Abstract
Levamisole is an anthelmintic drug restricted to veterinary use but is currently detected as the most widely used cocaine cutting agent in European countries. Levamisole-adulterated cocaine has been linked to acute kidney injury, marked by a decrease in glomerular filtration rate, which involves reduced renal blood flow, but data on the alteration of renovascular response produced by levamisole are scarce. Renal arteries were isolated from healthy rabbits and used for isometric tension recording in organ baths and protein analysis. We provide evidence that depending on its concentration, levamisole modulates renovascular tone by acting as a non-selective α-adrenergic receptor blocker and down-regulates α1-adrenoceptor expression. Furthermore, levamisole impairs the endothelium-dependent relaxation induced by acetylcholine without modifying endothelial nitric oxide synthase (eNOS) expression. However, exposure to superoxide dismutase (SOD) partially prevents the impairment of ACh-induced relaxation by levamisole. This response is consistent with a down-regulation of SOD1 and an up-regulation of NADPH oxidase 4 (Nox4), suggesting that endothelial NO loss is due to increased local oxidative stress. Our findings demonstrate that levamisole can interfere with renal blood flow and the coordinated response to a vasodilator stimulus, which could worsen the deleterious consequences of cocaine use.
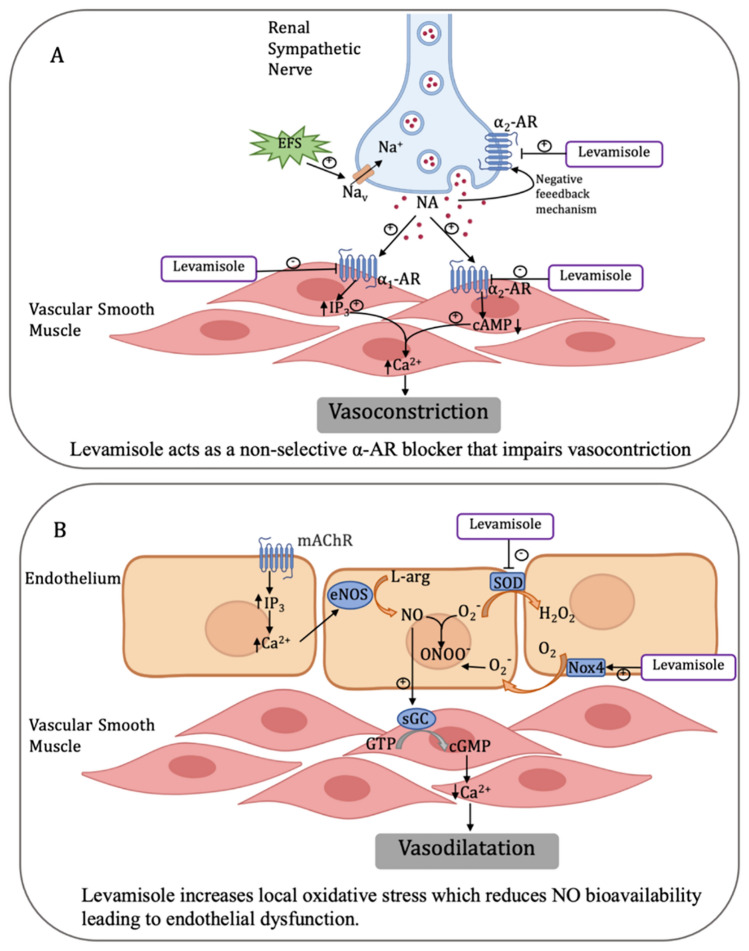
Graphical Abstract
EFS electric field stimulation, NA noradrenaline, AR adrenergic receptor, IP3 inositol 1, 4, 5-trisphosphate, cAMP cyclic adenosine monophosphate, mAChR muscarinic acetylcholine receptor, eNOS endothelial nitric oxide synthase, sGC soluble guanylyl cyclase, SOD superoxide dismutase, NOX4 NAPH oxidase 4
Keywords: Levamisole, Renovascular tone, Endothelial dysfunction, Oxidative stress
Introduction
Levamisole is an imidazothiol-derived substance approved as an anthelmintic for veterinary use. It was previously used as an immunomodulator and chemotherapy adjuvant in humans. Nevertheless, it was withdrawn from the market in the US and most European countries for its adverse effects, such as agranulocytosis, leukoencephalopathy, and vasculitis [1–3]. Levamisole received an orphan drug designation from the European Medical Agency (EMA) in 2005 for its potential benefit for the treatment of nephrotic syndrome based on the results in experimental models [4]. However, the application for marketing authorization was withdrawn by the manufacturer in 2017 after the Committee for Medicinal Products for Human Use (CHMP) manifested methodological concerns about the main study carried out in children [5]. Since 2004, levamisole has been detected as the most widely used cocaine cutting agent in European countries [6–8]. It has been suggested that cocaine adulteration with levamisole is related to its cheapness [9], its physicochemical properties [10], and its potentiating effect on cocaine. In fact, it has been shown that levamisole enhances the action of cocaine in vivo in planaria and rats [11, 12], which is attributed to its combined action on monoamine oxidase blockade and nicotinic receptor activation, thus increasing dopaminergic transmission. Levamisole also acutely blocks noradrenaline (NA) reuptake but with a much lower potency than cocaine [13]. Its active metabolite is aminorex, an amphetamine-like substance [13], so it could be assumed that levamisole is also used to extend the effects of cocaine.
Levamisole-adulterated cocaine is associated with neutropenia and agranulocytosis, vasculitis, retiform purpura and other forms of skin necrosis. However, besides cardiac and cerebral complications, it has also been related to renal complications such as acute kidney injury [14–17], nephrotic syndrome [15, 16, 18, 19] and chronic kidney disease [14, 20].
The hallmark of acute kidney injury is a reduction in glomerular filtration rate, which implies an underlying alteration of haemodynamic regulation, usually defined by a sustained increase in renovascular resistance in response to decreased renal perfusion pressure, further compromising blood flow [21, 22]. This response can be attributed to several factors, such as an imbalance between the release of vasoconstrictors and vasodilators, especially nitric oxide (NO), oxidative stress and O2 supply [23], which ultimately leads to endothelial dysfunction. In this regard, levamisole has been shown to produce apoptosis and reduce eNOS and endothelin-1 expression in HUVECs-human umbilical vein endothelial cells [24]. Apoptosis appears to be related to oxidative stress as it is counteracted by antioxidants such as glutathione and N-acetylcysteine [24]. Collectively, these findings suggest that levamisole may exert a significant effect on the endothelium, contributing to the detrimental effect on the cardiovascular system. Nevertheless, the possibility that levamisole may lead to altered renovascular response has not been seriously considered. Against that background, the aim of the present study was to test the following hypotheses in rabbit renal arteries: (1) levamisole increases adrenergic contractile response, (2) levamisole causes endothelial dysfunction by reducing NO bioavailability or affecting endothelium-derived hyperpolarization (EDH), and (3) loss of endothelial NO is related to an increase in the vascular oxidative stress.
Materials and Methods
Animal Procedures
16-Week-old male New Zealand white rabbits (San Bernardo S.L., Spain) weighing 3 to 3.5 kg were euthanized with sodium thiopental (60 mg/kg i.v.) and, after laparotomy, renal arteries were removed and placed in chilled (4 °C) Krebs–Henseleit solution containing (in mM): NaCl, 115; KCl, 4.6; MgCl2·6H2O, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 11.1 and disodium EDTA, 0.01 (pH 7.30–7.40 at 37 °C).
Preparation of Renal Arteries for Vascular Reactivity
Dissected renal arteries were cleaned and cut into 3 mm-rings. Each ring was mounted in an organ bath containing 4 ml of Krebs–Henseleit with two L-shaped stainless-steel wires, as previously described [25]. The solution was gassed with 95% O2 and 5% CO2 at 37 °C. Changes in isometric force generation were recorded at 100 Hz using a PowerLab/8e data acquisition system and LabChart software v7.2.5 (ADInstruments, New Zealand). Arteries were allowed to equilibrate for 2 h to a resting tension of 1 g. The contractile capacity of vascular smooth muscle was then evaluated by the maximal response to KCl (60 mM). Functional endothelium was assessed by pre-constriction to NA (10−7 to 3 × 10−7 M, ~ 75% of the maximal contraction to KCl 60 mM) followed by endothelium-dependent relaxation to acetylcholine (ACh, 10−6 M). Only arteries relaxing > 70% were used for experiments. Then, cumulative response to levamisole (10−6 to 10−3 M) was assessed in renal rings precontracted with NA (10−7- to 3 × 10−7 M). The concentrations of levamisole used in the study were in the range of 10−6 M to 10−3 M, which is similar to the levels found in plasma samples collected in vivo (peak concentration range 50–112 µg/l) [26–28] and post-mortem (peak concentration 1106 µg/l) [28] from cocaine users. In another set of experiments, relaxing response to levamisole was evaluated in the absence and presence of endothelium-derived factor inhibitors such as NG-nitro-l-arginine methyl ester (L-NAME, 10−4 M), indomethacin (Indo, 10−5 M) and tetraethylammonium (TEA, 10−3 M), and adrenoceptors (AR) blockers such as prazosin (10−6 M), yohimbine (10−6 M), and propranolol (10−6 M). In the case of prazosin, NA concentration (10−6 to 3 × 10−6 M) was adjusted to achieve the same degree of precontraction between comparison groups. To further determine the effects of levamisole on vascular α1-AR, curves to cumulative phenylephrine (PE, 10−9 to 3 × 10−5 M) were performed in the absence and presence of levamisole (10−5 to 10−3 M).
To examine endothelium-dependent relaxation–response, curves to cumulative ACh (10−9 to 3 × 10−6 M) were performed in rings precontracted with NA (10−6 to 3 × 10−6 M) in the absence and presence of levamisole (10−5 to 10−3 M). Levamisole-induced oxidative stress was tested in curves to cumulative ACh in the absence and presence of SOD (200 U/ml) and levamisole (10−3 M). Endothelium-independent relaxation was assessed by cumulative addition of sodium nitroprusside (10−9 to 3 × 10−6 M) in renal rings precontracted with NA (10−6 to 3 × 10−6 M) in the absence and presence of levamisole (10−3 M). For acetylcholine and sodium nitroprusside curves incubated with levamisole, the NA concentration for each vascular ring was adjusted to achieve the same degree of precontraction between comparison groups.
Measurement of Neurogenic Tone by Electrical Field Stimulation
For electric field stimulation (EFS) of sympathetic nerves to induce neurogenic tone, arteries were prepared as previously described [29]. EFS was applied by a Grass S88 stimulator (Grass Instruments) via two platinum electrodes positioned on each side and parallel to the axis of the arterial ring. Single square wave pulses (0.25 ms pulse duration, 8 Hz, at a supramaximal voltage of 20 V) were used. The train duration was 30 s with a stimulation interval of 5 min. In these conditions, EFS was applied to the renal rings before and after the addition of levamisole (10−6 to 10−3 M).
Western Blot Analysis
Renal arteries were incubated in the organ bath in control conditions and the presence of levamisole (10−5 to 10−3 M) for 30 min at 37 °C. Then, arteries were snap-frozen in liquid nitrogen and homogenized in lysis buffer (0.125 m Tris–HCl, pH 6.8, 2% SDS, 19% glycerol and 1% v/v protease inhibitors). Tissue lysates were centrifuged at 12,000 rpm for 20 min at 4 °C. Protein concentrations were determined using bicinchoninic acid protein assay (Thermo Scientific, USA). Proteins (30 μg) were separated on SDS‐PAGE gels and transferred to polyvinylidene fluoride membranes. Subsequently, the membranes were blocked for 1 h at room temperature in blocking buffer (5% bovine serum albumin, 0.1% Tween 20 in Tris-buffered solution), followed by incubation overnight at 4 °C with primary antibody against either α1A-adrenergic (NOVUS, #NB100 78585), α2A-adrenergic (NOVUS, #NBP2-22452), eNOS (Signalway, #21170-2), Cu/Zn–SOD (SOD1, Thermo Fisher, # MA1-105), Mn–SOD (SOD2, Thermo Fisher, #PA5-30604), Nox4 (Invitrogen, #PA5-72922), α‐tubulin (Santa Cruz Biotechnology, #sc 5286) and β‐actin (NOVUS, #NB-600-501SS). After incubation with corresponding secondary antibodies for 1 h at room temperature, bands were detected by chemiluminescence method (Amersham Biosciences) and visualized by the digital image system ImageQuant LAS 4000 (GE Healthcare). Protein expression was quantified densitometrically with ImageJ (National Institutes of Health, Bethesda, MD, USA). α‐Tubulin or β‐actin were used as protein loading controls.
Drugs and Solutions
Drugs were provided by Sigma Aldrich unless otherwise specified. Potassium chloride was obtained from Panreac. Indomethacin was dissolved in 100% ethanol. All other solutions were dissolved in distilled water. Stock solutions for organ baths were prepared daily in saline solution and kept on ice throughout the experiment. Levamisole and inhibitors were incubated for 30 min before measurements, except for SOD which was incubated for 45 min.
Statistical Analysis
Values were summarized as mean ± SEM of n arteries (one for each animal). Relaxations were expressed as a percentage of inhibition of agonist-induced response and contractions as a percentage relative to the value of the maximum contraction at KCl (60 mM). pEC50 values (negative log of concentration required to elicit 50% of the maximum effect) were determined from individual concentration–response curves by nonlinear regression analysis. The statistical analysis was made in Prism 8.0.2 (GraphPad Software, Inc., San Diego, CA, USA) using Student’s t-test, one-way analysis of variance (ANOVA), or two-way ANOVA with Bonferroni’s post-test as appropriate, where P < 0.05 was considered significant.
Results
Levamisole Induces Relaxation by Blocking α-Adrenergic Receptors
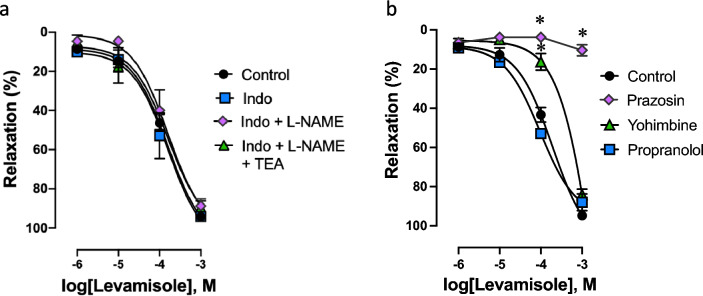
Relaxation of renal arteries was assessed after submaximal pre-constriction with NA (10−7 to 3 × 10−7 M). Levamisole produced concentration-dependent relaxation (Emax = 94.2 ± 1.2%, n = 4, Fig. 1a), and this response was not modified by inhibitors of prostanoids, NO and EDH production (Indomethacin, 94.1 ± 1.2%; Indomethacin + L-NAME, 88.7 ± 3.5%; Indomethacin + L-NAME + TEA, 89.6 ± 3.4%), suggesting the endothelium is not stimulated during relaxation to levamisole (Fig. 1a).
Fig. 1.
Levamisole induces relaxation in rabbit renal arteries precontracted with NA. a Relaxation–response curves to cumulative levamisole (10−6 to 10−3 M) in the absence (control) and presence of indomethacin (Indo, 10−5 M), a non-selective COX inhibitor, as well as consecutive additions of L-NAME (10−4 M), to block nitric oxide synthase and tetraethylammonium (TEA, 10−3 M), a calcium-activated K+ channels blocker. b Relaxation–response curves to cumulative levamisole in the absence (control) and presence of prazosin (10−6 M), to block α1-AR, yohimbine (10−6 M), to block α2-AR, and propranolol (10−6 M), to block β-AR. Data are means ± SEM (n = 3 to 6 arteries from different rabbits); *P < 0.05 vs. control
In order to evaluate if ARs were involved in this relaxing response, levamisole-induced relaxation was studied before and after treatment with AR blockers. Addition of the selective α1-AR blocker prazosin (10−6 M) completely prevented maximum relaxation to levamisole, while yohimbine (10−6 M), a selective α2-AR blocker, shifted to the right the concentration–response curve to levamisole, diminishing pEC50 (from 3.89 ± 0.05 to 3.22 ± 0.02, control vs. yohimbine P < 0.01, Fig. 1b). Propranolol, a non-selective β-AR blocker, did not attenuate relaxation to levamisole. These data suggest that levamisole-induced relaxation is mediated by α-AR blockage.
α1-AR Stimulation is Inhibited by Levamisole
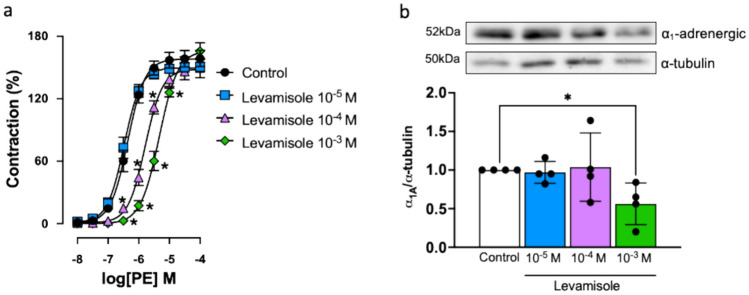
Contraction to PE (10−9 to 10−4 M) was studied in the absence and presence of levamisole to assess its effect during α1-AR activation in renal arteries. PE caused concentration-dependent contraction, which was right-shifted in the presence of levamisole (10−4 to 10−3 M, Fig. 2a; Table 1), confirming the α1-AR antagonistic effects of levamisole. Furthermore, consistent with this reduced response to PE, α1-AR expression was down-regulated in the presence of levamisole (10−3 M, Fig. 2b).
Fig. 2.
Levamisole impairs the activation of α1-AR. a Contraction–response curves to cumulative PE (10−8 to 10−4 M), an α1-AR agonist, in the absence (control) and presence of levamisole (10−5 to 10−3 M). Data are means ± SEM (n = 8 to 11 arteries from different rabbits). b Representative Western blot for α1-AR proteins in the absence (control) and presence of levamisole (Lev, 10−5 to 10−3 M); α-tubulin was used as internal control. Data are means ± SEM (n = 4 arteries from different rabbits); *P < 0.05, **P < 0.01 vs. control
Table 1.
pEC50 values and maximal responses (Emax) to phenylephrine in renal arteries in the different experimental conditions
| Phenylephrine | n | pEC50 | Emax |
|---|---|---|---|
| Control | 11 | 6.37 ± 0.2 | 157.1 ± 8 |
| Levamisole 10−5 M | 10 | 6.35 ± 0.3 | 150.5 ± 5 |
| Levamisole 10−4 M | 8 | 5.92 ± 0.3** | 150.3 ± 11 |
| Levamisole 10−3 M | 8 | 5.37 ± 0.2**** | 165.7 ± 8 |
Data are shown as mean ± SEM. Emax are expressed as a percentage of response to 60 mM KCl
n Number of rabbits, pEC50 − log M of substance causing 50% of the maximal contraction
**P < 0.01 and ****P < 0.0001 vs. control
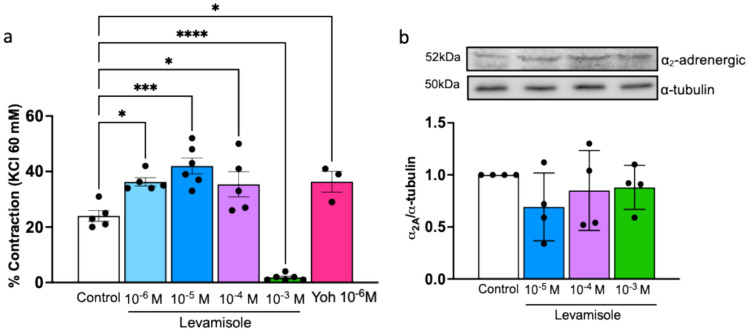
Levamisole Modulates EFS-Induced Contraction
EFS was performed on renal arteries before and after the addition of levamisole. Under control conditions, EFS caused a contractile response which was attenuated by the addition of tetrodotoxin (10−6 M) to block voltage-gated Na+ channels, guanethidine (10−6 M) to block transmission in post-ganglionic adrenergic nerves and prazosin (10−6 M) to block α1-AR (data not shown), indicating a neurogenic contractile response causing the release of NA from perivascular nerves which activates α1-AR, and not a direct stimulus on vascular smooth muscle.
EFS-induced contraction was further increased by the addition of cumulative levamisole (Control, 24 ± 1.94%; levamisole 10−6 M, 36.2 ± 1.9%; levamisole 10−5 M, 42 ± 2.8%; levamisole 10−4 M, 35.4 ± 4.5%, Fig. 3a). This increased contractile response may be attributed to a blockade of α2-AR, as it mirrored the effect induced by the selective α2-AR blocker yohimbine (Fig. 3a). Nevertheless, levamisole did not decrease α2-AR protein expression in renal arteries (Fig. 3b). Moreover, the highest concentration of levamisole completely blocked EFS-induced contraction (levamisole 10−3 M, 1.8 ± 0.4%, Fig. 3a), which is in line with its antagonistic effect on α1-AR. These data suggest that levamisole acts as a presynaptic α2-AR blocker, increasing the contractile response to adrenergic stimulation while, at high concentrations, it blocks the postsynaptic α1-AR, thus decreasing the vascular adrenergic response.
Fig. 3.
Levamisole enhances EFS-induced contraction but does not change α2-AR protein expression in rabbit renal arteries. a Bar graph summarizing EFS-induced contraction in the absence (control) and presence of levamisole (Lev, 10−6 to 10−3 M) and yohimbine (Yoh, 10−6 M). Data are means ± SEM (n = 3 to 6 arteries from different rabbits). b Representative Western blot for α2-AR proteins in the absence (control) and presence of levamisole (Lev, 10−5 to 10−3 M); α-tubulin was used as internal control. Data are means ± SEM (n = 4 arteries from different rabbits); *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 vs. control
Levamisole Induces Endothelial Dysfunction by Reducing NO Bioavailability
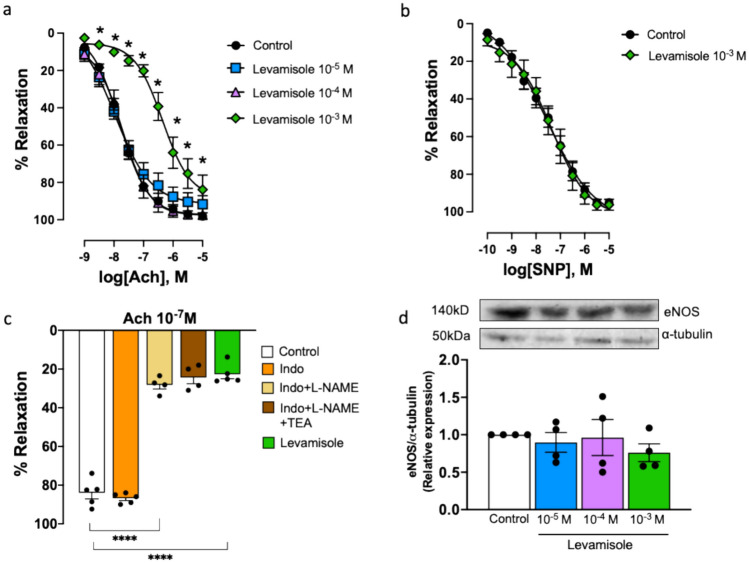
As expected, levamisole impaired endothelium-dependent relaxation induced by ACh (10−9 to 10−4 M) in renal arteries (Fig. 4a; Table 2), but it did not modify endothelium-independent relaxation to the NO donor sodium nitroprusside (SNP, 10−10 to 10−5 M, Fig. 4b).
Fig. 4.
Levamisole impairs endothelium-dependent relaxation but does not change eNOS protein expression in rabbit renal arteries. a Relaxation–response curves to cumulative acetylcholine (ACh, 10−9 to 10−5 M), a M3 muscarinic receptor agonist that activates endothelial NO production, in the absence (control) and presence of levamisole (10−5 to 10−3 M). Data are means ± SEM (n = 5 to 11 arteries from different rabbits). b Relaxation–response curves to cumulative sodium nitroprusside (SNP, 10−10 to 10−5 M), a nitric oxide donor independent of endothelial NO synthesis, in the absence (control) and presence of levamisole (10−3 M). Data are means ± SEM (n = 5 to 11 arteries from different rabbits). c Bar graph summarizing relaxation to ACh (10−7 M) in the absence (control) and presence of indomethacin (Indo, 10−5 M), a non-selective COX inhibitor, as well as consecutive additions of L-NAME (10−4 M), to block nitric oxide synthase and tetraethylammonium (TEA, 10−3 M), a calcium-activated K+ channels blocker, and levamisole (10−3 M). Data are means ± SEM (n = 4 to 5 arteries from different rabbits). d Representative Western blot for eNOS proteins in the absence (control) and presence of levamisole (Lev, 10−5 to 10−3 M); α-tubulin was used as internal control. Data are means ± SEM (n = 4 arteries from different rabbits). *P < 0.05 and ****P < 0.0001 vs. control
Table 2.
pEC50 values and maximal responses (Emax) to acetylcholine in renal arteries in the different experimental conditions
| Acetylcholine | n | pEC50 | Emax |
|---|---|---|---|
| Control | 11 | 7.84 ± 0.1 | 98.1 ± 1 |
| Levamisole 10−5 M | 5 | 7.93 ± 0.2 | 91.6 ± 4 |
| Levamisole 10−4 M | 5 | 7.70 ± 0.2 | 97.4 ± 2 |
| Levamisole 10−3 M | 6 | 6.48 ± 0.2**** | 83.9 ± 8 |
Data are shown as mean ± SEM. Emax is expressed as a percentage of response to 60 mM KCl
n Number of rabbits, pEC50 − log M of substance causing 50% of the maximal contraction
****P < 0.0001 vs control
ACh-induced relaxation is mostly dependent on NO in rabbit renal arteries, as was proved by a set of experiments where ACh (10−7 M), which stimulated sub-maximum relaxation, was studied before and after treatment with indomethacin, L-NAME and TEA (Fig. 4c). On this basis, the detrimental effect of levamisole could be due to a reduced NO bioavailability since impaired relaxation to ACh induced by levamisole evokes the same level of relaxation as the addition of NO blocker L-NAME (Fig. 4c). Nevertheless, intact renal arteries incubated with levamisole showed no evidence of a significant reduction in eNOS protein expression (Fig. 4d).
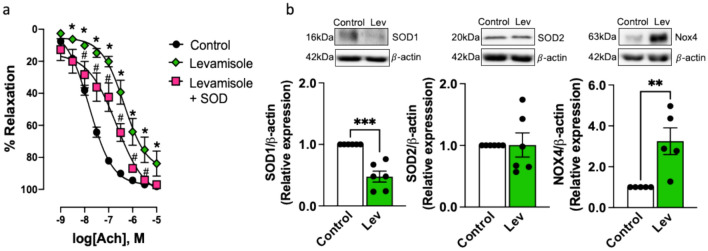
Vascular Oxidative Stress Underlines Loss of Endothelial NO Induced by Levamisole
To demonstrate the role of vascular oxidative stress in impaired endothelium-dependent relaxation in the presence of levamisole, we treated renal arteries with SOD (200 U/ml) and levamisole (10−3 M). As shown in Fig. 5a, SOD partially prevented impairment of ACh-induced relaxation by levamisole, diminishing pEC50 (from 7.84 ± 0.1 to 6.60 ± 0.29, Levamisole vs. Levamisole plus SOD P < 0.01). This was consistent with reduced SOD1 but not SOD2 protein expression (Fig. 5b). Furthermore, Nox4, a main source of ROS in kidney tissue [30], was upregulated in renal arteries incubated with levamisole (Fig. 5b). Taken together, these results demonstrate that impaired antioxidant capacity and stimulation of Nox4 underlie loss of NO induced by levamisole.
Fig. 5.
Oxidative stress underlies impaired endothelium-dependent relaxation by levamisole in rabbit renal arteries. a Relaxation–response curves to cumulative acetylcholine (ACh, 10−9 to 10−5 M), a M3 muscarinic receptor agonist that activates endothelial NO production, in the absence (control) and presence of levamisole (10−3 M) alone and levamisole plus SOD (200 U/ml). Data are means ± SEM (n = 5 to 11 arteries from different rabbits). b Representative Western blot for SOD1, SOD2 and Nox4 proteins in the absence (control) and presence of levamisole (Lev, 10−3 M); β-actin was used as internal control. Data are means ± SEM (n = 5 to 6 arteries from different rabbits). *P < 0.05 and ***P < 0.001 vs. control; #P < 0.05 vs. levamisole
Discussion
The main finding of the present study is that levamisole impairs or enhances vascular tone in rabbit renal arteries, likely through inhibition of α1 or α2-AR, respectively, and causes endothelial dysfunction. Moreover, levamisole causes a loss of endothelial NO-dependent relaxation without a reduction in eNOS protein expression.
To our knowledge, this is the first study to demonstrate that levamisole induces endothelial dysfunction in the renal artery. Although this effect has been previously reported by our group in rabbit aorta [31] and by others in mouse aorta [32], it is well known that the mechanism of vascular adaptation varies from one vascular bed to another [33]. Therefore, the vascular response to a stimulus may be heterogeneous between different vascular beds.
Our previous study [31] demonstrated that levamisole induces relaxation in aorta rings contracted with NA but not in those contracted with ET-1, U46619 or high-K+. These results suggest that levamisole interferes with the selective mechanisms of vascular adrenergic contraction, either by blocking intracellular signal transduction pathways involved in vascular contraction or even by antagonizing AR [34]. The latter mechanism was directly tested in the present study by using prazosin and yohimbine, an α1 and α2-AR blockers. Our observation that prazosin completely blocked levamisole-induced relaxation, while yohimbine partially prevented this response, argues for an involvement of α-AR in the vascular action of levamisole. Furthermore, an additional characteristic of the levamisole-induced relaxation is its independence from endothelium-derived vasodilators, such as prostanoids, NO or EDH, as the relaxing effect of levamisole was preserved in renal arteries previously incubated with indomethacin, L-NAME and TEA. Because PE is a selective agonist of α1-AR expressed in smooth muscle that causes contraction and levamisole right-shifted this response and down-regulated α1-AR expression, it is reasonable to suggest that in smooth muscle, levamisole acts as an α1-AR blocker. These observations were consistent with the previous finding that levamisole reduced renal constriction to exogenous NA, a non-selective α1-agonist, in isolated perfused rat kidneys [35].
The renal arteries are extensively innervated by sympathetic nerves, which play an important role in the regulation of vascular tone [36]. NA is the main neurotransmitter released by sympathetic nerve endings. Its release is controlled by pre-junctional α2-AR, which act as autoreceptors that inhibit NA release [36]. Sympathetic overactivity is frequently associated with the development of acute kidney injury [37], which causes a reduction of renal blood flow. Therefore, an important question is whether the ability of levamisole to attenuate the adrenergic response induced by post-junctional α2-AR could also affect the contribution of α2-AR on pre-junctional regulation. We showed that levamisole enhanced EFS-induced contraction, similar to observations made with yohimbine, a selective α2-AR blocker. These data are consistent with what occurs in other vascular beds, such as the rabbit aorta [31]. However, the highest concentration of levamisole abolished this response. This finding may be related to a threshold point for post-junctional α1-AR blockade induced by levamisole. Interestingly, in contrast to our previous study [31], a non-significant reduction in the expression of α2-AR was observed, further suggesting that intracellular signalling involved in the sympathetic response to levamisole in rabbit renal arteries may also be influenced by other mechanisms, such as the NA reuptake blockade proposed by Hofmaier et al. (13). In this regard, although we did not perform experiments comparing whether levamisole mimics the effect of NA reuptake inhibitors, it is worth noting that the vascular effects of levamisole observed in our EFS experiments could be in line with this idea. Therefore, levamisole could increase the amount of NA available and, consequently, its effect.
On the other hand, we observed significant differences between the changes produced by levamisole on ACh-mediated relaxation that could be related to a loss of NO, since we demonstrated that NO synthesis is crucial for endothelium-dependent relaxation in renal arteries. However, investigations into the loss of NO induced by levamisole are scarce. A prior study reported that levamisole reduces eNOS protein expression in HUVECs [24]. Here, we observed no significant difference in renal arteries in terms of eNOS expression in the presence of levamisole, suggesting that the reduced response to ACh in the presence of levamisole could be due to a decrease in NO bioavailability. Indeed, NO produced by eNOS can be scavenged by excess reactive oxygen species, ROS [38]. Therefore, NO bioavailability depends on the balance between ROS in the vascular wall and NO production [39]. In this case, we observed a significant difference in the response to ACh between control renal arteries and those incubated with the highest concentration of levamisole in the presence of SOD, supporting that NO is partly quenched by levamisole-induced excess ROS. However, the increase in ROS could simply reflect a decrease in antioxidant capacity, which was ruled out as protein expression of SOD1 and Nox4 is likewise altered in renal arteries incubated with levamisole. Therefore, although it appears that NO production is altered due to levamisole, we cannot confirm this since we did not measure eNOS activity or nitrate and nitrite levels in the vascular ring, but our experiments indicate that levamisole increases vascular oxidative stress, and this could lead to the loss of NO bioavailability.
Taken together, our results provide evidence that levamisole, depending on its concentration, modulates renovascular tone by acting as a non-selective α-AR blocker in rabbits. In addition, NO bioavailability is reduced in the presence of levamisole likely due to increased local oxidative stress, leading to endothelial dysfunction. Finally, it is important to note that the renal artery does not regulate renal blood flow per se, and this could implicate a limitation of this study. However, the renal artery is the major conduit of blood flow to the kidney and is highly innervated. Changes in its diameter and tone will therefore influence overall blood flow into the kidney, impacting the renal microvasculature. Furthermore, while acknowledging that the study of vascular responses is more readily conducted in the main renal artery compared to resistance vessels, that understanding the impact of levamisole on renal artery functional responses will guide subsequent experiments aimed at understanding how this drug, through adrenergic receptor modulation, both pre and postsynaptic, and endothelial function, could impact intrarenal perfusion and vascular resistance and modulate responses to vasoactive stimuli within the kidney, given they are part of the same arterial tree. Therefore, we can conclude that levamisole would interfere with the response to vasoactive stimuli in the renal arterial tree and worsen the deleterious consequences of cocaine use.
Acknowledgements
We thank Professor Luis Such and GRELCA Research Group, Department of Physiology, University of Valencia, Spain, for supporting animal model development.
Author Contributions
SG-O and PM participated in the conceptualization, investigation, methodology, data curation, formal analysis and wrote the main manuscript text. AS, MA, SV and PG participated in investigation, methodology and data curation. JMV and MDM participated in the conceptualization, formal analysis, project administration; supervision; writing-review and editing. All authors reviewed the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by Ministerio de Universidades (Spain) and the European Recovery, Transformation and Resilience Plan (Next Generation EU), Grant Number Margarita Salas UP2021-044 and MS21-162.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Animal experiments were authorised by the Ethics Committee of the University of Valencia under procedure 2017/VSC/PEA/00049 type 2. Animals were handled according to the University of Valencia ethical guidelines, the EU Directive 2010/63 and Spanish Royal Decree (RD) 1201/2005.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sol Guerra-Ojeda and Patricia Marchio contributed equally to this work and share first authorship.
Jose M. Vila and Maria D. Mauricio contributed equally to this work and share last authorship.
References
- 1.Khan, T. A., Cuchacovich, R., Espinoza, L. R., Lata, S., Patel, N. J., Garcia-Valladares, I., Salassi, M. M., & Sanders, C. V. (2011). Vasculopathy, hematological, and immune abnormalities associated with levamisole-contaminated cocaine use. Seminars in Arthritis and Rheumatism,41, 445–454. 10.1016/j.semarthrit.2011.04.010 10.1016/j.semarthrit.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 2.Dartevel, A., Chaigne, B., Moachon, L., Grenier, F., Dupin, N., Guillevin, L., Bouillet, L., & Mouthon, L. (2019). Levamisole-induced vasculopathy: A systematic review. Seminars in Arthritis and Rheumatism,48, 921–926. 10.1016/j.semarthrit.2018.07.010 10.1016/j.semarthrit.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 3.Larocque, A., & Hoffman, R. S. (2012). Levamisole in cocaine: Unexpected news from an old acquaintance. Clinical Toxicology,50, 231–241. 10.3109/15563650.2012.665455 10.3109/15563650.2012.665455 [DOI] [PubMed] [Google Scholar]
- 4.EMA EU/3/05/324. Retrieved March 6, 2023, from https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu305324
- 5.EMA Elmisol: Withdrawn application. Retrieved March 6, 2023, from https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/elmisol
- 6.Eiden, C., Diot, C., Mathieu, O., Mallaret, M., & Peyrière, H. (2014). Levamisole-adulterated cocaine: What about in European countries? Journal of Psychoactive Drugs,46, 389–392. 10.1080/02791072.2014.959215 10.1080/02791072.2014.959215 [DOI] [PubMed] [Google Scholar]
- 7.Hesse, M., Thomsen, K. R., Thylstrup, B., Andersen, C. U., Reitzel, L. A., Worm-Leonhard, M., & Lindholst, C. (2021). Purity of street-level cocaine across Denmark from 2006 to 2019: Analysis of seized cocaine. Forensic Science International,329, 111050. 10.1016/j.forsciint.2021.111050 10.1016/j.forsciint.2021.111050 [DOI] [PubMed] [Google Scholar]
- 8.Vonmoos, M., Hirsiger, S., Preller, K. H., Hulka, L. M., Allemann, D., Herdener, M., Baumgartner, M. R., & Quednow, B. B. (2018). Cognitive and neuroanatomical impairments associated with chronic exposure to levamisole-contaminated cocaine. Translational Psychiatry,8, 1–12. 10.1038/s41398-018-0279-3 10.1038/s41398-018-0279-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martello, S., Pieri, M., Ialongo, C., Pignalosa, S., Noce, G., Vernich, F., Russo, C., Mineo, F., Bernardini, S., & Marsella, L. T. (2017). Levamisole in illicit trafficking cocaine seized: A one-year study. Journal of Psychoactive Drugs,49, 408–412. 10.1080/02791072.2017.1361558 10.1080/02791072.2017.1361558 [DOI] [PubMed] [Google Scholar]
- 10.de Jong, M., Florea, A., de Vries, A.-M., van Nuijs, A. L. N., Covaci, A., Van Durme, F., Martins, J. C., Samyn, N., & De Wael, K. (2018). Levamisole: A common adulterant in cocaine street samples hindering electrochemical detection of cocaine. Analytical Chemistry,90, 5290–5297. 10.1021/acs.analchem.8b00204 10.1021/acs.analchem.8b00204 [DOI] [PubMed] [Google Scholar]
- 11.Tallarida, C. S., Egan, E., Alejo, G. D., Raffa, R., Tallarida, R. J., & Rawls, S. M. (2014). Levamisole and cocaine synergism: A prevalent adulterant enhances cocaine’s action in vivo. Neuropharmacology,79, 590–595. 10.1016/j.neuropharm.2014.01.002 10.1016/j.neuropharm.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tallarida, C. S., Tallarida, R. J., & Rawls, S. M. (2015). Levamisole enhances the rewarding and locomotor-activating effects of cocaine in rats. Drug and Alcohol Dependence,149, 145–150. 10.1016/j.drugalcdep.2015.01.035 10.1016/j.drugalcdep.2015.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmaier, T., Luf, A., Seddik, A., Stockner, T., Holy, M., Freissmuth, M., Ecker, G. F., Schmid, R., Sitte, H. H., & Kudlacek, O. (2014). Aminorex, a metabolite of the cocaine adulterant levamisole, exerts amphetamine like actions at monoamine transporters. Neurochemistry International,73, 32–41. 10.1016/j.neuint.2013.11.010 10.1016/j.neuint.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath, M. M., Isakova, T., Rennke, H. G., Mottola, A. M., Laliberte, K. A., & Niles, J. L. (2011). Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clinical Journal of American Society of Nephrology,6, 2799–2805. 10.2215/CJN.03440411 10.2215/CJN.03440411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ammar, A. T., Livak, M., & Witsil, J. C. (2015). Old drug new trick: Levamisole-adulterated cocaine causing acute kidney injury. The American Journal of Emergency Medicine,33(309), e3-309.e4. 10.1016/j.ajem.2014.08.015 10.1016/j.ajem.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 16.Sirvent, A. E., Enríquez, R., Andrada, E., Sánchez, M., Millán, I., & González, C. (2016). Necrotizing glomerulonephritis in levamisole-contaminated cocaine use. Nefrología (English Edition),36, 76–78. 10.1016/j.nefroe.2016.03.003 10.1016/j.nefroe.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 17.Álvarez Díaz, H., Marińo Callejo, A. I., García Rodríguez, J. F., Rodríguez Pazos, L., Gómez Buela, I., & Bermejo Barrera, A. M. (2013). ANCA-positive vasculitis induced by levamisole-adulterated cocaine and nephrotic syndrome: The kidney as an unusual target. American Journal of Case Reports,14, 557–561. 10.12659/AJCR.889731 10.12659/AJCR.889731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freyer, C. W., & Peters, M. (2012). Palpable purpura complicated by streptococcal toxic shock syndrome resulting in limb necrosis and amputation: A case of levamisole and cocaine coingestion. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy,32, e17–e23. 10.1002/PHAR.1012 10.1002/PHAR.1012 [DOI] [PubMed] [Google Scholar]
- 19.Marinelli, M. A., & McGhee, J. (2012). Clinical pathologic conference: A 51-year-old man with rash and joint pain. Academic Emergency Medicine,19, e41-44. 10.1111/j.1553-2712.2012.01398.x 10.1111/j.1553-2712.2012.01398.x [DOI] [PubMed] [Google Scholar]
- 20.Muñoz-Vahos, C. H., Herrera-Uribe, S., Arbeláez-Cortés, Á., Jaramillo-Arroyave, D., González-Naranjo, L. A., Vásquez-Duque, G., Restrepo-Escobar, M., Correa-Londoño, L. A., Arias-Restrepo, L. F., & Vanegas-García, A. L. (2019). Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy: A 30-case series. Journal of Clinical Rheumatology,25, e16–e26. 10.1097/RHU.0000000000000813 10.1097/RHU.0000000000000813 [DOI] [PubMed] [Google Scholar]
- 21.Prowle, J., Bagshaw, S. M., & Bellomo, R. (2012). Renal blood flow, fractional excretion of sodium and acute kidney injury: Time for a new paradigm? Current Opinion in Critical Care,18, 585–592. 10.1097/MCC.0b013e328358d480 10.1097/MCC.0b013e328358d480 [DOI] [PubMed] [Google Scholar]
- 22.Glodowski, S. D., & Wagener, G. (2015). New insights into the mechanisms of acute kidney injury in the intensive care unit. Journal of Clinical Anesthesia,27, 175–180. 10.1016/j.jclinane.2014.09.011 10.1016/j.jclinane.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 23.Aksu, U., Demirci, C., & Ince, C. (2011). The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Controversies in Acute Kidney Injury,174, 119–128. 10.1159/000329249 10.1159/000329249 [DOI] [PubMed] [Google Scholar]
- 24.Artwohl, M., Hölzenbein, T., Wagner, L., Freudenthaler, A., Waldhäusl, W., & Baumgartner-Parzer, S. M. (2000). Levamisole induced apoptosis in cultured vascular endothelial cells. British Journal of Pharmacology,131, 1577–1583. 10.1038/sj.bjp.0703660 10.1038/sj.bjp.0703660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerra-Ojeda, S., Marchio, P., Gimeno-Raga, M., Arias-Mutis, Ó. J., San-Miguel, T., Valles, S., Aldasoro, M., Vila, J. M., Zarzoso, M., & Mauricio, M. D. (2021). PPARγ as an indicator of vascular function in an experimental model of metabolic syndrome in rabbits. Atherosclerosis,332, 16–23. 10.1016/j.atherosclerosis.2021.08.006 10.1016/j.atherosclerosis.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 26.Eiden, C., Peyrière, H., Diot, C., & Mathieu, O. (2015). Prevalence of levamisole and aminorex in patients tested positive for cocaine in a French university hospital. Clinical Toxicology,53, 604–608. 10.3109/15563650.2015.1054499 10.3109/15563650.2015.1054499 [DOI] [PubMed] [Google Scholar]
- 27.Handley, S. A., Belsey, S. L., Couchman, L., & Flanagan, R. J. (2019). Plasma and urine levamisole in clinical samples containing benzoylecgonine: Absence of aminorex. Journal of Analytical Toxicology,43, 299–306. 10.1093/jat/bky102 10.1093/jat/bky102 [DOI] [PubMed] [Google Scholar]
- 28.Gameiro, R., Costa, S., Barroso, M., Franco, J., & Fonseca, S. (2019). Toxicological analysis of cocaine adulterants in blood samples. Forensic Science International,299, 95–102. 10.1016/j.forsciint.2019.03.005 10.1016/j.forsciint.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 29.Mauricio, M. D., Serna, E., Cortina, B., Novella, S., Segarra, G., Aldasoro, M., Martiénez-León, J. B., & Vila, J. M. (2007). Role of Ca2+-activated K+ channels on adrenergic responses of human saphenous vein. American Journal of Hypertension,20, 78–82. 10.1016/j.amjhyper.2006.06.011 10.1016/j.amjhyper.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 30.Sedeek, M., Nasrallah, R., Touyz, R. M., & Hébert, R. L. (2013). NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. Journal of American Society of Nephrology,24, 1512–1518. 10.1681/ASN.2012111112 10.1681/ASN.2012111112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra-Ojeda, S., Marchio, P., Aldasoro, M., Valles, S. L., Genovés, P., Mauricio, M. D., & Vila, J. M. (2022). Deleterious effects of levamisole, a cocaine adulterant, in rabbit aorta. Vascular Pharmacology,144, 106992. 10.1016/j.vph.2022.106992 10.1016/j.vph.2022.106992 [DOI] [PubMed] [Google Scholar]
- 32.Carmona-Rivera, C., Purmalek, M. M., Moore, E., Waldman, M., Walter, P. J., Garraffo, H. M., Phillips, K. A., Preston, K. L., Graf, J., Kaplan, M. J., et al. (2017). A role for muscarinic receptors in neutrophil extracellular trap formation and levamisole-induced autoimmunity. JCI Insights. 10.1172/jci.insight.89780 10.1172/jci.insight.89780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill, C. E., Phillips, J. K., & Sandow, S. L. (2001). Heterogeneous control of blood flow amongst different vascular beds. Medicinal Research Reviews,21, 1–60. 10.1002/1098-1128(200101)21:1%3c1::aid-med1%3e3.0.co;2-6 [DOI] [PubMed] [Google Scholar]
- 34.Ciccarelli, M., Sorriento, D., Coscioni, E., Iaccarino, G., & Santulli, G. (2017). Chapter 11—Adrenergic receptors. In J. C. Schisler, C. H. Lang & M. S. Willis (Eds.), Endocrinology of the heart in health and disease (pp. 285–315). Academic. ISBN 978-0-12-803111-7.
- 35.Jackson, E. K., Zhang, Y., & Cheng, D. (2017). Alkaline phosphatase inhibitors attenuate renovascular responses to norepinephrine. Hypertension,69, 484–493. 10.1161/HYPERTENSIONAHA.116.08623 10.1161/HYPERTENSIONAHA.116.08623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hering, L., Rahman, M., Potthoff, S. A., Rump, L. C., & Stegbauer, J. (2020). Role of Α2-adrenoceptors in hypertension: Focus on renal sympathetic neurotransmitter release, inflammation, and sodium homeostasis. Frontiers in Physiology,11, 566871. 10.3389/fphys.2020.566871 10.3389/fphys.2020.566871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grisk, O. (2020). The sympathetic nervous system in acute kidney injury. Acta Physiologica (Oxford),228, e13404. 10.1111/apha.13404 10.1111/apha.13404 [DOI] [PubMed] [Google Scholar]
- 38.Li, H., Horke, S., & Förstermann, U. (2014). Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis,237, 208–219. 10.1016/j.atherosclerosis.2014.09.001 10.1016/j.atherosclerosis.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 39.Förstermann, U. (2010). Nitric oxide and oxidative stress in vascular disease. Pflugers Archiv European Journal of Physiology,459, 923–939. 10.1007/s00424-010-0808-2 10.1007/s00424-010-0808-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.