Abstract
Patient: Male, 55-year-old
Final Diagnosis: Calcification of the bicuspid aortic valve
Symptoms: Cardiac insufficiency
Clinical Procedure: Double valve replacement for mechanical mitral and aortic valves
Specialty: Cardiac surgery
Objective:
Unusual setting of medical care
Background:
A blood cell saver, or autotransfusion system, is used to collect, wash, and return autologous blood collected from the surgical patient. This report describes a 55-year-old man who underwent combined mitral and aortic valve replacement surgery with cardiopulmonary bypass and had a successful outcome following intraoperative and postoperative autologous blood transfusion using a blood cell saver.
Case Report:
The patient did not accept blood transfusion for reasons of religious conscience and was in a critical condition, receiving palliative care. He needed combined mitral and aortic valve replacement surgery. The surgery was conducted using a cell saver (Sorin Xtra Autotransfusion System) in the intraoperative and postoperative periods for 24 h, to resolve this challenging case, from a technical and ethical point of view. The volume of red blood cells recovered intraoperatively was 1430 mL, with a hematocrit level of 40%, and 690 mL, with a hematocrit of 35%, in the postoperative period. Therefore, a significant volume of autologous blood was recovered. The autologous blood transfusion resulted in an excellent clinical outcome for the patient, who was discharged on the ninth postoperative day.
Conclusions:
We can conclude that the use of a blood cell saver in cardiac surgery, in both intra- and postoperative periods, resulted in the maintenance of adequate hemoglobin and hematocrit levels, no infection postoperatively, and rapid and complete recovery of the patient. Thus, the use of the blood cell saver guaranteed the individual’s autonomy to refuse blood products safely, with good clinical results, and without dependence on allogeneic blood transfusions.
Key words: Blood Cells, Thoracic Surgery
Introduction
In open heart surgery, cardiopulmonary bypass (CPB) is a necessity. However, CPB induces a significant systemic inflammatory response that is associated with adverse clinical outcomes, such as acute lung and kidney damage, which is also influenced by the duration of CPB [1–4]. This inflammatory reaction occurs due to mechanical shear stress and the contact of blood cells with the artificial surfaces of the CPB. Thus, cell lesions occur, induced by the activation of the vascular endothelium, polymorphonuclear cells, and platelets, with an increase in pro-inflammatory interleukins (IL-1, IL-2, IL-6, IL-8) and tumor necrosis factor (TNF)-α. With the release of proteolytic enzymes and reactive oxidative species into the circulation, damage occurs to the lung and kidney parenchyma [1,4–6]. This inflammatory response in cardiac surgery with CPB generates an acute response, sometimes resembling severe infection during the immediate postoperative period [7].
In this context, red blood cell (RBC) transfusions are frequently used to maintain hemodynamic stability during and after surgeries with CPB. However, as blood transfusion is a tissue transplant, it promotes or exacerbates the inflammatory response, together with a significant immunomodulatory effect, increasing hospital morbidity and mortality [8–13]. Patients who receive blood transfusions in cardiac surgery show greater activation of polymorphonuclear cells and higher levels of IL-6, with longer lengths of stay in the intensive care unit (ICU) and the necessity of postoperative mechanical ventilation [5,14].
Thus, with patient safety as a focus, efforts have been made to restrict the use of blood components through clinical and surgical strategies that together form part of a patient blood management (PBM) program. Based on 3 pillars, the PBM program aims to reduce/avoid blood transfusion use and improve clinical outcomes by reducing length of stay, infection, morbidity, and mortality [15,16]. The first pillar involves optimizing hematopoiesis, mainly in the preoperative period; the second pillar consists of minimizing blood loss and bleeding in the intraoperative and postoperative periods; and the third pillar involves using the patient’s physiological reserves and the patient tolerating anemia, mainly in the postoperative period [17–19].
In the second pillar of PBM, recovering the patient’s own blood cells by blood cell saver (BCS) equipment significantly reduces the need for blood transfusion and its related risks. In addition, it provides the patient with fresh RBCs without storage lesions, increasing their viability, maintaining their disc shape, and improving the supply of oxygen to the tissues [20–25]. In cardiac surgery, these advantages with the use of the BCS have been widely demonstrated [26,27].
The highlight of this case report is that, although the use of the BCS has been consolidated in the intraoperative period, blood recovery also occurred in the immediate postoperative period, showing an extension in the use of this strategy. The patient was in palliative care due to a critical condition and for not accepting blood transfusions for reasons of religious conscience, representing a challenging case from a technical and ethical point of view. Thus, this report describes a 55-year-old man with combined mitral and aortic valve replacement surgery with CPB and a successful outcome following intraoperative and postoperative autologous blood transfusion using a BCS.
Case Report
A 55-year-old male patient participated in a retrospective, non-randomized, observational cohort study with a primary endpoint to assess intra- and postoperative blood recovery after cardiac surgery with CPB (ethical approval no. 40567520.8.0000.0021 from the Ethics Committee of the Universidade Federal do Mato Grosso do Sul, Campo Grande, MS, Brazil). The patient signed an informed consent form and did not authorize receiving allogeneic blood transfusion during the procedure for reasons of religious conscience.
The preoperative diagnosis was calcification of the bicuspid aortic valve, with secondary moderate mitral regurgitation because of left ventricular dilation outcome to volume overload (cardiomegaly). The heart showed the left ventricle with global hypo-contractility and ejection fraction of 40%, and atrial fibrillation. The patient had a clinical indication for surgical treatment since 2016. However, because of the non-acceptance of receiving an allogeneic blood transfusion, he was evaluated at 3 cardiac surgery services but waited 5 years to get a service that respected his autonomy.
Finally, the procedure occurred in August 2021 at Hospital Unimed de Campo Grande (Campo Grande, MS, Brazil), with double valve replacement for mechanical mitral and aortic valves, using BCS equipment (Sorin Xtra Autotransfusion System; LivaNova Plc, London, UK), during the intraoperative and immediate postoperative period (for 24 h).
Following the principles of PBM, the patient was prepared for surgery by treating preoperative anemia until he reached adequate hemoglobin and hematocrit levels (hemoglobin of 15.5 g/dL and a hematocrit of 48.1%) in a few days. Studies show that anemia, bleeding/blood loss, and blood transfusions are independent risk factors for poor outcomes [28]. According to the World Health Organization, the hemoglobin level must be equal to or greater than 13.0 g/dL for men and 12. 0 g/dL for women [29]. During the preoperative preparation, the patient did not use warfarin, heparin, or other systemic anticoagulant drugs and did not present coagulation disorders or continuous or recurrent systemic sepsis.
In the surgery room, the bilateral spinal erector muscle plane blockade was performed with ropivacaine, and anesthetic induction was performed with fentanyl, propofol, lidocaine, and rocuronium. Anesthetic maintenance was performed with sevoflurane, lidocaine, clonidine, and magnesium, using an infusion pump. For cerebral and hemodynamic monitoring, the bilateral SedLine® monitor (Masimo Corporation, Irvine, CA, USA) and the Infinity® piCCO SmartPod® monitor (Dräger Ltda., São Paulo, SP, Brazil) were used, respectively. A transesophageal echocardiogram was performed intraoperatively. The access route used for double valve replacement surgery for mechanical mitral and aortic valves was median sternotomy, using systemic heparinization (300 U/kg) to obtain an activated clotting time higher than 400 s (controls were performed every 60 min). The CPB circuit was filled with 1500 mL of plasma Lyte (pH 7.4; Baxter, Deerfield, IL, USA). We used an adult membrane oxygenator (LivaNova Inspire™ 8F M; LivaNova, London, UK) and cardioplegic solution for myocardial protection (Del Nido solution: Plasma-Lyte A, 1000 mL; sodium bicarbonate 1 mEq/mL, 13 mL; mannitol 20%, 16.3 mL; magnesium sulfate 50%, 4 mL; lidocaine 1%, 13 mL; potassium chloride 2 mEq/mL, 13 mL) with 1000 mL infusion into the coronary ostia. CPB time was 120 min, with an aortic clamping time of 90 min and moderate hypothermia (between 28.0°C and 31.9°C). Following the principles of PBM, the patient underwent a procedure to avoid losing his own blood. Therefore, the BCS was used during the intraoperative and immediate postoperative (for 24 h) periods. The BCS (Figure 1) includes a specialized dual-lumen suction tube that allows a continuous flow of anticoagulant to the suction tip of the catheter, preventing the clotting of the aspirated blood. The vacuum aspiration pressure was kept between 60 mmHg and 100 mmHg to minimize hemolysis during intraoperative and postoperative aspiration. A solution of 25 000 IU of heparin in 1000 mL of 0.9% saline was used to anticoagulate the blood recovered from the cardiotomy reservoir. The flow of the anticoagulant was adjusted according to the rate of bleeding in the surgical field and the immediate postoperative period, and the blood was filtered. Once a sufficient volume of blood with anticoagulant had reached the cardiotomy reservoir (around 500 mL), processing began by draining the blood from the reservoir into the centrifuge chamber. RBCs and saline were then pumped into an infusion bag to be transfused into the patient (reinfusion within up to 4 h of blood processing).
Figure 1.
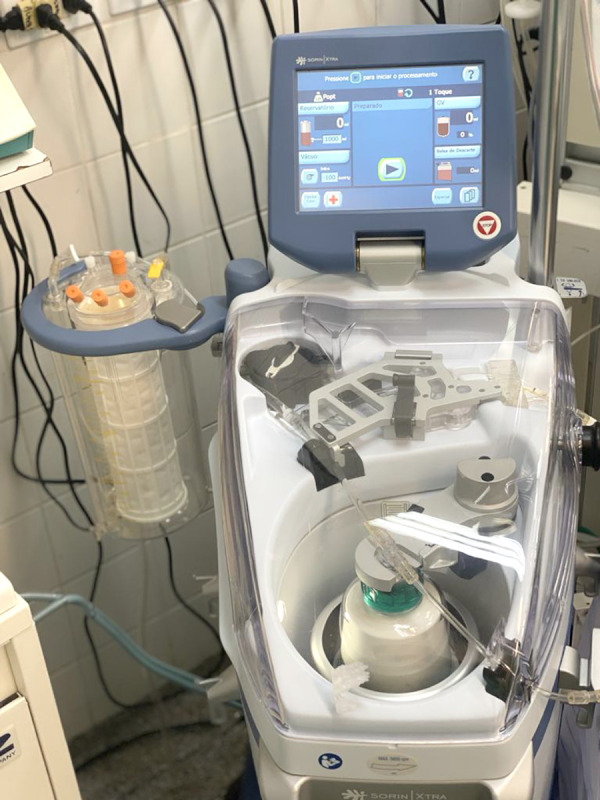
The blood cell saver equipment used.
After CPB, the heparin was completely neutralized with protamine. To neutralize 1 mg of heparin, 1 mg of protamine sulfate is necessary [30]. An additional 30% of the total protamine dose was used in the first postoperative hours to reduce blood loss and the need for blood transfusion [30]. The only hemostatic medication used during the procedure was tranexamic acid at a loading dose of 15 mg/kg during anesthetic induction and maintenance at 2 mg/kg/h during surgery. Tranexamic acid is an antifibrinolytic with proven efficacy in major surgeries. Current clinical practice guidelines recommend intraoperative use in cardiac procedures [31].
At the end of the procedure, the deep neuromuscular block was reversed with 10 mg of morphine and 2 g of dipyrone with sodium sugammadex (Bridion® 100 mg/mL, BU15; Pharma Manufacturing Services, Greenville, NC, USA), and then the patient was extubated. After 1 h of extubation, the patient was transferred to the ICU, conscious and oriented, with the BCS connected to the mediastinal drain in postoperative automatic mode.
Finally, the patient was discharged from the hospital on the ninth postoperative day, with a hemoglobin level of 11.6 g/dL and a hematocrit level of 35.1% (Figures 2, 3). The volume of RBCs recovered intraoperatively was 1430 mL, with a hematocrit of 40%, and in the postoperative period, it was 690 mL, with a hematocrit of 35%.
Figure 2.
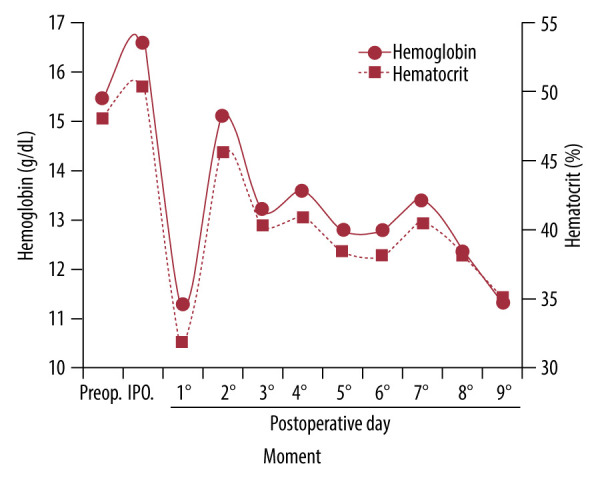
Variation in hemoglobin (g/dL) and hematocrit (%) levels. Assessment performed preoperatively (Preop), intraoperatively (IPO), and postoperatively (days 1 to 9).
Figure 3.
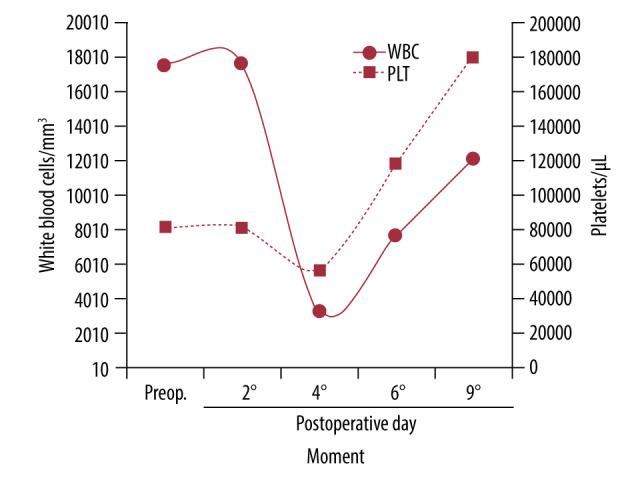
Variation of levels of white blood cells (mm³) and platelets (μL). Assessment performed preoperatively (Preop) and postoperatively (days 2,4,6, and 9).
Figure 4 shows the postoperative serum concentration of C-reactive protein (CRP) as an inflammatory parameter. The concentration preoperatively was 5.8 mg/L (less than 3.0 mg/L: normal level seen in most healthy adults; 10.0 to 100.0 mg/L: moderate elevation; more than 100.0 mg/L: marked elevation) [32]. Thus, the patient’s cardiovascular functions were recovered, and his autonomy in not accepting allogeneic blood transfusion was respected (Figure 5).
Figure 4.
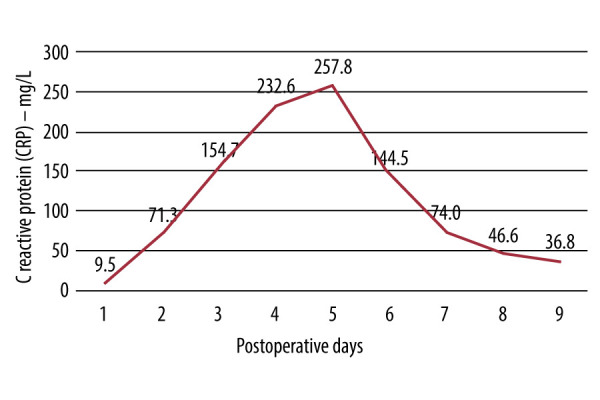
Kinetic curve of the serological concentration (mg/L) of C-reactive protein (CRP) in the postoperative period.
Figure 5.
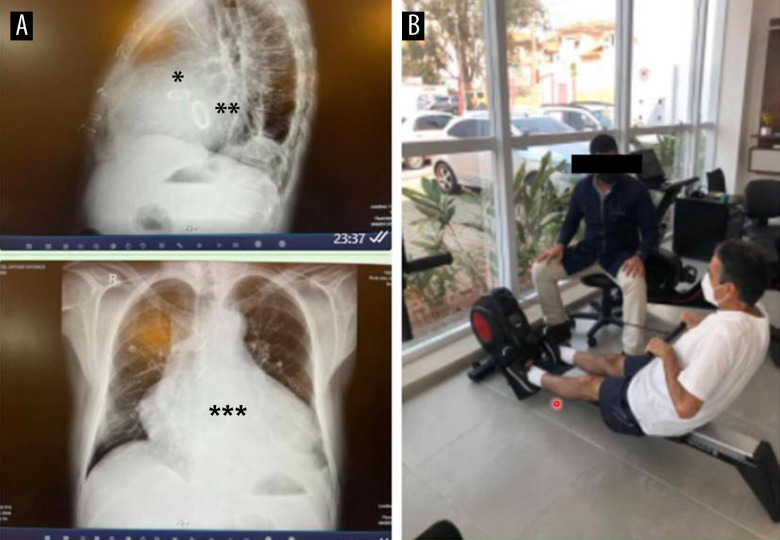
Chest X-rays (2 views) of the patient on the day of discharge (A). Note the mechanical prostheses in the aortic (*) and mitral (**) positions and the significant enlargement of the heart chambers (***). The same patient underwent cardiopulmonary rehabilitation 10 days after discharge (B).
There is no specific hemoglobin and hematocrit limit for PBM strategies in the perioperative period. The responsible physician must intervene with other strategies in critical hemorrhages or in severe symptomatic postoperative anemia, where the clinical need cannot be met with volume replacement or hematinic medication alone [28].
Discussion
In this case report, the first and second pillars of the PBM were used to treat preoperative anemia and reduce blood loss with the BCS. Thus, we observed that it is possible to use PBM strategies to avoid blood transfusions and their associated risks and to respect the autonomy of patients who refuse blood transfusions for reasons of religious conscience.
Massive blood loss during cardiac surgery is relatively common, and the risk is increased because of coagulopathies and platelet dysfunction resulting from CPB. The use of BCS intraoperatively in cardiac surgery has been demonstrated to decrease allogeneic blood transfusion by up to 40% [33] and is associated with lower rates of overall complications, including stroke, acute kidney injury, atrial fibrillation, and pulmonary complications [34]. The typical hematocrit of intraoperatively reinfused recovered blood is around 55% to 70%, similar to that found in allogeneic RBC units from the blood bank [17]. In addition, reinfused autologous RBCs maintain their biconcave and flexible shape and have more physiological concentrations of potassium, 2,3-diphosphoglycerate, and adenosine triphosphate, optimizing their viability and oxygen delivery [33].
Using BCS in the intraoperative and immediate postoperative periods is an effective and safe procedure for blood conservation, with clinically low residual heparin concentrations in the final product, which do not increase the risk of intraoperative and postoperative hemorrhage [35]. In fact, as observed in this case report, the use of BCS proved safe even for a patient who refused to receive a blood transfusion in a double valve replacement surgery and with severe heart disease. Good hemoglobin and hematocrit levels in the postoperative period were achieved, because it was possible to recover a significant blood volume intraoperatively and in the immediate postoperative period. This strategy was used on a patient who waited 3 years until he found a cardiac surgery team that adopted the PBM protocol, thus respecting his autonomy. The wait made his initial condition, congenital bicuspid aortic valve, evolve into dilation and worsening of ventricular function, and as a result, mitral valve insufficiency. Due to the patient’s critical condition, he was already under palliative care. Through the adoption of PBM principles by the surgeon responsible, focusing mainly on using an intra- and postoperative BCS (the use in the postoperative period is still little explored in cardiac surgery), it was possible to save the patient’s life and restore his cardiovascular capacity. In general, cardiac surgeries using cell savers are associated with a significant reduction in perioperative allogeneic RBC transfusions, with better results the more significant the intra-operative blood loss (by 52% for 3000 mL, 49% for 2000 mL, and 40% for blood loss of 1000 mL) [26,27]. A study comparing patients undergoing cardiac surgery with the use of BCS, compared with transfused patients, showed an increase in mortality, from 2% to 4%, higher incidence of reoperations, from 6% to 12%, but lower use of RBCs, from 4.31 to 1.25, and shorter average length of stay, from 10.8 to 7.4 days [36]. Another study using BCS in cardiac surgery showed that its use is cost-effective only in surgeries with CPB greater than 45 min [37].
An important aspect is the fact that, with BCS, allogeneic blood is replaced by autologous blood, which avoids the immunomodulatory/inflammatory effects that another individual’s blood can promote in the patient [38]. In fact, a recent study showed that BCS was associated with lower levels of postoperative IL-10, probably due to an overall decrease in the pro-inflammatory state and, consequently, less lung and kidney dysfunction due to the inflammation caused by the allogeneic transfusion [14]. In the present report, the kinetics of CRP in response to the procedure in the first week was similar to that of surgeries without extracorporeal circulation, with a peak between 72 and 120 h [39,40]. A CRP peak ≥100 mg/L, together with blood transfusion, significantly increases the risk of mortality [41]. In our case, the patient reached peaks twice as high, due to his critical condition, but he recovered completely.
Despite there being few studies, we identified a recent work that used BCS in the postoperative period (6 h) in cardiac surgery. In cardiac surgeries, postoperative atrial fibrillation affects between 19% and 50% of patients and is associated with increased length of stay, morbidity, and mortality. One of the factors contributing to postoperative atrial fibrillation is mediastinal bleeding and inflammation. However, in this study, the group of patients with BCS postoperative showed a decreased incidence of postoperative atrial fibrillation [22]. Another study in cardiac surgery and the use of BCS from 6 to 24 h postoperatively showed that its use significantly reduced the use of RBCs and did not increase infection rates, demonstrating the safety of the procedure in this regard [42].
Despite the advantages of BCS use, studies have demonstrated that the recovered blood is affected by important factors, such as negative pressure suction, filtration, and washing liquids, used during the autotransfusion process. These factors promote erythrocyte destruction, and researchers and manufacturers have made efforts to minimize these problems [43,44]. Furthermore, there is the problem of possible bacterial contamination of autologous blood, although this is also a problem in allogenic blood units. Bacterial contamination of autologous blood samples was observed in 42% of patients who underwent neurosurgery. Decontamination methods, such as white blood cell filtration and X-ray irradiation, reduced the bacterial load, but the contamination was significantly associated with the surgical approach. However, there were no postoperative infectious complications [45]. Another aspect related to risks in BCS use is in oncology cases, but recent studies have demonstrated that its safe use is possible [46].
Currently, we have seen progress in respecting patients’ self-determination when they refuse blood transfusions. This is because the most up-to-date scientific evidence, much of which is based on the principles and practices of PBM, shows that it is possible to achieve a balance between respect for the autonomy of the patient and the professional autonomy of the physician [47]. What has been observed for more than a decade is that patients who refuse blood transfusions for religious reasons (Jehovah’s Witnesses) have increasingly contributed to the advancement of transfusion practices by encouraging doctors to use therapeutic options aimed at their safety but with respect for their autonomy. There is no shortage of publications with scientific impact that make this appropriate citation [48]. The Joint Commission published a study in 2023 reviewing the prescription of blood products in 15 large hospitals. It concluded that only 14.52% of prescriptions were appropriate, reinforcing that overuse of blood transfusions increased the risk for patients and hospital costs [49].
Thus, it is clear that it is possible to advance in the transfusion practice to other options, even in critically ill patients [50,51]. Moreover, it is expected that with the aging of the population, blood components will be increasingly scarce [18]. Additionally, medical practice has evolved toward respecting patient autonomy. Cardiac surgery societies are increasingly adhering to the concept of individualized, shared, and humanized healthcare [52].
Conclusions
We can conclude that the use of a BCS in cardiac surgery, both in intra- and postoperative periods, resulted in the maintenance of adequate hemoglobin and hematocrit levels, no infection postoperatively, and rapid and complete recovery of the patient. Thus, the use of the BCS guaranteed the individual’s autonomy to refuse blood products safely, with good clinical results, and without dependence on allogeneic blood transfusions.
Acknowledgments
We thank Claudio Albernaz Cesar and Evandro Carlos Ribeiro Lopes (cardiovascular surgeons), Gustavo Barone Perez (anesthesiologist), and Priscila Aparecida Hilário Maya (nurse).
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Performed
This work was performed at the Faculdade de Medicina, PPG Saúde e Desenvolvimento na Região Centro-Oeste/FAMED, Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande, MS, Brazil.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121(4):1269–77. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- 2.Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999;68(3):1107–15. doi: 10.1016/s0003-4975(99)00781-x. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen K. Acute kidney injury in children undergoing surgery for congenital heart disease. Eur J Pediatr Surg. 2012;22(6):426–33. doi: 10.1055/s-0032-1322540. [DOI] [PubMed] [Google Scholar]
- 4.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 5.Liu KD, Altmann C, Smits G, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: A case-control study. Crit Care. 2009;13(4):R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Stadlbauer A, Heller A, et al. Impact of fluid balance and blood transfusion during extracorporeal circulation on outcome for acute type A aortic dissection surgery. J Cardiovasc Surg (Torino) 2022;63(6):734–41. doi: 10.23736/S0021-9509.22.12339-6. [DOI] [PubMed] [Google Scholar]
- 7.Auler Júnior JO, Chiaroni S. Circulação extracorpórea: Prevenção e manuseio de complicações. Rev Bras Anestesiol. 2000;50(6):464–69. [in Portuguese] [Google Scholar]
- 8.Fransen E, Maessen J, Dentener M, et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116(5):1233–39. doi: 10.1378/chest.116.5.1233. [DOI] [PubMed] [Google Scholar]
- 9.Garraud O, Tariket S, Sut C, et al. Transfusion as an inflammation hit: Knowns and unknowns. Front Immunol. 2016;7:534. doi: 10.3389/fimmu.2016.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Istaphanous GK, Wheeler DS, Lisco SJ, Shander A. Red blood cell transfusion in critically ill children: A narrative review. Pediatr Crit Care Med. 2011;12(2):174–83. doi: 10.1097/PCC.0b013e3181e30d09. [DOI] [PubMed] [Google Scholar]
- 11.Goodman AM, Pollack MM, Patel KM, Luban NL. Pediatric red blood cell transfusions increase resource use. J Pediatr. 2003;142(2):123–27. doi: 10.1067/mpd.2003.14. [DOI] [PubMed] [Google Scholar]
- 12.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36(9):2667–74. doi: 10.1097/CCM.0b013e3181844677. [Erratum in: Crit Care Med. 2008;36(11):3134] [DOI] [PubMed] [Google Scholar]
- 13.Kneyber MC, Hersi MI, Twisk JW, et al. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33(8):1414–22. doi: 10.1007/s00134-007-0741-9. [DOI] [PubMed] [Google Scholar]
- 14.Martinez MJ, Schwingshackl A, Romero T, et al. Cell saver blood transfusions may be associated with a decrease in inflammation and improved outcome measures in pediatric cardiac surgery patients. Perfusion. 2023;38(4):717–24. doi: 10.1177/02676591221078420. [DOI] [PubMed] [Google Scholar]
- 15.Santos AA, Silva JP, Silva Lda F, et al. Therapeutic options to minimize allogeneic blood transfusions and their adverse effects in cardiac surgery: A systematic review. Rev Bras Cir Cardiovasc. 2014;29(4):606–21. doi: 10.5935/1678-9741.20140114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shander A, Hardy JF, Ozawa S, et al. Collaborators A global definition of patient blood management. Anesth Analg. 2022;135(3):476–88. doi: 10.1213/ANE.0000000000005873. [DOI] [PubMed] [Google Scholar]
- 17.Isbister JP. The three-pillar matrix of patient blood management – an overview. Best Pract Res Clin Anaesthesiol. 2013;27(1):69–84. doi: 10.1016/j.bpa.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Farmer SL, Towler SC, Leahy MF, Hofmann A. Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA) Best Pract Res Clin Anaesthesiol. 2013;27(1):43–58. doi: 10.1016/j.bpa.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann A, Spahn DR, Holtorf AP, PBM Implementation Group Making patient blood management the new norm(al) as experienced by implementors in diverse countries. BMC Health Serv Res. 2021;21(1):634. doi: 10.1186/s12913-021-06484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank SM, Sikorski RA, Konig G, et al. Clinical utility of autologous salvaged blood: A review. J Gastrointest Surg. 2020;24(2):464–72. doi: 10.1007/s11605-019-04374-y. [DOI] [PubMed] [Google Scholar]
- 21.Sikorski RA, Rizkalla NA, Yang WW, Frank SM. Autologous blood salvage in the era of patient blood management. Vox Sang. 2017;112(6):499–510. doi: 10.1111/vox.12527. [DOI] [PubMed] [Google Scholar]
- 22.Koçyiğit M, Koçyiğit ÖI, Güllü AÜ, et al. Postoperative atrial fibrillation reduced by intraoperative and postoperative cell saver system in coronary artery bypass graft surgery. Turk J Anaesthesiol Reanim. 2022;50(3):173–77. doi: 10.5152/TJAR.2022.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Khabori M, Al Riyami A, Siddiqi MS, et al. Impact of cell saver during cardiac surgery on blood transfusion requirements: A systematic review and meta-analysis. Vox Sang. 2019;114(6):553–65. doi: 10.1111/vox.12824. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Chen L, Qian L, et al. Blood conservation strategies in cardiac valve replacement: A retrospective analysis of 1645 patients. Medicine (Baltimore) 2016;95(41):e5160. doi: 10.1097/MD.0000000000005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd TD, Geneen LJ, Bernhardt K, et al. Cell salvage for minimising perioperative allogeneic blood transfusion in adults undergoing elective surgery. Cochrane Database Syst Rev. 2023;9(9):CD001888. doi: 10.1002/14651858.CD001888.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neef V, Vo L, Herrmann E, et al. The association between intraoperative cell salvage and red blood cell transfusion in cardiac surgery – an observational study in a patient blood management centre. Anaesthesiol Intensive Ther. 2021;53(1):1–9. doi: 10.5114/ait.2021.103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quispe-Fernández LA, Carrillo-González ML, Gutiérrez-Ospina A, Gómez-Leandro II. Blood products with autologous transfusion versus allogeneic transfusion in cardiac surgery patients. Rev Med Inst Mex Seguro Soc. 2020;58(4):417–27. doi: 10.24875/RMIMSS.M20000066. [DOI] [PubMed] [Google Scholar]
- 28.Shander A, Corwin HL, Meier J, et al. Recommendations from the International Consensus Conference on Anemia Management in Surgical Patients (ICCAMS) Ann Surg. 2023;277(4):581–90. doi: 10.1097/SLA.0000000000005721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler AJ, Ahmad T, Phull MK, et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102(11):1314–24. doi: 10.1002/bjs.9861. [DOI] [PubMed] [Google Scholar]
- 30.Barroso RC, Mendonça JTD, Carvalho MR, et al. Assessment of protamine in the neutralization of heparin after extracorporeal circulation. Rev Bras Cir Cardiovasc. 2002;17(1):54–60. [Google Scholar]
- 31.Alaifan T, Alenazy A, Xiang Wang D, et al. Tranexamic acid in cardiac surgery: A systematic review and meta-analysis (protocol) BMJ Open. 2019;9(9):e028585. doi: 10.1136/bmjopen-2018-028585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nehring SM, Goyal A, Patel BC. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. C reactive protein. 2023. Available at: https:////pubmed.ncbi.nlm.nih.gov/28722873/ [PubMed] [Google Scholar]
- 33.Gross I, Seifert B, Hofmann A, Spahn DR. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015;55(5):1075–81. doi: 10.1111/trf.12946. [DOI] [PubMed] [Google Scholar]
- 34.Malhotra A, Islam MA, Tavilla G, et al. Autologous cell salvage in off-pump coronary artery bypass surgery reduces post-operative complications: A retrospective weighted-matching analysis. Gen Thorac Cardiovasc Surg. 2024 doi: 10.1007/s11748-024-02012-2. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019;17(1):27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almeida RM, Leitão L. The use of cell saver system in cardiac surgery with cardiopulmonary bypass. Rev Bras Cir Cardiovasc. 2013;28(1):76–82. doi: 10.5935/1678-9741.20130012. [DOI] [PubMed] [Google Scholar]
- 37.Steinbach M, Centenaro MH, Almeida RM. Benefit from using recycling red blood cells in cardiovascular surgery. Rev Bras Cir Cardiovasc. 2014;29(3):374–78. doi: 10.5935/1678-9741.20140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas LN, Garcia LO, Galvão ACS, et al. Blood transfusion-related immunomodulation. Clin Biomed Res. 2014;34(4):333–41. [Google Scholar]
- 39.Krumsdorf U, Chorianopoulos E, Pleger ST, et al. C-reactive protein kinetics and its prognostic value after transfemoral aortic valve implantation. J Invasive Cardiol. 2012;24(6):282–86. [PubMed] [Google Scholar]
- 40.Ruparelia N, Panoulas VF, Frame A, et al. Impact of clinical and procedural factors upon C reactive protein dynamics following transcatheter aortic valve implantation. World J Cardiol. 2016;8(7):425–31. doi: 10.4330/wjc.v8.i7.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sousa ALS, Carvalho LAF, Salgado CG, et al. C-reactive protein as a prognostic marker of 1-year mortality after transcatheter aortic valve implantation in aortic stenosis. Arq Bras Cardiol. 2021;117(5):1018–27. doi: 10.36660/abc.20190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulos L, Kuebler JD, Angona R, et al. Cell saver blood reinfusion up to 24 hours post collection in pediatric cardiac surgical patients does not increase incidence of hospital-acquired infections or mortality. J Extra Corpor Technol. 2021;53(3):161–69. doi: 10.1182/ject-2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruber M, Breu A, Frauendorf M, et al. Washing of banked blood by three different blood salvage devices. Transfusion. 2013;53(5):1001–9. doi: 10.1111/j.1537-2995.2012.03853.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, He J, Han X, et al. Application of an autotransfusion pressure control system in blood salvage. J Int Med Res. 2023;51(11):3000605231206963. doi: 10.1177/03000605231206963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kvan OK, Teryaeva NB, Sukhorukova MV, Lubnin AY. [Bacterial contamination of autologous blood in reinfusion in neurosurgery: A phenomenon or a problem?] Zh Vopr Neirokhir Im N N Burdenko. 2024;88(2):54–61. doi: 10.17116/neiro20248802154. [in Russian] [DOI] [PubMed] [Google Scholar]
- 46.Lugassy L, Marion S, Balthazar F, et al. Impact of blood salvage therapy during oncologic liver surgeries on allogenic transfusion events, survival, and recurrence, an ambidirectional cohort study. Int J Surg. 2024;110(6):3392–400. doi: 10.1097/JS9.0000000000001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolcato M, Shander A, Isbister JP, et al. Physician autonomy and patient rights: Lessons from an enforced blood transfusion and the role of patient blood management. Vox Sang. 2021;116(10):1023–30. doi: 10.1111/vox.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anthes E. Evidence-based medicine: Save blood, save lives. Nature. 2015;520(7545):24–26. doi: 10.1038/520024a. [DOI] [PubMed] [Google Scholar]
- 49.Jadwin DF, Fenderson PG, Friedman MT, et al. Determination of unnecessary blood transfusion by comprehensive 15-hospital record review. Jt Comm J Qual Patient Saf. 2023;49(1):42–52. doi: 10.1016/j.jcjq.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Long DA, Slaughter E, Mihala G, et al. Patient blood management in critically ill children undergoing cardiac surgery: A cohort study. Aust Crit Care. 2023;36(2):201–7. doi: 10.1016/j.aucc.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Helmer P, Hottenrott S, Steinisch A, et al. Avoidable blood loss in critical care and patient blood management: scoping review of diagnostic blood loss. J Clin Med. 2022;11(2):320. doi: 10.3390/jcm11020320. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grant MC, Crisafi C, Alvarez A, et al. Perioperative care in cardiac surgery: A joint consensus statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS) Ann Thorac Surg. 2024;117(4):669–89. doi: 10.1016/j.athoracsur.2023.12.006. [DOI] [PubMed] [Google Scholar]