Abstract
The herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) gene is essential for efficient spontaneous reactivation in the rabbit ocular model of HSV-1 latency and reactivation. LAT is also the only viral gene abundantly expressed during latency. Rabbits were ocularly infected with the wild-type HSV-1 strain McKrae or the McKrae-derived LAT null mutant dLAT2903. Serum neutralizing antibody titers were determined at various times during acute and latent infection. The neutralizing antibody titers induced by both viruses increased and were similar throughout the first 45 days after infection (P > 0.05). However, by day 59 postinfection (approximately 31 to 45 days after latency had been established), the neutralizing antibody titers induced by wild-type virus and dLAT2903 diverged significantly (P = 0.0005). The dLAT2903-induced neutralizing antibody titers decreased, while the wild-type virus-induced neutralizing antibody titers continued to increase. A rescuant of dLAT2903, in which spontaneous reactivation was fully restored, induced wild-type neutralizing antibody levels on day 59 postinfection. A second LAT mutant with impaired spontaneous reactivation had neutralizing antibody levels comparable to those of dLAT2903. In contrast to the results obtained in rabbits, in mice, neutralizing antibody titers did not increase over time during latency with any of the viruses. Since LAT is expressed in both rabbits and mice during latency, the difference in neutralizing antibody titers between these animals is unlikely to be due to expression of a LAT protein during latency. In contrast, LAT-positive (LAT+), but not LAT-negative (LAT−), viruses undergo efficient spontaneous reactivation in rabbits, while neither LAT+ nor LAT− viruses undergo efficient spontaneous reactivation in mice. Thus, the increase in neutralizing antibody titers in rabbits latently infected with LAT+ viruses may have been due to continued restimulation of the immune system by spontaneously reactivating virus.
Following initial exposure to an infectious virus, a neutralizing antibody response is mounted by the host. Significant serum neutralizing antibody titers usually become detectable approximately 1 week after exposure. Typically, the neutralizing antibody titer continues to increase until it reaches a peak approximately 3 to 6 weeks after exposure, and then it decreases, eventually stabilizing at a fairly low level. Subsequent reexposure to the virus results in a rapid increase in serum neutralizing antibody titer. If repeated reexposure occurs (as with booster vaccinations), the neutralizing antibody titer will usually increase to levels higher than the original peak values.
Following primary ocular infection with herpes simplex virus type 1 (HSV-1), the virus establishes a lifelong latent infection in the neurons of the trigeminal ganglia (TG). From time to time, the virus may reactivate, producing recurrent ocular infections. Serum neutralizing antibody titers develop following primary HSV-1 infection but may require several exposures to the virus (i.e., recurrences) to attain maximum levels (3). In addition, the average HSV-1 neutralizing antibody titer in individuals with recurrent herpetic disease is approximately two times higher than in seropositive individuals with no history of recurrent disease (16a).
During HSV-1 neuronal latency, the latency-associated transcript (LAT), is the only viral gene that is abundantly transcribed during latency (14). LAT transcription-negative mutants reactivate poorly by explant or induced reactivation in the mouse (6, 7, 15), by induced reactivation in the rabbit (1, 17), and by spontaneous reactivation in the rabbit (9, 11). Thus, LAT is essential for efficient, wild-type (wt) reactivation from sensory neurons. We were interested in determining the effects of LAT mutants on the development of serum neutralizing antibody titers, a question that to our knowledge, has not previously been addressed. In this report, we therefore infected rabbits with wt HSV-1 or various LAT mutants and examined serum neutralizing antibody titers over time.
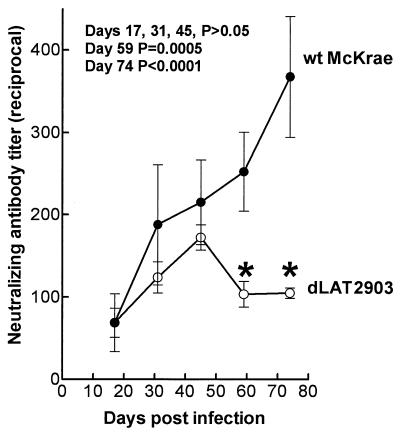
Rabbits were bilaterally ocularly infected with 2 × 105 PFU of wt HSV-1 strain McKrae in each eye as we previously described (9, 11). By day 18 to 20 postinfection, virus can no longer be detected in eyes or TG. By day 21 to 28 postinfection, all surviving rabbits have a latent infection in both TG and virus is detected only during sporadic spontaneous reactivation events. McKrae has a high level of spontaneous reactivation in the rabbit, with reactivated virus being detectable in approximately 10% of tears at any given time between 30 and 90 days postinfection (8–12). Rabbits were similarly infected with dLAT2903 (9), a McKrae-based LAT null mutant with reduced spontaneous reactivation. Serum was collected from five rabbits per group on days 17, 31, 45, 59, and 74 postinfection, and neutralizing antibody titers were determined on individual serum samples as described in the legend to Fig. 1. Between days 17 and 45, the average neutralizing antibody titers in the wt virus- and dLAT2903-infected rabbits increased over time and were similar (P > 0.05 on days 17, 31, and 45 by the Student t test [Fig. 1]). In the wt virus-infected rabbits, the average neutralizing antibody titer continued to increase after day 45. In contrast, in the dLAT2903-infected rabbits, the average neutralizing antibody titer decreased after day 45 and was significantly different from that of the wt on days 59 and 74 (P = 0.0005 and P < 0.0001, respectively, by the Student t test [Fig. 1]).
FIG. 1.
Serum neutralizing antibody titers in latently infected rabbits. Rabbits were ocularly infected with 2 × 105 PFU of wt McKrae or the LAT− mutant dLAT2903 in each eye as we previously described (9). All surviving rabbits harbor bilateral latent infections of both TG by day 21 to 28 postinfection. At the indicated times, serum was collected from five rabbits per group and their neutralizing antibody titers were individually determined as follows. wt HSV-1 (50 PFU) was incubated for 30 min at 37°C with twofold serial dilutions of individual serum samples, plated in triplicate on monolayers of RS cells in 12-well plates, overlaid with medium containing 1% methylcellulose, incubated for 3 days at 37°C, and stained with crystal violet, and then plaques were counted. The 50% plaque reduction titer for each individual serum sample was calculated by using the formula PDD50 = DL + [P50 − PL)(DH − DL)/(PH − PL)], where DL is the reciprocal of the lower dilution bracketing the 50% endpoint, PL is the number of plaques at the lower dilution bracketing the 50% endpoint, DH is the reciprocal of the higher dilution bracketing the 50% endpoint, PH is the number of plaques at the higher dilution bracketing the 50% endpoint, and P50 is the number of plaques at the 50% endpoint (5). Error bars indicate standard deviations. Asterisks indicate that the average neutralizing antibody titers induced by the LAT+ and LAT− viruses were significantly different (Student t test) at the indicated times.
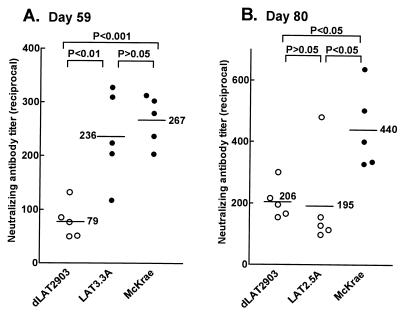
An additional, independent experiment is shown in Fig. 2A. Each datum point in this scattergram represents the neutralizing antibody titer from one rabbit. As in the previous experiment shown in Fig. 1, on day 59 postinfection, wt McKrae-infected rabbits had a significantly higher average neutralizing antibody titer than did dLAT2903-infected rabbits (P < 0.001 by analysis of variance [ANOVA] Tukey post test). To confirm that the reduced neutralizing antibody titers induced by dLAT2903 were due to the LAT mutation (i.e., lack of LAT or lack of spontaneous reactivation), the neutralizing antibody titers induced by LAT3.3A were also examined (Fig. 2A). LAT3.3A was constructed by inserting the LAT promoter and the first 1.5 kb of the primary 8.3-kb LAT transcript into an ectopic location in the LAT null mutant dLAT2903 between the UL37 and UL38 genes. This restored transcription of the first 1.5 kb of LAT and rescued spontaneous reactivation back to wt (11). On day 59 postinfection, the average neutralizing antibody titer in rabbits infected with LAT3.3A was similar to that of wt McKrae (P > 0.05 by ANOVA Tukey post test) and significantly greater than that of dLAT2903 (P < 0.01) (Fig. 2A). Thus, the lower neutralizing antibody titers in rabbits infected with dLAT2903 appeared to be due to the lack of transcription of the first 1.5 kb of LAT and/or the resulting impaired spontaneous reactivation.
FIG. 2.
Neutralizing antibody titers in rabbits latently infected with LAT3.3A or LAT2.5A. Rabbits were infected as described in the legend to Fig. 1. Serum was collected from five rabbits per group on day 59 or 80 postinfection, and neutralizing antibody titers were determined on individual serum samples. Each datum point represents a single serum sample. LAT3.3A was rescued from dLAT2903 by insertion of the LAT promoter and the first 1.5 kb of LAT into an ectopic location and has wt spontaneous reactivation (11). LAT2.5A is identical to LAT3.3A except that it contains only the first 661 LAT nucleotides and reactivates poorly (unpublished results). P values were determined by the ANOVA Tukey post test.
To examine a different LAT mutant with impaired spontaneous reactivation, neutralizing antibody induced by LAT2.5A was examined (Fig. 2B). LAT2.5A is similar to LAT3.3A, except that the ectopic insert contains only the first 661 nucleotides of the primary LAT transcript rather than the first 1,499 nucleotides. The spontaneous reactivation rate of LAT2.5A is indistinguishable from that of dLAT2903 (unpublished results). Consistent with this, on day 80 postinfection, the average neutralizing antibody titer in rabbits infected with LAT2.5A was similar to that of rabbits infected with dLAT2903 (P > 0.05 by ANOVA Tukey post test) and significantly less than that of rabbits infected with wt McKrae (P < 0.05) (Fig. 2B). Thus, two LAT-negative (LAT−) mutants with reduced spontaneous reactivation both had reduced neutralizing antibody titers during latency. Preliminary observations suggest that this is also the case with d34.5, an ICP34.5 (γ34.5) deletion mutant with reduced spontaneous reactivation (13) (data not shown).
The above results suggest that the average neutralizing antibody titer in rabbits infected with LAT-positive (LAT+) spontaneous reactivation-competent HSV-1 continued to increase during latency, while in rabbits infected with LAT− spontaneous reactivation-impaired mutants, the average neutralizing antibody titer decreased during latency. These results suggest two likely possibilities. Either the increasing neutralizing antibody titers seen during latency were due to continued restimulation of the immune system by spontaneously reactivating virus, or alternatively, the increasing neutralizing antibody titers were due to an immune response to a theoretical LAT protein continually produced during latency. To distinguish between these possibilities, we made use of a major difference between the rabbit and mouse ocular models of HSV-1 latency and reactivation. HSV-1 establishes latency in the TG of both mice and rabbits with similar levels of continued LAT expression. However, in mice, spontaneous reactivation is virtually undetectable (4, 16), regardless of the HSV-1 strain used, while spontaneous reactivation occurs in humans and in rabbits infected with HSV-1 strain McKrae.
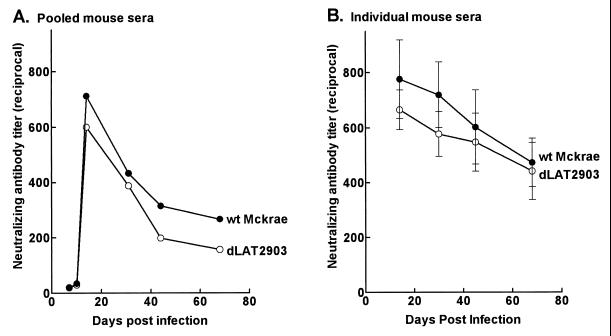
Mice were infected with wt McKrae or dLAT2903, and serum neutralizing antibody titers were determined as described above. Two independent experiments were performed. In the first, neutralizing antibody titers were determined at various times on pooled serum samples from four mice at each time point (Fig. 3A). With both viruses, the neutralizing antibody titers appeared similar, peaking around day 20 and then falling rapidly. In the second experiment, neutralizing antibody titers were determined on individual serum samples from five mice per group at each time point (Fig. 3B). Again, with both the wt and dLAT2903 viruses, the neutralizing antibody titer appeared to fall after day 20. At all time points during acute and latent infection, the neutralizing antibody titers for the wt virus- and dLAT2903-infected mice were similar (P > 0.05 by the Student t test). Thus, in an animal model that expresses LAT during latency but in which spontaneous reactivation is extremely rare, HSV-1 neutralizing antibody titers fell during latency regardless of whether the virus was LAT+ (wt) or LAT− (dLAT2903). This suggests that the increasing neutralizing antibody titers seen in rabbits latently infected with wt virus was due to continued restimulation of the immune system by reactivating virus, rather than an immune response to a theoretical LAT protein.
FIG. 3.
Neutralizing antibody in latently infected mice. (A) BALB/c mice were ocularly infected with 106 PFU of HSV-1 in each eye as previously described (2). Serum was collected from each of the four mice in a group at the indicated times and pooled, and neutralizing antibody titers were determined. (B) Swiss Webster mice were infected as described above, and sera were collected from five mice/group as indicated. Neutralization titers were determined on individual serum samples. The means and standard deviations are shown.
Despite the differences in average neutralizing antibody titers between LAT− and LAT+ viruses shown here during latency in rabbits, we were unable to detect any significant correlation between increased neutralizing antibody titers and increased detectable virus shedding in the tears for individual rabbits within each group. This suggests that the elevated neutralizing antibody titers induced by LAT+ viruses during latency were due to reactivation events other than those detectable by daily examination of tears for reactivated virus. Thus, spontaneous reactivation detected by shedding of reactivated virus in tears may grossly underestimate the amount of reactivation that occurs at the neuronal level. It is possible that the majority of neuronal reactivations in LAT+ viruses are terminated by viral or cell factors and/or immune factors prior to the presence of detectable amounts of infectious virus in the tears and that the host immune response is restimulated without detectable virus shedding.
To our knowledge, this is the first report comparing neutralizing antibody titers of LAT+ and LAT− viruses during latency in the rabbit. Our results suggest that during the first 2 to 3 months following acute infection, sporadic reactivations in the rabbit resulted in restimulation of the immune response and elevated serum neutralizing antibody titers. This is consistent with human infections in which individuals with clinical recurrences have average neutralizing antibody titers approximately two times those of seropositive individuals with no clinical recurrences (16a). This is similar to some human infections in which two or three exposures to the virus may be required for the development of maximum HSV-1 neutralizing antibody titers (3). In addition, the increased neutralizing antibody titers seen here with reactivation-competent viruses may provide a much less labor-intensive method of screening suspected reactivation-impaired mutants in the rabbit. It requires much less time and labor to determine serum neutralizing antibody titers at a single time point during latency (anywhere from 59 to 80 days postinfection) than it does to perform daily eye swabs for 3 to 4 weeks and individually analyze them for the presence of spontaneously reactivated virus. Perhaps more importantly, only 5 rabbits/group are required for the serum neutralizing antibody assays, while 10 or more rabbits/group are usually required for more direct analysis of spontaneous reactivation.
Acknowledgments
This work was supported by Public Health Service grants EY07566 and EY10243, the Discovery Fund for Eye Research, and the Skirball Program in Molecular Ophthalmology.
We thank Anita Avery for expert technical support.
REFERENCES
- 1.Bloom D C, Devi-Rao G B, Hill J M, Stevens J G, Wagner E K. Molecular analysis of herpes simplex virus type 1 during epinephrine-induced reactivation of latently infected rabbits in vivo. J Virol. 1994;68:1283–1292. doi: 10.1128/jvi.68.3.1283-1292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghiasi H, Nesburn A B, Kaiwar R, Wechsler S L. Immunoselection of recombinant baculoviruses expressing high levels of biologically active herpes simplex virus type 1 glycoprotein D. Arch Virol. 1991;121:163–178. doi: 10.1007/BF01316752. [DOI] [PubMed] [Google Scholar]
- 3.Gooding G W, Kibrick S. Pathogenesis of infection with herpes simplex virus with special reference to nervous tissue. In: Gajdusek D C, Gibbs J C J, Alpers M, editors. Slow, latent and temperate virus infections. U.S. Washington, D.C: Government Printing Office; 1965. pp. 143–144. [Google Scholar]
- 4.Harbour D A, Hill T J, Blyth W A. Recurrent herpes simplex in the mouse: inflammation in the skin and activation of virus in the ganglia following peripheral stimulation. J Gen Virol. 1983;64:1491–1498. doi: 10.1099/0022-1317-64-7-1491. [DOI] [PubMed] [Google Scholar]
- 5.Langford M P, Weigent D A, Stanton G J, Baron S. Virus plaque-reduction assay for interferon: microplaque and regular macroplaque reduction assays. Methods Enzymol. 1981;78:339–346. doi: 10.1016/0076-6879(81)78139-4. [DOI] [PubMed] [Google Scholar]
- 6.Leib D A, Bogard C L, Kosz-Vnenchak M, Hicks K A, Coen D M, Knipe D M, Schaffer P A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leib D A, Nadeau K C, Rundle S A, Schaffer P A. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci USA. 1991;88:48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perng G C, Chokephaibulkit K, Thompson R L, Sawtell N M, Slanina S M, Ghiasi H, Nesburn A B, Wechsler S L. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J Virol. 1996;70:282–291. doi: 10.1128/jvi.70.1.282-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perng G C, Dunkel E C, Geary P A, Slanina S M, Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perng G C, Ghiasi H, Slanina S M, Nesburn A B, Wechsler S L. High-dose ocular infection with a herpes simplex virus type 1 ICP34.5 deletion mutant produces no corneal disease or neurovirulence yet results in wild-type levels of spontaneous reactivation. J Virol. 1996;70:2883–2893. doi: 10.1128/jvi.70.5.2883-2893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perng G C, Ghiasi H, Slanina S M, Nesburn A B, Wechsler S L. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J Virol. 1996;70:976–984. doi: 10.1128/jvi.70.2.976-984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perng G C, Slanina S M, Ghiasi H, Nesburn A B, Wechsler S L. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J Virol. 1996;70:2014–2018. doi: 10.1128/jvi.70.3.2014-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perng G C, Thompson R L, Sawtell N M, Taylor W E, Slanina S M, Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J Virol. 1995;69:3033–3041. doi: 10.1128/jvi.69.5.3033-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rock D L, Nesburn A B, Ghiasi H, Ong J, Lewis T L, Lokensgard J R, Wechsler S L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987;61:3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimeld C, Hill T J, Blyth W A, Easty D L. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J Gen Virol. 1990;71:397–404. doi: 10.1099/0022-1317-71-2-397. [DOI] [PubMed] [Google Scholar]
- 16a.Spruance S L, Evans T G, McKeough M B, Thai L, Araneo B A, Daynes R A, Mishkin E M, Abramovitz A S. Th1/Th2-like immunity and resistance to herpes simplex labialis. Antiviral Res. 1995;28:39–55. doi: 10.1016/0166-3542(95)00037-m. [DOI] [PubMed] [Google Scholar]
- 17.Trousdale M D, Steiner I, Spivack J G, Deshmane S L, Brown S M, MacLean A R, Subak-Sharpe J H, Fraser N W. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol. 1991;65:6989–6993. doi: 10.1128/jvi.65.12.6989-6993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]