Abstract
The human T-cell leukemia virus type 1 (HTLV-1) transmembrane glycoprotein has a 24-amino-acid cytoplasmic domain whose function in the viral life cycle is poorly understood. We introduced premature-stop mutations and 18 single-amino-acid substitutions into this domain and studied their effects on cell-to-cell transmission of the virus. The results show that the cytoplasmic domain is absolutely required for cell-to-cell transmission of HTLV-1, through amino acids which cluster in a Y-S-L-I tyrosine-based motif. The transmission defect in two motif mutants did not result from a defect in glycoprotein incorporation or fusion. It appears that the Y-S-L-I tyrosine-based motif of the HTLV-1 glycoprotein cytoplasmic domain has multiple functions, including involvement in virus transmission at a postfusion step.
Retrovirus envelope glycoproteins consist of two subunits, the extracellular surface (SU) glycoprotein, responsible for attachment of the virus to a cell surface receptor, and the transmembrane (TM) glycoprotein, which anchors the SU-TM heterodimer at the surface of the infected cell or virion. The TM glycoprotein also mediates virus-to-cell and cell-to-cell fusion via a fusion peptide and a heptad repeat motif located at the N terminus of its extracellular domain.
All retrovirus TM glycoproteins have an intracellular domain. In lentiviruses, this cytoplasmic domain determines the rate of endocytosis of the glycoproteins at the plasma membrane (10, 27, 36, 37), permits interactions of the glycoproteins with the virus core (3), and is involved in incorporation of the glycoproteins into budding virus particles (9, 11, 12, 40, 41). The role of the cytoplasmic tail of the glycoprotein in the virus replication cycle is less clear in retroviruses which do not belong to the Lentivirus genus. However, the conservation of a cytoplasmic tail in these retroviruses must confer an evolutionary advantage, because the retroviral genome is very compact.
In the type C oncoviruses in which truncations of the TM cytoplasmic domain have been performed, i.e., Rous sarcoma virus and Moloney murine leukemia virus (Mo-MuLV), the domain is not required for incorporation of the glycoproteins into virions (29, 32, 34). This suggests that incorporation of the glycoproteins into the virus particles is a passive process which does not require interactions between the cytoplasmic domains of the glycoproteins and the Gag proteins. Hence, mutations of the cytoplasmic domains of Mo-MuLV or bovine leukemia virus (BLV) glycoproteins which reduce glycoprotein incorporation (14, 15, 19, 20) do so by hindering passive incorporation rather than by impairing active selection.
In Mo-MuLV, some deletions of the TM cytoplasmic domain which do not reduce incorporation do, nevertheless, affect infectivity (20, 32, 34), suggesting that the cytoplasmic domain is involved in other, perhaps early steps of the retrovirus cycle. However, the cytoplasmic domain of the Mo-MuLV glycoprotein has a special feature which most oncoviruses do not share: the TM cytoplasmic domain is truncated by the virus protease within the virus particle (16, 17).
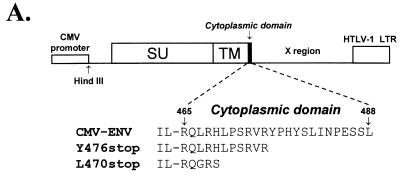
We have investigated the role of the oncoviral TM cytoplasmic domain in the virus life cycle by studying that of human T-cell leukemia virus type 1 (HTLV-1), which is a short domain of 24 amino acids. We first used the truncation mutants described in a previous study (30), modified by replacing the HTLV-1 promoter with the simian cytomegalovirus (CMV) promoter. This generated the L470stop (HTE-466 in reference 30, so named because it was constructed by inserting a linker at position 466) and the Y476stop (HTE-476 in reference 30) constructs, which have nonsense codons at positions 470 and 476, respectively (counting from the envelope initiator methionine) (Fig. 1A). The wild-type HTLV-1 envelope expressor construct was the CMV-ENV plasmid described in reference 5 (Fig. 1A).
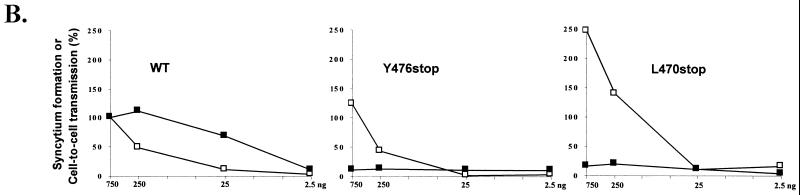
FIG. 1.
HTLV-1 envelope expressor plasmid and cytoplasmic domains of HTLV-1 wild-type and truncated glycoproteins (A), and comparison of syncytium formation and cell-to-cell transmission mediated by these glycoproteins (B). (A) The wild-type envelope expressor (CMV-ENV) used in this study. HindIII indicates that HTLV-1 sequences start at a HindIII site at position 4991 in Seiki’s sequence (38). Also shown are the cytoplasmic domains of the HTLV-1 wild-type glycoprotein and of the two truncated glycoproteins used in this study. LTR, long terminal repeat. (B) The results obtained for syncytium formation, shown as open symbols (□), and those for infectivity, shown as closed symbols (■). WT indicates the results obtained with the CMV-ENV wild-type construct; Y476stop and L470stop specify those obtained with the truncated glycoproteins. COS-1 cells were cotransfected with the pCS-HTLV-neo construct (0.75 μg) and various amounts of the glycoprotein expression plasmid. In each case, the total amount of DNA was kept constant at 1.5 μg by adding vector plasmid. To prevent cell growth, transfected cells were treated with mitomycin C 24 h after transfection. Half the treated cells were used for evaluation of syncytium formation, by coculture with 2 × 105 B5-Tat cells in a quantitative assay (5). The other half of the transfected cells were used to evaluate infectivity, by coculture with 2 × 105 B5 cells. G418-resistant colonies were counted after 2 to 3 weeks, and the infectivity index was calculated as described in reference 5. For both syncytium formation and infectivity assays, indices are expressed relative to 100%, which corresponds to the results obtained with 750 ng of wild-type glycoprotein expression vector.
HTLV-1 is transmitted almost exclusively via cell-to-cell contact in vivo (8, 21, 24, 28) and in vitro (25, 31). We evaluated the need for the TM cytoplasmic domain for transmission of HTLV-1, using an assay which measures quantitatively the cell-to-cell transmission of this virus in a single round of infection (5). This assay is based on trans complementation by envelope constructs of a defective HTLV-1 provirus in which the envelope gene was replaced by a selectable marker, the neomycin resistance gene. We first used transfection conditions similar to those employed in our previous studies (5). The HTLV-1 envelope constructs (0.75 μg) and the pCS-HTLV-neo defective provirus (0.75 μg) were used to cotransfect COS-LTRLacZ cells, which are COS-1 cells stably expressing the β-galactosidase gene under the control of the human immunodeficiency virus type 1 (HIV-1) long terminal repeat (obtained from M. Alizon, INSERM U332, Paris, France), or 293-TSA cells (obtained from J. Neyton, Ecole Normale Supérieure, Paris, France) seeded at a density of 3 × 105 per 60-mm-diameter dish the previous day. One day after transfection, the cells were treated with 10 μg of mitomycin (Amétycine; Laboratoires Choay, Paris, France)/ml for 3 h at 37°C to prevent growth. The cells were then washed with phosphate-buffered saline, trypsinized, and, along with 4 × 105 HeLa or B5 cells (a gift from D. Waters, Frederick Cancer Research and Development Center, Frederick, Md.), used to seed 60-mm-diameter dishes. The cells were cocultured for 2 days and then transferred to selection medium containing 125 μg (for B5 cells) or 0.5 mg (for HeLa cells) of G418 sulfate (Geneticin; Gibco)/ml. G418-resistant colonies were counted after 2 to 3 weeks.
Figure 1B is a plot of the infectivity index and the syncytium formation index, obtained in the same experiment using COS-LTRLacZ as transfected cells and B5 or B5-Tat cells as target cells. B5-Tat cells are stably transfected with a tat gene expressor (a gift from M. Sitbon, CNRS UMR 9942, Montpellier, France). This allows quantitative evaluation of syncytium formation, because the HIV-1 long terminal repeat is transactivated by the Tat protein upon envelope-induced fusion of the transfected COS-LTRLacZ cells with the B5-Tat cells, resulting in β-galactosidase synthesis. The enzymatic activity was evaluated by a chemilumiscence assay for use with cell lysates (Galacto-Light; Tropix). The indices were calculated as described in our previous report (5). Transfections with 750 ng of DNA for both the envelope and the defective provirus constructs showed that truncation of most (L470stop) or part (Y476stop) of the HTLV-1 cytoplasmic domain, although allowing very efficient cell-to-cell fusion (30), resulted in almost a complete loss of cell-to-cell transmission (less that 20% of the wild-type activity) regardless of the cells (HeLa or B5-Tat) used as targets and of the cells (COS-LTRLacZ or 293-TSA) used as virus-producing cells (Fig. 1B and data not shown).
Since the truncation of the cytoplasmic domain of HTLV-1 glycoprotein resulted in increased fusion of the mutated glycoproteins (30) (Fig. 1B), we tested whether the level of cell-to-cell transmission elicited by the truncated glycoproteins was artificially low due to cytopathic effects resulting in destruction of target cells in syncytia. We used transfection conditions that resulted in very low levels of syncytium formation while still allowing cell-to-cell transmission with the wild-type envelope construct. A constant amount of pCS-HTLV-neo plasmid was used to cotransfect COS-LTRLacZ cells with various amounts of the wild-type envelope construct. A slight increase in cell-to-cell transmission was correlated with lower levels of fusion when transfection was performed with 250 ng rather than 750 ng of DNA (Fig. 1B). Thus, high levels of fusion artificially caused a small (around 20%) decrease in cell-to-cell transmission. Transfection with 25 ng of DNA resulted in considerably less fusion, but we still detected cell-to-cell transmission. However, the truncated glycoproteins (Y476stop and L470stop) did not allow cell-to-cell transmission of the virus under the same conditions (Fig. 1B). Therefore, the loss of infectivity was not caused by the mutated glycoproteins being highly cytopathic but rather reflected a defect in the function of the mutated glycoproteins, preventing cell-to-cell transmission of the virus.
We then examined whether the loss of infectivity observed with the truncated mutants was caused by inefficient incorporation of truncated glycoproteins into the HTLV-1 virions. The incorporation of the mutated envelope glycoproteins into HTLV-1 virions was assayed as described previously (5), except that we used low concentrations of envelope expressors (0.5 μg) to minimize fusion and the release of cell membranes, vesicles, or blebs into the supernatants. Retrovirus particles were produced by cotransfecting 293-TSA cells with pCS-HTLV-neo and the envelope expression plasmids. The cells were metabolically labeled with 700 μCi of a [35S]cysteine-[35S]methionine mix (Amersham)/ml 24 h later. The cell supernatants were collected, centrifuged for 10 min at 1,200 × g to remove debris, and filtered through a 0.45-μm-pore-size filter. The amount of contaminating envelope proteins released into the supernatant by each mutant was evaluated by individually cotransfecting the envelope expression plasmids and a control plasmid. The supernatants were then layered onto discontinuous sucrose gradients (3 ml of 30% sucrose over 3 ml of 50% sucrose) and centrifuged for 2 h at 25,000 rpm, using an SW41 rotor. The virus sedimented at the interface between the two layers and was collected in a volume of 1 ml, to which 200 μl of 5× lysis buffer was added. The viral proteins were then immunoprecipitated with serum from HTLV-1-infected individuals (4, 35).
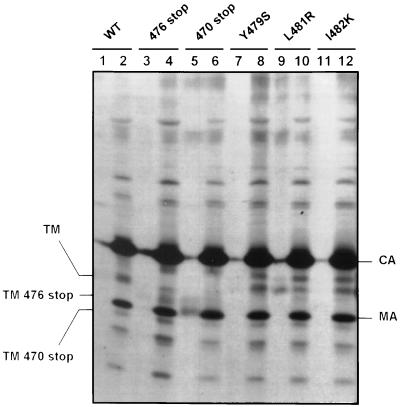
The protein with a short truncation of the cytoplasmic domain (Y476stop) was incorporated into the virions as efficiently as the wild-type glycoprotein, as shown by the comparable amounts of mature TM protein in the virus particles (Fig. 2; compare lane 4 with lane 2). Thus, the lack of infectivity in this case cannot be explained by an incorporation defect. The protein with the longest truncation (L470stop) was incorporated very inefficiently. The TM band was not detected in the virus particles (Fig. 2, lane 6), although it was visible in the cell supernatant, probably due to contamination by vesicles or blebs (Fig. 2, lane 5).
FIG. 2.
Incorporation of truncated or single-amino-acid-substituted glycoproteins into virions. Cells were transfected with the various constructs. The contamination of the virion preparations with vesicles or blebs was evaluated in each case by comparing the amounts of TM obtained when the envelope expressor plasmid was coexpressed with the pCS-HTLV-neo plasmid (even lanes) with the amounts of TM in contamination controls obtained when the envelope expressor plasmid was coexpressed with a mock plasmid (odd lanes). Virus particles released into the supernatants were purified on sucrose gradients, lysed, and immunoprecipitated with serum from HTLV-1-infected individuals, as described in reference 5. WT, wild type; CA, capsid; MA, matrix. Lanes: 1, wild-type glycoprotein expressor plus pcDNA3 mock plasmid (background level of envelope in the supernatant, in the absence of virus particle formation); 2, wild-type glycoprotein expressor plus pCS-HTLV-neo; 3, Y476stop plus pcDNA3; 4, Y476stop plus pCS-HTLV-neo; 5, L470stop plus pcDNA3; 6, L470stop plus pCS-HTLV-neo; 7, Y479S plus pcDNA3; 8, Y479S plus pCS-HTLV-neo; 9, L481R plus pcDNA3; 10, L481R plus pCS-HTLV-neo; 11, I482K plus pcDNA3; 12, I482K plus pCS-HTLV-neo.
We then performed single-amino-acid substitutions and tested their effects on the cell-to-cell transmission of HTLV-1 in an effort to identify the amino acids of the cytoplasmic domain of the HTLV-1 glycoproteins required for infectivity (Table 1). A total of 16 single-amino-acid substitutions were performed via oligonucleotide-directed mutagenesis of the sequence encoding the HTLV-1 envelope protein by the Kunkel method (4, 35). The mutated env fragments were then inserted into the CMV-ENV expression plasmid, sequenced, and used for transfection. The mutants were named “X amino acid position Y,” where X and Y are the wild-type and substituted amino acids, respectively, and amino acid position 1 corresponds to the initiator methionine of the HTLV-1 envelope protein. We also used two other single-amino-acid-substitution constructs which we described in a previous report (23). The effect of the mutations on HTLV-1 transmission was studied under the transfection conditions deduced from Fig. 1B, i.e., resulting in cell-to-cell transmission but very little syncytium formation (25 ng of DNA).
TABLE 1.
Effect of single-amino-acid substitutions in the HTLV-1 glycoprotein cytoplasmic domain on precursor cleavage, syncytium formation, and cell-to-cell transmissiona
| Proteinb | Precursor cleavage (%)c | Syncytium formation (% ± SE)df | Cell-to-cell transmission (% ± SE)ef |
|---|---|---|---|
| W.T. | 100 | 100 | 100 |
| R465L | 66 | 4 ± 2 | 47 ± 7 |
| R467Q | 84 | 26 ± 4 | 89 ± 36 |
| L470R | 85 | 60 ± 14 | 81 ± 12 |
| P471T | 90 | 196 ± 70 | 122 ± 37 |
| R473L | 87 | 103 ± 9 | 86 ± 4 |
| V474F | 81 | 108 ± 5 | 67 ± 18 |
| R475I | 96 | 49 ± 9 | 96 ± 6 |
| Y476S | 99 | 243 ± 108 | 81 ± 11 |
| P477T | 93 | 79 ± 9 | 72 ± 16 |
| H478V | 77 | 94 ± 36 | 73 ± 0 |
| Y479S | 96 | 146 ± 21 | 10 ± 6 |
| S480V | 79 | 64 ± 19 | 44 ± 1 |
| L481R | 77 | 168 ± 45 | 12 ± 2 |
| I482K | 63 | 166 ± 42 | 0 ± 0 |
| P484R | 78 | 101 ± 6 | 57 ± 26 |
| E485A | 84 | 73 ± 3 | 78 ± 10 |
| S486L | 71 | 86 ± 9 | 103 ± 13 |
| L488Q | 38 | 44 ± 8 | 62 ± 2 |
Data are the means of values from at least two or three independent experiments.
W.T., wild-type glycoprotein; otherwise the first letter represents the wild-type amino acid, the number refers to the amino acid position in the glycoprotein (number 1 corresponds to the initiator methionine), and the last letter indicates the name of the substituted amino acid.
Calculated as described in reference 5, using quantitative assays.
The assay for syncytium formation involves measuring the β-galactosidase activity induced by envelope-mediated fusion. For the wild-type construct, 1,000 to 1,500 syncytia were obtained per 3 × 105 cells transfected with 1 μg of envelope expressor plasmid, as counted in parallel experiments.
Cell-to-cell transmission with the wild-type glycoproteins resulted in 250 to 350 neomycin-resistant colonies per 3 × 105 cells transfected with 25 ng of envelope expressor plasmid.
The results shown were obtained with COS-LTRLacZ cells for transfections and B5-Tat cells as indicators.
Only 3 of the 18 amino acid substitutions (Y479S, L481R, and I482K) resulted in very low rates of cell-to-cell transmission, less than 20% of that of the wild-type protein (Table 1). The 15 other mutants had infectivity levels comparable to or within the range of half that of the wild-type protein. The three mutations resulting in the loss of infectivity clustered in a Y-S-L-I sequence (Fig. 1A), which fits the Y-X-X-hydrophobic amino acid consensus of tyrosine-based motifs. Such motifs are conserved among most type C retrovirus glycoprotein cytoplasmic domains (23).
The fusion capacities of the three mutants with impaired cell-to-cell transmission were augmented, as were those of the mutants with truncated cytoplasmic domains. We verified that the phenotypes of the three mutants were similar by using 293-TSA cells or COS-LTRLacZ as virus-producing cells and B5 or HeLa as indicator cells, although the increase in fusion was smaller with B5 cells (data not shown). The three mutants also exhibited correct precursor maturation (Table 1). The L481R glycoprotein mutant was not efficiently incorporated into the virions, since the amount of protein in purified virus particles was comparable to that of the control supernatant (Fig. 2, lanes 10 and 9, respectively). However, the amounts of TM glycoprotein in the virions of two of these mutants (Y479S and I482K) were similar to that for the wild-type glycoprotein (Fig. 2; compare lanes 8 and 12 with lane 2). Thus, these two mutants with single-amino-acid substitutions in the tyrosine-based motif have the same phenotype as the mutant with the shortest truncation of the cytoplasmic domain (Y476stop); i.e., they do not allow infection despite being fully mature, highly fusogenic, and competent for glycoprotein incorporation into virions.
Several conclusions may be drawn concerning the cytoplasmic domain of the HTLV-1 glycoproteins. The cytoplasmic domain is absolutely required for the cell-to-cell transmission of the virus, and a Y-S-L-I tyrosine-based motif plays a key role in this process. The event requiring the cytoplasmic domain or the tyrosine-based motif is distinct from fusion, as well as from the incorporation of glycoproteins into the virions, because some of the glycoproteins with truncations or mutations impairing transmission were both fusogenic and incorporated into virions.
Thus, there is not an association between high fusion competence and low infectivity for the mutants with truncated, or tyrosine-based motif-mutated, cytoplasmic domains. It cannot be argued, however, that their low infectivity results from their high fusion competence, which would cause cytopathic effects resulting in the destruction of target cells in syncytia. First, we defined transfection conditions which resulted in a very low level of syncytium formation. Infectivity remained very low for the truncated or the tyrosine-based motif-mutated glycoproteins under these conditions but was still detected for the wild-type glycoprotein. Second, there was a large increase in fusion with the P471T mutant, reaching 200% of that of the wild-type protein, but this was associated with augmented rather than diminished cell-to-cell transmission (Table 1). Thus, elevated levels of fusion can be associated with normal or high levels of cell-to-cell transmission, and hence the result obtained with the truncated or tyrosine-based motif-mutated glycoproteins is attributable to a defect in transmission.
Fusion is regulated by multiple determinants in the cytoplasmic domains of HTLV-1 glycoproteins. These include the tyrosine-based motif, as well as amino acids outside this motif (Table 1). A high-level fusion phenotype is consistently obtained with retroviral glycoproteins having a truncated cytoplasmic domain, provided that they are correctly transported to the cell surface. This is the case for the mammalian type C (34) and type D (2) retroviruses, as well as those of the Lentivirus genus (9, 13, 26, 42), and is also true for HTLV-1 truncated glycoproteins (30). Tyrosine-based motifs are involved in the ligand-induced internalization of some cell surface receptors via clathrin-coated pits (for a review, see reference 39). A membrane-proximal tyrosine is important for cell surface distribution (22) and for endocytosis (10, 36) of glycoproteins in HIV-1 and simian immunodeficiency virus. The HTLV-1 tyrosine-based motif is also likely to control the rate of endocytosis of the glycoproteins, thereby regulating the level of envelope-mediated fusion. We have shown that the glycoprotein cytoplasmic domain interacts with components of the clathrin adapter complexes (1). This suggests that during the course of evolution retroviruses have retained cytoplasmic domains which diminish the cell surface expression of their glycoproteins. This may help them to escape the host immune response in vivo. Alternatively, reducing cell surface expression may reduce the level of fusion that is detrimental to the virus, because fusion causes death of virus-producing cells or of potential target cells.
In contrast to what has been shown for Rous sarcoma virus (29) and Mo-MuLV (14, 15, 32, 34), complete truncation of the cytoplasmic domains of HTLV-1 glycoproteins resulted in their inefficient incorporation into the virion. This could indicate that incorporation involves interactions between the cytoplasmic domains of the glycoproteins and the Gag proteins anchored at the plasma membrane in HTLV-1, as in HIV-1 (3). Alternatively, HTLV-1 may incorporate its glycoproteins into budding virions via a common passive process like the other retroviruses with short cytoplasmic domains, as previously suggested (6, 18). The fact that truncations or single-amino-acid substitutions in the cytoplasmic domain can diminish incorporation, as shown here for HTLV-1 with the L470stop and L481R mutants and elsewhere for Mo-MuLV (20) and BLV (19), does not necessarily conflict with this view, but it suggests that some modified conformations of the cytoplasmic domain can hinder incorporation.
The tyrosine-based motif involved in HTLV-1 transmission is not, however, involved in the incorporation process, as is the tyrosine-based motif in the cytoplasmic domain of the BLV glycoproteins (19). Two HTLV-1 glycoproteins with substitutions in the tyrosine-based motif of the cytoplasmic domain were incorporated into virions without allowing viral transmission, regardless of the cell line used to produce the virus or as target cells. Very short truncations of the C terminus of the HIV-1 glycoproteins produce a similar phenotype (9, 41), as do mutations of the cytoplasmic domain of Mo-MuLV glycoproteins (20, 32, 33). Thus, in HTLV-1 in particular, and in retroviruses in general, the cytoplasmic domain has a function in viral transmission distinct from the defined envelope functions. This study shows that such a function can be attributed to the tyrosine-based motif of HTLV-1 glycoproteins and emphasizes that this motif has multiple functions. These include addressing virions to the basolateral face of polarized epithelial cells (23) and the endocytosis of glycoproteins, as discussed above.
What is the additional function of the cytoplasmic domain in virus transmission, and why is the tyrosine-based motif in the HTLV-1 cytoplasmic domain so important in this process? Recent data show that a membrane-proximal tyrosine-based motif favors cell-to-cell transmission of HIV-1 (7). The tyrosine-based motif in HTLV-1 might be essential for ensuring that the virus is released at the site of interactions between a virus-producing cell and a target cell, since HTLV-1 is mainly transmitted via cell-cell contacts. Alternatively, the tyrosine-based motif might be required to propel the virus core into the cytoplasm of the target cell. It could also be involved in the transduction of a signal, required for efficient infection, in the target cell. In Mo-MuLV, the tyrosine-based motif is removed after the truncation of the cytoplasmic domain in the virus particle, but a membrane-proximal domain, retained after the truncation, is also required for infectivity, probably at a postfusion step (20). Mo-MuLV and HTLV-1 glycoprotein cytoplasmic tails thus have similar functions, accomplished via different motifs. Further research is required to determine the exact nature of the process involving the cytoplasmic domains of oncoviruses in the early steps of infection.
Acknowledgments
We thank Y. Coste (CRTS, Montpellier, France) for regularly providing patients’ sera. The English text was edited by Owen Parkes.
This work was supported by a grant from the Association Nationale pour la Recherche sur le SIDA (ANRS; Paris, France) and by the Association pour la Recherche sur le Cancer (ARC; Villejuif, France).
REFERENCES
- 1.Berlioz-Torrent C, Shacklett B L, Erdtmann L, Delamarre L, Bouchaert I, Sonigo P, Dokhelar M C, Benarous R. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J Virol. 1999;73:1350–1361. doi: 10.1128/jvi.73.2.1350-1361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody B A, Rhee S S, Hunter E. Postassembly cleavage of a retroviral glycoprotein cytoplasmic domain removes a necessary incorporation signal and activates fusion activity. J Virol. 1994;68:4620–4627. doi: 10.1128/jvi.68.7.4620-4627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosson P. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 4.Delamarre L, Pique C, Pham D, Tursz T, Dokhélar M-C. Identification of functional regions in the human T-cell leukemia virus type 1 SU glycoprotein. J Virol. 1994;68:3544–3549. doi: 10.1128/jvi.68.6.3544-3549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delamarre L, Rosenberg A R, Pique C, Pham D, Dokhélar M-C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J Virol. 1997;71:259–266. doi: 10.1128/jvi.71.1.259-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denesvre C, Carrington C, Corbin A, Takeuchi Y, Cosset F-L, Schulz T, Sitbon M, Sonigo P. TM domain swapping of murine leukemia virus and human T-cell leukemia virus envelopes confers different infectious abilities despite similar incorporation into virions. J Virol. 1996;70:4380–4386. doi: 10.1128/jvi.70.7.4380-4386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschambeault J, Lalonde J-P, Cervantes-Acosta G, Lodge R, Cohen É, Lemay G. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J Virol. 1999;73:5010–5017. doi: 10.1128/jvi.73.6.5010-5017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donegan E, Lee H, Operskalski E A, Shaw G M, Kleinman S H, Busch M P, Stevens C E, Schiff E R, Nowicki M J, Hollingsworth C G, Mosley J W The Transfusion Safety Study Group. Transfusion transmission of retroviruses: human T-lymphotropic virus types I and II compared with human immunodeficiency virus type 1. Transfusion. 1994;34:478–483. doi: 10.1046/j.1537-2995.1994.34694295061.x. [DOI] [PubMed] [Google Scholar]
- 9.Dubay J W, Roberts S J, Hahn B H, Hunter E. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan M A, Carruth L M, Rowell J F, Yu X, Siliciano R F. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol. 1996;70:6547–6556. doi: 10.1128/jvi.70.10.6547-6556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed E O, Martin M A. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed E O, Martin M A. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabuzda D H, Lever A, Terwilliger E, Sodroski J. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1992;66:3306–3315. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granowitz C, Colicelli J, Goff S P. Analysis of mutations in the envelope gene of Moloney murine leukemia virus: separation of infectivity from superinfection resistance. Virology. 1991;183:545–554. doi: 10.1016/0042-6822(91)90983-i. [DOI] [PubMed] [Google Scholar]
- 15.Gray K D, Roth M J. Mutational analysis of the envelope gene of Moloney murine leukemia virus. J Virol. 1993;67:3489–3496. doi: 10.1128/jvi.67.6.3489-3496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green N, Shinnick T M, Witte O, Ponticelli A, Sutcliffe J G, Lerner R A. Sequence-specific antibodies show that maturation of Moloney leukemia virus envelope polyprotein involves removal of a COOH-terminal peptide. Proc Natl Acad Sci USA. 1981;78:6023–6027. doi: 10.1073/pnas.78.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson L E, Sowder R, Copeland T D, Smythers G, Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol. 1984;52:492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 19.Inabe K, Nishizawa M, Tajima S, Ikuta K, Aida Y. The YXXL sequences of a transmembrane protein of bovine leukemia virus are required for viral entry and incorporation of viral envelope protein into virions. J Virol. 1999;73:1293–1301. doi: 10.1128/jvi.73.2.1293-1301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Januszeski M M, Cannon P M, Chen D, Rozenberg Y, Anderson W F. Functional analysis of the cytoplasmic tail of Moloney murine leukemia virus envelope protein. J Virol. 1997;71:3613–3619. doi: 10.1128/jvi.71.5.3613-3619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinman S, Swanson P, Allain J P, Lee H. Transfusion transmission of human T-lymphotropic virus types I and II: serologic and polymerase chain reaction results in recipients identified through look-back investigations. Transfusion. 1993;33:14–18. doi: 10.1046/j.1537-2995.1993.33193142303.x. [DOI] [PubMed] [Google Scholar]
- 22.LaBranche C C, Sauter M M, Haggarty B S, Vance P J, Romano J, Hart T K, Bugelski P J, Marsh M, Hoxie J A. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J Virol. 1995;69:5217–5227. doi: 10.1128/jvi.69.9.5217-5227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodge R, Delamarre L, Lalonde J-P, Alvarado J, Sanders D A, Dokhélar M-C, Cohen É, Lemay G. Two distinct oncornaviruses harbor an intracytoplasmic tyrosine-based basolateral targeting signal in their viral envelope glycoprotein. J Virol. 1997;71:5696–5702. doi: 10.1128/jvi.71.7.5696-5702.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manns A, Wilks R J, Murphy E L, Haynes G, Figueroa J P, Barnett M, Hanchard B, Blattner W A. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer. 1992;51:886–891. doi: 10.1002/ijc.2910510609. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto K, Tomita N, Ishii A, Nishizaki T, Kitajima K, Tanaka T, Nakamura T, Watanabe S, Oda T. Transformation of ATLA-negative leukocytes by blood components from anti-ATLA-positive donors in vitro. Int J Cancer. 1984;33:721–725. doi: 10.1002/ijc.2910330603. [DOI] [PubMed] [Google Scholar]
- 26.Mulligan M J, Yamshchikov G V, Ritter G D, Jr, Gao F, Jin M J, Nail C D, Spies C P, Hahn B H, Compans R W. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J Virol. 1992;66:3971–3975. doi: 10.1128/jvi.66.6.3971-3975.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohno H, Aguilar R C, Fournier M C, Hennecke S, Cosson P, Bonifacino J S. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology. 1997;238:305–315. doi: 10.1006/viro.1997.8839. [DOI] [PubMed] [Google Scholar]
- 28.Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T-cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 1984;46:245–253. doi: 10.1111/j.1423-0410.1984.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 29.Perez L G, Davis G L, Hunter E. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J Virol. 1987;61:2981–2988. doi: 10.1128/jvi.61.10.2981-2988.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pique C, Pham D, Tursz T, Dokhélar M-C. The cytoplasmic domain of the human T-cell leukemia virus type I envelope can modulate envelope functions in a cell type-dependent manner. J Virol. 1993;67:557–561. doi: 10.1128/jvi.67.1.557-561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popovic M, Sarin P S, Robert-Guroff M, Kalyanaraman V S, Mann D, Minowada J, Gallo R C. Isolation and transmission of human retrovirus (human T-cell leukemia virus) Science. 1983;219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 32.Ragheb J A, Anderson W F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragheb J A, Anderson W F. Uncoupled expression of Moloney murine leukemia virus envelope polypeptides SU and TM: a functional analysis of the role of TM domains in viral entry. J Virol. 1994;68:3207–3219. doi: 10.1128/jvi.68.5.3207-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg A R, Delamarre L, Pique C, Pham D, Dokhélar M-C. The ectodomain of the human T-cell leukemia virus type 1 TM glycoprotein is involved in postfusion events. J Virol. 1997;71:7180–7186. doi: 10.1128/jvi.71.10.7180-7186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowell J F, Stanhope P E, Siliciano R F. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing with class II MHC. J Immunol. 1995;155:473–488. [PubMed] [Google Scholar]
- 37.Sauter M M, Pelchen-Matthews A, Bron R, Marsh M, LaBranche C C, Vance P J, Romano J, Haggarty B S, Hart T K, Lee W M, Hoxie J A. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J Cell Biol. 1996;132:795–811. doi: 10.1083/jcb.132.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Yuan X, Matsuda Z, Lee T-H, Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X, Yuan X, McLane M F, Lee T-H, Essex M. Mutations in the cytoplasmic domain of human immunodeficiency virus type 1 transmembrane protein impair the incorporation of Env proteins into mature virions. J Virol. 1993;67:213–221. doi: 10.1128/jvi.67.1.213-221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zingler K, Littman D R. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases Env incorporation into particles and fusogenicity and infectivity. J Virol. 1993;67:2824–2831. doi: 10.1128/jvi.67.5.2824-2831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]