.
| Methods | 6‐week, double‐blind, randomised, multi‐centre study, placebo wash‐out unclear | |
| Participants | Psychiatric inpatients meeting DSM‐III criteria for major depressive disorder, threshold baseline severity not reported Age: range (of all allocated patients) 18 to 82 years Sex: no data Exclusion criteria: recent alcohol or drug abuse, schizophrenia, organic brain syndrome, seizure disorder, pregnancy, severe medical illness, medical contraindications to amitriptyline chemotherapy |
|
| Interventions | Amitriptyline: number of participants unclear Placebo: number of participants unclear Amitriptyline dose: ascending dosage regimen, doses not reported |
|
| Outcomes | Primary outcome: plasma concentrations, HAM‐D Secondary outcome: Anxiety Rating Scale |
|
| Notes | Sponsor: NIH Research Scientist Development Award, Burroughs Wellcome Company (Bupropion) Response: no definition, no data Remission: no definition, no data 3‐arm study comparing bupropion to amitriptyline and placebo (total 61 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described, assumed, because it is a double‐blind study |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Multi‐centre double‐blind study", no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Multi‐centre double‐blind study", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs not indicated, unclear whether there were any |
| Selective reporting (reporting bias) | High risk | Only data for bupropion‐treated patients reported |
| Other bias | Low risk | No obvious other bias |
Raft 1981.
| Methods | 5‐week, double‐blind, randomised, single‐centre study, placebo wash‐out unclear | |
| Participants | Psychiatric outpatients meeting Feighner criteria for primary depression, threshold HAM‐D not reported, setting: outpatients of a pain clinic, randomised, 1‐week hospitalised, further outpatients Age: no data Sex: no data Exclusion criteria: not reported |
|
| Interventions | Amitriptyline: 12 participants Placebo: 7 participants Amitriptyline dose: range 100 mg to 300 mg, mean dose 235 mg, flexible dose regimen |
|
| Outcomes | Primary outcome: HAM‐D score (Hamilton 1960, probably 17‐item HAM‐D) Secondary outcome: platelet MAO‐activity for phenelzine |
|
| Notes | Sponsor: NIMH grant Response: no definition, no data Remission: no definition, no data 3‐arm study comparing phenelzine to amitriptyline and placebo (total 29 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In accordance with a random code" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "to receive on a double‐blind basis in accordance with a random code" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "to receive on a double‐blind basis in accordance with a random code". "The clinical investigators (DR, JD) were unaware of platelet MAO activity for each subject until completion of treatment, while the investigator responsible for enzyme assays (AM) had no contact with patients and was unaware of clinical data until the study had been completed." It is unclear whether the investigators were blind towards the treatment patients received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for drop‐out presented, most amitriptyline patients dropped out due to side effects, most likely only a completer analysis was presented |
| Selective reporting (reporting bias) | High risk | Missing standard deviations |
| Other bias | Low risk | No obvious other bias |
Reimherr 1990.
| Methods | 8‐week, double‐blind, randomised, multi‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depression, having a minimum score 18 of on the 18‐item HAM‐D Age: AMI mean 37.7; PBO mean 40.1 Sex: AMI M65, F84; PBO M72, F78 Exclusion criteria: patients not meeting DSM‐III criteria for MD, pregnant or lactating females, females of childbearing potential not presently using an adequate method of contraception; patients receiving concurrent psychotherapeutic medication or concomitant medications other than oestrogens, progesterone and diuretics, patients with other significant medical conditions; patients receiving another investigational drug within 4 weeks of enrolling in this study; patients with a history of serious intolerance or resistance to antidepressant medications; patients with an alcohol or drug abuse condition, patients with schizophrenia or schizoaffective disorder |
|
| Interventions | Amitriptyline: 149 participants Placebo: 150 participants Amitriptyline dose: 50 mg to 150 mg, mean 104 mg, flexible dosing schedule |
|
| Outcomes | Primary outcome: 17‐item HAM‐D, CGI‐Improvment Secondary outcome: change SCL |
|
| Notes | Sponsor: unclear Response: ≥ 50% decrease in HAM‐D total score between baseline and final visits Remission: no definition, no data 3‐arm study comparing sertraline to amitriptyline and placebo (total 448 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind", "in a double‐blind manner" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind", "in a double‐blind manner", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A similar number of participants in each group dropped out (˜50%), but more in the amitriptyline group due to adverse events. Details were reported. It appears that ITT analysis was done ("baseline to last visit"), however, there was an additional completer analysis |
| Selective reporting (reporting bias) | Low risk | No obvious selective reporting |
| Other bias | Low risk | No obvious other bias |
Rickels 1982.
| Methods | 6‐week, double‐blind, randomised, single‐centre study, placebo wash‐out unclear | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for unipolar major depressive disorder, being significantly depressed Age: total mean 40 ± 13 years Sex: total M69, F 133 Exclusion criteria: not reported |
|
| Interventions | Amitriptyline: 68 participants Placebo: 68 participants Amitriptyline dose: 100 mg to 200 mg, mean during final 2 weeks 140 mg |
|
| Outcomes | Primary outcome: 21‐item HAM‐D Secondary outcome: Global Improvement, Raskin, Hopkins Checklist, Covi |
|
| Notes | Sponsor: NIMH, Mead Johnson Research Centre Response: moderate or marked global improvement Remission: no definition, no data 3‐arm study comparing trazodone with amitriptyline and placebo (total 202 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no further detail |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical capsules", "neither patients nor physicians knew which medication patients were taking" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Identical capsules", "neither patients nor physicians knew which medication patients were taking", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs and reasons are presented. Results are based on participants who completed at least 2 weeks of treatment (modified ITT). |
| Selective reporting (reporting bias) | High risk | No standard deviations |
| Other bias | Low risk | No clear other bias |
Rickels 1985.
| Methods | 6‐week, double‐blind, randomised, 3‐centre study, a 4‐ to 7‐day placebo wash‐out, exclusion of placebo responders unclear | |
| Participants | Psychiatric outpatients meeting Feighner diagnostic criteria for primary depression, having a minimum score of 18 on the 21‐item HAM‐D Age: total mean 39 ± 11.7 Sex: total M171, F 333 Exclusion criteria: females of childbearing age without reliable contraception, psychopathic, psychotic, bipolar, involutional or schizoaffective depression, secondary depression, severe liver or kidney disease, uncontrolled cardiovascular, pulmonary, endocrinological or collagen disease, glaucoma or conditions contraindicated to TCA, patients sensitive to benzodiazepines or antidepressants, actively abusing alcohol or drugs, requiring other psychotropic or thyroid medications, anticholinergics, sympathomimetic amines, guanethidine, propanolol, methyl‐dopa |
|
| Interventions | Amitriptyline: 124 participants Placebo: 130 participants Amitriptyline dose: 50 mg to 225 mg, mean dose of final 2 weeks 148 mg |
|
| Outcomes | Primary outcome: 21‐item HAM‐D Secondary outcome: CGI, HSCL, Physician Global Rating of Depression |
|
| Notes | Sponsor: statistical analysis made by The Upjohn Co Response: percentage of patients with at least 50% improvement at treatment endpoint as indicated by HAM‐D score Remission: no definition, no data 4‐arm study comparing alprazolam, doxepin, amitriptyline and placebo (total 504 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical capsules", "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Identical capsules", "double‐blind", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "504 patients were randomly assigned" "605 patients entered he study, 101 were not included in efficacy evaluation, because they either did not fulfil the entrance criteria, wished to withdraw for non‐medical reasons, did not cooperate with the physician or were unavailable for follow‐up", thus the patients not co‐operating with the physician or unavailable for follow‐up might have been randomised, but there is no information in the study. They were rather evenly distributed between groups. The rest of the participants were included if they had been fin the study or at least 1 week and reasons for drop‐out were presented. More placebo‐treated patients dropped out due to inefficacy |
| Selective reporting (reporting bias) | High risk | Missing SDs. No overall response rates, only response rates grouped by investigator, but unclear how many participants were treated by each investigator. Response rates of the 3 investigators are quite inhomogeneous |
| Other bias | Low risk | No obvious other bias |
Roffman 1982.
| Methods | 4‐week, double‐blind, randomised, multi‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting DSM criteria for major depressive disorder (296.2 and 296.3), moderately ill, having a minimum score of 18 on the 17‐item HAM‐D Age: range for AMI and PBO 18 to 65 years Sex: AMI M53, F42; PBO M54, F40 Exclusion criteria: history or evidence of clinically significant: renal disease, BUN or creatinine elevations, hepatic disease, liver enzyme elevations, cardiovascular diseases, metabolic diseases, seizure disorders, hypersensitivity to TCA or related compounds, cerebrovascular disease, drug abuse, alcoholism or endocrine disease. Also patients with adjustment disorders, manic‐depressive illness, recurrent type schizophrenia and primary anxiety disorder were excluded. |
|
| Interventions | Amitriptyline: 95 participants Placebo: 94 participants Amitriptyline dose: range 75 mg to 150 mg |
|
| Outcomes | Primary outcome: HAM‐D Secondary outcome: CGI |
|
| Notes | Sponsor: Ciba‐Geigy Response: 50% reduction in Hamilton score from Visit 1 to endpoint visit or value of 12 or less in Hamilton Score Remission: no definition, no data 3‐arm study comparing oxaprotiline with amitriptyline and placebo The data from 30 participants with protocol violations were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Method not presented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 dropped out due to protocol violations after randomisation, reason and group of participants not reported, “in addition data from certain visits from 20 of the remaining 278 patients were also excluded from the efficacy analysis for the same reason” (protocol violations). Numbers do not add up |
| Selective reporting (reporting bias) | Low risk | No obvious selective reporting |
| Other bias | Low risk | No clear other bias |
Rowan 1980.
| Methods | 6‐week, randomised, double‐blind, single‐centre study, including a 5‐day inpatient period, followed by a 1‐week placebo wash‐out period, exclusion of placebo responder | |
| Participants | Psychiatric outpatients with major depression according to RDC and scoring a minimum of 7 points on the Raskin Three Area Depression Scale and 20.2 on the 17‐item HAM‐D Age range: total 21 to 65 years, mean total 37 years Gender: 71% of the total sample were female (sex and age of 2 patients, 1 in the AMI and 1 in the PBO group, are missing) Exclusion criteria: bipolar manic depressives, patients with physical illness, patients receiving already an antidepressant, depression subsidiary to another predominant syndrome |
|
| Interventions | Amitriptyline: 44 participants of the completers sample (Bhat 1984) Placebo: 45 participants of the completers sample (Bhat 1984) Amitriptyline dose range: 75 mg to 187.5 mg, flexible dosing |
|
| Outcomes | Primary outcome: Raskin Three Area total score Secondary outcome: global illness, global change, BPRS |
|
| Notes | Sponsor: medication provided by Warner‐Lambert Ltd., NIMH‐Grant Response: no definition, no data Remission: no definition, no data 3‐arm study comparing phenelzine to amitriptyline and placebo (total 176 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Modified randomisation procedure" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind", no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind", no further details, no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 45 patients of the total sample dropped out, "mainly due to non‐cooperation" (27.5% phenelzine, 29% amitriptyline, 19.6% placebo, number of dropped out placebo responder unclear), completer analysis |
| Selective reporting (reporting bias) | High risk | Data for scores and scales not reported, only comparison of results, no usable data, missing SDs |
| Other bias | Low risk | No obvious other bias |
Shipley 1981.
| Methods | 4‐week, double‐blind, probably randomised, single‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric inpatients meeting RDC criteria for affective disorder, having a minimum score of 30 on the 17‐item HAM‐D using the sum of 2 raters Age: AMI 39.0 ± 1.8; PBO 40.3 ± 2.9 Sex: AMI M22, F31; PBO M7, F16 Exclusion criteria: improvement during a 2‐week drug‐free period, diagnosis of concurrent medical disease |
|
| Interventions | Amitriptyline: 53 participants Placebo: 23 participants Amitriptyline dose: 200 mg, fixed dosing schedule |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcome: neuropsychological assessment, EEG, KDS‐COGDIS, SADS |
|
| Notes | Sponsor: Merck and by grants of NIMH Response: no definition, no data Remission: no definition, no data 2‐arm study, comparing amitriptyline with placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Four identical capsules daily", "double‐blind conditions", "both patient and staff remained blind to treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Four identical capsules daily", "double‐blind conditions", "both patient and staff remained blind to treatment", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs not reported. ITT analysis including all patients |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Reasons why group sizes are so different are unclear |
Smith 1990.
| Methods | 6‐week, double‐blind, randomised study, number of participating centres not reported, probably single‐centre, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depressive illness, having a minimum score 18 of on the 17‐item HAM‐D Age: total at least 18 years, total mean 43 years Sex: total M64, F86 Exclusion criteria: significant renal, hepatic, respirators, cardiovascular or cerebrovascular disease, narrow‐angle glaucoma, prostatic hypertrophy, seizure disorders, clinically relevant abnormal laboratory values or significantly abnormal ECG findings, primary diagnosis of schizophrenia, atypical depression, anxiety, adjustment or bipolar disorder, drug or alcohol abuse, suicidal tendencies, cognitive deficiencies |
|
| Interventions | Amitriptyline: 50 participants Placebo: 50 participants Amitriptyline dose: range 80 to 280 mg, modal dose 111 mg |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcome: Pulse, Zung, CGI‐I, EKG, Laboratory |
|
| Notes | Sponsor: 2 authors are representatives from Organon Inc. Response: at least 50% HAM‐D (17‐item) reduction from baseline Remission: no definition, no data 3‐arm study comparing mirtazapine, amitriptyline and placebo (total 150 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not presented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 60 drop‐outs (patients completing the trial N = 90 at week 6 of 150 patients), in the text only 53 drop‐outs are described, but these in a sufficiently detailed way. More participants in the placebo group dropped out due to inefficacy. Efficacy analysis included all patients who remained for at least 2 weeks in the study which was the vast majority (ITT) |
| Selective reporting (reporting bias) | High risk | Missing SDs, other outcomes seem to have all been reported |
| Other bias | Low risk | No obvious other bias |
Stratas 1984.
| Methods | 6‐week, double‐blind, randomised, single‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting RDC criteria for major depressive disorder, having a minimum score of 18 on the 21‐item HAM‐D Age: no data Sex: no data Exclusion criteria: physical or psychiatric disorders as a standard contraindication to TCA |
|
| Interventions | Amitriptyline: 12 participants Placebo: 10 participants Amitriptyline dose: 50 mg to 300 mg, mean 179 mg |
|
| Outcomes | Primary outcome: 18/21‐item HAM‐D Secondary outcome: global change |
|
| Notes | Sponsor: Marion Laboratories Inc. supplied dothiepin Response: no definition, no data Remission: no definition, no data 3‐arm study comparing dothiepin with amitriptyline and placebo (total 33 patients) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical appearing capsules", "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Identical appearing capsules", "double‐blind", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41 patients entered the study, only 33 were analysed for efficacy. 6 of the 7 patients who were not included were placebo responders or were otherwise not reaching the end of the wash‐out phase, so there is probably only one real drop‐out (non‐compliant patient). It is unclear to which group he belonged. |
| Selective reporting (reporting bias) | High risk | No means and no standard deviations for HAM‐D scores |
| Other bias | Low risk | No obvious other bias |
Thomson 1982.
| Methods | 12‐week, double‐blind, double‐dummy, randomised, multi‐centre study, placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting RDC criteria for major affective disorder, having a minimum score of 12 on the HAM‐D (unclear how many items) Age: range 18 to 65 years, median (total) 33 years Sex: total M25, F115 Exclusion criteria: receiving antidepressants in the previous 2 weeks, contraindication to TCA |
|
| Interventions | Amitriptyline: 31 participants Placebo: 28 participants Amitriptyline dose: fixed dosing schedule, dosage is described as 100 mg per day and in results as 150 mg per day |
|
| Outcomes | Primary outcome: first 18 items of the 21‐item HAM‐D, number of items unclear Secondary outcome: Present State Examination, 5‐point Global State of Depression, plasma tryptophane levels |
|
| Notes | Sponsor: Berk Pharmaceuticals Response: no definition, no data Remission: defined as a fall to 4 points or less on the total HAM‐D score 4‐arm study comparing amitriptyline with tryptophane, a combination of amitriptyline and tryptophane and with placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation to treatment was random, but balanced within each group practice", no further details |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐dummy technique for drug administration" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐dummy technique for drug administration", assessments were made by a research psychiatrist, not the treating psychiatrist, no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear whether randomisation was done before or after run‐in period so that some numbers are unclear. Clearly more drop‐outs due to inefficacy in the placebo group. Very much modified ITT (at least one assessment after 4 weeks). |
| Selective reporting (reporting bias) | High risk | No standard deviations, no numbers for all side effects |
| Other bias | Low risk | No obvious other bias |
van de Merwe 1984a.
| Methods | 4‐week, double‐blind, randomised, single‐centre study, placebo wash‐out "if indicated", exclusion of placebo responder unclear | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depressive disorder, threshold not reported Age: range 18 to 60 years, mean 36.5 ± 11.9 (for 20 patients starting active treatment) Sex: total M9, F11 (for 20 patients starting active treatment, sex of one early drop‐out is not reported) Exclusion criteria: patients with any cardiovascular or other psychiatric illness (this included organic brain disease, alcoholism, addiction or mental handicap), patients with antidepressive treatment or ECT in the recent past, severe depression with indication for ECT, patients with known enzyme inducing or inhibiting drugs or psychoactive medication, individuals unable to comprehend the purpose of the study or to comply with the programme, women of childbearing age without contraception |
|
| Interventions | Amitriptyline: 7 participants Placebo: 7 participants Amitriptyline dose: 95.3 mg |
|
| Outcomes | Primary outcome: cardiovascular effects and ECG Secondary outcome. plasma concentrations |
|
| Notes | Sponsor: 1 author is a representative of the Medical Research Department of Roussel Laboratories Limited Response: no definition, no data Remission: no definition, no data 3‐arm study comparing trazodone with amitriptyline and placebo (total 21 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised", "medication was pre‐packed labelled and sealed for each patient on a pre‐arranged randomised schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "medication was pre‐packed labelled and sealed for each patient on a pre‐arranged randomised schedule. Dispensing was done by the pharmacist at St. Bartholomew´s Hospital" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The matched white capsules contained either TZD 50mg, AMI 25mg or PBO", "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The matched white capsules contained either TZD 50mg, AMI 25mg or PBO", "double‐blind", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for 6 drop‐outs (out of 21 randomised) not specified, completer analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported, only cardiovascular effects were investigated, no psychiatric measurements were done |
| Other bias | Low risk | No obvious other risk of bias |
Wilcox 1994.
| Methods | 6‐week, double‐blind, randomised, 1‐centre study (with 3 different offices), placebo wash‐out with elimination of placebo responder | |
| Participants | Psychiatric outpatients meeting DSM‐III criteria for major depressive Illness, having a minimum score of 18 on the 17‐item HAM‐D and no decrease greater than 20% on the 21‐item HAM‐D by the end of the placebo wash‐out period Age: mean total 40 years Sex: AMI M26, F24; PBO M26, F23 Exclusion criteria: clinically significant renal, hepatic, respiratory, cardio‐ or cerebrovascular disease, narrow‐angle glaucoma, clinically significant prostatic hypertrophy, seizure disorders, drug allergies or hypersensitivity to TCA or related compounds, hyperthyroidism, history of blood dyscrasias from the use of TCA for prior depressive episodes, primary diagnosis of schizophrenia, anxiety, adjustment disorder or bipolar disorder, concomitant treatment with other psychotropic drugs, alcohol or drug abuse within previous 6 months, ECT within 3 months, MAO‐inhibitors within 17 days or other psychotropic drugs within 7 days of baseline, significant abnormal laboratory, ECG or physical examination findings at screening visit, active suicidal tendencies, cognitive deficiencies, HAM‐D‐21 fall of at least 20% during placebo wash‐out, women without acceptable birth control, pregnant or intend to become pregnant, nursing |
|
| Interventions | Amitriptyline: 50 participants Placebo: 49 participants Amitriptyline dose: 60 mg to 300 mg, mean overall dose 121.8 mg, semi‐flexible dosing schedule |
|
| Outcomes | Primary outcome: 17‐item HAM‐D Secondary outcome: 21‐item HAM‐D, MADRS, CGI‐Improvement, CGI‐Severity, SDS |
|
| Notes | Sponsor: Organon Inc. Response: at least 50% reduction from baseline on the 17‐item HAM‐D Remission: no definition, no data 3‐arm study comparing mianserin with amitriptyline and placebo One author is also a co‐author of Carman 1991 and representative of Organon. Due to identical parameters like equal numbers of patients randomised, same study design and duration, equal dosing and almost identical response rates we had the suspicion that both publications were describing the same trial, however there are also outcomes which are different such as the mean baseline HAM‐D and the mean dose. So we finally decided that these trials were independent and included both. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised", "randomly assigned", no furthers detail |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind", "identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind", "identical capsules", no details on blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for early drop‐outs (AMI 11, PBO 7, out of 150 randomised) not reported and data not included in results, further 10 patients were excluded from the analysis, because there were no post baseline data, ˜50% overall drop‐out rate with maybe more placebo‐treated patients dropping out due to inefficacy, modified ITT for efficacy (at least 1 post baseline examination at 14 days) |
| Selective reporting (reporting bias) | High risk | All outcomes are reported, but no SDs for HAM‐D |
| Other bias | Low risk | No obvious other bias |
AD = antidepressant; AMDP = Arbeitsgemeinschaft für Dokumentation in der Psychiatrie; AMI = amitriptyline; BDI = Beck Depression Inventory; BPRS = Brief Psychiatric Rating Scale; CGI = Clinical Global Impressions Scale; CGI‐I = Clinical Global Impressions Improvement Scale; DSM‐II = Diagnostic and Statistical Manual, 2nd edition; DSM‐III = Diagnostic and Statistical Manual, 3rd edition; DSM‐III = Diagnostic and Statistical Manual, 3rd edition, revised; ECG = electrocardiogram; ECT = electroconvulsive therapy; EEG = electroencephalogram; F = female; GAF = Global Assessment of Functioning; GAS = Global Assessment Scale; HAMA = Hamilton Anxiety Rating Scale; HAM‐D = Hamilton Depression Rating Scale; HARS = Hamilton Depression Rating Scale; HDRS = Hamilton Depression Rating Scale; HRQOL = Health Related Quality of Live; HSCL = Hopkins Symptom Checklist; IQ = intelligence quotient; ITT = intention‐to‐treat; KDSCOGDIS = forms assessing the severity of self reported symptoms of cognitive dysfunction; LOCF = last observation carried forward; M = male; MADRAS = Montgomery Asberg Depression Rating Scale; MAO‐I = monoamine oxidase inhibitor; MD = major depression; mg = milligram; NIH = National Institutes of Health; NIMH = National Institute of Mental Health; PBO = placebo; POMS = Profile of Mood States; RDC = Research Diagnostic Criteria; SADS = Schedule for Affective Disorders and Schizophrenia; SD = standard deviation; SDS = (Zung) Self‐Rating Depression Scale; SE = standard error of the mean; ß‐HCG = ß‐human‐cortico‐gonadal‐hormone; T3/4 = thyroxin 3/4; TCA = tricyclic antidepressant; TESS = Treatment Emergent Side Effects Scale; TZD = trazodone; ZDS: patient‐rated Zung Depression Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anon 1971 | No comparison with placebo |
| Barton 1972 | No comparison with placebo |
| Beasley 1990 | Not randomised |
| Bellack 1981 | No placebo group |
| Bente 1974 | No placebo group |
| Bergener 1968 | No placebo group |
| Branconnier 1982 | No operationalised criteria, geriatric depression, not further clarified |
| Browne 1963 | No operationalised criteria, all cases of depressive illness, diagnosed as reactive or endogenous depression |
| Bruck 1974 | No placebo group |
| Burrows 1980 | No operationalised criteria, moderate to severe depressive illness, not further classified |
| Claghorn 1984 | No operationalised criteria, neurotic depression |
| Coble 1981 | No amitriptyline group |
| Cosyns 1974 | No operationalised criteria, depressive illness according to DSM‐II |
| Davis 1967 | No operationalised criteria, no direct comparison amitriptyline versus placebo, depression not further classified |
| DiMascio 1974 | No placebo group, no operationalised diagnostic criteria |
| Downing 1972 | No operationalised criteria, reactive neurotic depression or mixed anxiety‐depressive reaction |
| Downing 1973 | No operationalised criteria, neurotic depression |
| Downing 1979 | No operationalised criteria, depression not further classified |
| Downing 1983 | No operationalised criteria, depression not further classified |
| Ellingson 1973 | No operationalised criteria, psychotic depression and psychoneuroses‐depressive states, not further classified |
| Fisch 1992 | Review |
| Friedman 1974 | No operationalised criteria, depression not further classified |
| Friedman 1975 | No operationalised criteria, neurotic or reactive depression, not further classified |
| Friedman 1976 | No operationalised criteria |
| Friedman 1980 | No placebo group |
| Garry 1963 | No operationalised criteria, chronic depression, depression not further classified |
| Gomez 1968 | No comparison with placebo |
| Guy 1982 | No comparison amitriptyline versus placebo, no operationalised criteria, neurotic, endogenous depression |
| Hazell 1995 | Child and adolescent depression |
| Hecht 1986 | No operationalised criteria, neurotic depression |
| Hollister 1964 | No operationalised criteria, all types of depressive syndromes |
| Houston 1983 | No operationalised criteria, depression not further classified |
| Hussain 1970 | No comparison of amitriptyline versus placebo |
| Imlah 1985 | No operationalised criteria, neurotic, non‐endogenous depression not further classified |
| Itil 1972 | No comparison of amitriptyline versus placebo, diagnostic criteria not indicated |
| Jacobson 1978 | No operationalised diagnostic criteria |
| Kasper 1995 | Review article |
| Kasper 1997 | Review article |
| Kasper 1997a | Review |
| Kiev 1974 | No operationalised criteria, "neurotic depression" |
| Klerman 1974 | No operationalised criteria |
| Laakmann 1995 | Depression according to ICD‐9 including depressive episodes within manic‐depressive illness and neurotic depression; exact numbers not indicated but 15% had neurotic depression making it likely that more than 20% had other diagnoses than major depressive disorder |
| Lipsedge 1970 | No placebo group |
| Malitz 1971 | No operationalised criteria, only target symptoms, not further classified |
| Master 1963 | No operationalised criteria, only target symptoms, not further classified |
| McCallum 1975 | No operationalised criteria, depression not further classified |
| McDonald 1966 | No operationalised criteria, no valid criteria |
| McLean 1992 | No comparison of amitriptyline versus placebo |
| Moll 1990 | Moclobemide versus various tricyclic antidepressants |
| Montgomery 1980b | No placebo group |
| Montgomery 1998 | Meta‐analysis |
| Morakinyo 1970 | No operationalised diagnostic criteria |
| Möller 1993 | No placebo group |
| Othmer 1983 | No placebo group |
| Othmer 1988 | No placebo group, less than 80 % with primary depression |
| Paykel 1973c | No operationalised diagnostic criteria |
| Podobnikar 1966 | No operationalised criteria, amitriptyline plus perphenazine versus placebo |
| Prusoff 1974 | No operationalised criteria |
| Rampello 1995 | Major depression or bipolar affective disorder (unclear how many) |
| Rickels 1970 | No operationalised criteria, neurotic depression |
| Rickels 1970b | No operationalised criteria, neurotic depression |
| Rickels 1974 | No operationalised criteria |
| Rickels 1981 | Amitriptyline versus limbitrol, no placebo group |
| Rockliff 1971 | Review article |
| Rosenberg 1974 | No operationalised criteria |
| Schou 1979 | Review article |
| Skarbek 1962 | No operationalised criteria, chronic depression, not further classified |
| Skarbek 1963 | No operationalised diagnostic criteria |
| Spiker 1988 | Retrospective study with rediagnosed and reviewed patients, who were already in part participants of Kupfer 1979 |
| Stabl 1989 | No amitriptyline group |
| Stahl 1997 | Meta‐analysis |
| Stein 1980 | Continuation study, no acute phase |
| Taverna 1969 | No operationalised diagnostic criteria |
| Taylor 1993 | Amitriptyline versus psychotherapeutic interventions, no placebo group |
| Wallerstein 1967 | No amitriptyline group |
| Warner 1988 | Review |
| Welner 1980 | Review |
| Wilson 1982 | No operationalised criteria, not further classified |
| Zis 1980 | No amitriptyline versus placebo |
| Zivkov 1995 | No placebo group |
Characteristics of studies awaiting assessment [ordered by study ID]
Kahn 2008.
| Methods | A double‐blind, random assignment, 6‐week trial |
| Participants | Outpatients ranging from 18 to 78 years |
| Interventions | 3‐arm study comparing mirtazapine versus amitriptyline versus placebo |
| Outcomes | Response defined as 50% reduction in symptom severity as measured by a 17‐item HAM‐D |
| Notes | The study of interest is the precursor study (lead‐in study) of the study described in the publication. The study of interest is also described shortly and even response rates are given. However, there is much missing information, which we could not complement from the replies of the author despite repeated enquiry. We decided to maintain this publication in the awaiting assessment section, because we cannot be certain whether the lead‐in study is summing up studies already included in the present review. |
Differences between protocol and review
In the protocol we had planned to use the mean standard deviation of the other studies in this review when the standard deviation of a study was missing. As we felt that the number of studies indicating a standard deviation was too small to provide a representative mean, we instead used the standard deviations from the MANGA project (Cipriani 2009) to the update of which this review will contribute.
Contributions of authors
Claudia Leucht: protocol development, search, study selection, data extraction, statistical analysis, interpretation of results, writing the review.
Stefan Leucht: protocol development, search, study selection, statistical analysis, interpretation of results, writing the review.
Maximilian Huhn: protocol development, search, study selection, data extraction, writing the review.
Sources of support
Internal sources
-
Klinikum rechts der Isar der Technischen Universität München, Germany.
The authors are employees of this hospital
External sources
-
German Ministry of Education and Research, Bundesministerium für Bildung und Forschung (BMBF) Grant number: 01KG1029, Germany.
Grant support
Declarations of interest
Stefan Leucht: has received honoraria for consulting/advisory boards from Alkermes, BristolMyersSquibb, EliLilly, Janssen, Johnson&Johnson, Medavante, Roche, lecture honoraria from AstraZeneca, BristolMyersSquibb, EliLilly, EssexPharma, Janssen, Johnson&Johnson, Lundbeck Institute, Pfizer and SanofiAventis, and EliLilly has provided medication for a trial with SL as the primary investigator.
Claudia Leucht: is Stefan Leucht's wife. Therefore, his conflicts of interest in part also apply to her. She has no other conflicts of interest.
Maximilian Huhn: none to declare.
New
References
References to studies included in this review
Amsterdam 1986 {published data only}
- Amsterdam JD, Case WG, Csanalosi E, Singer M, Rickels K. A double‐blind comparative trial of zimelidine, amitriptyline, and placebo in patients with mixed anxiety and depression. Pharmacopsychiatry 1986;19(3):115‐9. [DOI] [PubMed] [Google Scholar]
Bakish 1992 {published data only}
- Bakish D, Bradwejn J, Nair N, McClure J, Remick R, Bulger L. A comparison of moclobemide, amitriptyline and placebo in depression: a Canadian multicentre study. Psychopharmacology 1992;106(Suppl):S98‐101. [DOI] [PubMed] [Google Scholar]
- Bakish D, Wiens A, Ellis J, Alda M, Lapierre Y. A double‐blind placebo‐controlled comparison of moclobemide and amitriptyline in the treatment of depression. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 1992;37(Suppl 1):12‐7. [PubMed] [Google Scholar]
Bhatia 1991 {published data only}
- Bhatia SC, Hsieh HH, Theesen KA, Townley RG, Andersen JM, Weiss S, et al. Platelet alpha‐2 adrenoreceptor activity pre‐treatment and post‐treatment in major depressive disorder with melancholia. Research Communication in Chemical Pathology and Pharmacology 1991;74(1):47‐57. [PubMed] [Google Scholar]
Blashki 1971 {published data only}
- Blashki TG, Mowbray R, Davies B. Controlled trial of amitriptyline in general practice. British Medical Journal 1971;1(741):133‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blashki TG, Mowbray RM, Davies BM. A controlled trial of an antidepressant (amitriptyline) in general practice. V World Congress of Psychiatry, Ciudad de Mexico, 28 November ‐ 4 December 1971. [CN‐00216475]
- Blashki TG, Mowbray RM, Davies BM. A controlled trial of an antidepressant (amitriptyline) in general‐practice. V World Congress of Psychiatry, Ciudad de Mexico, 28 November ‐ 4 December 1971:409.
Bremner 1995 {published data only}
- Bremner JD. A double‐blind comparison of Org 3770, amitriptyline, and placebo in major depression. Journal of Clinical Psychiatry 1995;56(11):519‐25. [PubMed] [Google Scholar]
- Bremner JD. Double‐blind comparison of mirtazapine, amitriptyline and placebo in major depression. Nervenheilkunde 1996;15(8):533‐40. [Google Scholar]
Carman 1991 {published data only}
- Carman JS, Ahdieh H, Wyatt‐Knowles E, Warga E, Panagides J. A controlled study of mianserin in moderately to severely depressed outpatients. Psychopharmacology Bulletin 1991;27(2):135‐9. [PubMed] [Google Scholar]
Claghorn 1983 {published data only}
- Claghorn J, Gershon S, Goldstein BJ. Zimeldine tolerability in comparison to amitriptyline and placebo: findings from a multicentre trial. Acta Psychiatrica Scandinavica 1983;68(Suppl 308):104‐14. [DOI] [PubMed] [Google Scholar]
- Claghorn J, Gershon S, Goldstein BJ, Behrnetz S, Bush DF, Huitfeldt B. A double‐blind evaluation of zimelidine in comparison to placebo and amitriptyline in patients with major depressive disorder. Progress in Neuro‐Psychopharmacological and Biological Psychiatry 1983;7(2‐3):367‐82. [DOI] [PubMed] [Google Scholar]
Feighner 1979 {published data only}
- Feighner JP, Brauzer B, Gelenberg AJ, Gomez W, Kiev A, Kurland ML, et al. A placebo‐controlled multicenter trial of Limbitrol versus its components (amitriptyline and chlordiazepoxide) in the symptomatic treatment of depressive illness. Psychopharmacology 1979;61(2):217‐25. [DOI] [PubMed] [Google Scholar]
Gelenberg 1990 {published data only}
- Gelenberg AJ, Wojcik JD, Falk WE, Spring B, Brotman AW, Galvin Nadeau M. Clovoxamine in the treatment of depressed outpatients: a double‐blind, parallel‐group comparison against amitriptyline and placebo. Comprehensive Psychiatry 1990;31(4):307‐14. [DOI] [PubMed] [Google Scholar]
- Spring B, Gelenberg AJ, Garvin R, Thompson S. Amitriptyline, clovoxamine and cognitive function: a placebo‐controlled comparison in depressed outpatients. Psychopharmacology 1992;108(3):327‐32. [DOI] [PubMed] [Google Scholar]
Georgotas 1982 {published data only}
- Georgotas A, Krakowski M, Gershon S. Controlled trial of zimelidine, a 5‐HT reuptake inhibitor, for treatment of depression. American Journal of Psychiatry 1982;139(8):1057‐8. [DOI] [PubMed] [Google Scholar]
Hicks 1988 {published data only}
- Hicks F, Robins E, Murphy GE. Comparison of adinazolam, amitriptyline, and placebo in the treatment of melancholic depression. Psychiatry Research 1988;23(2):221‐7. [DOI] [PubMed] [Google Scholar]
Hormazabal 1985 {published data only}
- Hormazabal L, Omer LM, Ismail S. Cianopramine and amitriptyline in the treatment of depressed patients ‐ a placebo‐controlled study. Psychopharmacology 1985;86:205‐8. [DOI] [PubMed] [Google Scholar]
Hoschl 1989 {published data only}
- Hoschl C, Kozeny J. Verapamil in affective disorders: a controlled, double‐blind study. Biological Psychiatry 1989;25(2):128‐40. [DOI] [PubMed] [Google Scholar]
Jacobson 1990 {published data only}
- Bech P. Meta‐analysis of placebo‐controlled trials with mirtazapine using the core items of the Hamilton Depression Scale as evidence of a pure antidepressive effect in the short‐term treatment of major depression. International Journal of Neuropsychopharmacology 2001;4:337‐45. [DOI: 10.1017/S1461145701002565] [DOI] [PubMed] [Google Scholar]
- Jacobson AF, Dominguez RA, Goldstein B, Gandara J. Comparison of ORG‐3770 and amitriptyline in depressed outpatients. Psychopharmacology 1990;30:851. [Google Scholar]
Katz 1993 {published data only}
- Katz RJ, Lott M, Landau P, Waldmeier P. A clinical test of noradrenergic involvement in the therapeutic mode of action of an experimental antidepressant. Biological Psychiatry 1993;33:261‐6. [DOI] [PubMed] [Google Scholar]
Katz 1993a {published data only}
- Katz RJ, Lott M, Landau P, Waldmeier P. A clinical test of noradrenergic involvement in the therapeutic mode of action of an experimental antidepressant. Biological Psychiatry 1993;33:261‐6. [DOI] [PubMed] [Google Scholar]
Klieser 1988 {published data only}
- Klieser E, Lehmann E. Experimental comparison between the effect of standardized trazodone‐amitriptyline and placebo treatment in vitalized depressive patients. Psychopharmacology 1988;95(Suppl):3‐5. [DOI] [PubMed] [Google Scholar]
- Klieser E, Lehmann E. Experimental examination of trazodone. Clinical Neuropharmacology 1989;12(Suppl 1):18‐24. [DOI] [PubMed] [Google Scholar]
Kupfer 1979 {published data only}
- Kupfer DJ, Coble PA, Rubinstein D. Changes in weight during treatment for depression. Psychosomatic Medicine 1979;41(7):535‐44. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Hanin I, Spiker D. Amitriptyline plasma levels and clinical response in primary depression: II. Communications in Psychopharmacology 1978;2(5):441‐50. [0145‐5699] [PubMed] [Google Scholar]
Kusalic 1993 {published data only}
- Kusalic M, Engelsmann F, Bradwejn J. Thyroid functioning during treatment for depression. Journal of Psychiatry and Neuroscience 1993;18(5):260‐3. [PMC free article] [PubMed] [Google Scholar]
Langlois 1985 {published data only}
- Langlois R, Cournoyer G, Montigny C, Caille G. High incidence of multisystemic reactions to zimeldine. European Journal of Clinical Pharmacology 1985;28(1):67‐71. [DOI] [PubMed] [Google Scholar]
Lydiard 1997 {published data only}
- Lydiard RB, Stahl SM, Hertzman M, Harrison WM. A double‐blind, placebo‐controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. Journal of Clinical Psychiatry 1997;58(11):484‐91. [DOI] [PubMed] [Google Scholar]
McNair 1984a {published data only}
- McNair DM, Kahn RJ, Frankenthaler LM, Faldetta LL. Amoxapine and amitriptyline II Specificity of cognitive effects during brief treatment of depression. Psychopharmacology 1984; Vol. 83, issue 2:134‐9. [DOI] [PubMed]
- McNair DM, Kahn RJ, Frankenthaler LM, Faldetta LL. Amoxapine and amitriptyline. I. Relative speed of antidepressant action. Psychopharmacology 1984;83(2):129‐33. [DOI] [PubMed] [Google Scholar]
Mynors‐Wallis 1995 {published data only}
- Mynors‐Wallis LM, Gath DH. Predictors of treatment outcome for major depression in primary care. Psychological Medicine 1997;27:731‐736. [DOI] [PubMed] [Google Scholar]
- Mynors‐Wallis LM, Gath DH, Lloyd‐Thomas AR, Tomlinson D. Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. BMJ 1995;310(6977):441‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Organon 3‐020 unpublished {unpublished data only}
- Bech P. Meta‐analysis of placebo‐controlled trials with mirtazapine using the core items of the Hamilton Depression Scale as evidence of a pure antidepressive effect in the short‐term treatment of major depression. International Journal of Neuropsychopharmacology 2001;4:337‐45. [DOI: 10.1017/S1461145701002565] [DOI] [PubMed] [Google Scholar]
- Organon 3‐020. [personal communication from the pharmaceutical company]
Organon 84062 unpublished {published data only}
- Organon 84062. [personal communication from the pharmaceutical company]
Paykel 1988a {published data only}
- Hollyman JA, Freeling P, Paykel ES, Bhat A, Sedgwick P. Double‐blind placebo‐controlled trial of amitriptyline among depressed patients in general practice. Journal of the Royal College of General Practitioners 1988;38(314):393‐7. [PMC free article] [PubMed] [Google Scholar]
- Organon 84062.
- Paykel ES, Freeling P, Hollyman JA. Are tricyclic antidepressants useful for mild depression? A placebo controlled trial. Pharmacopsychiatry 1988;21(1):15‐8. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Hollyman JA, Freeling P, Sedgwick P. Predictors of therapeutic benefit from amitriptyline in mild depression: a general practice placebo‐controlled trial. Journal of Affective Disorders 1988;14(1):83‐95. [DOI] [PubMed] [Google Scholar]
Preskorn 1983 {published data only}
- Preskorn SH. Antidepressant response and plasma concentrations of bupropion. Journal of Clinical Psychiatry 1983;44(5 Pt 2):137‐9. [PubMed] [Google Scholar]
Raft 1981 {published data only}
- Raft D, Davidson J, Wasik J, Mattox A. Relationship between response to phenelzine and MAO inhibition in a clinical trial of phenelzine, amitriptyline and placebo. Neuropsychobiology 1981;7(3):122‐6. [DOI] [PubMed] [Google Scholar]
Reimherr 1990 {published data only}
- Lapierre YD. Controlling acute episodes of depression. International Clinical Psychopharmacology 1991; Vol. 6, issue Suppl 2:23‐35. [DOI] [PubMed]
- Reimherr FW, Byerley WF, Ward MF, Lebegue BJ, Wender PH. Sertraline, a selective inhibitor of serotonin uptake, for the treatment of outpatients with major depressive disorder. Psychopharmacology Bulletin 1988;24(1):200‐5. [PubMed] [Google Scholar]
- Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil TM, LaPierre YD, et al. Antidepressant efficacy of sertraline: a double‐blind, placebo‐ and amitriptyline‐controlled, multicenter comparison study in outpatients with major depression. Journal of Clinical Psychiatry 1990;51(Suppl B):18‐27. [PubMed] [Google Scholar]
Rickels 1982 {published data only}
- Rickels K, Case WG. Trazodone in depressed outpatients. American Journal of Psychiatry 1982;139(6):803‐6. [DOI] [PubMed] [Google Scholar]
Rickels 1985 {published data only}
- Rickels K, Feighner JP, Smith WT. Alprazolam, amitriptyline, doxepin, and placebo in the treatment of depression. Archives of General Psychiatry 1985;42(2):134‐41. [DOI] [PubMed] [Google Scholar]
Roffman 1982 {published data only}
- Roffman M, Gould E, Brewer S, Lau H, Sachais B, Dixon N, et al. Comparative anticholinergic activity of oxaprotiline and amitriptyline. Drug Development Research 1983;3(6):561‐6. [Google Scholar]
- Roffman M, Gould EF, Brewer SJ, Lau H, Sachais B, Dixon RB, et al. A double blind comparative study of oxaprotiline with amitriptyline and placebo in moderate depression. Current Therapeutic Research, Clinical and Experimental 1982;32(2):247‐56. [Google Scholar]
- Stassen HH, Angst J, Delina‐Stula A. Delayed onset of action of antidepressant drugs? Survey of recent results. European Psychiatry 1997; Vol. 12, issue 4:166‐76. [DOI] [PubMed]
- Stassen HH, Delini‐Stula A, Angst J. Time course of improvement under antidepressant treatment: a survival‐analytical approach. European Neuropsychopharmacology 1993;3:127‐35. [DOI] [PubMed] [Google Scholar]
Rowan 1980 {published data only}
- Bhat AV, Rowan PR, Paykel ES. Responses to phenelzine and amitriptyline absence of differential predictors by multiple regression analysis. Journal of Affective Disorders 1984;6(2):209‐18. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Rowan PR, Parker RR, Bhat AV. Response to phenelzine and amitriptyline in subtypes of outpatient depression. Archives of General Psychiatry 1982;39(9):1041‐9. [DOI] [PubMed] [Google Scholar]
- Rowan P, Paykel ES, Parker PR, West E. Comparative effects of phenelzine and amitriptyline: a placebo controlled trial. Neuropharmacology 1980;19(12):1223‐5. [DOI] [PubMed] [Google Scholar]
Shipley 1981 {published data only}
- Shipley JE, Kupfer DJ, Spiker DG, Shaw DH, Coble PA, Neil JF, et al. Neuropsychological assessment and EEG sleep in affective disorders. Biological Psychiatry 1981;16(10):907‐18. [PubMed] [Google Scholar]
Smith 1990 {published data only}
- Smith WT, Glaudin V, Panagides J, Gilvary E. Mirtazapine vs amitriptyline vs placebo in the treatment of major depressive disorder. Psychopharmacology Bulletin 1990;26(2):191‐6. [PubMed] [Google Scholar]
Stratas 1984 {published data only}
- Stratas NE. A double‐blind study of the efficacy and safety of dothiepin hydrochloride in the treatment of major depressive disorder. Journal of Clinical Psychiatry 1984;45(11):466‐9. [PubMed] [Google Scholar]
Thomson 1982 {published data only}
- Thomson J, Rankin H, Ashcroft GW, Yates CM, McQueen JK, Cummings SW. The treatment of depression in general‐practice: a comparison of L‐tryptophan, amitriptyline, and a combination of L‐tryptophan and amitriptyline with placebo. Psychological Medicine 1982;12(4):741‐51. [DOI] [PubMed] [Google Scholar]
van de Merwe 1984a {published data only}
- Merwe TJ, Silverstone T, Ankier SI, Warrington SJ, Turner P. A double‐blind non‐crossover placebo‐controlled study between group comparison of trazodone and amitriptyline on cardiovascular function in major depressive disorder. Psychopathology 1984;17(Suppl 2):64‐76. [DOI] [PubMed] [Google Scholar]
Wilcox 1994 {published data only}
- Wilcox CS, Cohn JB, Katz BB, Mijares CP, Guarino JJ, Panagides J, et al. A double‐blind, placebo‐controlled study comparing mianserin and amitriptyline in moderately depressed outpatients. International Clinical Psychopharmacology 1994;9(4):271‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anon 1971 {published data only}
- Anon. Multihospital controlled comparison of the therapeutic effects of four antidepressants. Activitas Nervosa Superior 1971;13(3):166‐7. [PubMed] [Google Scholar]
Barton 1972 {published data only}
- Barton JL, Snaith RP. A clinical assessment of a sustained release preparation of amitriptyline. Current Medical Research and Opinion 1972;1(3):123‐9. [DOI] [PubMed] [Google Scholar]
Beasley 1990 {published data only}
- Beasley CM, Jr, Sayler ME, Cunningham GE, Weiss AM, Masica DN. Fluoxetine in tricyclic refractory major depressive disorder. Journal of Affective Disorders 1990;20(3):193‐200. [DOI] [PubMed] [Google Scholar]
Bellack 1981 {published data only}
- Bellack AS, Hersen M, Himmelhoch J. Social skills training compared with pharmacotherapy and psychotherapy in the treatment of unipolar depression. American Journal of Psychiatry 1981;138(12):1562‐7. [DOI] [PubMed] [Google Scholar]
Bente 1974 {published data only}
- Bente D, Feder J, Helmchen H, Hippius H, Rosenberg L. Double blind test of three antidepressants. 3. Methodical problems. Arzneimittelforschung 1974;24(2):205‐7. [PubMed] [Google Scholar]
Bergener 1968 {published data only}
- Bergener M. Clinical examination of antidepressive acting substances in double‐blind test. Arzneimittelforschung 1968;18(2):245‐9. [PubMed] [Google Scholar]
Branconnier 1982 {published data only}
- Branconnier RJ, Cole JO, Ghazvinian S, Rosenthal S. Treating the depressed elderly patient: the comparative behavioral pharmacology of mianserin and amitriptyline. Advances in Biochemical Psychopharmacology 1982;32:195‐212. [PubMed] [Google Scholar]
Browne 1963 {published data only}
- Browne M, Kreeger L, Kazamia NG. A clinical trial of amitriptyline in depressive patients. British Journal of Psychiatry 1963;109:692‐4. [DOI] [PubMed] [Google Scholar]
Bruck 1974 {published data only}
- Bruck J, Söllner E, Tschabitscher H. Results of the clinical testing of amitriptylin and C 34,276‐Ba (dibenzobicyclooctadiene derivative) in a double‐blind study. Der Nervenarzt 1974;45(9):492‐6. [PubMed] [Google Scholar]
Burrows 1980 {published data only}
- Burrows GD, Norman TR, Davies BM. A comparative study of amoxapine and amitriptyline for depressive illness. Australian Family Physician 1980;9(11):762‐6. [PubMed] [Google Scholar]
Claghorn 1984 {published data only}
- Claghorn JL, Schroeder J, Goldstein BJ, 2nd. Comparison of the electrocardiographic effect of dothiepin and amitriptyline. Journal of Clinical Psychiatry 1984;45(7):291‐3. [PubMed] [Google Scholar]
Coble 1981 {published data only}
- Coble PA, Kupfer DJ, Shaw DH. Distribution of REM latency in depression. Journal of Neurology and Neurosurgery 1981;16(5):453‐66. [PubMed] [Google Scholar]
Cosyns 1974 {published data only}
- Cosyns P. A single daily dose of a sustained release form of amitriptyline in depressive illness. Acta Psychiatrica Belgica 1974;74(4):430‐6. [PubMed] [Google Scholar]
Davis 1967 {published data only}
- Davis WG. Treatment of anxiety and depression. A double‐blind study of a perphenazine and amitriptyline in combination. Rocky Mountain Medical Journal 1967;64(11):73‐6. [PubMed] [Google Scholar]
DiMascio 1974 {published data only}
- DiMascio A. Drug therapy of depressed outpatients. Psychopharmacology Bulletin 1974;10:57. [Google Scholar]
Downing 1972 {published data only}
- Downing RW, Rickels K. Predictors of amitriptyline response in outpatient depressives. Journal of Nervous and Mental Disease 1972;154(4):248‐63. [CN‐00007057] [DOI] [PubMed] [Google Scholar]
Downing 1973 {published data only}
- Downing RW, Rickels K. Predictors of response to amitriptyline and placebo in three outpatient treatment settings. Journal of Nervous and Mental Disease 1973;156(2):109‐29. [DOI] [PubMed] [Google Scholar]
Downing 1979 {published data only}
- Downing RW, Rickels K, Rickels LA, Downing D. Nonspecific factors and side effect complaints. Factors affecting the incidence of drowsiness in drug and placebo treated anxious and depressed outpatients. Acta Psychiatrica Scandinavica 1979;60(5):438‐48. [DOI] [PubMed] [Google Scholar]
Downing 1983 {published data only}
- Downing RW, Rickels K. Physician prognosis in relationship to drug and placebo response in anxious and depressed psychiatric outpatients. Journal of Nervous and Mental Disease 1983;171(3):182‐5. [CN‐00030322] [DOI] [PubMed] [Google Scholar]
Ellingson 1973 {published data only}
- Ellingson. BC‐105 vs amitriptyline vs placebo. Psychopharmacology Bulletin 1973;9:78‐82. [Google Scholar]
Fisch 1992 {published data only}
- Fisch C, Knoebel SB. Electrocardiographic findings in sertraline depression trials. Drug Investigations 1992;4(4):305‐12. [Google Scholar]
Friedman 1974 {published data only}
- Friedman AS. Drug and family therapy in depressed outpatients. Psychopharmacology Bulletin 1974;10:18‐9. [Google Scholar]
Friedman 1975 {published data only}
- Friedman AS. Interaction of drug therapy with marital therapy in depressive patients. Archives of General Psychiatry 1975;32(5):619‐37. [DOI] [PubMed] [Google Scholar]
Friedman 1976 {published data only}
- Friedman AS. Drug and family therapy in depressed outpatients. Psychopharmacology Bulletin 1976;12(3):82‐3. [0048‐5764] [Google Scholar]
Friedman 1980 {published data only}
- Friedman J, McCallum P, Meares R. Stimulus intensity control in depression: a study of the comparative effect of doxepin and amitriptyline on cortical evoked potentials.. Australian and New Zealand Journal of Psychiatry 1980;14(2):115‐9. [DOI] [PubMed] [Google Scholar]
Garry 1963 {published data only}
- Garry J, Leonard TJ. Trial of amitriptyline in chronic depression. British Journal of Psychiatry 1963;109:54‐5. [DOI] [PubMed] [Google Scholar]
Gomez 1968 {published data only}
- Gomez JR, Gomez G. The treatment of anxiety and depression in general‐practice using an amitriptyline‐perphenazine preparation. British Journal of Clinical Practice 1968;22(3):105‐9. [PubMed] [Google Scholar]
Guy 1982 {published data only}
- Guy W, Ban TA, McEvoy JP, Petrie WM, Wilson WH, Schaffer JD. A collaborative study of a new antidepressant, viloxazine, in neurotic and endogenous depressives. International Pharmacopsychiatry 1982;17(1):36‐42. [DOI] [PubMed] [Google Scholar]
Hazell 1995 {published data only}
- Hazell P, O'Connell D, Heathcote D, Robertson J, Henry D. Efficacy of tricyclic drugs in treating child and adolescent depression: a meta‐analysis. BMJ 1995;310(6984):897‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hecht 1986 {published data only}
- Hecht Orzack M, Cole JO, Friedman L, Bird M, McEachern J. Weight changes in antidepressants: a comparison of amitriptyline and trazodone. Neuropsychobiology 1986;15(Suppl 1):28‐30. [DOI] [PubMed] [Google Scholar]
Hollister 1964 {published data only}
- Hollister LE, Overall JE, Johnson M, Pennington V, Katz G, Shelton J. Controlled comparison of imipramine, amitriptyline and placebo in hospitalised depressed patients. Journal of Nervous and Mental Disease 1964;139(4):370‐5. [DOI] [PubMed] [Google Scholar]
Houston 1983 {published data only}
- Houston J, Berg I, Butler A, McGuire R. Amitriptyline for depressed women with young children in general practice. British Journal of Psychiatry 1983;142:103‐4. [PubMed] [Google Scholar]
Hussain 1970 {published data only}
- Hussain Z. Drugs in depressive illness. British Medical Journal 1970;1(707):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imlah 1985 {published data only}
- Imlah NW. An evaluation of alprazolam in the treatment of reactive or neurotic (secondary) depression. British Journal of Psychiatry 1985;146:515‐9. [DOI] [PubMed] [Google Scholar]
Itil 1972 {published data only}
- Itil TM, Cora R, Hsu W, Gig E, Saletu B. Clinical, toxicological and electroencephalographical effects of a new tricyclic antidepressant, OI‐77. Arzneimittelforschung 1972;22(12):2063‐6. [PubMed] [Google Scholar]
Jacobson 1978 {published data only}
- Jacobson AF. Doctor‐patient concordance in a placebo‐controlled trial of limbitrol versus its components. Psychopharmacology Bulletin 1978;14(3):61‐3. [PubMed] [Google Scholar]
Kasper 1995 {published data only}
- Kasper S. Clinical efficacy of mirtazapine: a review. 8th European College of Neuropsychopharmacology Congress, Venice, Italy. 30th September ‐ 4th October. 1995.
Kasper 1997 {published data only}
Kasper 1997a {published data only}
- Kasper S. Efficacy of antidepressants in the treatment of severe depression: the place of mirtazapine. Journal of Clinical Psychopharmacology 1997;17(Suppl 1):19S‐28S. [DOI] [PubMed] [Google Scholar]
Kiev 1974 {published data only}
- Kiev A. The role of chemotherapy in managing potentially suicidal patients. Diseases of the Nervous System 1974;35(3):108‐11. [PubMed] [Google Scholar]
Klerman 1974 {published data only}
- Klerman GL, Dimascio A, Weissman M, Prusoff B, Paykel ES. Treatment of depression by drugs and psychotherapy. American Journal of Psychiatry 1974;131(2):186‐91. [DOI] [PubMed] [Google Scholar]
Laakmann 1995 {published data only}
- Laakmann G, Faltermaier‐Temizel M, Bossert‐Zaudig S, Baghai T, Lorkowski G. Treatment of depressive outpatients with lorazepam, alprazolam, amitriptyline and placebo. Psychopharmacology 1995;120(1):109‐15. [DOI] [PubMed] [Google Scholar]
- Laakmann G, Faltermaier‐Temizel M, Bossert‐Zaudig S, Baghai T, Lorkowski G. Treatment of mild to moderate depressive syndrome. A placebo‐controlled double‐blind trial on therapy with benzodiazepines or antidepressants. Munchener Medizinische Wochenschrift 1996; Vol. 138, issue 5:39‐44.
Lipsedge 1970 {published data only}
- Lipsedge MS, Rees WL, Pike DJ. A double‐blind comparison of dothiepin and amitriptyline for the treatment of depression with anxiety. Psychopharmacologica 1971;19:153‐62. [DOI] [PubMed] [Google Scholar]
Malitz 1971 {published data only}
- Malitz S, Kanzler M. Are antidepressants better than placebo?. American Journal of Psychiatry 1971;127(12):1605‐11. [DOI] [PubMed] [Google Scholar]
Master 1963 {published data only}
- Master RS. Amitriptyline in depressive states. A controlled trial in India. British Journal of Psychiatry 1963;109:826‐9. [DOI] [PubMed] [Google Scholar]
McCallum 1975 {published data only}
- McCallum P, Meares R. A controlled trial of maprotiline (Ludiomil) in depressed outpatients. Medical Journal of Australia 1975;2(10):392‐3. [DOI] [PubMed] [Google Scholar]
McDonald 1966 {published data only}
- McDonald IM, Perkins M, Marjerrison G, Podilsky M. A controlled comparison of amitriptyline and electroconvulsive therapy in the treatment of depression. American Journal of Psychiatry 1966;122(12):1427‐31. [DOI] [PubMed] [Google Scholar]
McLean 1992 {published data only}
- McLean P, Taylor S. Severity of unipolar depression and choice of treatment. Behaviour Research and Therapy 1992;30(5):443‐51. [DOI] [PubMed] [Google Scholar]
Moll 1990 {published data only}
- Moll E, Hetzel W. Moclobemide (Ro 11‐1163) safety in depressed patients. Acta Paediatrica Scandinavica. Supplement 1990;82(360):69‐70. [CN‐00187843] [DOI] [PubMed] [Google Scholar]
Möller 1993 {published data only}
- Möller HJ, Berzewski H, Eckmann F, Gonzalves N, Kissling W, Knorr W, et al. Double‐blind multicenter study of paroxetine and amitriptyline in depressed inpatients. Pharmacopsychiatry 1993;26:75‐8. [DOI] [PubMed] [Google Scholar]
Montgomery 1980b {published data only}
- Montgomery SA, McAuley R, Montgomery DB. Pharmacokinetics and efficacy of maprotiline and amitriptyline in endogenous depression: a double‐blind controlled trial. Clinical Therapy 1980;3(4):292‐310. [PubMed] [Google Scholar]
Montgomery 1998 {published data only}
- Montgomery SA, Reimitz PE, Zivkov M. Mirtazapine versus amitriptyline in the long‐term treatment of depression: a double‐blind placebo‐controlled study. International Clinical Psychopharmacology 1998;13(2):63‐73. [DOI] [PubMed] [Google Scholar]
Morakinyo 1970 {published data only}
- Morakinyo VO. Amytriptyline and chlordiazepoxide (Limbitrol) in depressive states in Nigerians ‐ a double‐blind study. African Journal of Medical Sciences 1970;1(4):409‐14. [PubMed] [Google Scholar]
Othmer 1983 {published data only}
- Othmer E, Othmer SC, Stern WC, Wyck Fleet J. Long‐term efficacy and safety of bupropion. Journal of Clinical Psychiatry 1983;44(5):153‐6. [PubMed] [Google Scholar]
Othmer 1988 {published data only}
- Othmer SC, Thmer E, Preskorn SH, Mac D. Differential effect of amitriptyline and bupropion on primary and secondary depression: a pilot study. Journal of Clinical Psychiatry 1988;49(8):310‐2. [PubMed] [Google Scholar]
Paykel 1973c {published data only}
- Paykel ES, Prusoff BA, Klerman GL, Haskell D, DiMascio A. Clinical response to amitriptyline among depressed women. Journal of Nervous and Mental Disease 1973;156(3):149‐65. [CN‐00008451] [DOI] [PubMed] [Google Scholar]
Podobnikar 1966 {published data only}
- Podobnikar IG. Treatment of mixed states of anxiety and depression. Diseases of the Nervous System 1966;27(12):811‐3. [PubMed] [Google Scholar]
Prusoff 1974 {published data only}
- Prusoff B. Drug treatment of depressed outpatients. Psychopharmacology Bulletin 1974;10:59‐60. [Google Scholar]
Rampello 1995 {published data only}
- Rampello L, Nicoletti G, Raffaele R, Drago F. Comparative effects of amitriptyline and amineptine in patients affected by anxious depression. Neuropsychobiology 1995;31(3):130‐4. [DOI] [PubMed] [Google Scholar]
Rickels 1970 {published data only}
- Rickels K, Gordon PE, Jenkins BW, Perloff M, Sachs T, Stepansky W. Drug treatment in depressive illness. Diseases of the Nervous System 1970;31:30‐42. [PubMed] [Google Scholar]
Rickels 1970b {published data only}
- Rickels K, Hesbacher P, Downing RW. Differential drug effects in neurotic depression. Diseases of the Nervous System 1970;31(7):468‐75. [PubMed] [Google Scholar]
Rickels 1974 {published data only}
- Rickels K, Csanalosi I, Chung HR, Case WG, Pereira‐Ogan A, Downing RW. Amitriptyline in anxious‐depressed outpatients: a controlled study. American Journal of Psychiatry 1974;131(1):25‐30. [DOI] [PubMed] [Google Scholar]
Rickels 1981 {published data only}
- Rickels K. Limbitrol (amitriptyline plus chlordiazepoxide) revisited. Psychopharmacology 1981;75(1):31‐3. [DOI] [PubMed] [Google Scholar]
Rockliff 1971 {published data only}
- Rockliff BW. Measurement of drug effects in newly hospitalized depressives. Diseases of the Nervous System 1971;32(8):532‐7. [PubMed] [Google Scholar]
Rosenberg 1974 {published data only}
- Rosenberg L. [Doppelblindprüfung von drei Antidepressiva]. Arzneimittel Forschung (Drug Research) 1974;24(2):207‐13. [PubMed] [Google Scholar]
Schou 1979 {published data only}
- Schou M. Lithium as a prophylactic agent in unipolar affective illness: comparison with cyclic antidepressants. Archives of General Psychiatry 1979;36:849‐51. [DOI] [PubMed] [Google Scholar]
Skarbek 1962 {published data only}
- Skarbek A, Smedberg D. Amitriptyline: a controlled trial in chronic depressive states. British Journal of Psychiatry 1962;108:859‐61. [DOI] [PubMed] [Google Scholar]
Skarbek 1963 {published data only}
- Skarbek A. Trial of amitriptyline in chronic depression. Diseases of the Nervous System 1963;24(2):115‐9. [0012‐3714] [PubMed] [Google Scholar]
Spiker 1988 {published data only}
- Spiker DG, Kupfer DJ. Placebo response rates in psychotic and nonpsychotic depression. Journal of Affective Disorders 1988;14(1):21‐3. [DOI] [PubMed] [Google Scholar]
Stabl 1989 {published data only}
- Stabl M, Biziere K, Schmid‐Burgk W, Amrein R. Review of comparative clinical trials. Moclobemide vs tricyclic antidepressants and vs placebo in depressive states. Journal of Neural Transmission. Supplementum 1989;28:77‐89. [PubMed] [Google Scholar]
Stahl 1997 {published data only}
- Stahl SM, Wingard P. Meta‐analysis of randomized, double‐blind, placebo‐controlled studies of mirtazapine vs amitriptyline. Sixth World Congress of Biological Psychiatry, Nice, France. June 22‐27. 1997.
Stein 1980 {published data only}
- Stein MK, Rickels K, Weise CC. Maintenance therapy with amitriptyline: a controlled trial. American Journal of Psychiatry 1980;137(3):370‐1. [DOI] [PubMed] [Google Scholar]
Taverna 1969 {published data only}
- Taverna P, Ferrari G. Comparative evaluation of nortriptyline, amitriptyline and of a placebo in the treatment of anxiety and depressive disorders. Minerva Medica 1969;60(49):2417‐31. [PubMed] [Google Scholar]
Taylor 1993 {published data only}
- Taylor S, McLean P. Outcome profiles in the treatment of unipolar depression. Behaviour Research and Therapy 1993;31(3):325‐30. [DOI] [PubMed] [Google Scholar]
Wallerstein 1967 {published data only}
- Wallerstein E, Dykyj R, Nodine JH. Fluphenazine and amitriptyline in the anxious depressed patient. American Journal of Psychiatry 1967;124(3):397‐8. [DOI] [PubMed] [Google Scholar]
Warner 1988 {published data only}
- Warner MD, Peabody CA, Whiteford HA, Hollister LE. Alprazolam as an antidepressant. Journal of Clinical Psychiatry 1988;49(4):148‐50. [PubMed] [Google Scholar]
Welner 1980 {published data only}
- Welner A, Welner Z, Robins E. Effect of tricyclic antidepressants on individual symptoms. Journal of Clinical Psychiatry 1980;41(9):306‐9. [CN‐00023343] [PubMed] [Google Scholar]
Wilson 1982 {published data only}
- Wilson PH. Combined pharmacological and behavioural treatment of depression. Behaviour Research and Therapy 1982;20(2):173‐84. [DOI] [PubMed] [Google Scholar]
Zis 1980 {published data only}
- Zis AP, Grof P, Webster MA, Goodwin FK. Prediction of relapse in recurrent affective disorder. Psychopharmacology Bulletin 1980;16:47‐9. [PubMed] [Google Scholar]
Zivkov 1995 {published data only}
- Zivkov M, De JGD. Org 3770 versus amitriptyline: a 6‐week randomized double‐blind multicentre trial in hospitalized depressed patients. Human Psychopharmacology 1995;10(3):173‐80. [Google Scholar]
References to studies awaiting assessment
Kahn 2008 {published data only}
- Kahn AY, Catterson ML, Preskorn SH. Double‐blind cross‐over study of mirtazapine, amitriptyline and placebo in patients with major depressive disorder. Directions in Psychiatry 2008;28:15‐20. [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM III). Washington DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd edition ‐ Revised (DSM‐III‐R). Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV). Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Barbui 2000
- Barbui C, Hotopf M, Freemantle M, Boynton J, Churchill R, Eccles MP. Selective serotonin reuptake inhibitors versus tricyclic and heterocyclic antidepressants: comparison of drug adherence. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD002791] [DOI] [PubMed] [Google Scholar]
Barbui 2008
- Barbui C, Furukawav TA, Cipriani A. Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re‐examination of published and unpublished data from randomized trials. Canadian Medical Association Journal 2008;178:296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2004
- Berger M. Psychische Erkrankungen. Klinik und Therapie. 2. München: Urban & Fischer, 2004. [Google Scholar]
Brozek 2008
- Brozek J, Oxman AD, Schünemann H. GRADEpro. Version 3.2 for Windows. http://ims.cochrane.org/revman/gradepro 2008.
Cipriani 2009
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373:746‐58. [DOI] [PubMed] [Google Scholar]
Cuijpers 2010
- Cuijpers K, Smit F, Bohlmeijer E, Hollen SE, Andersson G. Efficacy of cognitive‐behaviour therapy and other psychological therapies for adult depression: meta‐analytic study of publication bias. British Journal of Psychiatry 2010;196:173‐8. [DOI] [PubMed] [Google Scholar]
Davis 1993
- Davis JM, Wang Z, Janicak PG. A quantitative analysis of clinical drug trials for the treatment of affective disorders. Psychopharmacology Bulletin 1993;29:175‐81. [PubMed] [Google Scholar]
Der‐Simonian 1986
- Der‐Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455‐63. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Feighner 1972
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26:57‐63. [DOI] [PubMed] [Google Scholar]
Furukawa 2003
- Furukawa TA, McGuire H, Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analysis can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Gayetot 2007
- Gayetot D, Ansseau M, Triffaux JM. When depression does not end. Resistant depression: recent clinical and therapeutic aspects. Review Medical de Liege 2007;62(2):103‐11. [PubMed] [Google Scholar]
Gloaguen 1998
- Gloaguen V, Cottraux J, Cucherata M, Blackburn J. A meta‐analysis of the effects of cognitive therapy in depressed patients. Journal of Affective Disorders 1998;49:59‐71. [DOI] [PubMed] [Google Scholar]
GPRD 2011
- Personal communication. GPRD. http://www.gprd.com/ 2011.
Greenberg 1992
- Greenberg RP, Bornstein RF, Greenberg MD, Fisher S. A meta‐analysis of antidepressant outcome under "blinder" conditions. Journal of Consulting and Clinical Psychology 1992;60:664‐9. [DOI] [PubMed] [Google Scholar]
Guaiana 2007
- Guaiana G, Barbui C, Hotopf M. Amitriptyline versus other types of pharmacotherapy for depression. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004186.pub2] [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions ‐ ECDEU Assessment Manual for Psychopharmacology (DHEW Publ No ADM 76‐338). Revised. Rockville MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH, 1976. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale of depression. Journal of Neurology 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 1995
- Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. British Medical Journal 1995 Jan 28;310((6974)):221‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org 2008.
Kawakami 2007
- Kawakami N. Epidemiology of depressive disorders in Japan and the world. Nippon Rinsho 2007;65(9):1578‐84. [PubMed] [Google Scholar]
Kirsch 2008
- Kirsch I, Deacon BJ, Huedo‐Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta‐analysis of data submitted to the Food and Drug Administration. PloS Medicine 2008;5:e45. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Davis JM, Engel RR, Kane JM, Kissling W, Engel RR. Defining 'response' in antipsychotic drug trials: recommendations for the use of scale‐derived cutoffs. Neuropsychopharmacology 2007;32:1903‐10. [DOI] [PubMed] [Google Scholar]
Leucht 2012
- Leucht S, Hierl S, Kissling W, Dold M, Davis JM. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta‐analyses. British Journal of Psychiatry 2012;200(2):97‐106. [DOI] [PubMed] [Google Scholar]
Lohse 2009
- Lohse MJ, Müller‐Oerlinghausen B. Psychopharmaka. In: Schwabe U, Pfaffrath D editor(s). Arzneiverordnungsreport 2009. Heidelberg: Springer, 2009:820‐64. [Google Scholar]
Luborsky 1962
- Luborsky L. Clinician's judgments of mental health. Archives of General Psychiatry 1962;7:407‐17. [DOI] [PubMed] [Google Scholar]
McAllister‐Williams 2008
- McAllister‐Williams RH. Do antidepressants work? A commentary on “Initial severity and antidepressant benefits: a meta‐analysis of data submitted to the Food and Drug Administration” by Kirsch et al. Evid Based Mental Health 2008;11(3):66‐8. [DOI: 10.1136/ebmh.11.3.66] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Moncrieff 2004
- Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003012.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Spitzer 1978
- Spitzer RL, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35:773‐82. [DOI] [PubMed] [Google Scholar]
Storosum 2001
- Storosum JG, Elferink AJ, Zwieten BJ, Brink W, Gersons BP, vanStrik R, et al. Short‐term efficacy of tricyclic antidepressants revisited: a meta‐analytic study. European Neuropsychopharmacology 2001;11:173‐80. [DOI] [PubMed] [Google Scholar]
Trikalinos 2004
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. EU‐PSI project. Effect sizes in cumulative meta‐analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology 2004;57:1124‐30. [DOI] [PubMed] [Google Scholar]
Turner 2008
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine 2008;358:252‐60. [DOI] [PubMed] [Google Scholar]
Walsh 2002
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 2002;287:1840‐7. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Centre, 1993. [Google Scholar]
WHO 1978
- World Health Organization. The Ninth Revision of the International Classification of Diseases and Related Health Problems (ICD‐9). Geneva: World Health Organization, 1978. [Google Scholar]
WHO 1992
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10). Geneva: World Health Organization, 1992. [Google Scholar]
WHO 2005
- World Health Organization. The European Health Report 2005. Geneva: World Health Organization, 2005. [Google Scholar]
Wing 1994
- Wing J. Measuring Mental Health Outcomes: a Perspective from the Royal College of Psychiatrists. London: BMJ Publishing, 1994. [Google Scholar]
Zimmerman 2004
- Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery‐Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. Journal of Psychiatry Research 2004;38:577‐82. [DOI] [PubMed] [Google Scholar]


