Abstract
Patient: Female, 39-year-old
Final Diagnosis: Late-developing mesh infection with preperitoneal abscess and cutaneous fistulization following endoscopic totally extraperitoneal hernia repair
Symptoms: Intermittent fever, progressive lower abdominal pain and fullness
Clinical Procedure: Ultrasound • surgical management • antibiotic therapy
Specialty: Surgery
Objective:
Diagnostic/therapeutic accidents
Background:
Endoscopic inguinal hernia repair has become the preferred technique currently. The use of mesh to facilitate a tension-free reinforcement has become the standard of care during endoscopic totally extraperitoneal (TEP), laparoscopic transabdominal pre-peritoneal, and open inguinal hernia repair. Although uncommon, late-developing mesh infections, defined as those occurring in the surgical site months or years after the procedure, can lead to severe complications. To achieve the best possible outcome for the patient, prompt imaging and a multidisciplinary approach to management, including complete surgical removal of the contaminated mesh and proper antibiotic therapy, are crucial.
Case Report:
A 39-year-old woman presented with a 1-month history of intermittent fever, progressive lower abdominal pain and fullness, and purulent discharge from the abdominal wall. Her medical history was significant for an endoscopic right TEP inguinal hernia repair performed 3 years earlier, which involved the use of an anatomic mesh and titanium screws. Physical examination and ultrasound findings revealed a large preperitoneal abscess with cutaneous fistulization, secondary to a deep-seated mesh infection. Pseudomonas aeruginosa was identified as the causative pathogen. She underwent a 2-step surgical procedure, including an initial fistulectomy followed by endoscopic abscess drainage and surgical excision of the infected mesh, combined with antimicrobial therapy, resulting in an excellent clinical response and complete resolution. This strategy also allowed for an effective assessment of the abdominal wall integrity.
Conclusions:
This case underscores the importance of considering late-developing mesh infections in patients presenting with abdominal symptoms who have previously undergone TEP hernia repair, even years after the initial surgery.
Key words: Surgical Mesh, Persistent Infection, Herniorrhaphy, Cutaneous Fistula
Introduction
Endoscopic inguinal hernia repair has emerged as the preferred technique among the more than 20 million procedures performed globally each year [1]. Although the endoscopic totally extraperitoneal (TEP) repair technique has demonstrated recurrence rates comparable to that of the traditional Lichtenstein method, patients who undergo TEP repair benefit from a decreased incidence of postoperative wound infections, enabling them to resume their normal activities more rapidly [2]. Furthermore, these procedures are associated with a reduced prevalence of chronic pain, resulting in enhanced patient comfort and improved quality of life [3].
Tension-free reinforcement of the posterior wall using mesh has become the standard of care in TEP inguinal hernia repair [4]. While complications related to mesh implantation are generally uncommon, they can still occur, including deep mesh infections that develop late after the procedure [5–9]. These conditions, characterized by their emergence in the surgical site months or even years after surgery, are identified through symptoms of infection and diagnostic imaging tests [10]. Traditional manifestations include chronic pain and erythematous, swollen skin with tenderness. In certain cases, purulent discharge is evident, and fistulas can be detected through physical examination [10].
The present case report details a rare instance of a late-onset deep mesh infection culminating in the development of a preperitoneal abscess, which manifested as a cutaneous fistula 3 years following an endoscopic TEP inguinal hernia repair. Prompt identification and proper intervention were critical to avoid additional complications.
Case Report
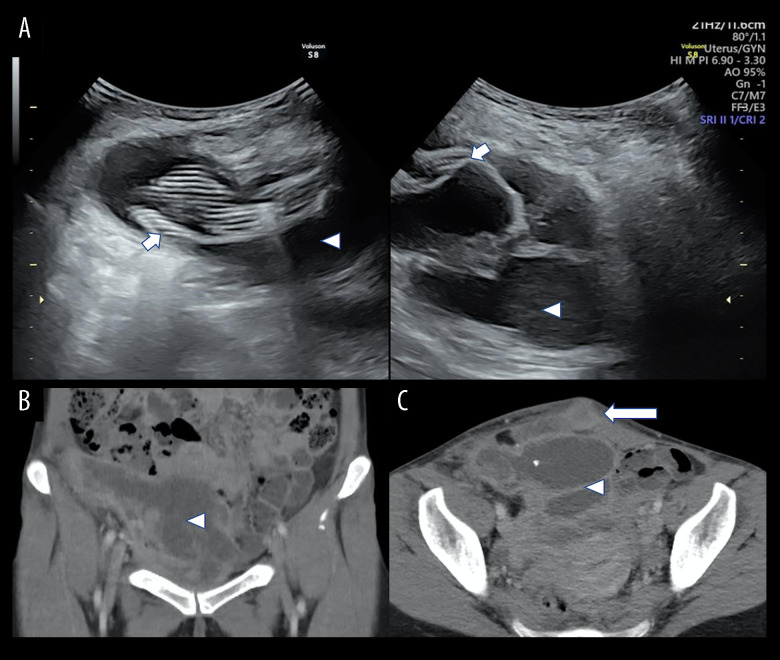
A 39-year-old woman, with a history of undergoing endoscopic TEP inguinal hernia repair using anatomical mesh and titanium screws 3 years earlier, reported experiencing intermittent fever, progressively worsening lower abdominal pain, and a sensation of fullness over the past month. Upon physical examination, the hypogastric area exhibited tenderness, erythematous swelling, and purulent discharge (Figure 1). Laboratory test results showed an increased C-reactive protein level at 12.9 mg/dL, although the white blood cell count remained within the normal range. Ultrasonography identified a 15.4-cm thick-walled, multiloculated cystic mass in the right lower quadrant of the abdomen. This lesion displayed acoustic shadowing and an undulating contour (Figure 2), suggesting a complex, possibly septated abscess or collection.
Figure 1.
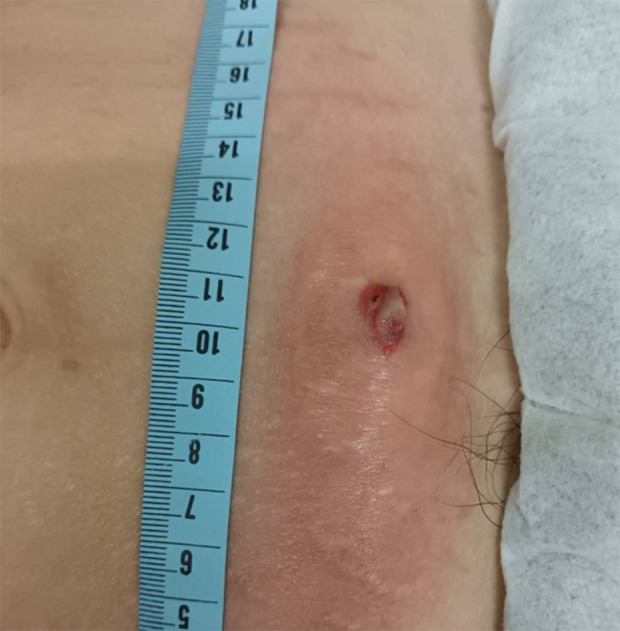
The hypogastric region exhibited notable erythema and swelling surrounding a purulent cutaneous fistula.
Figure 2.
(A) An ultrasound examination performed at presentation revealed a sizable (15.4×9.5 cm) preperitoneal multilocular abscess (arrowheads) with thick walls and internal striped artifacts, indicative of a mesh (short arrows). Enhanced computed tomography (CT) scan in (B) coronal view and (C) axial view showed complicated hypodense areas (arrows) occupying the preperitoneal space without intraperitoneal involvement. Note that the abscess-cutaneous fistula (long arrow) connected the skin opening with the deep-seated abscess.
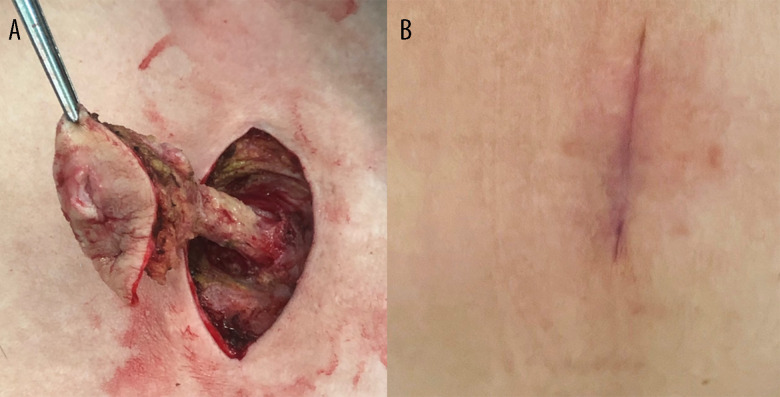
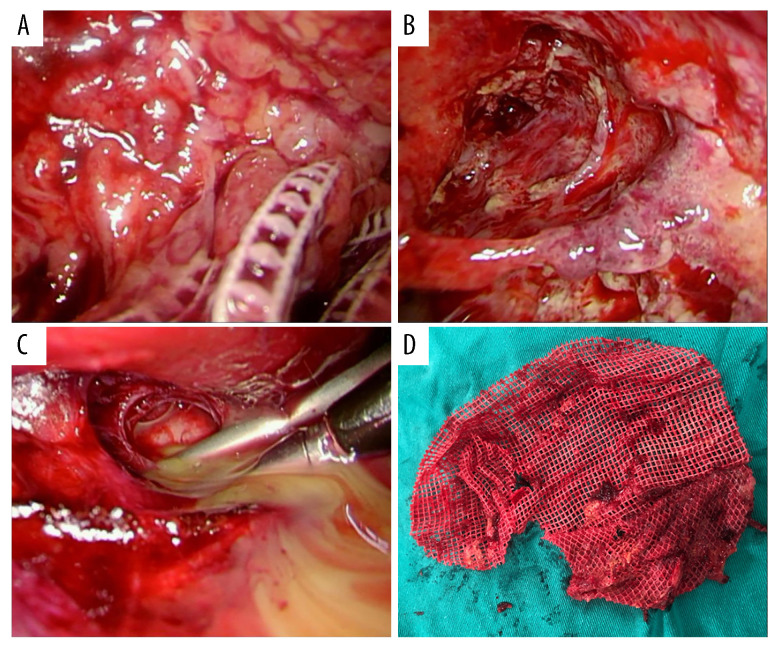
An enhanced computed tomography (CT) scan showed a complicated abscess occupying the preperitoneal space, without intraperitoneal violation. Based on the clinical presentation, physical examination, and imaging findings, a diagnosis of a large preperitoneal abscess with cutaneous fistulization was established. In addition, the patient’s surgical history of endoscopic TEP inguinal hernia repair raised suspicion that the abscess originated from a deep-seated mesh infection. To address the complex nature of the condition, a 2-step surgical approach was employed. The first phase involved performing a fistulectomy to treat the cutaneous fistula (Figure 3A), granting access to the preperitoneal space. A balloon trocar access was created to maximize visibility by limiting trocar intrusion into the abscess chamber, with the help of a 10-mm 30-degree laparoscope, following the extraction of 400 mL of pus from a tiny hole at the fistula end. As a functional port, a second 5-mm trocar was positioned in the right iliac region. During the second endoscopic phase, the prosthetic mesh and screws were extracted from soft tissues after the pyogenic membranes were debrided by sweeping the blunt-ended laparoscopic suction around the abscess chamber to break up loculations (Figure 4). Additionally, a silicone Penrose drainage tube was left inside the deepest part below the pubic symphysis to promote drainage of dirty remnants. Since we planned to place an open drainage tube in the deep dead space, we chose to primarily close the wound. The recovery of the wound was also closely monitored after the operation. If there was any sign of infection, the wound should be re-opened. The wounds were closed layer by layer (monofilament absorbable sutures for the buried fascial layer and non-absorbable nylon sutures for the outside skin). The healed operation scar 3 months after the fistulectomy is shown in Figure 3B. The patient was able to ambulate on the second day after the surgery.
Figure 3.
(A) Following a fish-mouth incision for fistulectomy, the entire fistula tract, located between the prosthetic mesh and the bilateral rectus abdominis muscles, was exposed along the skin incision. (B) The healed surgical scar is on the patient’s hypogastric region, following a fistulectomy to treat the cutaneous fistula. This photo was taken 3 months after surgery.
Figure 4.
(A) The infected mesh was embedded inside the suppurative granulomatous tissue and thick necrotic debris, necessitating generous debridement. (B) Deep-seated pus leaked from the septum between lobulated abscesses. (C) Excessive debridement resulted in a huge abscess cavity. (D) The extracted prosthetic mesh.
Bacterial cultures obtained from the abscess grew Pseudomonas aeruginosa, which was found to be sensitive to cefepime. Additional cultures for mycobacteria and fungi were negative. The patient was discharged on the seventh day after a full course of intravenous antibiotic treatment. Subsequent oral antibiotic administration was stopped under a complete resolution of clinical infection, and subsequent serial cultures did not exhibit any bacterial growth. The Penrose drain was “shortened” by gently withdrawing approximately 2 cm/3–5 days, thus allowing gradual healing of the huge preperitoneal space to avoid residual dead space. There has been no sign of re-infection to this day.
Discussion
We described a patient who presented with a preperitoneal abscess and cutaneous fistula as a late-developing complication 3 years following endoscopic TEP inguinal hernia repair. The noteworthy aspects of this case include the prolonged interval between the initial surgical intervention and the onset of the complication, as well as the effective use of a 2-step surgical approach to successfully resolve the abscess and fistula.
Late-developing mesh infections, initially described by Mann et al [5] in 1998, are infrequently encountered in clinical practice. In a large study by Delikoukos et al [11] involving 1452 patients who underwent groin hernioplasty using a tension-free polypropylene mesh technique, only 5 cases (0.35%) of late-developing infection were reported between 2 and 4.5 years postoperatively. Another study by Chen et al [12], which enrolled 2666 consecutive patients with abdominal wall hernia repairs, found an overall incidence of 0.30%, with 0.24% in inguinal hernia repairs and 0.78% in incisional hernia repairs. The interval from the operation ranged between 3 and 60 months [12].
Despite their rarity, late-onset deep mesh infections can be suspected based on typical symptoms and imaging findings. Ultrasonography can localize the mesh and its relationship to the infection site, as meshes often exhibit a characteristic linear geometry on ultrasound imaging [13]. Differential diagnosis should be considered [12], including superficial surgical site infections, intestinal leakage complications, and localized skin pathologies, such as carbuncles or cellulitis. Treatment of deep mesh infections generally requires complete surgical removal of the infected mesh [14]. In our case, a 2-step surgical approach was employed, consisting of initial fistulectomy followed by endoscopic abscess drainage and mesh removal.
The decision not to immediately reinforce the posterior wall after mesh removal is supported by the literature, as the risk of hernia recurrence is generally low [15]. If there is an obvious recurrence, repair is considered only after stabilization of active infection. Although re-endoscopic management of recurrent inguinal hernias has emerged as a promising idea with encouraging outcomes [16], repeat posterior surgery carries a higher risk of complications because of deformed anatomy; anterior mesh repair is recommended [17]. Therefore, it would make more sense to use traditional open inguinal repair to prevent a challenging scenario with inguinal repair (adhesion after severe infection can make the procedure even more difficult). The use of autologous tissue repair, such as the Shouldice procedure, or biocompatible mesh to minimize the risk of recurrent infections can be considered.
The pathogenesis of late-developing mesh infections remains unclear. Bacterial biofilm formation on the mesh surface can reduce effective mesh porosity and create a nidus for chronic, indolent infection [18]. Staphylococcus aureus, Enterobacter cloacae, and Pseudomonas aeruginosa, the latter of which was isolated in this case, are common culprits in mesh infections [12]. Additionally, persistent fluid collections around the mesh can provide an environment conducive to bacterial growth and the establishment of abscesses and subsequent fistulas. As demonstrated in the presented case, the results of an antibiogram performed on the causative pathogen can provide valuable guidance for the most appropriate antibiotics following surgery.
Regarding prevention, a mesh should be unpacked only right before implantation, to avoid bacterial adhesion in addition to standard operating room sterility protocols. Despite the fact there is increasing evidence that prophylactic antibiotic use does not enhance the outcome of endoscopic or laparoscopic herniorrhaphy [19,20], prophylactic antibiotics can still be important, nevertheless, especially for patients with recurrent hernias, the elderly, and immunocompromised individuals who are at a higher risk of surgical wound infection [17].
Conclusions
This case underscores the importance of considering late-developing mesh infections in patients presenting with abdominal symptoms who have previously undergone TEP hernia repair, even years after the initial surgery. By extension, delayed prosthesis infection could occur after any type of herniorrhaphy with prosthesis reinforcement. Early alert, accurate image assessment, and appropriate management are all essential for achieving optimal patient outcomes. Further research is needed to elucidate the precise mechanisms underlying the development of delayed mesh infections and to identify potential strategies for their prevention.
Acknowledgments
The authors wish to extend their sincere appreciation to Chien-Chih Chiu, the medical assistant in the operating room, for his outstanding dedication and invaluable assistance throughout the surgical procedure.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Yang XF, Liu JL. Laparoscopic repair of inguinal hernia in adults. Ann Transl Med. 2016;4:402. doi: 10.21037/atm.2016.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavriilidis P, Davies RJ, Wheeler J, et al. Total extraperitoneal endoscopic hernioplasty (TEP) versus Lichtenstein hernioplasty: A systematic review by updated traditional and cumulative meta-analysis of randomised-controlled trials. Hernia. 2019;23:1093–103. doi: 10.1007/s10029-019-02049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corthals S, van Cleven S, Uyttebroek O, et al. Quality of life after open versus laparoscopic preperitoneal mesh repair for unilateral inguinal hernias. Asian J Surg. 2021;44:1266–73. doi: 10.1016/j.asjsur.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Shah MY, Raut P, Wilkinson TRV, Agrawal V. Surgical outcomes of laparoscopic total extraperitoneal (TEP) inguinal hernia repair compared with Lichtenstein tension-free open mesh inguinal hernia repair: A prospective randomized study. Medicine (Baltimore) 2022;101:e29746. doi: 10.1097/MD.0000000000029746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann DV, Prout J, Havranek E, et al. Late-onset deep prosthetic infection following mesh repair of inguinal hernia. Am J Surg. 1998;176:12–14. doi: 10.1016/s0002-9610(98)00094-4. [DOI] [PubMed] [Google Scholar]
- 6.Samee A, Adjepong S, Pattar J. Late onset mesh infection following laparoscopic inguinal hernia repair. BMJ Case Rep. 2011;2011:bcr0920114863. doi: 10.1136/bcr.09.2011.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalilvand A, Sarker S, Fisichella PM. A rare case of mesh infection 3 years after a laparoscopic totally extraperitoneal (TEP) inguinal hernia repair. Surg Laparosc Endosc Percutan Tech. 2015;25:e69–71. doi: 10.1097/SLE.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 8.Colbran R, Nicol A, Ng J. Delayed mesh infection presenting as an abdominal mass after laparoscopic inguinal hernia repair. ANZ J Surg. 2020;90:2127–29. doi: 10.1111/ans.15742. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Matsumoto K, Terauchi T, et al. Delayed mesh infection after inguinal hernia repair: A case report. J Surg Case Rep. 2021;2021:rjab399. doi: 10.1093/jscr/rjab399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlosser KA, Warren JA. Hernia mesh complications: Management of mesh infections and enteroprosthetic fistula. Surg Clin North Am. 2023;103:1029–42. doi: 10.1016/j.suc.2023.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Delikoukos S, Tzovaras G, Liakou P, et al. Late-onset deep mesh infection after inguinal hernia repair. Hernia. 2007;11:15–17. doi: 10.1007/s10029-006-0131-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen T, Zhang YH, Wang HL, et al. Late-onset deep mesh infection: a study of eight cases detected from 2666 consecutive patients with abdominal wall hernia repairs. Chin Med J (Engl) 2016;129:1870–72. doi: 10.4103/0366-6999.186651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrilov SG, Son DA, Churikov DA, Shuliak GD. Ultrasound appearance of mesh after transabdominal preperitoneal inguinal hernia repair. J Laparoendosc Adv Surg Tech A. 2020;30:395–401. doi: 10.1089/lap.2019.0689. [DOI] [PubMed] [Google Scholar]
- 14.Falagas ME, Kasiakou SK. Mesh-related infections after hernia repair surgery. Clin Microbiol Infect. 2005;11:3–8. doi: 10.1111/j.1469-0691.2004.01014.x. [DOI] [PubMed] [Google Scholar]
- 15.Akyol C, Kocaay F, Orozakunov E, et al. Outcome of the patients with chronic mesh infection following open inguinal hernia repair. J Korean Surg Soc. 2013;84:287–91. doi: 10.4174/jkss.2013.84.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ertem M, Ozben V, Gok H, Ozveri E. Relaparoscopic treatment of recurrences after previous laparoscopic inguinal hernia repair. Minim Invasive Surg. 2013;2013:260131. doi: 10.1155/2013/260131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons MP, Aufenacker T, Bay-Nielsen M, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia. 2009;13:343–403. doi: 10.1007/s10029-009-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reśliński A, Mikucka A, Szmytkowski J, et al. In vivo biofilm on the surface of a surgical mesh implant. Pol J Microbiol. 2009;58:367–69. [PubMed] [Google Scholar]
- 19.Aufenacker TJ, Koelemay MJ, Gouma DJ, Simons MP. Systematic review and meta-analysis of the effectiveness of antibiotic prophylaxis in prevention of wound infection after mesh repair of abdominal wall hernia. Br J Surg. 2006;93(1):5–10. doi: 10.1002/bjs.5186. [DOI] [PubMed] [Google Scholar]
- 20.Kockerling F, Bittner R, Jacob D, et al. Do we need antibiotic prophylaxis in endoscopic inguinal hernia repair? Results of the Herniamed Registry. Surg Endosc. 2015;29(12):3741–49. doi: 10.1007/s00464-015-4149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]