Abstract
Left ventricular pseudoaneurysm is a rare complication of myocardial infarction and represent a myocardial rupture contained within a pericardial space limited by adhesions. Differentiating it from a left ventricular aneurysm can be a real diagnostic challenge. We report a case of a 50-year-old man admitted for symptoms of left heart failure. Transthoracic echocardiography and cardiac computed tomography scan incidentally showed a large lateral left ventricular pseudoaneurysm measuring 75/50 mm in diameter. Patch closure was carried out under cardiopulmonary bypass. Postoperative follow up was uneventful. This case demonstrates the increasing detection of «incidental» left ventricular pseudoaneurysm with more frequent use of multimodality imaging techniques including cardiac CT scan.
Keywords: Pseudoaneurysm, Myocardial infarction, Imaging, Left ventricle
Introduction
Left ventricular (LV) pseudoaneurysm is a rare complication of myocardial infarction (MI). It is defined as a contained rupture of the myocardium. LV pseudoaneurysms have a high risk of spontaneous rupture and death [1], requiring prompt surgical management.
Case presentation
A 50-year-old man was admitted to the department for management of a symptom of left heart failure. His cardiovascular risk factors were arterial hypertension and chronic smoking. His medical history included chest pain occurring 20 days before admission, which the patient had neglected. The baseline work-up including chest X-ray, electrocardiogram and transthoracic echocardiography (TTE), was suggestive of coronary artery disease complicated by a large lateral pseudoaneurysm of the left ventricle, confirmed by a cardiac computed tomography scan and measuring 75/50 mm in diameter (Fig. 1, Fig. 2). Subsequent coronary angiography revealed an atheromatous lesion with no significant stenosis. Left ventriculography showed an ejection fraction of 45% with an aneurysmal aspect of the lateral wall of the left ventricle, with no sign of rupture. The pseudoaneurysm was resected and the ventricular wall defect was closed with a Teflon patch reconstruction (Fig. 3, Fig. 4). Examination of the mitral subvalvular apparatus was normal. Postoperative follow was uneventful and TTE 1 month after surgery showed a LV ejection fraction of 52% with no mitral leak.
Fig. 1.
CT scan of a pseudoaneurysm in the lateral wall of the left ventricle (white arrow). Neck: transparietal defect in the lateral wall of the LV (black star).
Fig. 2.
CT scan with 3D reconstruction of the pseudoaneurysm in the lateral wall of the left ventricle (white arrow).
Fig. 3.
Intraoperative view of the neck of the pseudoaneurysm (black arrow).
Fig. 4.
Intraoperative view of the defect closure patch (black arrow).
Discussion
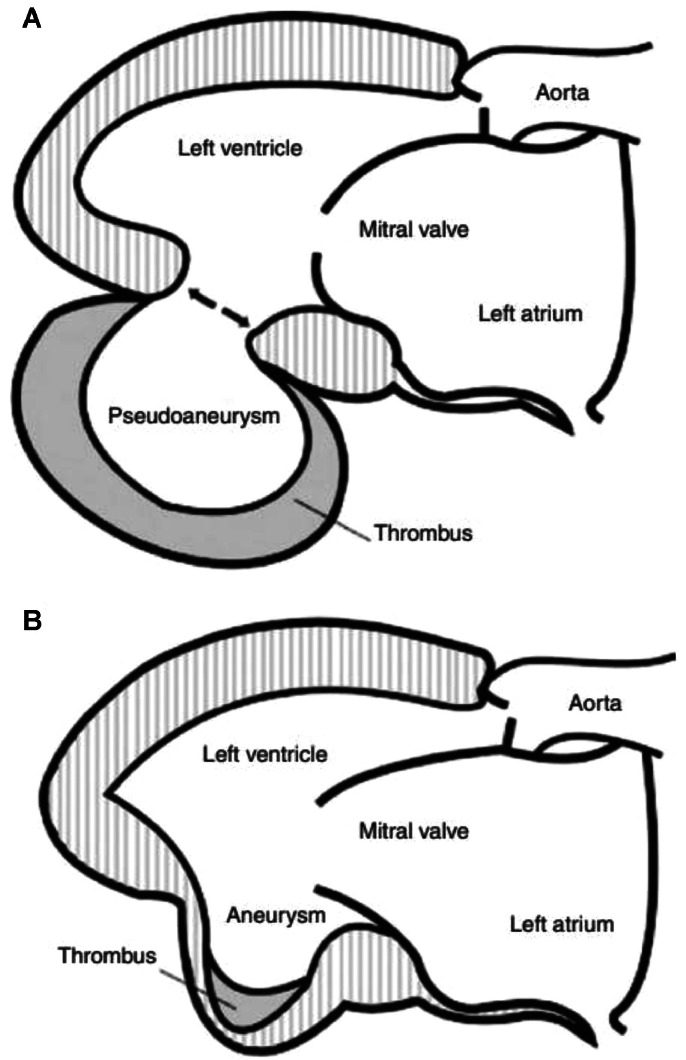
Left ventricular (LV) aneurysms are of 2 types : true and false (Fig. 5). True aneurysms are sequelae of transmural myocardial infarction (MI). False aneurysms (or pseudoaneurysms) are rare complications of MI and represent a myocardial rupture contained within a pericardial space limited by adhesions. Pseudoaneurysm does not contain all three layers of myocardium and is often covered with pericardium and mural thrombus [2,3]. The base of pseudoaneurysm typically includes a narrow neck opening into a saccular zone with a wall free of endocardium or myocardium [4]. Pseudoaneurysms are most commonly caused by MI, but also by mitral or aortic valve replacement, chest trauma and infection (abscess, endocarditis) [5]. Unlike true left ventricular aneurysms, false aneurysms have a high risk of spontaneous rupture and death [1] and therefore require rapid surgical management once the diagnosis has been confirmed, even in asymptomatic patients [6]. Indeed, left ventricular free wall rupture is a catastrophic complication occurring in 4% of patients after MI and in 23% of those who die of MI [7].
Fig. 5.
(A) Left ventricular pseudoaneurysm. (B) Left ventricular aneurysm.
Inferior MI is twice as common as anterior MI in the genesis of LV pseudoaneurysms [8]. This can be explained by the fact that anterior rupture is more likely to lead to hemopericardium and death than posterior rupture [9] and that the presence of an inflammatory reaction in the posterior pericardium leads to the formation of pericardial adhesions and therefore to the formation of a pseudoaneurysm rather than cardiac tamponade.
Clinical expression varies from one patient to another, but the literature shows that patients are most often asymptomatic or only mildly symptomatic (dyspnea, chest pain) [5,10,11]. It is therefore often discovered accidental during an episode of heart failure, a ventricular arrhythmia or after an embolic event [8]. Clinical examination may reveal signs of heart failure.
The electrocardiogram may show ST-segment elevation or non-ST-segment elevation, or Q waves of necrosis, but without sensitivity or specificity. Chest X-rays may show cardiomegaly at the level of the left lower arch.
Transthoracic echocardiography is the key diagnostic imaging modality. However, differentiation between a true and a pseudoaneurysm is an important factor in making treatment decisions and is sometimes difficult with TTE [8]. The echocardiographic signs in favor of a pseudoaneurysm are a clear discontinuity of the endocardial margin at the base of the pseudoaneurysm, a saccular shape of the aneurysm and a narrow neck compared to the diameter of the aneurysm [12]. Other echocardiographic parameters have been identified: the ratio of the diameter of the neck to the maximum diameter of the aneurysm, a ratio less than 0.5 is in favor of a pseudoaneurysm [13]. Other studies have shown a lack of specificity in this criteria [5]. Color Doppler flow has been used to detect turbulent flow through the neck of the pseudoaneurysm but the absence of flow does not exclude the diagnosis of a pseudoaneurysm, making this criterion specific but not sensitive.
Left ventriculography was the gold standard for differentiating true from LV pseudoaneurysms, with a diagnosis precision of > 85%. Pseudoaneurysms consists as a narrow neck leading to a saccular aneurysm [10]. Left ventriculography is combined with coronary angiography to explore coronary lesions. A pseudoaneurysm may be unrecognized if it is completely clothed with wall thrombus.
Cardiac computed tomography (CT) and nuclear magnetic resonance imaging (MRI) can be used to differentiating between the 2 forms of aneurysm and to better study the relationships and the anterior or posterior location of the pseudoaneurysm [14]. The presence of a neck that connects the aneurysm to the ventricular cavity, the larger size of the aneurysm and the posterior location are signs in favor of a LV pseudoaneurysm.
Once the diagnosis of LV pseudoaneurysm has been confirmed, surgery is usually required. Urgent surgery is recommended when a pseudoaneurysm is diagnosed within 2 to 3 months post myocardial infarction, as the risk of rupture is unpredictable [15]. However, when the diagnosis is made several years after MI or surgery, the indication for surgery is based on symptoms rather than the risk of rupture.
In the cases of preoperative hemodynamic instability, resuscitation measures must be taken, pharmacological support (digitalo-diuretics, positive inotropic drugs) and mechanical support, with the insertion of an intra-aortic counterpulsation balloon whenever possible, should be used to stabilize the patient's hemodynamic state. The size and location of the pseudoaneurysm determine the surgical procedure.
The preferred thoracic approach is a longitudinal median sternotomy with installation of extracorporeal circulation (ECC) between the aorta and the 2 vena cava. However, the left thoracotomy approach with a beating heart in the event of redux has been reported in the literature [16], thus improving LV hemodynamics by reducing its size and preventing long-term remodeling [17]. In the case of a small neck, direct ligation of the neck can be performed. In the case of a large neck or a neck located at the level of the basal segments of the LV wall, a synthetic patch (Dacron, Gore-Tex, Teflon) is used to avoid potential distortion of the valvular structures or excessive traction on the myocardium. This procedure may be combined with revascularization by coronary artery bypass grafting and/or mitral valve repair in the case of associated ischemic mitral insufficiency.
The timing of post-MI surgery remains a controversial issue. Surgery too early makes reconstruction difficult because of the fragility of the tissue, but some authors have reported that a delay of one month allows the tissue to heal as a result of fibrosis in the area affected [18]. Such a decision requires close monitoring of the patient, including clinical and regular echocardiography or cardiac CT scans.
Percutaneous closure is an alternative to surgery in patients at high surgical risk [19,20]. However, experience is still limited to rare cases. Selection of the device must be individualized according to the location and size of the pseudoaneurysm and adjacent structures.
Postoperative mortality in false LV aneurysms ranges from 13% to 29% [21]. The risk factors for early mortality are severe left ventricular failure, MI, and refractory arrhythmias. Risk factors for late mortality are incomplete revascularization and impaired LV systolic function.
Conclusion
Contained rupture of the left ventricle by adherent pericardium creates a pseudoaneurysm. This condition justifies urgent surgery. Given the risk of rupture of the false aneurysm leading to tamponade, shock or death, compared with the more benign natural history of true aneurysms, accurate diagnosis of these two entities is important.
Patient consent
Informed, written consent was obtained from the patient in accordance with COPE guidelines.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Antman EM. In: Braunwalds heart disease: a textbook of cardiovascular medicine. 7th ed. Zipes, et al., editors. Elsevier Saunders; Philadelphia: 2005. ST-elevation myocardial infarction: management; pp. 1167–1226. editors. [Google Scholar]
- 2.Stewart S, Huddle R, Stuard I, Schreiner BF, DeWeese JA. False aneurysm and pseudo-false aneurysm of the left ventricle (etiology, pathology, diagnosis, and operative management) Ann Thorac Surg. 1981;31:259–265. doi: 10.1016/s0003-4975(10)60938-1. [DOI] [PubMed] [Google Scholar]
- 3.Davidson KH, Parisi AG, Harrington JJ, Barsamian EM, Fishbein MC. Pseudo aneurysm of the left ventricle (an unusual echocardiographic presentation) Ann Intern Med. 1977;86:430–433. doi: 10.7326/0003-4819-86-4-430. [DOI] [PubMed] [Google Scholar]
- 4.March KL, Sawada SG, Tarver RD, Kesler KA, Armstrong WF. Current concepts of left ventricular pseudoaneurysm: pathophysiology, therapy, and diagnostic imaging methods. Clin Cardiol. 1989;12:531–540. doi: 10.1002/clc.4960120911. [DOI] [PubMed] [Google Scholar]
- 5.Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998;32:557–561. doi: 10.1016/s0735-1097(98)00290-3. [DOI] [PubMed] [Google Scholar]
- 6.Gobel FL, Visudh-Arom K, Edwards JE. Pseudoaneurysm of the left ventricle leading to recurrent pericardial hemorrhage. Chest. 1971;59:23–27. doi: 10.1378/chest.59.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Pollak H, Nobis H, Miczoc J. Frequency of left ventricular free wall ruptures complicating acute myocardial infarction since the advent of thrombolysis. Am J Cardiol. 1994;74:184–186. doi: 10.1016/0002-9149(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 8.Cho MN, Mehta SK, Matulevicius S, Weinstein D, Wait MA, McGuire DK. Differentiating true versus pseudo left ventricular aneurysm: a case report and review of diagnostic strategies. Cardiol Rev. 2006;14:e27–e30. doi: 10.1097/01.crd.0000233756.66532.45. [DOI] [PubMed] [Google Scholar]
- 9.Rittenhouse EA, Sauvage LR, Mansfield PB, Smith JC, Davis CC, Hall DG. False aneurysm of the left ventricle. Report of four cases and review of surgical management. Ann Surg. 1979;189:409–415. [PMC free article] [PubMed] [Google Scholar]
- 10.Yeo TC, Malouf JF, Oh JK, Seward JB. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Inter Med. 1998;128:299–305. doi: 10.7326/0003-4819-128-4-199802150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Moreno R, Gordillo E, Zamorano J, Almeria C, Garcia-Rubira JC, Fernandez-Ortiz A, et al. Long term outcome of patients with post infaction left ventricule pseudoaneurysm. Heart. 2003;89:1144–1146. doi: 10.1136/heart.89.10.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catherwood E, Mintz GS, Kotler MN, Parry WR, Segal BL. Two-dimensional echocardiographic recognition of left ventricular pseudoaneurysm. Circulation. 1980;62:294–303. doi: 10.1161/01.cir.62.2.294. [DOI] [PubMed] [Google Scholar]
- 13.Gatewood RP, Nanda NC. Differentiation of left ventricular pseudoaneurysm from true aneurysm with two dimensional echocardiography. Am J Cardiol. 1980;46:869–878. doi: 10.1016/0002-9149(80)90442-7. [DOI] [PubMed] [Google Scholar]
- 14.Ando S, Kadokami T, Momii H, Hironaga K, Kawamura N, Fukuyama T, et al. Left ventricular false-pseudo and pseudo aneurysm: serial observations by cardiac magnetic resonance imaging. Intern Med. 2007;46(4):181–185. doi: 10.2169/internalmedicine.46.1892. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Yang Y, Sun HS, Tang Y. Surgical treatment of left ventricular pseudoaneurysm. Chin Med J. 2018;131:1496–1497. doi: 10.4103/0366-6999.233954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domenico C, José T. Treatment of left ventricular pseudoaneurysm throught left thoracotomy on a beathing heart. The cardiothoracic surgery network.
- 17.Nwogu CE, Moran JM, Becker RM, Pezzella Surgical approach to myocardial rupture after acute myocardial infarction. Asian Cardiovasc Thorac Ann. 1998;6:108–114. [Google Scholar]
- 18.Kirklin JW, Barrat- Boyes BG. Churchill livingstone Inc.ed; 1993. Left ventricular aneurysm. Cardiac surgery new york; p. 384. [Google Scholar]
- 19.Madan T, Juneja M, Raval A, Thakkar B. Transcatheter device closure of pseudoaneurysms of the left ventricular wall: an emerging therapeutic option. Rev Port Cardiol. 2016;35 doi: 10.1016/j.repc.2015.06.013. 115e1–5. [DOI] [PubMed] [Google Scholar]
- 20.Dudiy Y, Jelnin V, Einhorn BN, Kronzon I, Cohen HA, Ruiz CE. Percutaneous closure of left ventricular pseudoaneurysm. Circ Cardiovasc Interv. 2011;4:322–326. doi: 10.1161/CIRCINTERVENTIONS.111.962464. [DOI] [PubMed] [Google Scholar]
- 21.Figueras J, Cortadellas J, Domingo E, Soler-Soler J. Survival following self-limited left ventricular free wall rupture during myocardial infarction. Management differences between patients with or without pseudoaneurysm formation. Int J cardiol. 2001;79:103–111. doi: 10.1016/s0167-5273(01)00415-6. [DOI] [PubMed] [Google Scholar]