Abstract
Adenovirus interaction with αv integrins is important for virus entry. We have examined the effects of adenovirus attachment on intracellular signaling in HeLa cells, with an emphasis on pathways known to be activated following integrin interaction with other ligands. We found no evidence for [Ca2+]c-mediated signaling or for tyrosine phosphorylation of pp125FAK, p130CAS, and paxillin. However, adenovirus attachment is known to activate phosphatidylinositol-3 kinase, which in turn may regulate endocytosis via rab5 GTPase. We found that adenovirus uptake was increased by overexpression of wild-type rab5 and decreased by dominant-negative rab5. These results indicate a role for rab5 in adenovirus entry.
Adenovirus infections are a common cause of human disease, and recombinant adenovirus vectors are frequently used for gene transfer. Uptake of adenovirus into cells by receptor-mediated endocytosis involves at least two viral proteins, the fiber and the penton base. The adenovirus fiber binds to fiber receptors on the cell surface (4, 5, 14, 23, 32), and the penton base binds to αv integrin receptors (1, 3, 22, 35, 36). Several intracellular molecular events downstream of this interaction correlate with adenovirus entry into cells. The penton base-αv integrin interaction activates the Raf-1/MAPK pathway, leading to increased interleukin-8 production (6). The penton base-αv integrin interaction also leads to phosphoinositide-3-OH kinase activation, which is essential for viral entry (19). In addition, focal adhesion kinase (pp125FAK) activation may occur following adenovirus surface binding (19). Finally, GTPase molecules of the dynamin and Rho families which modulate the actin cytoskeleton have been shown to mediate viral entry (18, 34). Despite the above advances, our understanding of the cell-mediated events that govern viral entry is incomplete.
αv integrins are coreceptors for adenovirus infection, and they also serve as receptors for proteins of the extracellular matrix and provide a physical connection to the cytoskeleton. Integrins play an important role in intracellular signal transduction, controlling gene expression and cell division (28). Previous studies have shown that ligand binding to αv integrins can activate several intracellular signaling processes. It can trigger a transient increase in intracellular calcium concentration ([Ca2+]c) which may regulate integrin uptake and recycling (17;27). Ligand binding to αv integrins also activates phosphatidylinositol-3 kinase [PI(3)kinase] (15), which in turn may regulate endocytosis via the rab5 GTPase (21). In addition, ligand binding can stimulate phosphorylation of several proteins, including focal adhesion kinase, pp125FAK. pp125FAK is autophosphorylated following integrin ligand binding, and the autophosphorylated protein regulates turnover of the focal adhesion contacts (8, 9, 16). Following pp125FAK activation, p130CAS and paxillin are phosphorylated (24, 26, 33). These intracellular events following integrin ligand binding lead to focal adhesion site formation and reorganization of the actin cytoskeleton; they are also potential regulators of adenovirus endocytosis and gene transfer.
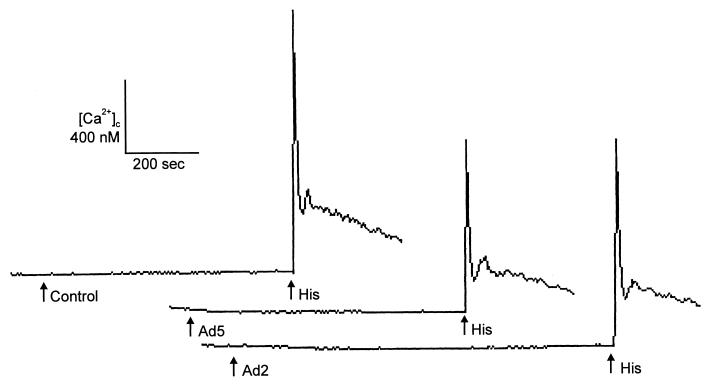
Effect of adenovirus on [Ca2+]c.
Binding of ligand to αv integrins transiently increases [Ca2+]c in several cell types (17, 27). Because an increase in [Ca2+]c can regulate αv integrin uptake and recycling (17), [Ca2+]c was an attractive candidate for involvement in adenovirus-mediated signal transduction. Therefore, we measured [Ca2+]c in HeLa cells loaded with fura-2-acetoxymethyl ester (5 μg/ml; fura-2 AM; Molecular Probes, Eugene, Oreg.) before and after virus binding with a Photoscan 2 spectrofluorometer (Photon Technologies International, New Brunswick, N.J.) (11). Figure 1 shows that 100 PFU/cell of Ad2/CMVβgal (37) or Ad5RSVβgal (10) did not alter [Ca2+]c. However, subsequent addition of 1 μM histamine triggered an immediate increase in [Ca2+]c in the continued presence of virus. As a further test of the potential role of Ca2+ in viral infection, we examined the effect of 0 to 50 nM calmidazolium (Calbiochem, San Diego, Calif.), an inhibitor of Ca2+-calmodulin (31). The cells were preincubated in medium with calmidazolium for 30 min, and then 10 PFU/cell of Ad2/CMVβgal was added in the continued presence of inhibitor for 30 min. Calmidazolium did not inhibit adenovirus-mediated β-galactosidase gene transfer, and at high concentrations there was a small increase in transgene expression (data not shown). These data suggest that changes in [Ca2+]c may not play an important role in adenovirus infection, even though calcium can be an important regulator in αv integrin-mediated signaling in response to other ligands (9, 28).
FIG. 1.
Effect of adenovirus (100 PFU/cell) on [Ca2+]c. HeLa cells were loaded with fura-2, and [Ca2+]c was monitored after adding Ad2/CMVβgal, Ad5RSVβgal, vehicle control, or histamine (1 μM) at the times indicated by the arrows. Data are examples from single experiments; each experiment was repeated at least three times.
Effect of adenovirus on protein tyrosine phosphorylation.
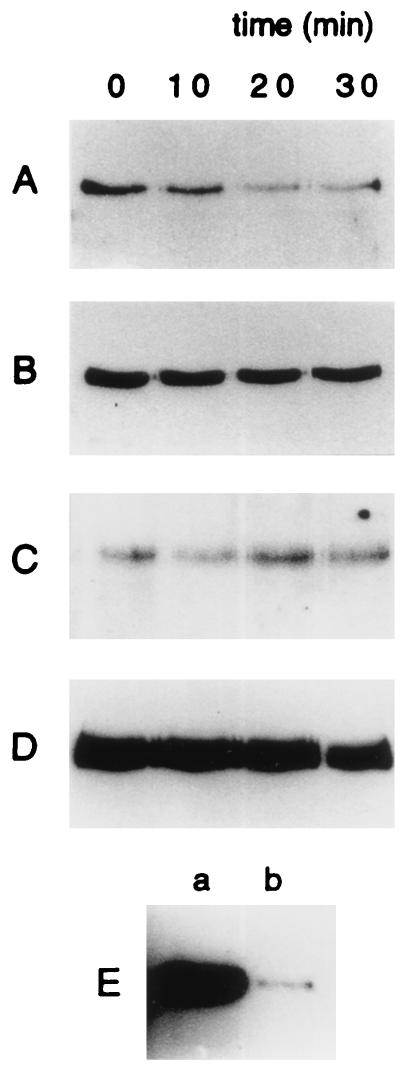
Previous studies have shown that focal adhesion kinase (pp125FAK) can be autophosphorylated and activated by αv integrins. There is evidence that adenovirus binding to the cell surface can activate pp125FAK phosphorylation although that may not be essential for viral entry (19). pp125FAK activation following αv integrin ligand binding is associated with increased tyrosine phosphorylation of p130CAS and paxillin (8, 25, 26, 33). We tested the effect of adenovirus on pp125FAK, p130CAS, or paxillin phosphorylation. For these studies, we used HeLa cells in suspension as well as serum-starved HeLa cells (0.3% fetal calf serum for 36 h) attached to culture dishes. Cells (2 × 106) were incubated with 50 PFU/cell of Ad2/CMVβgal (37) or Ad5RSVβgal (10) for 0 to 30 min at 37°C, washed with phosphate-buffered saline, and suspended in 500 μl of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1% deoxycholate, 50 mM NaF, 0.5 mM Na3VO4, 0.1 U/ml of aprotinin, 10 μg/ml of leupeptin, and 4 μg/ml of pepstatin). Monoclonal anti-pp125FAK, anti-p130CAS, or antipaxillin antibodies (2 μg; Transduction Laboratories, Lexington, Ky.) were added to the supernatants and incubated with shaking on ice for 1 h. GammaBind G Sepharose (30 μl; Pharmacia Biotech, Uppsala, Sweden) was added, and incubation on ice was continued for 2 h, after which the precipitate was carefully washed, solubilized in 2× sample buffer, boiled for 3 min, and run on an SDS–8 or 12% polyacrylamide gel. The proteins were transferred to polyvinylidene difluoride filters (Millipore). Filters were blocked for 2 h (3% bovine serum albumin in 10 mM Tris, pH 7.5, 100 mM NaCl, 1% Tween 20), incubated with PY20H peroxidase-conjugated antiphosphotyrosine antibody (Transduction Laboratories), and washed, and the bound antibody was detected by chemiluminescence (Pierce Chemical Co., Rockford, Ill.). Equal protein loading in pp125FAK, p130CAS, and paxillin phosphorylation experiments was verified by stripping and reblotting the filters with the appropriate monoclonal antibody.
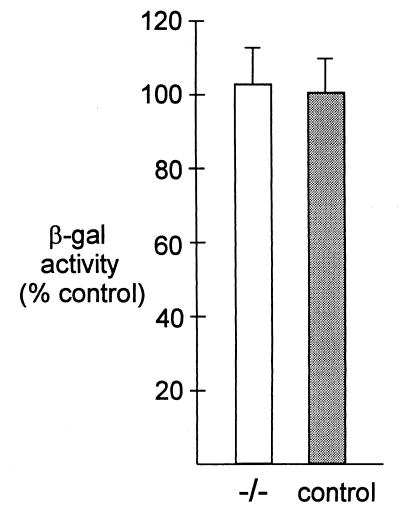
We found that adenovirus did not increase pp125FAK phosphorylation (Fig. 2A) in HeLa cells; instead there was a small decrease. Likewise, we saw no change in the tyrosine phosphorylation of pp125FAK following incubation of attached, serum-starved HeLa cells with Ad2/CMVβgal (Fig. 2B). Similar results were obtained with cells exposed to Ad5RSVβgal (data not shown). Incubation with adenovirus had no effect on the total amount of pp125FAK (not shown). In contrast, as previously reported (33), pp125FAK phosphorylation increased when attached HeLa cells were exposed to 10% fetal calf serum or when a suspension of HeLa cells was allowed to attach on vitronectin (Fig. 2E). We further found that adenovirus had no effect on p130CAS phosphorylation (Fig. 2C). We also showed that paxillin phosphorylation was not affected by the addition of adenovirus (Fig. 2D). We obtained similar results with attached, serum-starved cells (not shown). The role of pp125FAK in adenovirus infection was tested further with αv integrin-positive pp125FAK−/− cells, which are deficient in focal adhesion kinase pp125FAK (16). Figure 3 shows that β-galactosidase expression was similar in both pp125FAK−/− and control cell lines 24 h after infection with 10 PFU/cell of Ad2/CMVβgal. These results agree with the previously published data (19) and confirm that pp125FAK activity is not required for adenovirus infection and gene transfer.
FIG. 2.
Tyrosine phosphorylation of pp125FAK, p130CAS, and paxillin in HeLa cells following the addition of adenovirus. (A-D) Ad2/CMVβgal (50 PFU/cell) was added to the cells for the indicated times. (A, C, and D) Tyrosine-phosphorylated pp125FAK, p130CAS, and paxillin, respectively, using HeLa cells in suspension. (B) pp125FAK phosphorylation for attached serum-starved cells. (E) pp125FAK phosphorylation in attached (a) or in suspended cells (b). Each experiment was repeated at least three times.
FIG. 3.
Adenovirus-mediated gene expression in pp125FAK-deficient cells (−/−) and pp125FAK-positive control cells. Cells were infected with 10 PFU/cell of Ad2/CMVβgal at 37°C for 30 min. β-Galactosidase activity was measured 24 h later. Values are expressed as percentage of the control, and error bars represent the mean ± standard error of the mean. n is at least eight for each experiment.
Effect of rab5 GTPase activity on recombinant adenovirus β-galactosidase gene transfer.
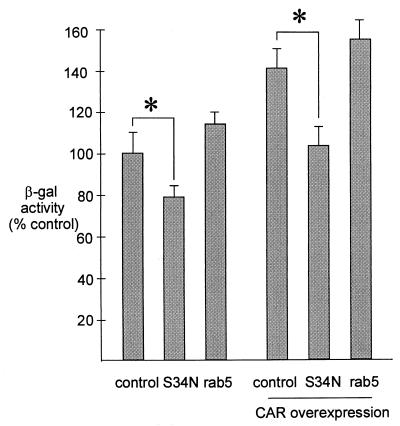
Ligand binding to αv integrins can activate PI(3)kinase, which in turn may regulate endocytosis via rab5 GTPase (21). To test the hypothesis of whether rab5 GTPase may play a role in adenovirus entry, we transfected HeLa cells with wild-type rab5 or dominant-negative rab5:S34N (30) cloned in pcDNA3.1(+) (InVitrogen, San Diego, Calif.) or with a control plasmid. We first showed that our rab5 expression constructs are functional by determining endocytosis of dextran-fluorescein isothiocyanate (FITC) (FD-10S; Sigma) in HeLa cells (300,000 cells/dish) cultured on glass coverslips. At 24 h after transfection, the cells were incubated for 15 min at 37°C with 330 μg/ml of dextran-FITC. We determined fluorescence intensity with videomicroscopy and showed that expression of wild-type rab5 significantly increased (190% of control value) uptake of dextran-FITC, whereas dominant-negative rab5:S34N expression decreased it (75% of control value). To test the effect of rab5 on recombinant adenovirus gene transfer, HeLa cells on 24-well plates were transfected (100,000 cells/well) with 6 μg of Lipofectin (Gibco-BRL) and 1 μg of plasmid. Twenty-four hours after transfection, the cells were incubated with 50 PFU/cell of Ad2/CMVβgal for 15 min and the adenovirus β-galactosidase activity was assayed with the Luminescent β-galactosidase Detection Kit II (Clontech, Palo Alto, Calif.) and luminometry (Luminoscan RS; Labsystems Oy, Helsinki, Finland) 24 h following infection. HeLa cells overexpressing wild-type rab5 displayed a slightly increased (118% of control; statistically not significant) level of adenovirus β-galactosidase expression. HeLa cells expressing rab5:S34N showed reduced β-galactosidase activity compared to the control (78% of control; P < 0.05) (Fig. 4). The cells were also transfected with a combination of coxsackievirus/adenovirus receptor (CAR) and rab5, rab5:S34N, or a control plasmid. The data indicate that the S34N-mutated rab5 significantly decreased (102% versus 141%; P < 0.05), while the wild-type rab5 only slightly increased (141% versus 156%; not significant), adenovirus-mediated gene transfer in HeLa cells overexpressing CAR (Fig. 4). In summary, HeLa cells with the most rab5 GTPase activity (wild-type overexpression) have approximately 40 to 50% more adenovirus β-galactosidase expression than the cells with partial inhibition of rab5 (dominant-negative overexpression). Interestingly, an increase caused by wild-type rab5 in the uptake of dextran-FITC was significantly greater than an increase observed in viral β-galactosidase expression, implying that endogenous levels of rab5 may be sufficient for internalization of all bound virus. We tested our lipofection efficiency with pEGFP-C1-plasmid (Clontech) coding for green fluorescent protein and showed that about 50% of the HeLa cells were transfected (data not shown). It is possible that our rab5 GTPase constructs would have a stronger effect on adenovirus β-galactosidase expression with more complete transfection efficiency into HeLa cells.
FIG. 4.
Adenovirus-mediated gene expression in HeLa cells or HeLa cells overexpressing CAR. The cells were transfected with wild-type rab5, dominant-negative rab5:S34N, or a control plasmid. Twenty-four hours later, the cells were incubated with 50 PFU/cell of Ad2/CMVβgal for 15 min and the adenovirus β-galactosidase activity was determined 24 h later. n equals 15 for each experiment. Error bars indicate standard errors of the mean, and an asterisk indicates P < 0.05 compared to the control.
Effect of rab5 on Cy3-labeled adenovirus endocytosis.
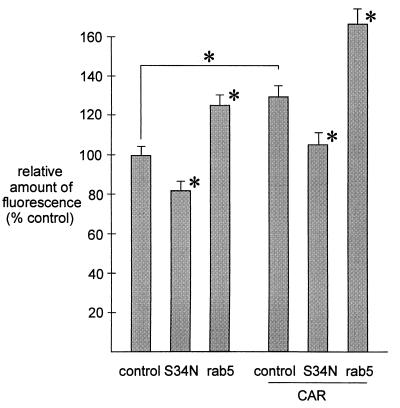
Because rab5 GTPase activity did show a regulatory effect on adenovirus β-galactosidase gene transfer, the effect was further explored with Cy3-labelled viruses. Recombinant adenovirus particles were fluorescently labeled by stirring 3 × 1011 virus particles with 200 μl of Fluorolink Cy3 (Amersham Life Science Inc., Arlington Heights, Ill.) in 100 mM sodium carbonate buffer, pH 9.3, for 30 min at room temperature with protection from light. Cy3-labelled adenovirus was dialyzed twice (Slide-A-Lyzer; Pierce) against a dialysis buffer (150 mM NaCl, 20 mM MgCl2, 10 mM Tris-HCl, pH 7.8, and 10% glycerol) (38). HeLa cells were cultured on microscope coverslips (300,000 cells/60-mm-diameter dish) and transfected (3 μg of plasmid and 18 μg of Lipofectin) with CAR and/or wild-type rab5, rab5:S34N, or a control plasmid. Distribution and intensity of endocytosed virus was determined in HeLa cells fixed in 4% paraformaldehyde by fluorescent laser-scanning confocal microscopy (Leica Aristoplan CLSM; Leica Lasertechnics GmbH, Heidelberg, Germany) and videomicroscopy of at least 150 randomly selected cells in each condition. The data were analyzed with image analysis software MCID-M2 (Imaging Research Inc., St. Catharines, Ontario, Canada). Efficiency of endocytosis was estimated by determining the average of the amount of fluorescently labelled adenovirus in each cell. Overexpression of wild-type rab5 increased endocytosis of labelled virus in HeLa cells compared to the control, and rab5:S34N expression decreased the amount and intensity of fluorescent endosomes (Fig. 5). The HeLa cells overexpressing CAR had an increased amount of endocytosed Cy3-adenovirus compared to the control, indicating that endocytosis depended on virus attachment to the CAR. In the presence of CAR overexpression, rab5:S34N decreased and wild-type rab5 further increased Cy3-adenovirus endocytosis (Fig. 5).
FIG. 5.
Videomicroscopic analysis of Cy3-labelled adenovirus uptake by HeLa cells overexpressing rab5, rab5:S34N, and/or CAR as described in the text. The cells were incubated with Cy3-labelled adenovirus (5,000 particles/cell) for 15 min at 37°C. At least 150 cells were analyzed on randomly selected microscopic fields in each experiment, and the experiments were repeated at least three times. The relative amount of fluorescence is indicated for each experiment. The error bars indicate standard errors of the mean, and an asterisk indicates P < 0.05 compared to the control.
Discussion.
Li et al. showed that adenovirus binding to cell surface αv integrins activates PI(3)kinase (19). It has also been shown that PI(3)kinase may regulate endocytosis via rab5 GTPase (21). Antibodies against rab5 and dominant-negative rab5 mutants have inhibitory effects on endosome fusion in vitro (12, 13, 20). Overexpression of wild-type rab5 causes enlargement of early endosomes and increases the rate of endocytosis (29). Overexpression of a dominant-negative rab5 mutant in intact cells causes fragmentation of early endosomes and reduces endocytosis (2, 7). Our data support the hypothesis that adenovirus endocytosis and gene transfer are regulated by rab5 GTPase. It is possible that adenovirus binding to the αv integrin activates PI(3)kinase, which in turn regulates rab5 GTPase and controls adenovirus entry into a cell. In our assay, an approximately 40 to 50% difference in β-galactosidase activity and an approximately 50% difference in fluorescently labelled adenovirus uptake was seen between conditions with the most and the least rab5 GTPase activity. A more pronounced effect might be expected if rab5 activity were completely eliminated. The data demonstrate that both intracellular and cell surface molecules are important mediators of viral infection. A complex series of intracellular events probably regulates viral endocytosis, exit from the endosome, cytoplasmic transport, and nuclear entry. Additional knowledge of these events will provide a better understanding of how adenovirus infect cells.
Acknowledgments
We thank Heikki Ruskoaho for generous support and helpful discussions. We thank Teresa Grunst for expert technical assistance. We thank Michael J. Welsh, Joseph Zabner, Joe Cotten, Kristiina Vuori, and Beverly Davidson for helpful discussions and careful revision of the manuscript. Marino Zerial is acknowledged for the generous gift of the rab5 cDNAs. We thank Richard Anderson and the University of Iowa Gene Transfer Vector Core for providing the recombinant adenovirus. We acknowledge the Veterans Affairs Medical Center (Iowa City, Iowa) Cell Fluorescence Core Facility for intracellular calcium measurements.
This work was supported by the Academy of Finland and the University Hospital of Oulu. J.M.B. is supported by grants from the National Institutes of Health and by an Established Investigator Award from the American Heart Association.
REFERENCES
- 1.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri M A, Li G, Colombo M I, Stahl P D. Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J Biol Chem. 1994;269:18720–18722. [PubMed] [Google Scholar]
- 3.Belin M T, Boulanger P. Involvement of cellular adhesion sequences in the attachment of adenovirus to the HeLa cell surface. J Gen Virol. 1993;74:1485–1497. doi: 10.1099/0022-1317-74-8-1485. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucci C, Parton R G, Mather I H, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 8.Burridge K, Turner C E, Romer L H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 10.Davidson B L, Doran S E, Shewach D S, Latta J M, Hartman J W, Roessler B J. Expression of Escherichia coli beta-galactosidase and rat HPRT in the CNS of Macaca mulatta following adenoviral mediated gene transfer. Exp Neurol. 1994;125:258–267. doi: 10.1006/exnr.1994.1028. [DOI] [PubMed] [Google Scholar]
- 11.Denning G M, Clark R A, Welsh M J. cAMP and inositol 1,4,5-trisphosphate increase Ca2+ in HT-29 cells by activating different Ca2+ influx pathways. Am J Physiol. 1994;267:C776–C783. doi: 10.1152/ajpcell.1994.267.3.C776. [DOI] [PubMed] [Google Scholar]
- 12.Gorvel J P, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 13.Hoffenberg S, Sanford J C, Liu S, Daniel D S, Tuvin M, Knoll B J, Wessling-Resnick M, Dickey B F. Biochemical and functional characterization of a recombinant GTPase, Rab5, and two of its mutants. J Biol Chem. 1995;270:5048–5056. doi: 10.1074/jbc.270.10.5048. [DOI] [PubMed] [Google Scholar]
- 14.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hruska K A, Rolnick F, Huskey M, Alvarez U, Cheresh D. Engagement of the osteoclast integrin αvβ 3 by osteopontin stimulates phosphatidylinositol 3-hydroxyl kinase activity. Endocrinology. 1995;136:2984–2992. doi: 10.1210/endo.136.7.7540546. [DOI] [PubMed] [Google Scholar]
- 16.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 17.Lawson M A, Maxfield F R. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 18.Li E, Stupack D, Bokoch G M, Nemerow G R. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li E, Stupack D, Klemke R, Cheresh D A, Nemerow G R. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, Barbieri M A, Colombo M I, Stahl P D. Structural features of the GTP-binding defective Rab5 mutants required for their inhibitory activity on endocytosis. J Biol Chem. 1994;269:14631–14635. [PubMed] [Google Scholar]
- 21.Li G, D’Souza-Schorey C, Barbieri M A, Roberts R L, Klippel A, Williams L T, Stahl P D. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayr G A, Freimuth P. A single locus on human chromosome 21 directs the expression of a receptor for adenovirus type 2 in mouse A9 cells. J Virol. 1997;71:412–418. doi: 10.1128/jvi.71.1.412-418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nojima Y, Mimura T, Morino N, Hamasaki K, Furuya H, Sakai R, Nakamoto T, Yazaki Y, Hirai H. Tyrosine phosphorylation of p130Cas in cell adhesion and transformation. Hum Cell. 1996;9:169–174. [PubMed] [Google Scholar]
- 25.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 26.Petch L A, Bockholt S M, Bouton A, Parsons J T, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 src substrate. J Cell Sci. 1995;108:1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz M A, Denninghoff K. αv integrins mediate the rise in intracellular calcium in endothelial cells on fibronectin even though they play a minor role in adhesion. J Biol Chem. 1994;269:11133–11137. [PubMed] [Google Scholar]
- 28.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 29.Stenmark H, Parton R G, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenmark H, Valencia A, Martinez O, Ullrich O, Goud B, Zerial M. Distinct structural elements of rab5 define its functional specificity. EMBO J. 1994;13:575–583. doi: 10.1002/j.1460-2075.1994.tb06295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takuwa N, Zhou W, Kumada M, Takuwa Y. Ca2+-dependent stimulation of retinoblastoma gene product phosphorylation and p34cdc2 kinase activation in serum-stimulated human fibroblasts. J Biol Chem. 1993;268:138–145. [PubMed] [Google Scholar]
- 32.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickham T J, Filardo E J, Cheresh D A, Nemerow G R. Integrin αvβ 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 37.Zabner J, Couture L A, Smith A E, Welsh M J. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: efficiency of adenovirus-mediated gene transfer in vitro. Hum Gene Ther. 1994;5:585–593. doi: 10.1089/hum.1994.5.5-585. [DOI] [PubMed] [Google Scholar]
- 38.Zabner, J. Personal communication.