Abstract
Background
Venous thromboembolism (VTE) is a preventable medical condition which has substantial impact on patient morbidity, mortality, and disability. Unfortunately, adherence to the published best practices for VTE prevention, based on patient centered outcomes research (PCOR), is highly variable across U.S. hospitals, which represents a gap between current evidence and clinical practice leading to adverse patient outcomes.
This gap is especially large in the case of traumatic brain injury (TBI), where reluctance to initiate VTE prevention due to concerns for potentially increasing the rates of intracranial bleeding drives poor rates of VTE prophylaxis. This is despite research which has shown early initiation of VTE prophylaxis to be safe in TBI without increased risk of delayed neurosurgical intervention or death. Clinical decision support (CDS) is an indispensable solution to close this practice gap; however, design and implementation barriers hinder CDS adoption and successful scaling across health systems. Clinical practice guidelines (CPGs) informed by PCOR evidence can be deployed using CDS systems to improve the evidence to practice gap. In the Scaling AcceptabLE cDs (SCALED) study, we will implement a VTE prevention CPG within an interoperable CDS system and evaluate both CPG effectiveness (improved clinical outcomes) and CDS implementation.
Methods
The SCALED trial is a hybrid type 2 randomized stepped wedge effectiveness-implementation trial to scale the CDS across 4 heterogeneous healthcare systems. Trial outcomes will be assessed using the RE2-AIM planning and evaluation framework. Efforts will be made to ensure implementation consistency. Nonetheless, it is expected that CDS adoption will vary across each site. To assess these differences, we will evaluate implementation processes across trial sites using the Exploration, Preparation, Implementation, and Sustainment (EPIS) implementation framework (a determinant framework) using mixed-methods. Finally, it is critical that PCOR CPGs are maintained as evidence evolves. To date, an accepted process for evidence maintenance does not exist. We will pilot a “Living Guideline” process model for the VTE prevention CDS system.
Discussion
The stepped wedge hybrid type 2 trial will provide evidence regarding the effectiveness of CDS based on the Berne-Norwood criteria for VTE prevention in patients with TBI. Additionally, it will provide evidence regarding a successful strategy to scale interoperable CDS systems across U.S. healthcare systems, advancing both the fields of implementation science and health informatics.
Trial registration
Clinicaltrials.gov – NCT05628207. Prospectively registered 11/28/2022, https://classic.clinicaltrials.gov/ct2/show/NCT05628207.
Keywords: Traumatic brain injury, Prophylaxis, Venous thromboembolism, Stepped wedge, Implementation science, Mixed methods, Clinical decision support, Randomized controlled trial, Learning health system, Health informatics
Contributions to the Literature.
This paper provides a study protocol for a new and novel stepped wedge study variation which includes external control sites to take into account external influences on the uptake of traumatic brain injury guidelines nationally
This paper provides a study design for one of the largest trauma pragmatic trials in the U.S. of 9 heterogenous hospitals
This study is also unique and first-in-kind feature as the guideline may change over time during the study due to the “living” nature of the guideline being implemented.
Introduction
Venous thromboembolism (VTE) is a preventable complication of traumatic brain injury (TBI), which has a substantial impact on patient morbidity, mortality, disability. It is also associated with significant economic burden > $1.5 billion per year [1, 2]. VTE is considered a preventable medical condition in the majority of cases [2, 3]. Unfortunately, adherence with patient centered outcomes research (PCOR)-informed VTE prevention best practices is highly variable and often poor across U.S. hospitals. Compliance with best practice is especially relevant in the case of TBI as 54% of TBI patients will develop a VTE if they do not receive appropriate anticoagulation [4]. The delivery of appropriate VTE prophylaxis to TBI patients is such an important quality measure that adherence is tracked nationally and benchmarked by the American College of Surgeons Trauma Quality Improvement Program (ACS-TQIP) [5]. We have previously shown that instituting a hospital-wide VTE prevention initiative modeled after the Berne-Norwood criteria for VTE prophylaxis in TBI was associated with significantly increased compliance with VTE-related process and improved outcome metrics [6]. Specifically, we observed improved adherence with the Berne-Norwood criteria [7, 8], reduced time to initiation of VTE prophylaxis, and reduced VTE events [9]. Multiple studies have shown that VTE prophylaxis in trauma patients not only reduces VTE events, but also significantly reduces mortality [10]. We noted the same reduction in mortality for TBI patients following the initiation of a VTE prophylaxis guideline for patients with TBI [11]. Unfortunately, despite widely published PCOR-informed best practice, nationally there is reluctance to initiate VTE prevention due to concerns for progression of intracranial hemorrhage. This is despite research which has shown early initiation of VTE prophylaxis to be safe in TBI without increased risk of delayed neurosurgical intervention or death [12–16].
Since approximately 40% of TBI patients do not receive DVT prophylaxis in a timely manner, there is a critical and timely need to close the gap between current PCOR evidence and clinical practice. [17–23]. Clinical decision support (CDS) systems are an indispensable solution to close this practice gap; however, design and implementation barriers hinder CDS adoption [24, 25]. Another significant challenge to the implementation of CDS is that health information technology (IT) needs a common language for PCOR evidence to translate it into practice across multiple organizations [26]. Because of these challenges, we will deploy CDS using fast healthcare interoperability resources (FHIR) standards to rapidly implement PCOR evidence into practice [27, 28]. We hypothesize that, FHIR standards will reduce CDS development and maintenance costs, increase PCOR uptake in rural and other underserved sites, and speed the development timeline to build a comprehensive suite of CDS for PCOR evidence [29].
Few studies have investigated specific barriers to and facilitating factors for adoption of interoperable FHIR-based CDS [30]. For example, many current studies investigating barriers and facilitators for interoperable CDS are limited to expert opinion [30, 31] or lack a formal implementation science framework-guided investigation [32, 33]. Barriers to and facilitating factors for adoption of interoperable CDS following real-life implementation and multicenter scaling guided by validated implementation science frameworks should be rigorously investigated. This study will facilitate comprehensive exploration of clinician and environmental (internal and external) contextual elements that influence interoperable CDS implementation success. In this study, we will scale and assess the effectiveness of a CDS system for a VTE prophylaxis guideline in patients with TBI and evaluate implementation across 9 sites within 4 U.S. trauma systems.
Methods
Study aims and implementation framework
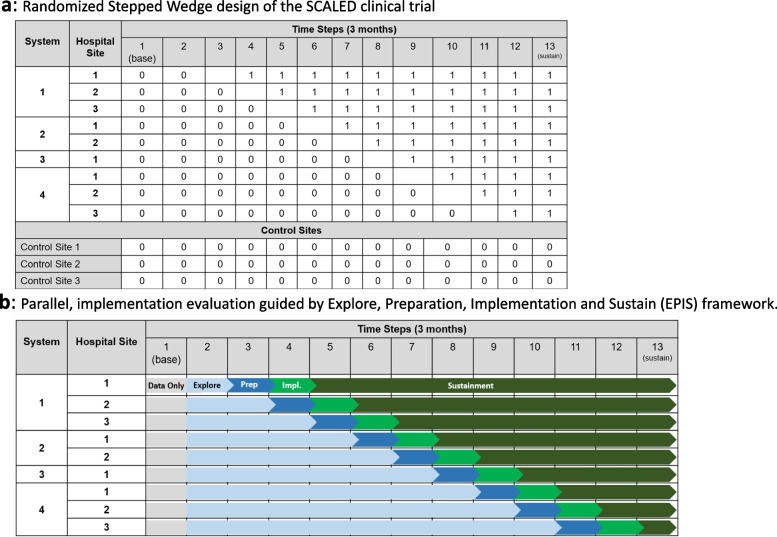
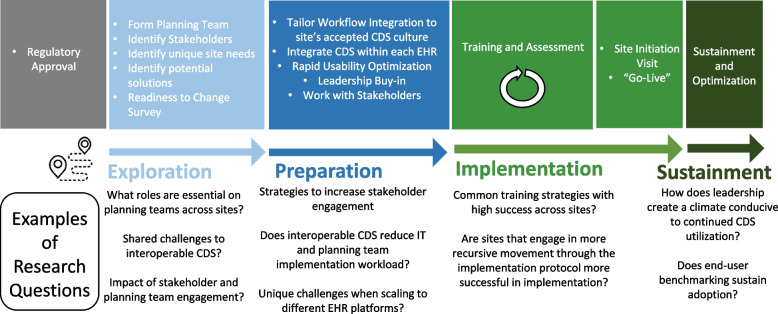
This trial consists of a stepped wedge hybrid effectiveness-implementation trial to scale the CDS system across 4 trauma systems and in parallel evaluate implementation strategy guided by the Exploration, Preparation, Implementation, and Sustainment (EPIS) implementation framework (Fig. 1a) [34]. We anticipate variability in CDS adoption across sites during the implementation trial. This variation represents a unique opportunity to study implementation at each site and understand what strategies, system factors, and engagement of specific stakeholders are associated with improved CDS adoption. We will rigorously evaluate each implementation phase, guided by The EPIS Implementation Framework [34], our determinant framework (Fig. 1b). We will apply the EPIS framework to guide assessment of implementation phases, barriers, and facilitators (Fig. 2) [34]. EPIS comprises 16 constructs over 4 domains (outer context, inner context, bridging factors, and innovation factors). We selected EPIS as our determinant framework as it includes clearly delineated implementation stages and allows for examination of change at multiple levels, across time, and through phases that build toward implementation. While EPIS was initially developed for implementation in public service, it has since been translated to healthcare, especially for complex multi-institutional healthcare interventions [34–36].
Fig. 1.
a Randomized Stepped Wedge design of the SCALED clinical trial. b Parallel, implementation evaluation guided by Explore, Preparation, Implementation and Sustain (EPIS) framework
Fig. 2.
Implementation evaluation across study sites
Trial overview, setting, and inclusion/exclusion criteria
This trial will be conducted at 4 healthcare systems with 1–3 hospitals per system and is projected to occur over a 3 to 4-year period. The trial uses a randomized stepped-wedge design to scale an interoperable CDS system for the Berne-Norwood TBI CPG. Figure 1a provides a schematic for the trial design. The order of health systems and sites will be randomly determined. This study will include a heterogeneous number of hospitals by trauma verification status, electronic health record (EHR) platform, bed size, and setting (Table 1). Our target population is adult patients admitted with an acute TBI defined as International Classification of Disease 10 Clinical Modification (ICD-10-CM): S06.1 – S06.9 or S06.A. Patients who die within 24 h of hospital admission and patients documented as “comfort cares” during the first 72 h of hospitalization will be excluded, as they would have a limited opportunity to receive adherence with the Berne-Norwood criteria. Additionally, patients with a pre-existing VTE or inferior vena cava (IVC) filter at the time of admission, and patients with a mechanical heart valve or ventricular assist device will be excluded from final analysis.
Table 1.
Implementation sites for SCALED Trial
| Setting | Trauma Level | EHR Platform | TBI patients annually | Bed Size | |
|---|---|---|---|---|---|
| M Health Fairview | |||||
| University of Minnesota Medical Center | University | 2 | Epic | 180 | 828 |
| Southdale Hospital | Community | 3 | Epic | 160 | 334 |
| Ridges Hospital | Community | 3 | Epic | 155 | 171 |
| Indiana University (IU) Health | |||||
| IU Health Methodist Hospital | University | 1 | Cerner | 520 | 625 |
| IU Health Bloomington | Community/Rural | 3 | Cerner | 203 | 297 |
| Geisinger Health | |||||
| Geisinger Medical Center | Rural | 1 | Epic | 942 | 552 |
| Geisinger Community Medical Center | Community | 2 | Epic | 292 | 297 |
| Geisinger Wyoming Valley | Rural | 2 | Epic | 211 | 300 |
| University of California – Davis | |||||
| UC-Davis | University | 1 | Epic | 1165 | 625 |
This study will also include up to 3 control sites (Fig. 1a), a feature not typically included with historic stepped-wedge trial designs, which will strengthen our ability to understand external influences on the study findings. These control sites, which do not receive the CDS intervention and do not have any planned initiatives around guideline implementation, will allow the study to assess baseline adherence and variation in clinical practice over the study period.
CDS Intervention
TBI diagnosis upon admission will activate an interoperable CDS system leveraging the Stanson Health (Charlotte, NC) CDS platform [37], which is being expanded to include interoperable offerings for TBI VTE prophylaxis. This system provides a knowledge representation framework to faithfully express the intent of the Berne-Norwood prevention criteria computationally (Table 2). The interoperable FHIR data standard will be used for bi-directional data transfer between each site’s EHR and the CDS platform. Workflow integration includes a combination of both passive and interruptive provider and trauma system leader information and “nudges”. Table 2 represents the Standards-based, Machine-readable, Adaptive, Requirements-based, and Testable (SMART) L2 layer [38] of the Berne-Norwood criteria.
Table 2.
Traumatic brain injury (TBI) venous thromboembolism (VTE) prevention clinical practice guideline. Modified Berne-Norwood criteria for VTE risk in TBI patients
| Low Risk | Moderate Risk | High Risk |
|---|---|---|
| • No moderate or high risk criteria | • Subdural or epidural hematoma > 8 mm | • Placement of an intracranial pressure monitor |
| • Contusion or intraventricular hemorrhage > 2 cm | • Craniotomy | |
| • Multiple contusions per lobe | • Evidence of progression at 72 h | |
| • Subarachnoid hemorrhage with abnormal CT | ||
| • Evidence of progression at 24 h | ||
| • Initiate pharmacologic prophylaxis if repeat head computed tomography (CT) stable at 24 h | • Initiate pharmacologic prophylaxis if head CT stable at 72 h | • Consider placement of an inferior vena cava filter |
CDS user-centered design
We will complete a rapid cycle CDS evaluation to optimize CDS workflow integration by conducting a user-driven simulation and expert-driven heuristic usability optimization as we have previously done [39]. For rapid cycle CDS evaluation, multidisciplinary trauma end-user “teams” will complete up to 3 scenarios designed to represent various extremes in TBI VTE prevention decision making. Simulation usability testing will be overseen by usability experts, who will catalogue usability issues that arise during simulation. Via consensus ranking, the development and planning teams will rank usability issues from 0 (cosmetic) to 5 (usability catastrophe). Using 10 predefined heuristics for usability design [40], we will conduct a heuristic evaluation of the CDS, then catalogue and rank usability issues. These results will inform CDS application design, optimized for TBI workflow integration.
Implementation strategy
Following CDS development, our healthcare system relies on a time-tested approach for the implementation and scaling of user-centered CDS: this approach is called the Scaling AcceptabLE cDs (SCALED) Strategy [41]. This framework integrates multiple evidence-based implementation strategies (Table 3).
Table 3.
Clinical decision support SCALED implementation strategy
| (1) Development of a local change team led by local champions at each implementation site |
|---|
| (2) Multidisciplinary Stakeholder engagement and training to optimize buy-in |
| (3) Readiness assessment and analysis |
| (4) Rapid cycle user-centered workflow and experience optimization at each site |
| (5) Multifaceted end-user training strategy |
| (6) Site Initiation Visit and “Go Live” Launch Event |
| (7) Maintenance elements as necessary (i.e. Booster Education sessions, Audit and Feedback) |
Study outcomes
The primary implementation outcome is patient-level adherence with the CPG: Specifically, did the patient received guideline-concordant care? Adherence will be measured as an all-or-none measure (binary endpoint at the encounter/patient-level). Thus, if a patient is low-risk for TBI progression, by 24 h they should have risk-specific VTE prevention ordered; if they receive this after 24 h, or if they receive the intermediate risk VTE prevention regimen, this would be deemed non-adherent. The primary effectiveness outcome is VTE (binary endpoint at the patient-encounter level). Safety outcomes evaluated include: TBI progression, in-hospital mortality, and bleeding events. A secondary hypothesis is that as the trial scales to additional sites, iterative implementations will be more efficient (reduced implementation time) and more effective (improved adoption). Secondary hypotheses will be evaluated using the RE2-AIM framework [42, 43] and are displayed in Table 4.
Table 4.
RE2-AIM implementation secondary outcomes
| Implementation Outcome | Measurement | Level |
| Reach | # of total patients that the CDS activated on / # of total eligible TBI patients | Patient – Level |
| Effectiveness | • VTE and Bleeding Event Rates (unadjusted and adjusted) | Patient—Level |
| • Transfusion Requirements (unadjusted and adjusted) | ||
| • Mortality (unadjusted and adjusted) | ||
| Equity | • Number of patients that receive adherence across demographic groups (race, ethnic, sex, and age) | Patient – Level |
| • Effectiveness outcomes (VTE, bleeding event rate, and mortality) across demographic groups | ||
|
Adoption Implementation |
Number of patients that received adherence with the CPG per site or provider/ number of eligible patients per site or provider | Hospital, Provider-Level |
| - All-or-none by implementation site | ||
| - All-or-none by trauma provider | ||
| - Adoption at hospital level by risk level (low, medium, high) | ||
| Fidelity to the CPG: | Patient—Level | |
| • Appropriate agent used (e.g. enoxaparin, unfractionated heparin) | ||
| • Appropriate dose delivered | ||
|
• Dose delivered at appropriate time after admission CDS provider training proficiency by implementation site |
||
| CDS order bundle utilization by implementation site | ||
| CDS IT integration time (in hours) | ||
| Maintenance | • Percent of patients that received the CPG at 6 months post implementation | Patient / Provider Level |
| • Provider adoption 6 months post implementation | ||
| • Average provider score on guideline proficiency quiz (compared to baseline) |
Clinical trial data collection methods
Data sources used in this trial include the Stanson Health CDS eCaseReport and site trauma registry. The eCaseReport is a living registry of all patients, and their associated clinical trial data elements, that were eligible for the CDS. All sites also maintain a trauma registry adhering to the National Trauma Data Standards [44], a requirement for ACS trauma center verification. This dataset is manually annotated by trained clinical abstractors. Data will be sent to the biostatistical team at 6-month intervals. Control and pre-implementation sites will provide their trauma registry in addition to supplemental standards-based EHR extraction of clinical trial data elements or manual abstraction. A data dictionary has been created for the study and will be made available on the trial webpage.
Multiple methods evaluation of implementation success at each EPIS phase
Survey instruments will be prepared using Likert-type scales. Outcomes will be calculated based on scoring guides for the following validated scales: Program Sustainability Assessment Tool (PSAT) [45], Clinical Sustainability Assessment Tool (CSAT) [46], Implementation Leadership Scale (ILS) [47], and Evidenced-based Practice Attitude Scale-36 (EBPAS-36) [48]. Two scales do not have scoring rubrics: the Organizational Readiness for Change Questionnaire [49, 50] and the Normalization Measure Development (NoMAD) Questionnaire [51–53]. Since both of these scales group questions into constructs, they will be analyzed by generating mean Likert scores and standard deviations per construct, and a mean across constructs, at each of the four implementation phases [54].
To deeply investigate barriers and facilitators of successful implementation, semi-structured qualitative interviews of key personnel (clinical leadership and end-users, IT leadership and staff) will be conducted at each of the 4 implementation phases. Studies suggest saturation of new ideas occurs after approximately 12 interviews [55]. Additional samples will be added as needed if thematic saturation is not achieved. Following informed consent, interviews will be performed by a trained qualitative research assistant, audio recorded, and transcribed verbatim. An interview guide, informed by the EPIS framework, was developed to collect key informant experiences with CDS implementation with a focus on inner and outer context factors [56]. A hybrid approach, primarily deductive and secondarily inductive, approach will be applied. All interviews will be independently double-coded and coding discrepancies will be resolved through discussion. A descriptive thematic analysis approach [57] will be used to characterize the codes into themes and sub-themes representing the barriers and facilitators to implementation success.
Results for all instruments will be primarily stratified according to site implementation success at each study phase. Additional stratifications may include respondent role, discipline, and hospital system. Bar charts displaying mean survey domains with integrative quotations from the qualitative analysis will be used to facilitate data visualization and understanding of key themes representing barriers and facilitators to successful CDSS implementation.
Statistical analysis
Mixed-effects logistic regression models will be fit to test whether or not CDS implementation changes the likelihood of a VTE event during TBI admission (effectiveness outcome) and the likelihood that the clinical guideline was followed (implementation outcome). The models for these outcomes include fixed-effects for month (when available, to account for secular trends) and an indicator variable for whether the center had the CDS integrated in the EHR. The primary test statistic will be a Wald test of the coefficient for this treatment indicator. We will include random center-specific intercepts to account for correlation within center. Assuming there are 9 sites enrolled with an average of 400 TBI admissions per year and the typical site has between 20%-40% adherence to the clinical guidelines, we will have > 80.0% and > 99.9% power to detect a 5 and 10 percentage point increase in the adherence. Similarly, assuming the typical site has between a VTE event rate of 5–6%, we will have > 80.0% power to detect a 40%-50% reduction in VTE consistent with our published data [11].
Study oversight
This study is overseen by the University of Minnesota Surgical Clinical Trials Office and by an independent Data Safety Monitoring Board (DSMB). Even though this intervention is deploying a TBI clinical guideline that is currently considered best practice, we believe the addition of a DSMB will improve trial safety, data quality, and trial integrity [58]. DSMB membership will be independent from the study investigators and will consist of 3 members including: 1 trauma surgeon, 1 informaticist, and 1 statistician. Annual reports including data from all sites, including control sites, will be shared with the DSMB to assure timely monitoring of safety and data quality. The trial will not be stopped early in the event of CDS efficacy because a critical secondary outcome focuses on studying implementation and effectiveness over time.
VTE guideline monitoring and maintenance
Given the potential for a changing evidence-base, it is possible that best practice VTE prevention guidance may change during the study period or afterwards. A critical element in improving adherence with PCOR evidence is updating guidance based on this evidence – in this study, this requires ensuring that the CDS system remains current.
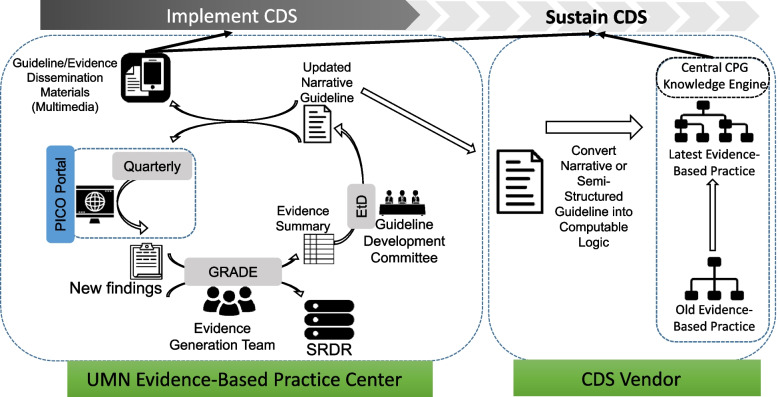
We will pilot a model for producing and maintaining TBI VTE prophylaxis 'Living Guidance and CDS' to ensure that the CDS remains current (Fig. 3). The University of Minnesota Evidence-based Practice Center (EPC) Evidence Generation team will conduct and maintain a “living” systematic review. Systematic review data will be uploaded to the AHRQ’s Systematic Review Data Repository (SRDR). “Living” implies that every 6 months the EPC team will evaluate and synthesize new evidence related to TBI VTE prophylaxis, update the existing systematic review and deliver it to a multi-stakeholder Guideline Committee. The Guideline Committee will then use the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) evidence-to-decision (EtD) framework to develop VTE prophylaxis guidelines for patients with TBI [59–61]. A computational representation of these guidelines will be updated and maintained within the CDS platform by Stanson Health, the CDS Vendor.
Fig. 3.
Pilot process for “Living Guideline”
Spreading successful results beyond study sites
The ultimate goal of this study is to spread successful CDS tools and strategies to broadly improve TBI VTE-related care processes and outcomes. The research outlined above will surface sharable insights about what information needs to be presented to which people in what formats through what channels at what times to reliably deliver guideline-based care – i.e., specific instantiations of the “CDS 5 Rights Framework” applied to this target [62]. We will use Health Service Blueprint tools to describe our recommended implementation approaches; these tools are being applied in an increasing number of public and private care delivery organizations as a structured approach to ‘get the CDS 5 Right right’ for various improvement targets. We will further adapt and apply Health Service Blueprint foundations supported by VA and AHRQ [63] to capture VTE care transformation guidance in Health Service Blueprint tooling [64]. Presenting recommended CDS-enabled workflow, information flow – as well as and related implementation considerations and broader healthcare ecosystem implications – in this structured format will help organizations beyond the initial study participants put study results into action efficiently and effectively.
Discussion
In this paper, we present the protocol for the SCALED trial, a stepped-wedge cluster randomized trial of a CDS intervention to improve adherence with VTE prevention best practices for patients with TBI. As a hybrid type 2 trial, this study will evaluate both implementation and effectiveness outcomes. In addition to investigating effectiveness, we will also be able to provide insight into the implementation challenges for deploying interoperable CDS across heterogenous health systems. In our pilot study [9], while patients who received guideline-concordant care had significantly improved outcomes, we noted that not all patients receive guideline concordant care following implementation. Additionally, best strategies for scaling interoperable CDS systems are poorly studied. Thus, this study represents one of the earliest implementation evaluations of scaling interoperable CDS systems across heterogeneous health systems.
This study has several strengths. First, it will rigorously test implementation of a CPG for VTE prevention across 9 U.S. trauma centers using a multi-faceted CDS platform supporting both passive and interruptive decision support. Second, it will rigorously investigate scalable and interoperable CDS strategies to deploy CPGs. Third, this study leverages a centralized eCaseReport generated by the CDS system, a solution which can drive data collection for future pragmatic trials. Importantly, this study takes place at trauma centers which are geographically distinct, utilize different EHR vendors, include both ACS-verified level 1 through level 3 trauma centers, and include rural, community, and university-based trauma centers. In addition to helping spread recommended care transformation strategies beyond additional study sites, documenting these approaches in Health Service Blueprint tools will also support creation of learning communities for sharing, implementing, and enhancing these strategies.
This study also has limitations. First, we are only investigating 4 trauma systems which already have fairly advanced informatics divisions and experience implementing interoperable CDS systems. Thus, these findings may not be broadly applicable to health systems with less informatics experience and expertise. Second, we are only investigating implementation across two EHR vendors: Epic and Cerner, thus these findings may not be applicable to health systems with different EHR vendors such as Meditech or Allscripts. However, the Health Service Blueprint implementation strategy representations should still enable users of other systems to glean valuable insights about components of the transformation approach less dependent on specific EHRs used.
In summary, this study will implement and scale a CDS-enabled care transformation approach across a diverse collaborative CDS community, serving as an important demonstration of this critical healthcare challenge. We will integrate lessons learned for a planned national scaling in collaboration with U.S. trauma societies. Finally, we will pilot an approach for the “Living Guideline” and use that to maintain evidenced-based decision logic within CDS platforms.
Authors’ contributions
CT conceived and jointly designed the study protocol and helped write and critically revise this protocol paper, SS conceived and jointly designed the study protocol and helped write and critically revise this protocol paper, DV jointly designed the study protocol and helped write and critically revise this protocol paper, LS jointly designed the study protocol and helped write and critically revise this protocol paper, CS jointly designed the study protocol and helped write and critically revise this protocol paper, EH jointly designed the study protocol and helped write and critically revise this protocol paper, SS jointly designed the study protocol and helped write and critically revise this protocol paper, CM jointly designed the study protocol and helped write and critically revise this protocol paper, RR jointly designed the study protocol and helped write and critically revise this protocol paper, VP jointly designed the study protocol and helped write and critically revise this protocol paper, PJ jointly designed the study protocol and helped write and critically revise this protocol paper, NL jointly designed the study protocol and helped write and critically revise this protocol paper, TT jointly designed the study protocol and helped write and critically revise this protocol paper, JO jointly designed the study protocol and helped write and critically revise this protocol paper, DT jointly designed the study protocol and helped write and critically revise this protocol paper, DV jointly designed the study protocol and helped write and critically revise this protocol paper, RC jointly designed the study protocol and helped write and critically revise this protocol paper, MB jointly designed the study protocol and helped write and critically revise this protocol paper, GM conceived and jointly designed the study protocol and helped write and critically revise this protocol paper.
Funding
This research was supported by the Agency for Healthcare Research and Quality (AHRQ), grant R18HS028583, the University of Minnesota Center for Learning Health System Sciences – a partnership between the University of Minnesota Medical School and the School of Public Health. The authors have no other conflicts of interest.
Availability of data and materials
Following trial completion data will be made available upon request through the University of Minnesota Data Repository.
Declarations
Ethics approval and consent to participate
This study protocol was given the determination of “Exempt” as secondary research for which consent is not required. The Mixed Methods investigation was given the determination of “Not Human Research” as a quality improvement activity.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3(8):1611–7. 10.1111/j.1538-7836.2005.01415.x [DOI] [PubMed] [Google Scholar]
- 2.Yorkgitis BK, Berndtson AE, Cross A, Kennedy R, Kochuba MP, Tignanelli C, Tominaga GT, Jacobs DG, Marx WH, Ashley DW, Ley EJ, Napolitano L, Costantini TW. American Association for the Surgery of Trauma/American College of Surgeons-Committee on Trauma Clinical Protocol for inpatient venous thromboembolism prophylaxis after trauma. J Trauma Acute Care Surg. 2022;92(3):597–604. 10.1097/TA.0000000000003475 [DOI] [PubMed] [Google Scholar]
- 3.Nicholson M, Chan N, Bhagirath V, Ginsberg J. Prevention of Venous Thromboembolism in 2020 and Beyond. J Clin Med. 2020;9(8):2467. 10.3390/jcm9082467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–6. 10.1056/NEJM199412153312401 [DOI] [PubMed] [Google Scholar]
- 5.Nathens AB, Cryer HG, Fildes J. The American College of Surgeons Trauma Quality Improvement Program. Surg Clin North Am. 2012;92(2):441–54, x−xi. 10.1016/j.suc.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, Dudley RA, Tignanelli CJ. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8(6):544–6. 10.1016/S2213-2600(20)30226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelan HA, Eastman AL, Madden CJ, Aldy K, Berne JD, Norwood SH, Scott WW, Bernstein IH, Pruitt J, Butler G, Rogers L, Minei JP. TBI risk stratification at presentation: a prospective study of the incidence and timing of radiographic worsening in the Parkland Protocol. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S122–7. 10.1097/TA.0b013e3182606327 [DOI] [PubMed] [Google Scholar]
- 8.Pastorek RA, Cripps MW, Bernstein IH, Scott WW, Madden CJ, Rickert KL, Wolf SE, Phelan HA. The Parkland Protocol’s modified Berne-Norwood criteria predict two tiers of risk for traumatic brain injury progression. J Neurotrauma. 2014;31(20):1737–43. 10.1089/neu.2014.3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tignanelli CJ, Gipson J, Nguyen A, Martinez R, Yang S, Reicks PL, Sybrant C, Roach R, Thorson M, West MA. Implementation of a Prophylactic Anticoagulation Guideline for Patients with Traumatic Brain Injury. Jt Comm J Qual Patient Saf. 2020;46(4):185–91. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs BN, Cain-Nielsen AH, Jakubus JL, Mikhail JN, Fath JJ, Regenbogen SE, Hemmila MR. Unfractionated heparin versus low-molecular-weight heparin for venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg. 2017;83(1):151–8. 10.1097/TA.0000000000001494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tignanelli CJ, Silverman GM, Lindemann EA, Trembley AL, Gipson JC, Beilman G, Lyng JW, Finzel R, McEwan R, Knoll BC, Pakhomov S, Melton GB. Natural language processing of prehospital emergency medical services trauma records allows for automated characterization of treatment appropriateness. J Trauma Acute Care Surg. 2020;88(5):607–14. 10.1097/TA.0000000000002598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Gearhart MM, Zurick A, Zuccarello M, James L, Luchette FA. Preliminary report on the safety of heparin for deep venous thrombosis prophylaxis after severe head injury. J Trauma. 2002;53(1):38–42; discussion 3. 10.1097/00005373-200207000-00008 [DOI] [PubMed] [Google Scholar]
- 13.Cothren CC, Smith WR, Moore EE, Morgan SJ. Utility of once-daily dose of low-molecular-weight heparin to prevent venous thromboembolism in multisystem trauma patients. World J Surg. 2007;31(1):98–104. 10.1007/s00268-006-0304-1 [DOI] [PubMed] [Google Scholar]
- 14.Norwood SH, Berne JD, Rowe SA, Villarreal DH, Ledlie JT. Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J Trauma. 2008;65(5):1021–6; discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 15.Scudday T, Brasel K, Webb T, Codner P, Somberg L, Weigelt J, Herrmann D, Peppard W. Safety and efficacy of prophylactic anticoagulation in patients with traumatic brain injury. J Am Coll Surg. 2011;213(1):148–53; discussion 53-4. 10.1016/j.jamcollsurg.2011.02.027 [DOI] [PubMed] [Google Scholar]
- 16.Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, Neal M, Pirouzmand F, Nathens AB. Timing of Pharmacologic Venous Thromboembolism Prophylaxis in Severe Traumatic Brain Injury: A Propensity-Matched Cohort Study. J Am Coll Surg. 2016;223(4):621-31e5. 10.1016/j.jamcollsurg.2016.06.382 [DOI] [PubMed] [Google Scholar]
- 17.Lau R, Stevenson F, Ong BN, Dziedzic K, Eldridge S, Everitt H, Kennedy A, Kontopantelis E, Little P, Qureshi N, Rogers A, Treweek S, Peacock R, Murray E. Addressing the evidence to practice gap for complex interventions in primary care: a systematic review of reviews protocol. BMJ Open. 2014;4(6): e005548. 10.1136/bmjopen-2014-005548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tignanelli CJ, Vander Kolk WE, Mikhail JN, Delano MJ, Hemmila MR. Noncompliance with American College of Surgeons Committee on Trauma recommended criteria for full trauma team activation is associated with undertriage deaths. J Trauma Acute Care Surg. 2018;84(2):287–94. 10.1097/TA.0000000000001745 [DOI] [PubMed] [Google Scholar]
- 19.Robbins AJ, Ingraham NE, Sheka AC, Pendleton KM, Morris R, Rix A, Vakayil V, Chipman JG, Charles A, Tignanelli CJ. Discordant Cardiopulmonary Resuscitation and Code Status at Death. J Pain Symptom Manage. 2021;61(4):770–780.e1. [DOI] [PMC free article] [PubMed]
- 20.Tignanelli CJ, Watarai B, Fan Y, Petersen A, Hemmila M, Napolitano L, Jarosek S, Charles A. Racial Disparities at Mixed-Race and Minority Hospitals: Treatment of African American Males With High-Grade Splenic Injuries. Am Surg. 2020;86(5):441–9. 10.1177/0003134820918262 [DOI] [PubMed] [Google Scholar]
- 21.Tignanelli CJ, Rix A, Napolitano LM, Hemmila MR, Ma S, Kummerfeld E. Association Between Adherence to Evidence-Based Practices for Treatment of Patients With Traumatic Rib Fractures and Mortality Rates Among US Trauma Centers. JAMA Netw Open. 2020;3(3): e201316. 10.1001/jamanetworkopen.2020.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliphant BW, Tignanelli CJ, Napolitano LM, Goulet JA, Hemmila MR. American College of Surgeons Committee on Trauma verification level affects trauma center management of pelvic ring injuries and patient mortality. J Trauma Acute Care Surg. 2019;86(1):1–10. 10.1097/TA.0000000000002062 [DOI] [PubMed] [Google Scholar]
- 23.Tignanelli CJ, Wiktor AJ, Vatsaas CJ, Sachdev G, Heung M, Park PK, Raghavendran K, Napolitano LM. Outcomes of Acute Kidney Injury in Patients With Severe ARDS Due to Influenza A(H1N1) pdm09 Virus. Am J Crit Care. 2018;27(1):67–73. 10.4037/ajcc2018901 [DOI] [PubMed] [Google Scholar]
- 24.Khairat S, Marc D, Crosby W, Al SA. Reasons For Physicians Not Adopting Clinical Decision Support Systems: Critical Analysis. JMIR Med Inform. 2018;6(2): e24. 10.2196/medinform.8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones EK, Ninkovic I, Bahr M, Dodge S, Doering M, Martin D, Ottosen J, Allen T, Melton GB, Tignanelli CJ. A novel, evidence-based, comprehensive clinical decision support system improves outcomes for patients with traumatic rib fractures. J Trauma Acute Care Surg. 2023;95(2):161–71. 10.1097/TA.0000000000003866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcos M, Maldonado JA, Martinez-Salvador B, Bosca D, Robles M. Interoperability of clinical decision-support systems and electronic health records using archetypes: a case study in clinical trial eligibility. J Biomed Inform. 2013;46(4):676–89. 10.1016/j.jbi.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 27.FHIR Clinical Guidelines. http://build.fhir.org/ig/HL7/cqf-recommendations/. Accessed 14 Sep 2021.
- 28.Mandel JC, Kreda DA, Mandl KD, Kohane IS, Ramoni RB. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc. 2016;23(5):899–908. 10.1093/jamia/ocv189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg HS, Paterno MD, Rocha BH, Schaeffer M, Wright A, Erickson JL, Middleton B. A highly scalable, interoperable clinical decision support service. J Am Med Inform Assoc. 2014;21(e1):e55-62. 10.1136/amiajnl-2013-001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcial LH, Blumenfeld B, Harle C, Jing X, Keller MS, Lee V, Lin Z, Dover A, Midboe AM, Al-Showk S, Bradley V, Breen J, Fadden M, Lomotan E, Marco-Ruiz L, Mohamed R, O’Connor P, Rosendale D, Solomon H, Kawamoto K. Barriers, Facilitators, and Potential Solutions to Advancing Interoperable Clinical Decision Support: Multi-Stakeholder Consensus Recommendations for the Opioid Use Case. AMIA Annu Symp Proc. 2019;2019:637–46. [PMC free article] [PubMed] [Google Scholar]
- 31.Lomotan EA, Meadows G, Michaels M, Michel JJ, Miller K. To Share is Human! Advancing Evidence into Practice through a National Repository of Interoperable Clinical Decision Support. Appl Clin Inform. 2020;11(1):112–21. 10.1055/s-0040-1701253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolin RH, Boxwala A, Shalaby J. A Pharmacogenomics Clinical Decision Support Service Based on FHIR and CDS Hooks. Methods Inf Med. 2018;57(S 02):e115–23. 10.1055/s-0038-1676466 [DOI] [PubMed] [Google Scholar]
- 33.Dorr DA, D’Autremont C, Pizzimenti C, Weiskopf N, Rope R, Kassakian S, Richardson JE, McClure R, Eisenberg F. Assessing Data Adequacy for High Blood Pressure Clinical Decision Support: A Quantitative Analysis. Appl Clin Inform. 2021;12(4):710–20. 10.1055/s-0041-1732401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA. Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement Sci. 2019;14(1):1. 10.1186/s13012-018-0842-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becan JE, Bartkowski JP, Knight DK, Wiley TRA, DiClemente R, Ducharme L, Welsh WN, Bowser D, McCollister K, Hiller M, Spaulding AC, Flynn PM, Swartzendruber A, Dickson MF, Fisher JH, Aarons GA. A model for rigorously applying the Exploration, Preparation, Implementation, Sustainment (EPIS) framework in the design and measurement of a large scale collaborative multi-site study. Health Justice. 2018;6(1):9. 10.1186/s40352-018-0068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idalski Carcone A, Coyle K, Gurung S, Cain D, Dilones RE, Jadwin-Cakmak L, Parsons JT, Naar S. Implementation Science Research Examining the Integration of Evidence-Based Practices Into HIV Prevention and Clinical Care: Protocol for a Mixed-Methods Study Using the Exploration, Preparation, Implementation, and Sustainment (EPIS) Model. JMIR Res Protoc. 2019;8(5): e11202. 10.2196/11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson JM, Witek MA, Hupert ML, Brady C, Pullagurla S, Kamande J, Aufforth RD, Tignanelli CJ, Torphy RJ, Yeh JJ, Soper SA. UV activation of polymeric high aspect ratio microstructures: ramifications in antibody surface loading for circulating tumor cell selection. Lab Chip. 2014;14(1):106–17. 10.1039/C3LC50618E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzag B, Tignanelli CJ, Smith GD. The effect of residual Ca2+ on the stochastic gating of Ca2+-regulated Ca2+ channel models. J Theor Biol. 2005;235(1):121–50. 10.1016/j.jtbi.2004.12.024 [DOI] [PubMed] [Google Scholar]
- 39.Jones EK, Hultman G, Schmoke K, Ninkovic I, Dodge S, Bahr M, Melton GB, Marquard J, Tignanelli CJ. Combined Expert and User-Driven Usability Assessment of Trauma Decision Support Systems Improves User-Centered Design. Surgery. 2022;172(5):1537–48. 10.1016/j.surg.2022.05.037 [DOI] [PubMed] [Google Scholar]
- 40.Jakob N. Enhancing the explanatory power of usability heuristics. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (CHI '94). New York: Association for Computing Machinery; 1994. p. 152–8. 10.1145/191666.191729.
- 41.Shah S, Switzer S, Shippee ND, Wogensen P, Kosednar K, Jones E, Pestka DL, Badlani S, Butler M, Wagner B, White K, Rhein J, Benson B, Reding M, Usher M, Melton GB, Tignanelli CJ. Implementation of an Anticoagulation Practice Guideline for COVID-19 via a Clinical Decision Support System in a Large Academic Health System and Its Evaluation: Observational Study. JMIR Med Inform. 2021;9(11): e30743. 10.2196/30743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingraham NE, Jones EK, King S, Dries J, Phillips M, Loftus T, Evans HL, Melton GB, Tignanelli CJ. Re-Aiming Equity Evaluation in Clinical Decision Support: A Scoping Review of Equity Assessments in Surgical Decision Support Systems. Ann Surg. 2023;277(3):359–64. 10.1097/SLA.0000000000005661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holtrop JS, Estabrooks PA, Gaglio B, Harden SM, Kessler RS, King DK, Kwan BM, Ory MG, Rabin BA, Shelton RC, Glasgow RE. Understanding and applying the RE-AIM framework: Clarifications and resources. J Clin Transl Sci. 2021;5(1): e126. 10.1017/cts.2021.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.https://www.facs.org/-/media/files/quality-programs/trauma/ntdb/ntds/data-dictionaries/ntds_data_dictionary_2022.ashx. Accessed 14 Sep 2021. ACoSNTDSDDA.
- 45.https://www.cdc.gov/pcd/issues/2014/13_0184.htm. Accessed 1/3/2021.
- 46.Malone S, Prewitt K, Hackett R, Lin JC, McKay V, Walsh-Bailey C, Luke DA. The Clinical Sustainability Assessment Tool: measuring organizational capacity to promote sustainability in healthcare. Implement Sci Commun. 2021;2(1):77. 10.1186/s43058-021-00181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aarons GA, Ehrhart MG, Farahnak LR. The Implementation Leadership Scale (ILS): development of a brief measure of unit level implementation leadership. Implement Sci. 2014;9(1):45. 10.1186/1748-5908-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rye M, Torres EM, Friborg O, Skre I, Aarons GA. The Evidence-based Practice Attitude Scale-36 (EBPAS-36): a brief and pragmatic measure of attitudes to evidence-based practice validated in US and Norwegian samples. Implement Sci. 2017;12(1):44. 10.1186/s13012-017-0573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holt DT, Armenakis AA, Feild HS, Harris SG. Readiness for Organizational Change. J Appl Behav Sci. 2007;43(2):232–55. 10.1177/0021886306295295 [DOI] [Google Scholar]
- 50.Weiner BJ. A theory of organizational readiness for change. Implement Sci. 2009;4:67. 10.1186/1748-5908-4-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodridge D, Rana M, Harrison EL, Rotter T, Dobson R, Groot G, Udod S, Lloyd J. Assessing the implementation processes of a large-scale, multi-year quality improvement initiative: survey of health care providers. BMC Health Serv Res. 2018;18(1):237. 10.1186/s12913-018-3045-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vis C, Ruwaard J, Finch T, Rapley T, de Beurs D, van Stel H, van Lettow B, Mol M, Kleiboer A, Riper H, Smit J. Toward an Objective Assessment of Implementation Processes for Innovations in Health Care: Psychometric Evaluation of the Normalization Measure Development (NoMAD) Questionnaire Among Mental Health Care Professionals. J Med Internet Res. 2019;21(2): e12376. 10.2196/12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NoMAD. https://www.implementall.eu/17-nomad.html. Accessed 1/2/2021.
- 54.Ng F, McGrath BA, Seth R, et al. Measuring multidisciplinary staff engagement in a national tracheostomy quality improvement project using the NoMAD instrument. Br J Anesth. 2019;123(4):e506. 10.1016/j.bja.2019.04.030 [DOI] [Google Scholar]
- 55.Guest G, Bunce A, Johnson L. How Many Interviews Are Enough?: An Experiment with Data Saturation and Variability. Field Methods. 2006;18:59–82. 10.1177/1525822X05279903 [DOI] [Google Scholar]
- 56.Beidas RS, Stewart RE, Adams DR, Fernandez T, Lustbader S, Powell BJ, Aarons GA, Hoagwood KE, Evans AC, Hurford MO, Rubin R, Hadley T, Mandell DS, Barg FK. A Multi-Level Examination of Stakeholder Perspectives of Implementation of Evidence-Based Practices in a Large Urban Publicly-Funded Mental Health System. Adm Policy Ment Health. 2016;43(6):893–908. 10.1007/s10488-015-0705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braun V, Clarke V. Thematic analysis. In Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA handbooks in psychology®. APA handbook of research methods in psychology, vol. 2. Research designs: Quantitative, qualitative, neuropsychological, and biological. American Psychological Association; 2012. p. 57–71.
- 58.Fiscella K, Sanders M, Holder T, Carroll JK, Luque A, Cassells A, Johnson BA, Williams SK, Tobin JN. The role of data and safety monitoring boards in implementation trials: When are they justified? J Clin Transl Sci. 2020;4(3):229–32. 10.1017/cts.2020.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AF, Group GW. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. 10.1136/bmj.i2016 [DOI] [PubMed] [Google Scholar]
- 60.Rosenbaum SE, Moberg J, Glenton C, Schunemann HJ, Lewin S, Akl E, Mustafa RA, Morelli A, Vogel JP, Alonso-Coello P, Rada G, Vasquez J, Parmelli E, Gulmezoglu AM, Flottorp SA, Oxman AD. Developing Evidence to Decision Frameworks and an Interactive Evidence to Decision Tool for Making and Using Decisions and Recommendations in Health Care. Glob Chall. 2018;2(9):1700081. 10.1002/gch2.201700081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schunemann HJ, Group GW. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. 10.1136/bmj.i2089 [DOI] [PubMed] [Google Scholar]
- 62.Osheroff JA. CDS and and the CDS & LHS 5 Rights. CDS/PI Collaborative: Getting Better Faster Together.
- 63.ACTS Project Team. Patient Journey and Service Blueprint How Tos. AHRQ evidence-based Care Transformation Support (ACTS) Home. [Online] October 2021. https://cmext.ahrq.gov/confluence/display/PUB/Patient+Journey+and+Service+Blueprint+How+Tos.
- 64.CDS Approach for Optimizing VTE Prophylaxis (VTEP) Society of Hospital Medicine (SHM) Recommendations1 Version 2; March, 2013. [online] https://www.healthit.gov/sites/default/files/cds/Detailed%20Inpatient%20CDS-QI%20Worksheet%20-%20VTE%20Example%20-%20Recommendations.xlsx.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Following trial completion data will be made available upon request through the University of Minnesota Data Repository.