Key Clinical Message
Limb‐shaking transient ischemic attack (TIA) is a rare manifestation of carotid‐occlusive damage. This case report highlights the importance of accurate diagnosis and treatment of bilateral ballism as limb‐shaking TIA. Superficial cephalic artery and mid‐large artery anastomosis was performed after the initial acute treatment, and involuntary limb shaking disappeared.
Keywords: carotid stenosis, diabetes mellitus, hypertension, limb shaking, transient ischemic attack
1. INTRODUCTION
The prevalence of transient ischemic attack (TIA) in a large‐scale study in the United States was estimated to be 2.3%, and the number of patients in the United States is estimated to be close to 5 million. 1 TIA has been defined as an episode of focal neurological dysfunction with abrupt onset and rapid resolution lasting less than 24 h that is due to altered circulation to a limited region of the brain. 2 TIA is commonly characterized by neurological deficits such as unilateral weakness, decreased sensation, or vision loss. Limb‐shaking TIA is a rare manifestation of carotid‐occlusive damage. The symptoms include seizure‐like activity which is often misdiagnosed as focal motor seizures. 3 It is critical to diagnose this uncommon form of TIA because early detection and treatment dramatically lower the probability of a catastrophic and incapacitating stroke in the future. 4 We present the case of a middle‐aged woman with recurrent limb‐shaking TIA for 4 months before diagnosis.
2. CASE HISTORY/EXAMINATION
A 59‐year‐old female had been visiting our hospital since 2003 and was taking metformin 1000 mg and pravastatin 10 mg for a diagnosis of type 2 diabetes and dyslipidemia she had received at that time. Her HbA1c was 7.3%, and her LDL‐C was 97 mg/dL in July 2022. There is no history of hypertension or smoking. She did not have seizure‐like activity in the past. In May 2022, she began to experience weakness in her legs while in the kitchen. It means that symptoms are posture‐related. At that time she visited a neurosurgery clinic near her place of residence. There, head computed tomography (CT) was performed with no specific findings. Her blood pressure was 137/64 mmHg, and her pulse was regular at 81 beats/min. The weakness in her legs occurred again in July 2022. When she presented at our institution for examination regarding the weakness, sciatica was suspected 7 years prior based on her relevant medical history. As a result, the Orthopedics Department was consulted and sciatica was ruled out. She visited our institution again in August 2022, at which time she complained not only of weakness in her legs but also in her bilateral upper limbs. She had jerky movement. In a further visit in September 2022, acute cerebral infarction was identified on head magnetic resonance imaging (MRI). On that day, she was admitted to the Department of Neurology.
3. METHODS
On admission, her blood pressure was 167/109 mmHg and her pulse was regular at 88 beats/min. The patient was alert. There were no bilateral cervical bruits, and her cranial nerves were intact on examination. She had no muscle weakness or spasms; her plantar flexor responses and sensory functions were normal. Her gait was intact. The patient had no consciousness disturbances, urinary incontinence, dystonic posture, or tongue biting during the attacks. The limb‐shaking episodes lasted less than 5 min. The frequency was twice every 7–10 days. The right side was involved, and the provoking factor was standing in the kitchen. During the episodes, she had weakness, but no numbness or difficulty speaking. Throughout these episodes, the patient did not lose consciousness, did not have urinary or fecal incontinence, and did not describe any other symptoms. Routine blood work was normal except for an HbA1c of 7.2%. (Table 1) Mean intima media thickness (IMT) was 1.12 mm on the right and 1.14 mm on the left on carotid artery ultrasonography. Carotid artery ultrasonography did not show obvious significant stenosis. There was also no significant increase in blood flow velocity (Table 2). Her chest x‐ray was normal, and ECG showed a normal sinus rhythm.
TABLE 1.
Laboratory data on admission.
| Urine test | Blood analysis | ||||
|---|---|---|---|---|---|
| SG | 1.014 | WBC | 5000/μL | AST | 22 IU/L |
| PH | 5.5 | Neut | 68.9% | ALT | 37 IU/L |
| Protein | − | Lymp | 23.2% | γGTP | 15 IU/L |
| Sugar | − | Mono | 4.5% | TG | 104 mEq/L |
| Keton | + | Eos | 1.3% | LDL‐C | 97 mEq/L |
| Bilirubin | − | Baso | 0.5% | Na | 140 mEq/L |
| Urobilinogen | 0.1 | RBC | 490 × 10000/μL | K | 3.7 mEq/L |
| Hb | 14.9 g/dL | Cl | 101 mEq/L | ||
| Ht | 44.5% | UN | 10.5 mg/dL | ||
| MCV | 90.7 fl | CRE | 0.41 mg/dL | ||
| MCH | 30.4 pg | eGFR | 118 mL/min/1.73 m2 | ||
| MCHC | 33.6 g/dL | Glu | 159 mg/dL | ||
| PLT | 228 × 1000/μL | HbA1c | 7.2% |
TABLE 2.
Results of carotid artery ultrasonography, MRA, MRI, and SPECT.
| Carotid artery ultrasonography | Mean intima media thickness (IMT) was 1.12 mm on the right and 1.14 mm on the left. There was not obvious significant stenosis. There was also no significant increase in blood flow velocity |
| MRA | Narrowing of the left internal carotid artery from the origin, suggesting potential obstruction or severe stenosis |
| Diffusion‐weighted MRI | Small high‐signal areas scattered in the left frontal watershed region of the head |
| SPECT | Decreased blood flow in the left anterior cerebral artery and middle cerebral arterial area |
Magnetic resonance angiography (MRA) of the brain revealed narrowing of the left internal carotid artery from the origin, suggesting potential obstruction or severe stenosis (Figure 1 and Table 2). Diffusion‐weighted MRI of the brain revealed small high‐signal areas scattered in the left frontal watershed region of the head (Figure 2 and Table 2). Unfortunately, we did not examine the brain electrical activity. Electroencephalography (EEG) is considered useful for the exclusion of epilepsy.
FIGURE 1.
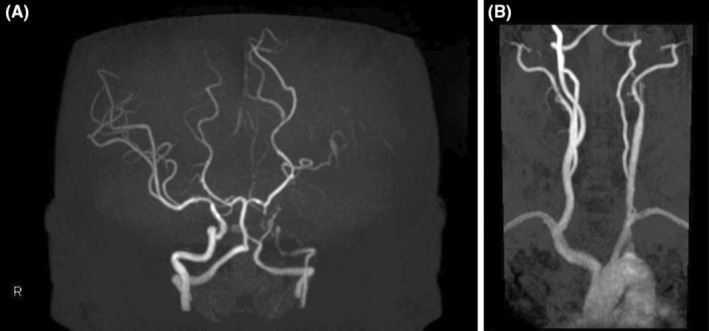
(A) MRA revealed narrowing of the left internal carotid artery from the origin. (B) MRA revealed narrowing of the left internal carotid artery from the origin.
FIGURE 2.
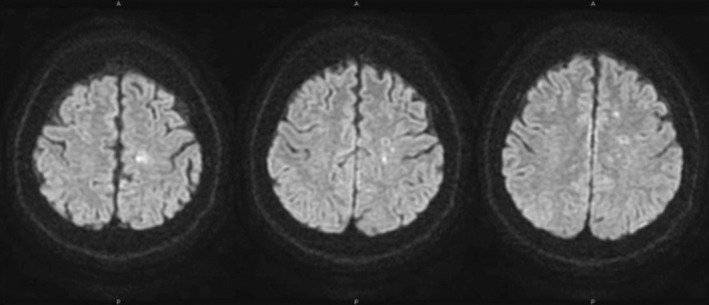
Diffusion‐weighted MRI revealed small high‐signal areas scattered in the left frontal watershed region of the head.
On arterial spin labeling (ASL) of the brain, we found a marked decrease in blood flow in the left middle cerebral arterial region. In the late phase, we found increased blood flow, likely originating from collateral circulation. The observed trembling of the upper and lower limbs led us to suspect limb‐shaking TIA.
For acute phase lesions, we infused argatroban 60 mg to prevent recurrence. The initial acute treatment plan included prescription of clopidogrel 300 mg, cilostazol 50 mg, and rosuvastatin 2.5 mg as initial start of acute treatment. The Japan Stroke Society Guideline 2021 for Treatment of Stroke recommended that intravenous administration of the selective thrombin inhibitor argatroban may be considered for non‐cardiogenic and non‐lacunar infarctions within 48 h of onset. Clopidogrel and cilostazol are recommended as effective antiplatelet agents for preventing recurrence of non‐cardiogenic cerebral infarction.
On single photon emission computed tomography (SPECT) in November 2022, we identified decreased blood flow in the left anterior cerebral artery and middle cerebral arterial area. (Table 2) Her blood pressure was 131/63 mmHg, and her pulse was regular at 79 beats/min. Superficial cephalic artery and mid‐large artery anastomosis (left STA‐MCA bypass surgery) was scheduled for January 2023. No more symptoms are present in the progress of post‐surgery status.
4. CONCLUSION AND RESULTS
It is important to differentiate this limb‐shaking TIA from other disorders such as focal motor seizures. Limb‐shaking TIA tends to transfer to cerebral infarction as does crescendo TIA. Early detection and treatment of the related carotid artery stenosis can not only eradicate the patient's TIA episodes but also lower the risk of future stroke. Multidisciplinary strategy incorporating neurology and possibly vascular surgery must be prioritized.
5. DISCUSSION
Limb shaking is a rare manifestation of TIA. Many papers have described the involuntary movements of limb shaking as shaking, twisting, and jerking of the extremities. Carotid endarterectomy and revascularization such as intracranial bypass surgery are regarded as effective treatment modalities. According to the Japan Stroke Society Guideline 2021 for Treatment of Stroke, carotid artery stenting (CAS) is appropriate for patients with carotid endarterectomy (CEA) risk factors for symptomatic internal carotid artery stenosis, in addition to the best medical treatment, including antiplatelet therapy.
The mechanism of limb shaking is reported to involve disturbances in the basal ganglia, which may play an important role in the cause of the limb shaking. 5 , 6 Cerebral blood flow in the basal ganglia and the frontal cortex may underlie the mechanism of limb shaking TIA. 7 , 8 With overactivity caused by reduced blood flow in the cerebral cortex being raised as the potential fundamental mechanism behind the limb shaking. 9
The relationship between diabetes and limb‐shaking TIA remains unclear. Vascular risk factors for atherosclerosis of large arteries, such as internal carotid artery, include diabetes mellitus, hypertension, and hyperlipidemia. We could not find reports and evidences about the relationship between diabetes and limb‐shaking TIA unfortunately. According to PubMed search, there were 12 case reports of limb‐shaking TIA from 2019 to 2024. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 (Table 3) There were five case reports of limb‐shaking TIA concomitant with diabetes mellitus. Rissardo et al. reported that hypertension was the most common comorbidity. 22 We considered the possibility of a connection among TIA symptoms, the extrapyramidal system, and neuropsychiatry. Im et al. reported that their observation supports the concept of a functional network between cortical areas and the basal ganglia or thalamus. 8 Patients with mental illnesses take dopamine receptor antagonists which may cause tremors that are difficult to distinguish from limb shaking.
TABLE 3.
Results of a literature search for the relationship between limb‐shaking TIA and diabetes mellitus on PubMed.
| Author | Published year | Diabetes | |
|---|---|---|---|
| 1 | Jiang et al. 10 | 2023 | DM |
| 2 | Handoko et al. 11 | 2023 | DM |
| 3 | Si et al. 12 | 2023 | N/A |
| 4 | Zhao et al. 13 | 2022 | N/A |
| 5 | Walls et al. 14 | 2022 | N/A |
| 6 | Shimizu et al. 15 | 2022 | N/A |
| 7 | Richardson et al. 16 | 2021 | DM |
| 8 | Ikeuchi et al. 17 | 2021 | DM |
| 9 | Tan et al. 18 | 2020 | N/A |
| 10 | Alkutbi et al. 20 | 2020 | N/A |
| 11 | Han et al. 21 | 2020 | DM |
| 12 | Ranasinghe et al. 22 | 2019 | N/A |
Abbreviations: DM, diabetes mellitus; N/A, not applicable.
In the present patient with TIA due to severe stenosis of the internal carotid artery, repeated limb shaking developed. This patient's clinical presentation was considered to warrant revascularization. Recognition of limb shaking as a relevant symptom could enable us to provide appropriate treatment as for acute phase cerebral infarction. Clinicians should always consider the existence of underlying carotid‐occlusive disease.
AUTHOR CONTRIBUTIONS
Go Yoshimichi: Conceptualization; writing – original draft.
FUNDING INFORMATION
There are no funding sources to disclose.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
Ethical approval is not required for this study in accordance with local or national guidelines.
CONSENT
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Yoshimichi G. A case of type 2 diabetes mellitus with limb‐shaking TIA . Clin Case Rep. 2024;12:e9249. doi: 10.1002/ccr3.9249
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
REFERENCES
- 1. Johnston SC, Fayad PB, Gorelick PB, et al. Prevalence and knowledge of transient ischemic attack among US adults. Neurology. 2003;60:1429‐1434. [DOI] [PubMed] [Google Scholar]
- 2. Panagos PD. Transient ischemic attack (TIA): the initial diagnostic and therapeutic dilemma. Am J Emerg Med. 2012;30:794‐799. [DOI] [PubMed] [Google Scholar]
- 3. Das A, Baheti NN. Limb‑shaking transient ischemic attack. J Neurosci Rural Pract. 2013;4(1):55‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salah Uddin ABM. Limb shaking transient ischemic attack—an unusual presentation of carotid occlusive disease. A case report and review of the literature. Parkinsonism Relat Disord. 2004;10:451‐453. [DOI] [PubMed] [Google Scholar]
- 5. Ninomiya S, Seno T, Fumoto N, et al. A case of the right internal carotid artery with limb shaking of the left arm and leg. Jpn J Stroke. 2014;36:42‐46. [Google Scholar]
- 6. Shimizu T, Hiroki M, Yamada Y, et al. Alternating paroxysmal hemibalism‐hemichorea in bilateral internal carotid artery stenosis. Intern Med. 2001;40:808‐812. [DOI] [PubMed] [Google Scholar]
- 7. Kim HY, Chung CS, Lee J, Han DH, Lee KH. Hyperventilation‐induced limb shaking TIA in moyamoya disease. Neurology. 2003;60:137‐139. [DOI] [PubMed] [Google Scholar]
- 8. Im S‐H, Oh CW, Kwon O‐K, Cho BK, Chung YS, Han DH. Involuntary movement induced by cerebral ischemia: pathogenesis and surgical outcome. J Neurosurg. 2004;100:877‐882. [DOI] [PubMed] [Google Scholar]
- 9. Muraga K, Suda S, Nagayama H, et al. Limb‐shaking TIA: cortical myoclonus associated with ICA stenosis. Neurology. 2016;86:307‐309. [DOI] [PubMed] [Google Scholar]
- 10. Jiang Q, Bai J, Nie S, Jin J, Qu L. Long‐segment common carotid occlusion presenting with limb‐shaking transient ischemic attack: case report. Front Surg. 2023;9:1028004. doi: 10.3389/fsurg.2022.1028004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Handoko KA, Hamdan M, Kurniawan D, Fatimah E. Limb shaking movement as a rare manifestation of transient ischemic attacks caused by carotid stenosis disease: a case report. Radiol Case Rep. 2023;8:2412‐2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Si L, Tu J, Lei H, Ji L, Zhang Z, Liu Z. A case of limb shaking transient ischaemic attack due to internal carotid artery dissection: an unusual presentation of fibromuscular dysplasia. BMC Neurol. 2023;23(1):91. doi: 10.1186/s12883-023-03130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Z, Qin J. Limb‐shaking transient ischemic attack in posterior circulation ischemia: a case report. J Int Med Res. 2022;50:3000605221142361. doi: 10.1177/03000605221142361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walls SP, Andre T, Adetunji A, Hama E. Transient ischemic attack shakes: a case report. Cureus. 2022;14:e28410. doi: 10.7759/cureus.28410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimizu T, Haro K, Tagawa M, et al. Bilateral ballism as limb‐shaking transient ischemic attacks treated with unilateral carotid artery stent placement. J Stroke Cerebrovasc Dis. 2022;31(11):106781. doi: 10.1016/j.jstrokecerebrovasdis.2022.106781 [DOI] [PubMed] [Google Scholar]
- 16. Richardson TE, Beech P, Cloud GC. Limb‐shaking TIA: a case of cerebral hypoperfusion in severe cerebrovascular disease in a young adult. BMC Neurol. 2021;21(1):60. doi: 10.1186/s12883-021-02296-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ikeuchi Y, Ashida N, Nishihara M, Hosoda K. Successful multiple burr hole openings for limb‐shaking transient ischemic attack due to moyamoya disease: illustrative case. J Neurosurg Case Lessons. 2021;2:CASE21401. doi: 10.3171/CASE21401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan CN, Taneja M, Venketasubramanian N. Limb‐shaking transient ischemic attacks in a patient with previous bilateral neck irradiation: the role of collateral flow. Case Rep Neurol. 2020;12(Suppl 1):84‐90. doi: 10.1159/000505391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alkutbi A, Elkady A. Limb‐shaking transient ischemic attacks masquerading as focal seizures. Cureus. 2020;12:e8157. doi: 10.7759/cureus.8157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han YY, Qi D, Chen XD, Song CJ. Limb‐shaking transient ischemic attack with facial muscles involuntary twitch successfully treated with internal carotid artery stenting. Brain Behav. 2020;10:e01679. doi: 10.1002/brb3.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ranasinghe T, Boo S, Adcock A, et al. Rare phenomenon of limb‐shaking TIA, resolved with intracranial wingspan stenting. Neurologist. 2019;24:37‐39. doi: 10.1097/NRL.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pitton RJ, Fornari CAL, et al. Limb‐shaking and transient ischemic attack: a systematic review. Neurologist. 2024;29:126‐132. doi: 10.1097/NRL.0000000000000526 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


