Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1), a complex retrovirus, encodes a hydrophobic 12-kD protein from pX open reading frame (ORF) I that localizes to cellular endomembranes and contains four minimal SH3 binding motifs (PXXP). We have demonstrated the importance of ORF I expression in the establishment of infection and hypothesize that p12I has a role in T-cell activation. In this study, we tested interleukin-2 (IL-2) receptor expression, IL-2-mediated proliferation, and Jak/Stat activation in T-cell lines immortalized with either wild-type or ORF I mutant clones of HTLV-1. All cell lines exhibited typical patterns of T-cell markers and maintained mutation fidelity. No significant differences between cell lines were observed in IL-2 receptor chain (α, β, or γc) expression, in IL-2-mediated proliferation, or in IL-2-induced phosphorylated forms of Stat3, Stat5, Jak1, or Jak3. The expression of ORF I is more likely to play a role in early HTLV-1 infection, such as in the activation of quiescent T cells in vivo.
Human T-cell lymphotropic virus type 1 (HTLV-1) is a complex retrovirus associated with adult T-cell leukemia/lymphoma and a variety of immune system-mediated diseases (10, 12, 14a). The virus contains, in addition to gag, pol and env genes, a regulatory region named pX that encodes several proteins from four open reading frames (ORFs), including Tax and Rex (4, 19, 30). Tax is a 40-kD phosphoprotein encoded by ORF IV that activates transcription from the viral promoter as well as from numerous cellular genes, including interleukin-2 (IL-2) and the IL-2 receptor (IL-2R) α chain (22). Tax-mediated activation of cellular cytokine and immediate-early genes, as well as inhibition of cell cycle regulatory proteins such as p53 and p16, is thought to contribute to the transforming potential of the virus (14a). The ORF III gene product, Rex, is a 27-kD phosphoprotein that regulates the cytoplasmic accumulation of unspliced and singly spliced viral mRNA and possibly cellular messages such as IL-2Rα (10, 12). Much less is known about the function of pX ORFs I and II.
The pX ORF I encodes conserved 152- and 99-amino-acid hydrophobic proteins known as p27I and p12I (18, 19). However, only p12I has been detected in eukaryotic expression systems, in which it is localized to endomembranes (18). Using the ACH molecular clone of HTLV-1, we have examined the role of ORF I in viral replication (6, 29). Abrogation of ORF I expression has no detectable effects on the ability of the virus to infect or immortalize primary cells in vitro (6, 9, 29). In vitro, however, peripheral blood mononuclear cells (PBMC) are cultured in the presence of mitogen and exogenous IL-2, which may obviate the need for any activating effects of p12I. In HeLa/Tat cells, it was shown that overexpressed p12I associated with IL-2R β and γ chains (24). We have shown ORF I expression to be critical for optimal infectivity of the virus in a rabbit model (6) and hypothesize that p12I influences early activation of T cells, allowing efficient transmission of the virus.
HTLV-1-immortalized cells are initially IL-2 dependent but may acquire independence from exogenous IL-2 supplementation over many months in culture (16). This IL-2 independence corresponds with gradual, constitutive activation of the Jak/Stat signaling proteins in all fully transformed HTLV-1-infected T-cell lines, as well as in adult T-cell leukemia/lymphoma cells (23, 31, 33). To test the effects of HTLV-1 ORF I expression, we examined IL-2R expression, basal and IL-2 responsive proliferation, and Jak/Stat activation in T-cell lines immortalized with either wild-type or mutant molecular clones of HTLV-1 lacking expression of ORF I.
Normal, uninfected PBMC were maintained in supplemented RPMI medium with 15% fetal bovine serum (FBS) and 10 U of human IL-2 (hIL-2) per ml (complete media) and were stimulated with 2 μg of phytohemagglutinin (PHA) per ml 72 h prior to electroporation (27). PBMC were transfected using 10 μg of the HTLV-1 molecular clone ACH or ACH.p12, a construct in which a PstI site corresponding to the splice acceptor for the third exon of ORF I has been deleted (6, 7, 17, 29). After continuous growth for 6 months, selected cultures were expanded in the presence of 10 U of hIL-2 per ml for 12 to 18 months (Table 1). Three lines, ACH.3, ACH.MR, and ACH.p12.3, became senescent after 18 to 24 months in culture. Immediately prior to experiments, cell lines were assayed for production of viral p19 matrix antigen by enzyme-linked immunosorbent assay (ELISA) (Cellular Products). MT-2 cells were grown as previously described (14).
TABLE 1.
Cell surface CD receptor expression of HTLV-I-immortalized PBMC
| Cell Line | Date transfected | % of cells positivea
|
||
|---|---|---|---|---|
| CD3 | CD4 | CD8 | ||
| ACH.1 | May 1997 | 98 | 11 | 68 |
| ACH.2 | February 1997 | 100 | 2 | 100 |
| ACH.3 | May 1997 | 96 | 89 | 15 |
| ACH.MR | February 1996 | 96 | 99 | 1 |
| ACH.p12.1 | February 1997 | 99 | 4 | 100 |
| ACH.p12.3 | May 1997 | 96 | 21 | 95 |
| ACH.p12.4 | May 1997 | 98 | 1 | 0 |
| ACH.p12.MR | February 1996 | 18 | 5 | 42 |
Results are expressed as the percent of positive cells determined by flow cytometric evaluation of cells directly labeled with PE-conjugated monoclonal antibodies to CD3, CD4, and CD8. 10,000 events were collected.
To confirm the ΔPstI mutation in each of the ACH.p12I cell lines, genomic DNA was harvested by affinity column separation (QIAamp; Qiagen) and examined for the presence of HTLV-1 provirus by PCR with a primer pair specific for the HTLV-1 pX ORF I region (6555 and 7492) (6). The amplified products were digested with PstI, which was predicted to yield fragments of 760 and 178 bp in the wild type but not in the ACH.p12I DNA. Products were separated in a 1.5% agarose gel and stained with ethidium bromide.
For detection of the p12I message in cell lines, total mRNA was harvested by affinity column separation (RNeasy; Qiagen) and was reverse transcribed using Moloney murine leukemia virus reverse transcription (Boehringer Mannheim). Resulting cDNA was amplified as described above using the primer pair RPX3 and IK4 (corresponding to nucleotides 5094 and 6876, respectively) which spanned the second ORF I splice junction and thus yielded a product of 232 bp, corresponding to the doubly spliced pX-Rex-ORF I transcript (6, 19). Primers that amplified a 183-bp transcript of the abl gene were used as a control (6).
Surface membrane receptor expression was determined by direct labeling of lymphocytes with fluorescein isothiocyanate-conjugated monoclonal antibodies against CD3 (UCHT-1; Sigma), HLA class I (W6/32; Sigma), or HLA-DR (HK14; Sigma), or with phycoerythrin (PE)-conjugated monoclonal antibodies against CD4 (Q4120; Sigma), CD8 (UCHT-4; Sigma), IL-2Rβ (Mik-β3; Pharmingen), or IL-2Rγ (TUGh4; Pharmingen). Expression of IL-2Rα and HTLV-1 Env was determined by labeling of lymphocytes with unconjugated monoclonal antibodies 22722.2 (R&D Systems) or IC11 (28), respectively, and followed with PE-conjugated goat anti-mouse immunoglobulin G polyclonal antibody (Sigma). Fluorescence was measured using a Coulter Epics Elite flow cytometer and was analyzed using EPICS Elite software, version 4.01 (Coulter Corp.). Group mean channel fluorescence between ACH- and ACH.p12I-transfected lines were statistically compared using Student’s t test.
To measure IL-2 responsiveness, cells were rested in complete RPMI medium without IL-2 overnight (approximately 16 h) prior to plating 100 μl of cells (106/ml) in duplicate wells of a 96-well plate containing varying concentrations of IL-2 (0 to 40 U/ml). Immediately after plating cells (baseline samples) or after 72 h in culture, cell proliferation was monitored by a tetrazolium dye-based assay (MTS; Promega). Data were expressed as fold increase in absorbance of 72-h samples compared to baseline samples. Group means were statistically compared using Student’s t test. Endogenous secretion of IL-2 by cell lines was measured by ELISA (R&D Systems).
To monitor Jak/Stat activation, cells were rested overnight in RPMI medium containing 1% FBS prior to stimulation. Prior to harvest, cells were plated in complete RPMI medium containing 15% FBS in 6-well plates in the presence or absence of 2 nM hIL-2. After a 15-min incubation, cells were washed, pelleted, and lysed by suspension at a concentration of 2.5 × 107/ml in standard radioimmunoprecipitation assay lysis buffer on ice for 2 h. Lysates were centrifuged, and 30 μg of supernatant protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis prior to transfer to nitrocellulose membranes and probing with antibodies against Stat3 (New England Biolabs), Stat5 (C-17; Santa Cruz Biotechnology), or phosphotyrosine-specific motifs of Stat proteins (phospho-Stat5 from Upstate Biotechnology and phospho-Stat3 from New England Biolabs). Development of immunoblots was performed with a chemiluminescent detection system according to the manufacturer’s protocol (New England Biolabs).
For immunoprecipitation, 100 to 300 μg of precleared protein was incubated at 4°C overnight with protein A-Sepharose beads conjugated with anti-Stat or anti-Jak polyclonal antibodies: Stat5b (C-17), Jak1 (HR-785), Jak3 (C-21) (Santa Cruz). Proteins were eluted from washed beads and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis prior to transfer and probing with antiphosphotyrosine antibody (4G10; Upstate Biotechnology). The nitrocellulose membranes were stripped and reprobed with antibodies against Stat5, Jak1, or Jak3.
To determine the effect of ORF I on IL-2R expression, IL-2-responsive proliferation, and Stat activation, eight cell lines were developed. Four were transfected with ACH and four with ACH.p12I. Upon outgrowth of cells in the presence of IL-2 (approximately 6 months posttransfection) and again immediately prior to further experiments (approximately 18 months posttransfection), the fidelity of the ΔPstI mutation in the ACH.p12I-transfected lines was examined by PCR. Proviral sequences were amplified by PCR from genomic DNA of all eight lines (Fig. 1). The 938-bp HTLV-1 ORF I-specific amplicons from all ACH lines were digested by PstI, whereas the DNA fragment amplified from ACH.p12I lines failed to digest, consistent with abrogation of the PstI site that coincides with the ORF I coding the exon splice acceptor (Fig. 1A). Compatible with the preservation of this mutation, the ACH.p12 lines failed to express ORF I (Fig. 1B). HTLV-1 p19 production was measured in cell culture supernatants by ELISA. Consistent with our previous studies, all eight lines produced abundant amounts of p19 (greater than 200 pg/ml) (6, 29).
FIG. 1.
Conservation of the ΔPstI mutation and absence of ORF I expression in PBMC immortalized with ACH.p12I. (A) PCR amplification of genomic DNA from ACH- and ACH.p12I-immortalized PBMC, HTLV-1-transformed MT-2, and naive PBMC. The native (−) or PstI-digested (+) 938-bp HTLV-1 ORF I/II-specific product is present in HTLV-1-positive cells and lacks the PstI site corresponding to the ORF I exon 3 splice acceptor site in ACH.p12I-immortalized cells. (B) Reverse transcription-PCR of total RNA from ACH- and ACH.p12I-immortalized PBMC, MT-2 cells, and naive PBMC. The 232-bp fragment specific for the pX-Rex-ORF I message is indicated and present in ACH-immortalized cells and MT-2 but is absent in ACH.p12I-immortalized cells and HTLV-1-negative PBMC. The constitutive 183-bp abl transcript is indicated and present in all samples.
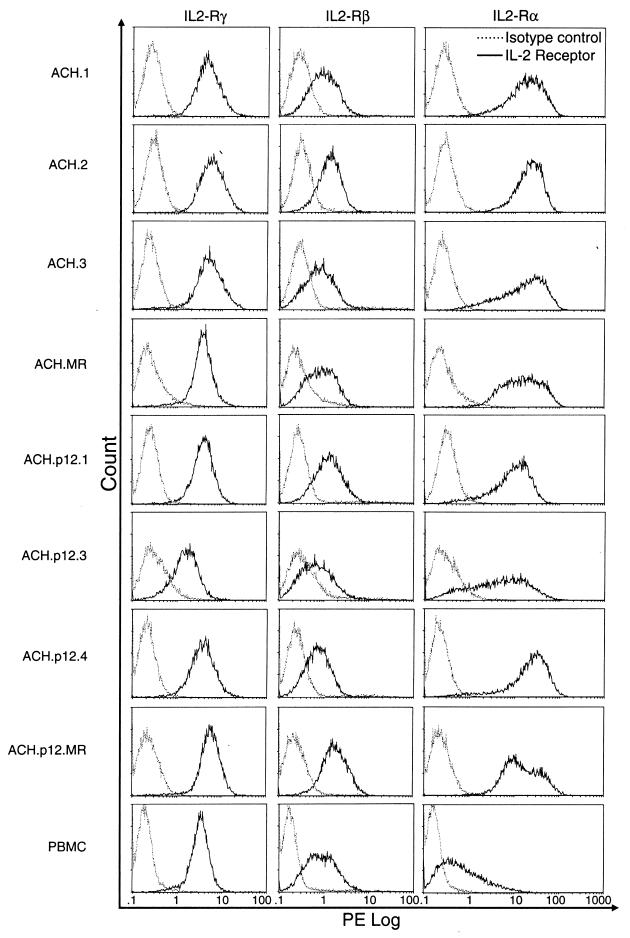
The cell lines were representative of all major phenotypes of T cells, including CD4+ and CD8+ cells, dual-positive cells, and dual-negative populations (Table 1). Major histocompatibility complex class I and II molecules and viral gp46 Env were expressed in 100% of the cells of all cell lines examined, with no significant differences in mean channel fluorescence between ACH- and ACH.p12I-transfected lines (data not shown). IL-2Rα was expressed at high levels in all HTLV-1-infected cells, in comparison to uninfected PBMC, compatible with upregulation by HTLV-1 Tax (Fig. 2). IL-2Rβ and -γ levels in the infected lines were comparable to that seen on uninfected, PHA-stimulated PBMC (Fig. 2). Levels did not vary among individual cell lines when reexamined 8 weeks later (data not shown). There were no significant differences in IL-2R expression levels between ACH- and ACH.p12I-immortalized lines.
FIG. 2.
Surface IL-2R chain expression on HTLV-1-immortalized cells as measured by flow cytometric detection of fluorescence intensity following labeling with PE-conjugated antibodies. The receptor chain detected by direct (IL-2β and -γc) or indirect (IL-2α) labeling with monoclonal antibodies is indicated along the top. For each IL-2R-chain-specific antibody (intensity histogram depicted as a solid line), fluorescence intensity with the isotype-matched control antibody is concurrently shown (intensity histogram depicted as a dotted line). Group mean channel fluorescence intensity of ACH- versus ACH.p12-immortalized PBMC were not different for IL-2R α, β, or γc chains (P > 0.05).
As expected, the IL-2-independent, HTLV-1-positive cell line MT-2 was unresponsive to IL-2 (Fig. 3). In contrast, uninfected PBMC are highly IL-2 dependent and only proliferated in the presence of IL-2. All ACH- and ACH.p12I-immortalized cell lines were intermediate in basal proliferative response compared with that of MT-2 and PBMC (Fig. 3). Five of the lines responded to increasing amounts of IL-2 with vigorous proliferation, albeit less than that seen with uninfected PBMC. In contrast, three lines (ACH.3, ACH.MR, and ACH.p12.3) failed to proliferate to a great extent even in the presence of large amounts of exogenous IL-2 and eventually ceased to proliferate in culture. There was no significant difference in proliferation rates or IL-2 responsiveness between lines immortalized with ACH versus ACH.p12I. Consistent with previous reports of HTLV-1-immortalized cell lines (16), no measurable endogenous IL-2 production could be detected from any of the lines (data not shown).
FIG. 3.
Basal and IL-2-responsive proliferation of HTLV-1-immortalized cells. Results are expressed as the relative increase in colorimetric conversion of MTS by viable cells, measured by absorbance before and after culturing for 72 h in the presence of varying amounts of IL-2. Data are the means of duplicate wells plated in a 96-well microtiter plate and are representative of three independent experiments. Group mean proliferations for ACH- versus ACH.p12-immortalized PBMC were not statistically different at any concentration of IL-2 (P > 0.05).
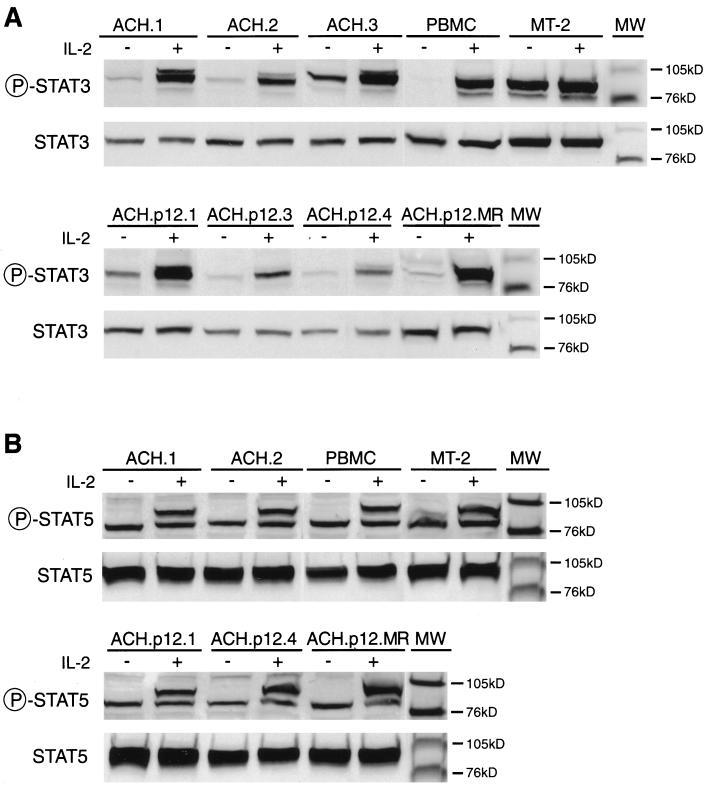
To determine if ablation of ORF I expression influenced IL-2 signaling, we measured basal and constitutive phosphorylation of Jak1, Jak3, Stat3, and Stat5 in ACH- and ACH.p12I-immortalized cell lines. Constitutive phosphorylation of Stat3 in the absence of IL-2 was detected in the HTLV-1-transformed MT-2 cell line but not in PHA-activated PBMC, as previously shown (23). All IL-2-dependent, HTLV-1-immortalized cell lines exhibited constitutive phosphorylation of Stat3 as well, although levels were much lower than those seen with MT-2 cells (Fig. 4A). This intermediate level of constitutive Stat3 phosphorylation is consistent with previous reports (23) and, importantly, did not differ consistently between lines immortalized with ACH versus ACH.p12I. In contrast, using a phospho-specific Stat5 antibody, no constitutive phosphorylation of Stat5 was detected in any of the IL-2-dependent, HTLV-1-immortalized cell lines or in PHA-activated PBMC (Fig. 4B). Although constitutive Stat5 phosphorylation was detected in MT-2 cells, levels were much lower relative to constitutive Stat3 phosphorylation.
FIG. 4.
Constitutive and IL-2-responsive Stat3 (A) and Stat5 (B) phosphorylation in HTLV-1-immortalized PBMC, as measured by immunoblot assay. Cells used are indicated at the top of each panel. Cells were treated with (+) or without (−) 2 nM IL-2. Native or phospho-specific Stat antibody types used for immunoblotting are indicated to the left of each panel. Molecular mass is indicated to the right (MW). The identity of the 85-kD band recognized by the phospho-specific Stat5 antibody is unknown.
Results obtained by immunoprecipitation were similar to those obtained by immunoblotting with the phospho-Stat5 antibody (Fig. 5A). Consistent with the above results, the HTLV-1-immortalized lines demonstrated partial basal phosphorylation of Jak3 (Fig. 5B and C). As was also expected, constitutive phosphorylation of Jak3 was strong in MT-2 and absent in uninfected PBMC. In contrast, the pattern of Jak1 phosphorylation was similar to that of Stat5, with slight constitutive activity in MT-2 but none detected in any other cell line examined. Importantly, no differences in basal phosphorylation of Jak1 or Jak3 were seen between PBMC immortalized with ACH versus ACH.p12I. IL-2-responsive phosphorylation of Jak1, Jak3, Stat3, and Stat5 was marked in all HTLV-1-immortalized cell lines, PHA-activated PBMC, and MT-2 cells examined by both immunoblotting with phospho-specific Stat antibodies and by immunoprecipitation (Fig. 4 and 5). As expected, levels of total (phosphorylated and nonphosphorylated) Jak1, Jak3, Stat3, and Stat5 remained constant before and after IL-2 stimulation (Fig. 4 and 5).
FIG. 5.
Constitutive and IL-2-responsive Jak and Stat phosphorylation in HTLV-1-immortalized PBMC, as measured by immunoprecipitation. Cells used are indicated at the top of each panel. Cells were treated with (+) or without (−) 2 nM IL-2. Immunoprecipitations were performed with anti-Stat3 (A), -Stat5 (B), -Jak1 (C), or -Jak3 (D) antibodies. Immunoblotting was performed with antiphosphotyrosine (4G10) or anti-Jak/Stat antibodies, as indicated to the left of each panel. Panel B sample ACH.p12.1 (prestimulated Jak-1) failed to load in the gel. Molecular mass is indicated to the right (MW).
The recent development of infectious molecular clones of HTLV-1 has enhanced efforts to evaluate the role of viral determinants in infection and transformation of T cells (7, 8, 17, 34). The availability of the ACH clone allowed our study of the role of ORF I of HTLV-1 in the infectivity of the virus, despite an apparent dispensability of this gene region during in vitro propagation of the virus (6, 9, 29). This is analogous to other viral regulatory genes, including homologous regions of HTLV-2 (5, 13) and bovine leukemia virus (32), as well as human immunodeficiency virus nef (15). Since experiments examining the role of ORF I in vitro have been conducted in the presence of IL-2, we postulated that these conditions obviate the role of ORF I in viral transcription and infectivity. In support of this, recent studies indicate that p12I associates with the IL-2 β and γc chains (24). Furthermore, p12I has homology to bovine papillomavirus E5, which has similar cellular distribution and activates the platelet-derived growth factor receptor (11). However, in contrast to the studies of p12I overexpression in Hela/Tat cells (24), we found that the differential expression of ORF I in the immortalized cell lines had no significant effect on surface IL-2R expression. Presumably, high levels of IL-2Rα expression in these lines, compared to uninfected PBMC, is due to the well-documented transcriptional effects of Tax on the IL-2Rα promoter (21). A functional role of ORF I proteins is suggested by our preliminary studies that demonstrated the inefficient transmission of HTLV-1 to resting T cells in limiting concentrations of IL-2 by ACH.p12I-immortalized cell lines (2).
The presence or absence of ORF I expression in the HTLV-1-immortalized cell lines used here had no apparent effects on basal or IL-2-responsive proliferation or signaling through the Jak/Stat pathway. All lines were consistent with an HTLV-1-immortalized population that is IL-2 dependent for continued proliferation but displaying some characteristics of partial IL-2 independence, including maintenance of viability and partial Jak3/Stat3 phosphorylation in the absence of IL-2. These characteristics were present in all HTLV-1-immortalized cell lines examined, regardless of the expression of ORF I, suggesting that ORF I gene products p12I and/or p27I do not greatly influence the progression of infected cells to this stage of immortalization. ORF I may be required for IL-2-independent transformation of HTLV-1-infected cells; however, this possibility seems remote since viral gene expression is not required for maintenance of the fully transformed phenotype (20). Nevertheless, it is intriguing that HTLV-1- but not HTLV-2-transformed lines consistently demonstrate constitutive activation of the Jak/Stat pathway, and the possibility that gene products unique to HTLV-1, such as p12I, are involved in this phenotype must be considered (23, 25).
We cannot rule out the use of cryptic splice sites to generate messages with p12I coding potential or the production of p12I from unspliced or singly spliced polycistronic messages for viral structural proteins, although no internal ribosomal entry sites have been described for HTLV-1. The conclusive detection of p12I in virally infected cells is dependent upon the generation of antibody reagents that recognize native p12I. Attempts to produce such reagents have not been successful due to the high hydrophobicity and poor immunogenicity of p12I (3, 19). Nevertheless, it is important to note that the Δp12I mutation used in this study is the same one that has previously been shown to reduce viral infectivity in vivo (6).
In summary, we demonstrate in this paper that ORF I expression does not overtly affect IL-2R expression or IL-2-mediated events during the late stages of HTLV-1-induced immortalization of infected cells. Furthermore, the early stages of acquisition of IL-2 independence do not require ORF I expression. The possibility exists that p12I/p27I are differentially expressed during the viral life cycle, as has been shown for human immunodeficiency virus Nef (1). Although ORF I transcripts were detected in the wild-type HTLV-1-immortalized cells, translational regulation may alter p12I/p27I expression at this stage of immortalization. Interestingly, p12I contains four minimal SH3 binding motifs (i.e., PXXP), which are common features of signal transduction molecules that modulate the Ras/mitogen-activated protein kinase and PI3-K signaling pathways (26). The function of ORF I genes may only be apparent during early events of viral replication, such as entry, uncoating, or integration. It therefore remains a possibility that ORF I expression modulates T-cell signaling at early stages of infection or in conditions of limited IL-2 concentrations more typical of the in vivo infection.
Acknowledgments
This work was supported, in part, by Public Health Service grants RR-14324, CA-55185, CA-16058, and CA-70259. M. D. Lairmore is supported by an Independent Scientist Career Award from the National Institutes of Health, AI-01474. B. Albrecht is supported by a fellowship from the Boehringer Ingelheim Corporation.
We thank Tim Vojt for preparation of figures and Richard K. Meister in the Center for Retrovirus Research Cytometry Laboratory for assistance with flow cytometric analysis.
REFERENCES
- 1.Ahmad N, Maitra R K, Venkatesan S. Rev-induced modulation of Nef protein underlies temporal regulation of human immunodeficiency virus replication. Proc Natl Acad Sci USA. 1989;86:6111–6115. doi: 10.1073/pnas.86.16.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht B, Ding W, Collins N, Wu X, Coggeshall K M, Green P L, Lairmore M D. Abstracts of Retrovirus Meeting 1999. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1999. Functional analysis of human T-cell lymphotropic virus type 1 p12 in vitro, abstr. 281; p. 281. [Google Scholar]
- 3.Cereseto A, Berneman Z, Koralnik I, Vaughn J, Franchini G, Klotman M E. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia. 1997;11:866–870. doi: 10.1038/sj.leu.2400665. [DOI] [PubMed] [Google Scholar]
- 4.Ciminale V, Pavlakis G N, Derse D, Cunningham C P, Felber B K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockerell G L, Rovnak J, Green P L, Chen I S Y. A deletion in the proximal untranslated pX region of human T-cell leukemia virus type II decreases viral replication but not infectivity in vivo. Blood. 1996;87:1030–1035. [PubMed] [Google Scholar]
- 6.Collins N D, Newbound G C, Albrecht B, Beard J L, Ratner L, Lairmore M D. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 7.Collins N D, Newbound G C, Ratner L, Lairmore M D. In vitro CD4+ lymphocyte transformation and infection in a rabbit model with a molecular clone of human T-cell lymphotropic virus type 1. J Virol. 1996;70:7241–7246. doi: 10.1128/jvi.70.10.7241-7246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derse D, Mikovits J, Polianova M, Felber B K, Ruscetti F. Virions released from cells transfected with a molecular clone of human T-cell leukemia virus type I give rise to primary and secondary infections of T cells. J Virol. 1995;69:1907–1912. doi: 10.1128/jvi.69.3.1907-1912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 10.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 11.Franchini G, Mulloy J C, Koralnik I J, Lo Monico A, Sparkowski J J, Andresson T, Goldstein D J, Schlegel R. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green P L, Chen I S Y. Molecular features of the human T-cell leukemia virus: mechanisms of transformation and leukemogenicity. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum Press; 1994. [Google Scholar]
- 13.Green P L, Ross T M, Chen I S Y, Pettiford S. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J Virol. 1995;69:387–394. doi: 10.1128/jvi.69.1.387-394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyot D J, Newbound G C, Lairmore M D. Signaling via the CD2 receptor enhances HTLV-1 replication in T lymphocytes. Virology. 1997;234:123–129. doi: 10.1006/viro.1997.8636. [DOI] [PubMed] [Google Scholar]
- 14a.Höllsberg P. Mechanisms of T-cell activation by human T-cell lymphotropic virus type 1. Microbiol Mol Biol Rev. 1999;63:308–333. doi: 10.1128/mmbr.63.2.308-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 16.Kimata J T, Ratner L. Temporal regulation of viral and cellular gene expression during human T-lymphocyte type 1-mediated lymphocyte immortalization. J Virol. 1991;65:4398–4407. doi: 10.1128/jvi.65.8.4398-4407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimata J T, Wong F, Wang J, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 18.Koralnik I J, Fullen J, Franchini G. The p12, p13, and p30 proteins encoded by human T-cell leukemia/lymphotropic virus type-1 open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koralnik I J, Gessain A, Klotman M E, Lo Monico A, Berneman Z N, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type 1. Proc Natl Acad Sci USA. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korber B, Okayama A, Donnelly R, Tachibana N, Essex M. Polymerase chain reaction analysis of defective human T-cell leukemia virus type 1 proviral genomes in leukemic cells of patients with adult T-cell leukemia. J Virol. 1991;65:5471–5476. doi: 10.1128/jvi.65.10.5471-5476.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung K, Nabel G J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 22.Mesnard J-M, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–284. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- 23.Migone T S, Lin J X, Cereseto A, Mulloy J C, Oshea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 24.Mulloy J C, Crowley R W, Fullen J, Leonard W J, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulloy J C, Migone T S, Ross T M, Ton N, Green P L, Leonard W J, Franchini G. Human and simian T-cell leukemia viruses type 2 (HTLV-2 and STLV-2pan-p) transform T cells independently of Jak/STAT activation. J Virol. 1998;72:4408–4412. doi: 10.1128/jvi.72.5.4408-4412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson B H, Willerford D M. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 27.Newbound G C, Andrews J M, O’Rourke J P, Brady J N, Lairmore M D. Human T-cell lymphotropic virus type 1 Tax mediates enhanced transcription in CD4+ T lymphocytes. J Virol. 1996;70:2101–2106. doi: 10.1128/jvi.70.4.2101-2106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palker T, Tanner M, Scearce R, Streilen R, Clark M, Haynes B. Mapping of immunogenic regions of human T-cell leukemia virus type 1 (HTLV-I) gp46 and gp21 envelope glycoproteins with env-encoded synthetic peptides and a monoclonal antibody to gp46. J Immunol. 1989;142:971–978. [PubMed] [Google Scholar]
- 29.Robek M D, Wong F H, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takemoto S, Mulloy J C, Cereseto A, Migone T S, Patel B K, Matsuoka M, Yamaguchi K, Takatsuki K, Kamihira S, White J D, Leonard W J, Waldmann T, Franchini G. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willems L, Kerkhofs P, Dequiedt F, Portetelle D, Mammerickx M, Burny A, Kettmann R. Attenuation of bovine leukemia virus by deletion of R3 and G4 open reading frames. Proc Natl Acad Sci USA. 1994;91:11532–11536. doi: 10.1073/pnas.91.24.11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Kang S H, Heidenreich O, Okerholm M, O’Shea J J, Nerenberg M I. Constitutive activation of different Jak tyrosine kinases in human T cell leukemia virus type 1 (HTLV-1) tax protein or virus-transformed cells. J Clin Investig. 1995;96:1548–1555. doi: 10.1172/JCI118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao T M, Robinson M A, Bowers F S, Kindt T J. Characterization of an infectious molecular clone of human T-cell leukemia virus type I. J Virol. 1995;69:2024–2030. doi: 10.1128/jvi.69.4.2024-2030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]