Abstract
Background
Biomarkers can be measured in various biological samples. Urine is among the most useful biofluids for routine testing, and several experimental and clinical studies support its role as a tool for the diagnosis and prevention of various diseases. The present systematic review aimed to examine periodontitis-specific urine biomarkers that could have a diagnostic relevance and to provide a qualitative assessment of the current literature.
Materials and Methods
Relevant studies identified from PubMed, Embase, Cochrane Library, and Scopus databases were examined to answer the following PECO question: “Could the concentration of specific metabolites in the urine be related to periodontal health and what is their diagnostic accuracy?”. Quality of included studies was rated using ROBINS-I tool. Meta-analysis was conducted on available quantitative data.
Results
After the screening of 768 titles, five studies were included in qualitative synthesis. The studies included referred to the evaluation of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and neopterin. Meta-analysis was conducted for neopterin concentration on data available in four studies involving 129 participants. Higher concentrations of neopterin were found in periodontitis-affected patients compared to controls and patients treated for periodontitis.
Conclusions
The literature appears controversial in attributing a role to neopterin and 8-OHdG as periodontal biomarkers, highlighting the need for further clinical studies on this topic. While some studies report variations in 8-OHdG and neopterin levels in periodontally affected patients versus either controls or periodontally treated patients, the level of evidence appears still limited to draw firm conclusions (PROSPERO CRD42020222681).
1. Introduction
Periodontitis is a multifactorial, chronic, inflammatory disease which recognizes as a major etiologic factor the presence of a dysbiotic plaque biofilm [1]. In terms of frequency, periodontitis is considered the most common chronic inflammatory disease in humans and the sixth most prevalent condition in the world [2]. Severe periodontitis is estimated to affect approximately 7%–11% of adults worldwide, while mild periodontitis affects around 50% of adults [2, 3].
The diagnosis of periodontitis relies on the evaluation of biometric clinical parameters, including probing depth (PD), clinical attachment level (CAL), and bleeding on probing (BOP) [4]. However, the assessment of the levels of inflammation through clinical parameters may not provide a complete overview of the current disease activity or future risk of breakdown [5].
From this perspective, an increasing application of biomarkers has been promoted to screen and predict the early onset of periodontitis. Biomarkers are biologic substances serving as indicators of biological health, pathogenic processes, environmental exposure, and pharmacologic responses to a therapeutic intervention [6]. Biomarkers can be measured in various biological samples, such as blood, urine, saliva, hair, faeces, cerebrospinal fluid, and body tissues. Urine is among the most useful biofluids for routine testing, and several experimental and clinical studies support its role as tool for the diagnosis, prevention, and treatment of various diseases, such as cancer, kidney diseases, infectious diseases, autoimmune diseases, and cardiovascular diseases [7].
Interestingly, evidence from the literature supports the assessment of biological diagnostic markers as indicators of periodontal status. Gingival crevicular fluid (GCF) and saliva have been claimed as potential repositories for the molecular changes associated with the destruction of periodontal tissues [8]. GCF is considered relevant for the assessment of the status of periodontal tissues, being rich in antibodies against oral microorganisms present in the dental biofilm, inflammatory mediators and tissue breakdown products [9]. Salivary biomarkers encompass a wide variety of inflammation-related cytokines and immunoglobulins, which can be indicative of the presence of periodontitis [10]. Consistently with the growing body of evidence on the relationship between periodontitis and systemic inflammation, the assessment of blood C-reactive protein (CRP) fluctuations in course of periodontitis, or following its treatment, have shed a light on the relationship of biomarkers in course of chronic periodontal diseases [11]. Although the relationship between blood and salivary biomarkers and periodontitis has been extensively studied, urine testing has only recently been suggested as a possible method for assessing periodontal health [12].
The aim of the present study was to systematically review the evidence on urine biomarkers in course of periodontitis and to assess their diagnostic relevance through a qualitative assessment of the current scientific literature.
2. Materials and Methods
2.1. Study Protocol
This research was conducted in accordance with the Cochrane Handbook and reported according to the PRISMA guidelines (Table 1) [36, 37, 38]. The protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO), registration number: CRD42020222681.
Table 1.
PRISMA checklist for systematic review reporting.
| Section/topic | # | Checklist item | Adherence | Pages |
|---|---|---|---|---|
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | Yes | 1 |
| Abstract | ||||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | Yes | 1 |
| Introduction | ||||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Yes | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | Yes | 4 |
| Methods | ||||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., web address), and, if available, provide registration information including registration number | Yes | 4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | Yes | 4–5 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | Yes | 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Yes | 6–7 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | Yes | 7 |
| Data collection process | 10 | Describe the method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Yes | 7 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | Yes | 7–8 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Yes | 8 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means); describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | Yes | 7–9 |
| Synthesis of results | 14 | — | Yes | 9 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | Yes | 9 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were prespecified | Yes | 9 |
| Results | ||||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | Yes | Table 2 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | Yes | Table 1 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see Item 12) | Yes | Figure 1 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot | Yes | Figures 2, 3, 4, and 5 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | Yes | 10–11 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | Yes | 10–11 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression (see Item 16)) | Yes | 10–11 |
| Discussion | ||||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policymakers) | Yes | 11 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review level (e.g., incomplete retrieval of identified research, reporting bias) | Yes | 13 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | Yes | 13 |
| Funding | ||||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | Yes | 13 |
The following focused question was phrased:
“Could the concentration of specific metabolites in the urine be related to periodontal health and what is their diagnostic accuracy?”
Articles to be included had to follow the following PECO:
(P) Population. Adult systemically healthy patients.
(E) Exposure. Patients or sites with a clinical or radiographic diagnosis of periodontitis.
(C) Comparison. Subjects or sites treated for periodontitis, or periodontally healthy subjects.
(O) Type of outcome measures. Changes in urinary metabolite concentration in periodontitis compared to treated groups or healthy subjects.
No time limitations were applied. Only articles in English were included.
2.2. Information Sources and Search
An electronic search was conducted by two independent reviewers (AF, LM) using PubMed, Embase, Cochrane Library, and Scopus for publications up to January 2022, using combinations of controlled terms (MeSH) and free text words. The search strategy was first designed for the MEDLINE database and was then modified for the other databases (Table 3). Additionally, a manual search of periodontology-related journals including the Journal of Dental Research, theJournal of Clinical Periodontology, the Journal of Periodontal Research, and the Journal of Periodontology was performed from 2010 to 2021. All references were exported and managed in the open-access platform Colandr (https://www.colandrapp.com) [39].
Table 3.
Electronic search strategy.
| Electronic search strategy |
|---|
| PubMed: (“periodontal disease”(Mesh) OR “periodontitis”(Mesh) OR “gingivitis”(Mesh) OR “periodontal disease” OR “periodontitis” OR “gingivitis”) AND (“urine” OR “urinary” OR “urinalysis”) Embase: (“periodontal disease”/exp OR “periodontitis”/exp OR gingivitis ∗) AND (“urine”/exp OR “urinary”/exp OR urinalysis ∗) Cochrane: #1 MeSH descriptor: (Periodontal diseases) explode all trees #2 MeSH descriptor: (Periodontitis) explode all trees #3 MeSH descriptor: (Gingivitis) explode all trees #4 #1 OR #2 OR #3 #5 #4 AND “urine” #6 #4 AND “urinary” #7 #4 AND “urinalysis” |
2.3. Eligibility criteria
The inclusion criteria for title and abstract analysis are the following:
Studies reporting on systemically healthy human subjects affected by periodontitis.
Collection of urine metabolomic biomarkers.
Study design: RCTs and clinical trials.
Exclusion criteria are as follows:
Studies reporting on periodontally affected adult patients with systemic diseases and/or pregnant or breastfeeding.
Study design: Case reports, literature reviews, editorials, animal studies, and in vitro experiments.
2.4. Study Selection
The titles and abstracts of potentially eligible studies were screened independently by two reviewers (AF and LM). Any disagreement was resolved by discussion with a third reviewer (EM). Cohen's K-score was calculated to assess the interreviewer reliability in the screening phase.
The reasons for exclusion of studies after full-text analysis were recorded and reported (Table 2). Relevant articles that met the inclusion criteria were analyzed in full-text.
Table 2.
Reasons for study exclusion following full-text analysis.
| Nonperiodontal patients | Absence of biomarkers | Other diseases/not periodontal biomarkers | Same data from other study |
|---|---|---|---|
| Cooke et al. [13] Ojeda et al. [14] | Matsumoto et al. [15] Yamori et al. [16] Yoshihara et al. [17] Yoshihara et al. [18] | Brotto et. al. [19] Grubbs et al. [20] Ioannidou et al. [21] Kang et al. [22] Liu K et al. [23] Mesa et al. [24] Nakajima et al. [12] Schulze-Späte et al. [25] Vachhani et al. [26] von Wowern et al. [27] Wangerin et al. [28] | Prasanna et al. [29] |
2.5. Data Extraction and Management
The following data were collected for each study (AF, LM): authors, study design, location, sample size, characteristics of patients (mean age, gender), periodontal diagnosis, treatment (if any), follow-up, urinary biomarkers and concentration levels, mean clinical outcomes (PPD, CAL), methodology used for sampling, storage, processing and detection of the urinary markers, and main findings.
2.6. Risk of Bias in the Included Studies and Quality Assessment
The quality assessment and the risk of bias of the included studies were performed independently by two calibrated reviewers (LM, AF). The risk of bias for nonrandomized studies was performed using ROBINS-I tool [40, 41]. In cases of critical or serious judgment, the study was considered at high risk of bias.
2.7. Data Synthesis
The urinary concentrations of biomarkers considered relevance for periodontal patients were reported. The concentration of each biomarker in periodontal patients at diagnosis/baseline and after treatment was expressed as mean and standard deviation.
A random-effect model was employed to estimate the average urinary mean concentration of each biomarker at diagnosis/baseline. Then, a random-effect model was applied to assess mean differences in urinary concentration of each biomarker between periodontal patients at diagnosis versus healthy controls, as well as between periodontal patients at diagnosis versus at first follow-up.
In all the models, a restricted maximum-likelihood estimator (REML) was used to estimate tau [2]. Tau [2] and prediction intervals were reported to represent the overall level of heterogeneity, as well as I2 to describe it. A value of I2 between 25% and 50% was considered as low heterogeneity, between 50% and 75% as medium heterogeneity, and >75% as high heterogeneity. The results of the meta-analyses were graphically represented using forest plots.
A meta-regression of the average urinary mean concentration of each biomarker at diagnosis/baseline was also performed, with mean age and gender as potential predictors.
The numerical synthesis of the results as well as the figures were developed using R studio and the metaphor package [42].
3. Results
3.1. Study Selection
In total, 768 articles were retrieved through the electronic search. Manual search did not retrieve further studies. After the removal of duplicates, 597 titles and abstracts were screened for eligibility and 25 papers were analyzed in full-text. Of these, 19 articles were excluded (Table 2), and six papers were included in the review (Figure 6). Inter-reviewer agreement was k = 0.92 for abstract screening and k = 0.97 for full-text analysis.
Figure 6.
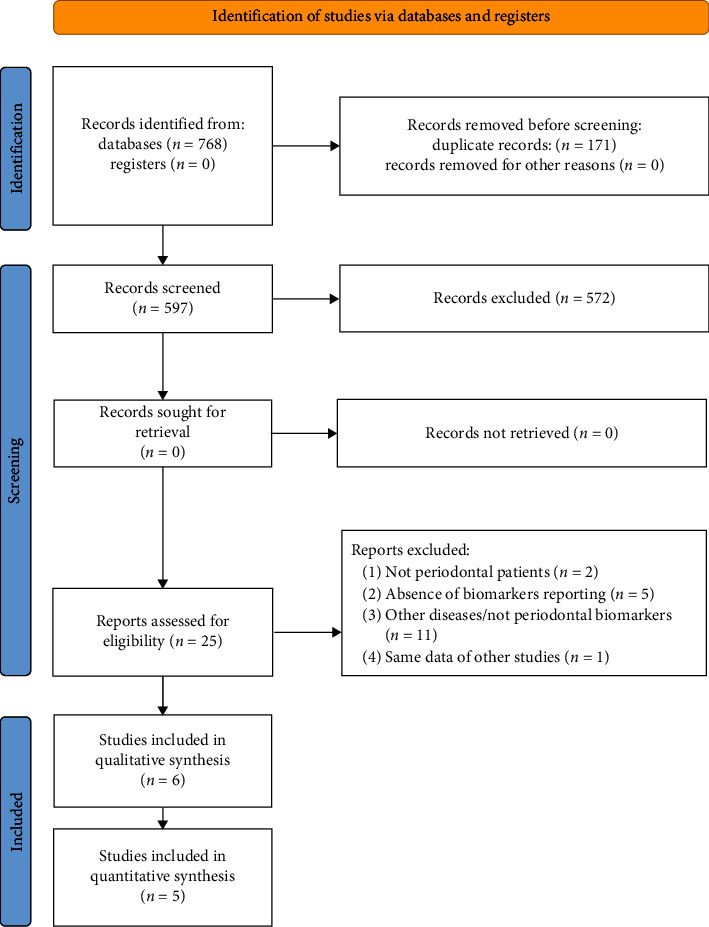
PRISMA flowchart. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers. From: MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit at http://www.prisma-statement.org/.
3.2. Study Characteristics
All the identified studies were published between 1997 and 2022 [30, 31, 32, 33, 34, 35]. Two studies were conducted in Turkey [30, 32], the others in Spain [35], India [33], Austria [34], and Czech Republic [31]. The sample size ranged between 16 and 70 individuals. All the papers reported observational studies. Two studies had a cross-sectional design [33, 35], three were case-control studies [30, 31, 32], and one was a cohort study [34].
Periodontitis case definitions and periodontal parameters were heterogeneous across the studies. Only two studies presented the classification for periodontal diseases currently in use [31, 35]. Two articles enrolled patients with chronic periodontitis (CP) [33, 34], and two studies referred to patients with aggressive periodontitis (AgP) [33, 34]. Three studies included a control group of healthy patients (Table 4) [30, 31, 32].
Table 4.
Synthesis of the included studies.
| Authors | Year | Study design | Follow-up (months) | Metabolites | Unite of measure | Sample size | Female (%) | Concentrations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case group | Control | ||||||||||||||
| Baseline | Follow-up time | Baseline | Follow-up time | ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||||
| Andreu et al. [30] | 2021 | Cross-sectional | — | 8-OHdG | Creatinine (µg/g) | 70 | 61.4 | 10.9 Stage II 9.17 Stage III 9.84 Stage IV | 3.05 2.05 3.21 | — | — | — | — | — | — |
| Bodur et al. [31] | 2003 | Observational study case control | 6 | Neopterin | Neopterin (μ mol)/creatinine (mol) | 16 | 68.8 | 168.1 | 20.21 | 310.1 | 39.82 | 188.5 | 30.98 | — | — |
| Heneberk et al. [32] | 2022 | Observational study case control | 3 | Neopterin | Neopterin (μ mol)/creatinine (mol) | 25 | — | 210.96 (183.60–282.91) | — | 237.87 (202.46–266.80) | — | 180.59 (133.97–220.22) | — | — | — |
| Ozmeriç et al. [33] | 2002 | Observational study case control | — | Neopterin | Neopterin (μ mol)/creatinine (mol) | 29 | 55.1 | 235.77 | 405.31 | — | — | 225.45 | 100.72 | — | — |
| Prasanna and Sumadhura [34] | 2019 | Cross-sectional interventional study | 3 | Neopterin | Neopterin (μ mol)/creatinine (mol) | 30 | 100.0 | 348.06 | 61.45 | 278.01 | 53.53 | — | — | — | — |
| Vrecko et al. [35] | 1997 | Observational cohort study | — | Neopterin | Neopterin (μ mol)/creatinine (mol) | 29 | 37.9 | 102 | 48 | — | — | — | — | — | — |
3.3. Synthesis of the Results
The studies included provided data on 8-hydroxy-2′-deoxyguanosine (8-OHdG) [35] and neopterin concentration [30, 31, 32, 33, 34] in a total of 199 patients (70 evaluated for 8-OHdG levels, 129 for neopterin levels). Meta-analysis was conducted only for neopterin, for which data were available in five studies involving 129 participants.
Gender was employed as a potential predictor for the meta-regression. Female gender in the sample was a significant predictor of the estimation of the average mean urinary concentration of neopterin (p=0.03). Female gender accounted for almost 60% of heterogeneity in the model (R2 = 59.39). Due to the limited number of observations and the missing values in the included studies, no meta-regression was conducted using the average age of the patients as potential predictor.
Regarding other confounders, only one study [35] included smokers, who represented 34.3% of the sample. Three studies [31, 32, 33] excluded smokers from the sample. The remaining two studies [30, 34] did not give any information on smoking status. Two studies [30, 33] included only systemically healthy patients. The remaining studies [31, 32, 34, 35] did not provide information on systemic health status.
3.4. Average Mean Concentration of 8-OHdG
The concentration of 8-OHdG was investigated in one study [35]. No differences were observed in 8-OHdG urine levels between groups of patients affected by different stages of periodontitis (10.9 ± 3.05 μg/g in Stage II; 9.17 ± 2.05 μg/g in Stage III; and 9.84 ± 3.21 μg/g in Stage IV).
3.5. Average Mean Concentration of Neopterin
An average mean urinary concentration of neopterin of 195.75 mmol neopterin/mol creatinine (95% CI [100.30 to 291.20]; p=0.03; I2 = 61%) was found in periodontal patients at diagnosis (Figure 2).
Figure 2.
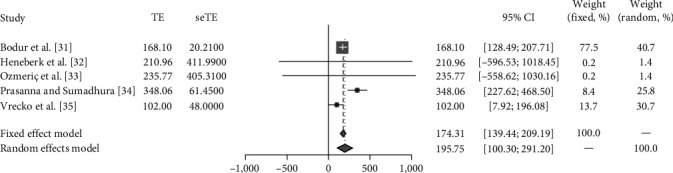
Meta-analysis of the four studies [31, 32, 33, 34, 35] reporting fluctuations in neopterin levels.
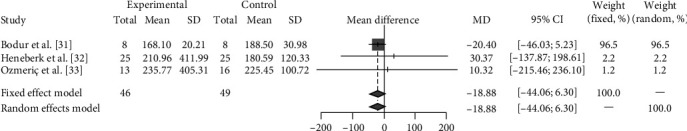
When comparing periodontal patients and controls, controls showed lower neopterin levels, although no statistically significant differences were reported (ns = 3; np = 95; WMD = −18.88 mmol neopterin/mol creatinine; 95% CI [−44.06; 6.30]; p=0.82; I2 = 31.75%) (Figure 3).
Figure 3.
Following periodontal treatment, a statistically significant decrease in neopterin levels was recorded (ns = 3; np = 63; WMD = −33.42 mmol neopterin/mol creatinine; 95% CI [−205.08; 138.23]; p < 0.01; I2 = 98%) (Figure 4).
Figure 4.
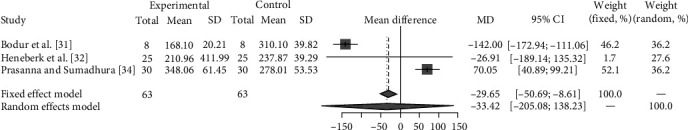
Meta-analysis of the studies [31, 32, 34] evaluating neopterin levels in periodontal patients pre- and post-treatment.
3.6. Risk of Bias Assessment
The risk of bias assessment for the included studies was completed independently by two reviewers (AF, LM) as part of the data extraction process using ROBINS-I tool and is summarized in Figures 1 and 5. One paper [32] showed a low risk of bias, one paper [35] a moderate risk of bias, and three papers [30, 31, 33] a high risk of bias, while one paper [34] showed a critical risk of performance bias due to missing data.
Figure 1.
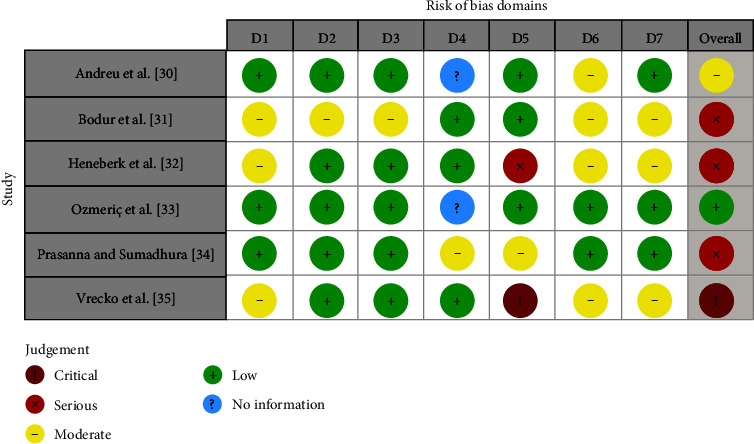
Risk of bias assessment for each of the included studies. Domains: D1, bias due to confounding; D2, bias due to selection of participants; D3, bias in classification of interventions; D4, bias due to deviations from intended interventions; D5, bias due to missing data; D6, bias in measurements of outcomes; D7, bias in selection of the reported result.
Figure 5.
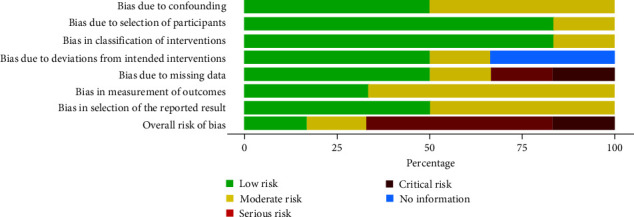
Risk of bias assessment with the ROBINS-I tool.
4. Discussion
The present systematic review highlights an overall paucity of literature on the association between urine biomarkers and periodontal health status. The results suggest the presence of higher urine biomarkers concentrations in periodontally affected patients compared to treated patients or healthy controls. Neopterin may be a relevant biomarker of periodontal status, as its concentration is significantly lower both in healthy subjects and in patients receiving periodontal treatment. The evidence on 8-OHdG is extremely scarce, and no statistically significant differences were detected depending on the stage of periodontitis. It should be noted, though, that only one study investigated this biomarker and presented an overall limited study sample. Such results should be interpretated with caution due to the weak evidence provided by the literature available.
Periodontal biomarkers are a trending topic in the current literature. The increasing attention toward periodontal medicine has fostered the research on the systemic implications of periodontal diseases. Importantly, a relationship has been found between periodontitis and cardiovascular diseases [43], diabetes [44], adverse pregnancy outcomes [45], and in general with systemic inflammation [46]. Indeed, untreated periodontitis has been associated with elevated systemic inflammation, which could contribute to the development of systemic health complications [47]. Among the inflammation biomarkers studied in relationship to periodontitis, serum C-reactive protein (CRP) is the most commonly examined. Evidence suggests that CRP levels tend to decrease following periodontal treatment [11]. Other biomarkers can be titrated in the saliva and gingival crevicular fluid, including macrophage inflammatory protein-1 alpha (MIP-1α), interleukin-1 beta (IL−1β), interleukin-6 (IL-6), and matrix metalloproteinase-8 (MMP-8) [48].
A growing interest in the role of neopterin and its relationship with periodontitis has been reported in the literature [49]. Neopterin is a pteridine deriving from guanosine triphosphate involved in cell-mediated immunity, and it is considered a biomarker for macrophage activation with a regulating effect on the bioavailability of nitric oxide [50]. Its effects include the enhancement of the cytotoxic potential of activated macrophages and the activation of reactive oxygen species [51]. A role in tumor development and growth has also been hypothesized due to the role of neopterin in inducing c-fos protooncogene [52].
Neopterin can be sampled in several bodily fluids with high-performance liquid chromatography or enzyme-linked immunosorbent assay methods [53]. In GCF sampling, neopterin titration may indicate the presence of inflammation along with the detection of T-cells and other inflammatory infiltrates [51]. In urinary samples, neopterin is titrated in a ratio with creatinine. Importantly, a variability in neopterin values has been found, showing higher values in women and older individuals. Moreover, a circadian oscillation has been observed with a peak between 7.00 and 12.00 am [54]. The role of gender as a predictor of mean urinary concentration of neopterin was also confirmed by the present analysis. In our meta-analysis, only studies reporting on urinary concentration of neopterin were evaluated. However, the limited literature available hinders the validation of a direct relationship between neopterin levels and periodontitis. While it could be possible that neopterin levels may be involved in periodontitis, presumably due to macrophage activation associated both with periodontitis and neopterin levels increase, it should not be forgotten that factors other than periodontitis may contribute to neopterin fluctuations, being a very sensitive and nonspecific biomarker of cellular immune system activation [55]. Moreover, literature is extremely controversial on the effects of periodontal treatment on urinary neopterin levels, and the increase in neopterin levels has been described following periodontal therapy [33] as well as the decrease in the neopterin/creatinine ratio [29, 34]. The evidence available highlights the unmet need for clinical trials investigating the actual relationship between neopterin levels and periodontitis.
Similarly to neopterin, 8-OHdG is a biomarker for oxidative stress and carcinogenesis, which has been employed for the risk assessment of cancer and degenerative diseases [56]. 8-OHdG role as a biomarker associated with periodontitis has been previously evaluated through salivary titration, showing positive correlation with the presence of chronic periodontitis and microbial parameters [57]. Moreover, increased levels of 8-OHdG have been reported in gingival crevicular fluid of periodontally affected sites [58]. However, the study included in the present review did not highlight a correlation between urine concentrations of 8-OHdG and periodontal disease activity.
Overall, the literature appears controversial in attributing a role to both neopterin and 8-OHdG as periodontal biomarkers, highlighting the need for further clinical studies on this topic. While some studies report variations in these biomarkers' levels in periodontally affected patients versus either controls or periodontally treated patients, the level of evidence appears still limited to draw firm conclusions [59].
The present review has some limitations. First, the paucity of literature on urinary biomarkers in periodontitis allowed to evaluate only neopterin and 8-OHdG. Second, only one study reported on 8-OHdG, providing insufficient evidence on this biomarker. Moreover, the quality of the studies included was deemed at moderate/high risk of bias, which can negatively affect the quality of the results. The studies reporting on neopterin levels in periodontal patients pre- and post-treatment evaluated patients after different follow-up periods. Indeed, the comparison between periodontally affected patients versus healthy or treated groups in terms of urinary metabolite concentration as an outcome may limit the assessment of the diagnostic accuracy of the investigated biomarkers. Moreover, it was not possible to assess the potential role of confounders contributing to systemic inflammation. The included studies enrolled relatively limited samples, thus highlighting the need for further evaluation on larger cohorts.
Nevertheless, a trend toward an increase in urinary neopterin can be hypothesized in the presence of untreated periodontitis, as the literature available suggests a normalization of neopterin levels following periodontal treatment. Conversely, the evidence on 8-OHdG is still extremely scarce and cannot be validated.
5. Conclusions
The evidence available from the present review aims at raising awareness on the potential role of urinary biomarkers for the diagnosis, prognosis, and monitoring of periodontitis, as to date there is insufficient evidence to draw firm conclusions. Further studies assessing urinary biomarker variations in the course of periodontitis compared to treated or healthy subjects are advised to enrich the body of literature on this topic.
Data Availability
The data presented in this study are openly available.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Adriano Fratini and Rossana Izzetti contributed to conceptualization, investigation, data curation, and writing—original draft preparation. Adriano Fratini and Stefano Gennai contributed to methodology and resources. Nicola Riccetti contributed to software and formal analysis. Enrico Marchetti contributed to validation and funding acquisition. Nicola Riccetti and Filippo Graziani contributed to writing—review and editing. Adriano Fratini, Rossana Izzetti, and Stefano Gennai contributed to visualization. Enrico Marchetti and Filippo Graziani contributed to supervision. Adriano Fratini contributed to project administration. All authors have read and agreed to the published version of the manuscript.
References
- 1.Papapanou P. N., Sanz M., Buduneli N., et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Periodontology . 1:S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 2.Kassebaum N. J., Bernabé E., Dahiya M., Bhandari B., Murray C. J. L., Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. Journal of Dental Research . 2014;93(11):1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassebaum N. J., Smith A. G. C., Bernabé E., et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of Dental Research . 2017;96(4):380–387. doi: 10.1177/0022034517693566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page R. C., Eke P. I. Case definitions for use in population-based surveillance of periodontitis. Journal of Periodontology . 2007;78(7S):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L., Henson B. S., Camargo P. M., Wong D. T. The clinical value of salivary biomarkers for periodontal disease. Periodontology 2000 . 2009;51(1):25–37. doi: 10.1111/j.1600-0757.2009.00315.x. [DOI] [PubMed] [Google Scholar]
- 6.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics . 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 7.Prasad S., Tyagi A. K., Aggarwal B. B. Detection of inflammatory biomarkers in saliva and urine: potential in diagnosis, prevention, and treatment for chronic diseases. Experimental Biology and Medicine . 2016;241(8):783–799. doi: 10.1177/1535370216638770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buduneli N., Kinane D. F. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. Journal of Clinical Periodontology . 2011;38(s11):85–105. doi: 10.1111/j.1600-051X.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 9.Barros S. P., Williams R., Offenbacher S., Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000 . 2016;70(1):53–64. doi: 10.1111/prd.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go H., Park T., Shin A. R., et al. Validity of a combination of periodontal pathogens and salivary biomarkers as predictors of periodontitis. Journal of Periodontal Research . 2022;57(5):1083–1092. doi: 10.1111/jre.13048. [DOI] [PubMed] [Google Scholar]
- 11.Luthra S., Orlandi M., Hussain S. B., et al. Treatment of periodontitis and C-reactive protein: a systematic review and meta-analysis of randomized clinical trials. Journal of Clinical Periodontology . 2023;50(1):45–60. doi: 10.1111/jcpe.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima M., Hosojima M., Tabeta K., et al. β 2 -microglobulin and neutrophil gelatinase-associated lipocalin, potential novel urine biomarkers in periodontitis: a cross-sectional study in Japanese. International Journal of Dentistry . 2019;2019:10. doi: 10.1155/2019/1394678.1394678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke M. S., Singh R., Hall G. K., et al. Evaluation of enzyme-linked immunosorbent assay and liquid chromatography-tandem mass spectrometry methodology for the analysis of 8-oxo-7,8-dihydro-2’-deoxyguanosine in saliva and urine. Free Radical Biology and Medicine . 2006;41(12):1829–1836. doi: 10.1016/j.freeradbiomed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Ojeda A. G., Wrobel K., Escobosa A. R., Garay-Sevilla M. E., Wrobel K. High-performance liquid chromatography determination of glyoxal, methylglyoxal, and diacetyl in urine using 4-methoxy-o-phenylenediamine as derivatizing reagent. Analytical Biochemistry . 2014;449:52–58. doi: 10.1016/j.ab.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto S., Matsuda M., Takekawa M., et al. Association between chronic periodontal disease and lower urinary tract symptoms in both sexes. Low Urinary Tract Symptoms . 2015;7(1):17–21. doi: 10.1111/luts.12042. [DOI] [PubMed] [Google Scholar]
- 16.Yamori M., Njelekela M., Mtabaji J., Yamori Y., Bessho K. Hypertension, periodontal disease, and potassium intake in nonsmoking, nondrinker African women on no medication. International Journal of Hypertension . 2011;2011 doi: 10.4061/2011/695719.695719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshihara A., Deguchi T., Hanada N., Miyazaki H. Renal function and periodontal disease in elderly Japanese. Journal of Periodontology . 2007;78(7):1241–1248. doi: 10.1902/jop.2007.070025. [DOI] [PubMed] [Google Scholar]
- 18.Yoshihara A., Hayashi Y., Miyazaki H. Relationships among bone turnover, renal function and periodontal disease in elderly Japanese. Journal of Periodontal Research . 2011;46(4):491–496. doi: 10.1111/j.1600-0765.2011.01365.x. [DOI] [PubMed] [Google Scholar]
- 19.Brotto R. S., Vendramini R. C., Brunetti I. L., Marcantonio R. A., Ramos A. P., Pepato M. T. Lack of correlation between periodontitis and renal dysfunction in systemically healthy patients. European Journal of Dentistry . 2011;5(1):8–18. [PMC free article] [PubMed] [Google Scholar]
- 20.Grubbs V., Plantinga L. C., Crews D. C., et al. Vulnerable populations and the association between periodontal and chronic kidney disease. Clinical Journal of the American Society Nephrology . 2011;6(4):711–717. doi: 10.2215/CJN.08270910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannidou E., Swede H., Dongari-Bagtzoglou A. Periodontitis predicts elevated C-reactive protein levels in chronic kidney disease. Journal of Dental Research . 2011;90(12):1411–1415. doi: 10.1177/0022034511423394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang S. H., Park J. W., Cho K. H., Do J.-Y. Association between periodontitis and low-grade albuminuria in non-diabetic adults. Kidney and Blood Pressure Research . 2017;42(2):338–346. doi: 10.1159/000477784. [DOI] [PubMed] [Google Scholar]
- 23.Liu K., Liu Q., Chen W., et al. Prevalence and risk factors of CKD in Chinese patients with periodontal disease. PLOS ONE . 2013;8(8) doi: 10.1371/journal.pone.0070767.e70767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mesa F., Magán-Fernández A., Muñoz R., et al. Catecholamine metabolites in urine, as chronic stress biomarkers, are associated with higher risk of chronic periodontitis in adults. Journal of Periodontology . 2014;85(12):1755–1762. doi: 10.1902/jop.2014.140209. [DOI] [PubMed] [Google Scholar]
- 25.Schulze-Späte U., Turner R., Wang Y., et al. Relationship of bone metabolism biomarkers and periodontal disease: the osteoporotic fractures in men (MrOS) study. The Journal of Clinical Endocrinology & Metabolism . 2015;100(6):2425–2433. doi: 10.1210/jc.2014-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vachhani K. S., Bhavsar N. V. Effects of non-surgical periodontal therapy on serum inflammatory factor high-sensitive C-reactive protein, periodontal parameters and renal biomarkers in patients with chronic periodontitis and chronic kidney disease. Dental and Medical Problem . 2021;58(4):489–498. doi: 10.17219/dmp/136034. [DOI] [PubMed] [Google Scholar]
- 27.von Wowern N., Westergaard J., Kollerup G. Bone mineral content and bone metabolism in young adults with severe periodontitis. Journal of Clinical Periodontology . 2001;28(6):583–588. doi: 10.1034/j.1600-051x.2001.028006583.x. [DOI] [PubMed] [Google Scholar]
- 28.Wangerin C., Pink C., Endlich K., et al. Long-term association of periodontitis with decreased kidney function. American Journal of Kidney Diseases . 2019;73(4):513–524. doi: 10.1053/j.ajkd.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Prasanna J. S., Sumadhura C., Karunakar P., Rekharani K., Himabindu G., Manasa A. Correlative analysis of plasma and urine neopterin levels in the pre- and post-menopausal women with periodontitis, following nonsurgical periodontal therapy. Journal of Indian Society of Periodontology . 2017;21(4):276–284. doi: 10.4103/jisp.jisp_278_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreu R., Santos-del-Riego S., Payri F. Serum inflammatory and prooxidant marker levels in different periodontal disease stages. Healthcare . 2021;9(8) doi: 10.3390/healthcare9081070.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodur A., Baydar T., Ozmeric N., et al. Neopterin profile to evaluate the effectiveness of treatment in aggressive periodontitis. Pteridines . 2003;14(3):77–81. [Google Scholar]
- 32.Heneberk O., Vernerova A., Kujovska Krcmova L., Wurfelova E., Radochova V. Neopterin levels in periodontitis and after nonsurgical periodontal therapy: evaluation of gingival crevicular fluid, oral fluid, serum and urinary samples—a case-control study. Biomedicines . 2022;10(12) doi: 10.3390/biomedicines10123200.3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozmeriç N., Baydar T., Bodur A., et al. Level of neopterin, a marker of immune cell activation in gingival crevicular fluid, saliva, and urine in patients with aggressive periodontitis. Journal of Periodontology . 2002;73(7):720–725. doi: 10.1902/jop.2002.73.7.720. [DOI] [PubMed] [Google Scholar]
- 34.Prasanna J. S., Sumadhura C. Biochemical analysis of three biological fluids and its response to non-surgical periodontal therapy in pre and postmenopausal women with periodontitis. Journal of Menopausal Medicine . 2019;25(3):149–157. doi: 10.6118/jmm.18179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrecko K., Staedtler P., Mischak I., Maresch L., Reibnegger G. Periodontitis and concentrations of the cellular immune activation marker neopterin in saliva and urine. Clinica Chimica Acta . 1997;268(1-2):31–40. doi: 10.1016/S0009-8981(97)00154-X. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J. P. T., Altman D. G., Gotzsche P. C., et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ . 2011;343(oct18 2) doi: 10.1136/bmj.d5928.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ . 2009;339(jul21 1) doi: 10.1136/bmj.b2700.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moher D., Liberati A., Tetzlaff J., Altman D. G., for the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ . 2009;339(jul21 1) doi: 10.1136/bmj.b2535.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng S. H., Augustin C., Bethel A., et al. Using machine learning to advance synthesis and use of conservation and environmental evidence. Conservation Biology . 2018;32(4):762–764. doi: 10.1111/cobi.13117. [DOI] [PubMed] [Google Scholar]
- 40.Sterne J. A. C., Hernán M. A., Reeves B. C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ . 2016;355 doi: 10.1136/bmj.i4919.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schünemann H. J., Cuello C., Akl E. A., et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology . 2019;111:105–114. doi: 10.1016/j.jclinepi.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software . 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 43.Sanz M., Marco Del Castillo A., Jepsen S., et al. Periodontitis and cardiovascular diseases: consensus report. Journal of Clinical Periodontology . 2020;47(3):268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanz M., Ceriello A., Buysschaert M., et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. Journal of Clinical Periodontology . 2018;45(2):138–149. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 45.Sanz M., Kornman K., on behalf of working group 3 of the joint EFP/AAP workshop Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Periodontology . 2013;84(4S) doi: 10.1902/jop.2013.1340016. [DOI] [PubMed] [Google Scholar]
- 46.Graziani F., Gennai S., Marruganti C., et al. Acute-phase response following one-stage full-mouth versus quadrant non-surgical periodontal treatment in subjects with comorbid type 2 diabetes: a randomized clinical trial. Journal of Clinical Periodontology . 2023;50(4):487–499. doi: 10.1111/jcpe.13760. [DOI] [PubMed] [Google Scholar]
- 47.D’Aiuto F., Gkranias N., Bhowruth D., et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinology . 2018;6(12):954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]
- 48.KC S., Wang X. Z., Gallagher J. E. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: systematic review. Journal of Clinical Periodontology . 2020;47(3):289–308. doi: 10.1111/jcpe.13218. [DOI] [PubMed] [Google Scholar]
- 49.Heneberk O., Wurfelova E., Radochova V. Neopterin, the cell-mediated immune response biomarker, in inflammatory periodontal diseases: a narrative review of a more than fifty years old biomarker. Biomedicines . 2023;11(5) doi: 10.3390/biomedicines11051294.1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zembron-Lacny A., Dziubek W., Tylutka A., et al. Assessment of serum neopterin as a biomarker in peripheral artery disease. Diagnostics . 2021;11(10) doi: 10.3390/diagnostics11101911.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pradeep A. R., Kumar M. S., Ramachandraprasad M. V., Shikha C. Gingival crevicular fluid levels of neopterin in healthy subjects and in patients with different periodontal diseases. Journal of Periodontology . 2007;78(10):1962–1967. doi: 10.1902/jop.2007.070096. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann G., Wirleitner B., Fuchs D. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflammation Research . 2003;52(8):313–321. doi: 10.1007/s00011-003-1181-9. [DOI] [PubMed] [Google Scholar]
- 53.Moutereau S., Ech Chad N., Devanlay M., Esmilaire L., Loric S. Improved neopterin ELISA kit: a good compromise between HPLC results and clinical practice. Clinical Chemistry and Laboratory Medicine . 2011;49(3):553–554. doi: 10.1515/CCLM.2011.071. [DOI] [PubMed] [Google Scholar]
- 54.Murr C., Widner B., Wirleitner B., Fuchs D. Neopterin as a marker for immune system activation. Current Drug Metabolism . 2002;3(2):175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Haq A., Kusnierz-Cabala B., Darczuk D., et al. Interleukin-6 and neopterin levels in the serum and saliva of patients with Lichen planus and oral Lichen planus. Journal of Oral Pathology & Medicine . 2014;43(10):734–739. doi: 10.1111/jop.12199. [DOI] [PubMed] [Google Scholar]
- 56.Valavanidis A., Vlachogianni T., Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. Journal of Environmental Science and Health, Part C . 2009;27(2):120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 57.Yang X., Li C., Pan Y. The influences of periodontal status and periodontal pathogen quantity on salivary 8-hydroxydeoxyguanosine and interleukin-17 levels. Journal of Periodontology . 2016;87(5):591–600. doi: 10.1902/jop.2015.150390. [DOI] [PubMed] [Google Scholar]
- 58.Baima G., Corana M., Iaderosa G., et al. Metabolomics of gingival crevicular fluid to identify biomarkers for periodontitis: a systematic review with meta-analysis. Journal of Periodontal Research . 2021;56(4):633–645. doi: 10.1111/jre.12872. [DOI] [PubMed] [Google Scholar]
- 59.Kiss S. Szeged University (Hungary) proquest dissertations & theses. 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are openly available.


