Abstract
To understand transplant center recommendations on return-to-school timing and related support for hematopoietic cell transplant (HCT) survivors, we conducted a two-phase, cross-sectional, web-based survey: In Phase I, medical directors of pediatric HCT centers from the National Marrow Donor Program/ Be The Match Registry were asked regarding the availability of a return to school standardized operating procedure (SOP). In Phase II, HCT physician members of the Pediatric Transplantation and Cellular Therapy Consortium were approached to study inter-physician practice variability regarding return to school post-HCT, factors affecting their decision-making, and support provided by HCT centers for return to school. Out of 46 respondents in Phase I (55% response rate), 28 (61%) reported having a SOP. Wide variations in recommendations were noted in 12 received SOPs. In Phase II, 122 physicians (60 centers) responded (30.6% response rate). The majority (60%) recommended autologous HCT recipients return to school within 6 months post-HCT but 65% recommended allogeneic HCT recipients return to school after 6 months or once off immunosuppression. Our findings indicate a -;lack of consensus within and across HCT centers regarding recommended return to school timing and underscore need for a guideline to standardize this process to ensure patient safety and re-integration into school.
Introduction:
Allogeneic hematopoietic cell transplant (HCT) is increasingly used as a curative option for children and adolescents with malignant and non-malignant hematologic conditions.1 However, it is also an intense process during children’s formative years and negatively impacts their physical, emotional, and socio-environmental domains of quality of life (QOL).2 Pediatric HCT recipients remain out of school for a prolonged period of time during their treatment course and recovery, and in part due to their immune compromised status and potential risk of infections.3, 4 Returning to school post-HCT is an important milestone for survivors as it provides a sense of normalcy, a self-esteem boost, and social support while they cope with treatment-related toxicities.5 School also provides an essential framework for social, intellectual, and academic development of children and is critical to obtain the skillsets necessary to enter higher educational training and/ or the workforce. Therefore, early school reintegration could be potentially beneficial for HCT survivors, despite the current literature lacking data on recommended timing of return to school post-HCT for survivors.
Educational disruption is commonly seen while patients undergo peri-transplant hospitalization,6, 7 and returning to school after prolonged absence(s) can be challenging for patients and caregivers. Literature thus far has been predominantly focused on the school and educational challenges of childhood and adolescent cancer survivors treated with conventional (non-HCT) therapy, including physicians’ perspectives on the return to school process after completion of therapy.8–10 However, patients receiving HCT have prolonged hospitalizations and their therapy results in prolonged and severe immune deficiency. For this reason, return to school guidance in HCT patients must be considered differently from their oncology peers. Unfortunately, information by HCT physicians on how HCT centers provide support for the return to school process is lacking.
To address the knowledge gap, our primary aim was to assess the availability of any standardized operating procedure (SOP) documents at HCT centers to guide physicians regarding autologous and allogeneic HCT recipients’ return to school in a traditional classroom setting. Additionally, we aimed to identify inter-physician practice variations in guiding HCT recipients when to return to school and factors associated with their decision-making process.
Materials/ Subjects and Methods:
This study was approved by the National Marrow Donor Program (NMDP) Institutional Review Board. We conducted a two-phase cross-sectional web-based survey of HCT physicians in the United States (US) through the Center for International Blood and Marrow Transplant Research (CIBMTR) Health Services Research Program. The overall survey development and dissemination plan is shown in Figure 1. The surveys were developed in consultation with content experts in pediatric HCT and health services research. In Phase I, medical directors of pediatric HCT programs listed in the NMDP/ Be The Match Registry were approached between January and February 2020 by email to answer a single question evaluating the existence of an SOP advising physicians when to recommend autologous and allogeneic HCT recipients should return to school after HCT (Supplemental File: Appendix A). The centers that reported having a SOP were requested to provide a copy of the SOP as able to share. In Phase II, pediatric HCT physicians identified through the Pediatric Transplantation and Cellular Therapy Consortium (PTCTC) membership directory were approached to study: 1) inter-physician practice variability within and across transplant centers to guide patients regarding when to return to in-person school post-HCT; 2) factors considered before a patient’s return to in-person school; and 3) topics addressed to help with survivors’ return to school. Additionally, demographics of HCT physicians, such as age, sex, and years of experience, were collected. The Phase II survey was initially piloted with three pediatric HCT experts before its dissemination. These physicians were excluded at the time of the survey dissemination. Similar to Phase I, HCT physicians were approached by email to fill out a web-based survey between December 2020 and January 2021. In Phase II, HCT physicians answered up to a maximum of 22 questions (Supplemental File: Appendix B). An additional questionnaire to understand return to school practices during the Coronavirus disease-19 (COVID-19) pandemic was also offered to HCT physicians during Phase II, results of which have previously been published.11 Both phases of the survey were hosted by Alchemer (Louisville, CO), a secure online survey platform. Weekly follow-up emails were sent after the initial survey was sent in both phases.
Figure 1.
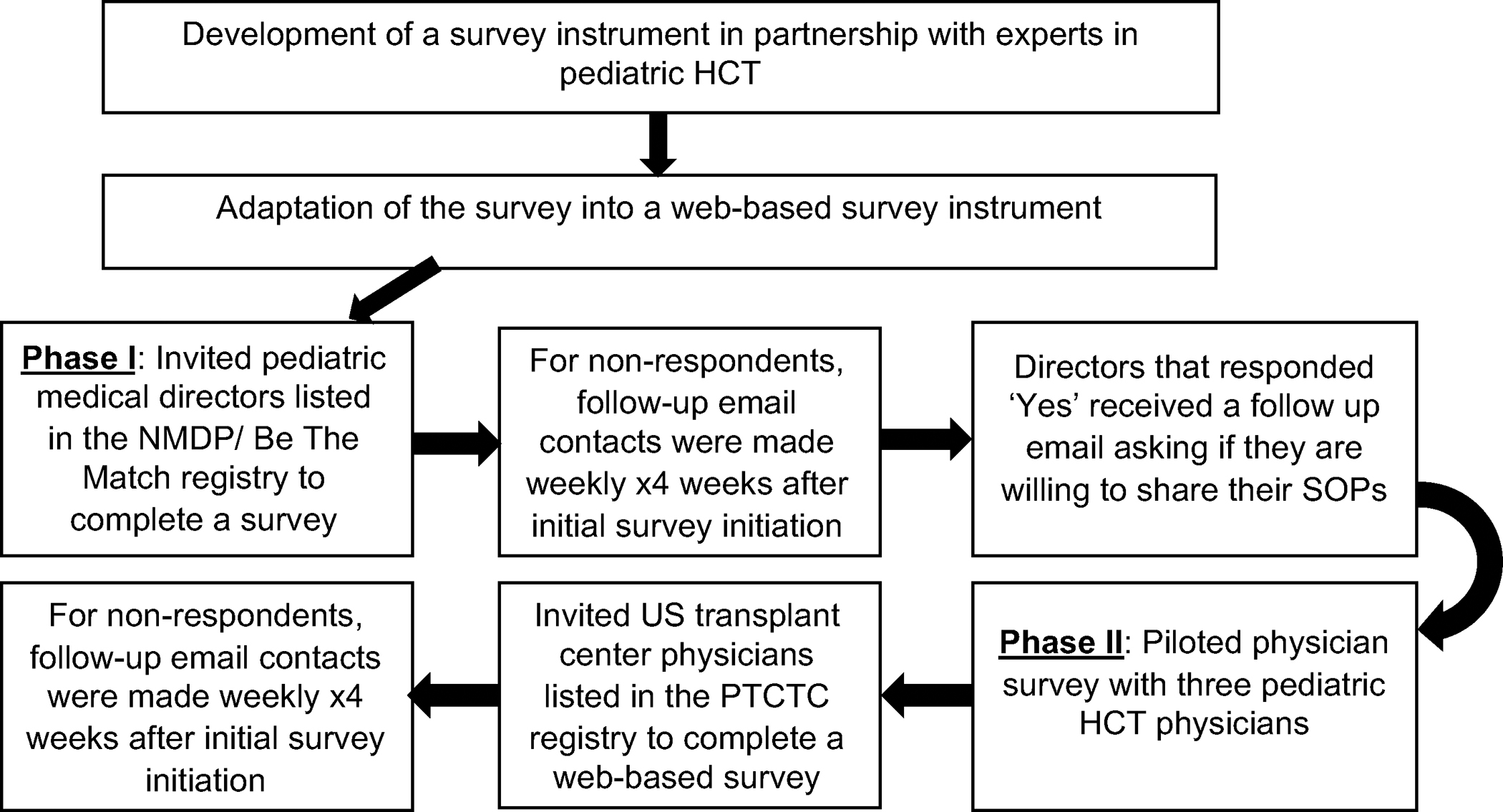
Survey development and administration plan
Descriptive analysis of survey data was performed, noting the frequency and percentages of survey responses. The answers were stratified by physicians’ demographic characteristics available through the survey and transplant center characteristics, such as location and average number of transplants performed per center as available through the CIBMTR.
Results:
Phase I:
Of 84 total pediatric HCT centers, 46 HCT center directors responded to Phase I (55% response rate). Twenty-eight (61%) reported having an SOP advising clinicians practicing at the transplant centers regarding return to school timing and decision-making for HCT recipients. Twelve SOPs (43%) were received and reviewed. The details of SOP responses are provided in Table 1. Wide variations existed in the recommendations regarding the need for specific assessments prior to return to school, the acceptable extent of immune suppression, and overall timing.
Table 1:
Return to school SOPs provided by HCT center directors
| Centers | Description |
|---|---|
| 1 | Allogeneic: At least 9 months before returning to school |
| 2 |
Both Allogeneic and Autologous: At least 6–12 months before returning to school Allogeneic: Ok to go back to school after 1 year even on IST |
| 3 |
Autologous: Minimum 3 months Allogeneic related donor: Minimum 6 months Allogeneic unrelated donor: Minimum 12 months Allogeneic: Check lymphocyte subsets in to verify adequate immunologic function to return |
| 4 |
Autologous: At least 3 months Allogeneic: At least 6 months and receiving vaccines |
| 5 | Both Allogeneic and Autologous: At 1-year post-BMT |
| 6 | Allogeneic: off or tapering GVHD meds, CD4+ >200/uL, and receiving vaccines |
| 7 |
Autologous: ALC >1000/uL, CD4 =200/uL Allogeneic: no GVHD ≥Grade II, ALC >1000/uL, CD4 >400/uL, weaning immunosuppression |
| 8 |
Allogeneic and Autologous: Response to mitogen & antigen-stimulated lymphocyte proliferation & ready to receive vaccines Allogeneic: No active, uncontrolled GVHD |
| 9 | Multiple factors are considered; psychosocial readiness, fatigue, immune suppression, etc. |
| 10 | Allogeneic: At least 6 months, receiving vaccines, central catheter removed, and no active GVHD requiring >0.5 mg/kg/day prednisone or ≥2 lines of immunosuppressive therapy |
| 11 | Allogeneic: off immune suppression, without active GVHD; with normal ANC and lymphocyte counts; CD4 >400/uL and immunoglobulin levels within normal range for age; receiving vaccines |
| 12 |
Autologous: after day 50 or at the discretion of primary oncologist and ANC >2000/uL Allogeneic: at least 6–12 months and off systemic immunosuppression |
IST= immune suppressive therapy; BMT= bone marrow transplant; GVHD= graft vs. host disease; ALC=absolute lymphocyte count; ANC=absolute neutrophil count
Half of the centers (n=6) recommended immunologic assessment prior to return to school. Specifically, Centers 6, 7, 11, and 12 recommended certain levels for absolute neutrophil count, absolute lymphocyte counts, and/ or CD4 T-lymphocyte counts; however, thresholds for these parameters differed. Five of the responding centers (42%) also recommended the initiation of re-immunization as one of the criteria for return to school. Additionally, Center 8 recommended assessing a response to mitogen & antigen-stimulated lymphocyte proliferation. Only one of the responding centers (Center 9) reported assessing physical and psychosocial readiness prior to return to school. Eight centers (67%) documented recommendations regarding the acceptable extent of immune suppression prior to return to school in their SOPs. Centers 11 and 12 required that allogeneic HCT recipients be off all immune suppression, while Centers 6 and 7 allowed allogeneic HCT recipients to return to school with tapering immune suppression, and Centers 8 and 10 were comfortable with allogeneic HCT recipients returning to school as long as graft vs. host disease (GVHD) was not active and/ or uncontrolled, which was defined by Center 10 as requiring greater than 0.5 mg/kg/day of prednisone or ≥2 lines of immunosuppressive therapy. Five of the SOPs (42%) provided predominantly timing-based return-to-school guidance; the recommended timing for return to school varied between 3–12 months for autologous HCT recipients and between 6–12 months for allogeneic HCT recipients. Center 2 recommended return to school at 1-year post-HCT for allogeneic HCT recipients even if they were on immune suppressive therapy.
Phase II:
In Phase II, 122 HCT physicians from 60 HCT centers responded to the survey (30.6% response rate) between December 14, 2020, and January 1, 2021. Table 2 shows the characteristics of the study population. Fifty-five percent (n=67) of the respondents were females, and 70% of physicians had more than ten years of experience. Respondents practiced across 32 states of the US with equal representation from Midwest (28%), South (28%), West (24%), and Northeast (20%) regions. Sixty-eight percent (n=83) practiced at HCT centers that, on average, performed up to 60 transplants per year.
Table 2:
Characteristics of Phase II Survey Respondents (N=122)
| Characteristics | N (%) |
|---|---|
| Age group, n (%) | |
| 30–40 | 20 (16.4) |
| 41–50 | 58 (47.5) |
| >50 | 43 (35.2) |
| Missing | 1 (0.8) |
| Years in Practice, n (%) | |
| 0–10 | 36 (29.5) |
| 11–20 | 53 (43.4) |
| >20 | 33 (27) |
| Sex, n (%) | |
| Male | 55 (45.1) |
| Female | 67 (54.9) |
| Region, n (%) | |
| Midwest | 35 (28.7) |
| Northeast | 24 (19.7) |
| South | 34 (27.9) |
| West | 29 (23.8) |
| Average pediatric transplants performed at center*, n (%) | |
| ≤25 | 40 (32.8) |
| 26–60 | 43 (35.2) |
| >60 | 39 (32.0) |
3-year average total of allogeneic and autologous HCT for ages < 21
Regarding recommended timing of return to school, variations in inter-physician practice were noted (Figure 2). The majority (60%) of respondents recommended that recipients of autologous HCT should return to school within 6 months post-HCT. For allogeneic HCT recipients, 65% recommended returning to school only after 6 months post-HCT or once off immune suppression. A lack of consensus was noted regarding the timing of return to school between HCT physicians practicing at the same center. When restricting the analysis to centers with more than one respondent (n=29 centers), physicians from only four centers (14%) showed agreement in recommended return to school timing. In the remaining centers, inter-physician practice variability was evident regardless of the availability of SOP (Figure 3a–b).
Figure 2:
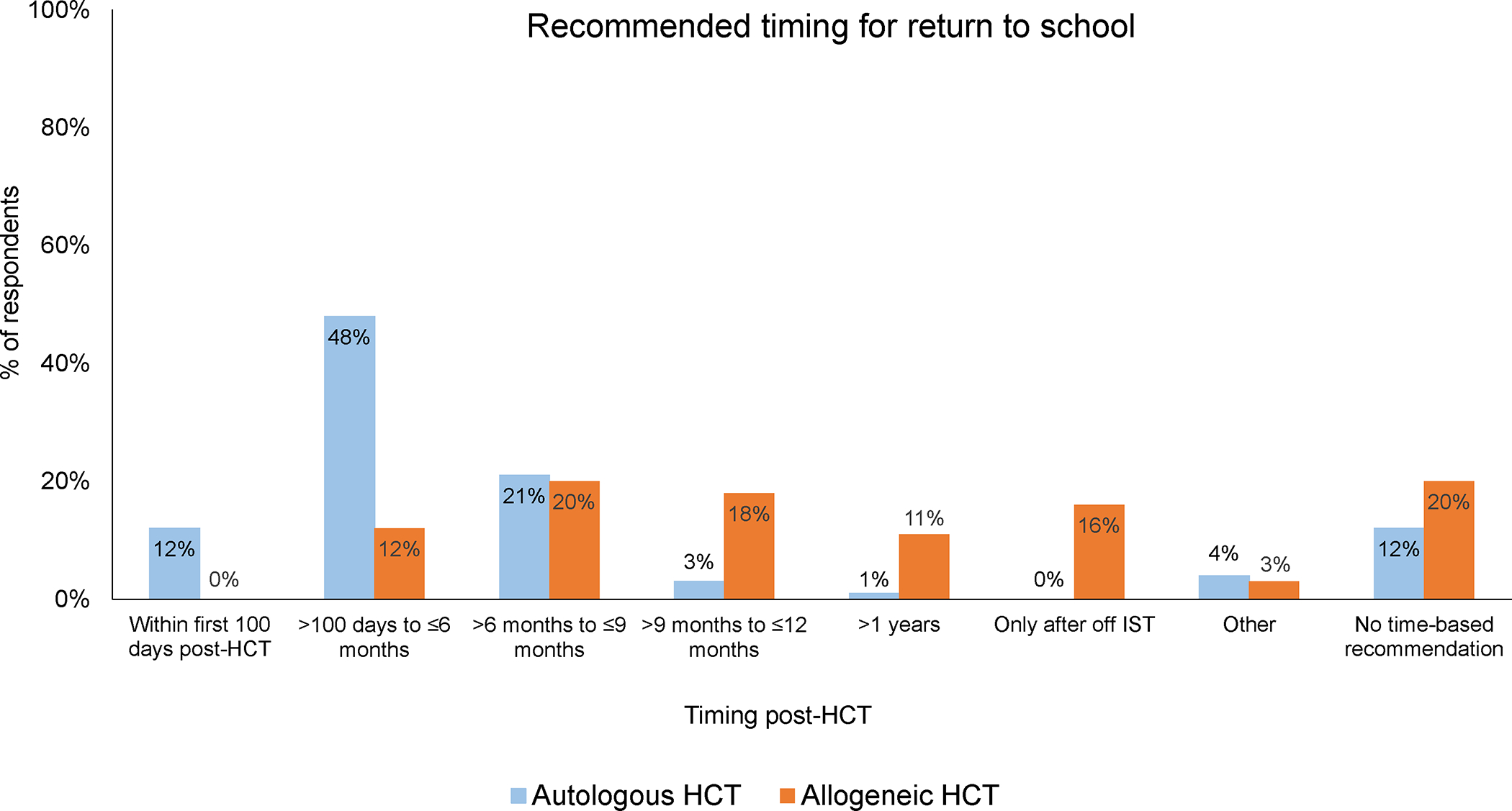
Variations physician practices regarding recommended return to school timings
Figure 3:
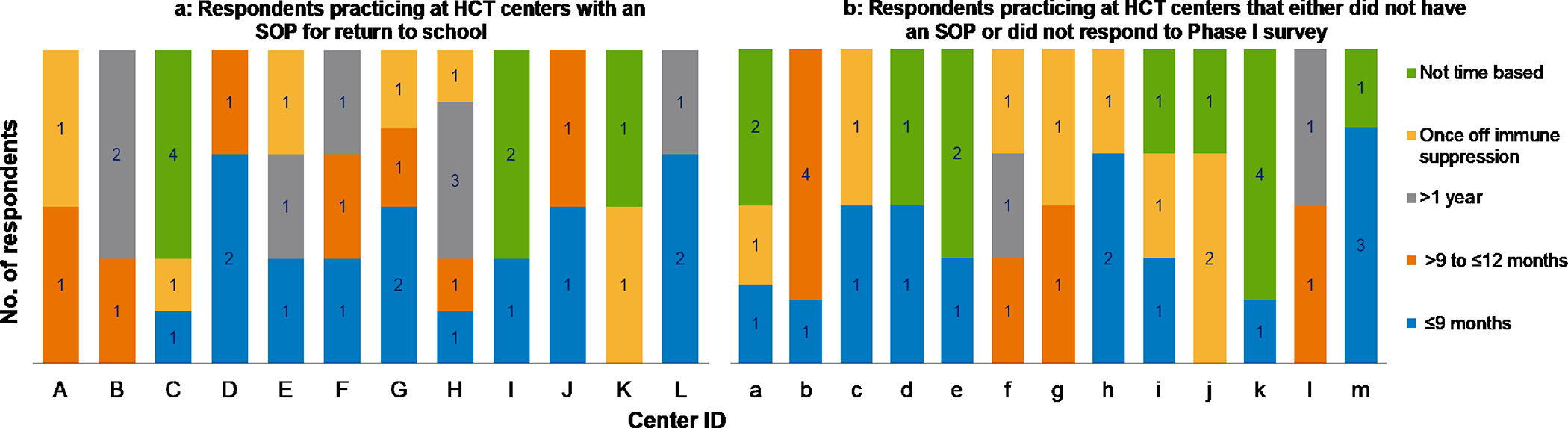
Variations in recommended return to school timing among HCT physicians at the same HCT centers according to the availability of a standardized operating procedure for return to school
Figure 3a: Respondents practicing at HCT centers with an SOP for return to school
Figure 3b: Respondents practicing at HCT centers that either did not have an SOP or did not respond to Phase I survey
When asked about the timing of the first detailed discussion regarding return to school with HCT recipients and/or caregivers, 65% of HCT physicians reported having their first detailed discussion with autologous HCT recipients and/or caregivers within 6 months post-HCT. In comparison, only 42% of respondents reported having the discussion with allogeneic HCT recipients and/or caregivers within 6 months post-HCT, but 77% had the discussion by 12 months post-HCT. Next, we asked participants how the return to school information is provided to HCT recipients and/or caregivers. Respondents stated that the discussion with a physician (92%) or advanced practice provider (61%) were the most common sources of return to school information delivery. Discussion with a physician remained the most common form of information delivery regardless of respondents’ age, sex, year of experience, HCT center location, or average number of transplants performed.
We also asked participants about the factors considered by them before recommending when HCT recipients should return to school post-HCT. The top three factors taken into consideration by physicians were ongoing health complications (88%), disease status (61%), and primary oncologist’s preference (47%) for autologous HCT recipients and GVHD or immune suppression (93%), immune reconstitution (89%), and ongoing health complications (87%) for allogeneic HCT recipients. Again, these findings remained consistent when stratified by the physician and center characteristics. Next, we asked physicians what topics are addressed by the HCT centers in preparation of a recipient’s return to school. Eighty percent of respondents reported addressing the need for an individualized education plan/ 504 plan prior to HCT recipients’ return to school post-HCT, whereas 75% reported addressing the need for homebound schooling/ tutoring. Physicians aged 41–50, from mid to large-size programs (averaging >25 transplants per year) and having 11–20 years of experience also reported directly communicating with school officials.
Lastly, we asked physicians to respond to five questions on a Likert scale ranging from strongly agree to strongly disagree. Overall, more than half of the respondents either agreed or strongly agreed that return to school is difficult for patients post-HCT (75%), physical demands make a return to school difficult for patients post-HCT (63%), emotional distress makes return to school difficult for patients post-HCT (67%), cognitive concerns make return to school difficult for patients post-HCT (63%), and lack of support from school and/or other students make return to school difficult for patients post-HCT (57%).
Discussion:
To our knowledge, this is the first study to describe the current pediatric HCT center practices and HCT physicians’ perspective on return to school after HCT. While three-quarters of the respondents stated that returning to school after HCT can be challenging for survivors, we noted a limited availability of standardized return to school recommendations within HCT centers. The existing recommendations varied in terms of timing and criteria for returning to in-person school. We also found a significant heterogeneity in return to school timing recommended by HCT physicians practicing at the same center regardless of the availability of standardized guidelines. Additionally, variations in factors affecting physicians’ decision-making and the type of support provided by HCT centers were also noted.
Majority of survey respondents felt that the process of returning to school can be challenging for HCT recipients, which is consistent with the prior studies that have assessed school and educational challenges in HCT survivors.7, 12–15 Despite this evidence, return to school support was not consistently provided by physicians as reported in our analysis. Additionally, there is a limited availability of online resources from national organizations to guide HCT recipients, caregivers, and healthcare and school professionals regarding the return to school process.16 In contrast, organizations such as the American Cancer Society and Leukemia & Lymphoma Society have developed materials to educate patients and caregivers, and other involved stakeholders regarding the return to school process for cancer survivors after completion of therapy.17–19 It is known that HCT survivors are at a higher risk of chronic health conditions and worse QOL compared to childhood cancer survivors treated with non-HCT therapy, likely due to their exposure to prolonged immune suppression and the cumulative impact of treatment exposures prior to and during HCT.20 These data along with our study findings emphasize the importance of providing better return to school guidance to HCT recipients.
The significant variations in the return to school guidance noted in our analysis likely stems from the lack of evidence-based data on this topic among HCT survivors. Several centers recommended assessments related to immune reconstitution prior to return to school, however, it is unclear whether they are based on any evidence. Prior studies have shown that HCT recipients remain at a higher risk of infections compared to cancer survivors treated with non-HCT therapy and general population even beyond 2 years post-HCT,21 albeit, it is unclear how these risks have changed with improvements in supportive care and use of antimicrobial prophylaxis. Additionally, chronic GVHD and ongoing immune suppression are known risk factors for fatal infections.22 On the contrary, from the studies focusing on childhood cancer survivors, it is also evident that extended school absences could significantly impact survivors’ their academic attainment, their future employment opportunities, and become productive members of the society.23, 24 Therefore, these data warrant serious consideration of school reintegration benefits in relation to the infection risks. While our study findings provide valuable information to HCT physicians regarding the current return to school practices across the country, given the wide variations in timing, assessments, and support, it does not help fill the unmet need. However, our study findings provide an opportunity to highlight the potential implications of variations in return to school practices at patient- and caregiver-, other stakeholder-, and center-level, which could further emphasize the importance of having standardized return to school practices across the country.
While our current analysis did not focus on the perspectives of patients and caregivers on the return to school practices, we recently performed a single center qualitative analysis of HCT recipients and their caregivers to understand the barriers and facilitators of return to school process after HCT.15 Availability and lack thereof communication and coordination from both healthcare and school professionals were reported as facilitators and barriers for the return to school process, which highlights the significant burden from return to school practice variations on caregivers. Caregivers also recommended having direct communication between family, school, and healthcare professionals prior to return to school and having a point of contact (return to school navigator) to improve the process for future patients15, which aligns with the recommendations provided through the psychosocial standard of care.25
Our findings have several implications on clinicians as well. Our analysis noted that HCT physicians play an important role in the return to school process since they reported being primarily responsible for providing schooling information to patients and families. This finding is consistent with another cross-sectional survey of pediatric oncologists practicing at Children’s Oncology Group member institutions.8 This survey was primarily focused on assessing physicians’ knowledge and training regarding cognitive and school-related challenges faced by childhood cancer survivors. The authors found that more than half of the physicians reported receiving no training, and two-thirds somewhat understood the return to school challenges faced by childhood cancer survivors. While the majority of HCT physicians in our analysis agreed that return to school process could be challenging for HCT survivors, our survey lacked information on their knowledge and training regarding the return to school process. Future work aimed towards understanding and improving HCT physicians’ understanding of HCT survivors’ return to school challenges would be beneficial.
Return to school is a complex process, and support from all stakeholders, including healthcare professionals and school professionals, is required for a successful return to school experience. While the respondents reported addressing several of the key topics, such as the need for an individualized education plan, homebound schooling, and gradual return to school approach, variations in available support were noted with low volume centers (≤25 procedures per year) reporting a lesser return to school support compared to higher volume centers. Additionally, physicians were less likely to address the need for physical or occupational therapy and discussion of legal rights related to school services. Only 65% of respondents reported direct communication with school officials prior to survivors’ return to in-person school. School professionals play an important role in the return to school process, and prior studies focusing on the return to school of childhood and adolescent cancer survivors treated with conventional (non-HCT) therapy have reported significant challenges related to limited communication between healthcare professionals and school officials.8–10 These findings provide a potential opportunity to improve communication between all involved stakeholders.
The inconsistent availability of and variability in return to school SOP also highlights the importance of improving return to school process at the HCT center level given the potential impact on quality of care. There is a growing need for quality improvement in the field of HCT and cellular therapy to improve both short- and long-term outcomes.26 At the center level, the process could possibly be improved using the Model for Improvement methodology, which includes rapid PDSA (Plan, Develop, Study, and Act) cycles to improve the process. On a broader scale, the use of learning health networks could also be considered, which include healthcare professionals, researchers, patients, and caregivers with a common goal of driving innovation and improving outcomes through sharing knowledge in real time and solving complex problems.27
Based on the findings from our study and their potential implications, as a next step, we envision development of standardized return to school guidelines. Such effort would require involvement of all stakeholders (physicians/ advance practice providers specializing in HCT and infectious diseases, with content specific expertise, nursing, social workers, psychologists, educators, and HCT recipients/ caregivers) to develop evidence or consensus-based recommendations for return to school timing, assessments, and support. Similar work was recently completed for the return to work process for HCT recipients with the support of the American Society of Transplantation and Cellular Therapy.28 Similar to the effort to improve the return to work process post-HCT, the development of standardized return to school guidelines would also require a thorough literature search on all relevant aspects of this topic. Subsequently, using the evidence as per available literature and expert-opinion based guidance, the recommendations should be made regarding the ideal timing of return to school post-HCT considering the risks and benefits, optimal assessments to understand survivors’ readiness to return to school from physical, psychological, and neurocognitive perspective, and type of support that should be provided to patients and caregivers prior to school reentry. In addition to the development of the guidelines, it would be equally important to ensure its dissemination to all stakeholders such as having patient and caregiver facing resources, practical checklists for healthcare professionals, educational materials for school professionals and classmates, and online resources from national organizations directed towards all stakeholders. . Lastly, emphasis should also be placed on ongoing evaluations to assess and ensure its uptake. Table 3 provides an outline of the proposed process of standardizing the return to school process for HCT recipients.
Table 3:
Proposed process to standardize return to school recommendations for patients undergoing hematopoietic cell transplant
| Stakeholder Involvement to develop evidence/ consensus-based recommendations for return to school timing, assessments, and support | • Hematopoietic cell transplant (HCT) specialists • Infectious disease specialists • Advance practice providers • Nursing • Social work • Educators • Psychologists • HCT recipients/caregivers |
| Dissemination of recommendations | • Patient and caregiver facing resources • Practical checklists for healthcare professionals • Standardized communication between healthcare and school professionals • Educational materials for school professionals and classmates • Online resources from national organizations targeted towards all stakeholders |
| Parameters for process/ outcomes assessment | • Target population adherence to return to school guidance • Increase in knowledge of patients/ caregivers/ teachers/ classmates • Patients’/ caregivers’ ability advocate/ access return to school resources • Assessment of patients’ quality of life through patient-reported outcomes |
There are certain limitations of our study that need to be acknowledged. While we noted a modest response rate of 30%, potentially limiting the generalizability of our findings, the variations in return to school timing and support were quite evident from the responses. However, our findings could be biased if only those who believed that returning to school is challenging and were more likely to have responded to the survey. While our analysis included respondents from several institutions across the United States, given the relatively small sample size, we were unable to perform a multivariable analysis. The survey was limited to HCT physicians, and therefore we were unable to understand the perspectives of other healthcare professionals, such as advance practice providers, nurse coordinators, social workers, and psychologists, who play key roles in the post-HCT return to school process. Lastly, while this survey was conducted during the COVID-19 pandemic, respondents were asked to specifically provide answers without considering the school closures and COVID-19 transmission risk among HCT recipients. In fact, a separate survey was administered at the same time focusing on post-HCT return to school practices during the COVID-19 pandemic, and the findings have been previously published.11
Notwithstanding, our analysis provides an important insight into the post-HCT return to school process from HCT physicians’ perspective. Using the data from this study and with help of all involved stakeholders, such as educators, social workers, and psychologists, we anticipate the development of standardized return to school recommendations. Additionally, the results of this study will help increase awareness of clinicians regarding school-related challenges for recipients of HCT, as well as increase their engagement in developing and testing future interventions to improve the return to school experience, and in turn quality of life, of HCT survivors.
Supplementary Material
Acknowledgement:
The authors wish to acknowledge the physicians who participated in this study. We additionally wish to sincerely thank Dr. Linda Burns for her valuable support throughout this study. Without her help, the current analysis would not have been possible.
Footnotes
Competing interests: All authors have no conflict of interest to disclosre relevant to this work.
Data Availability Statement:
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Reference:
- 1.Phelan R, Chen M, Bupp C, Bolon YT, Broglie L, Brunner-Grady J et al. Updated Trends in Hematopoietic Cell Transplantation in the United States with an Additional Focus on Adolescent and Young Adult Transplantation Activity and Outcomes. Transplant Cell Ther 2022. e-pub ahead of print 20220418; doi: 10.1016/j.jtct.2022.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevans M, El-Jawahri A, Tierney DK, Wiener L, Wood WA, Hoodin F et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biology of Blood and Marrow Transplantation 2017; 23(4): 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin DK Jr., Miller WC, Bayliff S, Martel L, Alexander KA, Martin PL. Infections diagnosed in the first year after pediatric stem cell transplantation. Pediatr Infect Dis J 2002; 21(3): 227–234. doi: 10.1097/00006454-200203000-00013 [DOI] [PubMed] [Google Scholar]
- 4.Brauer ER, Pieters HC, Ganz PA, Landier W, Pavlish C, Heilemann MV. “From Snail Mode to Rocket Ship Mode”: Adolescents and Young Adults’ Experiences of Returning to Work and School After Hematopoietic Cell Transplantation. J Adolesc Young Adult Oncol 2017; 6(4): 551–559. e-pub ahead of print 2017/06/09; doi: 10.1089/jayao.2017.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonen H, Petry K. How do children with a chronic or long-term illness perceive their school re-entry after a period of homebound instruction? Child Care Health Dev 2012; 38(4): 490–496. e-pub ahead of print 2011/07/05; doi: 10.1111/j.1365-2214.2011.01279.x [DOI] [PubMed] [Google Scholar]
- 6.Bhatia M, Kolva E, Cimini L, Jin Z, Satwani P, Savone M et al. Health-related quality of life after allogeneic hematopoietic stem cell transplantation for sickle cell disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015; 21(4): 666–672. doi: 10.1016/j.bbmt.2014.12.007 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt NS, Brazauskas R, Tecca HR, Vogel J, Mattila D, Lee SJ et al. Female Sex is Associated With Poor Health-related Quality of Life in Children at 12 Months Post-Hematopoietic Cell Transplantation. J Pediatr Hematol Oncol 2019; 41(3): 233–237. e-pub ahead of print 2018/06/21; doi: 10.1097/MPH.0000000000001239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruble K, Pare-Blagoev J, Cooper S, Jacobson LA. Pediatric oncology provider perspectives and practices: Supporting patients and families in schooling after cancer diagnosis. Pediatr Blood Cancer 2020; 67(4): e28166. e-pub ahead of print 20200113; doi: 10.1002/pbc.28166 [DOI] [PubMed] [Google Scholar]
- 9.Ruble K, Pare-Blagoev J, Cooper S, Martin A, Jacobson LA. Parent perspectives on oncology team communication regarding neurocognitive impacts of cancer therapy and school reentry. Pediatr Blood Cancer 2019; 66(1): e27427. e-pub ahead of print 2018/08/31; doi: 10.1002/pbc.27427 [DOI] [PubMed] [Google Scholar]
- 10.Pare-Blagoev EJ, Ruble K, Bryant C, Jacobson L. Schooling in survivorship: Understanding caregiver challenges when survivors return to school. Psychooncology 2019; 28(4): 847–853. e-pub ahead of print 20190307; doi: 10.1002/pon.5026 [DOI] [PubMed] [Google Scholar]
- 11.Bhatt NS, Meyer C, Mau LW, Broglie L, Devine S, Choi SW et al. Return-to-School Practices for Pediatric Hematopoietic Cell Transplantation Recipients during the COVID-19 Pandemic. Transplant Cell Ther 2022; 28(1): 54 e51–54 e54. e-pub ahead of print 20210917; doi: 10.1016/j.jtct.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke SA, Skinner R, Guest J, Darbyshire P, Cooper J, Vora A et al. Clinical outcomes and health-related quality of life (HRQOL) following haemopoietic stem cell transplantation (HSCT) for paediatric leukaemia. Child Care Health Dev 2011; 37(4): 571–580. e-pub ahead of print 2010/12/15; doi: 10.1111/j.1365-2214.2010.01182.x [DOI] [PubMed] [Google Scholar]
- 13.Nespoli L, Verri AP, Locatelli F, Bertuggia L, Taibi RM, Burgio GR. The impact of paediatric bone marrow transplantation on quality of life. Qual Life Res 1995; 4(3): 233–240. e-pub ahead of print 1995/06/01; doi: 10.1007/bf02260862 [DOI] [PubMed] [Google Scholar]
- 14.Felder-Puig R, Peters C, Matthes-Martin S, Lamche M, Felsberger C, Gadner H, Topf R. Psychosocial adjustment of pediatric patients after allogeneic stem cell transplantation. Bone Marrow Transplant 1999; 24(1): 75–80. e-pub ahead of print 1999/08/06; doi: 10.1038/sj.bmt.1701853 [DOI] [PubMed] [Google Scholar]
- 15.Bhatt NS, Shipman KJ, Rosenberg AR, Jenssen KM, Ballard SA, Baker KS, Barton KS. Barriers and facilitators of the return-to-school process affecting adolescent recipients of allogeneic hematopoietic cell transplantation: A qualitative interview study of caregivers. Pediatr Blood Cancer 2023: e30510. e-pub ahead of print 20230621; doi: 10.1002/pbc.30510 [DOI] [PubMed] [Google Scholar]
- 16.Going back to school. Be The Match. Accessed January 26, 2023, https://bethematch.org/patients-and-families/transplant-for-children-and-teens/going-back-to-school/#:~:text=Depending%20on%20the%20type%20of,before%20returning%20to%20the%20classroom. [Google Scholar]
- 17.Returning to School. Leukemia & Lymphoma Society. Accessed January 26, 2023, https://www.lls.org/children-and-young-adults/long-term-and-late-effects-treatment-childhood-cancer-survivors/returning [Google Scholar]
- 18.Returning to School After Cancer Treatment. American Cancer Society. Accessed January 26, 2023, https://www.cancer.org/treatment/children-and-cancer/when-your-child-has-cancer/after-treatment/returning-to-school.html [Google Scholar]
- 19.Ruble KJ, Pare-Blagoev EJ, Cooper SL, Jacobson LA. Assessment of Online Resources for Returning to School During and After Treatment of Childhood Cancer. J Cancer Educ 2020; 35(5): 876–884. doi: 10.1007/s13187-019-01537-y [DOI] [PubMed] [Google Scholar]
- 20.Armenian SH, Sun CL, Kawashima T, Arora M, Leisenring W, Sklar CA et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 2011; 118(5): 1413–1420. e-pub ahead of print 2011/06/10; doi: 10.1182/blood-2011-01-331835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foord AM, Cushing-Haugen KL, Boeckh MJ, Carpenter PA, Flowers MED, Lee SJ et al. Late infectious complications in hematopoietic cell transplantation survivors: a population-based study. Blood Adv 2020; 4(7): 1232–1241. doi: 10.1182/bloodadvances.2020001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norkin M, Shaw BE, Brazauskas R, Tecca HR, Leather HL, Gea-Banacloche J et al. Characteristics of Late Fatal Infections after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2019; 25(2): 362–368. e-pub ahead of print 2018/10/06; doi: 10.1016/j.bbmt.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogensen H, Tettamanti G, Frederiksen LE, Talback M, Harkonen J, Modig K et al. Educational attainment in survivors of childhood cancer in Denmark, Finland, and Sweden. Br J Cancer 2023. e-pub ahead of print 20231122; doi: 10.1038/s41416-023-02499-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devine KA, Christen S, Mulder RL, Brown MC, Ingerski LM, Mader L et al. Recommendations for the surveillance of education and employment outcomes in survivors of childhood, adolescent, and young adult cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Cancer 2022. e-pub ahead of print 20220418; doi: 10.1002/cncr.34215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson AL, Christiansen HL, Elam M, Hoag J, Irwin MK, Pao M et al. Academic Continuity and School Reentry Support as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer 2015; 62 Suppl 5: S805–817. e-pub ahead of print 2015/12/25; doi: 10.1002/pbc.25760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia M, Lehmann L, Auletta J, Beatty L, Bhatt N, Blacken R et al. Quality Improvement in Hematopoietic Stem Cell Transplant and Cellular Therapy: Using the Model for Improvement to impact Outcomes. Transplant Cell Ther 2022; 28(5): 233–241. e-pub ahead of print 20220210; doi: 10.1016/j.jtct.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 27.Ardura M, Hartley D, Dandoy C, Lehmann L, Jaglowski S, Auletta JJ, Transplant-Associated Learning Network T. Addressing the Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Hematopoietic Cell Transplantation: Learning Networks as a Means for Sharing Best Practices. Biol Blood Marrow Transplant 2020; 26(7): e147–e160. e-pub ahead of print 20200424; doi: 10.1016/j.bbmt.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salit RB, Schoeppner K, De Biase C, Mohammed J, Gonzales AL, Hashmi SK et al. American Society for Transplantation and Cellular Therapy Return to Work Guidance Committee Recommendations for Health Care Providers Who Take Care of Hematopoietic Cell Transplantation Patients. Transplant Cell Ther 2022; 28(12): 822–828. e-pub ahead of print 20220930; doi: 10.1016/j.jtct.2022.09.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


