Abstract
Introduction
Quality of surgical care is understudied for lobular inflammatory breast cancer (IBC), which is less common, more chemotherapy-resistant, and more mammographically occult than ductal IBC. We compared guideline-concordant surgery (modified radical mastectomy [MRM] without immediate reconstruction following chemotherapy) for lobular versus ductal IBC.
Methods
Female individuals with cT4dM0 lobular and ductal IBC were identified in the National Cancer Database (NCDB) from 2010–2019. Modified radical mastectomy receipt was identified via codes for “modified radical mastectomy” or “mastectomy” and “≥10 lymph nodes removed” (proxy for axillary lymph node dissection). Descriptive statistics, chi-square tests, and t-tests were used.
Results
A total of 1456 lobular and 10,445 ductal IBC patients were identified; 599 (41.1%) with lobular and 4859 (46.5%) with ductal IBC underwent MRMs (p = 0.001). Patients with lobular IBC included a higher proportion of individuals with cN0 disease (20.5% lobular vs. 13.7% ductal) and no lymph nodes examined at surgery (31.2% vs. 24.5%) but were less likely to be node-negative at surgery (12.7% vs. 17.1%, all p < 0.001). Among those who had lymph nodes removed at surgery, patients with lobular IBC also had fewer lymph nodes excised versus patients with ductal IBC (median [interquartile range], 7 (0–15) vs. 9 (0–17), p = 0.001).
Conclusions
Lobular IBC patients were more likely to present with node-negative disease and less likely to be node-negative at surgery, despite having fewer, and more frequently no, lymph nodes examined versus ductal IBC patients. Future studies should investigate whether these treatment disparities are because of surgical approach, pathologic assessment, and/or data quality as captured in the NCDB.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-024-15540-1.
Inflammatory breast cancer (IBC) is rare, constituting only 1–5% of breast cancers, yet contributes to approximately 10% of breast cancer-related mortality in the United States.1,2 Treatment for IBC has improved over recent decades with increasingly effective systemic therapy, particularly with regards to targeted therapies (e.g., trastuzumab, pertuzumab) for HER2-positive (HER2+) disease.3-5
Current guideline-concordant care (GCC) for IBC dictates a course of treatment that typically begins with neoadjuvant chemotherapy (NACT) followed by modified radical mastectomy (MRM) without immediate reconstruction; postmastectomy radiation therapy (PMRT); and appropriate adjuvant therapy by tumor subtype (e.g., endocrine therapy for hormone receptor-positive [HR+] disease) and response to NACT.6 Inflammatory breast cancer is a clinical diagnosis and can manifest as any histology but most commonly presents as ductal histology in nearly 90% of cases.5,7,8 Thus, most literature and treatment recommendations are based on cumulative experience with ductal IBC. Furthermore, receipt of guideline-concordant surgery for lobular IBC is relatively understudied. We compare receipt of guideline-concordant surgery (i.e., MRM without immediate reconstruction after chemotherapy) for lobular versus ductal IBC.
Methods
Female patients diagnosed with cT4dM0 ductal or lobular breast cancer between 2010 and 2019 from the National Cancer Database (NCDB) were identified. Patients missing survival status or with metastatic disease (M1) were excluded.
Guideline-concordant surgery was defined as modified radical mastectomy (MRM) without immediate reconstruction following neoadjuvant chemotherapy. We defined MRM as any of the following: total (simple) mastectomy with ≥10 lymph nodes removed, total simple mastectomy with ≥10 lymph nodes removed without removal of uninvolved contralateral breast, total simple mastectomy with ≥10 lymph nodes removed with removal of uninvolved contralateral breast, MRM, MRM without removal of uninvolved contralateral breast, MRM with removal of uninvolved contralateral breast, and radical mastectomy, NOS. The specific codes that were used to identify potentially guideline-concordant surgeries are summarized in Appendix 1 and Appendix 2. A sizable proportion of patients with primary surgery site codes for MRM were documented as having no lymph nodes removed despite axillary lymph node dissection (ALND) being a standard component of MRMs. For most analyses, we limited our cohort of guideline-concordant surgery recipients to those who had ≥10 lymph nodes removed and examined, given common use of this criterion to define ALND (see Limitations). However, we also performed sensitivity analyses in which we did not exclude MRM recipients with <10 lymph nodes from being categorized as having received guideline-concordant surgery to account for potential miscoding of ALND and under-capture of lymph node yield.
Demographic factors for patients who met inclusion criteria were abstracted. Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. Chi-square tests and t-tests were used to test for differences in categorical and continuous variables, respectively. Effective sample sizes are included for all tables and figures. No adjustments were made for multiple comparisons. P < 0.05 was deemed significant for all analyses, which were conducted using SAS version 9.4 and R version 4.2.2.
This study operated under a waiver of HIPAA after being reviewed by the University of Pennsylvania Institutional Review Board (Protocol #831190). NCDB guidelines were followed to protect patient privacy, and counts < 20 were not reported. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines for reporting observational studies.9
Results
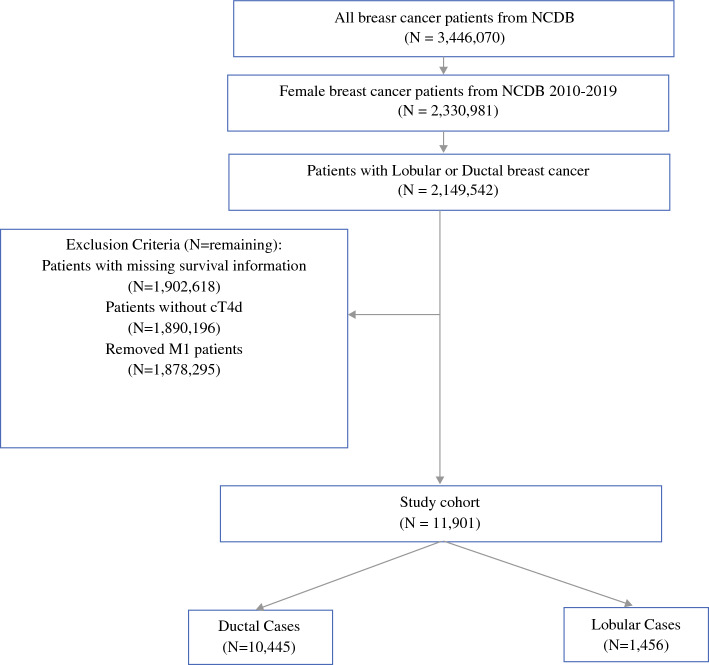
Of the 3,446,070 breast patients identified in the NCDB from 2010–2019, only 11,901 female patients with nonmetastatic ductal (n = 10,445) or lobular (n = 1,456) IBC were included in our analytic cohort (Fig. 1).
Fig. 1.
Consort diagram for analytical cohort using the national cancer database, 2010–2019. NCDB National Cancer Database
Patient characteristics are summarized in Table 1. Patients with lobular IBC were older compared with patients with ductal IBC (56.7% lobular vs. 43.9% ductal ≥ 60 years; p < 0.001); 75.3% of patients with lobular IBC and 68.7% of patients with ductal IBC were non-Hispanic (NH) white, and 15.1% of patients with lobular IBC and 10.4% of patients with ductal IBC were NH black. Biomarker subtypes were less evenly distributed within the lobular cohort, which had significantly higher rates of HR+/HER2− disease (55.3% lobular vs. 34.9% ductal) and lower rates of HER2+ disease (13.4% lobular vs. 24.6% ductal) and TNBC (13.4% lobular vs. 24.6% ductal, p < 0.001). Patients with lobular IBC also were more likely to have tumors >5 cm at the time of diagnosis (68.3% lobular vs. 61.4% ductal, p < 0.001).
Table 1.
Patients with ductal and lobular inflammatory breast cancer in the National Cancer Database, 2010–2019
| Ductal | Lobular | All patients | p | ||
|---|---|---|---|---|---|
| n = 10,445 | n = 1456 | N = 11,901 | |||
| Age median (IQR) | 57 (48–66) | 62 (52–71) | 58 (49–67) | < 0.001 | |
| Age | <50 | 2835 (27.1) | 294 (20.2) | 3129 (26.3%) | < 0.001 |
| 50–59 | 3,019 (28.9) | 336 (23.1) | 3,355 (28.2%) | ||
| 60–69 | 2,573 (24.6) | 415 (28.5) | 2,988 (25,1%) | ||
| 70–79 | 1,265 (12.1) | 240 (16.5) | 1,505 (12.6%) | ||
| >80 | 753 (7.2) | 171 (11.7) | 924 (7.8%) | ||
| Race/ethnicity | AI/AN | 43 (0.4) | 10 (0.7) | 53 (0.4%) | < 0.001 |
| Hispanic | 741 (7.1) | 79 (5.4) | 820 (6.9%) | ||
| NH Asian/PI | 305 (2.9) | 25 (1.7) | 330 (2.8%) | ||
| NH black | 2031 (10.4) | 220 (15.1) | 2,251 (18.9%) | ||
| NH white | 7177 (68.7) | 1097 (75.3) | 8274 (69.5%) | ||
| Unknown | 148 (1.4) | 25 (1.7) | 173 (1.5%) | ||
| Subtype | HER2+ | 3604 (34.5) | 369 (25.3) | 3973 (33.4%) | < 0.001 |
| HR+/HER2− | 3646 (34.9) | 805 (55.3) | 4451 (37.4%) | ||
| TNBC | 2566 (24.6) | 195 (13.4) | 2761 (23.2%) | ||
| Unknown | 629 (6.0) | 87 (6.0) | 716 (6.0%) | ||
| Tumor size (cm) | <1 | 346 (3.3) | 36 (2.5) | 382 (3.2%) | < 0.001 |
| 1–1.9 | 860 (8.2) | 115 (7.9) | 975 (8.2%) | ||
| 2–2.9 | 998 (9.6) | 97 (6.7) | 1,095 (9.2%) | ||
| 3–3.9 | 956 (9.2) | 127 (8.7) | 1083 (9.1%) | ||
| 4–4.9 | 873 (8.4) | 86 (5.9) | 959 (8.1%) | ||
| >5 | 6,412 (61.4) | 995 (68.3) | 7407 (62.2%) | ||
| Node-negative | No** | 5895 (56.4) | 798 (54.8) | 6693 (56.2%) | < 0.001 |
| Yes** | 1848 (17.7) | 185 (12.7) | 2033 (17.1%) | ||
| Nodes not examined | 2556 (24.5) | 454 (31.2) | 3010 (25.3%) | ||
| Unknown | 146 (1.4) | 19 (1.3) | 165 (1.4%) | ||
| Lymph node count | No nodes examined | 2556 (24.5) | 454 (31.2) | 3010 (25.3%) | < 0.001 |
| <10 | 2,248 (21.5) | 310 (21.3) | 2,558 (21.5%) | ||
| ≥10 | 3711 (35.5) | 455 (31.3) | 4,166 (35%) | ||
| Unknown | 1930 (18.5) | 237 (16.3) | 2167 (18.2%) | ||
| Types of breast surgery | Modified radical mastectomy | 4230 (40.5%) | 519 (35.6%) | 4749 (39.9%) | 0.002 |
| Total mastectomy | 1768 (16.9%) | 244 (16.8%) | 2012 (16.9%) | ||
| Other surgery | 1,280 (12.3%) | 192 (13.2%) | 1,472 (12.4%) | ||
| None | 3167 (30.3%) | 501 (34.4%) | 3668 (30.8%) | ||
| Guideline-concordant surgery (GCS) vs. other types of surgery | GCS (i.e., MRM and/or TM with ≥10 LN excised) | 4859 (46.5%) | 599 (41.1%) | 5458 (45.9%) | < 0.001 |
| Other surgery | 2,419 (23.2%) | 356 (24.5%) | 2775 (23.3%) | ||
| None | 3167 (30.3%) | 501 (34.4%) | 3668 (30.8%) | ||
| Clinical N classification | cN0 | 1430 (13.7%) | 298 (20.5%) | 1728 (14.5%) | < 0.001 |
| cN1 | 5230 (50.1%) | 684 (47.0%) | 5914 (49.7%) | ||
| cN2 | 1600 (15.3%) | 200 (13.7%) | 1800 (15.1%) | ||
| cN3 | 1903 (18.2%) | 226 (15.5%) | 2129 (17.9%) | ||
| Unknown | 282 (2.7%) | 48 (3.3%) | 330 (2.8%) | ||
| Pathological T classification | pT0 | 1404 (13.50%) | 136 (9.3%) | 1540 (12.9%) | < 0.001 |
| pT1 | 943 (9.0%) | 95 (6.5%) | 1,038 (8.7%) | ||
| pT2 | 796 (7.6%) | 116 (8.0%) | 912 (7.7%) | ||
| pT3 | 595 (5.7%) | 178 (12.2%) | 773 (6.5%) | ||
| pT4 | 1702 (16.3%) | 232 (15.9%) | 1934 (16.3%) | ||
| Other | 5005 (47.9%) | 699 (48.0%) | 5704 (47.9%) | ||
| Pathological N classification | pN0 | 2547 (24.4%) | 272 (18.7%) | 2819 (23.7%) | < 0.001 |
| pN1 | 1684 (16.1%) | 195 (13.4%) | 1879 (15.8%) | ||
| pN2 | 1,303 (12.5%) | 218 (15.0%) | 1,521 (12.8%) | ||
| pN3 | 827 (7.9%) | 151 (10.4%) | 978 (8.2%) | ||
| Other | 4084 (39.1%) | 620 (42.6%) | 4704 (39.5%) | ||
| Received radiation | Administered | 3228 (31.0%) | 478 (32.8%) | 3716 (31.2%) | 0.334 |
| None | 5669 (54.3%) | 777 (53.4%) | 6446 (54.2%) | ||
| Possible inappropriate administration (e.g., whole breast) | 789 (7.6%) | 113 (7.8%) | 902 (7.6%) | ||
| Other | 749 (7.25%) | 88 (6%) | 837 (7%) | ||
| Received endocrine therapy* | < 0.001 | ||||
| Yes | 2786 (76.4%) | 655 (81.4%) | 3441 (77.3%) | ||
| No | 860 (23.6%) | 150 (18.6%) | 1010 (22.7%) | ||
*HR + patients only; N = 4,451
**Lymph nodes removed at surgery and examined by pathologist.
IQR Interquartile range; AI/AN American Indian/Alaska Native; NH Non-Hispanic; TNBC Triple-negative breast cancer; MRM Modified radical mastectomy
Among patients undergoing axillary surgery, higher proportion of patients with lobular IBC presented with cN0 disease (20.5% lobular vs. 13.7% ductal), whereas a lower proportion were node-negative at surgery compared with ductal IBC patients (12.7% lobular vs. 17.1% ductal; both p < 0.001). Furthermore, more patients with lobular IBC had no nodes examined from surgery compared with patients with ductal IBC (31.2% lobular vs. 24.5% ductal, p < 0.001). Of these 2556 patients, 645 had breast surgery and no nodes examined: 155 were coded as having technically undergone MRM, and 294 underwent total mastectomy. Fewer patients with lobular IBC underwent MRM (35.6% lobular vs. 40.5% ductal), and a higher proportion of patients with lobular IBC had no surgery compared with patients with ductal IBC (34.4% lobular vs. 30.3% ductal; p = 0.002). Overall, 19.5% of patients with lobular IBC and 20.2% of patients with ductal IBC received all components of GCC (i.e., neoadjuvant chemotherapy, MRM without immediate reconstruction, postmastectomy radiation therapy, and if HR+, endocrine therapy).
The distribution of patients undergoing MRM by lymph node count is summarized in Table 2. Fewer patients with lobular IBC had ≥10 nodes examined compared with patients with ductal disease (31.3% lobular vs. 35.5% ductal, p < 0.001), but the proportion of patients with one to nine nodes retrieved (21.3% lobular vs. 21.5% ductal) or with an unknown number of lymph nodes retrieved (16.3% lobular vs. 18.5% ductal) were similar between the two groups. The median number of lymph nodes excised was lower among patients with lobular IBC compared with ductal IBC (median (interquartile range [IQR]), 7 (0−15) lobular vs. 9 (0−17) ductal, p = 0.001). Overall, fewer patients with lobular IBC received guideline-concordant surgery, i.e., MRM as defined by coding or definition of total mastectomies with ≥10 nodes removed (41.1% lobular vs. 46.5% ductal, p < 0.001); this finding remained true in sensitivity analyses in which we did not apply lymph node criteria restrictions (Table 2).
Table 2.
Modified radical mastectomies and variations based on lymph node count restriction in the National Cancer Database, 2010–2019
| Ductal | Lobular | p | ||
|---|---|---|---|---|
| n = 10,445 (%) | n = 1,456 (%) | |||
| Lymph nodes excised count | No LN examined | 2556 (24.5%) | 454 (31.2%) | < 0.001 |
| 1–9 LN examined | 2248 (21.5%) | 310 (21.3%) | ||
| ≥10 LN examined | 3711 (35.5%) | 455 (31.3%) | ||
| Unknown | 1930 (18.5%) | 237 (16.3%) | ||
| Median no. lymph nodes excised (IQR) | 9 (0–17) | 7 (0–15) | 0.001 | |
| Surgery types | Modified radical mastectomy | 4,230 (40.5%) | 519 (35.6%) | 0.002 |
| Total mastectomy | 1768 (16.9%) | 244 (16.8%) | ||
| Other surgery | 1280 (12.3%) | 192 (13.2%) | ||
| None | 3167 (30.3%) | 501 (34.4%) | ||
| Inclusive surgery types | MRM or TM with ≥ 10 LN excised | 4859 (46.5%) | 599 (41.1%) | < 0.001 |
| Other surgery | 2419 (23.2%) | 356 (24.5%) | ||
| None | 3167 (30.3%) | 501 (34.4%) | ||
IQR Interquartile range; LN Lymph node; MRM Modified radical mastectomy; TM Total mastectomy
Discussion
In this study of patients with nonmetastatic IBC, we found that more patients with lobular IBC presented with clinically node-negative disease, but fewer of these patients were node-negative at surgery, despite higher rates of omitted axillary surgery compared with patients with ductal IBC. This is the first study to our knowledge that compares guideline-concordant surgical management for lobular and ductal IBC.
Our findings suggest there are differences in the standardization of surgical treatment among patients with different subtypes of IBC. It is unclear whether these differences are due to disparate surgical approaches, variable preoperative and/or pathologic assessment, or differences in data quality as captured at institutions contributing to the NCDB. Lobular histology is frequently understaged on conventional preoperative imaging (i.e., mammograms and ultrasounds). Thus, some patients with lobular IBC may in fact have node-positive disease that was not detected due to not receiving diagnostic breast MRI.10-13 These findings suggest a potential role for alternative staging methods that may be more sensitive to lobular disease, such as 18F-fluoroestradiol (FES) PET, for patients who are HR+.14
The lower rate of guideline-concordant MRM and lower lymph node retrieval rates among patients with lobular IBC may be related to chemotherapy response, which can decrease lymph node yield; however, it is still unclear how this may differentially impact ductal versus lobular histologic subtypes. While literature on surgical approaches to IBC is limited, more aggressive surgical management may be associated with lower locoregional recurrence and more extensive axillary surgery with improved survival in node-positive patients.15-17 Our findings suggest the need for more accurate preoperative staging to ensure that patients with lobular IBC are receiving the appropriate surgery to maximize their chances of survival, particularly as surgical de-escalation of the axilla accelerates, despite the absence of evidence that it is safe in IBC.15,18,19 The high proportion of patients with lobular IBC who have no nodes examined in surgery is concerning for a potentially vital missed treatment opportunity and may represent poor adherence to current guidelines. Furthermore, inadequate surgery of the axilla can result in false reassurance of the degree of responsiveness to neoadjuvant chemotherapy. Accurate surgical staging is critical for therapeutic planning, especially in considering the role of adjuvant therapies for what is often chemotherapy-resistant disease.15
The differences we appreciated in lobular versus ductal IBC from patient demographics to clinical characteristics suggests a need to delineate IBC subtypes in research and treatment. Given the generally low prevalence of lobular IBC compared with ductal IBC, and the relatively low incidence of IBC within breast cancer pathology, lobular-specific guidelines are limited. We hypothesize that the surgical disparities in lobular IBC may be partially attributable to operative challenges in identifying, retrieving, and pathologically assessing the recommended number of nodes in addition to challenges with determining operative candidacy for MRM to begin with. Surgeon intent is not captured in the NCDB; thus, it also is difficult to assess how many operations were intended to be performed with higher lymph node yield. It is impossible to know whether omission of axillary surgery was because of on-treatment disease progression that prompted a change from planned MRM to planned palliative mastectomy. Future studies should seek to delineate histologic IBC types and future efforts should be devoted to developing treatment guidelines optimized for specific tumor biology and patient characteristics. Additional studies using multicenter data where surgeon intent can be captured may help to understand how much these treatment disparities are because of operative challenges and histology-specific difficulties with pathologic assessment.
Our findings highlight the importance of detailed and accurate data reporting for surgical procedures. The NCDB was used for this analysis due to its historically robust oncologic data.20,21 Yet, as documented by Rubenstein et al.’s comparison of national databases for breast surgical oncology, the NCDB uses a unique coding system which has potential for coding errors, as seen with lymph node coding prior to 2012.22,23 This is unlike the data included in databases such as the National Surgical Quality Improvement Program (NSQIP), where multiple Current Procedural Terminology (CPT) codes can be assigned to indicate surgery type, outcomes, and indications for the primary procedure; however, NSQIP does not capture cancer-related data as robustly as the NCDB.22 Assessing surgical standards of care for lobular versus ductal IBC using an additional database, such as the Surveillance, Epidemiology, and End Results (SEER) Program, may clarify the surgical differences appreciated in our analysis.
Limitations
Limitations of our study include those that are inherent to retrospective studies using the NCDB, including selection bias and incomplete data reporting. Inflammatory breast cancer is a clinical diagnosis that can be difficult to diagnose and distinguish from locally advanced breast non-IBC, and this diagnostic challenge may have impacted the IBC data quality in the NCDB.24 Nevertheless, the treatment for both types of disease is similar, suggesting that the rates of guideline-concordant surgery that we observed likely are not significantly affected by any diagnostic misclassification that may have occurred. Similarly, the NCDB does not have a mechanism for central pathology review, necessitating reliance on hospital-level clinical data which may result in inaccurate categorization (e.g., locally advanced vs. noninflammatory breast cancer) and inability to confirm histology. We made the assumption that ≥10 lymph nodes represented ALND and <10 lymph nodes represented sentinel lymph node biopsy; yet, we recognize that there are potential coding errors in the NCDB, acknowledge that our selected categories do not reflect surgical intent, and acknowledge these categories may be impacted by response to NACT.25,26 To mitigate these potential errors, we conducted sensitivity analyses with and without lymph node count restrictions among patients who were coded as having received MRMs.
Finally, one of the reasons for nonreceipt of surgery may be progression to or delayed recognition of metastatic disease during neoadjuvant treatment, which may be more common with lobular IBC, prompting the conduct of palliative mastectomy with intentional omission of axillary surgery. Thus, in large part to optimize surgical care, we need to optimize systemic therapy for what is often chemotherapy-resistant disease. Indeed, our study highlights the persistent challenge of durable clinical response to systemic therapy for HR+ disease, which makes up a majority of lobular IBC and non-IBC. Fortunately, even in the time elapsed since the end of our study’s inclusion period, there have been significant advances in systemic therapy for patients with HR+ disease, including increased use of CDK4/6 inhibitors and emerging therapies from clinical trials, such as I-SPY.27,28 We hope that neoadjuvant use of some of these treatments and concomitant improvement in response among patients with IBC may facilitate more receipt of guideline-concordant surgery.
Conclusions
Patients with lobular IBC were more likely to present with node-negative disease and less likely to be node-negative at surgery despite having fewer, and more frequently no, lymph nodes examined compared with ductal IBC patients. Future studies should investigate whether these treatment disparities are the result of differences in surgical approach, preoperative staging, pathologic assessment, and/or data quality as captured in the NCDB. Such knowledge would inform development of potentially more tailored treatments for particular histological subtypes of IBC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Disclosure
Dr. Fayanju is supported by the National Institutes of Health (NIH) under Award Numbers 7K08CA241390-03 (PI: Fayanju) and P50CA244690 (PIs: Bekelman), the Breast Cancer Research Foundation, and philanthropic funds from the Haas family. She also reports research support unrelated to this work from Gilead Sciences, Inc. This work also was supported by the NIH under Award Number 2P30CA016520-45 (PI: Vonderheide). S. M. Thomas is supported by the NIH under award numbers 5P30-CA014236-50 (PI: Kastan), 5UL1TR002553-05 (PI: Li), and 5R01DA047301-05 (PI: Vilardaga). S.M.T. also reports foundational funding from the V Foundation and the Fullerton Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the national cancer institute. JNCI J Natl Cancer Inst. 2005;97(13):966–75. 10.1093/jnci/dji172. 10.1093/jnci/dji172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menta A, Fouad TM, Lucci A, et al. Inflammatory breast cancer. Surg Clin North Am. 2018;98(4):787–800. 10.1016/j.suc.2018.03.009. 10.1016/j.suc.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 3.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7. 10.1016/S1470-2045(14)70080-4. 10.1016/S1470-2045(14)70080-4 [DOI] [PubMed] [Google Scholar]
- 4.Hieken TJ, Murphy BL, Boughey JC, Degnim AC, Glazebrook KN, Hoskin TL. Influence of biologic subtype of inflammatory breast cancer on response to neoadjuvant therapy and cancer outcomes. Clin Breast Cancer. 2018;18(4):e501–6. 10.1016/j.clbc.2017.10.003. 10.1016/j.clbc.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Kupstas AR, Hoskin TL, Day CN, Boughey JC, Habermann EB, Hieken TJ. Biological subtype, treatment response and outcomes in inflammatory breast cancer using data from the National Cancer Database. Br J Surg. 2020;107(8):1033–41. 10.1002/bjs.11469. 10.1002/bjs.11469 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN). NCCN Guidelines Version 4.2023 Inflammatory Breast Cancer. NCCN Guidel Recomm. Published online March 23, 2023:IBC-2.
- 7.Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60(6):351–75. 10.3322/caac.20082. 10.3322/caac.20082 [DOI] [PubMed] [Google Scholar]
- 8.Raghav K, French JT, Ueno NT, Lei X, Krishnamurthy S, Reuben JM, Valero V, Ibrahim NK. Inflammatory breast cancer: a distinct clinicopathological entity transcending histological distinction. PLOS ONE. 2016;11(1):e0145534. 10.1371/journal.pone.0145534. 10.1371/journal.pone.0145534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elm EV, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. 10.1136/bmj.39335.541782.AD. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michael M, Garzoli E, Reiner CS. Mammography, sonography and MRI for detection and characterization of invasive lobular carcinoma of the breast. Breast Dis. 2009;30(1):21–30. 10.3233/BD-2009-0279. 10.3233/BD-2009-0279 [DOI] [PubMed] [Google Scholar]
- 11.Vijayaraghavan GR, Vedantham S, Santos-Nunez G, Hultman R. Unifocal invasive lobular carcinoma: tumor size concordance between preoperative ultrasound imaging and postoperative pathology. Clin Breast Cancer. 2018;18(6):e1367–72. 10.1016/j.clbc.2018.07.017. 10.1016/j.clbc.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovis KK, Lee JM, Hippe DS, et al. Accuracy of preoperative breast MRI versus conventional imaging in measuring pathologic extent of invasive lobular carcinoma. J Breast Imaging. 2021;3(3):288–98. 10.1093/jbi/wbab015. 10.1093/jbi/wbab015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parvaiz MA, Yang P, Razia E, et al. Breast MRI in invasive lobular carcinoma: a useful investigation in surgical planning? Breast J. 2016;22(2):143–50. 10.1111/tbj.12566. 10.1111/tbj.12566 [DOI] [PubMed] [Google Scholar]
- 14.Covington MF, Hoffman JM, Morton KA, et al. Prospective pilot study of 18 F-fluoroestradiol PET/CT in patients with invasive lobular carcinomas. Am J Roentgenol. 2023;221(2):228–39. 10.2214/AJR.22.28809. 10.2214/AJR.22.28809 [DOI] [PubMed] [Google Scholar]
- 15.Fayanju OM, Ren Y, Greenup RA, et al. Extent of axillary surgery in inflammatory breast cancer: a survival analysis of 3500 patients. Breast Cancer Res Treat. 2020;180(1):207–17. 10.1007/s10549-020-05529-1. 10.1007/s10549-020-05529-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosso KJ, Tadros AB, Weiss A, et al. Improved locoregional control in a contemporary cohort of nonmetastatic inflammatory breast cancer patients undergoing surgery. Ann Surg Oncol. 2017;24(10):2981–8. 10.1245/s10434-017-5952-x. 10.1245/s10434-017-5952-x [DOI] [PubMed] [Google Scholar]
- 17.Adesoye T, Irwin S, Sun SX, Lucci A, Teshome M. Contemporary surgical management of inflammatory breast cancer: a narrative review. Chin Clin Oncol. 2021;10(6):57. 10.21037/cco-21-113. 10.21037/cco-21-113 [DOI] [PubMed] [Google Scholar]
- 18.Stearns V, Ewing CA, Slack R, Penannen MF, Hayes DF, Tsangaris TN. Sentinel lymphadenectomy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Ann Surg Oncol. 2002;9(3):235–42. 10.1007/BF02573060. 10.1007/BF02573060 [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi H, Woodward WA, Valero V, et al. Inflammatory breast cancer: what we know and what we need to learn. The Oncologist. 2012;17(7):891–9. 10.1634/theoncologist.2012-0039. 10.1634/theoncologist.2012-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722. 10.1001/jamaoncol.2016.6905. 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 21.Mohanty S, Bilimoria KY. Comparing national cancer registries: the national cancer data base (NCDB) and the surveillance, epidemiology, and end results (SEER) program. J Surg Oncol. 2014;109(7):629–30. 10.1002/jso.23568. 10.1002/jso.23568 [DOI] [PubMed] [Google Scholar]
- 22.Rubenstein RN, Nelson JA, Azoury SC, et al. Breast surgical oncology epidemiologic research: a guide and comparison of four national databases. Ann Surg Oncol. 2023;30(4):2069–84. 10.1245/s10434-022-12890-6. 10.1245/s10434-022-12890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American college of Surgeons NCD. National Cancer Database Participant User File, 2017 Data Dictionary, Includes Patients Diagnosed in 2004–2017.; 2020:139-144. Accessed May 7, 2023. https://www.facs.org/media/khro23pr/puf_data_dictionary_2017.pdf
- 24.Edge SB, Compton CC. AJCC cancer staging manual, 7th edn. Springer; 2010. [DOI] [PubMed]
- 25.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–53. 10.1200/JCO.2008.19.5750. 10.1200/JCO.2008.19.5750 [DOI] [PubMed] [Google Scholar]
- 26.Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB). Ann Surg. 2018;268(4):591–601. 10.1097/SLA.0000000000002953. 10.1097/SLA.0000000000002953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chien J, Esserman L, Elias A, Wei M, Plourde P, Portman D. P132 (trial in progress): a phase 2, open-label, randomized multicenter trial to evaluate neoadjuvant lasofoxifene in molecularly-selected HR+/HER2− Clinical Stage 2/3 breast cancer. Breast. 2023;68:S66. 10.1016/S0960-9776(23)00249-7 [DOI] [Google Scholar]
- 28.Potter DA, Herrera-Ponzanelli CA, Hinojosa D, et al. Recent advances in neoadjuvant therapy for breast cancer. Fac Rev. 2021;10:2. 10.12703/r/10-2. 10.12703/r/10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.