Abstract
Obsessive–compulsive disorder (OCD) is characterized by intrusive thoughts and repetitive, compulsive behaviors, with childhood trauma recognized as a contributing factor to its pathophysiology. This study aimed to delineate brain functional aberrations in OCD patients and explore the association between these abnormalities and childhood trauma, to gain insights into the neural underpinnings of OCD. Forty-eight drug-naive OCD patients and forty-two healthy controls (HC) underwent resting-state functional magnetic resonance imaging and clinical assessments, including the Yale–Brown Obsessive Compulsive Scale (Y–BOCS) and Childhood Trauma Questionnaire-Short Form (CTQ-SF). Compared to HCs, OCD patients exhibited significantly decreased amplitude of low-frequency fluctuations (ALFF) in the right cerebellum, decreased regional homogeneity (ReHo) in the right cerebellum and right superior occipital lobes (FWE-corrected p < 0.05), which negatively correlated with Y–BOCS scores (p < 0.05). Furthermore, cerebellar ALFF negatively correlated with the CTQ emotional abuse subscale (r = − 0.514, p < 0.01). Mediation analysis revealed that cerebellar ALFF mediated the relationship between CTQ-emotional abuse and Y–BOCS (good model fit: R2 = 0.231, MSE = 14.311, F = 5.721, p < 0.01; direct effect, c′ = 0.153, indirect effect, a*b = 0.191). Findings indicated abnormal spontaneous and regional cerebellar activity in OCD, suggesting childhood trauma impacts OCD symptoms through cerebellar neural remodeling, highlighting its importance for clinical treatment selection.
Keywords: Obsessive–compulsive disorder, Resting-state functional connectivity, Childhood trauma, Amplitude of low-frequency fluctuation, Regional homogeneity
Subject terms: Obsessive compulsive disorder, Obsessive compulsive disorder, Human behaviour
Introduction
Obsessive–compulsive disorder (OCD), is characterized by obsessions and compulsions, resulting in impaired quality of life1. OCD has a lifetime prevalence of 2–3% among the general population2. The pathophysiology of OCD remains unclear after extensive research, which is still under debate due to inconsistent findings. Prior research on the pathophysiology of OCD has reported some inconsistent findings, including: different mechanisms of inherited pathologies, inconsistent structural/functional neuroimaging results, and divergent neurochemical hypotheses2–4. Numerous neuroimaging studies suggest structural and functional abnormalities play a crucial role in OCD pathophysiology2,5. However, inconsistent results have been reported regarding both structural and functional alterations, which have not sufficiently explained the biological basis of OCD2,3. More studies are warranted to elucidate the neural mechanisms of OCD.
Resting-state functional magnetic resonance imaging (rs-fMRI) has been widely used to describe the features underlying the functional organization of brain, and to measure the inter-regional temporal correlations6. Specifically, rs-fMRI can provide information on regional brain function in living humans, such as amplitude of low-frequency fluctuation (ALFF) which reflects the intensity of spontaneous brain activity6,7. In addition, regional homogeneity (ReHo) measures local synchronization of brain activity, assessing regional brain functional connectivity8,9. Secondly, functional connectivity (FC) provides information of the integration level to show abnormal brain network topology across brain regions7. Regional and remote functional connectivity has proven to be mutually complementary in characterizing brain functional connectomes9. Network-based analyses are an interesting alternative to explore FC abnormalities in networks like the default mode network10, which provides new insights into brain functional activity. Regarding FC and network analysis findings in OCD patients from fMRI studies, several studies have reported abnormal functional connectivity patterns in OCD patients, particularly involving the fronto-striatal, fronto-parietal, limbic, and default mode networks)11–13. Graph theory-based network analyses have revealed disrupted global and local efficiency, clustering, and small-worldness properties in OCD, indicating suboptimal brain network organization14.
Rs-fMRI has been increasingly used to identify potential biomarkers for different psychiatric conditions, such as depression and OCD15. Numerous fMRI studies revealed abnormal FC between OCD and controls in regions like the frontal cortex, thalamus and cerebellum16,17, meta-analysis results indicated hypoconnectivity within corticostriato-thalamo-cortical circuit, frontoparietal and salience network, and between salience, frontoparietal and default-mode network5,11. Abnormal ReHo values in the frontal gyrus, and cerebellum were found in the patients with OCD18–20. Altered ALFF value of the posterior cingulate cortex, hippocampal gyrus, and cerebellum network in OCD21,22. However, fMRI findings remain inconsistent across studies. Regarding brain networks, the cortico-striato-thalamo-cortical (CSTC) circuit has been associated with OCD12,13,23,24. More evidence has emerged regarding abnormal cerebellar-cerebral functional connectivity in OCD25–27. Beyond CSTC, other networks involving the default mode network (DMN), frontoparietal networks (FPN), and salience network (SN)11,28, have been linked to OCD. These networks are not specific to OCD, but also contribute to multiple psychiatric disorders29. The inconsistent fMRI findings could be attributed to heterogeneity within OCD patients.
By controlling for the clinical heterogeneity of OCD, insight may be gained in exploring its pathophysiology30. Empirical evidence suggests that childhood trauma may be a vulnerability factor of OCD31,32. Childhood trauma involves physical, psychological, and/or sexual abuse or neglect during childhood33. Childhood trauma correlates with symptoms severity in OCD patients34–36. However, whether childhood trauma contributes to OCD onset remains inconsistent, with some studies demonstrating increased childhood trauma before onset37,38, but others not reaching this conclusion39,40. The mechanisms linking childhood trauma and OCD remain unclear and further study is needed to elucidate the area. Previous studies suggested this relationship is mediated by other factors, like alexithymia41, anxiety/depression symptoms42, serotonin transporter levels43. Additionally, studies have examined gene-environment interactions, where certain gene polymorphisms may moderate the association between childhood trauma and OCD symptoms31,44, rather than acting as mediators. Prior studies have examined the association between childhood trauma and brain structural and functional activity. Patients with higher levels of exposure to childhood trauma showed increased brain grey matter volume in the caudate nucleus45. One study focused on thalamic functional connectivity by comparing thalamic functional connectivity between patients with varying trauma levels in OCD patients46. To date, no study has specifically examined the associations between childhood trauma and whole-brain activity and functional connectivity in OCD patients. More studies are warranted to firmly establish the evidence by exploring how childhood trauma affects brain abnormalities in OCD patients.
In summary, this study aimed to characterize regional brain activity and functional connectivity alterations in OCD patients using two approaches: ALFF and ReHo. We also explored associations between brain functional changes and childhood trauma in OCD patients to understand the neural mechanisms. We hypothesized OCD patients would show impaired functional activity and connectivity compared to healthy controls, which would be associated with childhood trauma. This study provides empirical evidence for further insights into the pathogenesis of OCD.
Methods
Participants
Forty-eight drug-naive OCD patients were recruited from outpatient clinics of Ningxia Medical University General Hospital, and forty-two healthy controls (HC) were recruited from the local community. The study was approved by the Ethics Committee of Ningxia Medical University General Hospital (KYLL-2022-1101), adhering to the principles of the Declaration of Helsinki.
The OCD group inclusion criteria were as follows: (1) 18–60 years old; (2) meet the diagnostic criteria of OCD in DSM-5; (3) Yale–Brown OCD Scale (Y–BOCS) total score > 16; (4) no family history of mental illness; (5) right-handed; (6) have not taken any psychiatric drugs; (7) OCD treatment naïve. The HC group inclusion criteria were as follows: age, gender and education match those of the OCD group and meet inclusion criteria (1), (4), (5) (6) above.
The exclusion criteria for the two groups of participants were as follows: (1) presence of serious physical diseases; (2) history of suicide; (3) presence of other mental disorders, such as schizophrenia, bipolar disorder, mental retardation, and alcohol dependence.
Clinical assessment
We conducted psychiatric interviews with participants using the MINI-International Neuropsychiatric Interview (M.I.N.I)47 to exclude other psychiatric disorders. We employed the Chinese version of the Yale–Brown Obsessive Compulsive Scale (Y–BOCS)48 to measure the overall severity of OCD symptoms. The 24-item Hamilton Depression Rating Scale (HAMD)49 and the 14-item Hamilton Anxiety Rating Scale (HAMA)50 were used to assess depressive and anxiety symptoms. The Chinese version of Childhood Trauma Questionnaire-Short Form (CTQ-SF)51 was used to evaluate childhood trauma. The CTQ-SF is a 28-item self-report questionnaire that retrospectively assesses five types of childhood maltreatment: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. The CTQ-SF has demonstrated good psychometric properties, including high internal consistency, test–retest reliability, and convergent and discriminant validity51. We calculated the total and subscale CTQ scores by summing the scores of all items, with higher scores indicating greater exposure to childhood trauma.
Data acquisition and preprocessing
All brain imaging was performed on a GE 3.0-T imaging system (SIGNA EXCITE 3.0T HDMR) with an 8-channel head coil. Resting-state BOLD-fMRI date was acquired using a gradient-recalled echo echo-planar imaging (GRE-EPI) sequence (repetition time 3000 ms, echo time 35 ms, flip angle = 90°, FOV = 240 × 240 mm2, matrix = 64 × 64, slice thickness = 5 mm, 35 contiguous axial slices with no slice gap, and volumes = 240). During the 12-min scan, the participants were instructed to relax, stay awake, and let their minds wander freely without focusing on any particular thought or task. They were asked to keep their eyes closed to minimize visual input and facilitate the resting state. Earplugs and foam padding were used to reduce scanner noise and minimize head movements.
The preprocessing was performed using the SPM12 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm/) toolbox, and Data Processing Assistant for Resting-State fMRI (DPARSFA version 4.4, http s://rfmri.org/dpabi) based on MATLAB R2013b. The first 3 volumes were deleted and followed slice-timing and realignment. Subjects were excluded by head motion of > 3 mm in maximum displacement or > 3°rotation in angular motion (5 OCD patients were unqualified; 4 controls were unqualified). Next the high-resolution T1weight images were co-registered to the mean realigned fMRI images and segmented into gray matter, white matter, cerebrospinal fluid (CSF). Then, the images were then spatially normalized to the Montreal Neurological Institute (MNI) space and resliced by 3 × 3 × 3 mm3@@52. Next, functional images were trended and temporal band pass filtered between 0.01 and 0.08 Hz53. Subsequently, several nuisance variances including 24 head motion parameters were regressed by multiple linear regression analysis. Functional images were spatially smoothed with a Gaussian kernel of full-width at half maximum of 6 mm. Framewise displacement (FD) was calculated for each time point, and mean FD value exceeding 0.5 mm was excluded. The quality control procedures involved: (a) Excluding participants with excessive head motion (as mentioned earlier); (b) Calculating FD and excluding participants with mean FD exceeding 0.5 mm; (c) Visually inspecting the preprocessed functional images for potential artifacts or abnormalities. Finally, after MRI quality control, 43 OCD patients and 38 controls were included in the analysis.
Image analysis
The data after magnetic resonance preprocessing were analyzed by the Statistical Parametric Mapping toolbox (SPM12), the Resting-State fMRI Data Analysis Toolkit (REST, www.restfmri.net), and Data Processing Assistant for Resting-State fMRI (DPARSFA). The brain regions were represented based on the use of predefined automated anatomical labeling (AAL)54.
ALFF analysis
We calculated the amplitude of low-frequency fluctuations (ALFF). The time series of each voxel were transformed into the frequency domain using fast Fourier transform to obtain the power spectrum. The averaged square root of power in the frequency band (0.01–0.08 Hz) was taken as the ALFF. For each voxel, the ALFF was standardized by dividing the global mean ALFF value for each subject.
ReHo analysis
The ReHo brain map was constructed by calculating the Kendall coefficient of the time series consistency between each voxel and its neighboring 26 voxels. And then the ReHo was standardized by dividing the global mean ReHo value for each subject to get Kendall's coefficient of concordance, KCC-ReHo. Finaly, The KCC-ReHo value of all single voxel directions was calculated and normalized to the KCC-ReHo z value55.
Statistical analysis
Two sample t tests and Chi-square tests were used to compare the demographic and clinical characteristics of the OCD and HC groups. The statistical analysis of fMRI data was performed in SPM12. We examined the differences in regional functional activity (ALFF map) and connectivity (ReHo map) of the two groups using two sample t-test analysis at the whole-brain voxel level controlling for gender, age, education level, and head movement, running under MATLAB. Statistical significance was set at a voxel height threshold of p < 0.001 and cluster-level threshold of p < 0.05 with family-wise error (FWE) correction. And then, mean ALFF/ReHo values of these impaired brain regions were extracted and their correlations with patients’ OCD symptoms/ CTQ scores were evaluated. Correlation analyses were performed between the severity of obsessive–compulsive symptoms and childhood trauma in the OCD group, with gender, age, education level, and head motion as covariates. To address the issue of multiple comparisons, we applied the False Discovery Rate (FDR) correction using the Benjamini–Hochberg method56. This correction was implemented using the p.adjust function with the "BH" method in R software. Finally, a mediating effect model was constructed using PROCESS version 15.0 to examine how childhood trauma impact the clinical symptoms to further understand the neural mechanism of OCD.
Ethics approval and consent to participate
The study conformed to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Ningxia Medical University General Hospital (KYLL-2022-1101). Informed consent was obtained from all subjects and/or their legal guardian(s).
Results
Demographic characteristics of participants
Demographic and clinical features were presented in Table 1. The OCD group (N = 43; age, 27.81 ± 9.35; male/female = 20/23; education years, 16.40 ± 5.30) did not differ from HC group (N = 38; age, 28.87 ± 7.23; male/female = 15/23; education years, 17.29 ± 5.58) in terms of gender, age, or educational years (all p > 0.05). Antipsychotic-naive OCD patients are included in our study to avoid the effect of medicine on results. In the 43 OCD patients, the total Y–BOCS was 23.86 ± 4.27, corresponding to moderate and severe OCD symptoms, with obsessive and compulsive subscale scores of 11.77 ± 3.01 and 12.33 ± 2.23, respectively. OCD patients reported mild anxiety and depression levels (HAMA, 7.60 ± 3.50, HAMD, 7.65 ± 3.76, p < 0.001). OCD patients also reported more emotional abuse (t79 = 8.851, p < 0.001), physical abuse (t79 = 6.896, p < 0.001), and emotional neglect (t79 = 2.153, p = 0.034) than HC based on the CTQ scores.
Table 1.
Demographic information of drug-naive OCD patients and healthy controls.
| OCD (N = 43) | HC (N = 38) | t/x2 | p values | |
|---|---|---|---|---|
| Age (years) | 27.81 ± 9.35 | 28.87 ± 7.23 | 0.562 | 0.576 |
| Gender (male/female) | 20/23 | 15/23 | 0.407 | 0.523 |
| Education (years) | 16.40 ± 5.30 | 17.29 ± 5.58 | 0.727 | 0.469 |
| Y–BOCS total | 23.86 ± 4.27 | 2.87 ± 4.43 | 21.699 | < 0.001 |
| Y–BOCS obsessions | 11.77 ± 3.01 | 1.29 ± 2.10 | 17.942 | < 0.001 |
| Y–BOCS compulsions | 12.33 ± 2.23 | 1.58 ± 2.62 | 19.944 | < 0.001 |
| HAMD | 26.40 ± 10.90 | 3.76 ± 3.46 | 12.257 | < 0.001 |
| HAMA | 31.40 ± 14.51 | 3.68 ± 3.97 | 11.395 | < 0.001 |
| CTQ total | 45.07 ± 10.90 | 30.11 ± 5.52 | 7.639 | < 0.001 |
| CTQ-emotional abuse | 14.58 ± 5.30 | 6.45 ± 2.11 | 8.851 | < 0.001 |
| CTQ-physical abuse | 11.16 ± 4.26 | 6.13 ± 1.51 | 6.896 | < 0.001 |
| CTQ-sexual abuse | 5.65 ± 1.25 | 5.32 ± 0.74 | 1.444 | 0.153 |
| CTQ-emotional neglect | 7.35 ± 2.64 | 6.08 ± 2.65 | 2.153 | 0.034 |
| CTQ-physical neglect | 6.33 ± 2.36 | 6.13 ± 1.61 | 0.426 | 0.671 |
OCD obsessive–compulsive disorder; HC healthy control; Y–BOCS Yale–Brown Obsessive Compulsive Scale; HAMD Hamilton Depression Scale; HAMA Hamilton Anxiety Scale; CTQ Childhood Trauma Questionnaire; M ± SD Mean ± Standard deviation.
Between-group differences in ALFF and ReHo between OCD and HC
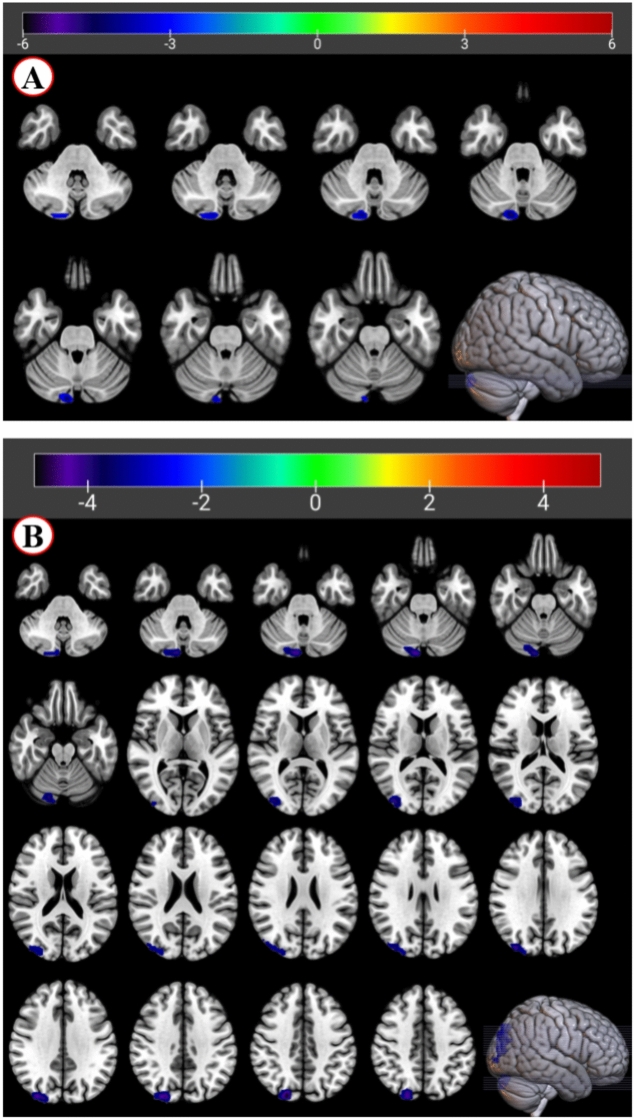
Compared with the HC group, the ALFF values of OCD group decreased in the right cerebellum. Similarly, there was a significant group difference of ReHo value in the right cerebellum and the right superior occipital (OCD < HC) (depicted in Fig. 1 and Table 2).
Figure 1.
fMRI shows brain regions with abnormal ALFF and ReHo values in the OCD group compared with the HC group. Note: voxel p < 0.001, cluster p < 0.05, FWE corrected. (A) Brain regions showing significant differences in ALFF values between the OCD and HC groups. The color bar represents the t-value range, with red indicating higher ALFF values in the OCD group and blue indicating lower ALFF values in the OCD group compared to the HC group. The ALFF of the OCD group was lower than that of HC in the Cerebellum_Crus2_R (MNI coordinates, 12, -87, -30). (B) Brain regions showing significant differences in ReHo values between the OCD and HC groups. The color bar represents the z-score range, with warm colors (yellow–red) indicating higher ReHo values in the OCD group and cool colors (blue) indicating lower ReHo values in the OCD group compared to the HC group. The ReHo of the OCD group was lower than that of HC in the Cerebellum_Crus2_R (MNI coordinates, 9, − 87, -30), and the Occipital_Sup_R (MNI coordinates, 21, − 84, 39).
Table 2.
Brain area with abnormal ALFF and ReHo value in OCD patients.
| Brain area | Cluster size | MNI | t | p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ALFF value | ||||||
| Cerebelum_Crus2_R | 45 | 12 | − 87 | − 30 | − 5.25 | < 0.001 |
| ReHo value | ||||||
| Cerebelum_Crus2_R | 114 | 9 | − 87 | − 30 | − 4.78 | < 0.001 |
| Occipital_Sup_R | 253 | 21 | − 84 | 39 | − 5.01 | < 0.001 |
ALFF amplitude of low-frequency fluctuation; ReHo regional homogeneity; MNI Montreal neurological institute template.
Relationships among childhood trauma, brain functional activity, and symptom severity
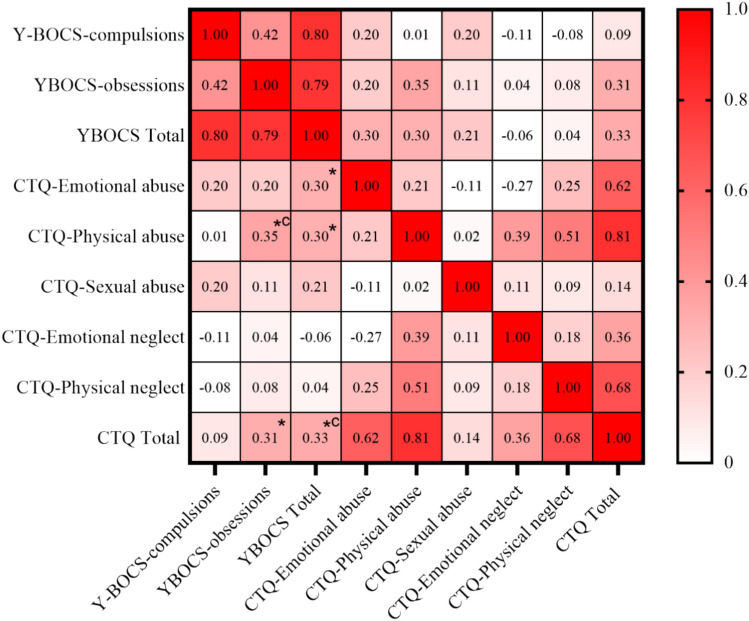
In OCD patients, moderate positive correlations were found between various CTQ scores and Y–BOCS scores through multiple correlation analysis (CTQ emotional abuse- Y–BOCS total, r = 0.30, p = 0.047; CTQ physical abuse- Y–BOCS total, r = 0.30, p = 0.047; CTQ physical abuse- Y–BOCS obsession, r = 0.35, p = 0.021; CTQ total- Y–BOCS obsession, r = 0.31, p = 0.040; CTQ total- Y–BOCS total, r = 0.33, p = 0.032). After applying FDR correction, two correlations remained statistically significant: CTQ physical abuse- Y–BOCS obsession (r = 0.35, p = 0.040) and CTQ total- Y–BOCS total (r = 0.33, p = 0.046). These results suggest a potential relationship between childhood trauma levels and OCD symptom severity, though this relationship appears less robust after correction for multiple comparisons (see Fig. 2).
Figure 2.
The correlation matrix of Y–BOCS scores and CTQ scores. Note: *, uncorrected p < 0.05; *c FDR corrected p < 0.05. The correlation between CTQ-emotional abuse with Y–BOCS total is significant (r = 0.30, p = 0.047); The correlation between CTQ-physical abuse with Y–BOCS -obsessions and Y–BOCS total is significant (r = 0.35, p = 0.021; r = 0.30, p = 0.047; respectively); The correlation between CTQ total with Y–BOCS -obsessions and Y–BOCS total is significant (r = 0.31, p = 0.040; r = 0.33, p = 0.032; respectively). After applying FDR correction, two correlations remained statistically significant: CTQ physical abuse- Y–BOCS obsession (r = 0.35, p = 0.040) and CTQ total- Y–BOCS total (r = 0.33, p = 0.046).
When assessing the association between fMRI values and OCD symptoms, negative correlation between ALFF/ReHo value and Y–BOCS scores were found. (see in Fig. 3) The ALFF value of the Cerebelum_Crus2_R brain area in the OCD group was negatively correlated with the total score of Y–BOCS (r = − 0.372, p = 0.014). No linear correlation was observed among the ALFF value and subscales of Y–BOCS. The ReHo value of the Cerebelum_Crus2_R brain area in the OCD group was negatively correlated with the total score of Y–BOCS and the score of the compulsion subscale (r = − 0.415, − 0.432; p < 0.01). The Y–BOCS total and the compulsion subscale scores of the OCD group was also negatively correlated with the ReHo value of the Occipital_Sup_R (r = − 0.307, − 0.404; p = 0.045, 0.007).
Figure 3.
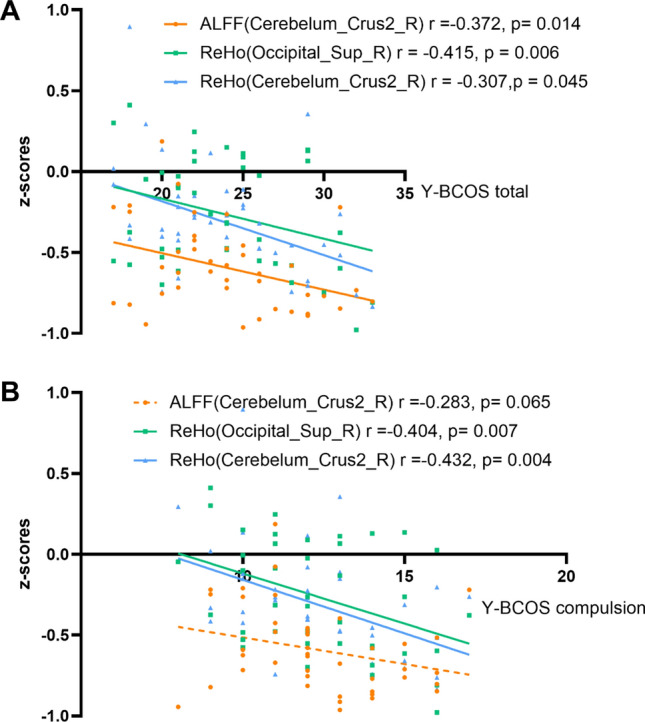
The correlation between fMRI and OCD symptoms. Note: (A) Correlation between the ALFF/ReHo value of the impaired brain areas and the total score of Y–BOCS in the OCD group. (B) Correlation between the ALFF/ReHo value of the impaired brain areas and the score of the compulsion subscale in the OCD group.
We conducted the correlation analysis between childhood trauma and ALFF/ReHo value in the brain regions of interest. As demonstrated in Fig. 4, In the OCD patients, the ALFF value of the cerebellum was negatively correlated with the CTQ emotion abuse subscale (r = − 0.514, p = 0.004). When considering all participants together, we found a significant negative correlation between the ALFF value of the cerebellum and the CTQ emotional abuse subscale (r = − 0.505, p < 0.001). However, when analyzing the healthy control group separately, we did not observe any significant correlations between CTQ scores and ALFF/ReHo values in the brain regions of interest.
Figure 4.
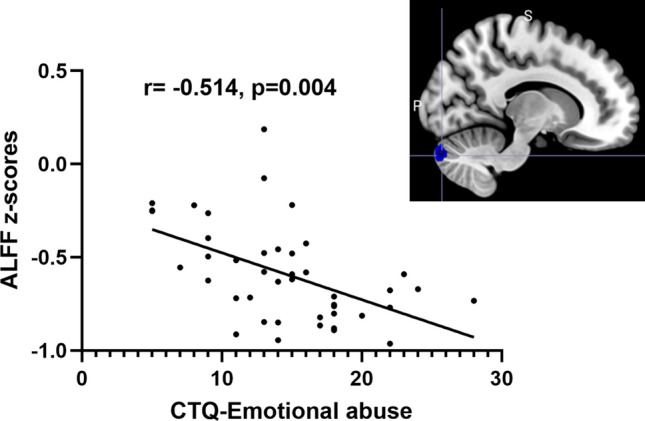
The CTQ emotional abuse subscale correlated with the ALFF value of cerebellum. Note: CTQ: Childhood Trauma Questionnaire; ALFF: amplitude of low-frequency fluctuation; the CTQ emotional abuse was negatively correlated with the ALFF value of cerebellum (r = − 0.514, p = 0.004).
As mentioned before, the cerebellum was found to be correlated to the CTQ emotional abuse and Y–BCOS total scores. To further understand the relationship among them, the mediation analysis was used to show how childhood trauma impact the OCD symptoms. We found that the ALFF value of the cerebellum mediated the relationship between CTQ-emotional abuse and YBCOS total scores (model fit is good, R2 = 0.231, MSE = 14.311, F = 5.721, p < 0.01). It indicated that cerebellar activity alterations may represent one potential neural substrate linking childhood trauma and OCD symptoms The CTQ emotional abuse predicted the OCD symptoms via a totally mediation effect of cerebellum (direct effect, c′ = 0.153, p = 0.247, indirect effect, a*b = 0.191) (See in Fig. 5 and Table 3). These data suggested that the cerebellum functional activity had a total mediating effect on the association between the childhood trauma and OCD symptoms.
Figure 5.
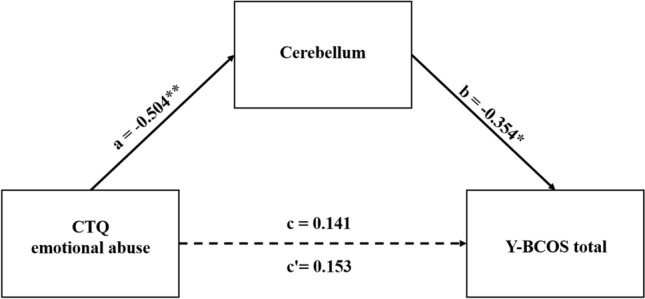
The CTQ emotional abuse- cerebellum-YBCOS total scores mediation model. Note: *p < 0.05, **p < 0.01; CTQ emotional abuse: measured by the CTQ subscale; cerebellum: the ALFF value of the Cerebelum_Crus2_R, which was the differential brain area of OCD and HC group; Y–BCOS total: measured by the Y–BCOS subscale. The model fit is good, R2 = 0.231, MSE = 14.311, F = 5.721, p < 0.01; a, b, c variables’ value represented the standard coefficient. c′ direct effect of X on Y, c indirect effect of X on Y.
Table 3.
The mediation analysis results.
| Direct effect | Indirect effect | Coeff | SE | t | p | LLCI | ULCI | |
|---|---|---|---|---|---|---|---|---|
| Constant | – | – | 17.78 | 1.92 | 9.25 | < 0.001*** | 13.89 | 21.67 |
| CTQ | 0.14 | 0.19 | 0.15 | 0.13 | 1.18 | 0.25 | − 0.11 | 0.42 |
| Cerebellum | 0.35 | – | − 6.07 | 2.83 | − 2.15 | 0.04* | − 11.79 | − 0.35 |
CTQ childhood trauma questionnaire emotional abuse subscale; Cerebellum the ALFF value of the Cerebelum_Crus2_R.; LLCI lower limit of confidence interval; ULCI upper limit of confidence interval.
Discussion
Our main findings showed that OCD patients exhibited significantly decreased ALFF value in the right cerebellum, decreased ReHo value in the right cerebellum and right superior occipital lobes, compared to HC; furthermore, the ALFF and ReHo values were negatively correlated with OCD symptoms. In addition, by revealing the relationship among alterations of brain function, childhood trauma, and OCD symptoms, the mediation analysis results further indicated that cerebellum mediated the association between childhood trauma and OCD symptoms. Although some prior studies have explored the association between trauma and neuroimaging alterations45,46, none have used mediation analysis to elucidate how trauma may lead to OCD symptoms through its impact on brain function. The present study is the first to reveal, from the perspective of mediation analysis, that alterations in cerebellar function mediate the relationship between childhood trauma and OCD symptoms, thereby shedding light on the mechanism by which trauma influences OCD.
The first core region closely associated with OCD symptoms in our study was the cerebellum. We found decreased ALFF and ReHo values in the OCD patients, the ALFF and ReHo values of the cerebellum were further negatively correlated with the OCD symptoms, revealing cerebellum was the neural underpinnings of OCD.
The cerebellum comprises distinct subregions that are implicated in diverse processes beyond its traditional role in motor control57,58. The posterior cerebellar regions, including the posterior lobe (hemispheric portions VII-X) and vermis, are crucially involved in cognitive functions such as executive function (working memory, cognitive flexibility, attention)59,60, emotion regulation61, and behavioral regulation62. Focusing on our findings, we will elaborate on the potential roles of the posterior cerebellar abnormalities in the cognitive deficits observed in OCD. Decreased ALFF and ReHo values in these regions, which negatively correlated with symptom severity, may reflect disruptions in the cerebellar modulation of executive function, habit formation, and inhibitory control over repetitive behaviors—core impairments in OCD19,20,63–65. Machine-learning technique identified cerebellum as one essential neuroimaging biomarkers of OCD66. Specifically, the posterior cerebellum is implicated in shifting cognitive set and suppressing prepotent responses, processes that are compromised in OCD patients’ inability to disengage from intrusive thoughts and compulsions. Moreover, we will discuss the cerebellum's functional connectivity with large-scale cortical networks, such as the fronto-parietal and cingulo-opercular networks implicated in cognitive control and salience processing67,68. Disruptions in the cerebellum’s integration within these networks may contribute to the pathophysiology of OCD by impairing the coordination of cognitive resources necessary for flexible behavioral regulation. Future studies should examine whether this fMRI regional characteristic of the cerebellum affects other brain regions of large-scale networks responsible for OCD symptoms.
The decreased ReHo values observed in the superior occipital lobes in the OCD group should be carefully interpreted. Few neuroimaging studies found the superior occipital abnormalities of OCD. Previous study suggested the lower FC values of the superior occipital cortex in OCD69. A diffusion tensor imaging study showed impaired white matter integrity of OCD in the superior occipital lobes70. These findings raise the question of how the superior occipital lobes are involved in OCD psychopathology. The superior occipital lobes are located in the visual cortex, which processes information about the location of objects in space. It may entail symptoms of compulsion, such as an intense need to organize and arrange objects and spaces. The question of the superior occipital connectivity in OCD should be further explored in future studies, with a more specific assumption and larger sample sizes.
This study indicated that OCD patients experienced more childhood trauma, including physical abuse, emotional abuse and emotional neglect; further, childhood trauma was positively correlated with OCD symptom severities. Consistent with previous studies indicating a relationship between traumatic experiences and OCD symptoms34,71,72. Other factors that may have an impact on the link between childhood trauma and OCD include anxiety/depression symptoms42, alexithymia41, serotonin transporter levels43, as well as gene polymorphisms in the BDNF, MAOA, and COMT31,44. In line with a prior structural MRI investigation that found a positive correlation between the right cerebellar grey matter volume and physical neglect episodes73, the current study found that childhood trauma negatively linked with the cerebellum neural activity in OCD patients. While, there was different proposal, fMRI study reporting that history of early life trauma positively correlates with DMN networks, rather than cerebellum74. More research is needed to clarify the neuroimaging effects of childhood trauma on OCD severity.
The main goal of our study was to assess the relationship between OCD patients’ brain functions and their experiences of childhood trauma. The results of the present research demonstrated a negative correlation between the cerebellum and childhood trauma, as well as a correlation between the cerebellum and the severity of OCD symptoms. Moreover, our results show, for the first time, that cerebellum mediated the impact of childhood trauma on OCD symptom. The effect of childhood trauma on brain functional connectivity in OCD has only been examined in one study to date, and the results show that OCD patients with high and low levels of childhood trauma had distinct alterations in the thalamus, prefrontal cortex, and caudate46. The current study suggested that trauma childhood trauma impact OCD symptoms via cerebellum neural remodeling. In other words, the patients with OCD who encountered with childhood trauma exhibit specific neural mechanism, i.e., alterations in the cerebellum related to the experiences of childhood trauma. Our findings suggest a potential pathway whereby childhood trauma may contribute to altered cerebellar activity, which in turn could impair cognitive functions involved in the development of OCD symptoms. However, this proposed mechanism is speculative and requires rigorous testing in future longitudinal and experimental studies to establish causal relationships and delineate the precise neural processes involved.
Limitations
This study has a number of limitations. Firstly, the small sample size might have limited the statistical power to perform a stratification based on OCD subtypes. Future studies would benefit of assessing OCD subtypes since different symptomatic profiles seem to be associated with distinct neuroimaging findings. A notable limitation of this study is the lack of an a priori power analysis to determine the appropriate sample size. The absence of a predetermined sample size based on expected effect sizes limits our ability to confidently generalize our findings and may affect the robustness of our conclusions. Recently a growing importance of pre-registering study protocols in neuroimaging research to enhance transparency and reduce potential biases. These practices would have strengthened our study design and should be considered essential components of future research in this field. Secondly, this study was not a longitudinal follow-up study to explore whether the impact of trauma on the OCD brain persist or whether the abnormalities gradually recover as the traumatic event disappears. Thirdly, after applying FDR correction for multiple comparisons, some of our initially significant correlations did not retain statistical significance. This change in results highlights a potential limitation in our study's statistical power. Our sample size, while adequate for our primary analyses, may have been insufficient to detect smaller effect sizes when correcting for multiple comparisons. Finally, Alexithymia is a known confounding factor that would influence the relationship between childhood trauma and OCD symptoms, and its absence in our study design is a limitation. In the further study, we will apply a specific measure for alexithymia (such as the TAS-20).to construct a larger model exploring the roles of alexithymia in the relationship between childhood trauma, cerebellar function, and obsessive–compulsive symptoms.
Conclusions
The study indicated that abnormal spontaneous neural activity and regional functional activity in the cerebellum might be neural underpinnings of OCD. Furthermore, our findings revealed that the cerebellum mediated the impact of childhood trauma on OCD symptoms. It suggested that childhood trauma impacts OCD symptoms through cerebellar neural remodeling, which should be considered when selecting clinical treatment strategies.
Abbreviations
- OCD
Obsessive–compulsive disorder
- rs-fMRI
Resting-state functional connectivity
- HC
Healthy control
- ALFF
Amplitude of low-frequency fluctuation
- ReHo
Regional homogeneity
- CTQ
Childhood Trauma Questionnaire
- Y–BCOS
Yale–Brown Obsessive Compulsive Scale
Author contributions
Concept and design: Manxue Zhang, Jianqun Fang Acquisition, analysis, or interpretation of data: Manxue Zhang, Chujun Wu, Shihao Lu, Yunyun Du, Shaoxia Wang, Rui Ma Drafting of the manuscript: Manxue Zhang. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Manxue Zhang Obtained funding: Jianqun Fang Administrative, technical, or material support: Manxue Zhang, Chujun Wu, Shaoxia Wang, Yanrong Wang, Jianqun Fang. Supervision: Yanrong Wang, Jianqun Fang.
Funding
This work was supported by Natural Science Foundation of Ningxia Program (No. 2022AAC02067). It provided the fund for the investigation, editing and the subject's allowance. We gratefully acknowledge the participants of this study for generously donating their time.
Data availability
We guarantee the authenticity of the data, but do not disclose the data, if necessary, you can email fjq7887215@163.com to obtain the data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Association, A. P. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™ 5th edn, Vol. 25 (American Psychiatric Association, 2013). [Google Scholar]
- 2.Stein, D. J. et al. Obsessive–compulsive disorder. Nat. Rev. Dis. Prim.10.1038/s41572-019-0102-3 (2019). 10.1038/s41572-019-0102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad, M. R. & Rauch, S. L. Obsessive–compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn. Sci.16, 43–51. 10.1016/j.tics.2011.11.003 (2012). 10.1016/j.tics.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandelow, B. et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry18, 162–214. 10.1080/15622975.2016.1190867 (2017). 10.1080/15622975.2016.1190867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu, J. et al. Abnormal resting-state functional connectivity in patients with obsessive–compulsive disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev.135, 104574. 10.1016/j.neubiorev.2022.104574 (2022). 10.1016/j.neubiorev.2022.104574 [DOI] [PubMed] [Google Scholar]
- 6.Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci.8, 700–711. 10.1038/nrn2201 (2007). 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- 7.Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med.34, 537–541. 10.1002/mrm.1910340409 (1995). 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 8.Jiang, L. & Zuo, X. N. Regional homogeneity: A multimodal, multiscale neuroimaging marker of the human connectome. Neurosci. Rev. J. Bring. Neurobiol. Neurol. Psychiatry22, 486–505. 10.1177/1073858415595004 (2016). 10.1177/1073858415595004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepulcre, J. et al. The organization of local and distant functional connectivity in the human brain. PLoS Comput. Biol.6, e1000808. 10.1371/journal.pcbi.1000808 (2010). 10.1371/journal.pcbi.1000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu, J., Potenza, M. N. & Calhoun, V. D. Spatial ICA reveals functional activity hidden from traditional fMRI GLM-based analyses. Front. Neurosci.7, 154. 10.3389/fnins.2013.00154 (2013). 10.3389/fnins.2013.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gürsel, D. A., Avram, M., Sorg, C., Brandl, F. & Koch, K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: A meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev.87, 151–160. 10.1016/j.neubiorev.2018.01.016 (2018). 10.1016/j.neubiorev.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 12.Haber, S. N. Corticostriatal circuitry. Dialogues Clin. Neurosci.18, 7–21. 10.31887/DCNS.2016.18.1/shaber (2016). 10.31887/DCNS.2016.18.1/shaber [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira, P. S. et al. The resting-brain of obsessive–compulsive disorder. Psychiatry Res. Neuroimaging290, 38–41. 10.1016/j.pscychresns.2019.06.008 (2019). 10.1016/j.pscychresns.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Zhang, T. et al. Abnormal small-world architecture of top-down control networks in obsessive–compulsive disorder. J. Psychiatry Neurosci. JPN36, 23–31. 10.1503/jpn.100006 (2011). 10.1503/jpn.100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodward, N. D. & Cascio, C. J. Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry72, 743–744. 10.1001/jamapsychiatry.2015.0484 (2015). 10.1001/jamapsychiatry.2015.0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banca, P. et al. Imbalance in habitual versus goal directed neural systems during symptom provocation in obsessive–compulsive disorder. Brain J. Neurol.138, 798–811. 10.1093/brain/awu379 (2015). 10.1093/brain/awu379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein, G. A. et al. Abnormal striatal resting-state functional connectivity in adolescents with obsessive–compulsive disorder. Psychiatry Res. Neuroimaging247, 49–56. 10.1016/j.pscychresns.2015.11.002 (2016). 10.1016/j.pscychresns.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan, X. et al. Regional homogeneity in patients with obsessive–compulsive disorder and depression: A resting state functional magnetic resonance imaging study. Neurosci. Lett.817, 137528. 10.1016/j.neulet.2023.137528 (2023). 10.1016/j.neulet.2023.137528 [DOI] [PubMed] [Google Scholar]
- 19.Hu, X. et al. Localized connectivity in obsessive–compulsive Disorder: An investigation combining univariate and multivariate pattern analyses. Front. Behav. Neurosci.13, 122. 10.3389/fnbeh.2019.00122 (2019). 10.3389/fnbeh.2019.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qing, X., Gu, L. & Li, D. Abnormalities of localized connectivity in obsessive–compulsive disorder: A voxel-wise meta-analysis. Front. Hum. Neurosci.10.3389/fnhum.2021.739175 (2021). 10.3389/fnhum.2021.739175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomiyama, H. et al. Posterior cingulate cortex spontaneous activity associated with motor response inhibition in patients with obsessive–compulsive disorder: A resting-state fMRI study. Psychiatry Res. Neuroimaging334, 111669. 10.1016/j.pscychresns.2023.111669 (2023). 10.1016/j.pscychresns.2023.111669 [DOI] [PubMed] [Google Scholar]
- 22.Xu, Y. et al. Static and temporal dynamic changes of intrinsic brain activity in pediatric and adults OCD. J. Affect. Disord.311, 416–424. 10.1016/j.jad.2022.05.101 (2022). 10.1016/j.jad.2022.05.101 [DOI] [PubMed] [Google Scholar]
- 23.Nakao, T., Okada, K. & Kanba, S. Neurobiological model of obsessive–compulsive disorder: Evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin. Neurosci.68, 587–605. 10.1111/pcn.12195 (2014). 10.1111/pcn.12195 [DOI] [PubMed] [Google Scholar]
- 24.Menzies, L. et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive–compulsive disorder: The orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev.32, 525–549. 10.1016/j.neubiorev.2007.09.005 (2008). 10.1016/j.neubiorev.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, T. et al. Altered resting-state cerebellar-cerebral functional connectivity in obsessive–compulsive disorder. Psychol. Med.49, 1156–1165. 10.1017/s0033291718001915 (2019). 10.1017/s0033291718001915 [DOI] [PubMed] [Google Scholar]
- 26.Moody, T. D. et al. Mechanisms of cognitive-behavioral therapy for obsessive–compulsive disorder involve robust and extensive increases in brain network connectivity. Transl. Psychiatry7, e1230. 10.1038/tp.2017.192 (2017). 10.1038/tp.2017.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, W. et al. Disrupted pathways from frontal-parietal cortex to basal ganglia and cerebellum in patients with unmedicated obsessive compulsive disorder as observed by whole-brain resting-state effective connectivity analysis–a small sample pilot study. Brain Imaging Behav.15, 1344–1354. 10.1007/s11682-020-00333-3 (2021). 10.1007/s11682-020-00333-3 [DOI] [PubMed] [Google Scholar]
- 28.Stern, E. R., Fitzgerald, K. D., Welsh, R. C., Abelson, J. L. & Taylor, S. F. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive–compulsive disorder. PloS ONE7, e36356. 10.1371/journal.pone.0036356 (2012). 10.1371/journal.pone.0036356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci.15, 483–506. 10.1016/j.tics.2011.08.003 (2011). 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 30.Bragdon, L. B. & Coles, M. E. Examining heterogeneity of obsessive–compulsive disorder: Evidence for subgroups based on motivations. J. Anxiety Disord.45, 64–71. 10.1016/j.janxdis.2016.12.002 (2017). 10.1016/j.janxdis.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 31.McGregor, N. W. et al. Modification of the association between early adversity and obsessive–compulsive disorder by polymorphisms in the MAOA MAOB and COMT genes. Psychiatry Res.246, 527–532. 10.1016/j.psychres.2016.10.044 (2016). 10.1016/j.psychres.2016.10.044 [DOI] [PubMed] [Google Scholar]
- 32.Real, E. et al. Stressful life events at onset of obsessive–compulsive disorder are associated with a distinct clinical pattern. Depress. Anxiety28, 367–376. 10.1002/da.20792 (2011). 10.1002/da.20792 [DOI] [PubMed] [Google Scholar]
- 33.Miller, A. B., Esposito-Smythers, C., Weismoore, J. T. & Renshaw, K. D. The relation between child maltreatment and adolescent suicidal behavior: A systematic review and critical examination of the literature. Clin. Child Fam. Psychol. Rev.16, 146–172. 10.1007/s10567-013-0131-5 (2013). 10.1007/s10567-013-0131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgado, P., Freitas, D., Bessa, J. M., Sousa, N. & Cerqueira, J. J. Perceived stress in obsessive–compulsive disorder is related with obsessive but not compulsive symptoms. Front. Psychiatry4, 21. 10.3389/fpsyt.2013.00021 (2013). 10.3389/fpsyt.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvi, Y. et al. Relations between childhood traumatic experiences, dissociation, and cognitive models in obsessive compulsive disorder. Int. J. Psychiatry Clin. Pract.16, 53–59. 10.3109/13651501.2011.617458 (2012). 10.3109/13651501.2011.617458 [DOI] [PubMed] [Google Scholar]
- 36.Semiz, U. B., Inanc, L. & Bezgin, C. H. Are trauma and dissociation related to treatment resistance in patients with obsessive–compulsive disorder?. Soc. Psychiatry Psychiatr. Epidemiol.49, 1287–1296. 10.1007/s00127-013-0787-7 (2014). 10.1007/s00127-013-0787-7 [DOI] [PubMed] [Google Scholar]
- 37.Cromer, K. R., Schmidt, N. B. & Murphy, D. L. An investigation of traumatic life events and obsessive–compulsive disorder. Behav. Res. Therapy45, 1683–1691. 10.1016/j.brat.2006.08.018 (2007). 10.1016/j.brat.2006.08.018 [DOI] [PubMed] [Google Scholar]
- 38.Murayama, K. et al. Impacts of stressful life events and traumatic experiences on onset of obsessive–compulsive disorder. Front. Psychiatry11, 561266. 10.3389/fpsyt.2020.561266 (2020). 10.3389/fpsyt.2020.561266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coles, M. E., Johnson, E. M. & Schubert, J. R. Retrospective reports of the development of obsessive compulsive disorder: Extending knowledge of the protracted symptom phase. Behav. Cogn. Psychother.39, 579–589. 10.1017/s135246581100004x (2011). 10.1017/s135246581100004x [DOI] [PubMed] [Google Scholar]
- 40.Ivarsson, T., Saavedra, F., Granqvist, P. & Broberg, A. G. Traumatic and adverse attachment childhood experiences are not characteristic of OCD but of depression in adolescents. Child Psychiatry Hum. Dev.47, 270–280. 10.1007/s10578-015-0563-x (2016). 10.1007/s10578-015-0563-x [DOI] [PubMed] [Google Scholar]
- 41.Carpenter, L. & Chung, M. C. Childhood trauma in obsessive compulsive disorder: The roles of alexithymia and attachment. Psychol. Psychother.84, 367–388. 10.1111/j.2044-8341.2010.02003.x (2011). 10.1111/j.2044-8341.2010.02003.x [DOI] [PubMed] [Google Scholar]
- 42.Çoban, A. & Tan, O. Attention deficit hyperactivity disorder, impulsivity, anxiety, and depression symptoms mediating the relationship between childhood trauma and symptoms severity of obsessive–compulsive disorder. Noro Psikiyatr. Ars.57, 37–43. 10.29399/npa.23654 (2020). 10.29399/npa.23654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lochner, C. et al. Investigating the possible effects of trauma experiences and 5-HTT on the dissociative experiences of patients with OCD using path analysis and multiple regression. Neuropsychobiology56, 6–13. 10.1159/000109971 (2007). 10.1159/000109971 [DOI] [PubMed] [Google Scholar]
- 44.Hemmings, S. M. et al. BDNF Val66Met modifies the risk of childhood trauma on obsessive–compulsive disorder. J. Psychiatr. Res.47, 1857–1863. 10.1016/j.jpsychires.2013.08.012 (2013). 10.1016/j.jpsychires.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 45.Benedetti, F. et al. Caudate gray matter volume in obsessive–compulsive disorder is influenced by adverse childhood experiences and ongoing drug treatment. J. Clin. Psychopharmacol.32, 544–547. 10.1097/JCP.0b013e31825cce05 (2012). 10.1097/JCP.0b013e31825cce05 [DOI] [PubMed] [Google Scholar]
- 46.Chu, M. et al. The impact of childhood trauma on thalamic functional connectivity in patients with obsessive–compulsive disorder. Psychol. Med.52, 2471–2480. 10.1017/s0033291720004328 (2020). 10.1017/s0033291720004328 [DOI] [PubMed] [Google Scholar]
- 47.Si, T. M. et al. Evaluation of the reliability and validity of Chinese version of the Mini-International Neuropsychiatric Interview in patients with mental disorders. Chin. Ment. Health J.23(7), 493–497 (2009). [Google Scholar]
- 48.Goodman, W. K. et al. The Yale–Brown obsessive compulsive scale II. Validity. Arch. Gen. Psychiatry46, 1012–1016. 10.1001/archpsyc.1989.01810110054008 (1989). 10.1001/archpsyc.1989.01810110054008 [DOI] [PubMed] [Google Scholar]
- 49.Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry23, 56–62. 10.1136/jnnp.23.1.56 (1960). 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol.32, 50–55. 10.1111/j.2044-8341.1959.tb00467.x (1959). 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- 51.Bernstein, D. P. et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl.27, 169–190. 10.1016/s0145-2134(02)00541-0 (2003). 10.1016/s0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- 52.Ashburner, J. & Friston, K. J. Unified segmentation. NeuroImage26, 839–851. 10.1016/j.neuroimage.2005.02.018 (2005). 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 53.Wen, M. et al. More than just statics: Temporal dynamic changes of intrinsic brain activity in cigarette smoking. Addict. Biol.26, e13050. 10.1111/adb.13050 (2021). 10.1111/adb.13050 [DOI] [PubMed] [Google Scholar]
- 54.Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage15, 273–289. 10.1006/nimg.2001.0978 (2002). 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 55.Zuo, X. N. et al. Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. NeuroImage65, 374–386. 10.1016/j.neuroimage.2012.10.017 (2013). 10.1016/j.neuroimage.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glickman, M. E., Rao, S. R. & Schultz, M. R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol.67, 850–857. 10.1016/j.jclinepi.2014.03.012 (2014). 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 57.Buckner, R. L., Krienen, F. M. & Yeo, B. T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci.16, 832–837. 10.1038/nn.3423 (2013). 10.1038/nn.3423 [DOI] [PubMed] [Google Scholar]
- 58.Ramnani, N. The primate cortico-cerebellar system: Anatomy and function. Nat. Rev. Neurosci.7, 511–522. 10.1038/nrn1953 (2006). 10.1038/nrn1953 [DOI] [PubMed] [Google Scholar]
- 59.Vaghi, M. M. et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive–compulsive disorder: Evidence from resting-state functional connectivity. Biol. Psychiatry81, 708–717. 10.1016/j.biopsych.2016.08.009 (2017). 10.1016/j.biopsych.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buckner, R. L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron80, 807–815. 10.1016/j.neuron.2013.10.044 (2013). 10.1016/j.neuron.2013.10.044 [DOI] [PubMed] [Google Scholar]
- 61.Frazier, M. R. et al. A missing link in affect regulation: The cerebellum. Soc. Cogn. Affect. Neurosci.17, 1068–1081. 10.1093/scan/nsac042 (2022). 10.1093/scan/nsac042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gruner, P., Anticevic, A., Lee, D. & Pittenger, C. Arbitration between action strategies in obsessive–compulsive disorder. Neurosci. Rev. J. Bring. Neurobiol. Neurol. Psychiatry22, 188–198. 10.1177/1073858414568317 (2016). 10.1177/1073858414568317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anticevic, A. et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive–compulsive disorder. Biol. Psychiatry75, 595–605. 10.1016/j.biopsych.2013.10.021 (2014). 10.1016/j.biopsych.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu, J. et al. Temporal variability of regional intrinsic neural activity in drug-naïve patients with obsessive–compulsive disorder. Hum. Brain Mapp.42, 3792–3803. 10.1002/hbm.25465 (2021). 10.1002/hbm.25465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, H. et al. Altered functional connectivity between the cerebellum and the cortico-striato-thalamo-cortical circuit in obsessive–compulsive disorder. Front. Psychiatry10.3389/fpsyt.2019.00522 (2019). 10.3389/fpsyt.2019.00522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu, W. et al. Disrupted pathways from the frontal-parietal cortices to basal nuclei and the cerebellum are a feature of the obsessive–compulsive disorder spectrum and can be used to aid in early differential diagnosis. Psychiatry Res.293, 113436. 10.1016/j.psychres.2020.113436 (2020). 10.1016/j.psychres.2020.113436 [DOI] [PubMed] [Google Scholar]
- 67.Sha, Z. et al. Functional disruption of cerebello-thalamo-cortical networks in obsessive–compulsive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging5, 438–447. 10.1016/j.bpsc.2019.12.002 (2020). 10.1016/j.bpsc.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kashyap, R. et al. Individual-fMRI-approaches reveal cerebellum and visual communities to be functionally connected in obsessive compulsive disorder. Sci. Rep.10.1038/s41598-020-80346-6 (2021). 10.1038/s41598-020-80346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian, L. et al. Abnormal functional connectivity of brain network hubs associated with symptom severity in treatment-naive patients with obsessive–compulsive disorder: A resting-state functional MRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry66, 104–111. 10.1016/j.pnpbp.2015.12.003 (2016). 10.1016/j.pnpbp.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 70.Fan, Q. et al. Abnormalities of white matter microstructure in unmedicated obsessive–compulsive disorder and changes after medication. PloS ONE7, e35889. 10.1371/journal.pone.0035889 (2012). 10.1371/journal.pone.0035889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin, H. et al. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive–compulsive disorder. J. Child Psychol. Psychiatry Allied Discip.48, 157–166. 10.1111/j.1469-7610.2006.01687.x (2007). 10.1111/j.1469-7610.2006.01687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ay, R. & Erbay, L. G. Relationship between childhood trauma and suicide probability in obsessive–compulsive disorder. Psychiatry Res.261, 132–136. 10.1016/j.psychres.2017.12.054 (2018). 10.1016/j.psychres.2017.12.054 [DOI] [PubMed] [Google Scholar]
- 73.Brooks, S. J. et al. Early-life adversity and orbitofrontal and cerebellar volumes in adults with obsessive–compulsive disorder: Voxel-based morphometry study. Br. J. Psychiatry J. Ment. Sci.208, 34–41. 10.1192/bjp.bp.114.162610 (2016). 10.1192/bjp.bp.114.162610 [DOI] [PubMed] [Google Scholar]
- 74.Mao, Y., Xiao, H., Ding, C. & Qiu, J. The role of attention in the relationship between early life stress and depression. Sci. Rep.10, 6154. 10.1038/s41598-020-63351-7 (2020). 10.1038/s41598-020-63351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We guarantee the authenticity of the data, but do not disclose the data, if necessary, you can email fjq7887215@163.com to obtain the data.