Abstract
Sarcocystis spp. cause pigeon protozoan encephalitis, a neuronal disease. A female pigeon exhibiting torticollis had a necrotic area in the cerebral hemisphere surrounded by lesions with perivascular cuffing, gliosis, granulomatous foci, and meningitis. Non-necrotic lesions were also observed in the brainstem. Intact and degenerative schizonts were observed within the neuropils and neurons in the lesions. Deoxyribonucleic acid (DNA) was extracted from paraffin-embedded brain tissues and genetically analyzed after gel electrophoresis to determine Sarcocystis spp. using specific primer sets for 28S ribosomal ribonucleic acid and internal transcribed spacer region-1. DNA sequencing confirmed a significant homology with S. calchasi. This is the first report of meningoencephalitis with malacia caused by S. calchasi in a rock pigeon in Japan.
Keywords: malacia, pigeon protozoan encephalitis, rock pigeon, Sarcocystis calchasi
Apicomplexan parasites of the genus Sarcocystis cause diseases in various species, though they have a highly pathogenic predilection for the central nervous system of wild birds [1, 10, 13]. Sarcosystis falcatula infection induces neuronal signs in free-ranging horned owls [16] and free-ranging eagles [17] in the USA. Olias et al. reported Sarcocystis spp., which exhibited only 51% nucleotide sequence similarity in the internal transcribed spacer region (ITS-1) with S. falcatula in Germany [7]. Sarcocystis spp. was identified as Sarcocystis calchasi, which causes pigeon protozoan encephalitis (PPE), a novel neuronal disease in domestic pigeons [8]. Spontaneous PPE associated with S. calchasi has also been reported in white-winged doves and domestic pigeons in the USA [3, 18] and in a rock pigeon in Japan [15]. Protozoa, schizonts, and merozoites have been observed in brain lesions associated with lymphohistiocytic, lymphoplasmacytic, or granulomatous encephalitis and meningoencephalitis [3, 7, 15, 18]. Herein, we encountered the first case of PPE in a rock pigeon with granulomatous meningoencephalitis and malacia in Japan, best to our knowledge, and confirmed S. calchasi caused the lesion.
A female wild rock pigeon (Columba livia) was picked from Western Tokyo because it exhibited torticollis. The pigeon was diagnosed with a poor prognosis and euthanized using pentobarbital anesthesia by a veterinarian. Macroscopically, the left cerebral hemisphere had collapsed and softened (Fig. 1). Tissue specimens including brain, lung, heart, spleen, and liver were fixed in 10% neutral buffered formalin, embedded in paraffin, and sliced into 3 µm-thick sections, which were then stained with hematoxylin-eosin (HE), and Luxol Fast Blue (LFB)-Periodic acid-Schiff (PAS). Immunohistochemical staining using anti-CD3 antibody, anti-paired box 5 (PAX5) antibody, anti-glial fibrillary acidic protein (GFAP) antibody, and anti-ionized calcium-binding adapter molecule 1 (Iba1) antibody was conducted on brain lesions to identify T and B lymphocytes, microglia, and astrocytes, respectively (Supplementary Table 1).
Fig. 1.
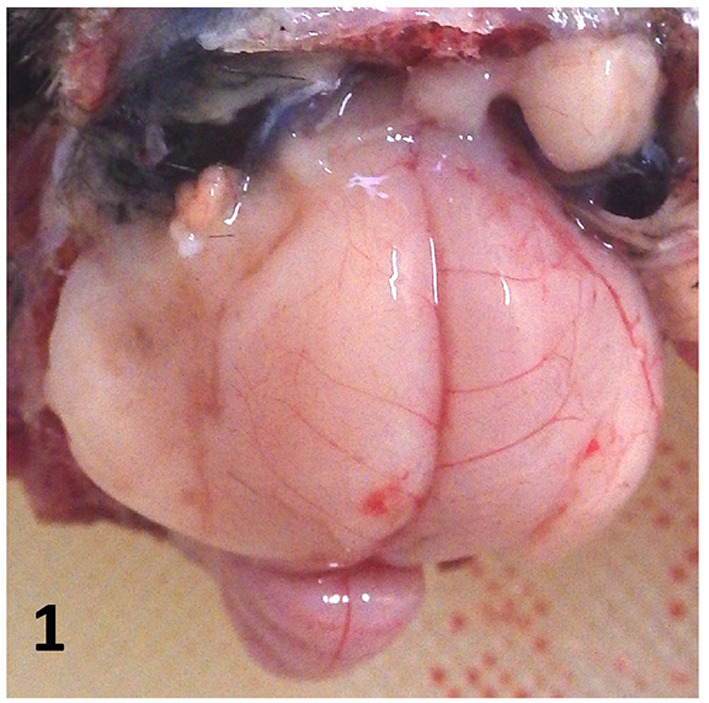
Macroscopic image of the brain. The left cerebral hemisphere is collapsed, suggesting cerebral malacia.
Histopathologically, we observed an extensive necrotic area in the left cerebral hemisphere in the HE- and LFB-PAS-stained sections, which were notably coarsened, with aggregation of fat granule cells and neovascularization in the central parts (Fig. 2). The necrotic area contained Iba-1-positive microglia/macrophages and GFAP-positive reactive astrocytes (Fig. 3). It was surrounded by relatively intact neuropils with perivascular cuffing, gliosis, and granulomatous lesions (Fig. 4). Perivascular cuffing and gliosis with heterophils and mononuclear cells were observed in the right cerebral hemisphere and brainstem. Mononuclear cells had infiltrated the meninges of the cerebrum and brainstem (Fig. 5). Iba1-positive microglia/macrophages and CD3-positive T lymphocytes were observed in the non-necrotic areas and meninges, and a smaller population of PAX5-positive B lymphocytes was also observed in these lesions (Supplementary Figs. 1–4).
Fig. 2.
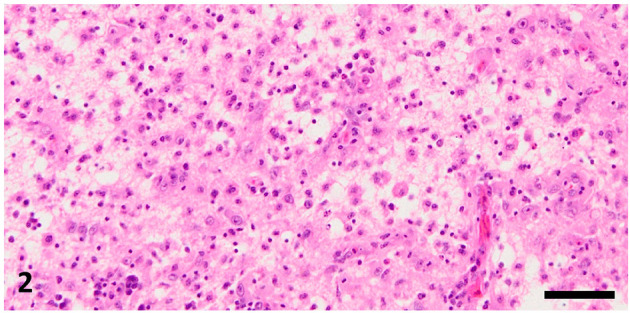
Necrotic area with fat granule cells in the left cerebral hemisphere. Hematoxylin and eosin stain, bar=50 µm.
Fig. 3.
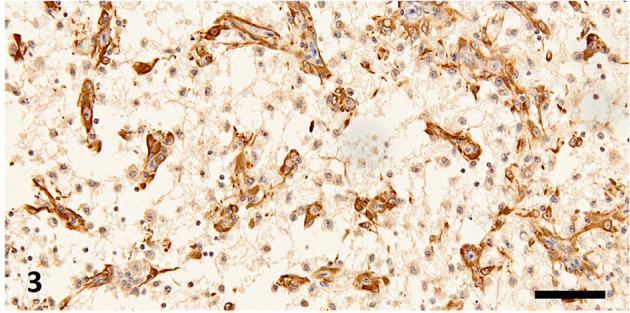
Necrotic area in the left cerebral hemisphere. Reactive astrocytes are observed in the necrotic neuropils. Immunohistochemistry for glial fibrillary acidic protein, bar=50 µm.
Fig. 4.
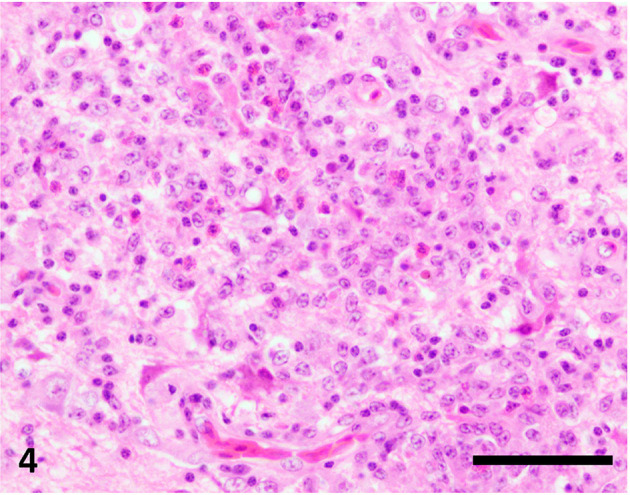
Granulomatous lesions in the right cerebral hemisphere. Microglia, lymphocytes, and heterophils are observed. Hematoxylin and eosin stain, bar=50 µm.
Fig. 5.
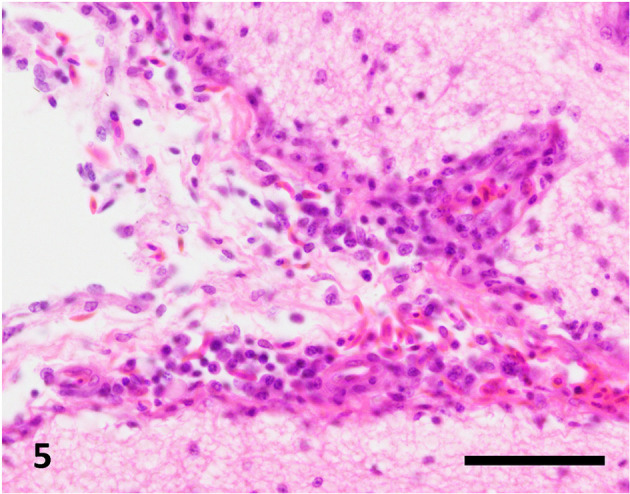
Mononuclear cell infiltration in the meninges of the right cerebral hemisphere. Hematoxylin and eosin stain, bar=50 µm.
Subsequently, we identified pathogens associated with neuropathological changes in HE- and LFB-PAS-stained sections. In the non-necrotic lesions, immature to mature schizonts (Fig. 6) were scattered within the neuropil and neurons. Intact schizonts were PAS-negative; however, degenerative schizonts contained PAS-positive granules observed in the neuropils and microglia. Protozoa were not identified in either malacia or severe granulomatous lesions.
Fig. 6.
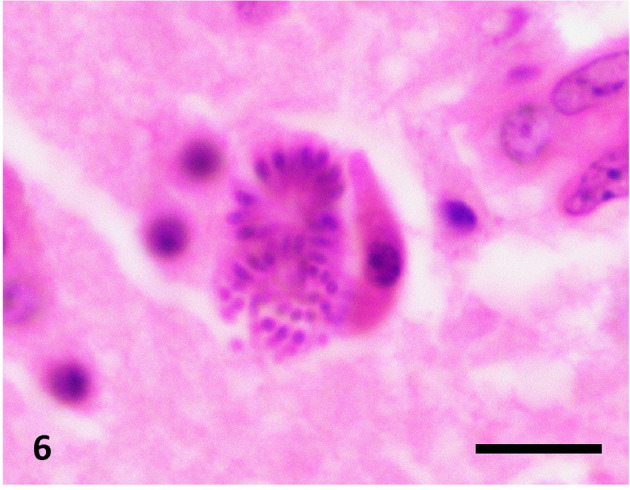
A schizont with merozoites. Hematoxylin and eosin stain, bar=20 µm.
Some sarcocysts and mononuclear cell infiltrates were observed in the heart. Mononuclear cell infiltrates and necrotic foci were observed in the liver, and diffuse mononuclear cell infiltration was observed in the kidney; however, protozoa were not identified in these organs.
We extracted deoxyribonucleic acid (DNA) from formalin-fixed paraffin-embedded (FFPE) sections of the cerebrum, cerebellum, midbrain, medulla oblongata, heart, kidneys, lungs, liver, and spleen using NucleoSpin® DNA FFPE XS (MACHEREY-NAGEL GmbH & Co., KG, Duren, Germany). The extracted DNA samples were amplified using specific primer sets for the ITS-1 region and complete D2 in the conserved regions of the 28S ribosomal ribonucleic acid (rRNA) of Sarcocystis spp. (Supplementary Table 2). For ITS1, polymerase chain reaction (PCR) was conducted using the primer pairs SCa1 and SCa2 for initial amplification, and the amplicons were amplified using the primer pair SCa1/SNca3 [11, 14]. For the 28S rRNA, PCR was conducted using the primer pairs SAD2F and SAD2R [18]. According to the manufacturer’s instructions, real-time PCR was performed using the StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). Specific bands of 350 and 220 bp were detected in the midbrain and cerebrum for the 28S rRNA and in the heart, medulla oblongata, midbrain, and cerebrum for ITS-1, respectively (Figs. 7and 8, Supplementary Figs. 5 and 6). Mixtures of the midbrain and cerebrum samples for 28S rRNA and mixtures of the heart and cerebrum samples for ITS-1 were isolated and purified for sequencing. Sequences were aligned using the MUSCLE algorithm implemented in the MEGA X software (Megasoftware. net). Evolutionary history was deduced using the maximum-likelihood method. The Tamura 3 parameter model with gamma distribution and the Hasegawa-Kishino-Yano model with gamma distribution were selected as the nucleotide substitution models that best fit the aligned sequence dataset. These models were used to construct phylogenetic trees for the 28S rRNA and ITS-1. A bootstrap test with 1,000 replicates was carried out to evaluate the robustness of the implied phylogeny [2, 4]. For each Toxoplasma gondii was selected as an outgroup for recombinant DNA sequencing. ITS-1 and 28S rRNA nucleotide sequences were deposited in the DNA Data Bank of Japan under accession numbers LC796271 and LC796270, respectively.
Fig. 7.

Agarose gel electrophoresis (2%) shows the expected sizes (350 bp) of PCR products from DNA using the primer pairs SAD2F/SAD2R for 28S rRNA. Lane M, DNA ladder; Lane 1, heart; Lane 2, medulla oblongata; Lane 3, midbrain; Lane 4, cerebrum; Lane 5, kidney; Lane 6, lung; Lane 7, liver; Lane 8, spleen. PCR, polymerase chain reaction; DNA, deoxyribonucleic acid; rRNA, ribosomal ribonucleic acid.
Fig. 8.
Phylogenetic analysis for 28S rRNA using the maximum likelihood method with 1,000 bootstrap replications. Values <50% are not shown. The nucleotide sequences of 28S rRNA detected in this study were contained within Sarcocystis calchasi and formed a separate branch within a group encompassing Sarcocystis spp. obtained from birds as intermediate hosts or definitive hosts. Toxoplasma gondii was included as the outer group. Bootstrap values are shown on the interior branch nodes, and scale bars indicate the number of substitutions per site. Individual Sarcocystis spp. are named as follows: accession number, definitive or intermediate host, and species of Sarcocystis (country). A red circle represents the Sarcocystis spp. identified as LC796270 in this study. rRNA, ribosomal ribonucleic acid.
Genetic analysis of the FFPE sections of the brain and heart, but not of other organs, detected two sets of specific bands by gel electrophoresis, and S. calchasi was identified by BLAST analysis of the nucleotide sequences of the PCR products (Figs. 7 and 8, Supplementary Figs. 5 and 6). Using the 350 bp sequences from 28S rRNA, BLAST analysis showed 99.68 and 99.66% similarity with S. calchasi from roller pigeons (KU220951) as well as white-winged doves and Eurasian collared doves (KT945019), respectively, and 99.68% similarity with Sarcocystis spp. from racing pigeons (FJ232949) (Fig. 8). Using the 220 bp sequences from ITS1, BLAST analysis showed 96.32% similarity with S. calchasi from Accipiter gentilis, Accipiter nisus (OQ848675), and racing pigeons (FJ232948) (Supplementary Fig. 6). Based on phylogenetic analysis, 350 bp sequences from 28S rRNA and 220 bp sequences from ITS1 formed one cluster with those of S. calchasi identified in Columbiformes as intermediate hosts. The 350 bp sequences from 28S rRNA clustered with 95% bootstrap support in the tree branch corresponding to S. calchasi, whereas the 220 bp sequences from ITS1 showed 52% bootstrap support within the same branch. Other Sarcocystis spp. grouped outside this branch. Hence, DNA sequencing of the rock pigeon examined here confirmed significant homology to S. calchasi. The sequences were classified into a group distinctively identified as S. calchasi, and distinct from S. falcatula and S. halieti. However, all three species are known to induce encephalitis and meningitis in the central nervous system, and myositis in the skeletal muscles [3, 6, 15,16,17]. Newcastle disease virus, avian herpesvirus, and avian influenza virus genes were not detected in the FFPE sections of the brain samples.
S. calchasi and S. falcatula are highly pathogenic to the brains of intermediate hosts, including pigeons, owls, and eagles [3, 7, 12, 15,16,17] (Supplementary Table 3). S. halieti can also migrate to the brain and induce cerebral damage in a juvenile owl [6]. These Sarcocystis spp. induced similar pathological changes, that is, lymphohistiocytic or granulomatous encephalitis and meningoencephalitis in the cerebrum, cerebellum, or brain stem [3, 6, 7, 12, 15,16,17]. However, some variations in pathological changes were identified, depending on the pathogen and the intermediate host. T lymphocyte-mediated inflammation was mainly observed in the brains of pigeons infected with S. calchasi [12, 15], whereas plasma cells and Mott cells were highly observed in eagles [3]. More severe damage, that is necrotizing encephalitis or malacia, was found in the brains of pigeons infected with Sarcocystis spp., which could potentially be S. calchasi [7], and in the great horned owl infected with S. falcatula [16]. Necrotic changes were also experimentally induced by oral inoculation with 102–4 sporocysts of S. calchasi derived from the northern goshawk [12]. In Japan, multifocal inflammatory changes, including mononuclear cell infiltration, perivascular cuffing, and meningitis, have been reported in the brain of a rock pigeon [15]; however, necrotic changes have not been reported. Although the location of the brain lesion has not been reported in detail, it has been reported to occur in the cerebellum and brainstem in spontaneous cases of S. calchasi infection [7]. It is considered rare for a lesion confined to the left cerebral hemisphere, as in the present case.
Previous studies reported neurological signs and brain lesions in detail in pigeons infected with S. calchasi in the USA, Germany, and Japan [3, 8, 15, 18]. The S. calchasi-infected pigeons exhibited neurological signs such as polyuria, diarrhea, torticollis, tremors, and paralysis, which appeared more severe than that of the pigeon with torticollis examined in this study. Previous studies also reported the biphasic pathogenesis of brain lesions in PPE, classified into early acute and late chronic stages [9, 10]. One group of infected pigeons died of multi-organ failure caused by many protozoan infections in the acute phase. In contrast, other groups of infected pigeons died of neuronal and cardiac dysfunction caused by a low number of protozoan infections in the chronic phase. Encephalitis develops slowly through a delayed-type hypersensitivity against schizonts and immature sarcocysts [5, 9, 10, 12]. Thus, the chronic stage is characterized by a marked upregulation of interferon-γ expression, accompanied by massive mononuclear cell infiltration, consistent with the T cell-mediated granulomatous lesions in the present case and other reports [12, 15]. The pathogenesis of meningoencephalitis with malacia caused by Sarcocystis spp. is not fully understood; therefore, the relationship between antigens derived from degraded protozoa and the progress of granulomatous encephalitis should be explored further in future studies.
POTENTIAL Conflicts of Interest
The authors have nothing to disclose.
Supplementary Material
REFERENCES
- 1.Dubey JP, Hamir AN. 2000. Immunohistochemical confirmation of Sarcocystis neurona infections in raccoons, mink, cat, skunk, and pony. J Parasitol 86: 1150–1152. doi: 10.1645/0022-3395(2000)086[1150:ICOSNI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa M, Kishino H, Yano T. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22: 160–174. doi: 10.1007/BF02101694 [DOI] [PubMed] [Google Scholar]
- 3.Hodo CL, Whitley DB, Hamer SA, Corapi WV, Snowden K, Heatley JJ, Hoffmann AR. 2016. Histopathologic and molecular characterization of Sarcocystis calchasi encephalitis in white-winged doves (Zenaida asiatica) and eurasian collared doves (Streptopelia decaocto), east-central Texas, USA, 2010–13. J Wildl Dis 52: 395–399. doi: 10.7589/2015-10-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier K, Olias P, Enderlein D, Klopfleisch R, Mayr SL, Gruber AD, Lierz M. 2015. Parasite distribution and early-stage encephalitis in Sarcocystis calchasi infections in domestic pigeons (Columba livia f. domestica). Avian Pathol 44: 5–12. doi: 10.1080/03079457.2014.978263 [DOI] [PubMed] [Google Scholar]
- 6.Maier-Sam K, Kaiponen T, Schmitz A, Schulze C, Bock S, Hlinak A, Olias P. 2021. Encephalitis associated with Sarcocystis halieti infection in a free-ranging little owl (Athene noctua). J Wildl Dis 57: 712–714. doi: 10.7589/JWD-D-20-00184 [DOI] [PubMed] [Google Scholar]
- 7.Olias P, Gruber AD, Heydorn AO, Kohls A, Mehlhorn H, Hafez HM, Lierz M. 2009. A novel Sarcocystis-associated encephalitis and myositis in racing pigeons. Avian Pathol 38: 121–128. doi: 10.1080/03079450902737847 [DOI] [PubMed] [Google Scholar]
- 8.Olias P, Olias L, Lierz M, Mehlhorn H, Gruber AD. 2010a. Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from the wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrowhawk (Accipiter nisus). Vet Parasitol 171: 7–14. doi: 10.1016/j.vetpar.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 9.Olias P, Gruber AD, Heydorn AO, Kohls A, Hafez HM, Lierz M. 2010b. Unusual biphasic disease in domestic pigeons (Columba livia f. domestica) following experimental infection with Sarcocystis calchasi. Avian Dis 54: 1032–1037. doi: 10.1637/9303-031110-Reg.1 [DOI] [PubMed] [Google Scholar]
- 10.Olias P, Gruber AD, Kohls A, Hafez HM, Heydorn AO, Mehlhorn H, Lierz M. 2010c. Sarcocystis species lethal for domestic pigeons. Emerg Infect Dis 16: 497–499. doi: 10.3201/eid1603.090860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olias P, Olias L, Krücken J, Lierz M, Gruber AD. 2011. High prevalence of Sarcocystis calchasi sporocysts in European Accipiter hawks. Vet Parasitol 175: 230–236. doi: 10.1016/j.vetpar.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 12.Olias P, Meyer A, Klopfleisch R, Lierz M, Kaspers B, Gruber AD. 2013. Modulation of the host Th1 immune response in pigeon protozoal encephalitis caused by Sarcocystis calchasi. Vet Res 44: 10. doi: 10.1186/1297-9716-44-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson EJ, Wünschmann A, Dubey JP. 2007. Sarcocystis sp.-associated meningoencephalitis in a bald eagle (Haliaeetus leucocephalus). J Vet Diagn Invest 19: 564–568. doi: 10.1177/104063870701900519 [DOI] [PubMed] [Google Scholar]
- 14.Trupkiewicz JG, Calero-Bernal R, Verma SK, Mowery J, Davison S, Habecker P, Georoff TA, Ialeggio DM, Dubey JP. 2016. Acute, fatal Sarcocystis calchasi-associated hepatitis in Roller pigeons (Columba livia f. dom.) at Philadelphia Zoo. Vet Parasitol 216: 52–58. doi: 10.1016/j.vetpar.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 15.Ushio N, Watanabe K, Chambers JK, Shibato T, Nakayama H, Uchida K. 2015. Sarcocystis calchasi encephalitis in a rock pigeon. J Vet Med Sci 77: 1523–1526. doi: 10.1292/jvms.15-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wünschmann A, Rejmanek D, Cruz-Martinez L, Barr BC. 2009. Sarcocystis falcatula-associated encephalitis in a free-ranging great horned owl (Bubo virginianus). J Vet Diagn Invest 21: 283–287. doi: 10.1177/104063870902100223 [DOI] [PubMed] [Google Scholar]
- 17.Wünschmann A, Rejmanek D, Conrad PA, Hall N, Cruz-Martinez L, Vaughn SB, Barr BC. 2010. Natural fatal Sarcocystis falcatula infections in free-ranging eagles in North America. J Vet Diagn Invest 22: 282–289. doi: 10.1177/104063871002200222 [DOI] [PubMed] [Google Scholar]
- 18.Wünschmann A, Armien AG, Reed L, Gruber AD, Olias P. 2011. Sarcocystis calchasi-associated neurologic disease in a domestic pigeon in North America. Transbound Emerg Dis 58: 526–530. doi: 10.1111/j.1865-1682.2011.01254.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



