Abstract
Per- and polyfluoroalkyl substances (PFAS) are a class of synthetic chemicals with several applications. Multiple adverse health effects are reported for longer carbon chain (≤C8) PFAS. Shorter carbon chain PFAS, [e.g., hexafluoropropylene oxide dimer acid (HFPO–DA; GenX) and perfluorobutanesulfonic acid (PFBS)] were introduced as alternatives. Past studies indicate that longer-chain PFAS are neurotoxic targeting the dopamine pathway, but it is not known if shorter-chain PFAS act similarly. This study aimed to evaluate developmental neurotoxicity and tissue uptake of GenX and PFBS using the zebrafish (Danio rerio). First, acute toxicity was assessed by measuring LC50 at 120 h postfertilization (hpf). Body burden was determined after embryonic exposure (1–72 hpf) to sublethal concentrations of GenX or PFBS by LC-ESI-MS/MS. Locomotor activity using a visual motor response assay at 120 hpf and dopamine levels at 72 hpf was assessed after embryonic exposure. PFBS was more acutely toxic and bioaccumulative than GenX. GenX and PFBS caused hyperactivity at 120 hpf, but stronger behavioral alterations were observed for PFBS. An increase in whole organism dopamine occurred at 40 ppb of GenX, while a decrease was observed at 400 ppb of PFBS. Differences detected in dopamine for these two PFAS indicate differential mechanisms of developmental neurotoxicity.
Keywords: behavior, dopamine, HFPO–DA, perfluoroalkyl substances, perfluorobutanesulfonic acid, polyfluoroalkyl substances, toxicokinetics, zebrafish
Graphical Abstract

INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are used in many consumer and industrial processes1 but persist in the environment and bioaccumulate in humans and wildlife.2,3 The longer chain PFAS [e.g., perfluorooctanesulfonic acid (PFOS) and perfluoroocatonic acid (PFOA)] have long half-lives in humans and are associated with many adverse human health effects.4,5 In order to overcome concerns associated with longer chain PFAS, chemicals with a shorter alkyl chain [e.g., perfluorobutanesulfonic acid (PFBS), C4HF9O3S] or a shorter chain with one or more ether group(s) [e.g., hexafluoropropylene oxide dimer acid (HFPO–DA), known as GenX, C6HF11O3] were introduced into industry, but assessments indicate the presence of these PFAS alternatives in the environment. For example, GenX and PFBS are detected in environmental water samples and in treated drinking water with half-lives reported as stable (i.e., no degradation in water at temperatures up to 50 °C regardless of pH).6–8 A study by Guillette et al. also showed that the serum level of GenX in juvenile Striped Bass (Morone saxatilis) was 136 times higher than the concentration in surface water, indicating that GenX can accumulate in tissue similar to other PFAS.9
Researchers have started to investigate the toxicity of GenX and PFBS due to concerns of their persistence in the environment and based on these studies, the United States Environmental Protection Agency (US EPA) released the human lifetime health advisory level for GenX and PFBS of 10 parts per trillion (ppt) and 2000 ppt, respectively.10 In addition, the US EPA introduced a hazard index of 1.0 for PFAS mixtures in drinking water consisting of PFBS, GenX, and two other PFAS (i.e., perfluorononanoic acid-PFNA and perfluorohexanesulfonic acid-PFHxS). GenX exposure is reported to cause hepatic, reproductive, developmental, thyroid, hematological, and immune effects in rats and mice.11–13 PFBS [as sulfonic acid (CAS 375–73–50), potassium salt (CAS 29420–49–3), or deprotonated anionic form PFBS– (CAS 45187–15–3)] causes multiple adverse effects in animals such as decreased concentration of thyroid hormones, decreased sperm health, delayed eye opening, and decreased body weight of offspring, alongside increased liver weight, as per a US EPA review.14
Currently, there is limited information regarding neurotoxic potential for most PFAS beyond the connection of neuroendocrine control of the thyroid pathway.14 In addition, the US EPA identified significant knowledge gaps for GenX and PFBS neurotoxic risk.14,15 Given evidence of developmental neurotoxicity of the two legacy PFAS in which GenX and PFBS were introduced to replace (i.e., PFOA and PFOS, respectively) and the suggested effects of the legacy PFAS on dopaminergic neuron development (e.g., altered dopamine levels, behavioral changes, and altered expression of genes associated with dopaminergic neuronal development), it is important to investigate the developmental neurotoxicity of these two PFAS replacements. Dopamine plays a fundamental role in neurodevelopment in cell differentiation and circuit formation and throughout life as a neurotransmitter and neurohormone, regulating movement, memory, and motivation. In addition, altered dopamine levels are associated with several neurological and mental health diseases.16–18 A recent study showed that predifferentiation exposure to GenX in dopaminergic-like neurons using an SH-SY5Y cell line caused neurological alterations that are associated with neurodevelopment and neurodegeneration including changes in intracellular calcium, tyrosine hydroxylase (TH), and alpha synuclein (αSyn) expression.19 Moreover, assessing the internal dose after PFAS exposure is highly important to anchor the observed effects to an internal dose given the remaining questions on the toxicokinetics of each PFAS in different organisms. This need for internal dose measurements was recently emphasized to assist regulatory agencies in their interpretations of toxicity data.20
The zebrafish continue to grow in popularity as a model for developmental neurotoxicity studies after decades of use in developmental and neurobiology research. For example, assessing the behavior of zebrafish is widely used in neuropharmacology for evaluating the neurotoxicity of drugs.21–24 In addition, zebrafish have orthologs to 70% of human genes with this number increasing to 82% when considering disease-related genes.25
In this study, we hypothesized that GenX and PFBS will cause developmental neurotoxicity by altering locomotor behavior outcomes in developing zebrafish larvae and altering dopaminergic neuron development. In order to test this hypothesis, developing zebrafish were exposed throughout embryogenesis (1–72 h postfertilization, hpf) to GenX or PFBS. Locomotor activity was determined at 120 hpf and dopamine levels in whole eleuthero-embryos at 72 hpf. Body burden was assessed at 72 hpf (at the cessation of exposure and assessment time of dopamine levels) and at 120 hpf (at the time of locomotor activity assessment and 48 h after PFAS exposure was ceased).
MATERIALS AND METHODS
Zebrafish Husbandry.
Adult zebrafish (Danio rerio) of the wild-type AB strain were housed in a Z-Mod system (Aquatic Habitats, Apopka, FL, USA) on a 14:10 light-dark cycle for breeding. Water was maintained at 28 ± 1 °C, pH at 7.0–7.2, conductivity at 550 μS, and dissolved oxygen at 7.3–8.3 ppm. Water quality was assessed twice a day. Adult fish were fed a mixture of brine shrimp (Artemia International LLC., Fairview, TX, USA), Golden Pearls 500–800 μm (Artemia International LLC., Fairview, TX, USA), and Zeigler adult zebrafish food (Zeigler Bros Inc., Gardners, PA, USA). Adult fish were bred in spawning tanks according to established protocols.26,27 Embryos were collected within 1 hpf. The embryos were rinsed with embryo water (filtered reverse osmosis water of pH 7.2 and conductivity at 550 μS) and randomly distributed in groups of 50 into 100 × 20 mm polystyrene Petri dishes for each treatment group. A biological replicate was blocked as either the single Petri dish (e.g., visual motor response assay) or the pooling of fish from two Petri dishes when pools consisted of >50 fish (e.g., body burden). All embryos used in experiments were incubated at 28 °C through 72 or 120 hpf on the same light-dark cycle as the adults. Protocols were approved by the Purdue University Animal Care and Use Committee (no. 1110000088) and all fish were treated humanely with regard to prevention and alleviation of suffering.
Chemical Treatments for Zebrafish Assays.
GenX (CAS# 13252–13–6, 97% purity, Matrix Scientific, Columbia, SC, USA) and PFBS potassium salt (PFBS) (CAS# 29420–49–3, 98% purity, Sigma, St. Louis, MO, USA) were used in the study (Table S1). The potassium salt completely dissociates in aqueous solutions (pH 4–9), with the sulfonate being the predominant form and is the preferential form used in laboratory studies.14 The chemicals were solubilized in reverse osmosis water and all within solubility limits. Stock solutions were neutralized to pH 7–7.5 with 5 M sodium hydroxide.28
Acute Developmental Toxicity Assay.
To assess the lethal concentration at which 50% mortality was observed (LC50) of the test chemicals, three biological replicates (embryos from three different clutches) of 50 embryos per treatment were placed into a Petri dish and dosed with 20 mL of a range of concentrations of each PFAS within 1 h after spawning.26,29 Selected concentrations were 0, 4000, 6000, 8000, and 10,000 ppm (ppm, mg/L) (12,119–30,298 μM) of GenX and 0, 1000, 1200, 1300, 1500, and 2000 ppm (3332– 6664 μM) of PFBS. All solutions were adjusted to a neutral pH (7–7.5).28 The developing zebrafish were exposed to the test chemicals until 120 hpf. The negative control was filtered water only. Mortality rates were monitored every 24 h. Mortality rates of treatment groups were normalized to the control treatment group.
PFAS Body Burden and Water Concentration Determination.
Sample Collection for the PFAS Body Burden and Dosing Solution Concentrations.
PFAS body burden was assessed at two time points: 72 and 120 hpf. The rationale for these tissue collection time points is aligned with the exposure duration and conditions for the subsequent assessment of dopamine at 72 hpf and behavioral analysis at 120 hpf (i.e., 1–72 hpf PFAS exposure, cessation of exposure at 72 hpf, and development in water only until 120 hpf) (Figure S1). In addition, concentration ranges aligned with exposure concentrations of these two time point assessments (i.e., 0, 0.4, 4, 40, and 400 ppb at 72 hpf for dopamine level analysis and 0, 4, 40, 400, and 4000 ppb at 120 hpf for behavior analysis). Concentrations were chosen to encompass a log scale of sublethal concentrations with the inclusion of concentrations above an earlier US EPA provisional health advisory limit for PFOA at 0.4 ppb.30 Four biological replicates were completed for each treatment group at each time point.
For dosing, embryos were rinsed with filtered fish water (reverse osmosis water of pH 7.2 and conductivity of 550 μS/cm) and randomly distributed into groups of 50 in Petri dishes at 1 hpf. First, fish were exposed to 0, 0.4, 4, 40, or 400 ppb (ppb, μg/L) of GenX or PFBS from 1 to 72 hpf (end of embryogenesis) at 28 °C. Each treatment group of each biological replicate had two Petri dishes with 50 fish to total 100 fish exposed (i.e., fish were pooled from the two Petri dishes to attain a single biological replicate). Dosing solutions were collected at the initiation of exposure (1 hpf) and stored at 4 °C until concentrations of GenX or PFBS were measured. At 72 hpf, eleuthero-embryos were rinsed twice with filtered water and euthanized by cooling for ~2–7 h at 4 °C. For each treatment group of each biological replicate, 70 fish were pooled (35 from each Petri dish) into a microcentrifuge tube. The weight of the tubes before the addition of the fish was measured. Samples were freeze-dried, the weight of tubes (containing samples) was measured, and the samples were stored at −20 °C until further preparation for chemical analysis.
The second set of PFAS body burden analyses was completed similarly but aligned with the exposure scenario of the behavioral assessments. Zebrafish embryos were exposed to 0, 4, 40, 400, or 4000 ppb GenX or PFBS from 1 to 72 hpf at 28 °C. At 72 hpf, fish were rinsed with filtered fish water and incubated in filtered fish water only for an additional 2 days for depuration (120 hpf). At 120 hpf, 70 larvae were collected, as mentioned above. Water samples from the dosing solutions in the Petri dishes were collected at three time points: (1) at 1 hpf (at the initiation of PFAS exposure), (2) at 72 hpf (end of PFAS exposure), and (3) at 120 hpf (48 h following cessation of PFAS exposure). These collection time points were chosen to (1) confirm the expected PFAS concentration of the dosing solution at exposure initiation, (2) PFAS concentration at the cessation of the exposure period, and (3) to determine PFAS depuration. Water samples were stored in the dark at 4 °C until further processing.
PFAS Body Burden Analysis.
Analytical standards and isotopically labeled PFAS were purchased from the Wellington laboratory (Overland Park, KS, USA). Methanol, ammonium acetate, tetrahydrofuran, and isopropyl alcohol at Optima HPLC or higher grade were purchased from Fisher Scientific (Hampton, NH, USA). Ultrapure water was prepared using Barnstead laboratory water purification (Thermo Scientific, Waltham, MA, USA). Tissue samples were spiked with 25 μL of an internal standard solution in methanol (200 ng/mL of each mass-labeled PFAS). Extraction was performed by adding 600 μL of tetrahydrofuran and 200 μL of ultrapure water. Samples were vortexed for 10 min, sonicated for 30 min, and centrifuged at 17,000g for 20 min. The supernatant was transferred to a 1.5 mL glass injection vial, gently dried with nitrogen, and solvent-exchanged to 500 μL of 50:50 v/v methanol: water. The supernatant was then vortexed, transferred to a 1.5 mL microcentrifuge tube, and centrifuged for 10 min at 17,000g. The supernatant (200 μL) was transferred back to the 1.5 mL glass injection vials and stored at 4 °C until analysis.
Quality assurance and quality control were conducted to meet acceptance criteria described in Table B-15 in the Quality Systems Manual (QSM) for Environmental Laboratories published by the Department of Defense (DoD) and Department of Energy (DOE). Process blanks (spiked with internal standard), spiked control samples (spiked with internal standard and native compounds), and solvent blanks (no native or mass-labeled PFAS) were prepared with samples to confirm any background PFAS as well as extraction efficiency of sample processing. Due to the size of the sample matrix, the spike was not included in the individual batches. The extraction efficiency is reported previously.31,65,66 PFAS analysis was performed on a Triple Quadrupole Liquid Chromatograph Mass Spectrometer system equipped with an electrospray ionization source (LC–ESI–MS/MS) in negative ion mode.31,65,66 To retain potential contamination from the LC system, a delay column (Restek, Bellefonte, PA, USA; PFAS Delay Column, 5 μm, 50 × 2.1 mm) was installed between LC pumps and autosampler. The analytical column was Kinetex 5 μm EVO C18 (Phenomenex, Torrance, CA, USA; 100 Å, LC Column 100 × 2.1 mm) with a guard filter (Phenomenex, Torrance, CA, USA; KrudKatcher ULTRA HPLC In-Line Filter, 2.0 μm Depth Filter × 0.004 in. ID), and the column temperature was maintained at 40 °C. The injection volume was 10 μL. A combined flow rate of 0.4 mL/min was used for mobile phases A (20 mM ammonium acetate in water) and B (methanol). The gradient started at 10% B, increased to 50% in the first 0.5 min, 99% at 8 min and maintained for 5 min, decreased back to 10% in 0.5 min, and maintained for 20 min. The acquired data set on scheduled multiple reaction monitoring (MRM) mode was processed using LabSoutions software (Shimadzu, Columbia, MD, USA) for quantitative analysis. The limit of quantification (LOQ) and limit of detection (LOD) of PFBS and GenX were 0.04–0.06 and 0.01–0.02 ppb.
PFAS Dosing Solution Concentration Analysis.
Prior to analysis, the 400 and 4000 ppb dosing solutions collected at initiation of exposure (1 hpf) and at cessation of exposure (72 hpf) were diluted by adding 50 μL of the PFAS dosing solution with 450 μL of ultrapure water for the 400 ppb solutions (1:10) or by adding 10 μL of the PFAS dosing solution with 490 μL of ultrapure water for the 4000 ppb solutions (1:50). The dosing solution (500 μL at either a full concentration or diluted concentration) was transferred into a 1.5 mL plastic injection vial. Methanol (475 μL) and 25 μL of an internal standard solution in methanol were added (about 200 ng/mL of each mass-labeled PFAS). Samples were vortexed and stored at 4 °C until analysis. PFAS analysis was performed by LC–ESI–MS/MS in negative ion mode as described for body burden.31,65,66
Calculation of the Bioconcentration Factor (BCF).
BCFs were calculated using the tissue dose at 72 hpf and the measured concentrations of corresponding dosing solutions at 1 hpf as follows32:
| (1) |
where Czebrafish is the dry weight concentration of GenX or PFBS in zebrafish larvae (ng/g), and Cmedia represents the concentrations of PFAS exposure media at 1 hpf (ppb), respectively.
Visual Motor Response Behavior Assay.
Larval locomotion was assessed to determine whether embryonic exposure to sublethal concentrations of GenX or PFBS resulted in behavioral alterations. A visual motor response test was performed using a Noldus DanioVision Observation Chamber (Noldus Information Technology, Wageningen, Netherlands). In each biological replicate, 50 zebrafish embryos were exposed to 0, 4, 40, 400, or 4000 ppb of GenX (0.012–12.12 μM) or PFBS (0.013–13.33 μM) within 1 hpf. 0 ppb represents the negative control treatment consisting of filtered water. At 72 hpf, exposure to the chemicals was terminated by rinsing the fish twice with filtered water. Fish were then incubated in water only at 28 °C until 120 hpf when behavioral analysis was completed similar to our past studies.33,34 Subsamples from each replicate were placed in separate wells in a 96-well plate. Grossly malformed or dead larvae were excluded, which averaged 1–2 per group as the background rate (including 0 ppb). The wells were filled with 500 μL of filtered water, the plate was placed in the DanioVision observation chamber, and the temperature was maintained at 28 °C throughout the experiment using the Noldus temperature control unit. After a 10 min dark acclimation period, the test was started by exposing larvae to 10 min of alternating periods of dark and light for a total of 50 min (dark-light-dark-light-dark).33,34 Infrared light that is not visible to zebrafish larvae was used for tracking movement.35 During the light phase, a 5000 lx light was activated under the DanioVision observation chamber. The infrared movement traces were recorded at a rate of 25 fps with a Basler GenICam acA 1300–60 gm camera. Tracks were smoothed via a minimum distanced moved profile set to >0.2 mm. The exposure to dark or light was controlled by EthoVision 12 software. All behavioral experiments were performed from 11 am–2 pm to minimize circadian variability in movement. Total distance moved, mean velocity, and cumulative time spent moving were calculated using EthoVision 12 software. Seven biological replicates were completed for GenX with 15–19 subsamples per treatment per replicate to total 129–132 fish per treatment group. Six biological replicates were performed for PFBS with 18–19 subsamples per treatment in each replicate to total 111–113 fish per treatment group.
Assessment of Dopamine Concentrations.
A total of 50 zebrafish embryos were randomly sorted into a Petri dish and dosed with 0, 0.4, 4, 40, or 400 ppb GenX or PFBS from 1 to 72 hpf at 28 °C. One or two plates were used for collections for each treatment group. At 72 hpf, fish were rinsed twice with filtered water and then pooled in a 1.5 mL tube (35 or 70 total). Eleuthero-embryos were homogenized with a pestle in 500 μL of 1× phosphate buffered saline (PBS) and stored at −20 °C. After two freeze–thaw cycles to break down tissue, the homogenates were centrifuged for 5 min at 5000g at 2–8 °C. The supernatant was removed and assayed immediately or stored at −20 °C. Whole organism dopamine levels were measured using a competitive inhibition enzyme-linked-immunoassay (ELISA) (CSB-eq 027496FI; Cusabio, Houston, TX, USA) following manufacturer instructions. The plate design included a standard curve supplied with the kit. Each standard curve and experimental sample was run in duplicate (technical replicates). The optical density (OD) of each well was assessed by using a microplate reader set to 450 nm. Optical density at 630 nm was set to account for background. The duplicate readings for each standard and sample were averaged. The average OD of the blank wells was subtracted from the OD of the standards and samples. The standard curve was created using GraphPad Prism (8.4.1) using the four-parameter logistic (4-PL) curve fit. Dopamine concentrations of the samples were calculated from the standard curve. Five (PFBS) or ten (GenX) biological replicates were assessed for each treatment group.
Statistical Analysis.
For acute toxicity assessment, LC50 and associated 95% confidence limits at 120 hpf in zebrafish larvae were determined using nonlinear regression with a hill slope curve fitting using GraphPad Prism 8.4.1 (Boston, MA, USA). In the visual motor response assay, the total distance moved, mean velocity, and time spent moving were analyzed. A Grubb’s outlier test (GraphPad Prism 8.4.1., Boston, MA, USA) was used to detect outliers within a treatment group of each outcome, normal distribution of the data confirmed, and a repeated measures analysis of variance (ANOVA) using SAS 9.4 software (Cary, NC, USA) (α = 0.05) was completed. The phase (dark or light) and chemical treatments were the independent variables, and locomotor activity was the dependent variable. Behavior data are reported with F statistic expressed as F[(df of variable), df (error)] = F value and the p-value. A one-way ANOVA with SAS 9.4 software (Cary, NC, USA) was used to analyze survival rates and dopamine concentration with a least significant difference post hoc test when a significant ANOVA was observed (α = 0.05). All data are presented as the mean ± standard deviation.
RESULTS AND DISCUSSION
Acute Toxicity at 120 hpf of GenX and PFBS.
There is relatively limited information on the developmental toxicity, especially the developmental neurotoxicity, of the emerging PFAS, GenX and PFBS, which are the shorter-chain replacements of PFOA and PFOS, respectively. In this study, the acute toxicity of GenX and PFBS was first assessed by exposing zebrafish to a range of each chemical beginning at 1 hpf and monitoring the survival rates through 120 hpf. The 120 hpf LC50 of GenX was 8617 (95% CI: 8280–8974) ppm. There was a significant decrease in survival in the 2000 ppm PFBS treatment group at 120 hpf (p = 0.002); however, the average percent survival was 84.4%. Thus, the 120 hpf-LC50 for PFBS is greater than 2000 ppm (Figure S2). The 120 hpf LC50 for PFBS was greater than 2000 ppm. No higher exposure concentrations were tested since this concentration is magnitudes above environmental concentrations and is similar to previously published data.36,37 This 120 hpf-LC50 of GenX is substantially different from another study that reported it at 105 ppm38 and also a reported 24 hpf-LC50 of GenX at 56.11 ppm.39 Our previous study showed that this discrepancy in reported LC50s in the literature is due to a lack of neutralizing the acidic dosing solution that results when working with higher concentrations of these perfluoroalkyl acids.28 As such, some studies inaccurately report much more toxic LC50s and can vary depending on the buffering capacity of the dosing solution being used by the group if not accounting for the acidic conditions created, which are toxic to the developing zebrafish. Overall, these results are in agreement with previous studies that show the acute toxicity of PFAS is dependent on the chain length, where longer-chain chemicals have higher acute toxicity compared to shorter-chain chemicals.36,37,40 In addition, these results are in agreement with the 120 hpf-LC50 values reported in developing zebrafish in studies with similar exposure paradigms.
Further evaluations in this study were at sublethal concentrations more likely to represent environmental exposures, including those in a higher range to investigate dose response. Initially, these test concentrations were chosen as a log scale inclusive of concentrations above an earlier US EPA provisional health advisory limit for PFOA at 0.4 ppb (400 ppt).30 In 2022, the US EPA revised to a decreased interim lifetime health advisory level of 0.004 ppt for PFOA, alongside the release of 10 ppt for GenX and 2000 ppt for PFBS.10 Studies are continuing to provide more information on the concentration range of PFAS as drinking water sampling sites are expanded, and technological improvements have advanced detection capabilities and decreased limits of detection. For example, private drinking water wells in the Cape Fear River, NC, USA area measured up to 4000 ppt (4 ppb) GenX stemming from a PFAS manufacturing facility6 and PFBS was detected at up to 0.3 ppb in public drinking water in Minnesota (USA).41 Moreover, in a study completed by the United States Geological Services (USGS) sampling public and private drinking water, the hazard index of 1 for the sum of GenX, PFBS, PFNA, and PFHxS was exceeded in 4.6% of the samples.42 It must also be remembered that drinking water is not the only exposure source for GenX and PFBS with diet and indoor air as additional primary exposure sources. To further address the body burden of GenX and PFBS of the exposure parameters in this study, whole organism internal dose and concentration of dosing solutions was completed.
GenX and PFBS Body Burden and Dosing Solution Concentration Assessment.
Body Burden at 72 hpf and Concentrations of Dosing Solutions at Exposure Initiation.
The body burden of GenX and PFBS in our exposure scenario from 1 to 72 hpf without replenishing dosing solutions was first measured immediately after exposure at 72 hpf. There was a dose-dependent accumulation of GenX and PFBS in zebrafish eleuthero-embryos immediately following the exposure period (Figure 1A). For GenX, only the 40 and 400 ppb GenX treatment groups had measurable concentrations of 38.5 and 639.2 ng/g, respectively. The control (0 ppb), 0.4, and 4 ppb treatment groups were below the LOQ. PFBS was more bioaccumulative with higher tissue concentrations observed (Figure 1A). PFBS concentrations in fish in 40 ppb treatment averaged 166.1 ng/g, and in the 400 ppb group measured at 1921.8 ng/g. The PFBS control (0 ppb), 0.4, and 4 ppb groups were also below the LOQ.
Figure 1.
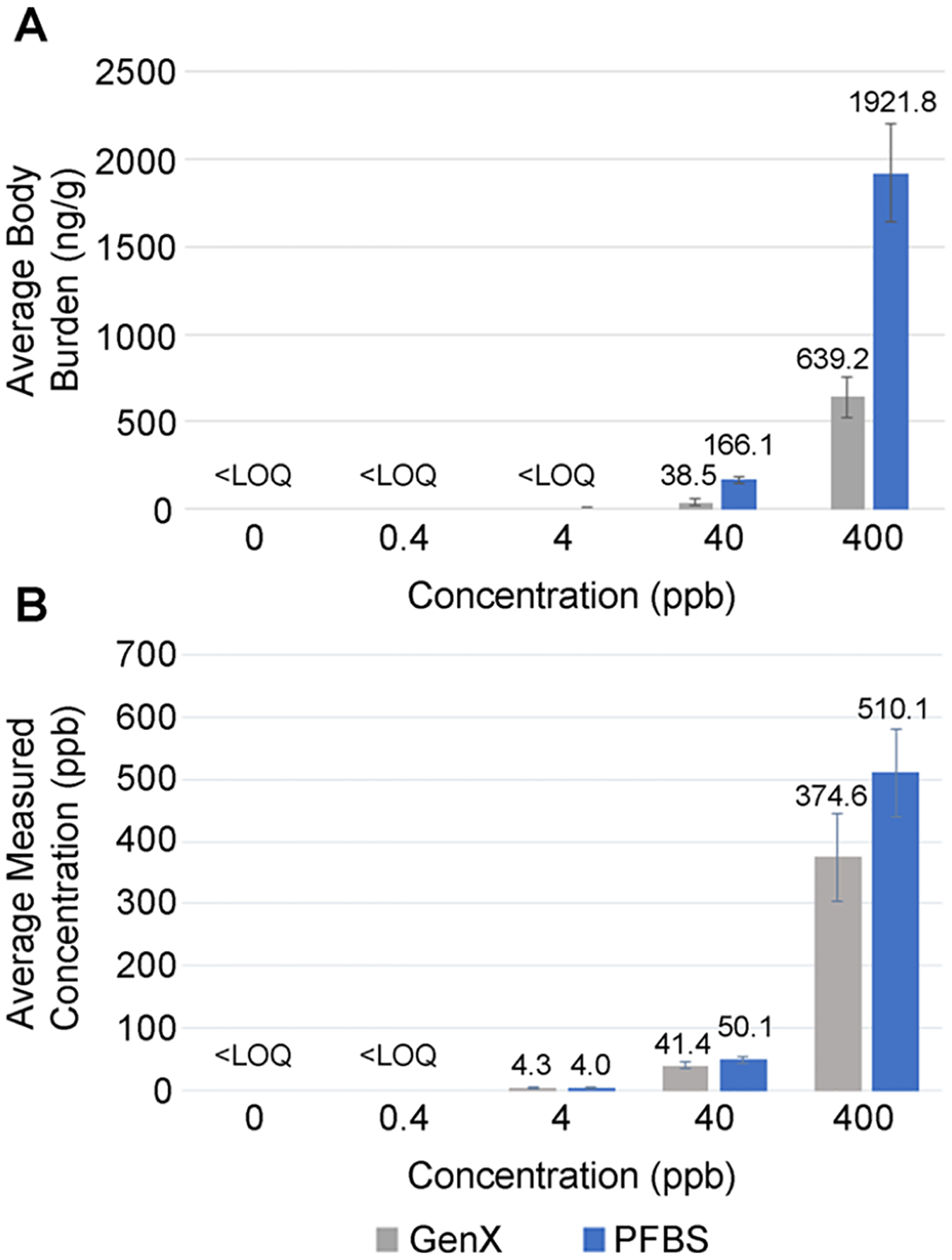
Body burden at 72 hpf and concentration of dosing solution at exposure initiation for GenX and PFBS. (A) Body burden of whole pooled eleuthero-embryos at 72 hpf at the cessation of chemical exposure (N = 4 with 70 subsamples pooled per treatment per replicate). (B) Concentration of dosing solutions at the initiation of chemical exposure (at 1 hpf, N = 4). Error bars are the standard deviation.
Concentrations of GenX and PFBS in test solutions were close to those expected at the beginning of the exposure period (1 hpf) (Figure 1B). The negative control treatment (0 ppb GenX or PFBS) and the 0.4 ppb treatments for each GenX and PFBS were below the LOQ (Figure 1B). A concentration-dependent increase in the treatment groups was observed with GenX-measured concentrations of 4.3, 41.4, and 374.6 ppb close to the 4, 40, and 400 ppb expected concentrations, respectively (Figure 1B). A similar result was detected in the PFBS dosing solutions with the observed concentrations at 4.0, 50.1, and 510.1 ppb for the 4, 40, and 400 ppb expected concentrations, respectively (Figure 1B).
Generally, PFAS bioaccumulation potential follows the chain length rule (i.e., the longer the chain length, the greater the risk for bioaccumulation and respective toxicity), but this rule does not hold when comparing sulfonate-containing PFAS to those with a carboxylic group. The higher internal dose for PFBS than GenX at similar nominal concentrations aligns with other sulfonate group-containing PFAS having a higher potential to accumulate compared to longer chain carboxylic-group-containing PFAS in multiple organisms. For example, Gomis et al. reported the lowest-observed effect level (LOEL) of GenX was lower than the LOEL of PFOA for liver enlargement in rats when comparing the carboxylic C6 (GenX) and C8 (PFOA) PFAS.43 This group showed that different toxicokinetic properties among the PFAS explained observed toxicity differences when different chain-length carboxylic PFAS are administered at the same dose.43 In contrast, zebrafish larvae at 144 hpf had higher concentrations of PFHxS (C6) compared to PFOA (C8) or PFHxA (C6) when exposed to the same nominal concentrations, demonstrating greater bioaccumulation potential for sulfonate-containing PFAS.44 Menger et al. showed that the bioconcentration factor for perfluorosulfonic acids (PFSAs) was higher than perfluorocarboxyl acids (PFCAs).45 Furthermore, Vogs et al. found that the bioconcentration factors of different PFAS in 120 hpf zebrafish larvae vary and the difference in potency was reduced by 3 orders of magnitude when considering internal doses.46 To further address this question, bioconcentration factors were determined.
Determination of the BCF.
Since the tissue doses for the 0.4 and 4 ppb treatment groups were below the LOQ, only measurements of tissue dose for 40 and 400 ppb were used to determine BCF (Table 1). The BCF for PFBS was higher than that for GenX. The BCF values for PFBS at 40 and 400 ppb were relatively close, while the BCF for GenX at 400 ppb is almost double that at 40 ppb (Table 1). Gaballah et al. revealed that 1 μM (500.1 ppb) and 1.8 μM (900.2 ppb) of PFOS had almost the same BCF at 1374 L/kg in zebrafish larvae; however, those exposed to 3.2 μM (1600.4 ppb) had a lower BCF of 684.03 L/kg.44 In addition, other studies report an inverse relationship between water concentration of PFAS and BCF, which has been hypothesized to be based on saturation of the active transporter at high doses that leads to a decreased BCF.20 This effect may explain the small difference between the BCF at 40 and 400 ppb PFBS and the larger difference for 40 and 400 ppb GenX.
Table 1.
Calculated BCFs of GenX and PFBSa
| chemical | nominal concentration (μg/L) | measured water (media) concentration (μg/L) | body burden (ng/g) | average BCF (L/kg) |
|---|---|---|---|---|
| GenX | 40 | 41.42 ± 4.63 | 38.51 ± 16.85 | 0.93 ± 0.38 |
| 400 | 374.63 ± 70.68 | 639.22 ± 112.61 | 1.72 ± 0.25 | |
| PFBS | 40 | 50.07 ± 3.92 | 166.14 ± 17.16 | 3.35 ± 0.55 |
| 400 | 510.00 ± 28.79 | 1921.79 ± 282.98 | 3.80 ± 0.72 |
All measurements are expressed as average ± standard deviation.
Body Burden at 120 hpf and Concentrations of Dosing Solutions at 1, 72, and 120 hpf.
In addition to measuring the dose immediately after the PFAS exposure at 72 hpf, body burden at 120 hpf at the end of the depuration period (i.e., 2 days after cessation of exposure) was also measured to anchor dose under this exposure scenario to when the behavior outcomes were completed. Overall, body burden only slightly decreased for GenX and PFBS indicating minimal elimination during the 48-h period and trends remained the same for concentration-dependency, LOQ, and higher doses for PFBS (Figure 2A). For GenX, only the 40, 400, and 4000 ppb GenX treatment groups had measurable concentrations at 26.4, 546.9, and 7396.83 ng/g, respectively. The control (0 and 4 ppb) treatment groups were below the LOQ. 4, 40, and 400 ppb PFBS exposures were detected in 120 hpf larval tissues at 99.42, 1806.85, and 15011.56 ppb, respectively (Figure 2A). The PFBS negative control (0 ppb) and 4 ppb treatment groups were also below the LOQ. This data is in contrast to another study that showed GenX was completely eliminated at 168 hpf in larvae exposed to 3.03 μM (1 ppm) or 20 μM (6 ppm), respectively, through 120 hpf.39 Differences may be due to the sensitivity of the equipment, which continues to rapidly increase, or the biological maturation of metabolism capacities at the different developmental ages assessed.
Figure 2.

Body burden at 120 hpf and concentrations of dosing solutions at the initiation of exposure, cessation of exposure, and time of behavior analysis. (A) Body burden of whole pooled larvae at 120 hpf aligning with time of behavior analysis 48 h after chemical exposure ceased (N = 3–4 with 70 larvae pooled per treatment per replicate). (B) Concentration of GenX dosing solution at the initiation of exposure (1 hpf), at the end of chemical exposure (72 hpf), and 48 h after the chemical exposure ceased (at 120 hpf when behavior analysis was completed, N = 2–4). (C) Concentration of PFBS dosing solution at the initiation of exposure (1 hpf), at the end of chemical exposure (72 hpf), and 48 h after the chemical exposure ceased (at 120 hpf when behavior analysis was completed, N = 3–4). Error bars are standard deviation.
In this set of exposures, dosing solutions were collected at the initiation of exposure (1 hpf), at the end of the PFAS exposure period (72 hpf), and at the time point behavioral analysis was completed (120 hpf), which was 48 h after the PFAS exposure ceased. Similar to the first measurements reported in Figure 1B, the concentrations of the dosing solutions at the beginning of the exposure period (1 hpf) and concentrations at the end of the exposure period (72 hpf) remained close to the nominal concentrations, although they were never replenished (Figure 2B,C). Water samples from the depuration period (72–120 hpf) showed much lower concentrations (Figure 2B, C) with only the 400 and 4000 ppb GenX and 4000 ppb PFBS groups having measurable concentrations at 120 hpf (0.16, 1.51, and 1.74 ppb, respectively).
Researchers are starting to explore mechanisms to explain the different toxicokinetic properties among PFAS. Some studies report variable abilities to bind to tissue proteins. Luebker et al. showed that PFOS and PFOA can bind to the secondary binding sites in rat liver fatty acid binding protein (L-FABP), but in a competitive binding assay, PFOS showed a higher inhibition level of ligand binding to L-FABP compared to PFOA.47 In addition, using molecular docking analysis, it was determined that PFOS bound to L-FABP by creating three hydrogen bonds compared to two hydrogen bonds by PFOA, indicating stronger binding interactions for the sulfonated PFAS.48 These results help explain the higher accumulation potential of the sulfonate group containing PFAS. Overall, these studies highlight the importance of assessing the body burden to account for differences in PFAS toxicokinetics and to provide a more informed comparison of toxicity. Also, the determination of the internal dose accounts for differences in exposure regimes in different studies. For example, some studies use static exposure (e.g., what was done in the current study), and others use daily renewal of dosing solutions, which will likely change the internal dose and thus assist in defining differences that may be reported among these studies.
Locomotor Activity of Zebrafish Larvae at 120 hpf with Embryonic GenX or PFBS Exposure.
There is relatively limited information on the developmental toxicity, especially the developmental neurotoxicity, of the emerging PFAS, GenX and PFBS, although they are the shorter-chain replacements of PFOA and PFOS, respectively. The developing central nervous system is especially sensitive to toxicant exposure. The locomotor activity of zebrafish larvae can be used to determine neuroactive and neurotoxic chemicals with the neuronal pathways and neurotransmission systems conserved throughout vertebrate evolution.21,49 A recent study concluded that a chemical can be suggested as a developmental neurotoxicant if it induces a significant effect compared to the negative control in multiple lighting phases of the visual motor response assay or if multiple concentrations of a chemical caused a significant effect within one lighting phase.44 Locomotor activity has been included after developmental PFAS exposure in multiple studies but is limited in specifically investigating GenX and PFBS at lower concentration ranges.44,45,50–53
In the current study, zebrafish were exposed throughout embryogenesis (1–72 hpf) to relatively low concentrations compared to other studies, and the locomotor activity of larvae was assessed at 120 hpf. Overall, zebrafish larvae moved more in dark periods compared to light periods as expected with different trends in behavioral alterations observed for GenX and PFBS (Figure 3). The GenX embryonic exposure resulted in a significant effect on the interaction between the treatment group and phase (dark or light condition) in all tested outcomes including total distance moved and velocity [(F16, 2552) = 3.36, p < 0.05 for both outcomes] and time spent moving [(F16, 2552 = 3.29, p < 0.05] (Figure 3A, C, E). There was no significant difference in the overall treatment group effect for each outcome signifying overall alterations were dependent on phase [total distance moved and velocity [(F4, 638) = 1.14, p = 0.331] and time spent moving [(F4, 638) = 0.83), p = 0.50]]. Phase-specific effects in the treatment groups included an increase in the total distance moved, mean velocity, and time spent moving during the first dark phase of larvae exposed to 40, 400, or 4000 ppb GenX. This effect persisted only in the 400 ppb treatment group for total distance moved and velocity in the second dark phase (Figure 3A, C). In addition, an increased time spent moving was also seen in the larvae exposed to 4000 ppb (Figure 3E). These results are different from what was reported by two other groups for GenX. Gaballah et al. did not observe locomotor changes in zebrafish larvae at 144 hpf when exposed to 4.4 to 80 μM GenX (1527 to 27,766 ppb) for 6 days.44 Only the highest treatment group in the current study overlapped with this previous study, and there were differences in exposure period, age of assessment, and replenishment of solutions every 24 h (Table S2). Alternatively, Rericha et al. found that exposure to 0.76 μM (250 ppb) GenX from 6 to 120 hpf caused hypoactivity in the larval photomotor response test in the light phase, which has different light/dark phases compared to the visual motor response assay (Table S2).53
Figure 3.

Visual motor response behavior assay in zebrafish larvae at 120 hpf following an embryonic exposure (1–72 hpf) to GenX (A, C, E) or PFBS (B, D, F). N = 7 biological replicates for GenX with 15–19 subsamples per treatment per replicate to total 129–132 fish per treatment group. N = 6 for PFBS exposure with 18–19 subsamples per treatment per replicate to total 111–113 fish per treatment group. Error bars represent standard deviation. *p < 0.05 compared to the control treatment group within each phase. (D1: first dark phase; L1: first light phase; D2: second dark phase; L2: second light phase; D3: third dark).
The embryonic PFBS exposure resulted in a significant overall effect of the treatment group [(F4, 549) = 21.01, p < 0.05] for the total distance moved and mean velocity (same values for each outcome) (Figure 3B, D). In addition, PFBS exposure caused a significant overall effect of treatment [(F4, 549) = 18.55, p < 0.05] for time spent moving (Figure 3F). In the three dark phases, all treatment groups (4, 40, 400, and 4000 ppb) showed hyperactivity with an increase in total distance moved and mean velocity (Figure 3B, D). A similar effect was observed for time spent moving, except that only the 40, 400, and 4000 ppb groups were hyperactive in the first dark phase (Figure 3F). In the light phases, all treatment groups except the 4 ppb exposure group were hyperactive for all three outcomes. A near-to-significant interaction of treatment and phase (dark or light) was observed for the total distance moved [(F16, 2196) = 1.6, p = 0.06], mean velocity [(F16, 2196) = 1.6, p = 0.05], or time spent moving [(F16, 2196) = 1.5, p = 0.09], indicating similar effects were observed among the phases. Rericha et al. also observed hyperactivity in light phases in the photomotor response assay, but in contrast to our results, other studies showed that developmental PFBS exposure in zebrafish did not induce behavioral changes.44,45 In addition, Ulhaq et al. observed a biphasic alteration in activity with PFBS exposure but exposures were at much higher concentrations than used in the present study.50 Similar to the discrepancies for GenX, the different behavioral outcomes observed in this study compared to others can be explained by variations in exposure concentrations, duration of exposure, age of the larvae at the time of assessment, removal of chorion after 24 h, and lighting regime during the experiments (Table S2). In addition, some studies maintained zebrafish at 26 °C during development,44,45,50 which results in slower development compared to those studies that maintain zebrafish at 28 °C given temperature influence on developmental rate [The Zebrafish Book54 standardizes developmental staging at 28 °C55].
Dopamine Levels in Eleuthero Embryos.
Previous studies reported that behavioral abnormalities in zebrafish larvae can be associated with dopamine deficits or alterations in dopaminergic neuron development.17,18,56 Dopamine concentration in zebrafish eleuthero-embryos was increased in the 40 ppb GenX exposed group (13% increase, p < 0.05, Figure 4A). While an increasing trend was observed in the 400 ppb GenX group, this difference failed to reach a significant difference from that of the negative control treatment (0 ppb). Similarly, nonmonotonic alterations in brain-transcriptome profiles in Drosophila melanogaster exposed to GenX are also reported.57 Alternatively, a decreasing trend was observed for the PFBS exposure groups with the 400 ppb treatment group significantly different from the negative control treatment (0 ppb) (9% decrease, p < 0.05, Figure 4B).
Figure 4.

Average whole-body dopamine content in eleutheroembryos exposed to (A) GenX or (B) PFBS from 1 to 72 hpf. N = 5 (for PFBS) and N = 10 (for GenX) with pools of 35−70 fish per treatment group per biological replicate. Error bars are standard deviation. *p < 0.05.
This result is interesting in showing potential for two different mechanisms of action for each chemical on the dopaminergic pathway, which agrees with an earlier study that modeled the developmental neurotoxicity of a few PFAS in differentiating PC12 cells.58 The study in PC12 cells concluded that PFAS will not have one shared mechanism of developmental neurotoxicity and observed variations in the promotion and/or suppression of dopamine and acetylcholine neurotransmitter phenotypes. Specifically, they observed PFBS to suppress dopamine neuron differentiation (around a 35% decrease), which aligns with the findings of our study reporting decreased dopamine for PFBS.
While studies are limited that have included GenX or PFBS, others have observed dopamine-related alterations with other PFAS in multiple organisms. Reports include decreased dopamine and dopamine turnover in Northern leopard frogs with developmental exposure to PFOA (1000 ppb) or PFOS (100 and 1000 ppb)16; dopaminergic neuropathology in C. elegans with a 72 h PFOS exposure59; alterations in dopamine in planarians with PFOS exposure60; perturbations to dopaminergic gene transcription in the mouse cerebral cortex and hippocampus with a postnatal PFOS exposure in mice61; multiple modifications in the dopaminergic system in the adult male rat amygdala, prefrontal cortex, and hippocampus with PFOS exposure for 28 days62; and impairment of human dopaminergic neurons with PFOA exposure for 24 h at 10 ppb63; among others (reviewed in ref 64).
Overall, the results of this study confirm that PFBS is more acutely toxic compared to GenX in developing zebrafish. Assessment of the tissue dose showed variation in accumulation between GenX and PFBS with dosing solutions at similar exposure concentrations. These findings emphasize the importance of assessing the internal dose based on different toxicokinetic properties among the PFAS. In addition, an embryonic exposure to GenX or PFBS induced behavioral changes in zebrafish larvae. PFBS had more persistent behavioral alterations at lower exposure concentrations compared to GenX, highlighting that sulfonate-containing PFAS are triggering stronger behavioral alterations compared to carboxylic-containing PFAS and confirming that the functional group is playing an important role in the induction of PFAS toxicity. Furthermore, different mechanisms of developmental neurotoxicity are hypothesized for these two PFAS based on opposite directional effects observed for dopamine concentration alterations for each. Future work is needed to further investigate the molecular mechanisms driving developmental neurotoxicity on the dopaminergic system, along with addressing brain-specific outcomes, given only whole-body assessments were completed here for dopamine levels. In addition, further studies can be aimed at determining if other neurotransmitter systems are also impacted.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute for Occupational Safety and Health through the Pilot Research Project Training Program of the University of Cincinnati Education and Research Center Grant (OH008432), the Illinois Occupational and Environmental Health and Safety Education and Research Center at the University of Illinois at Chicago (OH008672), a Purdue Research Foundation Research Grant, and National Institute of Environmental Health Sciences (ES031646).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c05023.
Additional information provided on the experimental design and analysis time points, lethality curves, physicochemical properties, and comparative behavioral analysis from the literature for larval zebrafish (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.est.3c05023
Notes
The authors declare no competing financial interest.
Contributor Information
Ola Wasel, School of Health Sciences, Purdue University, West Lafayette, Indiana 47907, United States.
Hanna King, School of Health Sciences, Purdue University, West Lafayette, Indiana 47907, United States.
Youn J. Choi, Department of Agronomy and Environmental and Ecological Engineering, Purdue University, West Lafayette, Indiana 47907, United States
Linda S. Lee, Department of Agronomy and Environmental and Ecological Engineering, Purdue University, West Lafayette, Indiana 47907, United States
Jennifer L. Freeman, School of Health Sciences, Purdue University, West Lafayette, Indiana 47907, United States
REFERENCES
- (1).Glüge J; Scheringer M; Cousins IT; Dewitt JC; Goldenman G; Herzke D; Lohmann R; Ng CA; Trier X; Wang Z An Overview of the Uses of Per- And Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process Impacts 2020, 22 (12), 2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Giesy JP; Kannan K Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol 2001, 35 (7), 1339–1342. [DOI] [PubMed] [Google Scholar]
- (3).Panieri E; Baralic K; Djukic-Cosic D; Djordjevic AB; Saso L PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10 (2), 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Olsen GW; Burris JM; Ehresman DJ; Froehlich JW; Seacat AM; Butenhoff JL; Zobel LR Half-Life of Serum Elimination of Perfluorooctanesulfonate,Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect 2007, 115 (9), 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).ATSDR. Toxicological Profile for Perfluoroalkyls; Atlanta, GA, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed 2021-08-25). [Google Scholar]
- (6).Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett 2016, 3 (12), 415–419. [Google Scholar]
- (7).Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol 2015, 49 (19), 11622–11630. [DOI] [PubMed] [Google Scholar]
- (8).Brandsma SH; Koekkoek JC; van Velzen MJM; de Boer J The PFOA Substitute GenX Detected in the Environment near a Fluoropolymer Manufacturing Plant in the Netherlands. Chemosphere 2019, 220, 493–500. [DOI] [PubMed] [Google Scholar]
- (9).Guillette TC; McCord J; Guillette M; Polera ME; Rachels KT; Morgeson C; Kotlarz N; Knappe DRU; Reading BJ; Strynar M; Belcher SM Elevated Levels of Per- and Polyfluoroalkyl Substances in Cape Fear River Striped Bass (Morone Saxatilis) Are Associated with Biomarkers of Altered Immune and Liver Function. Environ. Int 2020, 136, No. 105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).US Environmental Protection Agency Drinking Water Health Advisories for PFAS Fact Sheet for Public Water Systems. https://www.epa.gov/system/files/documents/2022-06/drinking-water-ha-pfas-factsheet-water-system.pdf.
- (11).Rushing BR; Hu Q; Franklin JN; McMahen RL; Dagnino S; Higgins CP; Strynar MJ; DeWitt JC Evaluation of the Immunomodulatory Effects of 2,3,3,3- Tetrafluoro-2-(Heptafluoropropoxy)-Propanoate in C57BL/6 Mice. Toxicol. Sci 2017, 156 (1), 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Conley JM; Lambright CS; Evans N; Strynar MJ; McCord J; McIntyre BS; Travlos GS; Cardon MC; Medlock-Kakaley E; Hartig PC; Wilson VS; Gray LE Adverse Maternal, Fetal, and Postnatal Effects of Hexafluoropropylene Oxide Dimer Acid (GenX) from Oral Gestational Exposure in Sprague-Dawley Rats. Environ. Health Perspect 2019, 127 (3), 37008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Blake BE; Cope HA; Hall SM; Keys RD; Mahler BW; McCord J; Scott B; Stapleton HM; Strynar MJ; Elmore SA; Fenton SE Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice Following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ. Health Perspect 2020, 128 (2), 27006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).US Environmental Protection Agency. Human Health Toxicity Values for Perfluorobutane Sulfonic Acid and Related Compound Potassium Perfluorobutane Sulfonate; U.S. Environmental Protection Agency: Washington, DC, EPA/600/R-20/345F, 2021. [PubMed] [Google Scholar]
- (15).US Environmental Protection Agency. Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252–13–6 and CASRN Also Known as “GenX Chemicals; U.S. Environmental Protection Agency: Washington, DC, EPA Document Number: 822R-21–010, 2021. [Google Scholar]
- (16).Foguth RM; Flynn RW; de Perre C; Iacchetta M; Lee LS; Sepúlveda MS; Cannon JR Developmental Exposure to Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Selectively Decreases Brain Dopamine Levels in Northern Leopard Frogs. Toxicol. Appl. Pharmacol 2019, 377, No. 114623. [DOI] [PubMed] [Google Scholar]
- (17).Yu T; Zhou G; Cai Z; Liang W; Du Y; Wang W Behavioral effects of early-life exposure to perfluorooctanoic acid might synthetically link to multiple aspects of dopaminergic neuron development and dopamine functions in zebrafish larvae. Aquat. Toxicol 2021, 238, No. 105926. [DOI] [PubMed] [Google Scholar]
- (18).Wu L; Dang Y; Liang L-X; Gong Y-C; Zeeshan M; Qian Z; Geiger SD; Vaughn MG; Zhou Y; Li Q-Q; Chu C; Tan Y-W; Lin L-Z; Liu R-Q; Hu L-W; Yang B-Y; Zeng X-W; Yu Y; Dong G-H Perfluorooctane Sulfonates Induces Neurobehavioral Changes and Increases Dopamine Neurotransmitter Levels in Zebrafish Larvae. Chemosphere 2022, 297, No. 134234. [DOI] [PubMed] [Google Scholar]
- (19).Wu S; Xie J; Zhao H; Sanchez O; Zhao X; Freeman JL; Yuan C Pre-Differentiation GenX Exposure Induced Neurotoxicity in Human Dopaminergic-like Neurons. Chemosphere 2023, 332, No. 138900. [DOI] [PubMed] [Google Scholar]
- (20).Tal T; Vogs C Invited Perspective: PFAS Bioconcentration and Biotransformation in Early Life Stage Zebrafish and Its Implications for Human Health Protection. Environ. Health Perspect 2021, 129 (7), No. 071304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Basnet RM; Zizioli D; Taweedet S; Finazzi D; Memo M Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Irons TD; MacPhail RC; Hunter DL; Padilla S Acute Neuroactive Drug Exposures Alter Locomotor Activity in Larval Zebrafish. Neurotoxicol. Teratol 2010, 32 (1), 84–90. [DOI] [PubMed] [Google Scholar]
- (23).de Esch C; Slieker R; Wolterbeek A; Woutersen R; de Groot D Zebrafish as Potential Model for Developmental Neurotoxicity Testing. A Mini Review. Neurotoxicol. Teratol 2012, 34 (6), 545–553. [DOI] [PubMed] [Google Scholar]
- (24).Kiper KG; Freeman JL Zebrafish as a Tool to Assess Developmental Neurotoxicity. In Neuromethods; Humana Press Inc., 2019; 145, 169–193. [Google Scholar]
- (25).Howe K; Clark MD; Torroja CF; Torrance J; Berthelot C; Muffato M; Collins JE; Humphray S; McLaren K; Matthews L; McLaren S; Sealy I; Caccamo M; Churcher C; Scott C; Barrett JC; Koch R; Rauch GJ; White S; Chow W; Kilian B; Quintais LT; Guerra-Assunção JA; Zhou Y; Gu Y; Yen J; Vogel JH; Eyre T; Redmond S; Banerjee R; Chi J; Fu B; Langley E; Maguire SF; Laird GK; Lloyd D; Kenyon E; Donaldson S; Sehra H; Almeida-King J; Loveland J; Trevanion S; Jones M; Quail M; Willey D; Hunt A; Burton J; Sims S; McLay K; Plumb B; Davis J; Clee C; Oliver K; Clark R; Riddle C; Eliott D; Threadgold G; Harden G; Ware D; Mortimer B; Kerry G; Heath P; Phillimore B; Tracey A; Corby N; Dunn M; Johnson C; Wood J; Clark S; Pelan S; Griffiths G; Smith M; Glithero R; Howden P; Barker N; Stevens C; Harley J; Holt K; Panagiotidis G; Lovell J; Beasley H; Henderson C; Gordon D; Auger K; Wright D; Collins J; Raisen C; Dyer L; Leung K; Robertson L; Ambridge K; Leongamornlert D; McGuire S; Gilderthorp R; Griffiths C; Manthravadi D; Nichol S; Barker G; Whitehead S; Kay M; Brown J; Murnane C; Gray E; Humphries M; Sycamore N; Barker D; Saunders D; Wallis J; Babbage A; Hammond S; Mashreghi-Mohammadi M; Barr L; Martin S; Wray P; Ellington A; Matthews N; Ellwood M; Woodmansey R; Clark G; Cooper J; Tromans A; Grafham D; Skuce C; Pandian R; Andrews R; Harrison E; Kimberley A; Garnett J; Fosker N; Hall R; Garner P; Kelly D; Bird C; Palmer S; Gehring I; Berger A; Dooley CM; Ersan-Ürün Z; Eser C; Geiger H; Geisler M; Karotki L; Kirn A; Konantz J; Konantz M; Oberländer M; Rudolph-Geiger S; Teucke M; Osoegawa K; Zhu B; Rapp A; Widaa S; Langford C; Yang F; Carter NP; Harrow J; Ning Z; Herrero J; Searle SMJ; Enright A; Geisler R; Plasterk RHA; Lee C; Westerfield M; de Jong PJ; Zon LI; Postlethwait JH; Nüsslein-Volhard C; Hubbard TJP; Crollius HR; Rogers J; Stemple DL The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496 (7446), 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Peterson SM; Zhang J; Weber G; Freeman JL Global Gene Expression Analysis Reveals Dynamic and Developmental Stage-Dependent Enrichment of Lead-Induced Neurological Gene Alterations. Environ. Health Perspect 2011, 119 (5), 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Peterson SM; Zhang J; Freeman JL Developmental Reelin Expression and Time Point-Specific Alterations from Lead Exposure in Zebrafish. Neurotoxicol. Teratol 2013, 38, 53–60. [DOI] [PubMed] [Google Scholar]
- (28).Wasel O; Thompson KM; Gao Y; Godfrey AE; Gao J; Mahapatra CT; Lee LS; Sepúlveda MS; Freeman JL Comparison of Zebrafish in Vitro and in Vivo Developmental Toxicity Assessments of Perfluoroalkyl Acids (PFAAs). J. Toxicol. Environ. Health A 2021, 84 (3), 125–136. [DOI] [PubMed] [Google Scholar]
- (29).Weber G; Sepúlveda MS; Peterson SM; Lewis S; Freeman JL Transcriptome Alterations Following Developmental Atrazine Exposure in Zebrafish Are Associated with Disruption of Neuroendocrine and Reproductive System Function, Cell Cycle, and Carcinogenesis. Toxicol. Sci 2013, 132 (2), 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).US Environmental Protection Agency. PFAS Laws and Regulations. https://www.epa.gov/pfas/pfas-laws-and-regulations (accessed 2021-08-29).
- (31).Hoover GM; Chislock MF; Tornabene BJ; Guffey SC; Choi YJ; de Perre C; Hoverman JT; Lee LS; Sepúlveda MS Uptake and Depuration of Four Per/Polyfluoroalkyl Substances (PFASS) in Northern Leopard Frog Rana Pipiens Tadpoles. Environ. Sci. Technol. Lett 2017, 4 (10), 399–403. [Google Scholar]
- (32).Han J; Gu W; Barrett H; Yang D; Tang S; Sun J; Liu J; Krause HM; Houck KA; Peng H A Roadmap to the Structure-Related Metabolism Pathways of per- and Polyfluoroalkyl Substances in the Early Life Stages of Zebrafish (Danio Rerio). Environ. Health Perspect 2021, 129 (7), 77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Horzmann KA; Reidenbach LS; Thanki DH; Winchester AE; Qualizza BA; Ryan GA; Egan KE; Hedrick VE; Sobreira TJP; Peterson SM; Weber GJ; Wirbisky-Hershberger SE; Sepúlveda MS; Freeman JL Embryonic Atrazine Exposure Elicits Proteomic, Behavioral, and Brain Abnormalities with Developmental Time Specific Gene Expression Signatures. J. Proteomics 2018, 186, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ahkin Chin Tai JK; Horzmann KA; Franco J; Jannasch AS; Cooper BR; Freeman JL Developmental Atrazine Exposure in Zebrafish Produces the Same Major Metabolites as Mammals along with Altered Behavioral Outcomes. Neurotoxicol. Teratol 2021, 85, No. 106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).DanioVision. Benefits of Using DanioVision https://www.noldus.com/daniovision/benefits (accessed 2023-09-03).
- (36).Hagenaars A; Vergauwen L; de Coen W; Knapen D Structure-Activity Relationship Assessment of Four Perfluorinated Chemicals Using a Prolonged Zebrafish Early Life Stage Test. Chemosphere 2011, 82 (5), 764–772. [DOI] [PubMed] [Google Scholar]
- (37).Ulhaq M; Carlsson G; Örn S; Norrgren L Comparison of Developmental Toxicity of Seven Perfluoroalkyl Acids to Zebrafish Embryos. Environ. Toxicol. Pharmacol 2013, 36 (2), 423–426. [DOI] [PubMed] [Google Scholar]
- (38).Gebreab KY; Eeza MNH; Bai T; Zuberi Z; Matysik J; O’Shea KE; Alia A; Berry JP Comparative Toxicometabolomics of Perfluorooctanoic Acid (PFOA) and next-Generation Perfluoroalkyl Substances. Environ. Pollut 2020, 265, No. 114928. [DOI] [PubMed] [Google Scholar]
- (39).Satbhai K; Vogs C; Crago J Comparative Toxicokinetics and Toxicity of PFOA and Its Replacement GenX in the Early Stages of Zebrafish. Chemosphere 2022, 308, No. 136131. [DOI] [PubMed] [Google Scholar]
- (40).Mahapatra CT; Damayanti NP; Guffey SC; Serafin JS; Irudayaraj J; Sepúlveda MS Comparative in Vitro Toxicity Assessment of Perfluorinated Carboxylic Acids. J. Appl. Toxicol 2017, 37 (6), 699–708. [DOI] [PubMed] [Google Scholar]
- (41).Minnesota Department of Health. PFBS and Drinking water. https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/pfbsinfo.pdf (accessed 2023-09-03).
- (42).Smalling KL; Romanok KM; Bradley PM; Morriss MC; Gray JL; Kanagy LK; Gordon SE; Williams BM; Breitmeyer SE; Jones DK; DeCicco LA; Eagles-Smith CA; Wagner T Per- and Polyfluoroalkyl Substances (PFAS) in United States Tapwater: Comparison of Underserved Private-Well and Public-Supply Exposures and Associated Health Implications. Environ. Int 2023, 178, No. 108033. [DOI] [PubMed] [Google Scholar]
- (43).Gomis MI; Vestergren R; Borg D; Cousins IT Comparing the Toxic Potency in Vivo of Long-Chain Perfluoroalkyl Acids and Fluorinated Alternatives. Environ. Int 2018, 113, 1–9. [DOI] [PubMed] [Google Scholar]
- (44).Gaballah S; Swank A; Sobus JR; Howey XM; Schmid J; Catron T; McCord J; Hines E; Strynar M; Tal T Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ. Health Perspect 2020, 128 (4), 47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Menger F; Pohl J; Ahrens L; Carlsson G; Örn S Behavioural Effects and Bioconcentration of Per- and Polyfluoroalkyl Substances (PFASs) in Zebrafish (Danio Rerio) Embryos. Chemosphere 2020, 245, No. 125573. [DOI] [PubMed] [Google Scholar]
- (46).Vogs C; Johanson G; Näslund M; Wulff S; Sjödin M; Hellstrandh M; Lindberg J; Wincent E Toxicokinetics of Perfluorinated Alkyl Acids Influences Their Toxic Potency in the Zebrafish Embryo (Danio Rerio). Environ. Sci. Technol 2019, 53 (7), 3898–3907. [DOI] [PubMed] [Google Scholar]
- (47).Luebker DJ; Hansen KJ; Bass NM; Butenhoff JL; Seacat AM Interactions of Flurochemicals with Rat Liver Fatty Acid-Binding Protein. Toxicology 2002, 176 (3), 175–185. [DOI] [PubMed] [Google Scholar]
- (48).Jones PD; Hu W; de Coen W; Newsted JL; Giesy JP Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem 2003, 22 (11), 2639. [DOI] [PubMed] [Google Scholar]
- (49).Horzmann KA; Freeman JL Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4 (3), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Ulhaq M; Örn S; Carlsson G; Morrison DA; Norrgren L Locomotor Behavior in Zebrafish (Danio Rerio) Larvae Exposed to Perfluoroalkyl Acids. Aquat. Toxicol 2013, 144–145, 332–340. [DOI] [PubMed] [Google Scholar]
- (51).Khezri A; Fraser TWK; Nourizadeh-Lillabadi R; Kamstra JH; Berg V; Zimmer KE; Ropstad E A Mixture of Persistent Organic Pollutants and Perfluorooctanesulfonic Acid Induces Similar Behavioural Responses, but Different Gene Expression Profiles in Zebrafish Larvae. Int. J. Mol. Sci 2017, 18 (2), 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Truong L; Rericha Y; Thunga P; Marvel S; Wallis D; Simonich MT; Field JA; Cao D; Reif DM; Tanguay RL Systematic Developmental Toxicity Assessment of a Structurally Diverse Library of PFAS in Zebrafish. J. Hazard. Mater 2022, 431, No. 128615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Rericha Y; Cao D; Truong L; Simonich M; Field JA; Tanguay RL Behavior Effects of Structurally Diverse Per- And Polyfluoroalkyl Substances in Zebrafish. Chem. Res. Toxicol 2021, 34 (6), 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Westerfield M The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.; University. of Oregon Press: Eugene, 2000. [Google Scholar]
- (55).Kimmel CB; Ballard WW; Kimmel SR; Ullmann B; Schilling TF Stages of Embryonic Development of the Zebrafish. Dev. Dyn 1995, 203 (3), 253–310. [DOI] [PubMed] [Google Scholar]
- (56).Spulber S; Kilian P; Ibrahim WNW; Onishchenko N; Ulhaq M; Norrgren L; Negri S; Di Tuccio M; Ceccatelli S PFOS Induces Behavioral Alterations, Including Spontaneous Hyperactivity That Is Corrected by Dexamfetamine in Zebrafish Larvae. PLoS One 2014, 9 (4), No. e94227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Vu JP; McLamb F; Feng Z; Griffin L; Gong S; Shea D; Szuch MA; Scott S; Gersberg RM; Bozinovic G Locomotion and Brain Gene Expression Exhibit Sex-Specific Non-Monotonic Dose-Response to HFPO-DA during Drosophila Melanogaster Lifespan. Neurotoxicology 2023, 96, 207–221. [DOI] [PubMed] [Google Scholar]
- (58).Slotkin TA; MacKillop EA; Melnick RL; Thayer KA; Seidler FJ Developmental Neurotoxicity of Perfluorinated Chemicals Modeled in Vitro. Environ. Health Perspect 2008, 116 (6), 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Sammi SR; Foguth RM; Nieves CS; De Perre C; Wipf P; McMurray CT; Lee LS; Cannon JR Perfluorooctane Sulfonate (PFOS) Produces Dopaminergic Neuropathology. Toxicol. Sci 2019, 172 (2), 417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Yuan Z; Shao X; Miao Z; Zhao B; Zheng Z; Zhang J Perfluorooctane Sulfonate Induced Neurotoxicity Responses Associated with Neural Genes Expression, Neurotransmitter Levels and Acetylcholinesterase Activity in Planarians Dugesia Japonica. Chemosphere 2018, 206, 150–156. [DOI] [PubMed] [Google Scholar]
- (61).Hallgren S; Viberg H Postnatal Exposure to PFOS, but Not PBDE 99, Disturb Dopaminergic Gene Transcription in the Mouse CNS. Environ. Toxicol. Pharmacol 2016, 41, 121–126. [DOI] [PubMed] [Google Scholar]
- (62).Salgado R; López-Doval S; Pereiro N; Lafuente A Perfluorooctane Sulfonate (PFOS) Exposure Could Modify the Dopaminergic System in Several Limbic Brain Regions. Toxicol. Lett 2016, 240 (1), 226–235. [DOI] [PubMed] [Google Scholar]
- (63).di Nisio A; Pannella M; Vogiatzis S; Sut S; Dall’Acqua S; Rocca MS; Antonini A; Porzionato A; de Caro R; Bortolozzi M; de Toni L; Foresta C Impairment of Human Dopaminergic Neurons at Different Developmental Stages by Perfluoro-Octanoic Acid (PFOA) and Differential Human Brain Areas Accumulation of Perfluoroalkyl Chemicals. Environ. Int 2022, 158, No. 106982. [DOI] [PubMed] [Google Scholar]
- (64).Foguth R; Sepuvelda MS; Cannon J Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health. Toxics 2020, 8 (2), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Choi YJ; Lee LS; Hoskins TD; Gharehveran MM; Sepulveda MS Occurrence and implications of per and polyfluoroalkyl substances in animal feeds used in laboratory toxicity testing. Sci. Total Environ 2023, 867 (1), No. 161583. [DOI] [PubMed] [Google Scholar]
- (66).Hoskins TD; Flynn RW; Coogan GSM; Catlin AC; de Perre C; Gharehveran MM; Choi YJ; Lee LS; Hoverman JT; Sepulveda MS Chronic exposure to a PFAS mixture resembling AFFF-impacted surface water decreases body size in Northern Leopard Frogs (Rana pipiens). Environ. Sci. Technol 2023, 57, 14797–14806, DOI: 10.1021/acs.est.3c01118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


