Abstract
Youth with intellectual and developmental disabilities typically have higher rates of tics and stereotypies compared to children with otherwise typical development. Differentiating between these two pediatric movement disorders can be challenging due to overlapping clinical features, but is relevant due to distinct treatment modalities. The current study evaluated sensitivity and specificity of a tic screening measure, the Motor or Vocal Inventory of Tics (MOVeIT) in a pediatric sample enriched for stereotypy and tics. Children (n=199, age 2-15 years old) receiving care in a developmental-behavioral pediatrics clinic underwent a gold-standard diagnostic assessment by a tic expert; these evaluations were compared to the MOVeIT. The MOVeIT demonstrated good sensitivity (89.8%) and relatively lower specificity (57.1%) compared to tic expert for detecting tics in the overall sample. Specificity of the MOVeIT to identify tics improved to 75% when excluding children with co-occurring stereotypy. For children with tics and co-occurring stereotypy, sensitivity remained high (91.9%) but specificity was low (39.1%). The area under the curve (AUC) value to detect tics on the MOVeIT compared to the tic expert gold standard was significantly higher for children without stereotypy (AUC=85.7%) than those with stereotypy (AUC=64.3%, p <0.01). Overall, the ability to detect tics was better in those without co-occurring stereotypy symptoms. Further work is needed to establish the utility of the MOVeIT in populations where there is a high likelihood of co-occurring tics and stereotypy and in general population settings. Accurate distinction between tics and stereotypy will guide choices for intervention and anticipatory guidance for families.
Keywords: Autism spectrum disorder, developmental disability, screening tools, sensitivity, specificity, stereotypy, stereotypic movement disorder, tic disorders, tics, Tourette
BACKGROUND
Tics are involuntary, discrete, repetitive movements and sounds that wax and wane; severity ranges from minor to severe and disabling. Up to 20-25% of typically developing children in the United States may have tics at some point (Black et al., 2016; Scahill et al., 2014). Persistent tic disorders involve the presence of motor and/or vocal tics that have been present for at least one year, developed before 18 years of age, and are not a result of medications, drugs, or other health conditions (American Psychiatric Association, 2013). Children with persistent tic disorders can experience adverse academic and social impacts, behavior problems and mood disorders, and some tics may cause self-injury or pain (Abwender et al., 1996; Charania et al., 2022; Conelea et al., 2011; Gorman et al., 2010; Kurlan et al., 2002; Kurlan et al., 2001; Mathews et al., 2004; McGuire et al., 2013; Storch et al., 2007; Sukhodolsky et al., 2003; Zinner et al., 2012).
Accurate, early recognition of tics could improve rapid diagnosis for tic disorders and may serve as a flag for providers to increase surveillance for potential co-occurring conditions (Hirschtritt et al., 2015). However, tics and tic disorders are under-recognized, which can delay access to care (Arman et al., 2009; Debes et al., 2008; Janik et al., 2007; Shilon et al., 2008; Wand et al., 1992). Tics may also be challenging to differentiate from stereotypies, another relatively common type of abnormal movement in childhood. Stereotypies are repetitive, rhythmic, and non-meaningful movements (e.g., hand flapping, rocking). A primary stereotypic movement disorder may be diagnosed when repetitive movements develop in early childhood, cause interference with daily activities or lead to self-injury, and are not principally due to the effects of a medication or drug or another developmental disorder or behavioral health condition (American Psychiatric Association, 2013). Stereotyped movements and repetitive behaviors are included in the diagnostic criteria for autism spectrum disorders if the social communication criteria are met (American Psychiatric Association, 2013). Of note, when stereotypic movements, actions, or speech are present in the setting of ASD, a stereotypic movement disorder may not be separately diagnosed (American Psychiatric Association, 2013). Additional common co-occurring conditions for individuals who may have stereotypy overlap with those of tic disorders, including attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder (OCD), tics, developmental coordination disorder, and learning disorders (Katherine, 2018).
On the surface, both tics and stereotypies are characterized by involuntary, patterned, repetitive movements that may impair function (Singer et al., 2016), and both may increase at times of stress or anxiety (Martino & Hedderly, 2019; Singer, 2013). However, there are also key differences that can help differentiate the abnormal movements from each other (Barry et al., 2011; Katherine, 2018; Mahone et al., 2004), including differing natural history. Age of onset is typically earlier for stereotypies (12 months to 3 years) (Harris et al., 2008; Katherine, 2018; Mahone et al., 2004; Oakley et al., 2015) compared to tics which have an average age of onset from 4-8 years old (Hirschtritt et al., 2015; Openneer et al., 2022). Lifetime persistence may differ as well. Stereotypies typically continue through life (Péter et al., 2017), including in children without intellectual or developmental disabilities (Harris et al., 2008); by contrast, among children with persistent tic disorders, tics may worsen between 9-12 years of age and may improve or resolve for many individuals in late adolescence or early adulthood, though periodic recurrence of tics can occur through the lifespan (Black et al., 2021; Bloch & Leckman, 2009; Lowe et al., 2019).
Despite these differences, transient tics and stereotypy are both seen in children with otherwise typical development, and persistent tic disorders and stereotypic movement disorder both occur at higher rates among children with intellectual and developmental disabilities (IDDs). For example, the base rate for persistent tic disorders in children with ASD and a range of other developmental delays may be as high as 20-25% (Baron-Cohen et al., 1999; Canitano & Vivanti, 2007; MacDonald et al., 2007; Ringman & Jankovic, 2000). Less is known about the prevalence of tic disorders in individuals with intellectual disability (ID) in particular, but Bitsko et al (2014) reported that among children identified with diagnosed Tourette syndrome in a national survey, 12% had a co-occurring diagnosis of ID. Rates of stereotypy include an estimated median prevalence of nearly 90% for individuals with ASD (Chebli et al., 2016), and among children with a variety of IDD diagnoses, prevalence estimates for stereotypy range from 20% to over 90% (Chebli et al., 2016; Melo et al., 2020).
In addition, both tics and stereotypy may interfere with participation in educational, social, and community activities, increase social isolation, cause injury, and herald anxiety or other behavioral health diagnoses (Cook & Rapp, 2020; Cunningham & Schreibman, 2008; Hirschtritt et al., 2015; Singer, 2013). However, clinical management for stereotypy and tics differ; accurate assessment is not only critical to guide the treatment approach, but also to determine whether intervention is warranted. Both tics and stereotypies only need treatment if they are bothersome or cause interference or impairment to the child. Treatment options for tics include a tic-specific behavior therapy and pharmacologic interventions (Mills & Hedderly, 2014; Piacentini et al., 2010; Pringsheim et al., 2019; Ringman & Jankovic, 2000; Wolicki et al., 2020) or rarely, deep brain stimulation (Deeb & Malaty, 2020). Stereotypies are rarely responsive to pharmacologic management (Carrasco et al., 2012; Popow et al., 2021; Rajapakse & Pringsheim, 2010). When stereotypy does cause interference or impairment, behavioral strategies include reinforcing success with desired behaviors (e.g., redirection, differential reinforcement of other/incompatible behavior) rather than isolated targeted reduction of the stereotypic movement symptoms (Miller et al., 2006; Singer et al., 2018; Specht et al., 2017). By contrast, the most effective behavior therapy for tics, comprehensive behavioral intervention for tics (CBIT), does focus on reducing tic frequency and severity through habit reversal techniques.
Due to the differences in the expected clinical courses (Bloch & Leckman, 2009; Katherine, 2018; Leckman, 2003; Mahone et al., 2004; Pappert et al., 2003) and approaches to symptom management, there is a clinical imperative to differentiate between tics and stereotypy to best inform discussions and decisions regarding treatment, and to provide anticipatory guidance to families regarding expected course and surveillance for possible co-occurring conditions. The Motor or Vocal Inventory of Tics, 14-item (MOVeIT-14) is a new screening measure that has demonstrated initial good sensitivity and specificity for identifying tics and tic disorders in a case-control study in which the cases were a well-defined group of children with confirmed tic disorder diagnoses (Adams et al., 2023; Lewin et al., under review for this issue). However, little is known so far about its ability to distinguish tics from other common abnormal movements of childhood, particularly stereotypy. In the present study, the primary question was whether the MOVeIT-14 could specifically identify tics among children attending a specialty developmental-behavioral pediatrics (DBP) clinic who may be more likely to present with stereotypy, or with a mixed presentation of tics and stereotypy. We therefore examined the use of the MOVeIT-14 to screen for tics in a sample of children enriched for stereotypy and tic disorders, namely, children served by a DBP clinic.
METHOD
Participants.
All study visits were conducted between April – September 2018. Eligible participants were male and female children between the ages of 2 – 15 years who were clinical patients of the DBP program at Golisano Children’s Hospital at Strong in Rochester, New York. The DBP program provides multidisciplinary care for children with IDDs including but not limited to those with diagnoses of ASD, cerebral palsy, and other causes of developmental delay. The age range was selected based on the natural history of stereotypies which are often established by 2 years of age or younger, and tics, which may emerge as early as 3 years of age, have peak frequency/severity by about age 12 years, and in many cases, begin to subside by the early- to mid-teen years (Leckman, 2003; Leckman et al., 1998). Eligible children were new or returning patients of the DBP clinic with an upcoming in-person clinical appointment. Approximately 1-2 months prior to their child’s scheduled appointment, parents were sent recruitment letters via postal mail or through the patient portal section (“MyChart”) of the healthcare system’s electronic health record system (Epic). Parents were also informed of the research opportunity during reminder phone calls for their child’s upcoming clinic appointment. Study announcements were also physically displayed in the DBP clinic and on the clinic’s community-facing website.
All parents completed an informed consent process to provide parental permission for their child’s participation in the study and received remuneration for their participation. The University of Rochester Institutional Review Board (Research Subjects Review Board; RSRB) granted a waiver of documentation of informed consent for this minimal risk study. Consent was therefore obtained verbally (via telephone or in person) by the study coordinator, or through a RSRB-approved REDCap-based study information sheet. All parents were provided with a copy of the study information sheet for their records. The research was approved by the University of Rochester’s RSRB (Study #70865).
Motor or Vocal Inventory of Tics (MOVeIT), parent-proxy version.
The parent-proxy MOVeIT is a brief tic screening questionnaire completed by parents to report on whether their child displays behaviors that might be tics. The MOVeIT was developed to improve the identification of tics and tic disorders in non-specialty clinical and research settings where expert assessment of tics and tic disorders may not be available, with a goal of reaching at least 85% sensitivity compared with expert assessment (Adams et al., 2023; Lewin et al., under review for this issue). Items describe various motor and vocal tics and are ranked on a 3-point scale (never =0, sometimes =1, often =2) to describe the frequency with which the potential tics occur. Item content addresses common motor tics (e.g., blinking, shrugging shoulders, jerking arms or legs) and phonic tics (grunting, coughing, sniffing). The MOVeIT also captures some aspects of tic phenomenology, including their involuntary nature and repetitiveness, though does not capture all information needed to formally evaluate the presence/absence of a tic disorder. There are two parent-proxy versions, containing 14 or 10 items (MOVeIT-14; MOVeIT-10). The MOVeIT-10 contains a subset of the MOVeIT-14 items, but the two versions do not differ with respect to content, i.e., descriptions of common motor and vocal tics such as sniffing, coughing, or shrugging shoulders are included in both. The items excluded from the 10-item version had lower endorsement among youth with tics (or their parents) and higher endorsement among youth without tics (or their parents) compared to other items and were repetitive with other items that were retained ((Lewin et al., under review for this issue). For the current project, all parents completed all 14 items, which also permitted analysis of the 10-item version. Total score on the MOVeIT-14 ranges from 0-28, and on the MOVeIT-10 from 0-20, with higher scores on each version indicating greater endorsement of potential tics (i.e., more tics, and/or more frequent tics). The MOVeIT-10 was developed as a shorter version to improve efficiency of assessment and has teacher proxy and child self-report versions, with identical content as the parent proxy MOVeIT-10 questionnaire. For the current project, we focused exclusively on the MOVeIT-14 and MOVeIT-10 parent proxy version, permitting enrollment of children with limited ability to self-report their tic experiences, including those who were nonverbal, and also enabling all data collection to occur through the DBP clinic setting rather than also requiring engagement of classroom teachers. A supplemental file is provided with this publication that presents a sample copy of the current version of the parent MOVeIT-14.
Study Procedures.
Parents who consented to their child’s study participation were asked to complete the MOVeIT-14 within several weeks of their child’s upcoming clinically scheduled appointment with a DBP provider. The MOVeIT-14 was completed either by telephone through an interview with the study coordinator, or as an electronic form available through the patient portal section (“MyChart”) of the institution’s electronic health record system (Epic). We also provided paper copies of the MOVeIT-14 at the clinic site in case parents were unable to complete the form prior to their child’s appointment date. During the clinical appointment, but after all clinical concerns were addressed, DBP providers used the information in the completed MOVeIT-14 to guide their own clinical screening for the presence of tics and a tic disorder, benchmarked against Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) diagnostic criteria (American Psychiatric Association, 2013). This model was chosen to emulate the likely use of the MOVeIT-14 in clinical practice to guide providers in screening for tics. The DBP provider also reviewed and documented whether criteria were met for stereotypy and a DSM-5 defined stereotypic movement disorder. Prior to any enrollment of participants, tic expert study personnel met with participating DBP providers to review criteria for tic disorders, provide training on assessment of tics and tic disorders, and discuss how DBP providers and tic experts differentiate between tics and stereotypy. Tic experts were clinicians in the URMC Pediatric Neurology Movement Disorder program (pediatric neurologists; pediatric neurology nurse practitioner; clinical child psychologist). Though this study was focused on screening for tics, our rationale for also screening for stereotypy was to test the sensitivity of the MOVeIT-14 to reliably detect tics but with sufficient specificity that stereotypies were not identified using this screening tool. Results of the DBP provider assessment will be discussed in a forthcoming manuscript.
After conclusion of the child’s clinical appointment with a DBP provider, a tic expert met with the parent and child to conduct a conventional diagnostic evaluation, to determine the presence/absence of tics and/or stereotypy symptoms and review of all criteria required to establish a DSM-5 diagnosis of a tic disorder or stereotypic movement disorder, respectively. In our Pediatric Neurology-based practice this included a clinical neurological exam though this may not be a component of tic/stereotypy evaluation by other clinical specialists. Tic experts were blinded to the parents’ responses on the MOVeIT-14 and to the DBP assessment. A future paper will discuss the sensitivity/specificity of DBP assessment of tics (which incorporated information from the MOVeIT); the current paper focuses on the parent MOVeIT-14 responses alone in comparison to the tic experti’s blinded assessment for tics. Assessment by tic experts was conducted either immediately after the clinical appointment (before the child/parent were dismissed from clinic), or within several weeks after the appointment, if a tic expert was unavailable or if the family left clinic before the tic expert could meet with them. Those latter assessments were conducted by the tic experts in a phone interview, using a method established based on consultation with our lead tic expert (JWM). This method consisted of standard clinical interviewing with an emphasis on eliciting detailed description of the behaviors in question and review of clinical characteristics of tics and stereotypies, and review of DSM-5 criteria for tic disorder and stereotypic movement disorder. If needed to aid parents’ understanding of the behaviors (tics, stereotypies) of interest, the tic expert described examples of some common tics (e.g., eye blinking, sniffing) and stereotypies (e.g., hand flapping, rocking). A set of sensitivity analyses to evaluate potential differences by assessment mode compared (1) the distribution of tic and stereotypy symptoms and diagnoses by mode of tic expert assessment using chi-square tests and (2) MOVeIT scores using ANOVA interaction terms by symptom or diagnosis category and tic expert assessment mode; see below for details on primary ANOVA analyses.
Analysis plan.
The primary aim of the study was to determine the sensitivity and specificity of the parent proxy MOVeIT-14 to identify tics in a sample of children with high potential for tics and/or stereotypies, compared to the tic expert’s clinical assessment, which served as the gold standard. As noted above, the DBP assessment of tics as compared to the tic expert is not the focus of the current analyses but will be addressed in future. As a secondary goal, we compared the MOVeIT-14 to the current shorter version, the MOVeIT-10, to assess whether they would perform similarly. We also calculated descriptive results for sample characteristics (age, sex, race/ethnicity), and the number of children identified with one or more of the following: tics, tic disorders, stereotypy, and stereotypic movement disorder). A review of DBP clinic billing code records for a one-year period prior to the start of this project (May 01, 2016-April 30, 2017) indicated that tics and related tic disorders were coded in approximately 5% of patients seen in that clinic, stereotypies in 6-7%, and both disorders noted as present in about 3% of children. During this time period, over half (58%) of children seen in the DBP clinic had a primary diagnosis of ASD. In discussion with DBP clinic providers, we established that both tics and stereotypy were likely underestimated because the DBP clinic staff typically only formally code these symptoms when they are clinically impairing. In addition, because stereotyped behaviors are included as part of the diagnostic criteria for autism spectrum disorder (ASD; Part B: restrictive and repetitive behavior symptoms), children with a primary ASD diagnosis would not separately have had stereotypy coded for a clinical visit. Therefore, based upon the extant literature and the diagnostic makeup of the DBP clinic, we anticipated a minimum of 5-10% of the sample would have at least one tic (current or past).
Analyses for descriptive statistics and group comparisons were performed using Statistica version 13.3 (TIBCO Software Inc., 2017). One-way Analysis of Variance (ANOVA) tests were used to compare differences in MOVeIT-14 and MOVeIT-10 scores by group, with group defined based on symptom status: tics only; stereotypic movements only; both tics and stereotypy symptoms; neither tics or stereotypy. Where appropriate, post-hoc Scheffé tests were used for pairwise comparisons of MOVeIT-14 scores and of MOVeIT-10 scores by symptom status.
MedCalc version 20.106 (MedCalc Software Ltd., 2022) was used for receiver operating characteristic (ROC) curve analyses and calculation of the area under the curve (AUC; a priori significance set at p=0.05). Potential optimal cutoff scores on the MOVeIT-14 and MOVeIT-10 were identified to differentiate between individuals with and without tics. Once an optimal MOVeIT score threshold was determined, we calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for identifying the presence of tics based on this threshold. We used the prevalence estimate of tics by tic expert evaluation from this sample (29.6%) in our calculations of PPV and NPV. We intentionally focused on identifying the presence of any tics, rather than a persistent tic diagnosis for calculation of PPV, NPV, and accuracy because the MOVeIT is designed as a tic symptom screener rather than for diagnostic evaluation. That is, though the MOVeIT asks respondents to endorse the presence or absence of tics, it does not assess for other criteria of tic disorders including age of onset, persistence, or absence of other medical reasons for tics. The tic expert assessed for the presence of tic symptoms and also whether diagnostic criteria for a tic disorder were met. The latter determination (diagnosis of a tic disorder) was not a primary focus of the present study. However, as a supplemental analysis, we evaluated MOVeIT-14 and MOVeIT-10 scores for study participants by tic disorder and/or stereotypic movement disorder diagnosis group status.
Results
Sample characteristics
A total of N=254 children participated in the study. Some participants did not complete one or two of the core assessments (MOVeIT-14, DBP provider assessment, tic expert assessment). Table 1 shows the number of participants by the assessments completed and summarizes the reasons for non-completion of study assessments. Altogether, a total of 199 children (78.3% of the total sample) had a parent-completed MOVe-IT and were also evaluated by the tic expert, and this is the sample used for the current paper’s analyses. There were no significant differences between the group that completed all three assessments compared to the group that only completed the MOVeIT and tic expert assessment, based on age, sex, race or ethnicity of the child (Table 2). Table 2 also presents sample characteristics for the final analyzed sample (N=199). As expected, in particular because of the higher prevalence of ASD in males compared to females and related predominance of male patients in the DBP clinic, most children included in the analytic sample were male (n=153, 76.9%).
Table 1.
Number of child participants in a developmental-behavioral pediatrics clinic evaluated with the Motor or Vocal Inventory of Tics (MOVeIT), by a developmental-behavioral pediatrics provider, and/or by a tic expert
| N (%) of total sample | |
|---|---|
| Total Sample | 254 (100%) |
| Individual Assessment Completed | |
| Parent Motor or Vocal Inventory of Tics (MOVeIT-14)1 | 252 (99.2%) |
| Developmental-Behavioral Pediatrics Provider (DBP)2 Assessment | 218 (85.8%) |
| Tic Expert3 Assessment | 201 (79.1%) |
| Paired Assessments Completed | |
| Parent MOVeIT-14 + Tic Expert | 199* (78.3%) |
| Parent MOVeIT-14 + DBP | 216 (85.0%) |
| Tic Expert + DBP | 182 (71.7%) |
| All Assessments: Parent MOVeIT-14 + DBP + Tic Expert | 180 (70.9%) |
Sample size which is the focus of analyses for the current paper
Reasons for missing assessments:
Parent MOVeIT: Two parents did not complete the MOVeIT-14 (14-item version) despite reminders by the study coordinator but participated in the tic expert and DBP assessments. These participants were not included in the present study.
DBP: 36 children did not undergo the DBP assessment due to lack of time in the visit, or because the DBP provider either forgot the child was enrolled in study or forgot to conduct the assessment and could not reach the parent later by phone. Three additional children, included in the count above, completed evaluation of tics, but not stereotypy, with the DBP provider.
Tic Expert assessment: Some children did not undergo tic expert assessment because they and their parent left clinic before this activity could be conducted and could not be reached later by phone. These participants were not included in the present study.
Table 2.
Demographic characteristics of total sample, children who completed all three assessments (Parent MOVeIT*, Tic Expert, Developmental-Behavioral Pediatrician) and children who only completed the MOVeIT and Tic Expert assessment
| Total Sample (N=254) | Children with all 3 assessments (N=180) | Children with MOVeIT + TE* (N=199) (analytic sample) | |
|---|---|---|---|
| Age in Years, Mean (SD) | 8.1 (3.4) | 8.0 (3.3) | 8.1 (3.4) |
| Range | 2.0 – 16.0 | 2.1 – 15.8 | 2.0 – 16.0 |
| Sex (n, %) | |||
| Females | 59 (23.2%) | 42 (23.3%) | 46 (23.1%) |
| Males | 195 (76.8%) | 138 (76.7%) | 153 (76.9%) |
| Race (n, %) | |||
| American Indian | 2 (0.8%) | 2 (1.1%) | 2 (1.0%) |
| Asian | 4 (1.6%) | 2 (1.1%) | 3 (1.5%) |
| African American | 20 (7.9%) | 15 (8.3%) | 16 (8.0%) |
| White | 210 (82.7%) | 154 (85.6%) | 169 (84.9%) |
| Other, More than One Race | 8 (3.1%) | 2 (1.1%) | 3 (1.5%) |
| Not reported1 | 10 (3.9%) | 5 (2.8%) | 6 (3.0%) |
| Ethnicity: (n, %) | |||
| Hispanic | 13 (5.1%) | 8 (4.4%) | 10 (5.0%) |
| non-Hispanic | 224 (88.2%) | 163 (90.6) | 178 (89.4%) |
| Not reported1 | 17 (6.7%) | 9 (5.0%) | 11 (5.5%) |
MOVeIT: Motor or Vocal Inventory of Tics; TE: Tic Expert
Not reported: Parent opted to not share information about child’s race and/or ethnicity
Tic and Stereotypy Symptoms and Diagnosis
Of the N=199 children whose data were analyzed for the current paper, a total of n=59 children (29.6%) had any current or past tics according to tic expert evaluation, and a total of n=56 (28.1%) had a persistent tic disorder based on the tic expert assessment: persistent motor tic disorder, n=12; persistent vocal tic disorder, n=10; Tourette syndrome, n=25; provisional tic disorder, n=9. A total of n=106 (53.3%) had stereotypic movements or behaviors (stereotypy), and 22 (11.1%) children were determined to have a stereotypic movement disorder based on tic expert assessment. Also, n=8 (4.0%) children were deemed by the tic expert to meet DSM-5 diagnostic criteria for both a stereotypic movement disorder and a co-occurring tic disorder: persistent motor tic disorder, n=2; persistent vocal tic disorder, n=1; Tourette syndrome, n=5.
Of the 199 children whose data were evaluated for the current paper, n=116 (58.3%) were able to complete in-person assessments with the tic expert; the remaining n=83 (41.7%) were assessed by the tic expert by phone. The percent of children in the symptom and diagnostic groups did not differ significantly by mode of assessment (Supplemental Table S1).
Parent MOVeIT-14 and MOVeIT-10 scores by symptoms
Table 3 presents summary data (mean, standard deviation, median, range) for the Parent MOVeIT-14 and MOVeIT-10 total scores. MOVeIT scores are presented for the total group and based on whether the child was deemed by the tic expert to have any tic symptoms (with or without co-occurring stereotypy), any stereotypy symptoms (with or without tics), both tic and stereotypy symptoms, or neither. As expected, the group of children with any tic symptoms, with or without co-occurring stereotypy symptoms (n=59, 29.6%) had significantly higher mean Parent MOVeIT-14 (p<.001) and MOVeIT-10 (p < .001) scores than children without any tic symptoms (n=140). One-way ANOVA tests revealed a significant difference by symptom status (tics only, stereotypy only, both tics and stereotypy, or neither) for both the MOVeIT-14 total score: F(3, 195) = 18.75, p < .001 and the MOVeIT-10 total score: F(3, 195) = 18.46, p <.001. Post-hoc testing (Scheffé; results shown in Table 4) identified that the mean MOVeIT-14 score for the group with neither tic nor stereotypy symptoms was significantly lower than for each of the other three groups. There was only one other significant difference among the 3 symptom groups (tics only, stereotypy only, tics + stereotypy symptoms); the mean MOVeIT-14 total score for the group with stereotypy symptoms only was significantly lower than for the group with both tics and stereotypy. The mean MOVeIT-10 total score for the group with neither tic nor stereotypy symptoms was significantly lower than for each of the other three groups, but there were no significant differences among the 3 symptom groups (tics only, stereotypy only, tic + stereotypy symptoms) for either the MOVeIT-14 or MOVe-IT 10 scores.
Table 3.
Parent Motor or Vocal Inventory of Tics (MOVeIT-14 and MOVeIT-10)* scores by symptom status (N=199)
| All Participants (N=199) | ||
|---|---|---|
|
| ||
| MOVeIT-14 Mean (SD) Median, Range |
MOVeIT-10 Mean (SD) Median, Range |
|
| All children (N=199) | 9.2 (8.1) | 7.0 (6.1) |
| 7.0, 0–28 | 6.0, 0–20 | |
| Children with tics, with or without co-occurring stereotypy (n=59) | 13.8 (7.5) | 10.3 (5.5) |
| 12.0, 0-28 | 10.0, 0-20 | |
| Children with tics only (n=22) | 12.9 (6.8) | 9.9 (5.3) |
| 11.0, 2-26 | 10.5, 1-18 | |
| Children with stereotypy, with or without co-occurring tics (n=106) | 11.6 (8.3) | 8.8 (6.1) |
| 11.0, 0-28 | 8.0, 0-20 | |
| Children with stereotypy only (n=69) | 10.1 (8.2) | 7.9 (6.1) |
| 10.0, 0-27 | 8.0, 0-20 | |
| Children with co-occurring tics and stereotypy (n=37) | 14.3 (8.0) | 10.6 (5.6) |
| 13.0, 0-28 | 10.0, 0-20 | |
| Children without tics (with or without co-occurring stereotypy, n=140) | 7.3 (7.6) | 5.6 (5.8) |
| 4.0, 0-28 | 4.0, 0-28 | |
| Children with neither tics nor stereotypy (n=71) | 4.5 (5.8) | 3.4 (4.4) |
| 2.0, 0-28 | 2.0, 0-20 | |
Motor or Vocal Inventory of Tics: 14-item=MOVeIT-14; 10-item=MOVeIT-10
Table 4.
Post-hoc (Scheffé) comparisons for differences between symptom groups on Parent Motor or Vocal Inventory of Tics (MOVeIT-14 and MOVeIT-10)* total scores
| MOVeIT-14 | |||
|---|---|---|---|
| Symptom Group | Mean Difference with Group | p value | 95.0% CI** |
| Group 1: Tics only | Group 2=2.8 | 0.49 | −2.2, 7.8 |
| Group 3=−1.4 | 0.92 | −6.9, 4.1 | |
| Group 4=8.5 | <0.001 | 3.5, 13.4 | |
| Group 2: Stereotypy only | Group 3=−4.2 | <0.05 | −8.3, 0.0 |
| Group 4=5.7 | < 0.001 | 2.3, 9.1 | |
| Group 3: Tics and stereotypy | Group 4=9.8 | <0.001 | 5.7, 14.0 |
| Group 4: Neither | -- | -- | -- |
| MOVeIT-10 | |||
| Symptom Group | Mean Difference with Group | p value | 95.0% CI |
| Group 1: Tics only | Group 2 = 2.0 | 0.53 | −1.8, 5.7 |
| Group 3 = −0.7 | 0.97 | −4.8, 3.4 | |
| Group 4 = 6.4 | <0.001 | 2.7, 10.1 | |
| Group 2: Stereotypy only | Group 3 = −2.7 | 0.12 | −5.8, 0.4 |
| Group 4 = 4.5 | < 0.001 | 1.9, 7.0 | |
| Group 3: Tics and stereotypy | Group 4 = 7.1 | <0.001 | 4.0, 10.2 |
| Group 4: Neither | -- | -- | -- |
Motor or Vocal Inventory of Tics: 14-item=MOVeIT-14; 10-item=MOVeIT-10
CI: Confidence interval
We also examined Parent MOVeIT-14 and MOVeIT-10 scores by diagnosis group (stereotypic movement disorder only, tic disorder only, both diagnoses, or neither). Table S2 (supplement) presents summary data (mean, standard deviation, median, range) for Parent MOVeIT-14 and MOVeIT-10 total scores according to diagnosis group. The Parent MOVeIT-14 and MOVeIT-10 total scores for the group with neither diagnosis were significantly lower than that of the other 3 groups, whose MOVeIT scores were not significantly different from one another (detailed results available upon request).
Regarding the potential influence of mode of tic expert assessment on the MOVeIT total score results, interactions between mode (in person, phone) and tic or stereotypy symptom and diagnosis groups (tics only, stereotypy only, both, neither) were tested using factorial ANOVA tests. All interaction tests resulted in non-significant p-values (p-value range: 0.24–0.35), indicating that the differences in MOVeIT total scores by symptom or diagnosis group were not different for those whose tic assessment was completed in person than those whose assessment was completed by phone (see supplemental Table S3a–d for details).
ROC Curve Analyses for the Parent MOVeIT-14 and MOVeIT-10
Figure 1 presents the ROC curves with 95% confidence intervals (CI) for identifying any tic symptoms on the Parent MOVeIT-14 (Figure 1a) and MOVeIT-10 (Figure 1b) in the analytic sample for the present paper (N=199). These ROC curves were similar; for the MOVeIT-14, AUC = 74.5% (p < .001), and for MOVeIT-10, AUC = 73.5% (p < .001). Table 5 presents the values and 95% confidence intervals for the Parent MOVeIT-14 and MOVeIT-10 AUC and the sensitivity, specificity, and optimal threshold scores for differentiating between children with and without tic symptoms. On the Parent MOVeIT-14, a total score >5 was identified as the optimal threshold, and on the Parent MOVeIT-10, the optimal threshold was a total score >4. Table 5 also shows the PPV, NPV, and accuracy and 95% confidence intervals for identification of tics based on these threshold scores in this population.
Figure 1.
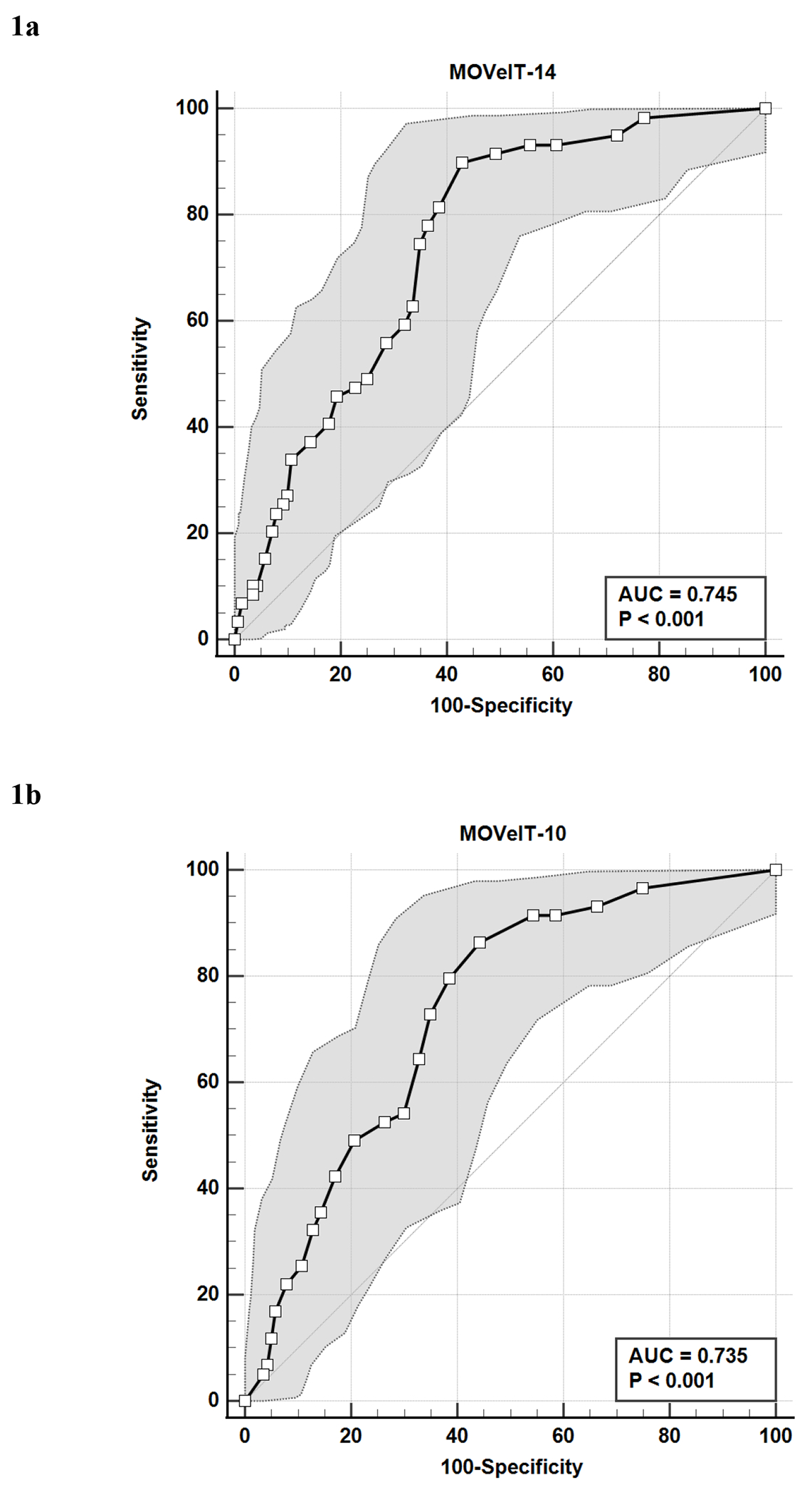
a. Receiver Operating Characteristic (ROC) Curve analysis with 95% Confidence Intervals of Parent 14-item Motor or Vocal Inventory of Tics (MOVeIT-14) total score for distinguishing between participants (n=199) with and without tics based on tic expert assessment.
b. Receiver Operating Characteristic (ROC) Curve analysis with 95% Confidence Intervals of Parent 10-item Motor or Vocal Inventory of Tics (MOVeIT-10) total score for distinguishing between participants (n=199) with and without tics based on tic expert assessment.
Table 5.
Area under the Curve (AUC) values, Optimal Cutoff Score, Sensitivity, Specificity, Positive Predictive Value (PPV), and Negative Productive Value (NPV) for the Parent Motor or Vocal Inventory of Tics (MOVeIT-14 and MOVeIT-10)* for identifying tic symptoms (n=199)
| MOVeIT-14 value (95% CI**) |
MOVeIT-10 value (95% CI**) |
|
|---|---|---|
| AUC | 74.5% (67.9, 80.4%) | 73.5% (66.8, 79.5%) |
| Optimal cutoff score | >5 (>4, >7) | >4 (>3, >5) |
| Sensitivity | 89.8% (79.2, 96.2%) | 86.4% (75.0, 94.0%) |
| Specificity | 57.1% (48.5, 65.5%) | 55.7% (47.1, 64.1%) |
| PPV | 46.9% (41.7, 52.1%) | 45.1% (40.0, 50.3%) |
| NPV | 93.0% (86.0, 96.7%) | 90.7% (83.5, 95.0%) |
| Accuracy | 66.8% (59.8, 73.3%) | 64.8% (57.7, 71.4%) |
Motor or Vocal Inventory of Tics: 14-item=MOVeIT-14; 10-item=MOVeIT-10
CI: Confidence interval
We also examined performance of the Parent MOVeIT-14 and MOVeIT-10 for identifying tic symptoms in children with (n = 106) and without (n = 93) co-occurring stereotypy symptoms. Figures 2a and 2b show comparisons of independent ROC curves for the MOVeIT-14 (Figure 2a) and MOVeIT-10 (Figure 2b) for determining the presence or absence of tics among children with and without stereotypy symptoms, with the presence of tics and/or stereotypy both established by the tic expert evaluation. For the Parent MOVeIT-14, the AUC value for children without stereotypy symptoms was = 85.7% and for children with stereotypies the AUC = 64.3%. The difference between the AUC for these ROC curves was significant (difference between areas =0.21; z=3.14, p<0.01; 95% CI: 0.08, 0.35). A similar pattern was observed for the Parent MOVeIT-10, with an AUC = 84.2% for children without stereotypy symptoms and an AUC = 62.8% for children with stereotypy symptoms; this difference was statistically significant (difference between areas=.21, z=3.03, p<.01, 95% CI: 0.08, 0.35). The optimal threshold score for detection of tic symptoms on the Parent MOVeIT-14, regardless of the presence or absence of stereotypy, was >5, but the 95% confidence interval band around this threshold value was narrower for the group without stereotypy symptoms (95% CI: >1, >6) than for the group without stereotypy symptoms (95% CI: >4, >16). Similarly for the Parent MOVeIT-10 the optimal threshold score for children without stereotypy was >4 (95% CI: >1, >6) and for the children with stereotypy, the optimal threshold was >5 (95% CI: >4, >17). Table 6 presents the values and 95% confidence intervals for the AUC and optimal threshold scores for the Parent MOVeIT-14 and MOVeIT-10 as well as the sensitivity, specificity, PPV, NPV, and accuracy when using these optimal threshold scores stratified by the presence or absence of stereotypy symptoms in this population.
Figure 2.
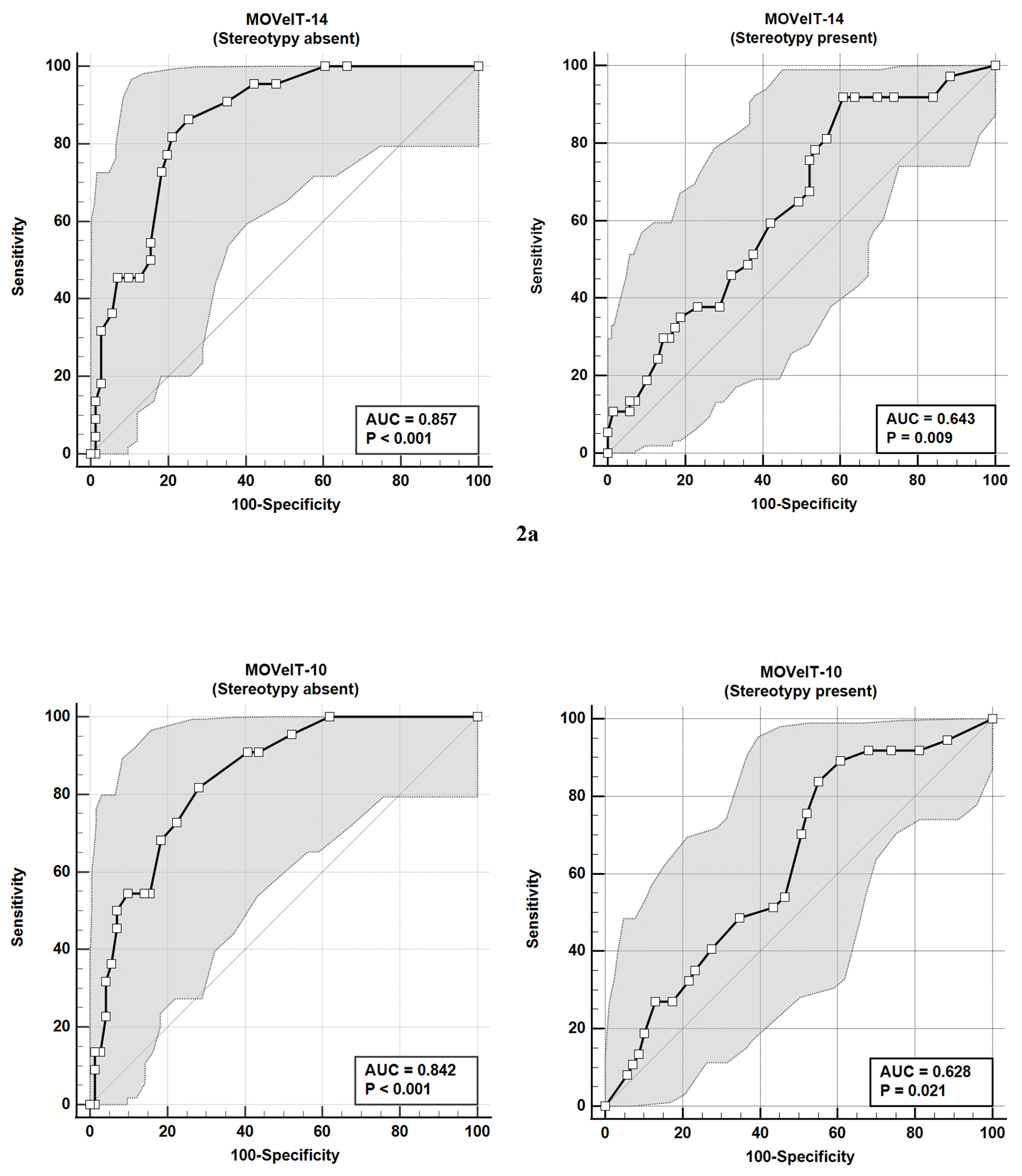
a. Receiver Operating Characteristic (ROC) Curve analysis and 95% Confidence Intervals of Parent 14-item Motor or Vocal Inventory of Tics (MOVeIT-14) scores for distinguishing between participants with and without tics based on tic expert assessment, according to absence or presence of stereotypy symptoms.
b. Receiver Operating Characteristic (ROC) Curve analysis and 5% Confidence Intervals of Parent 10-item Motor or Vocal Inventory of Tics (MOVeIT-10) scores for distinguishing between participants with and without tics based on tic expert assessment, according to absence or presence of stereotypy symptoms.
Table 6.
Motor or Vocal Inventory of Tics (MOVeIT): Area under the Curve (AUC) values, Optimal Cutoff Score, Sensitivity, Specificity, Positive Predictive Value (PPV), and Negative Productive Value (NPV) for the MOVeIT-14 and MOVeIT-10* for identifying tic symptoms in children without (n=93) stereotypy symptoms versus children with stereotypy symptoms (n=106).
| MOVeIT-14* | MOVeIT-10* | |||
|---|---|---|---|---|
|
without stereotypy value (95% CI**) |
with stereotypy value (95% CI**) |
without stereotypy value (95% CI**) |
with stereotypy value (95% CI**) |
|
| AUC | 85.7% (76.9, 92.1%) | 64.3% (54.4, 73.4%) | 84.2% (25.1, 90.9%) | 62.8% (52.9, 72.0%) |
| Optimal cutoff score | >5 (>1, >6) | >5 (>4, >16) | >4 (>0, >9) | >5 (>4, >17) |
| Sensitivity | 86.4% (65.1, 97.1%) | 91.9% (78.1, 98.3%) | 81.8% (59.7, 94.8%) | 83.8% (68.0, 93.8%) |
| Specificity | 74.7% (62.9, 84.2%) | 39.1% (27.6, 51.6) | 71.8% (59.9, 81.9%) | 44.9% (32.9, 57.4%) |
| PPV | 58.9% (48.2, 68.8%) | 38.8% (33.9, 44.0%) | 55.0% (44.5, 65.0%) | 39.0% (33.1, 45.2%) |
| NPV | 92.9% (81.8, 97.4%) | 92.0% (78.9, 97.2%) | 90.4% (79.3, 95.8%) | 86.8% (75.2, 93.5%) |
| Accuracy | 78.1% (68.3, 86.0%) | 54.7% (44.8, 64.4%) | 74.8% (64.7, 83.2%) | 56.4% (46.5, 66.0%) |
MOVeIT: Motor or Vocal Inventory of Tics: 14-item=MOVeIT-14; 10-item=MOVeIT-10
CI: confidence interval
Discussion
The purpose of these analyses was to assess the ability of tic screening measures (MOVeIT-14 and MOVeIT-10) to identify tics in a population of children enriched for tics and stereotypies. The MOVeIT was developed to improve identification of tics using a short screener and performed well (>85% sensitivity and specificity) in a population of children recruited for a tic specialty clinic (Adams et al., 2023). In this sample of children seen for developmental and behavioral concerns, tics and stereotypic movements were common (30% and 53%, respectively). Children with tics had significantly higher MOVeIT-14 and MOVeIT-10 scores than children without tics, and optimal thresholds were determined for differentiating between children with and without tic symptoms. The ability to detect tics was better in those without stereotypy symptoms compared to those with stereotypy symptoms. Although the presence of stereotypy symptoms had little to no impact on the optimal cutoff scores, the group with stereotypy symptoms had greater variability in their scores. Also, the MOVeIT-14 and MOVeIT-10 performed comparably; the latter’s shorter length could allow further efficiency in screening for tics.
The MOVeIT-14 and MOVeIT-10 did not include some items that may distinguish tics from stereotypies with respect to clinical phenomenology and the patient experience. Tics commonly exhibit a waxing and waning course (Barry et al., 2011; Katherine, 2018; Mahone et al., 2004; Openneer et al., 2022), but stereotypies tend to persist. Also, in tic disorders, the presence of a premonitory urge and the ability to suppress tics are common (Banaschewski et al., 2003; Leckman et al., 1993). The premonitory urge is an aversive sensation that may be focal (e.g., a scratch in the throat for a throat-clearing tic) or generalized (e.g., feeling of tension in the body). It tends to build up and is relieved by performance of the tic. Although approximately 80% of individuals with tic disorder describe a premonitory urge, it is typically not described until an average of 3 years after onset of tics (Leckman et al., 1993). By contrast, a premonitory urge is not present for stereotypies; indeed, they may be experienced as enjoyable or unnoticed by the patient rather than unwanted (Katherine, 2018). In addition, many children can suppress their tics for a short period of time. Though children with stereotypies can often be distracted out of their movements, they typically do not intentionally suppress them (Katherine, 2018; Mahone et al., 2004). The MOVeIT-14 and MOVeIT-10 include questions about abnormal, repetitive movements/sounds that are undesired and involuntary (“hard to keep from doing”). However, they do not evaluate for age of onset of movements/sounds, changes in movements/sounds over time, presence of a premonitory urge, or the ability to suppress movements/sounds. Thus, it is possible that the absence of questions evaluating these parameters contributed to the lower specificity and PPV of the MOVeIT-14 and MOVe-IT-10 in those with stereotypy symptoms.
Although there were not significant differences in MOVeIT scores among children with either a tic disorder, stereotypic movement disorder, or both diagnoses, we noted a pattern of a higher mean total Parent MOVeIT-14 and MOVeIT-10 score for children with a diagnosis of a stereotypic movement disorder. This may seem unexpected, as MOVeIT scores might be higher when both motor and vocal symptoms are endorsed, and a diagnosis of a stereotypic movement disorder would by definition not involve repetitive sounds. However, there are slightly fewer unique items that ask about sounds than movements on both the Parent MOVeIT-14 (5 motor and 4 vocal items) and MOVeIT-10 (4 motor and 2 vocal items), and remaining items in each version can be positively endorsed whether only motor, only vocal, or both types of symptoms are present (see supplemental file for item content). Hence the unique contribution of vocal tic symptoms to the total MOVeIT score is constrained by the number of related items on the questionnaire. Also, although the MOVeIT is intended to screen for tics, the instrument does not explicitly ask respondents to consider whether their child’s repetitive movements or sounds are actually tics vs. stereotypy or another type of symptom. Thus, it is possible that some parents may have endorsed repetitive sounds that are neither tics nor stereotypy, but nonetheless were present in the setting of the latter symptoms or diagnosis. Finally, since the MOVeIT item scores are constructed from rank-ordered ratings (i.e., ‘never’; ‘sometimes’; ‘often’) it may be the case that items assessing only movements, or movements and/or sounds received higher rankings than items that assessed only repetitive sounds. Overall, however, the distribution of Parent MOVeIT-14 and MOVeIT-10 scores across these positive diagnosis groups was similar, and the range of values across the groups (shown in Table S1) were likewise similar.
Although these instruments were designed to detect tics in children, identifying stereotypy symptoms may also be beneficial. When stereotypies do not cause functional impairment they may not warrant intervention (Katherine, 2018). However, individuals who experience impairment from stereotypies can be referred for appropriate treatment (Barry et al., 2011; Katherine, 2018). In particular, stereotyped movements that are self-injurious require functional analysis to determine appropriate behavioral intervention. In our sample, stereotypy symptoms were present in 53% of participants and self-injury attributed to stereotyped behavior by the clinician (or as reported to the clinician by the parent) was recorded in 13% (n=26) of participating children, but a primary stereotypic movement disorder diagnosis (as defined by DSM-5 criteria; (American Psychiatric Association, 2013) was present in only 11% of participants. We did not collect information on participants’ specific underlying IDD diagnosis (such as ASD) but the greater proportion of children in the sample with stereotypy symptoms vs. a stereotypic movement disorder suggests that for many participants, stereotypies were one component of a broader intellectual or developmental disability.
There were limitations to this study. First, we had missing data for at least one measure for 22% of participants, but this was not unexpected given the real-world environment (a busy clinical setting) in which the study was based. This analysis only included data from those who completed both the MOVeIT-14 and tic expert assessment. There were no group differences between the entire sample and those included in the analysis in terms of age, sex, race, or ethnicity of the child. However, it is possible that missing data affected the results of our study. Second, this sample was not representative of the general population. The vast majority of participants were male, white, and non-Hispanic. Also, we studied a population with a higher level of clinical need compared to the general population, and therefore, results will not be generalizable to populations of typically developing children. Given the dependence of PPV and NPV on the underlying prevalence, these estimates are expected to be elevated compared to what would be expected in a non-specialty setting. This limits the generalizability of our results. Also, in the real-world setting in which this study was based, a developmental-behavioral pediatrics clinic, some families did not remain on site for the tic expert assessment after completing their clinical appointment with their DBP provider. In order to ensure a complete dataset for these individuals, the tic expert assigned to a respective study visit called the child’s parent to complete their assessment by phone. While a phone-based evaluation is not a conventional approach to evaluating pediatric movements, the proportion of children identified as having tic and/or stereotypy symptoms, or as having a tic disorder and/or stereotypic movement disorder, did not differ based on the tic expert’s assessment mode (in person vs. in phone). Next, the reference standard assessment of tics, by the tic expert, explicitly considered both current and past history of tics. Though the MOVeIT does not explicitly specify a time frame for parents’ endorsement of their child’s tics, items are worded in the present tense (see supplement). This might lead to an underestimation of any history of tics by the MOVeIT, compared to the tic expert’s evaluation, but would not impact screening for current tics which might have the greatest relevance in a clinical setting. Additionally, some items ask parents to infer their child’s internal experience of tics, e.g., “My child feels like they have to make a noise……”. Among children with no or minimal verbal skills it may be difficult for parents (or clinicians) to always know what a child is thinking or feeling with respect to tics or other abnormal movements. In fact, some parents did convey that such items were difficult to answer, for this reason. Finally, we provided additional training on assessment of tics and stereotypy to study personnel to augment the training conventionally provided in DBP training programs.
Despite these limitations, this study has several strengths. Although tics and stereotypies are not uncommon, they are more commonly seen in individuals with IDDs (Baron-Cohen et al., 1999; Canitano & Vivanti, 2007; MacDonald et al., 2007; Ringman & Jankovic, 2000). Thus, the focus of this study in a DBP clinic setting allows for identification of more individuals with tics than in the general population. Additionally, this clinic setting has more non-verbal children than general pediatric populations. Use of a parent report allowed us to include children regardless of their verbal abilities. Of note, children with no or limited verbal skills may be unable to indicate the presence of a premonitory urge. This is not a requirement for the diagnosis of a tic disorder, but can be helpful in clarifying clinical phenomenology and for initiating ‘awareness training’, an early step in habit reversal training for tic management (Woods et al., 2008). Finally, the tic experts were blinded to the parent MOVeIT responses, thus decreasing bias in the study.
In the future, we plan to build upon these results to test the ability of these instruments (and revised versions of these instruments) to perform in the general community. As part of the methods of this study, DBP providers reviewed parents’ MOVeIT-14 responses and used this information to guide evaluation for tics and tic disorder, similar to how the MOVeIT-14 would be used in a real-world clinical setting. This approach reflects what would occur in clinical practice in the future. Future work could also examine whether the instruments are able to differentiate between tics and compulsions, which commonly co-occur with tic disorders (Hirschtritt et al., 2015) and have overlapping features, i.e., repetitive and stereotyped actions.
Tics and tic disorders are common in childhood (Tinker et al., 2022) but are likely under- diagnosed. Tics are not currently screened for as standard of care in general pediatric practices (Hagan et al., 2017). Pediatric neurologists, DBP specialists, psychologists and psychiatrists have expertise to recognize tics and stereotypies, diagnose their related disorders, and guide treatment decisions. Unfortunately, many patients and families have limited access to such subspecialists (American Psychological Association, 2018; Basco & Rimsza, 2013; Dall et al., 2013; Kang et al., 2016; Satiani et al., 2018). Additionally, despite growth in the pediatric neurology and DBP professions there remains a national shortage of both providers; there is an approximately 20% shortfall predicted by 2025 in the ability to meet clinical need for pediatric neurology in most U.S. states (Majersik et al., 2021) and a similar anticipated shortfall in the DBP talent pool as well with far more anticipated retirements by senior clinicians than there are incoming new providers based on the existing training pipeline (Bridgemohan et al., 2018). The primary pediatric setting may thus be well-suited to conduct initial screening for tics and stereotypy, as a first step to expand access for diagnosis and treatment. Understanding how the MOVeIT performs in a DBP setting can inform these next steps by illuminating the strengths and limitations of the measure for differentiating among common abnormal movements of childhood.
Early detection of tic disorders in children has the potential to improve early access to treatment for tics and to increase identification of common co-occurring conditions. Accurate and feasible tic screening instruments can facilitate diagnosis and management of youth with tic disorders, yet will also need to effectively differentiate between tics and stereotypies, the most common other abnormal movements of childhood. This study demonstrates that the MOVeIT-14 can accurately detect tics in a sample of children enriched for presence of tics but lacks sensitivity to distinguish tics from stereotypy in children at risk for both of these common pediatric movement disorders.
Supplementary Material
Acknowledgments
We thank the children and their parents for participation in this study. We gratefully acknowledge the contributions of the clinical providers in the Developmental and Behavioral Pediatrics clinic who engaged with the study: Lynn Cole, DNP; Jenniffer Herrera, MD; Angela Liberatore, NP; Lorna Patanella, NP; Jessica Reiffer, MD Melissa Ryan, NP; Johanna Stump-Siembor, NP.
This study was supported by the Association of University Centers on Disabilities (AUCD) cooperative agreement which was funded by the Centers for Disease Control and Prevention (Grant award U38OT000140). Dr. Mink received salary support from IDDRC grant P50HD103536 for preparation of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Interest Statement
None of the authors have conflicts of interest related to the subject matter of this manuscript.
References
- Abwender D, Como P, Kurlan R, Parry K, Fett K, Cui L, Plumb S, & Deely C (1996). School problems in Tourette’s syndrome [R (ab)]. Arch Neurol, 53(6), 509–511. [DOI] [PubMed] [Google Scholar]
- Adams H, Augustine E, Bonifacio K, Collins A, Danielson M, Mink J, Morrison P, van Wijngaarden E, Vermilion J, Vierhile A, & Bitsko R (2023). Evaluation of new instruments for screening and diagnosis of tics and tic disorders in a well characterized sample of youth with tics and recruited controls. Evidence-based Practice in Child and Adolescent Mental Health. 10.1080/23794925.2023.2178040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- American Psychological Association. (2018). A Summary of Psychologist Workforce Projections: Addressing Supply and Demand from 2015-2030.
- Arman S, Golmirzaei J, Naeini AE, & Azhar MM (2009). The evaluation of relationship between group A streptococcal infection with tic disorders in children. Saudi Med J, 30(9), 1180–1185. [PubMed] [Google Scholar]
- Banaschewski T, Woerner W, & Rothenberger A (2003). Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Dev Med Child Neurol, 45(10), 700–703. 10.1017/s0012162203001294 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Scahill V, Izaguirre J, Hornsey H, & Robertson M (1999). The prevalence of Gilles de la Tourette syndrome in children and adolescents with autism: a large scale study. Psychol Med, 29(5), 1151–1159. [DOI] [PubMed] [Google Scholar]
- Barry S, Baird G, Lascelles K, Bunton P, & Hedderly T (2011). Neurodevelopmental movement disorders - an update on childhood motor stereotypies. Dev Med Child Neurol, 53(11), 979–985. [DOI] [PubMed] [Google Scholar]
- Basco WT, & Rimsza ME (2013). Pediatrician workforce policy statement. Pediatrics, 132(2), 390–397. 10.1542/peds.2013-1517 [DOI] [PubMed] [Google Scholar]
- Black KJ, Black ER, Greene DJ, & Schlaggar BL (2016). Provisional Tic Disorder: What to tell parents when their child first starts ticcing. F1000Res, 5, 696. 10.12688/f1000research.8428.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KJ, Kim S, Yang NY, & Greene DJ (2021). Course of tic disorders over the lifespan. Current Developmental Disorders Reports, 8(2), 121–132. 10.1007/s40474-021-00231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, & Leckman J (2009). Clinical course of Tourette syndrome. Journal of Psychosomatic Research, 67(6), 497–501. 10.1016/j.jpsychores.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgemohan C, Bauer NS, Nielsen BA, DeBattista A, Ruch-Ross HS, Paul LB, & Roizen N (2018). A Workforce Survey on Developmental-Behavioral Pediatrics. Pediatrics, 141(3). 10.1542/peds.2017-2164 [DOI] [PubMed] [Google Scholar]
- Canitano R, & Vivanti G (2007). Tics and Tourette syndrome in autism spectrum disorders. Autism, 11(1), 19–28. 10.1177/1362361307070992 [DOI] [PubMed] [Google Scholar]
- Carrasco M, Volkmar FR, & Bloch MH (2012). Pharmacologic treatment of repetitive behaviors in autism spectrum disorders: evidence of publication bias. Pediatrics, 129(5), e1301–1310. 10.1542/peds.2011-3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charania SN, Danielson ML, Claussen AH, Lebrun-Harris LA, Kaminski JW, & Bitsko RH (2022). Bullying victimization and perpetration among US children with and without Tourette Syndrome. Journal of Developmental & Behavioral Pediatrics, 43(1), 23–31. 10.1097/dbp.0000000000000975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli S, Martin V, & Lanovaz M (2016). Prevalence of stereotypy in individuals with developmental disabilities - a systematic review. Rev J Autism Dev Disord, 3(2), 107–118. [Google Scholar]
- Conelea CA, Woods DW, Zinner SH, Budman C, Murphy T, Scahill LD, Compton SN, & Walkup J (2011). Exploring the impact of chronic tic disorders on youth: Results from the Tourette Syndrome Impact Survey. Child Psychiatry and Human Development, 42(2), 219–242. [DOI] [PubMed] [Google Scholar]
- Cook JL, & Rapp JT (2020). To What Extent Do Practitioners Need to Treat Stereotypy During Academic Tasks? Behav Modif, 44(2), 228–264. 10.1177/0145445518808226 [DOI] [PubMed] [Google Scholar]
- Cunningham AB, & Schreibman L (2008). Stereotypy in Autism: The Importance of Function. Res Autism Spectr Disord, 2(3), 469–479. 10.1016/j.rasd.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall T, Storm M, Chakrabarti R, Drogan O, Keran C, Donofrio P, Henderson V, Kaminski J, Stevens J, & Vidic T (2013). Supply and demand analysis of the current and future US neurology workforce. Neurology, 81(5), 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes NM, Hjalgrim H, & Skov L (2008). Limited knowledge of Tourette syndrome causes delay in diagnosis. Neuropediatrics, 39(2), 101–105. 10.1055/s-2008-1081457 [DOI] [PubMed] [Google Scholar]
- Deeb W, & Malaty I (2020). Deep Brain Stimulation for Tourette Syndrome: Potential Role in the Pediatric Population. Journal of Child Neurology, 35(2), 155–165. 10.1177/0883073819872620 [DOI] [PubMed] [Google Scholar]
- Gorman DA, Thompson N, Plessen KJ, Robertson MM, Leckman JF, & Peterson BS (2010). Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: Controlled study. British Journal of Psychiatry, 197(1), 36–44. 10.1192/bjp.bp.109.071050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan J, Shaw J, & Duncan P (Eds.). (2017). Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. (4th ed.). American Academy of Pediatrics. [Google Scholar]
- Harris KM, Mahone EM, & Singer HS (2008). Nonautistic motor stereotypies: clinical features and longitudinal follow-up. Pediatr Neurol, 38(4), 267–272. 10.1016/j.pediatrneurol.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, King RA, Sandor P, McMahon WM, Lyon GJ, Cath DC, Kurlan R, Robertson MM, Osiecki L, Scharf JM, & Mathews CA (2015). Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry, 72(4), 325–333. 10.1001/jamapsychiatry.2014.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik P, Kalbarczyk A, & Sitek M (2007). Clinical analysis of Gilles de la Tourette syndrome based on 126 cases. Neurologia i neurochirurgia polska, 41(5), 381–387. [PubMed] [Google Scholar]
- Kang P, Bale J, Mintz M, Joshi S, Gilbert D, Radabaugh C, & Ruch-Ross H (2016). The child neurology clinical workforce in 2015: Report of the AAP/CNS Joint Taskforce. Neurology, 87, 1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katherine M (2018). Stereotypic movement disorders. Seminars in Pediatric Neurology, 25, 19–24. 10.1016/j.spen.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen E, Eapen S, & McDermott M (2002). The behavioral spectrum of tic disorders: A community-based study. Neurology, 59, 414–420. [DOI] [PubMed] [Google Scholar]
- Kurlan R, McDermott M, Deeley C, Como P, Brower C, Eapen S, Andresen E, & Miller B (2001). Prevalence of tics in schoolchildren and association with placement in special education [Empirical Study; Quantitative Study]. Neurology, 57(8), 1383–1388. [DOI] [PubMed] [Google Scholar]
- Leckman J (2003). Phenomenology of tics and natural history of tic disorders. Brain Development, 25, S24–S28. 10.1016/s0387-7604(03)90004-0 [DOI] [PubMed] [Google Scholar]
- Leckman J, Walker D, & Cohen D (1993). Premonitory urges in Tourette’s syndrome. American Journal of Psychiatry, 150(1), 98–102. 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- Leckman J, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim Y-S, & Peterson B (1998). Course of tic severity in Tourette Syndrome: The first two decades. Pediatrics, 102, 14–19. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Brennan EA, Kudryk K, & Murphy TK (under review for this issue). Development of a rapid screening instrument for tic disorders in primary care settings – the MOVeIT- 14. Evidence-based Practice in Child and Adolescent Mental Health. [Google Scholar]
- Lowe TL, Capriotti MR, & McBurnett K (2019). Long-Term Follow-up of Patients with Tourette’s Syndrome. Mov Disord Clin Pract, 6(1), 40–45. 10.1002/mdc3.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R, Green G, Mansfield A, Geckeler A, Gardenier N, Anderson J, Holcomb W, & Sanchez J (2007). Stereotypy in young children with autism and typically developing children. Research in Developmental Disabilities, 28, 266–277. 10.1016/j.ridd.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Mahone E, Bridges D, Prahme C, & Singer H (2004). Repetitive arm and hand movements (complex motor stereotypies) in children. Journal of Pediatrics, 145(3), 391–395. 10.1016/j.jpeds.2004.06.014 [DOI] [PubMed] [Google Scholar]
- Majersik J, Ahmed A, Chen I, & al e. (2021). A shortage of neurologists we must act now: a report from the AAN 2019 Transforming Leaders Program. Neurology, 96(24), 1122–1134. 10.1212/WNL.0000000000012111 [DOI] [PubMed] [Google Scholar]
- Martino D, & Hedderly T (2019). Tics and stereotypies: A comparative clinical review. Parkinsonism and Related Disorders, 59, 117–124. 10.1016/j.parkreldis.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Mathews CA, Waller J, Glidden D, Lowe TL, Herrera LD, Budman CL, Erenberg G, Naarden A, Bruun RD, Freimer NB, & Reus VI (2004). Self injurious behaviour in Tourette syndrome: correlates with impulsivity and impulse control. Journal of Neurology, Neurosurgery & Psychiatry, 75(8), 1149–1155. 10.1136/jnnp.2003.020693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Hanks C, Lewin AB, Storch EA, & Murphy TK (2013). Social deficits in children with chronic tic disorders: phenomenology, clinical correlates, and quality of life. Comprehensive psychiatry, 54(7), 1023–1031. 10.1016/j.comppsych.2013.04.009 [DOI] [PubMed] [Google Scholar]
- MedCalc Software Ltd. (2022). MedCalc Statistical Software (Version 20.106) [Computer software]. MedCalc. [Google Scholar]
- Melo C, Ruano L, Jorge J, Ribeiro T, Oliveira G, Azevedo L, & Temudo T (2020). Prevalence and determinates of motor stereotypies in autism spectrum disorder: A systematic review and meta-analysis. Autism, 24(3), 560–590. [DOI] [PubMed] [Google Scholar]
- Miller JM, Singer HS, Bridges DD, & Waranch HR (2006). Behavioral therapy for treatment of stereotypic movements in nonautistic children. Journal of Child Neurology, 21(2), 119–125. 10.1177/08830738060210020701 [DOI] [PubMed] [Google Scholar]
- Mills S, & Hedderly H (2014). A guide to childhood motor stereotypies, tic disorders, and the Tourette spectrum for the primary care practitioner. Ulster Medical Journal, 83(1), 22–30. [PMC free article] [PubMed] [Google Scholar]
- Oakley C, Mahone M, Morris-Berry C, Kline T, & Singer H (2015). Primary complex motor stereotypies in older children and adolescents: Clinical features and longitudinal follow-up. Pediatr Neurol, 52, 398–403. [DOI] [PubMed] [Google Scholar]
- Openneer TJC, Huyser C, Martino D, Schrag A, Hoekstra PJ, & Dietrich A (2022). Clinical precursors of tics: an EMTICS study. J Child Psychol Psychiatry, 63(3), 305–314. 10.1111/jcpp.13472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappert E, Goltz C, Louis ED, Blasucci L, & Leungans S (2003). Objective assessment of longitudinal outcomes in Gilles de la Tourette Syndrome. Neurology, 61(7), 936–940. [DOI] [PubMed] [Google Scholar]
- Péter Z, Oliphant ME, & Fernandez TV (2017). Motor stereotypies: a pathophysiological review. Front Neurosci, 11 ((Article 171)), 1–6. 10.3389/fnins.2017.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini J, Woods D, & Scahill L (2010). Behavior therapy for children with Tourette disorder - A randomized controlled trial. JAMA, 303(19), 1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popow C, Ohmann S, & Pilener P (2021). Practitioner’s review: medication for children and adolescents with autism spectrum disorder (ASD) and comorbid conditions. Neuropsychiatrie, 35, 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Holler-Managan Y, Okun MS, Jankovic J, Piacentini J, Cavanna AE, Martino D, Müller-Vahl K, Woods DW, Robinson M, Jarvie E, Roessner V, & Oskoui M (2019). Comprehensive systematic review summary: Treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology, 92(19), 907–915. 10.1212/wnl.0000000000007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse T, & Pringsheim T (2010). Pharmacotherapeutics of Tourette syndrome and stereotypies in autism. Seminars in Pediatric Neurology, 17, 254–260. 10.1016/j.spen.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Ringman JM, & Jankovic J (2000). Occurrence of tics in Asperger’s syndrome and autistic disorder. Journal of Child Neurology, 15(6), 394–400. 10.1177/088307380001500608 [DOI] [PubMed] [Google Scholar]
- Satiani A, Niedermier J, Satiani B, & Svendsen D (2018). Projected workforce of psychiatrists in the United States: a population analysis. Psychiatric Services, 69, 710–713. 10.1176/appi.ps.201700344 [DOI] [PubMed] [Google Scholar]
- Scahill L, Specht M, & Page C (2014). The Prevalence of Tic Disorders and Clinical Characteristics in Children. J Obsessive Compuls Relat Disord, 3(4), 394–400. 10.1016/j.jocrd.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilon Y, Pollak Y, Benarroch F, & Gross-Tsur V (2008). Factors influencing diagnosis delay in children with Tourette syndrome. Eur J Paediatr Neurol, 12(5), 398–400. 10.1016/j.ejpn.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Singer HS (2013). Motor control, habits, complex motor stereotypies, and Tourette syndrome. Annals of the New York Academy of Sciences(1304), 22–31. 10.1111/nyas.12281 [DOI] [PubMed] [Google Scholar]
- Singer HS, Mink JW, Gilbert DL, & Jankovic J (2016). Chapter 8: Motor Stereotypies. Academic Press. 10.1016/B978-0-12-411573-6.00008-5 [DOI] [Google Scholar]
- Singer HS, Rajendran S, Waranch HR, & Mahone EM (2018). Home-Based, Therapist-Assisted, Therapy for Young Children With Primary Complex Motor Stereotypies. Pediatr Neurol, 85, 51–57. 10.1016/j.pediatrneurol.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht MW, Mahone EM, Kline T, Waranch R, Brabson L, Thompson CB, & Singer HS (2017). Efficacy of parent-delivered behavioral therapy for primary complex motor stereotypies. Dev Med Child Neurol, 59(2), 168–173. 10.1111/dmcn.13164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch E, Merlo L, Lack C, Milson V, GR G, Goodman W, & Murphy T (2007). Quality of life in youth with Tourette’s Syndrome and Chronic Tic Disorder. Journal of Clinical Child and Adolescent Psychology, 36(2), 217–227. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, Zhang H, Peterson BS, King RA, Lombroso PJ, Katsovich L, Findley D, & Leckman JF (2003). Disruptive behavior in children with Tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. Journal of the American Academy of Child & Adolescent Psychiatry, 42(1), 98–105. 10.1097/00004583-200301000-00016 [DOI] [PubMed] [Google Scholar]
- TIBCO Software Inc. (2017). Statistica (data analysis software system (Version 13.3) [Computer software]. http://statistica.io.
- Tinker SC, Bitsko RH, Danielson ML, Newsome K, & Kaminski JW (2022). Estimating the number of people with Tourette syndrome and persistent tic disorder in the United States. Psychiatry Res, 314, 114684. 10.1016/j.psychres.2022.114684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand R, Shady G, Broder R, Furer P, & Staley D (1992). Tourette syndrome: issues in diagnosis. Neuroscience and Biobehavioral Reviews, 16(4), 449–451. 10.1016/s0149-7634(05)80186-1 [DOI] [PubMed] [Google Scholar]
- Wolicki SB, Bitsko RH, Holbrook JR, Danielson ML, Zablotsky B, Scahill L, Walkup JT, Woods DW, & Mink JW (2020). Treatment use among children with Tourette syndrome living in the United States, 2014. Psychiatry Res, 293, 113400. 10.1016/j.psychres.2020.113400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW, Piacentini JC, Chang SW, Deckersbach T, Ginsburg GS, Peterson AL, Scahill LD, Walkup JT, & Wilhelm D (2008). Managing Tourette Syndrome. A behavioral intervention for children and adults: therapist guide. Oxford University Press. [Google Scholar]
- Zinner SH, Conelea CA, Glew GM, Woods DW, & Budman CL (2012). Peer victimization in youth with Tourette syndrome and other chronic tic disorders. Child Psychiatry and Human Development, 43(1), 124–136. 10.1007/s10578-011-0249-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


