Abstract
Advances in human genome editing, in particular the development of the clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 method, have led to increasing concerns about the ethics of editing the human genome. In response, the US National Academy of Sciences and the National Academy of Medicine constituted a multidisciplinary, international committee to review the current status and make recommendations. I was a member of that committee, and the core of this review reflects the committee’s conclusions. The committee’s report, issued in February 2017, recommends the application of current ethical and regulatory standards for gene therapy to somatic (nonheritable) human genome editing. It also recommends allowing experimental germline genome editing to proceed if (a) it is restricted to preventing transmission of a serious disease or condition, (b) the edit is a modification to a common DNA sequence known not to be associated with disease, and (c) the research is conducted under a stringent set of ethical and regulatory requirements. Crossing the so-called red line of germline genome editing raises important bioethical issues, most importantly, serious concern about the potential negative impact on individuals with disabilities. This review highlights some of the major ethical considerations in human genome editing in light of the report’s recommendations.
Keywords: human genome editing, germline (heritable) human genome editing, somatic (nonheritable) human genome editing, disability, genetic, mutation
INTRODUCTION
Ethical considerations and concerns about altering human DNA extend back at least 48 years, when, at the beginning of the revolution in molecular biology, Bernard Davis raised the prospect of “modifying the pattern of genes of a human being” (1, p. 1279). Since then, an enormous amount has been written on this topic from a wide breadth of viewpoints, including bioethics, religion, anthropology, philosophy (utilitarian and deontologic), law, sociology, and medicine (1–12). This body of commentary has been complemented by numerous attempts to sample public opinion, including the views of the general public and of individuals with specialized scientific and medical knowledge (13, 14).
Precedents abound regarding general acceptance of modifying a single person’s nonheritable (somatic) DNA in order to achieve a definable medical benefit with a favorable benefit-to-risk ratio. These include transplanting a solid organ from another individual and replacing an individual’s bone marrow with donor cells, both of which involve a recipient whose cells contain a different DNA sequence, as well as the addition of genes containing a DNA sequence different from that of the recipient in gene therapy to treat serious medical disorders. Robust ethical safeguards and regulatory policies have been developed to ensure that these procedures are conducted in accord with societal values, including nation-specific variations to accommodate different cultural and religious views (4, 15–19).
In contrast, the ethics of heritable (germline) gene modification lacks societal consensus and thus has been contentious, with some countries outlawing it under any circumstances and others placing barriers of varying heights. For example, 29 countries have signed and ratified the European Oviedo Convention (https://www.coe.int/en/web/bioethics/oviedo-convention), which specifically outlaws heritable genome editing (20, 21). In the United States, the Food and Drug Administration (FDA) requires an Investigational New Drug (IND) exemption for clinical trials involving transfer and gestation of a DNA-edited embryo in accord with Title 21 of the Code of Federal Regulations, Part 313 (21 CFR Part 312). The Research Technology Subcommittee of the US House of Representatives Committee on Science, Space, and Technology held a hearing on this topic in June 2015 (22). Subsequently, Congress passed an omnibus spending bill that explicitly prevented the FDA from using any of its resources to consider an IND application involving germline DNA modification (23). As a result, while not illegal in the United States, germline editing cannot proceed at present. Independently, National Institutes of Health (NIH) Director Francis Collins decided in 2015 that the NIH would not fund any use of gene-editing technologies in human embryos, thus reflecting the intent of the congressional Dickey-Wicker Amendment (H.R. 2880, Section 128) and the policies of the NIH Recombinant DNA Advisory Committee (24). In summarizing the subject, Dr. Collins, who had previously expressed concerns about human enhancement to the President’s Council on Bioethics in 2002 (25), noted that in addition to problems with informed consent, the “concept of altering the human germline in embryos for clinical purposes has been debated over many years from many different perspectives, and has been viewed almost universally as a line that should not be crossed.”
Dramatic technical advances in genome modification, particularly the introduction of the CRISPR/Cas9 method (26, 27), created a sense of heightened urgency in developing regulatory and ethical guidelines to address both somatic and germline genome editing. The nucleating event was the first report of modifying the DNA of a nonviable human embryo using CRISPR/Cas9 by Chinese scientists in 2015 (28). Soon thereafter, in December 2015, an international group of scientific leaders in the field joined with the Chinese Academy of Sciences and the United Kingdom Royal Society to sponsor a summit at the US National Academies of Science (29). That meeting culminated in a joint statement that included the following: “It would be irresponsible to proceed with any clinical use of germline editing unless and until the relevant safety and efficacy issues have been resolved … and there is broad societal consensus about the appropriateness of the proposed application” (30). The statement also highlighted the need for appropriate regulatory oversight.
In parallel, the US National Academy of Sciences (NAS) and the National Academy of Medicine (NAM) cosponsored the creation of an international committee composed of individuals from diverse disciplines to review the current state of the field and make recommendations about policies and procedures governing human genome editing. The committee issued its report in February 2017 (31). The author of this review was a member of that committee, and the core of this review reflects the conclusions of the committee’s report (32).
OVERARCHING PRINCIPLES
As a subset of human subjects research, human genome editing performed anywhere in the world should conform to international conventions and norms for protecting human rights as they relate to human experimentation. This is of particular importance in human genome editing because differences in cultural and religious values and priorities essentially foreclose the possibility of gaining international agreement on a single set of rules and regulations. If, however, rules and regulations are widely divergent from country to country with regard to regulatory oversight and conformity to international bioethical standards, both scientists and patients may choose to migrate to countries with less stringent regulations or ethical standards. To address these challenges, the NAS/NAM committee report builds on a long tradition in developing international standards of behavior, including the 1948 Universal Declaration of Human Rights by the United Nations (33) and the 1979 Belmont Report by the US National Commission for the Protection of Human Subjects in Biomedical and Behavioral Research (34). The report proposed seven overarching principles: promoting well-being, transparency, due care, responsible science, respect for persons, fairness, and transnational cooperation. These have all been extensively analyzed in the bioethical literature and so are not further discussed in this review.
NONHERITABLE (SOMATIC) HUMAN GENOME EDITING
The bioethical and regulatory framework developed for gene therapy (which involves adding genetic information to a person’s cells) in many countries is largely applicable to nonheritable human genome editing. In the United States, this framework includes review by an Institutional Review Board, the FDA via an IND, and, under certain circumstances, the Recombinant DNA Advisory Committee (17–19, 35). The central issues are the likely benefit-to-risk ratio and potential alternative therapies (36). In addition, when the gene therapy procedure involves novel aspects, the FDA requires long-term follow-up observations extending out as far as 15 years to mitigate potential risks (17, 31). Agreement to such long-term follow-up is included in the informed consent document signed by the participant, but of course participants are free to withdraw from the study at any time. The FDA approved the first human gene therapy product in 2017 to treat Leber’s hereditary blindness (37).
Even after FDA approval, postmarket requirements of approved gene therapy and genome editing products are likely to be rigorous, including documentation of the proficiency of physicians in the proper use of the technology and entry of data into patient registries. The potential for less-well-regulated off-label uses of these products is of considerable concern, especially uses other than treating or preventing a serious illness. However, the specificity of the approved product may limit the risks (31). Special requirements are in place to insure that gene therapy products do not inadvertently integrate into germline DNA (16), and these requirements will also likely be applicable to nonheritable human genome editing studies.
The current jurisdictional uncertainty about the FDA’s authority to regulate some stem cell therapies, especially when cells are taken from a person, altered, and returned to the same person, is a potential vulnerability if extended to nonheritable human genome editing (31). Consequently, the FDA’s authority and its willingness to enforce its authority in human genome editing are issues highlighted by the NAS/NAM report.
Editing of somatic cells of fetuses in utero may be medically justified when a disease has early onset and irreversible effects. Moreover, the plasticity of fetal cells may provide important scientific benefits when genome editing is performed in utero (38). Such use would, however, raise the ethical issue of being unable to obtain consent from the fetus. Under NIH guidelines (45 CFR Part 46, Subpart B), fetal research must hold the prospect of direct benefit to the fetus, and both maternal and paternal (if available) consent is required. For comparison, in some cases of fetal surgery, or when maternal health is involved, maternal consent is sufficient. Editing the cells of a fetus in utero may, however, increase the risks of unintended germline editing, especially if the editing occurs before germ cells are sequestered from somatic cells (31).
In summary, the NAS/NAM report (31) identified a number of diseases that might benefit from somatic genome editing (see table 4–1, p. 92) and recommended that (a) the current ethical and regulatory mechanisms for reviewing and approving gene therapy protocols are also appropriate for somatic gene therapy protocols that involve human genome editing and for somatic genome editing protocols that do not involve gene therapy, and (b) such protocols should currently be limited to those that are designed to “treat or prevent disease and disability.” Any expansion of the use of somatic cell genome editing for other purposes should be based on “inclusive public policy debates” (31, p. 110).
HERITABLE (GERMLINE) HUMAN GENOME EDITING
Heritable human genome editing has the potential to (a) prevent the transmission of genetic variants unequivocally known to be associated with a serious illness or condition, (b) lessen the likelihood of developing a serious illness or condition, and (c) enhance a human’s function beyond typical human capabilities.
In the first category, when preimplantation genetic diagnosis is not applicable (39), a couple may face the difficult choice of either not having biologically related children or knowing unequivocally that they will pass along a genetic variant responsible for a disorder such as sickle cell disease or Huntington disease. Germline genome editing thus has the potential to give this couple another option. The second category applies to couples who are predicted to pass along a genetic variant that would substantially increase the risk of a disease, such as BRCA1 variants associated with an increased risk of developing breast cancer. The second category also potentially encompasses population-based public health efforts to reduce the risks of future diseases or disabilities. For example, inactivation of a particular gene, which can be readily accomplished with CRISPR/Cas9, can lower cholesterol levels (40), blood pressure (41), or the likelihood that an individual will be infected by HIV (42). The third category of germline genome edits includes those designed to enhance human function, for example, increasing muscle mass to increase strength (43, 44). Table 1 contains a partial list of the various objections that have been raised to performing germline genome editing, divided into those that are relevant to all three categories of potential applications and those that are only relevant to applications other than preventing the transmission of a variant known to be associated with a serious illness or condition.
Table 1.
Concerns about heritable (germline) human genome editing
| Concerns common to all applications | Concerns specific to applications other than preventing transmission of genetic variants known to be associated with serious illness or disability |
|---|---|
|
| |
| Disrespect of DNA as human heritage Challenging God’s role in creation Lack of informed consent by the child and future generations affected by the editing Negative impact on individuals with disabilities related to genetic variants Perceptions of parental negligence for deciding against performing genome editing |
Commodification of children Creation of social pressure to modify children to maintain a level playing field with children modified by other parents Exacerbation of social inequality based on access to the technology Unknown and unpredictable risks of creating novel genome modifications Potential to create harm that will extend to multiple generations Potential for state-imposed eugenic applications Potential for criminal applications |
OBJECTIONS COMMON TO ALL FORMS OF HUMAN GERMLINE GENOME EDITING
Disrespect of Human DNA as a Human Heritage
The idea has been advanced that humans have an obligation to preserve and protect human DNA from any alteration because it reflects our uniquely human collective heritage (45), perhaps analogous to our obligation to protect other common goods, like public lands, as heritages. In assessing this objection, it is important to appreciate the intrinsic dynamism of human DNA. The overall intergenerational mutation rate of human DNA, that is, the frequency with which a germline DNA base is different in a child relative to a parent, is approximately 12 times per billion (46). This is a net rate that reflects both inaccurate replication and nonreplicative DNA alterations, for example, from exposure to a toxic chemical or spontaneous deamination of 5-methylcytosine at CpG sites (47), as well as the effectiveness of the cell’s elaborate mechanisms to repair DNA that has not been accurately replicated. Since humans have approximately three billion base pairs in their genome, it is likely that every time a cell divides, the new cell’s DNA differs in sequence from that of the parent cell.
While our medical orientation predisposes us to consider DNA mutations as threats to health, from the standpoint of evolutionary biology, it is vital for humans to have mutable DNA so that our species can improve its fitness to survive in the current environment or adapt to changing environments. Indeed, from this perspective, mutations are not errors or a reflection of the infidelity of the replicating molecular machinery but rather an intrinsic and important aspect of our species’ evolutionary success. The mutation rate is perhaps better characterized as an exploration rate than as an error rate. As Lewis Thomas wrote (48, p. 23), “The capacity to blunder slightly is the real marvel of DNA. Without this special attribute, we would still be anaerobic bacteria and there would be no music.”
If we consider our DNA a human heritage, modifying a variant associated with a serious illness or condition to a sequence that is present in many other individuals in the same group and is not associated with a disease or disability (that is, a reference sequence) would not introduce a novel DNA sequence, and thus would not alter humankind’s DNA. It is not clear whether individuals who express concern about DNA as heritage view disease-associated variants as part of that heritage. If they do, then any modification would trigger this objection.
Challenging God’s Role in Creation
The NAS/NAM committee heard presentations from a broad range of religious leaders who provided diverse views about human germline genome editing (31). Since one possible method of germline genome editing involves editing an embryo’s DNA prior to implantation, and since this would almost certainly result in some embryos not being implanted, religions that reject all research or artificial reproductive methods that may result in the nonimplantation or destruction of an embryo oppose such germline genome editing. It is possible, however, that in the future human germline genome editing may be performed on human gametes produced by in vitro gametogenesis from induced pluripotent stem cells derived from human somatic cells, such as those from the skin (48–51). In fact, this approach may be preferable from both a scientific and a regulatory standpoint because editing of the cells in an embryo may result in only some of the embryo’s cells being successfully edited (mosaicism), and it is difficult or impossible to be sure that all of the cells in the embryo are successfully edited (52). In contrast, editing sperm precursor cells in vitro, followed by in vitro expansion, allows for the testing of large numbers of sperm precursors after editing and affords a more robust assessment of the success of the edit. Gamete precursor editing has already been successfully performed in mice, but many obstacles remain in translating this technology to humans (48–51). If these are overcome, however, it could obviate the religious objections related to embryo destruction.
The mainstream western Judeo-Christian tradition recognizes the legitimacy and importance of medical interventions to protect human health based on the belief that humans are made in God’s image and therefore humans have an obligation to protect God’s creation. Similarly, religions in this tradition tend to emphasize the importance of having children, in some cases even making it an obligation (53–56). Thus, with the exception of those religions that oppose embryo editing or in vitro fertilization, they are unlikely to oppose human germline genome editing performed to prevent the transmission of a serious disease or disability, especially when the disability results in infertility.
Religious concerns are likely to be much more intense if human germline genome editing is used to enhance human performance or even reduce the risk of developing a disease by making novel changes in the DNA sequence. Polls of public opinion on genome editing have repeatedly identified a substantial percentage of people who express serious concern about such edits (13, 14, 57). For example, a Pew Research Center poll of US adults in 2016 found that 46% of individuals felt that genome editing to give babies a much-reduced disease risk “crosses a line, is meddling with nature,” and an even higher percentage of religious respondents expressed this view (13, 14, 57).
Lack of Informed Consent by the Child and Future Generations Affected by the Edits
Informed consent by the individual agreeing to participate in a research study is the cornerstone principle of human experimentation, since that individual has to accept the potential risks in order to have the opportunity to enjoy the potential benefits (58). This principle cannot be fully met when the research participant is a child below the age of decision-making capacity. In the United States, the decision is left to one or preferably both parents, and added safeguards are introduced. These include the requirement for a chance of direct benefit to the child from participating if the research involves more than minimal risk and, with few exceptions, obtaining the child’s assent to participate if the child is old enough to have an understanding of the procedures involved (21 CFR 46, Subpart D). When the research involves a fetus, assent is not possible, and there is a requirement to try to obtain consent from both parents (45 CFR 46, Subpart B).
Heritable human genome editing has similarities to fetal research, but it differs in that there is a potential for all future generations of the offspring of the individual whose genome is edited to be affected by the edit, without their consent or the consent of their parents. Once again, if the edit is performed to prevent the transmission of a serious disease or disability by changing a DNA sequence to one known to be common and not associated with a disease, the concern may be mitigated. However, it is not eliminated, since this is striking new bioethical grounds without precedent. These concerns are likely to be more intense if the goal is to enhance human capabilities and/or introduce novel DNA sequences. Thus, there is an immediate need to develop an ethical framework, policies, and procedures for multigenerational consent for experimental procedures that may alter germline DNA.
Negative Impact on Individuals with Disabilities Related to Genetic Variants
Historically, individuals with genetic disorders that produce phenotypes identifiable as abnormal by the general public have been stigmatized, bullied, discriminated against, and even physically assaulted. Such treatment can have a serious psychological impact on individuals with the disorders, especially children, arousing feelings of insecurity and anxiety about self-image and self-worth that often lead to social isolation. Great advances have been made in the United States in countering the stigmatization and unfair treatment of individuals with genetic disorders, including the passage of the Americans with Disabilities Act in 1990 and the growth of both disease-specific patient advocacy groups and umbrella organizations representing large numbers of patient advocacy groups (59, 60). Advocates for individuals with these disorders also have changed the fundamental discourse on disabilities, with some members of the deaf community, for example, contending that deafness is not a disability but rather a manifestation of human diversity and that it is important to protect and preserve the deaf community’s culture. This view is manifested by documented examples of couples’ use of preimplantation genetic diagnosis to insure that they had a deaf child to carry on the culture (61). It is thus vital to develop and sustain a dialogue between healthcare professionals and advocates for individuals with disabilities, especially since studies indicate that healthcare professionals tend to overestimate the impact of some disabilities on life satisfaction of children and their families (62).
Public accommodations and public education have dramatically improved conditions for individuals with genetic disorders that compromise ordinary function, both in schools and in the workplace (63, 64). Nonetheless, residual stigmatization remains, and given people’s innate fear of those who are different from themselves, the advances are fragile. Thus, the emphasis that human genome editing places on “correcting” mutations has the potential for the unintended consequence of stigmatizing and marginalizing individuals with genetic disorders. It is vital, therefore, to redouble our efforts to protect against such stigmatization.
One place to start is with careful attention to the language used to describe human genome editing. The word “editing” itself is value laden, implying correction or improvement in a text, whereas a dispassionate analysis of the science is much more nuanced. For example, the prevalence of the gene for hemoglobin S is considerably higher in regions of the world where malaria is endemic because heterozygosity for hemoglobin S and hemoglobin A confers protection from death from malaria, although homozygosity for hemoglobin S dramatically reduces life span without modern therapy (65, 66). Thus, in a region where malaria is prevalent, making the single DNA base pair conversion from hemoglobin S to hemoglobin A in a heterozygous person would not be “correcting” a mutation; rather, it would be eliminating a potentially important genetic adaptation. Whenever possible, therefore, it is important to consider the impact of language on those with genetic disorders and select terms that are as value free as possible. In our hemoglobin example, modifying the single base pair from encoding hemoglobin S to encoding hemoglobin A could be termed “converting to a reference sequence.” The word “mutation” itself, while value free when used in scientific writing, has a negative connotation for much of the lay public. Use of the term “variant,” which has much less of a negative connotation, coupled with education to inform the public that all human beings have some genetic variants relative to even group-specific reference sequences as part of human evolution, may provide a more appropriate and supportive context for avoiding stigmatization of individuals with genetic disorders.
Perceptions of Parental Negligence for Deciding Against Performing Genome Editing
Once heritable genome editing becomes available, parents who choose not to have their progeny undergo the procedure may be viewed as being negligent for failing to perform the editing if they have a child with a genetic disorder (67). This may have serious social and psychological effects and may even have legal implications.
OBJECTIONS SPECIFIC FOR APPLICATIONS OTHER THAN PREVENTING TRANSMISSION OF GENETIC VARIANTS KNOWN TO BE ASSOCIATED WITH A SERIOUS DISEASE OR CONDITION
Traditional bioethical formulations of genome modification have separated interventions designed to treat an illness from those designed to enhance human function, placing these along one axis, and separated somatic (nonheritable) from germline (heritable) modifications on the other axis (Figure 1a) (67–68b). The binary division into treatment versus enhancement, however, omits the application of genome editing for the prevention of illness via risk reduction. In fact, the identification of risk factors for disease, many of which are under genetic control, and the design of interventions to lower those risks have been major contributors to improved human life expectancy over the past half century, with better control of hypertension and hypercholesterolemia being two vivid examples (69, 70). Moreover, as associations between genetic variants and disease risk have been identified, measures to prevent the development of the disease despite the genetic predisposition have been devised, such as prophylactic bilateral mastectomy to prevent the development of breast cancer in women with deleterious BRCA1 variants (71). What distinguishes these preventive efforts from those related to genetic variants with large phenotypic effects and near-complete penetrance (i.e., Mendelian disorders), such as sickle cell disease and Huntington disease, is that they operate at a probabilistic (stochastic) level rather than a deterministic level. As a result, it is not possible to be certain that a given individual at increased risk will actually develop the disorder. At the population level, however, it is virtually certain that modifying the genomes of a large number of individuals with the variants would result in a decrease in the incidence of the illness.
Figure 1.
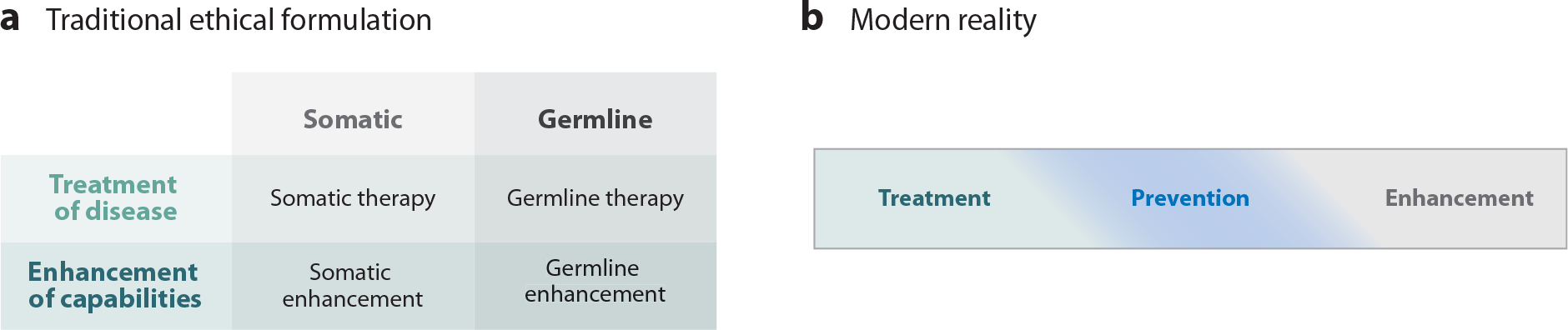
Ethical formulations of human genome editing. (a) Traditional ethical formulation (adapted from 67a and 68b with permission) with treatment/enhancement dichotomy and somatic/germline dichotomy. (b) Modern reality formulation, in which disease prevention occupies a middle ground, melding at its borders into both treatment and enhancement.
Preventing disease by modifying risk-associated variants thus occupies a middle ground between treatment and enhancement and bleeds into both of those categories (Figure 1b) (68). For example, based on both the reduced risk of cardiovascular disease in association with reduced cholesterol levels in individuals heterozygous for inactivating variants in the gene PCSK9 (40) and the demonstrated risk reduction afforded by drugs that antagonize the enzymatic activity of PCSK9 (72), I suspect that most people would consider inactivating one PCSK9 gene in a patient with a history of several heart attacks and elevated cholesterol levels refractory to currently available drugs a treatment. Performing the same genetic modification in the patient’s younger brother, who has not yet had a heart attack but has an elevated cholesterol level, would probably be considered an act of prevention. But what about performing the same intervention on the patient’s 21-year-old healthy son, who does not have an elevated cholesterol level, in order to decrease his future risk of cardiovascular disease? Is that prevention, enhancement, or both?
Views on Human Enhancement
While as noted, there is a broad consensus supporting the use of nonheritable (somatic) genetic modifications to treat patients with serious illness, there is nearly universal discomfort about using genetic modifications for human enhancement, despite the recognition that to some extent genetic enhancement is an essential feature underlying all of evolution (2, 10, 24, 25). Individuals who espouse the principles of transhumanism are an exception, contending that humans are obligated to improve themselves by any means available (73–75).
The psychological anxiety elicited by the concept of enhancement may be rooted in two mutually conflicting aspects of our evolutionary background, namely, the desire for personal success and the need for communal cooperation. If one views human evolution exclusively from the standpoint of the “selfish gene” (76), one will emphasize the importance of individual reproductive success, which undoubtedly benefits from enhancements in attractiveness and performance, with the latter translating in our modern world into material success and financial security. It is not surprising, therefore, that most nongenetic enhancements in society are directly or indirectly connected to appearance, which translates into attractiveness for preferred mate selection, or to physical or mental abilities and knowledge that are likely to result in professional success. The strength of the desire for such enhancements is attested to by the risks individuals are willing to accept (e.g., those of cosmetic surgery), as well as the costs (e.g., that of a college education), in exchange for the perceived benefits. Thus, there is compelling evidence that individuals have a strong desire to compete with others for success and to stand out from the crowd.
More recently, however, in order to explain the biologic advantages conferred by humans’ large brains and ability to communicate by language, evolutionary biologists have emphasized the benefits of group cooperation, as for example in communal hunting. Such cooperation requires conformity to social norms of group cohesion and mechanisms to enforce such norms (77), and storytelling made possible by human language may play an important role (78). A reasonably fair distribution of resources is one of the cardinal elements in such group behavior, and so it is of especial interest that children as young as six years of age are willing to forego receiving candy in order to prevent others from benefiting from an unfair distribution of candy (79).
The tension between these principles—individual achievement and recognition versus group cooperative success and social cohesion—is perhaps most dramatically seen in the field of sports. There is enormous admiration for superior individual athletic performance, coupled with adulation for the athlete, but this is balanced by admiration for teamwork and team effort. There is near universal disdain if athletes are self-aggrandizing at the expense of their teammates, or worse yet if it turns out that the superior performance was aided by performance-enhancing drugs. Individuals have literally gone from being heroes to heels overnight (80). Thus, while it is impossible to predict how public opinion will evolve with regard to the application of human genome editing for enhancement, concerns that some individuals will have an unfair advantage in social and economic competition is likely to be a central element in public perception. For example, if individuals learn which children underwent genome editing for enhancement, the children are likely to be viewed as having an unfair advantage, perhaps eliciting the kind of response generated by athletes who have surreptitiously used performance-enhancing drugs.
Commodification of Children
Thoughtful critics of heritable human genome editing have noted that if one has the ability to modify the genes of one’s children, that is, to produce “designer” babies, there is a risk that the relationship between parents and children will undergo radical changes, with parents expressing their values, priorities, and aspirations in the genetic makeup of their children. Thus, children will not be embraced as precious gifts regardless of their genetic makeup, but rather will become extensions of their parents’ clever ability to design them (12, 81, 82) and “a commodity that is to be ordered at will” (83). This commodification may have profound implications, including parental guilt if the design does not turn out well, especially when the children reach an age when they can question their parents’ choices.
Creation of Social Pressure to Modify Children to Maintain a Level Playing Field with Children Modified by Other Parents
It seems self-evident that if even a small percentage of parents decide to modify their child’s DNA to reduce the child’s risk of developing diseases or enhance the child’s functional capabilities, then other parents will feel pressure to modify their children’s DNA in order to level the playing field on which they will compete. This can set off a parental genetic “arms race” in which riskier and riskier edits are attempted.
Exacerbation of Social Inequality
If genome editing is successful in advantaging those who undergo the procedure, by definition it is likely to result in inequality. Since the wealthy are most likely to gain access to the technology, the new biologic inequality would be grafted onto the current wealth inequality, which has been increasing at an alarming rate over the past several decades and threatens social cohesion (84).
Unknown and Unpredictable Risks of Creating Novel Genome Variants
Converting a variant known to be associated with a disease or disability to a reference sequence common in the individual’s genetic group that is known not to be associated with a disease or disorder is highly unlikely to have unanticipated effects. However, DNA edits that create novel DNA sequences that have not previously been identified in humans will necessarily confer a risk of unforeseen and unintended consequences. Such sequences are likely to be introduced when editing by CRISPR/Cas9 or similar technology is used to inactivate a gene, as is currently commonly performed in animals, since the procedure relies on the double-strand break being repaired by nonhomologous end joining, which introduces unpredictable sequences that may disrupt the normal transcription and/or translation of the gene (85). This concern would also extend to any off-target effects of genome editing, which involve making double-strand breaks in the DNA at sites other than the intended site of editing. It also extends to the undesirable production of long DNA deletions and complex DNA rearrangements from on-site breaks that may alter gene function or expression (86). While remarkable advances have been made in improving the specificity of the editing techniques and in identifying off-target effects since the technology was first developed (87), it may not be possible to achieve certainty. An off-target edit in an unexpected gene thus may have important consequences. The history of the unpredicted and unexpected insertional mutagenesis leading to hematopoietic malignancies associated with the vectors used in early gene therapy trials provides a compelling case for scientific and medical humility (88).
Potential for Harm That Will Extend to Multiple Generations
If a heritable gene edit does harm, there is the potential for that harm to extend beyond the initial individual to their progeny. This would complicate family planning and might lead to a decision not to have children or undergo assisted reproduction in combination with preimplantation genetic diagnosis, with their associated risks and costs, to prevent transmission of the harmful DNA edit. In cases where preimplantation genetic diagnosis would not be able to prevent transmission of the harmful edit, the individual would have to consider undergoing another editing procedure to correct the harmful edit.
Potential for State-Imposed Eugenic Applications
Totalitarian and democratic regimes have demonstrated their willingness to impose control over human reproduction for political purposes (89–91). There is real concern, therefore, that if heritable human genome editing is able to produce war fighters with enhanced functional capabilities, whether physical or mental (as for cyber warfare or disinformation campaigns), it will unleash a genome editing arms race with serious national security consequences. Nonmilitary state-imposed eugenics is also a potential concern; for example, a state may decide to enhance worker productivity and reduce healthcare costs by requiring heritable genome editing to reduce blood pressure, cholesterol levels, and the risk of HIV infection, all of which can be achieved by inactivating currently known genes.
Potential for Criminal Applications
A 2016 announcement of a new television series in development, starring Jennifer Lopez and titled C.R.I.S.P.R., stated that each episode will explore a bio-attack and crime related to genome editing. Story lines include a genetic assassination attempt on the president and the framing of an unborn child for murder. The villain is a diabolical genius scientist with a “twisted God complex” (92). If Hollywood can dream up criminal applications, it is not farfetched to think that real criminals can as well.
NAS/NAM COMMITTEE RECOMMENDATIONS ON HERITABLE HUMAN GENOME EDITING
The NAS/NAM committee report details the statements by international groups expressing reservations about considering heritable human genome editing, including the clear legal barrier created by the European Oviedo Convention (5, 6, 8, 20). Despite these concerns, the report recommends that clinical trials be permitted to proceed for limited purposes under strict controls. The NAS/NAM report (31) recommends that clinical trials using heritable genome editing be permitted only within a robust and effective regulatory framework that includes the following conditions:
the absence of reasonable alternatives;
restriction to preventing a serious disease or condition;
restriction to editing genes that have been convincingly demonstrated to cause or to strongly predispose to that disease or condition;
restriction to converting such genes to versions that are prevalent in the population and are known to be associated with ordinary health with little or no evidence of adverse effects;
the availability of credible preclinical and/or clinical data on risks and potential health benefits of the procedures;
ongoing, rigorous oversight during clinical trials of the effects of the procedure on the health and safety of the research participants;
comprehensive plans for long-term, multigenerational follow-up that still respect personal autonomy;
maximum transparency consistent with patient privacy;
continued reassessment of both health and societal benefits and risks, with broad ongoing participation and input by the public; and
reliable oversight mechanisms to prevent extension to uses other than preventing a serious disease or condition.
In making this recommendation, the committee emphasized the complexity of balancing individual-level benefits and societal-level risks. On one side, the report acknowledges the desire of a couple to use the technology to have an unaffected biologically related child who would have a level playing field on which to compete against other children; on the other side, it acknowledges potential societal harms, which may include those detailed above, as well as concern that the desire for a biologically related child involves an outdated notion of kinship in an era when adoption, same-sex marriage, donor gametes, surrogacy, and step-parenting are increasing (93). We currently have limited ethical frameworks and mechanisms for balancing benefits that accrue primarily to individuals against what some perceive as societal harms, as attested to by the ongoing intense debate about abortion. In the realm of human experimentation, it is unclear how much latitude Institutional Review Boards have in adjudicating group cultural risks of research beyond the direct risks to individuals, and there are few precedents and little experience in weighing such complex factors (94). From a US legal standpoint, if the state decides to restrict a person’s freedom to choose an activity, it needs to present at least a rational basis for its action if ordinary liberties are restricted, but it needs a much more compelling justification if it denies or closely regulates fundamental liberties, especially those enumerated in the Bill of Rights (31). The procreative rights of parents fall within a disputed area between these categories, often receiving a form of intermediate scrutiny, and so it is uncertain how courts may rule if parents challenge state restrictions on their perceived right to utilize heritable genome editing (95). As noted above, there is a theoretical possibility that parents may be considered legally negligent if they have an affected child with a genetic disorder that might have been avoided by genome editing.
Perhaps most important, from a policy standpoint, is the serious concern that genome editing will diminish support for laws protecting individuals with disabilities. In this regard, it is comforting that the same era that has seen improved genetic screening and preimplantation genetic diagnosis has also seen strong support for laws protecting individuals with disabilities. It is notable, therefore, that even within the disability community, people hold widely varying views of the benefits and risks of genome editing technology (96–98).
Finally, some individuals oppose performing any heritable human genome editing, even when they do not oppose its use to prevent transmission of a serious disease or condition, because they fear a “slippery slope” in which its permitted use for a desirable purpose will one day expand to include its use for undesirable purposes, most notably enhancement (52). Similar arguments were advanced to challenge the introduction of in vitro fertilization and preimplantation genetic diagnosis, but in fact, neither technique expanded into the disconcerting realms predicted. Whether heritable human genome editing will follow these precedents or slide down a slippery slope is currently unknowable.
CONCLUSION
The NAS/NAM report supports crossing what has been a bright red line by recommending that clinical trials of heritable human genome editing be allowed. However, by restricting its use to preventing a serious disease or condition, limiting the editing procedure to prevent the creation of novel DNA sequences, and requiring a robust bioethical and regulatory framework, the report focuses the technology on the goal of having healthy babies, not designer babies. Public reaction to the report has been generally favorable, and its publication has triggered multiple proposals to widen the opportunities for broad, ongoing societal input (99, 100). An excerpt from an editorial in the Washington Post soon after the publication (101) provides one eloquent example.
“A line would have to be drawn between heritable changes that are clearly valuable and those that risk unnecessarily humiliating people, destabilizing society and changing the nature of humanity. The panel attempted to draw a preliminary line—and put it in the right place The debate will not—and should not—end there. But before society has a full chance to process these questions, the panel’s approach is the right one. The goal should be to stop crippling diseases, not to build designer babies.”
ACKNOWLEDGMENTS
I thank Harriet Rabb, Dr. Eli Adashi, and Zachary Shapiro for valuable comments. This work was supported in part by grant UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Davis BD. 1970. Prospects for genetic intervention in man. Science 170:1279–83 [DOI] [PubMed] [Google Scholar]
- 2.Pres. Counc. Bioeth. 2003. Beyond Therapy: Biotechnology and the Pursuit of Happiness. Washington, DC: Harper Perennial. 352 pp. [Google Scholar]
- 3.Kohn DB, Porteus MH, Scharenberg AM. 2016. Ethical and regulatory aspects of genome editing. Blood 127:2553–60 [DOI] [PubMed] [Google Scholar]
- 4.Juengst ET. 1991. Germ-line gene therapy: back to basics. J. Med. Philos. 16:587–92 [DOI] [PubMed] [Google Scholar]
- 5.Group Hinxton. 2015. Statement on genome editing technologies and human germline genetic modification. http://www.hinxtongroup.org/hinxton2015_statement.pdf [DOI] [PubMed] [Google Scholar]
- 6.Hirsch F, Levy Y, Chneiweiss H. 2017. CRISPR-Cas9: a European position on genome editing. Nature 541:30. [DOI] [PubMed] [Google Scholar]
- 7.Cox DB, Platt RJ, Zhang F. 2015. Therapeutic genome editing: prospects and challenges. Nat. Med. 21:121–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Counc. Europe 2015. Statement on genome editing technologies. https://www.coe.int/en/web/bioethics/news/-/asset_publisher/EV74osp47zWZ/content/gene-editing [Google Scholar]
- 9.Chan S, Donovan PJ, Douglas T, et al. 2015. Genome editing technologies and human germline genetic modification: the Hinxton Group consensus statement. Am. J. Bioeth. 15:42–47 [DOI] [PubMed] [Google Scholar]
- 10.Pres. Comm. Stud. Bioeth. Issues. 2015. Gray matters: topics at the intersection of neuroscience, ethics, and society. https://bioethicsarchive.georgetown.edu/pcsbi/sites/default/files/GrayMatter_V2_508.pdf [Google Scholar]
- 11.Ramsey P 1971. The ethics of a cottage industry in an age of community and research medicine. N. Engl. J. Med. 284:700–6 [DOI] [PubMed] [Google Scholar]
- 12.Fletcher J 1971. Ethical aspects of genetic controls. Designed genetic changes in man. N. Engl. J. Med. 285:776–83 [DOI] [PubMed] [Google Scholar]
- 13.Funk C, Kennedy B, Podrebarac Sciupac E. 2016. U.S. public wary of biomedical technologies to ‘enhance’ human abilities. Pew Res. Cent. Internet and Technol. Rep. http://www.pewinternet.org/2016/07/26/u-s-public-wary-of-biomedical-technologies-to-enhance-human-abilities/ [Google Scholar]
- 14.Nuffield Counc. Bioeth. 2016. Public dialogue on genome editing: Why? When? Who? Rep. Worksh. Public Dialogue for Genome Editing. http://nuffieldbioethics.org/wp-content/uploads/Public-Dialogue-on-Genome-Editing-workshop-report.pdf [Google Scholar]
- 15.Kessler DA, Siegel JP, Noguchi PD, et al. 1993. Regulation of somatic-cell therapy and gene therapy by the Food and Drug Administration. N. Engl. J. Med. 329:1169–73 [DOI] [PubMed] [Google Scholar]
- 16.EMEA (Eur. Med. Agency). 2006. ICH considerations: general principles to address the risk of inadvertent germline integration of gene therapy vectors. Int. Counc. Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Rep. CHMP/ICH/469991/2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002679.pdf [Google Scholar]
- 17.FDA. 2006. Guidance for industry: gene therapy clinical trials—observing subjects for delayed adverse events. Guidance for industry, US Dep. Health Hum. Serv., Food and Drug Admin., Cent. Biologics Eval. Res. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm078719.pdf [Google Scholar]
- 18.FDA. 2015. Considerations for the design of early-phase clinical trials of cellular and gene therapy products. Guidance for industry, US Dep. Health Hum. Serv., Food and Drug Admin., Cent. Biologics Eval. Res. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM564952.pdf [Google Scholar]
- 19.FDA. 1991. Points to consider in human somatic cell therapy and gene therapy 1991. Hum. Gene Ther. 2:251–56 [DOI] [PubMed] [Google Scholar]
- 20.Andorno R 2005. The Oviedo convention: a European legal framework at the intersection of human rights and health law. J. Int. Biotechnol. Law 2:133–43 [Google Scholar]
- 21.Ishii T 2015. Germline genome-editing research and its socioethical implications. Trends Mol. Med. 21:473–81 [DOI] [PubMed] [Google Scholar]
- 22.Comm. Sci., Space, Technol. 2015. Subcommittee examines human genetic engineering. Press Release, Jun. 16, Comm. Sci., Space, Technol., Washington, DC. https://science.house.gov/news/press-releases/subcommittee-examines-human-genetic-engineering [Google Scholar]
- 23.Cohen IG, Adashi EY. 2016. The FDA is prohibited from going germline. Science 353:545–46 [DOI] [PubMed] [Google Scholar]
- 24.Collins FS. 2015. Statement on NIH funding of research using gene-editing technologies in human embryos. https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologies-human-embryos [Google Scholar]
- 25.Collins FS. 2002. Genetic enhancements: current and future prospects. Paper presented at Pres. Counc. Bioeth., Washington, DC, Dec. 12–13. https://bioethicsarchive.georgetown.edu/pcbe/transcripts/dec02/session5.html [Google Scholar]
- 26.Wright AV, Nunez JK, Doudna JA. 2016. Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. Cell 164:29–44 [DOI] [PubMed] [Google Scholar]
- 27.Lander ES. 2016. The heroes of CRISPR. Cell 164:18–28 [DOI] [PubMed] [Google Scholar]
- 28.Liang P, Xu Y, Zhang X, et al. 2015. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 6:363–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NASEM (Natl. Acad. Sci. Eng. Med.). 2015. International Summit on Human Gene Editing: A Global Discussion. Dec. 1–13, Washington, DC. http://nationalacademies.org/gene-editing/Gene-Edit-Summit/ [Google Scholar]
- 30.Baltimore D, Baylis F, Berg P, et al. 2015. On human gene editing: international summit statement. News release, Dec. 3, International Summit on Human Gene Editing. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a [Google Scholar]
- 31.Comm. Hum. Gene Editing: Sci., Med., Ethical Consid. 2017. Human Genome Editing: Science, Ethics, and Governance. Washington, DC: Natl. Acad. Press [Google Scholar]
- 32.Hynes RO, Coller BS, Porteus M. 2017. Toward responsible human genome editing. JAMA 317:1829–30 [DOI] [PubMed] [Google Scholar]
- 33.United Nations. 1948. Universal declaration of human rights. http://www.un.org/en/udhrbook/pdf/udhr_booklet_en_web.pdf [Google Scholar]
- 34.Natl. Comm. Prot. of Hum. Subj. of Biomed. and Behav. Res. 1979. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. US Dep. Health, Educ., and Welf. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html [Google Scholar]
- 35.IOM (Inst. Med.). 2014. Oversight and Review of Clinical Gene Transfer Protocols: Assessing the Role of the Recombinant DNA Advisory Committee. Washington, DC: Natl. Acad. Press; [PubMed] [Google Scholar]
- 36.Califf RM. 2017. Benefit-risk assessments at the US Food and Drug Administration: finding the balance. JAMA 317:693–94 [DOI] [PubMed] [Google Scholar]
- 37.FDA. 2017. FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. News Release, Dec. 19, US Food and Drug Admin., Washington, DC. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm589467.htm [Google Scholar]
- 38.McClain LE, Flake AW. 2016. In utero stem cell transplantation and gene therapy: recent progress and the potential for clinical application. Best Pract. Res. Clin. Obstet. Gynaecol. 31:88–98 [DOI] [PubMed] [Google Scholar]
- 39.Lander ES. 2015. Brave new genome. N. Engl. J. Med. 373:5–8 [DOI] [PubMed] [Google Scholar]
- 40.Cohen J, Pertsemlidis A, Kotowski IK, et al. 2005. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37:161–65 [DOI] [PubMed] [Google Scholar]
- 41.Ji W, Foo JN, O’Roak BJ, et al. 2008. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat. Genet. 40:592–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutter G, Bodor J, Ledger S, et al. 2015. CCR5 targeted cell therapy for HIV and prevention of viral escape. Viruses 7:4186–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SM. 2017. This guy says he’s the first person to attempt editing his DNA with CRISPR. BuzzFeed News, Oct. 14. https://www.buzzfeed.com/stephaniemlee/this-biohacker-wants-to-edit-his-own-dna?utm_term=.fskkY6Ywx#.ce0N2R28a [Google Scholar]
- 44.Wang X, Niu Y, Zhou J, et al. 2018. CRISPR/Cas9-mediated MSTN disruption and heritable mutagenesis in goats causes increased body mass. Anim. Genet. 49:43–51 [DOI] [PubMed] [Google Scholar]
- 45.UNESCO. 2017. Universal declaration on the human genome and human rights: adopted on 11 November 1997. In Universal Declaration on the Human Genome and Human Rights (1997) and International Declaration on Human Genetic Data (2003). Paris: United Nations Educ., Sci. and Cult. Organ. http://unesdoc.unesco.org/images/0025/002539/253908e.pdf [Google Scholar]
- 46.Scally A 2016. The mutation rate in human evolution and demographic inference. Curr. Opin. Genet. Dev. 41:36–43 [DOI] [PubMed] [Google Scholar]
- 47.Gao Z, Wyman MJ, Sella G, et al. 2016. Interpreting the dependence of mutation rates on age and time. PLOS Biol. 14:e1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas L 1979. The Medusa and the Snail. New York: Viking [Google Scholar]
- 49.Cohen IG, Daley GQ, Adashi EY. 2017. Disruptive reproductive technologies. Sci. Transl. Med. 9:372. [DOI] [PubMed] [Google Scholar]
- 50.Hikabe O, Hamazaki N, Nagamatsu G, et al. 2016. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 539:299–303 [DOI] [PubMed] [Google Scholar]
- 51.Ishikura Y, Yabuta Y, Ohta H, et al. 2016. In vitro derivation and propagation of spermatogonial stem cell activity from mouse pluripotent stem cells. Cell Rep. 17:2789–804 [DOI] [PubMed] [Google Scholar]
- 52.Lanphier E, Urnov F, Haecker SE, et al. 2015. Don’t edit the human germ line. Nature 519:410–11 [DOI] [PubMed] [Google Scholar]
- 53.Dorff EN. 1998. Matters of Life and Death: A Jewish Approach to Modern Medical Ethics Philadelphia: Jewish Publ. Soc. [Google Scholar]
- 54.Dorff EN, Zoloth L. 2015. Jews and Genes: The Genetic Future in Contemporary Jewish Thought. Philadelphia: Jewish Publ. Soc. [Google Scholar]
- 55.Napier S Dignitas Personae on gene therapy and enhancement: a commentary on Dignitas Personae, Part Three, nn 24–27. https://www.ncbcenter.org/resources/information-topic/dignitas-personae/gene-therapy [Google Scholar]
- 56.Mena A 2017. Catholics shouldn’t totally reject human gene editing—but it still has ethical problems. News Release, Apr. 11, Catholic News Agency, Denver, CO https://www.catholicnewsagency.com/news/catholics-shouldnt-totally-reject-human-gene-editing–but-it-still-has-ethical-problems-29961 [Google Scholar]
- 57.Blendon RJ, Gorski MT, Benson JM. 2016. The public and the gene-editing revolution. N. Engl. J. Med. 374:1406–11 [DOI] [PubMed] [Google Scholar]
- 58.Shuster E 1997. Fifty years later: the significance of the Nuremberg Code. N. Engl. J. Med. 337:1436–40 [DOI] [PubMed] [Google Scholar]
- 59.Moitra K, Garcia S, Jaldin M, et al. 2017. ABCC6 and pseudoxanthoma elasticum: the face of a rare disease from genetics to advocacy. Int. J. Mol. Sci. 18:1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terry SF. 2017. The study is open: participants are now recruiting investigators. Sci. Transl. Med. 9(371):eaaf1001. [DOI] [PubMed] [Google Scholar]
- 61.Mand C, Duncan RE, Gillam L, et al. 2009. Genetic selection for deafness: the views of hearing children of deaf adults. J. Med. Ethics 35:722–28 [DOI] [PubMed] [Google Scholar]
- 62.Parens E, Asch A. 2000. Prenatal Testing and Disability Rights. Washington, DC: Georgetown Univ. Press [Google Scholar]
- 63.Global Genes. 2018. Advocating for your child with a rare disease at their school. RARE Toolkit, Parent Information Cent. Special Education, New England Genetics Collaborative, Durham, NH. https://globalgenes.org/wp-content/uploads/2015/08/GG_toolkit_educational-advocacy_web-hyperlinked.pdf [Google Scholar]
- 64.Sundar V 2017. Operationalizing workplace accommodations for individuals with disabilities: a scoping review. Work 56:135–55 [DOI] [PubMed] [Google Scholar]
- 65.Bunn HF. 2013. The triumph of good over evil: protection by the sickle gene against malaria. Blood 121:20–25 [DOI] [PubMed] [Google Scholar]
- 66.Piel FB, Patil AP, Howes RE, et al. 2010. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat. Commun. 1:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daniels N 2000. Normal functioning and the treatment-enhancement distinction. Cambridge Q. Healthcare Ethics 9:309–22. [DOI] [PubMed] [Google Scholar]
- 67a.Walters L, Palmer JG. 1997. The Ethics of Human Gene Therapy. New York: Oxford Univ. Press [Google Scholar]
- 68.Juengst ET. 1997. Can enhancement be distinguished from prevention in genetic medicine? J. Med. Philos. 22:125–42. [DOI] [PubMed] [Google Scholar]
- 68a.Evans JH. 2002. Playing God? Human Genetic Engineering and the Rationalization of Public Bioethical Debate. Chicago: Univ. Chicago Press. [DOI] [PubMed] [Google Scholar]
- 68b.Evans JH, Schairer CE. 2009. Bioethics and human genetic engineering. In Handbook of Genetics and Society, ed. Atkinson P, Glasner P, Lock M, pp. 349–66. New York: Routledge [Google Scholar]
- 69.Wadhera RK, Steen DL, Khan I, et al. 2016. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 10:472–89 [DOI] [PubMed] [Google Scholar]
- 70.Oparil S, Acelajado MC, Bakris GL, et al. 2018. Hypertension. Nat. Rev. Dis. Primers 4:18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carbine NE, Lostumbo L, Wallace J, et al. 2018. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst. Rev. 4:CD002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt AF, Pearce LS, Wilkins JT, et al. 2017. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 4:CD011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris J 2007. Enhancing Evolution: The Ethical Case for Making People Better. Princeton, NJ: Princeton Univ. Press [Google Scholar]
- 74.Bostrom N 2005. In defense of posthuman dignity. Bioethics 19:202–14 [DOI] [PubMed] [Google Scholar]
- 75.Porter A 2017. Bioethics and transhumanism. J. Med. Philos. 42:237–60 [DOI] [PubMed] [Google Scholar]
- 76.Dawkins R 1989. The Selfish Gene. Oxford/New York: Oxford Univ. Press [Google Scholar]
- 77.Buckholtz JW, Marois R. 2012. The roots of modern justice: cognitive and neural foundations of social norms and their enforcement. Nat. Neurosci. 15:655–61 [DOI] [PubMed] [Google Scholar]
- 78.Smith D, Schlaepfer P, Major K, et al. 2017. Cooperation and the evolution of hunter-gatherer storytelling. Nat. Commun. 8:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McAuliffe K, Jordan JJ, Warneken F. 2015. Costly third-party punishment in young children. Cognition 134:1–10 [DOI] [PubMed] [Google Scholar]
- 80.Fotheringham W 2015. Timeline: Lance Armstrong’s journey from deity to disgrace. Guardian, Mar. 8 [Google Scholar]
- 81.Sandel MJ. 2013. The case against perfection. In Society, Ethics, and Technology, ed. Winston M, Edelbach R, pp. 343–54. Boston: Cengage Learning [Google Scholar]
- 82.Meilaender G 2008. Chapter 11: Human dignity: exploring and explicating the council’s vision. In Human Dignity and Bioethics: Essays Commissioned by the President’s Council on Bioethics. Washington, DC: Pres. Counc. Bioeth. https://bioethicsarchive.georgetown.edu/pcbe/reports/human_dignity/chapter11.html [Google Scholar]
- 83.Catholic News Agency. 2009. New ‘designer babies’ make life a commodity, bioethicist says. News Release, Catholic News Agency, Mar. 4, Denver, CO. https://www.catholicnewsagency.com/news/new_designer_babies_make_life_a_commodity_bioethicist_says [Google Scholar]
- 84.Cent. Genet. Soc. 2015. Extreme genetic engineering and the human future: Reclaiming emerging biotechnologies for the common good. Rep., Cent. Genet. Soc., Berkeley, CA. https://1bps6437gg8c169i0y1drtgz-wpengine.netdna-ssl.com/wp-content/uploads/wpallimport/files/archive/FOE_ExtremeGenEngineering_10.pdf [Google Scholar]
- 85.Rouet P, Smih F, Jasin M. 1994. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 14:8096–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kosicki M, Tomberg K, Bradley A. 2018. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotech. 36:765–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen JS, Dagdas YS, Kleinstiver BP, et al. 2017. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 550:407–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nienhuis AW, Dunbar CE, Sorrentino BP. 2006. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 13:1031–49 [DOI] [PubMed] [Google Scholar]
- 89.Proctor R 1988. Racial Hygiene: Medicine Under the Nazis. Boston: Harvard Univ. Press. 414 pp. [Google Scholar]
- 90.Greenhalgh S 2008. Just One Child: Science and Policy in Deng’s China. Berkeley/Los Angeles: Univ. Calif. Press. 404 pp. [Google Scholar]
- 91.Black E 2003. War Against the Weak: Eugenics and America’s Campaign to Create a Master Race. New York: Four Walls Eight Windows [Google Scholar]
- 92.Goldberg L 2016. Jennifer Lopez sets futuristic bio-terror drama at NBC (exclusive). Hollywood Reporter, Oct. 8. https://www.hollywoodreporter.com/live-feed/jennifer-lopez-sets-futuristic-bio-939509 [Google Scholar]
- 93.Franklin S 2013. Biological Relatives: IVF, Stem Cells, and the Future of Kinship. Durham/London: Duke Univ. Press. 364 pp. [Google Scholar]
- 94.Klitzman RL. 2013. How IRBs view and make decisions about social risks. J. Empir. Res. Hum. Res. Ethics 8:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murray M, Luker K. 2014. Cases on Reproductive Rights and Justice. St. Paul, MN: Foundation Press. 949 pp. [Google Scholar]
- 96.Hernandez B, Keys CB, Balcazar FE. 2004. Disability rights: attitudes of private and public sector representatives. J. Rehabil. 70:28–37 [Google Scholar]
- 97.Makas E 1988. Positive attitudes toward disabled people: disabled and nondisabled persons’ perspectives. J. Soc. Issues 44:49–61 [Google Scholar]
- 98.Steinbach RJ, Allyse M, Michie M, et al. 2016. “This lifetime commitment”: public conceptions of disability and noninvasive prenatal genetic screening. Am. J. Med. Genet. A 170A:363–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scheufele DA, Xenos MA, Howell EL, et al. 2017. U.S. attitudes on human genome editing. Science 357:553–54 [DOI] [PubMed] [Google Scholar]
- 100.Jasanoff S, Hurlbut JB. 2018. A global observatory for gene editing. Nature 555:435–37 [DOI] [PubMed] [Google Scholar]
- 101.Board Ed. 2017. A way forward in gene editing. Washington Post, Feb. 28. https://www.washingtonpost.com/opinions/a-way-forward-in-gene-editing/2017/02/18/2c9a2036-f550-11e6-b9c9-e83fce42fb61_story.html?utm_term=.27c34bd8ea51 [Google Scholar]


