Abstract
Prion infections can present without clinical manifestations. B-cell deficiency may be a model for subclinical transmissible spongiform encephalopathy, since it protects mice from disease upon intraperitoneal administration of scrapie prions; however, a proportion of B-cell-deficient mice accumulate protease-resistant prion protein in their brains. Here, we have characterized this subclinical disease. In addition, we have studied the possibility that a neurotoxic factor secreted by B cells may contribute to pathogenesis.
Prion diseases are transmissible neurodegenerative disorders of rodents, ruminants, and humans. According to the protein-only hypothesis (21) the infectious principle is a pathological isoform (termed PrPSc) of the normal, host-encoded prion protein (termed PrPC): PrPSc is thought to replicate by converting PrPC into further PrPSc. PrPC is necessary for prion replication (7, 24), and differences in the amino acid sequences of PrPC were shown to be the main determinants of interspecies transmission barriers (25). Upon intracerebral (i.c.) or peripheral exposure, replication of prion infectivity occurs in the lymphoreticular system (LRS) of experimental animals long before becoming detectable in the central nervous system (CNS) (10, 12).
LRS colonization is important for the preneural phase of scrapie pathogenesis. Peripheral inoculation of SCID (2), RAG-1−/− (18), and RAG-2−/− (26) mice which lack mature lymphocytes and follicular dendritic cells (FDCs), as well as μMT mice (15) which lack mature B cells and immunoglobulins, fails to establish prion replication in the LRS and does not lead to manifest scrapie for >600 days (16). However, upon i.c. challenge with a mouse-adapted scrapie strain, immunodeficient mice succumb to scrapie similarly to wild-type controls. SCID mice resist infection with bovine spongiform encephalitis (BSE) even upon i.c. challenge (5). Hence, lymphocytes and FDCs are involved in prion transfer from peripheral sites to the CNS and in trespassing species barriers.
The brains of B-cell-deficient animals were sampled randomly at late time points (222 to 504 days) after inoculation with scrapie prions and assayed for protease-resistant PrPSc and/or infectivity: 5 of 14 mice (13 to 65% within a confidence interval of 95%) tested positive by either Western blot analysis, infectivity bioassay, or both (16). However, clinically apparent scrapie was never detected in B-cell-deficient mice (n = 27), even 665 days after intraperitoncal (i.p.) inoculation with prions (16), suggesting that a neurotoxic or catalytic factor secreted by B cells may contribute to pathogenesis and lead to the manifestation of symptoms. If this were true, i.c. inoculation of μMT or RAG-1−/− mice with inoculum derived from μMT or RAG-2−/− brains would not contain such a hypothetical cofactor and therefore should not lead to scrapie in the recipient mice.
We therefore prepared prion inocula from brains of two RAG-2−/− and two μMT mice which had been scrapie inoculated i.p. (Table 1). Brain homogenates (1%) were prepared as described previously (8) and inoculated i.c. into μMT and Rag-1−/− mice, which were kept under pathogen-free conditions.
TABLE 1.
Origin of scrapie inoculum, occurrence of clinical scrapie, and latency of disease in immunodeficient mice inoculated with prions derived from asymptomatic immunodeficient micea
| Inoculumb and days of inoculation | Recipient (i.c.) | Average incubation period (days) ± SD |
|---|---|---|
| RAG-2−/− | ||
| Day 286 | RAG-1−/− | 182 ± 35 |
| Day 342 | 155 ± 2.2 | |
| μMT | ||
| Day 375 | μMT | 161 ± 5.5 |
| Day 432 | 149 ± 9.4 |
Note that in each group, four of four mice developed scrapie.
One percent brain homogenate.
One RAG-2−/− mouse and one μMT mouse contained levels comparable to terminally-scrapie-sick wild-type mice, as assayed by titration of infectivity by incubation time (22) in PrP-overexpressing tga20 indicator mice (11). The brain infectivity titer in the second RAG-2−/− mouse was approximately 2 log 50% lethal dose units lower. The second μMT mouse was not tested for infectivity, but showed PrPSc levels exceeding the ones of the infected wild-type control upon Western blotting (16). For the latter, 80 μg of total protein was electrophoresed through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide), transferred to nitrocellulose membranes, probed with monoclonal antibody 6H4 (17) to mouse PrP, and developed by enhanced chemiluminescence (Amersham).
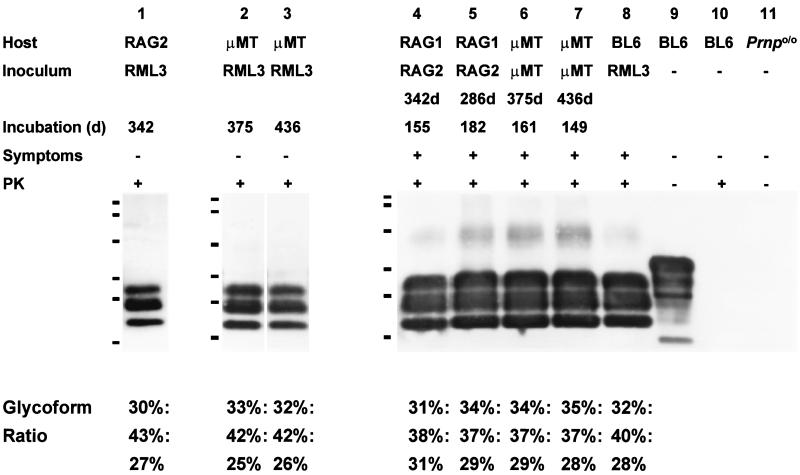
Sixteen mice were inoculated i.c. with prions derived from brains of asymptomatic μMT and Rag-2−/− mice which had been challenged i.p.: all mice developed weight loss, hind limb paresis, ataxia, apathy, and hunched posture, with incubation periods shown in Table 1, and were killed when terminally sick. To confirm the diagnosis of scrapie, we investigated the accumulation of PrPSc in one brain for each group of mice by Western blot and histoblot analyses, and assessed spongiosis and gliosis in paraffin-embedded brain sections of all mice. PrPSc (Fig. 1) and/or transmissible spongiform encephalopathy-characteristic histopathology (not shown) was demonstrated in all brains of clinically sick mice.
FIG. 1.
Western blot analysis of brains of immunodeficient mice challenged i.p. with RML prions and not exhibiting clinical symptoms (lanes 1 to 3) and of immunodeficient mice challenged i.c. with inocula derived from asymptomatic RAG-2−/− and μMT mice (lanes 4 to 7). The latter mice were terminally sick. The lower tabulation indicates the relative percent contribution of un-, mono-, and diglycosylated prion protein bands after proteinase K (PK) digestion to the total PrPSc immunoreactivity present on the blot. The predominance of the monoglycosylated form in all mice, as well as the very similar glycoform ratio in all samples tested, indicates that no alteration of the strain properties detectable by glycotype analysis has taken place during the passage of CD-1 mouse-derived RML prions onto RAG-2−/− and μMT mice (genetic background, C57/BL6), and upon a second passage onto further RAG-1−/− and μMT mice. Lane 8, brain of a terminally scrapie sick C57/BL6 mouse inoculated with RML prions; lanes 9 and 10, brain of a noninfected C57/BL6 mouse, showing no anti-PrP immunoreactive bands upon treatment with proteinase K; lane 11, brain of a Prnp0/0 mouse.
We noted a statistically significant tendency towards shorter incubation periods when compared with the incubation periods of RAG-2−/− and μMT mice primarily inoculated i.c. with standard Rocky Mountain Laboratory (RML) prions (Student’s t test, P < 0.001). For example, the inoculum derived from a μMT mouse sacrificed 436 days after i.p. inoculation which did not display clinical symptoms provoked terminal stage scrapie with an average of 149 days, whereas μMT mice i.c. challenged with RML prions (derived from terminally sick CD1 wild-type animals) succumbed with an average of 181 days.
PrPSc was demonstrated in all brains tested by Western blotting (Fig. 1). Glycoform ratios were calculated by densitometric analysis with ImageQuaNT software, and evidenced a characteristic pattern of proteinase K-resistant un-, mono-, or diglycosylated PrP (9), which was similar to the patterns of RML-inoculated C57/BL6, RAG-2−/−, and μMT mice (Fig. 1).
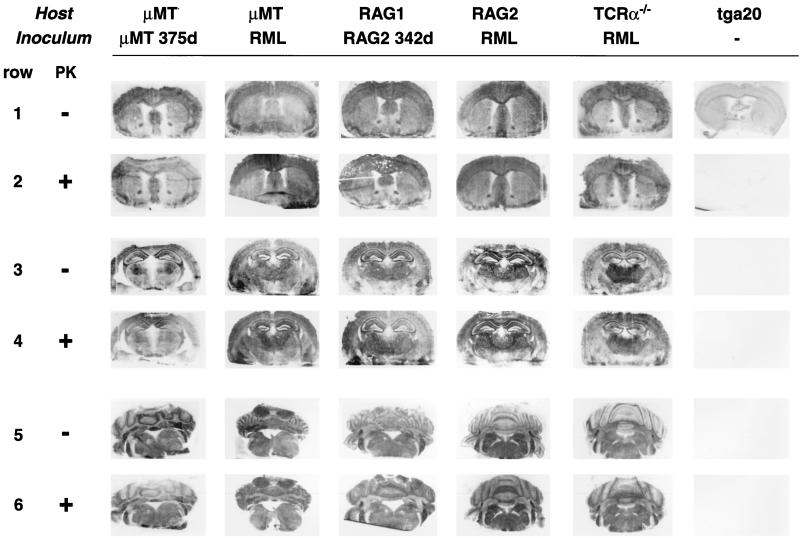
For histoblots (27), frozen sections (8 μm) were mounted on nitrocellulose and subjected to limited proteolysis (proteinase K at 100 μg/ml, 37°C, 4 h). Detection was accomplished with monoclonal antibody 6H4 to PrP (17) (1:2,000) as described previously (3). Histoblots of forebrain with cortex and basal ganglia, rostral hippocampus with thalamus and brain stem, and cerebellum did not reveal differences in the distribution of proteinase K-resistant PrP in infected yet clinically healthy immunodeficient animals compared to terminally-scrapie-sick μMT and RAG mice inoculated i.c. with RML prions, and with one RML-inoculated, terminally scrapie sick TcRα−/− mouse (20) lacking all T-cell receptor alpha/beta (TCR-α/β)-expressing lymphocytes (Fig. 2).
FIG. 2.
Histoblot analysis of distribution patterns of proteinase K (PK)-resistant PrPSc in different brain regions of immunodeficient mice. Coronal sections through the forebrain including the basal ganglia (rows 1 and 2), through the rostral hippocampus and thalami (rows 3 and 4), and through the caudal regions including brain stem and cerebellum (rows 5 and 6) show indistinguishable patterns of PrPSc distributions in RAG-2−/− and μMT mice inoculated i.c. with RML prions (columns 1 and 3, respectively) and in RAG-1−/− and μMT mice inoculated i.c. with inocula derived from asymptomatic RAG-2−/− and μMT mice (columns 2 and 4, respectively), as well as in TCRα−/− mice succumbing from scrapie upon i.c. inoculation with RML prions (column 5). 375d and 342d, days of inoculation.
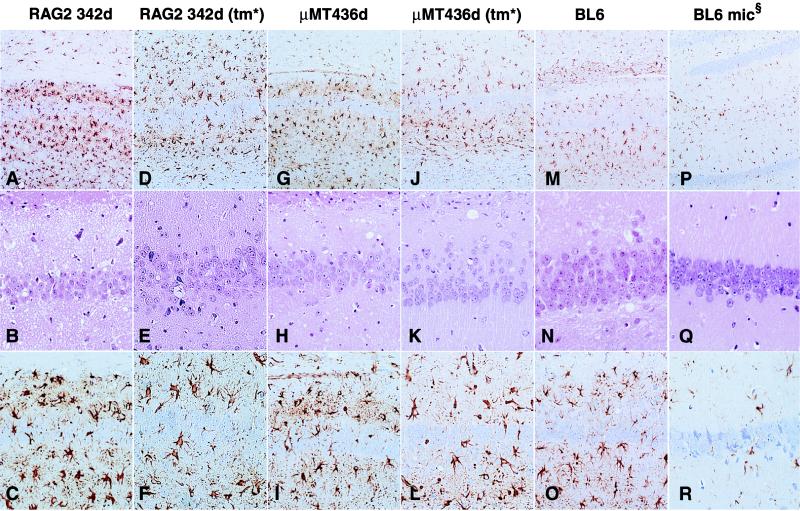
Upon formic acid inactivation (6) and paraffin embedding, brain sections were stained with hematoxylin-eosin and with antibodies to glial fibrillary acidic protein. Gliosis (a nonspecific but early indicator of brain damage) was visualized by the presence of large immunostained reactive astrocytes. All scrapie-sick mice displayed characteristic vacuolar changes and strong reactive gliosis with a regional lesion profile very similar to that of RAG-2−/−, μMT, and C57/BL6 mice inoculated i.c. with RML prions (Fig. 3).
FIG. 3.
Brain histopathology of asymptomatic RAG-2−/− (A to C) and μMT mice (G to I) inoculated i.p. with scrapie prions, and of RAG-1−/− (D to F) and μMT mice (J to L) inoculated i.c. with inocula derived from asymptomatic RAG-2−/− and μMT mice, respectively. The hippocampal formation shows the typical morphology of scrapie with vacuolar changes in both asymptomatic (B and H) and in terminally sick (E and K) immunodeficient mice. Despite some interindividual variations in the density of microvacuolation (middle row, hematoxylin & eosin), immunostaining for glial fibrillary acidic protein (top and bottom rows) reveals extensive, diffuse gliosis surrounding the pyramidal cell ribbon in all mice (original magnification and stain, upper row, ×100; middle and bottom rows, ×200). (M to O) RML-inoculated, terminally sick C57/BL6 mouse. (P to R) Mock-inoculated, age-matched C57/BL6 mouse. An asterisk indicates transmission (tm) of sample RAG-2 342d into RAG-1−/− and of sample μMT 436d (inoculated on days 342 and 436, respectively) into μMT mice. mic§, i.c. mock inoculation.
In a former study, we discovered that mice lacking differentiated B lymphocytes can harbor high titers of prion infectivity and PrPSc in their brains following peripheral challenge with an RML standard inoculum, yet do not develop clinical symptoms of scrapie. This surprising finding led us to hypothesize that once prions have reached the CNS, a neurotoxic or catalytic cofactor produced by B lymphocytes could be responsible for, or at least dramatically accelerate, the decline of CNS neurons and lead to the manifestation of symptoms in concert with the infectious agent. There are examples for similar pathogenetic mechanisms: in human immunodeficiency virus infection of the CNS, for example, pathogenesis is mediated not only by viral products (28), but also by nitric oxide produced by infected microglial cells (13).
Because B-cell-deficient mice develop scrapie upon i.c. infection with a standard RML inoculum with the same latency and efficiency as wild-type control mice (16), a possible neurotoxic cofactor would have to be present in infectious brain homogenates derived from immunocompetent animals. If so, i.c. inoculation of B-cell-deficient mice with an inoculum derived from brains of subclinically infected B-cell-deficient mice should result in greatly (or indefinitely) prolonged incubation times, since the primary i.p. inoculation of B-cell-deficient mice would dilute the cofactor.
However, the results of the present study confirm that B lymphocytes and their secreted products do not influence scrapie pathogenesis if the agent is directly inoculated into the brain. This remains true even after prions have been passaged twice in B-cell-deficient mice. We can also exclude the involvement a cofactor produced by T lymphocytes, which are known to be able to invade CNS parenchyma when activated (4, 19), since RAG-1−/− and RAG-2−/− mice are devoid of both B and T cells.
We have considered the possibility that the agent may have undergone a “strain shift” changing its pathogenic properties (1), because it was passaged twice in immunodeficient hosts and it induced disease with shortened incubation times upon its second passage. However, this is very unlikely since (i) the glycoform distribution of PrPSc, (ii) the regional patterns of PrPSc deposition as assessed by histoblotting, and (iii) the lesion profiles in the brains of RAG-1−/− and μMT mice inoculated with prions from immunodeficient mice are indistinguishable from those of RML-inoculated TcRα−/−, RML-inoculated RAG-2−/−, μMT, and wild-type mice.
While it is remarkable that immunodeficient mice harboring brain infectivity titers equaling or even exceeding those of terminally sick wild-type controls can be perfectly healthy on clinical examination, there are further documented examples of chronic subclinical infections with prions. Mice heterozygous for a disrupted PrP gene show high prion and PrPSc levels, but a delayed onset of disease (7). Hamster prions can persist and perhaps even replicate in a clinically silent fashion when transmitted to mice (23). In the latter experiment, mice inoculated with hamster prions clinically resisted infection, yet when brain homogenates of these mice were transmitted to further animals, hamsters (but not mice) developed scrapie.
However, subclinical infection may also occur when prions are being passaged within the same species. Although many patients were presumably exposed to Creutzfeldt-Jakob disease-contaminated batches of growth hormone and gonadotropins, only about 100 (14) developed overt disease. This may be interpreted as a sign that the “take” of infection occurred only in few individuals, perhaps because the inoculum was small, or that incubation times vary vastly so that many individuals may have developed subclinical infection.
Also the fact that only single cows developed clinically overt BSE in exposed herds raises the possibility that subclinical infection of cattle with BSE may occur more frequently than suspected. Given the health hazards posed by BSE, the phenomenon of subclinical prion infections deserves in-depth investigations: B-cell-deficient mice may serve as a suitable model system. The results presented here argue against a direct effect of B lymphocytes or their secreted products on establishment of subclinical prion infection and raise the possibility that secondary events, such as maturation of cells induced by B lymphocytes—e.g., FDCs in lymphoid organs or immune cells resident in the CNS—may contribute to scrapie neurotoxicity.
Acknowledgments
We thank A. Burlet, M. König, and N. Wey for technical help; C. Weissmann for critical reading; and K. Rajewsky for μMT mice.
This work is supported by the Kanton of Zürich; the Bundesämter für Gesundheit, Veterinärwesen, Bildung und Wissenschaft; and grants from the Swiss National Research Program (NFP38/NFP38+ to A.A. and R.M.Z.).
REFERENCES
- 1.Aguzzi A. Protein conformation dictates prion strain. Nat Med. 1998;4:1125–1126. doi: 10.1038/2621. [DOI] [PubMed] [Google Scholar]
- 2.Bosma M J, Carroll A M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 3.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 4.Brinkman C J J, Ter Laak H J, Hommes O R, Poppema S, Delmotte P. T-lymphocyte subpopulations in multiple-sclerosis lesions. N Engl J Med. 1982;307:1644–1645. doi: 10.1056/NEJM198212233072613. [DOI] [PubMed] [Google Scholar]
- 5.Brown K L, Stewart K, Bruce M E, Fraser H. Severely combined immunodeficient (SCID) mice resist infection with bovine spongiform encephalopathy. J Gen Virol. 1997;78:2707–2710. doi: 10.1099/0022-1317-78-10-2707. [DOI] [PubMed] [Google Scholar]
- 6.Brown P, Wolff A, Gajdusek D C. A simple and effective method for inactivating virus infectivity in formalin-fixed tissue samples from patients with Creutzfeldt-Jakob disease. Neurology. 1990;40:887–890. doi: 10.1212/wnl.40.6.887. [DOI] [PubMed] [Google Scholar]
- 7.Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Collinge J, Sidle K C, Meads J, Ironside J, Hill A F. Molecular analysis of prion strain variation and the aetiology of ’new variant’ CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 10.Eklund C M, Kennedy R C, Hadlow W J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967;117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser H, Dickinson A G. Pathogenesis of scrapie in the mouse: the role of the spleen. Nature. 1970;226:462–463. doi: 10.1038/226462a0. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 14.Johnson R T, Gibbs C J., Jr Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994–2004. doi: 10.1056/NEJM199812313392707. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 16.Klein M A, Frigg R, Flechsig E, Raeber A J, Kalinke U, Bluethmann H, Bootz F, Suter M, Zinkernagel R M, Aguzzi A. A crucial role for B cells in neuroinvasive scrapie. Nature. 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 17.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 18.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 19.Nyland H, Matre R, Mork S, Bjerke J R, Naess A. T-lymphocyte subpopulations in multiple-sclerosis lesions. N Engl J Med. 1982;307:1643–1644. doi: 10.1056/NEJM198212233072613. [DOI] [PubMed] [Google Scholar]
- 20.Philpott K L, Viney J L, Kay G, Rastan S, Gardiner E M, Chae S, Hayday A C, Owen M J. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 21.Prusiner S B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 22.Prusiner S B, Cochran S P, Groth D F, Downey D E, Bowman K A, Martinez H M. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982;11:353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- 23.Race R, Chesebro B. Long-term persistence of scrapie infectivity in brain and spleen tissue of a clinically resistant species: implications for control of BSE. Nature. 1998;392:770. doi: 10.1038/33834. [DOI] [PubMed] [Google Scholar]
- 24.Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 25.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Waelchli M, Torchia M, Groth D, Carlson G, DeArmond S J, Westaway D, Prusiner S B. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989;59:847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 26.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 27.Taraboulos A, Jendroska K, Serban D, Yang S L, DeArmond S J, Prusiner S B. Regional mapping of prion proteins in brain. Proc Natl Acad Sci USA. 1992;89:7620–7624. doi: 10.1073/pnas.89.16.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyss Coray T, Masliah E, Toggas S M, Rockenstein E M, Brooker M J, Lee H S, Mucke L. Dysregulation of signal transduction pathways as a potential mechanism of nervous system alterations in HIV-1 gp120 transgenic mice and humans with HIV-1 encephalitis. J Clin Investig. 1996;97:789–798. doi: 10.1172/JCI118478. [DOI] [PMC free article] [PubMed] [Google Scholar]