Abstract
During the replication cycle of murine leukemia virus (MLV), Pol is normally synthesized as part of a Gag-Pol fusion protein. In this study, the ability of free MLV Pol to be incorporated into virions was examined. When MLV Gag and MLV Pol were coexpressed from separate plasmids in cells, reverse transcriptase (RT) activity associated with Gag core particles at a slightly lower level than did RT activity generated from wild-type Gag-Pol expression. Particles produced in this manner were somewhat less infectious than those produced with wild-type Gag-Pol. A smaller amount of MLV Pol also associated with heterologous human immunodeficiency virus type 1 Gag cores.
The genomes of all retroviruses contain gag, pol, and env genes, which encode the major structural and enzymatic proteins necessary for formation of virions and completion of the viral replication cycle (reviewed in reference 8). During virus production in infected cells, the Gag precursor protein is processed to produce the matrix (MA), capsid (CA), and nucleocapsid (NC) proteins that comprise the virion core. The Pol precursor is processed to produce the functional viral enzymes protease (PR), reverse transcriptase (RT), and integrase (IN). Though much is known about viral replication and virion structure, there are still relatively few details known about the molecular interactions among viral proteins which occur during virion assembly, maturation, and release from cells.
Pol protein is normally produced only in the form of the Gag-Pol fusion precursor protein, either by infrequent read-through suppression of Gag termination or by ribosomal frameshifting during translation of viral mRNA, at a level estimated to be 5 to 10% of the amount of free Gag produced (11, 16, 34). It is generally accepted, though it has not been shown definitively, that retroviral pol gene products are incorporated into forming virions as part of Gag-Pol fusion proteins via interactions of the Gag portion of the Gag-Pol precursor with other Gag molecules constituting the virion core (3, 10, 15, 26, 29). Subsequent proteolytic processing of Gag and Gag-Pol precursors by the virus-encoded protease leads to formation of mature, functional virions made up of condensed viral cores consisting of NC, MA, and CA and active PR, RT, and IN surrounded by the viral envelope obtained during virus budding (8, 33).
The amount of Gag-Pol that is produced relative to the level of free Gag appears to be important to the process of virus assembly. For murine leukemia virus (MLV), a mutation that resulted in production of 100% Gag-Pol and no free Gag prevented the proteolytic processing of Gag-Pol and the assembly of virions (11). Similar mutations in human immunodeficiency virus (HIV) and spleen necrosis virus also prevented virion formation (17, 18, 24, 34). It was suggested that the stoichiometry of Gag and Gag-Pol production is important in virion formation because overproduction of Gag-Pol may impede core formation by preventing appropriate Gag molecular interactions. Conversely, the presence of the Gag-Pol precursor is not necessary for virion core formation, since expression of Gag alone in cells is sufficient for the formation and release of virus-like particles, though such particles are noninfectious since they lack a viral genome, pol-encoded enzymatic functions, and the envelope glycoprotein (13, 32).
The experiments described herein were designed to determine whether MLV Pol not generated from the Gag-Pol precursor can associate with assembling virions. The ability of MLV Gag and Pol proteins generated from separate expression plasmids to assemble into infectious virus particles was examined. Then, as a first step in studying the feasibility of engineering a chimeric MLV/HIV vector system, the studies were extended by using HIV Gag and MLV Pol to determine if MLV Pol could associate with heterologous HIV Gag virion cores.
MLV Pol associates with MLV Gag virion cores.
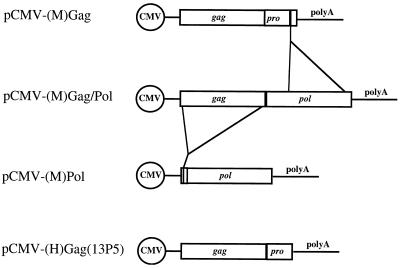
The plasmid pCMV-(M)Gag/Pol expresses the MLV gag and pol genes as the wild-type Gag and Gag-Pol precursors from the human cytomegalovirus (CMV) promoter. The introduction of this plasmid into cells results in efficient production of MLV virus-like cores; when it is coexpressed with amphotropic MLV envelope or vesicular stomatitis virus G protein and an MLV vector, infectious virus is produced (31). pCMV-(M)Gag/Pol was modified to generate constructs capable of expressing either the MLV gag or pol gene separately (Fig. 1). A deletion of approximately 2,700 bp between the two KpnI sites in the pol gene resulted in the plasmid pCMV-(M)Gag, a construct designed to produce MLV Gag protein; this construct also produces the viral protease to allow proper processing of the precursor Gag protein. A deletion of approximately 1,500 bp between the upstream AflII and NruI sites of the gag gene followed by linker insertion at those sites in pCMV-(M)Gag/Pol resulted in the construct pCMV-(M)Pol, which is capable of expressing MLV Pol; this construct also contains the C terminus of the NC protein, preserved to maintain the appropriate protease cleavage site of the amino terminus of the Pol precursor.
FIG. 1.
Gag- and Pol-expressing constructs. pCMV-(M)Gag/Pol is the parental construct expressing wild-type MLV Gag-Pol from the CMV promoter. pCMV-(M)Gag, which contains the MLV gag and pro genes, was constructed by deletion of the majority of the pol gene (2,718 bp). The terminal 255 bp of the IN domain remain. pCMV-(M)Pol expresses MLV Pol and was constructed by deleting 1,476 bp from the gag gene. Twenty-three base pairs of the amino terminus of the MA domain and 102 bp of the C terminus of the NC domain remain. pCMV-(H)Gag(13P5) expresses HIV Gag and PR from the CMV promoter.
To determine if proteins expressed from these constructs could form virions, either plasmid pCMV-(M)Gag/Pol or both pCMV-(M)Gag and pCMV-(M)Pol, together with pCMV-ampho (a construct expressing the amphotropic MLV envelope from the CMV promoter, previously referred to as pHCMV-ampho-env [30]), were transfected into 293/LZRNL cells (293 cells stably expressing LZRNL [36], an MLV vector with the marker genes lacZ and neo [encoding neomycin resistance]) maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 0.1 mg of streptomycin/ml. For transfections, 106 293 cells were seeded in 60-mm-diameter plates. The next day, cells were transfected by the calcium phosphate coprecipitation method as described elsewhere (5), except that DNA was incubated with the cells for 8 h prior to changing of the medium and glycerol shock was not used. A total of 15 μg of DNA was used in each transfection. Equivalent amounts of the plasmids of interest were used in all experiments; if necessary, the total amount of DNA used was equalized by the addition of carrier plasmid DNA. Twenty-four hours posttransfection, the cell medium was changed. Approximately 60 hours posttransfection, virus was harvested for use in vector titer determinations and RT activity assays by collecting the medium, centrifuging it briefly to remove cell debris, and filtering it through a 0.45-μm-pore-size filter.
To determine the amount of RT activity associated with virus particles produced from these transfections, virus was pelleted from harvested conditioned medium by centrifugation as previously described (31). For each sample, 18 μl of virus pellet, prepared as noted above and resuspended in phosphate-buffered saline (PBS) to a volume of 30 μl, was incubated at 37°C for 60 min with 30 μl of RT buffer: 50 mM Tris-HCl (pH 8.3), 10 mM dithiothreitol, 1 mM MnCl2, 60 mM NaCl, 0.02 mM dTTP, 2.5 μCi of [3H]dTTP, 0.25% Nonidet P-40, and 5 μg (0.04 U) of poly(rA) · poly(dT)10. Thirty microliters of each sample was spotted onto DE81 paper (Whatman International) in duplicate and air dried. The filters were washed twice in 5% Na2HPO4 for 15 min, twice in H2O for 15 min, and once in 100% ethanol for 10 min. The filters were dried, and incorporated 3H was measured by scintillation counting with a Beckman LS6500 scintillation counter. RT activity was detected only in virus from cells that had been transfected with constructs that expressed both Gag and Pol (Table 1), indicating that export of RT to the culture supernatant is dependent on association of Pol with virion cores. The amount of RT activity present in the virions produced from separate MLV Gag- and Pol-expressing plasmids was approximately one-third that of the wild-type Gag-Pol expression level.
TABLE 1.
Virion-associated RT activity and titer of infectious virus upon separate MLV Gag and MLV Pol expressiona
| Viral proteins expressed in cells | Virion-associated RT activity (cpm) | Vector titer/ml | Relative vector titer per equivalent RT activity |
|---|---|---|---|
| None | 24 ± 2 | 0 | 0 |
| MLV Gag-Pol (w.t.) | 2,160 ± 463 | 7,400 ± 1,400 | 1.00 |
| MLV Gag | 19 ± 4 | 0 | 0 |
| MLV Pol | 30 ± 1 | 0 | 0 |
| MLV Gag plus MLV Pol | 840 ± 72 | 850 ± 280 | 0.35 |
293/LZRNL cells (293 cells stably expressing the MLV vector LZRNL) were transfected with pCMV-ampho and constructs expressing MLV Gag and MLV Pol as indicated. Supernatant was collected from cells and either centrifuged to pellet virions prior to RT assay or used to infect 208F cells. Infected cells were fixed, stained with X-Gal, and scored by microscopy for blue foci to determine the vector titer. When normalized for the amount of RT activity present in virus preparations, the MLV Gag plus MLV Pol virus was one-third as infectious as the wild-type virus. The mean titers ± standard deviations of four trials are shown. w.t., wild type.
For infections, virus-containing supernatant in the presence of 8 μg of Polybrene/ml was added to 1.4 × 105 208F cells, maintained in the same medium as 293 cells, plated the day before in 60-mm-diameter dishes. Thirty-six to 48 h postinfection, the vector titer was determined by fixing the cells and assaying for β-galactosidase activity. Cells infected with the LZRNL vector were expected to form blue-staining foci due to expression of the lacZ marker. Infected cells were washed in PBS and then fixed with 1.25% glutaraldehyde for 30 min at room temperature. The cells were then washed four times in PBS and incubated overnight at 37°C with PBS containing 50 mM ferricyanide, 50 mM ferrocyanide, 400 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml, and 1 mM MgCl2. The stain was removed, the cells were washed with PBS, and blue foci were scored by microscopy. The relative titer of infectious virus produced by separate expression of Gag and Pol was 12% (850 of 7,400 foci were blue) compared to the titer of virus resulting from wild-type Gag-Pol expression (Table 1). When normalized for the amount of RT activity present in the supernatant, however, the relative infectivity of the Gag- plus Pol-produced virus was approximately one-third that of the wild-type virus. Similar titers were seen when the LZRNL vector titer was assayed by determination of G418 resistance, demonstrating that the vector produced in this system was capable of forming a stably integrated provirus, thereby completing the replication cycle.
These results demonstrate that infectious virus can be formed from the separate Gag- and Pol-expressing plasmids. To simplify analysis of virions produced from separate Gag and Pol proteins, transient-transfection experiments using pCMV-(M)Gag/Pol, pCMV-(M)Gag, and pCMV-(M)Pol, but omitting a packageable viral vector and envelope glycoprotein, were performed. This approach to studying virus assembly is feasible because neither packageable viral RNA nor envelope glycoprotein is necessary to produce virus-like particles when wild-type gag and pol genes are expressed in cells (31, 32). As described above, a total of 15 μg of DNA containing equivalent amounts of each plasmid of interest was used; when necessary, the total amount of DNA was held constant by addition of carrier DNA. Sixty to 72 h posttransfection, culture supernatant was collected and, as before, virus was pelleted by centrifugation. Cell lysates of the transfected cells also were prepared by washing cells four times in PBS, centrifuging briefly to pellet the cells, resuspending the cells in 100 μl of PBS, and lysing them by repetitive cycles of freezing and thawing.
RT assays were performed on both cell lysates and pelleted virions. The results (Table 2) show that under these transfection conditions, pCMV-(M)Pol efficiently expressed MLV RT in transfected cells and that pelletable RT activity was detected in the cell supernatant only when MLV Gag and Pol were coexpressed in cells, again suggesting an association between free Pol protein and Gag virion cores. In these cotransfection experiments, the amount of pelletable RT obtained from Gag and Pol coexpression was similar to that from the wild-type Gag-Pol, indicating that under these conditions the association of independently produced Gag and Pol is reasonably efficient. The difference in the pelleted RT activities obtained in these cotransfection experiments and the multiple-plasmid transfection experiments (Table 1) might reflect the higher efficiency of DNA couptake when only two plasmids are used (data not shown) and the larger amount of Gag- and Pol-expressing plasmids used in the cotransfection experiments.
TABLE 2.
RT activity and p24 levels in cell lysates and virion pellets after transfection of Gag- and Pol-expressing plasmidsa
| Viral protein(s) expressed in cells | RT activity (cpm) in:
|
Relative virion RT/MLV Gag content | p24 level (pg/ml) in:
|
||
|---|---|---|---|---|---|
| Cell lysate | Virion pellet | Cell lysate | Virion pellet | ||
| MLV Gag and MLV Pol vs MLV Gag-Polb | |||||
| None | 70 ± 59 | 132 ± 19 | 0.00 | <25 | <25 |
| MLV Gag-Pol (w.t.)d | 570 ± 24 | 28,543 ± 2,700 | 1.00 | N/Ae | N/A |
| MLV Gag | 85 ± 36 | 123 ± 13 | 0.00 | N/A | N/A |
| MLV Pol | 619 ± 78 | 140 ± 23 | 0.00 | <25 | <25 |
| MLV Gag plus MLV Pol | 873 ± 106 | 34,213 ± 2,620 | 0.76 | N/A | N/A |
| HIV Gag and MLV Pol vs MLV Gag-Polc | |||||
| HIV Gag | 42 ± 19 | 137 ± 15 | N/A | 129 ± 87 | 68 ± 31 |
| HIV Gag plus MLV Pol | 622 ± 118 | 7,773 ± 1,120 | N/A | 178 ± 31 | 298 ± 37 |
293 cells were transfected with plasmids expressing the proteins indicated. Cell lysates and virus pellets were prepared approximately 60 h posttransfection. The data represent the averages of values from at least three separate trials ± standard deviations.
To normalize the amount of RT activity in virus preparations to the amount of MLV Gag in the same preparation, the ratio of RT activity to band intensity of viral protein seen by Western blot analysis (Fig. 3) was calculated.
Transfections were done in parallel with those described above. A p24 ELISA assay was performed to quantitate HIV Gag expression and release from cells. p24 values are for samples diluted 1:12,500 prior to ELISA analysis; 25 pg/ml was the cutoff value for the linear range of the assay.
w.t., wild-type.
N/A, not applicable.
To examine Gag expression and virion content, Western blot analysis of transfected-cell lysates and of pelleted virions was performed. Twenty microliters of virus pellet or cell lysate resuspended in PBS was used for each sample. After 6 μl of loading buffer (Tris-HCl, glycerol, sodium dodecyl sulfate [SDS], dithiothreitol, bromophenol blue [1]) was added to each sample, the samples were boiled for 10 min and then electrophoresed through an SDS–10% polyacrylamide gel. Following electrophoresis, the proteins were transferred to an Immobilon-P membrane (Millipore Corp., Bedford, Mass.). The membrane was incubated overnight at 4°C, in blocking reagent (5% nonfat dry milk–2% bovine serum albumin in Tris-buffered saline [TBS]). The membrane was incubated for 2 h with primary antibody (goat anti-MLV serum, lot no. 76S000127 and 77S000186; Quality Biotech, Camden, N.J.) diluted 1:1,000 in blocking reagent which had been diluted 1:1 with TBS. The membrane was washed three times with blocking reagent and incubated for 1 h with secondary antibody (horseradish peroxidase [HRP]-conjugated donkey anti-goat immunoglobulin G; Santa Cruz Biotechnology, Santa Cruz, Calif.) diluted 1:1,000 in blocking agent which had been diluted 1:1 in TBS. The membrane was washed once in blocking reagent, once in 0.5% Tween–TBS, and twice in TBS (10 min per wash). Secondary antibodies bound to viral proteins were detected by using an enhanced chemiluminescence kit (ECL; Amersham) in accordance with the manufacturer’s instructions.
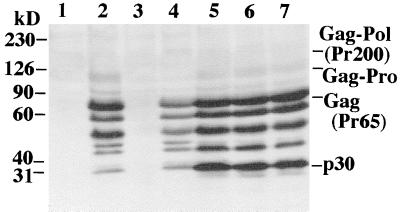
As can be seen in Fig. 2, pCMV-(M)Gag (lane 2) and the wild-type Gag-Pol construct (lane 4) express and process MLV Gag similarly. Fully processed and partially processed protein intermediates are evident. The anti-MLV serum used does not recognize the Pol protein (lane 3). Cell lysates from three representative trials of cells cotransfected with pCMV-(M)Gag and pCMV-(M)Pol are shown in lanes 5 to 7. The Gag-protease precursor can be seen in cells transfected with pCMV-(M)Gag (lanes 2 and 5 to 7). The wild-type MLV Gag-Pol precursor (Pr200) can be seen only in lane 4 (and is more easily visualized with a longer exposure of the membrane to film) at a predicted smaller amount compared to the processed proteins. Also, with a longer exposure, the p15 (MA) band can be seen in lanes expressing Gag. The amount of Gag in lanes 5 to 7 is slightly increased compared to that of the wild-type Gag-Pol.
FIG. 2.
Western blot analysis of cell lysates expressing MLV Gag and Pol. Lysates of cells transfected with the indicated plasmids were subjected to SDS-polyacrylamide gel electrophoresis and blotted, and the blots were incubated with anti-MLV serum and probed with an HRP-conjugated secondary antibody. Gag precursor, fully processed, and partially processed proteins can be seen. Cells were transfected with no DNA (lane 1), pCMV-(M)Gag (expresses MLV Gag-protease [Gag-Pro]) (lane 2), pCMV-(M)Pol (expresses MLV Pol) (lane 3), pCMV-(M)Gag/Pol (expresses wild-type MLV Gag and Gag-Pol) (lane 4), or pCMV-(M)Gag and pCMV-(M)Pol (lanes 5 to 7). The positions of molecular mass markers and selected viral proteins are indicated. kD, kilodaltons.
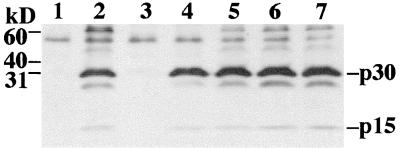
Western blot analysis of pelleted virions, using the same antibodies as employed for the cell lysate analysis, demonstrated that the protein contents of the various samples were similar (Fig. 3). The Gag plus Pol samples (lanes 5 to 7) did have a slightly higher protein content (confirmed and quantitated by densitometric analysis of band intensity [data not shown]) than those with wild-type Gag-Pol (lane 4), suggesting that more virus particles were produced from these transfected cells. The approximately 60- and 50-kDa bands evident in lanes 2 and 5 to 7 represent partially processed Gag precursor (Pr65). They may be somewhat more evident in these lanes than in the lane with wild-type Gag-Pol (lane 4) because of the increased amount of virus protein present (faint bands become evident in lane 4 upon longer exposure of the membrane to film). However, it is possible that these bands represent an increase in the relative amount of partially processed Gag produced by pCMV-(M)Gag within virions due to a decreased efficiency of Gag precursor processing by the viral protease compared to protease activity in the context of wild-type Gag-Pol. A similar situation was reported in HIV virions resulting from trans complementation with Vpr-RT and Vpr-IN fusion proteins (35). It is conceivable that subtle alterations in protein conformation which may occur when virions are not formed from wild-type Gag-Pol precursors could affect protease activity or accessibility to precursor cleavage sites.
FIG. 3.
Western blot analysis of virions. SDS-polyacrylamide gel electrophoresis analysis of protein isolated from pelleted virions and probed with anti-MLV serum and an HRP-conjugated secondary antibody. Fully processed and partially processed Gag proteins can be seen. Virions were isolated from the supernatant of cells transfected with no DNA (lane 1), pCMV-(M)Gag (expresses MLV Gag-protease) (lane 2), pCMV-(M)Pol (expresses MLV Pol) (lane 3), pCMV-(M)Gag/Pol (expresses wild-type MLV Gag and Gag-Pol) (lanes 4), or pCMV-(M)Gag and pCMV-(M)Pol (lanes 5 to 7). The positions of molecular mass markers and viral proteins are indicated. kD, kilodaltons.
Densitometric analysis of Western blot band intensity was used to normalize the amounts of RT activity in the virus pellets of the wild-type Gag-Pol and Gag plus Pol samples relative to the amounts of Gag present in these pellets. The ratios of pelletable RT activity to densitometric units of p15 and p30 band intensity for the pelleted virus samples were calculated and are shown in Table 2. When normalized for the amount of Gag present in virion preparations (a reflection of the number of virions formed and released), the amount of RT in the Gag plus Pol virus pellet is three-fourths that of wild-type Gag-Pol, indicating that free Pol associates with Gag at a slightly lower level than when Pol is incorporated into virions as the Gag-Pol precursor. It is important to remember that this value represents the average for the population of virions, not individual particles. It does not address the fact that there may be a heterogeneous population of particles, containing different numbers of RT molecules.
These results indicate that MLV Pol produced independently from the Gag-Pol precursor can associate with MLV Gag virion cores. In Western blot analyses of cell lysates, no Gag-Pol protein, which theoretically could result from recombination between the Gag- and Pol-expressing plasmids used in this system, was detected in cotransfected cells. As noted above, the wild-type Gag-Pol precursor (Pr200) was identified only in the lysate of cells transfected with the wild-type gag-pol construct. Longer exposure of the membrane to film failed to detect any Pr200 in cells transfected with separate Gag- and Pol-expressing constructs, though the Gag-protease precursor protein produced from pCMV-(M)Gag was readily apparent. In addition, further experiments (data not shown) involving serial dilution of cell lysate containing wild-type Gag-Pol with cell lysate from control cells demonstrated an ability to detect Pr200 even if the amount present was 1/10 to 1/20 of that present in the wild-type samples. Together, these observations indicate that recombination, leading to expression of wild-type protein, is not occurring at a high enough level in this system to account for the results seen. Also, the ability of MLV Pol to associate with a heterologous Gag protein (see below), in which there is no sequence identity that might support recombination, further supports the observation that Pol can associate with virion cores independently of the Gag-Pol precursor.
MLV Pol can associate with HIV Gag virion cores.
In the above-described system, MLV Pol was able to associate with virion cores composed of MLV Gag. If also observed with Gag from other retroviruses, this ability could facilitate the generation of chimeric retroviral vector systems by allowing substitution of whole retroviral genes between viruses. In particular, an MLV/HIV chimeric vector and packaging system may offer unique opportunities to study virus assembly and to characterize further the required elements that allow HIV to infect nondividing cells (7, 14, 19). If the ability to infect nondividing cells could be transferred to an MLV-based vector, such a vector might offer advantages over the use of an HIV vector system in clinical applications. For these reasons, the methods described above were used to determine whether MLV Pol could associate with HIV Gag virion cores.
In addition to containing the gag, pol, and env genes, the HIV genome encodes a number of accessory proteins not found in MLV (21). Normally, HIV gag and pol expression is dependent on the viral Rev protein, which facilitates transport of viral RNA from the nucleus to the cytoplasm by overcoming the effects of negative regulatory elements present in gag-pol transcripts. Also, some of the HIV accessory proteins are incorporated into virions and play various roles in the viral replication cycle, though their roles and mechanisms of action are not all clear. To facilitate efficient Gag production and to avoid confounding variables due to the presence of accessory proteins, an HIV gag-pro construct containing silent mutations in negative regulatory elements was used to express Gag. This construct (p55BM13P5) has been shown to express and process Gag protein and to form virion cores in the absence of Rev (28). In the experiments described here, the HIV gag-pro coding region was expressed from the CMV promoter. Thus, this construct [pCMV-(H)Gag(13P5)] allows Gag expression and processing in the absence of all HIV accessory proteins and keeps the HIV Gag-MLV Pol experimental system as similar as possible to the MLV Gag-MLV Pol system.
293 cells were transfected with pCMV-(H)Gag(13P5) and pCMV-(M)Pol in parallel with the MLV Gag plus MLV Pol experiments. Cell lysates and virus pellets were prepared as described above for RT assay and protein analysis. HIV Gag expression and virion core production were quantitated by measuring p24 (CA) levels present in cell lysates and virus pellets, using a standard enzyme-linked immunosorbent assay (ELISA) performed by the University of California—San Diego Center for AIDS Research. Samples were prepared for analysis by diluting them 12,500-fold in a buffer containing 0.5% Triton X-100, pH 7.4.
The results (Table 2) show that when pCMV-(H)Gag(13P5) was introduced into cells, p24 was expressed and incorporated into virions that could be pelleted by centrifugation. Coexpression of MLV Pol in cells did not adversely affect HIV Gag expression. Coexpression of HIV Gag and MLV Pol resulted in RT activity in cells at a level equivalent to that achieved when MLV Pol was expressed alone (Table 2), showing that expression of HIV Gag does not inhibit MLV Pol expression or activity.
MLV RT activity also was detected in pelleted virions, at about one-fourth the level of wild-type MLV Gag-Pol, suggesting that MLV Pol could associate with HIV Gag cores and that the RT retained enzymatic activity (Table 2). A direct comparison of the MLV RT activity in these virions and of the HIV RT activity in native virions is not possible for two reasons. First, it is not possible to express HIV RT efficiently in the absence of at least some of the HIV accessory proteins (28); the presence of these HIV proteins would confound analysis of experiments designed to compare the ability of MLV Pol to associate with MLV or HIV Gag, since MLV does not encode accessory proteins. More importantly, the optimal assay conditions for HIV RT and MLV RT differ (8), precluding a direct comparison of the two enzyme activities.
Attempts to determine if virus containing HIV Gag and MLV Pol is infectious were inconclusive. In its simplest form, a viral vector used in such a study would have to be composed of both HIV and MLV cis-acting sequences. Since the virion cores are composed of HIV Gag, the vector must contain the HIV encapsidation sequence (22) recognized by the gag-encoded NC protein. In addition, since the enzymatic activities of such a virus would be supplied by MLV Pol, other cis-acting sequences, including the primer binding site and att sites, would need to be of MLV origin. Attempts were made to propagate such a vector, using the HIV Gag plus MLV Pol system, by transfecting pCMV-(H)Gag(13P5), pCMV-(M)Pol, pCMV-ampho, and a vector construct into 293 cells, infecting 208F cells with the collected supernatant, and assaying for vector propagation by a focal β-galactosidase assay. However, the chimeric vectors studied so far have shown poor gene expression from the viral long terminal repeat, limiting their utility. Because the development of a chimeric vector containing cis-acting sequences from more than one type of retrovirus is liable to be difficult, and no appropriate alternative vector exists, the infectivity of the virions produced by HIV Gag and MLV Pol cannot be assessed at this time. Nonetheless, the above results show that in this system, MLV Pol can associate with heterologous HIV cores and the viral polymerase retains enzymatic activity, as assessed by RT assay.
The precise steps and timing of virion assembly, processing, and maturation are complex and incompletely understood. The main determinants driving core formation involve interactions among at least the CA domains (3, 10, 15, 26, 29) (with possible MA and NC contributions [4, 9, 37]) of the Gag precursor protein, which presumably associate to form the virion core. In type C retroviruses and lentiviruses, it is believed that Pol is incorporated into virions as part of the Gag-Pol precursor. Activation of the viral protease, most likely during core formation at the cell membrane for type C retroviruses, ensues, allowing processing of the Gag and Pol proteins (33). The RT and IN proteins may play roles in the timing of protease activation and the efficiency with which PR processes the Gag and Gag-Pol precursors (6). In addition, it is likely that during encapsidation of genomic viral RNA, RT and NC play roles in selection and annealing of the tRNA used to prime reverse transcription (2, 27), further illustrating the complex roles Gag and Pol play during virion assembly.
An experimental system that allows virion incorporation of HIV type 1 RT and IN that have been fused to Vpr, an HIV accessory protein normally incorporated into virus particles, has been described (12, 35). As assayed by the ability of the fusion proteins to trans complement RT and IN mutations in the HIV genome, Vpr-RT and Vpr-IN were able to mediate incorporation of RT and IN into virus particles, demonstrating an ability to separate HIV Pol assembly from function. In addition, a small amount of IN could be incorporated into virions even when not expressed as a Vpr fusion protein.
The experiments described herein do not address how Pol incorporation occurs during wild-type virus replication and do not contradict the as-yet-unconfirmed likelihood that Pol is normally incorporated as the Gag-Pol precursor. The results described herein, however, do show that to maintain appropriate RT activity, it is sufficient for MLV Pol to associate with virions but, as with HIV RT and IN, it is not necessary for incorporation to occur as part of the Gag-Pol protein. One interpretation of the results seen upon using the described system is that the RT activity in virions produced from separately expressed MLV Gag and Pol was lower than that produced from Gag-Pol expression in virions because independently synthesized Pol might not be incorporated into virus particles as efficiently or in the same stoichiometry as with Gag-Pol expression. The observed difference in virus infectivity may reflect the existence of a heterogeneous population of virions containing various amounts of RT. Alternatively, it may be that free Pol, compared to Gag-Pol, results in an altered molecular organization within virions (with regard to the viral genome, tRNA primer, and viral enzymes and structural proteins) that is subsequently reflected in decreased particle infectivity.
There are two possible explanations for the observation that functional Pol could associate with virion cores. One scenario is that Pol incorporation simply is a result of the experimental system used in this study, in which Gag and Pol were expressed independently of CMV promoters as opposed to the MLV long terminal repeat. It may be that in this artificial scenario, Pol nonspecifically associates with virion cores differently than it does during wild-type replication and that this difference is irrelevant to wild-type virus replication. Nevertheless, these results show that trans complementation is possible and demonstrate that at least some of MLV Pol’s enzymatic functions are not dependent on the protein being expressed and incorporated into virions as the Gag-Pol protein. Alteration of the wild-type MLV genome to allow separate gag and pol gene expression with no Gag-Pol production would permit further study to determine the relevance, if any, of these findings to the natural viral replication cycle.
Alternatively, it is possible that in this system Pol incorporation into particles is occurring via specific interactions between the free Gag and Pol molecules. Such interactions have been postulated to exist in spumaviruses, which incorporate free Pol into virus particles (in spumaviruses, no Gag-Pol precursor is produced [20]). If they exist for MLV and HIV, these interactions are likely to be at least partially conserved among retroviruses, as indicated by the incorporation of MLV Pol into HIV Gag cores. Although it is nearly certain that under wild-type conditions the major determinant of Pol incorporation into virions occurs via molecular interactions of the Gag CA domain of Gag and Gag-Pol (3, 10, 15, 26, 29), it is conceivable that there are other, minor interactions between Gag and Pol that are unmasked in this system when the more native interactions are prevented. Such interactions might assist in Gag-Pol incorporation into virions or promotion and stabilization of the preferred molecular organization within virions. If specific interactions among precursor molecules during retroviral particle assembly can be identified, it is foreseeable that inhibitors of such interactions, capable of interfering with the production of infectious virus from cells, might be developed. Further study will be necessary to determine if specific Gag and Pol interactions are occurring, to define their roles in virion formation, and to identify potential intermolecular PR or C-terminal NC interactions that might play roles in the association of free Pol with virion cores. In addition to providing information regarding virus structure and assembly, the development of an MLV/HIV chimeric vector system may also help to identify the mechanism of and minimal requirements for infection of nondividing cells. This information might allow transfer of the ability to infect nondividing cells to the MLV-based vectors systems, which have been more thoroughly studied, and accepted as relatively safe for clinical use, than the more recently developed lentivirus vectors (23, 25).
Acknowledgments
G.L.B. and L.Y. contributed equally to the work presented.
We thank J. Corbeil and the University of California—San Diego Center for AIDS Research for performing the p24 quantitation and G. N. Pavlakis for supplying the plasmid p55BM13P5.
G.L.B. was supported in part by a grant from the Bank of America-Giannini Foundation. These studies also were funded by DNAVEC Research, Inc., NIH grants HL53620 and DK49023, and the Charles H. and Anna S. Stern Foundation.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 2.Barat C, Schatz O, LeGrice S, Darlix J-L. Analysis of the interactions of HIV-1 replication primer tRNA3Lys with nucleocapsid protein and reverse transcriptase. J Mol Biol. 1993;231:185–190. doi: 10.1006/jmbi.1993.1273. [DOI] [PubMed] [Google Scholar]
- 3.Borsetti A, Öhagen Å, Göttlinger H G. The C-terminal half of the human immunodeficiency virus type 1 Gag precursor is sufficient for efficient particle assembly. J Virol. 1998;72:9313–9317. doi: 10.1128/jvi.72.11.9313-9317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowzard J B, Bennett R P, Krishna N K, Ernst S M, Rein A, Wills J W. Importance of basic residues in the nucleocapsid sequence for retrovirus Gag assembly and complementation rescue. J Virol. 1998;72:9034–9044. doi: 10.1128/jvi.72.11.9034-9044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchschacher G L, Jr, Panganiban A T. Human immunodeficiency virus vectors for inducible expression of foreign genes. J Virol. 1992;66:2731–2739. doi: 10.1128/jvi.66.5.2731-2739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukovsky A, Göttlinger H. Lack of integrase can markedly affect human immunodeficiency virus type 1 particle production in the presence of an active viral protease. J Virol. 1996;70:6820–6825. doi: 10.1128/jvi.70.10.6820-6825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubei A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin J M. Retroviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1767–1847. [Google Scholar]
- 9.Deminie C A, Emerman M. Incorporation of human immunodeficiency virus type 1 Gag proteins into murine leukemia virus virions. J Virol. 1993;67:6499–6506. doi: 10.1128/jvi.67.11.6499-6506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfman T, Bukovsky A, Öhagen Å, Höglund S, Göttlinger H G. Functional domains of the capsid protein of human immunodeficiency virus type 1. J Virol. 1994;68:8180–8187. doi: 10.1128/jvi.68.12.8180-8187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felsenstein K M, Goff S P. Expression of the gag-pol fusion protein of Moloney murine leukemia virus without gag protein does not induce virion formation or proteolytic processing. J Virol. 1988;62:2179–2182. doi: 10.1128/jvi.62.6.2179-2182.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher T M, III, Soares M A, McPhearson S, Hui H, Wiskerchen M, Muesing M A, Sham G M, Leavitt A D, Boeke J D, Hahn B H. Complementation of integrase function in HIV-1 virions. EMBO J. 1997;16:5123–5138. doi: 10.1093/emboj/16.16.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, DeWilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 14.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang M, Martin M A. Incorporation of Pr160gag-pol into virus particles requires the presence of both the major homology region and adjacent C-terminal capsid sequences within the Gag-Pol polyprotein. J Virol. 1997;71:4472–4478. doi: 10.1128/jvi.71.6.4472-4478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacks T, Power M D, Masiarz F R, Luciw P A, Barr P J, Varmus H E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988;331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- 17.Karacostas V, Wolffe E J, Nagashima K, Gonda M A, Moss B. Overexpression of the HIV-1 Gag-Pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993;193:661–671. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y-M, Tian C-J, Yu X-F. A bipartite membrane-binding signal in the human immunodeficiency virus type 1 matrix protein is required for the proteolytic processing of Gag precursors in a cell type-dependent manner. J Virol. 1998;72:9061–9068. doi: 10.1128/jvi.72.11.9061-9068.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Löchelt M, Flügel R M. The human foamy virus pol gene is expressed as a Pro-Pol polyprotein and not as a Gag-Pol fusion protein. J Virol. 1996;70:1033–1040. doi: 10.1128/jvi.70.2.1033-1040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luciw P A. Human immunodeficiency viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1881–1952. [Google Scholar]
- 22.McBride M S, Panganiban A T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Morrow C D. Overexpression of the gag-pol precursor from human immunodeficiency virus type 1 proviral genomes results in efficient proteolytic processing in the absence of virion production. J Virol. 1991;65:5111–5117. doi: 10.1128/jvi.65.9.5111-5117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poeschla E M, Wong-Staal F, Looney D J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 26.Reicin A S, Ohagen A, Yin L, Hoglund S, Goff S P. The role of Gag in human immunodeficiency virus type 1 virion morphogenesis and early steps of the viral life cycle. J Virol. 1996;70:8645–8652. doi: 10.1128/jvi.70.12.8645-8652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rong L, Liang C, Hsu M, Kleiman L, Petitjean P, de Rocquigny H, Roques B P, Wainberg M A. Roles of the human immunodeficiency virus type 1 nucleocapsid protein in annealing and initiation versus elongation in reverse transcription of viral negative-strand strong-stop DNA. J Virol. 1998;72:9353–9358. doi: 10.1128/jvi.72.11.9353-9358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider R, Campbell M, Nasioulas G, Felber B K, Pavlakis G N. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartzberg P, Colicelli J, Gordon M L, Goff S P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984;49:918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S, Cantwell M, Kipps T J, Friedmann T. Efficient infection of a human T-cell line and of human primary peripheral blood leukocytes with a pseudotyped retrovirus vector. Proc Natl Acad Sci USA. 1996;93:11842–11847. doi: 10.1073/pnas.93.21.11842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S, Murai F, Miyanohara A, Friedmann T. Noninfectious virus-like particles produced by Moloney murine leukemia virus-based retrovirus packaging cells deficient in viral envelope become infectious in the presence of lipofection reagents. Proc Natl Acad Sci USA. 1997;94:10803–10808. doi: 10.1073/pnas.94.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith A J, Cho M-I, Hammarskjöld M-L, Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into viruslike particles. J Virol. 1990;64:2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogt V M. Proteolytic processing and particle maturation. Curr Top Microbiol Immunol. 1996;214:95–132. doi: 10.1007/978-3-642-80145-7_4. [DOI] [PubMed] [Google Scholar]
- 34.Weaver T A, Talbot K J, Panganiban A T. Spleen necrosis virus gag polyprotein is necessary for particle assembly and release but not for proteolytic processing. J Virol. 1990;64:2642–2652. doi: 10.1128/jvi.64.6.2642-2652.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Liu H, Xiao H, Conway J A, Hunter E, Kappes J C. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J. 1997;16:5113–5122. doi: 10.1093/emboj/16.16.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L, Yee J K, Wolff J A, Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Qian H, Love Z, Barklis E. Analysis of the assembly function of the human immunodeficiency virus type 1 Gag protein nucleocapsid domain. J Virol. 1998;72:1782–1789. doi: 10.1128/jvi.72.3.1782-1789.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]