Respiratory syncytial virus (RSV) is the leading viral cause of acute lower respiratory tract infections, including bronchiolitis and pneumonia, in children under 5 years of age globally.1 Historically, RSV-associated hospitalization rates among American Indian and Alaska Native (AI/AN) children have been among the highest in the world.2–7 Contemporary estimates of RSV-acute respiratory infection (ARI) are needed to inform RSV prevention strategies for AI/AN children.
METHODS
We conducted active, facility-based surveillance for ARI among hospitalized AI/AN children < 5 years in Chinle, Arizona (Navajo Nation), Whiteriver, Arizona (White Mountain Apache Tribal lands), and Anchorage, and the Yukon-Kuskokwim (YK) Delta region of Alaska in November 2019 to May 2020. We identified all potentially eligible individuals through on-site surveillance. Participants who met the WHO case definition for extended severe ARI were enrolled following parental informed consent.8 Study-specific midturbinate nasal swabs were stored in universal transport medium at −80°C until testing via single-plex reverse-transcription quantitative polymerase chain reaction (RT-qPCR) at Vanderbilt University Medical Center for RSV A and B. Demographic and clinical data were collected by questionnaire and chart review.
Overall and age-stratified incidence rates per 1000 for all-cause ARI and RSV-associated hospitalization were calculated using the Poisson distribution (SAS v9.4, Cary, NC). Numerators of enrolled children meeting the ARI case definition were adjusted to account for eligible but not enrolled cases (Supplemental Table 2). The age-stratified Indian Health Service (IHS) User Population was used as the denominator. This study was reviewed by the Centers for Disease Control and Prevention and relevant institutional review boards (IRBs) and was conducted consistent with applicable federal law and Centers for Disease Control and Prevention policy*. See Supplemental Information for additional details.
RESULTS
This study enrolled 324 children hospitalized with ARI (Chinle: 82, Whiteriver: 73, Anchorage: 33, YK Delta: 136) (Supplemental Figs 2 and 3, Supplemental Table 3 and 4). The mean age was 16.8 months in Southwest sites and 10.6 months in Alaska sites (P < .01). Household density was highest in YK Delta (2.4 persons per room), as was the proportion of homes without running water (35.3%).
All children enrolled had a RSV test result available; 171 (53%) tested RSV-positive. One-third of RSV-associated hospitalizations occurred in infants < 6 months old (Table 1). Among RSV-positive children, mean length of stay ranged from 3.5 days (Chinle) to 5.7 days (YK Delta) and use of supplemental oxygen ranged from 70% (Anchorage, elevation 102 feet) to 100% (Whiteriver, elevation 5290 feet). Among children < 6 months old, the annual RSV-associated hospitalization rates per 1000 children were: Anchorage 35.7 (95% confidence interval 20.4–62.6); Chinle 83.0 (52.0–132.5); Whiteriver 70.4 (36.3–136.6); and YK Delta 132.3 (98.2–178.1). Among children < 5 years old, rates were: Anchorage 7.7 (5.3–11.1); Chinle 27.2 (21.4–34.4); Whiteriver 25.4 (18.7–34.5); and YK Delta 32.7 (26.9–39.7) (Fig 1; Supplemental Table 5).
TABLE 1.
Demographics and Clinical Characteristics of AI/AN Children < 5 y of Age Hospitalized With RSV-associated Acute Respiratory Infection by Site, November 2019 to May 2020
| Variablef | Chinle, Arizona N = 50 | Whiteriver, Arizona N = 32 | Anchorage, Alaska N = 20 | Yukon-Kuskokwim Delta, Alaska N = 69 |
|---|---|---|---|---|
| Male, n (%) | 28 (56.0) | 14 (43.8) | 10 (50.0) | 34 (49.3) |
| Age in months, mean (range) | 17.3 (0–54) | 14.9 (0–41) | 10.6 (1–54) | 9.8 (0–56) |
| Age group (months), n (%) | ||||
| 0 to <3 | 4 (8.0) | 2 (6.3) | 8 (40.0) | 15 (21.7) |
| 3 to <6 | 8 (16.0) | 4 (12.5) | 3 (15.0) | 14 (20.3) |
| 6 to <12 | 10 (20.0) | 9 (28.1) | 0 | 22 (31.9) |
| 12 to <24 | 15 (30.0) | 11 (34.4) | 8 (40.0) | 11 (15.9) |
| 24 to <36 | 7 (14.0) | 4 (12.5) | 0 | 5 (7.3) |
| 36 to <48 | 3 (6.0) | 2 (6.3) | 0 | 1 (1.5) |
| 48 to <60 | 3 (6.0) | 0 | 1 (5.0) | 1 (1.5) |
| Other children <5 y in home, n (%) | 48 (96.0) | 28 (87.5) | 19 (95.0) | 52 (75.4) |
| Currently breastfed (children <2 y)a, n (%) | 10 (27.0) | 5 (19.2) | 6 (31.6) | 23 (37.1) |
| Tobacco smoker in the home, n (%) | 2 (4.0) | 1 (3.1) | 9 (45.0) | 31 (44.9) |
| Running water in the home, n (%) | 41 (82.0) | 32 (100) | 18 (90.0) | 39 (56.5) |
| Any underlying conditionb, n (%) | 22 (45.8) | 10 (33.3) | 15 (79.0) | 46 (71.9) |
| History of prematurity (<37 wk) (children <2 y), n (%) | 3 (8.1) | 6 (23.1) | 5 (26.3) | 15 (24.2) |
| Any underlying condition or history of prematurity, n (%) | 23 (46.9) | 13 (40.6) | 16 (80.0) | 49 (73.1) |
| Received palivizumab (children <2 y)c, n (%) | 0 | 1 (3.9) | 0 | 3 (4.8) |
| Length of hospital stay (days), mean (range) | 3.5 (1–14) | 4.5 (1–11) | 4.3 (1–13) | 5.7 (1–45) |
| Lowest oxygen saturation (percent)d, mean (range) | 86.2 (77–93) | 83.0 (71–87) | 89.1 (75–98) | 89.0 (40–97) |
| Supplemental oxygen during hospitalization, n (%) | 46 (92.0) | 32 (100) | 14 (70.0) | 50 (72.5) |
| RSV subtypee | ||||
| RSV A | 30 | 24 | 15 | 48 |
| RSV B | 5 | 1 | 2 | 12 |
For age group restricted variables, percentages calculated out of total RSV-associated hospitalized children by age group.
Underlying conditions included chronic pulmonary and/or airway, cardiac, gastrointestinal, kidney, endocrine, neurologic and/or neuromuscular, hematologic and/or oncologic, genetic and/or metabolic, or immunocompromised conditions.
Received Palivizumab during the months of September 2019 through May 2020.
Documented in the first 24 h of admission.
Subtyping not available on all samples.
Variables with missing data: other children < 5 (Chinle n = 1, Whiteriver n = 1, Anchorage n = 1, Yukon-Kuskokwim (YK) Delta n = 5); currently breastfed (Chinle n = 1, Whiteriver n = 2, Anchorage n = 1, YK Delta n = 4); smoker in home (Chinle n = 1, Anchorage n = 1, YK Delta n = 4); running water (Chinle n = 1, Anchorage n = 1, YK Delta n = 4); under-lying conditions or prematurity (YK Delta n = 1); length of stay (YK Delta n = 5); oxygen saturation (Whiteriver n = 1, Anchorage n = 1, YK Delta n = 4); supplemental oxygen (Chinle n = 4; Anchorage n = 4, YK Delta n = 13).
FIGURE 1.
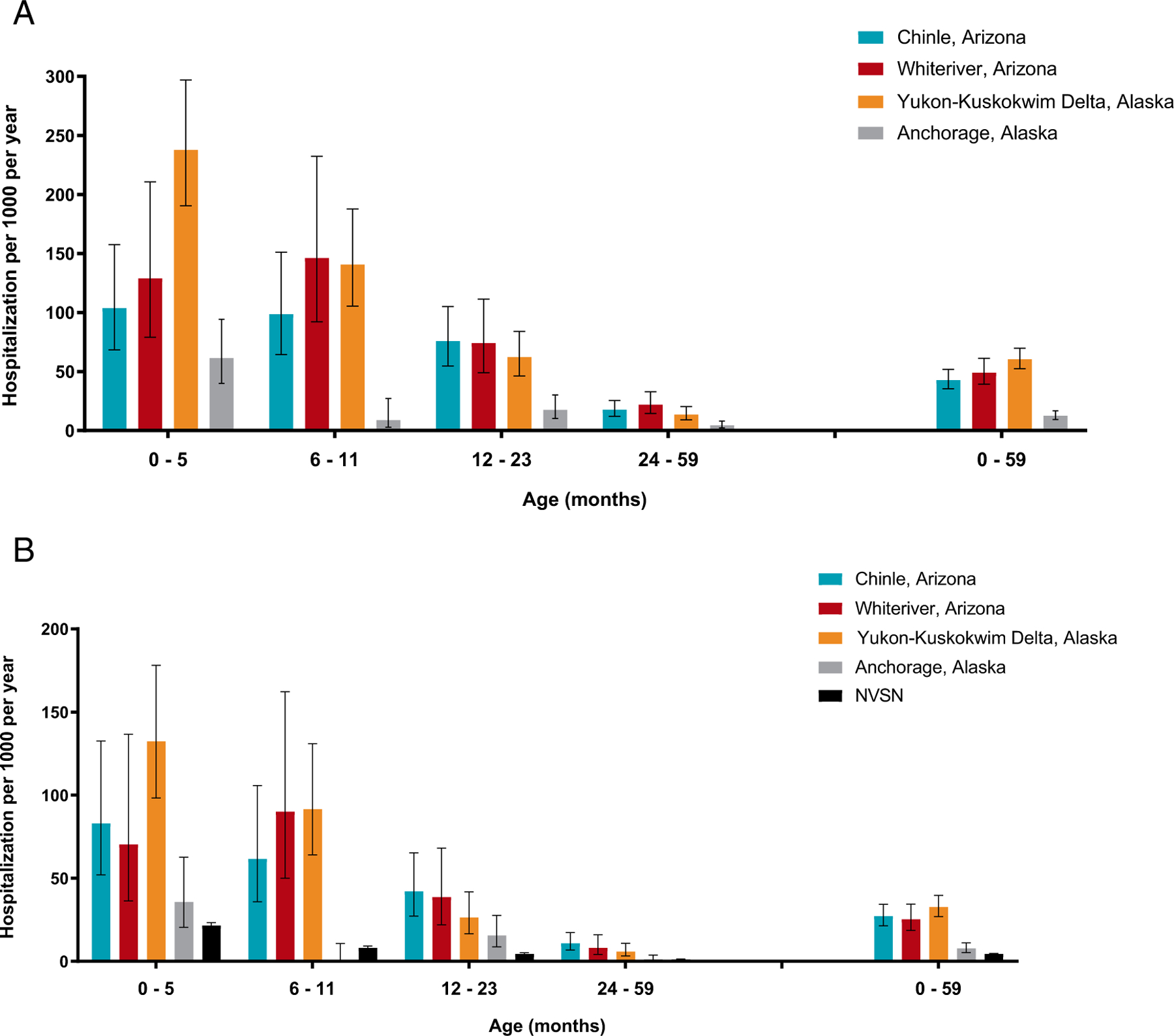
Incidence among AI/AN children < 5 years of age of (A) all-cause acute respiratory infection (ARI) hospitalization and (B) RSV-associated ARI hospitalization, November 2019 to May 2020. NVSN for comparison.9
DISCUSSION
AI/AN children in these communities experienced a high burden of RSV. Hospitalization rates among children < 5 years old were 1.7 to 7.1 times higher than recent estimates from the methodologically similar US New Vaccine Surveillance Network (NVSN).9,10 These are the first active, population-based surveillance hospitalization data with laboratory-confirmed RSV among AI/AN children in over 15 years.
Similarities in duration of hospitalization and supplemental oxygen use compared with NVSN suggest that disease severity was similar in this population, consistent with previous findings.4 As with prior studies, rates among AN children in Anchorage, the only urban site, were much lower than in YK Delta, the most rural, and were more similar to rates observed in NVSN.5,9,11 This corroborates findings from other studies, implicating socioeconomic determinants of health (eg, lack of running water, household overcrowding, poor indoor air quality) as a root cause of elevated RSV hospitalizations among AI/AN children living in tribal lands.2,6
In this study the greatest burden of RSV was seen among full term infants < 6 months old. This underscores the importance of prevention strategies that are highly effective in early life (e.g., maternal immunization, monoclonal anti-bodies) and available to all infants. Substantial RSV burden is also seen between 6 and 36 months of life, indicating that active immunization in older infants and toddlers would also have clinical and public health value.12
The number of children hospitalized with ARI fell sharply after coronavirus disease 2019 (COVID) pandemic mitigation measures were implemented in spring 2020; therefore, our observations of ARI in the 2019 to 2020 season may underestimate the pre-COVID-19 rates, particularly in Alaska where the respiratory season typically extends into late April and May.
These findings show that AI/AN children continue to experience high rates of RSV-associated ARI hospitalization. Improvements in the socioeconomic determinants of health for AI/AN children and RSV-prevention products are urgently needed.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the study participants and their families, the IHS and Tribal Health Organization partners, the dedicated research team at each of the study sites, and the Halasa Laboratory at Vanderbilt University Medical Center.
FUNDING:
This work was supported by the Centers for Disease Control and Prevention (grant number U01 IP001116-02-00 to Dr Hammitt). The other authors received no additional funding. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Indian Health Service.
ABBREVIATIONS
- AI/AN
American Indian or Alaska Native
- ARI
acute respiratory infection
- IHS
Indian Health Service
- IRB
Institutional Review Board
- NVSN
New Vaccine Surveillance Network
- RSV
respiratory syncytial virus
- RT-qPCR
reverse transcription quantitative polymerase chain reaction
- YK
Yukon Kuskokwim
Footnotes
See e.g., 45 C.F.R. part 46.102(l)(2).
CONFLICT OF INTEREST DISCLOSURES: Dr Halasa reports research funding to her institution from Sanofi and Quidell; she also reports receipt of an educational grant from Genetech, Inc. Dr Hammitt reports research funding to her institution from Pfizer, Inc. and Merck and Co. Dr Atwell is employed by Johns Hopkins University when this work was conducted. She is now employed by Pfizer Vaccines and may receive stock or stock options. Dr Singleton reports research funding to her institution from Merck and Co. The other authors have no relevant conflicts to disclose.
REFERENCES
- 1.Li Y, Wang X, Blau DM, et al. ; Respiratory Virus Global Epidemiology Network; RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulkow LR, Singleton RJ, Karron RA, Harrison LH; Alaska RSV Study Group. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics. 2002;109(2):210–216 [DOI] [PubMed] [Google Scholar]
- 3.Bockova J, O’Brien KL, Oski J, et al. Respiratory syncytial virus infection in Navajo and White Mountain Apache children. Pediatrics. 2002;110(2 Pt 1):e20. [DOI] [PubMed] [Google Scholar]
- 4.Karron RA, Singleton RJ, Bulkow L, et al. ; RSV Alaska Study Group. Severe respiratory syncytial virus disease in Alaska native children. J Infect Dis 1999;180(1):41–49 [DOI] [PubMed] [Google Scholar]
- 5.Holman RC, Curns AT, Cheek JE, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114(4):e437–e444 [DOI] [PubMed] [Google Scholar]
- 6.Foote EM, Singleton RJ, Holman RC, et al. Lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general United States child population. Int J Circumpolar Health. 2015;74:29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien KL, Chandran A, Weatherholtz R, et al. ; Respiratory Syncytial Virus (RSV) Prevention study group. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo--controlled trial. Lancet Infect Dis 2015;15(12):1398–1408 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization RSV surveillance case definitions. Available at: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance/case-definitions. Accessed January 20, 2023
- 9.Jones J ACIP General Meeting Evidence to Recommendations framework for nirsevimab. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/RSV-Pediatric-04-Jones-508.pdf. Accessed March 15, 2023
- 10.Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics. 2020;146(1):e20193611. [DOI] [PubMed] [Google Scholar]
- 11.Bruden DJ, Singleton R, Hawk CS, et al. Eighteen years of respiratory syncytial virus surveillance: changes in seasonality and hospitalization rates in southwestern Alaska Native children. Pediatr Infect Dis J 2015;34(9):945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karron RA. Preventing respiratory syncytial virus (RSV) disease in children. Science. 2021;372(6543):686–687 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


