Key Clinical Message
This case report describes a 52‐year‐old patient presenting with recurrent episodes of pancreatitis and renal stones. Further investigation revealed hypocalcemia and elevated parathyroid hormone (PTH) levels, leading to diagnosis of a parathyroid adenoma. This case highlights the importance of considering primary hyperparathyroidism in patients with recurrent pancreatitis and renal stones, as early diagnosis and surgical intervention can prevent recurrence and reduce morbidity.
Abstract
Primary Hyperparathyroidism secondary to Parathyroid adenoma, rarely presents as acute pancreatitis. A 38‐year‐young male with a history of recurrent renal stones referred from a local center, presented to the emergency services, with a diagnosis of acute pancreatitis and bilateral renal stones. Laboratory evaluation showed an elevated calcium level, elevated PTH levels, low vitamin D, and low phosphorus levels. CT scan done outside was suggestive of acute pancreatitis along with bilateral renal calculi. USG neck and MIBI scan done as a part of hypercalcemia evaluation showed presence of a right parathyroid adenoma. Parathyroid adenoma was later removed, and calcium and parathyroid levels were normal on subsequent follow ups.
Keywords: hypercalcemia, pancreatitis, parathyroid adenoma, primary hyperparathyroidism, renal stones
1. INTRODUCTION
Acute pancreatitis includes inflammation and injury to the pancreas, which may lead to sepsis and multi organ dysfunction. 1 Pancreatitis is considered as a major public health concern worldwide and the incidence has increased in the last 30 years, with the highest increment seen in South Asia. 1 Gall stones and alcohol contribute as the major etiological factor of acute pancreatitis, together contributing approximately 2/3rd of the total cases. 2 Hypercalcemia is an uncommon cause of pancreatitis that causes calcium deposition in the pancreatic duct and calcium activation of trypsinogen. 3 Hypercalcemia classically presents as bone pain, constipation, renal stones, fatigue and neurological disturbances such as depression and confusion. Primary hyperparathyroidism is a common cause of hypercalcemia, and usually results due to a solitary parathyroid adenoma, accounting to around 80%–85% of the total cases of primary hyperparathyroidism. 4 However, parathyroid adenoma presenting as acute pancreatitis is a rare condition, contributing to around 1.5%–7% of the total etiology of acute pancreatitis. 5 , 6 Only few cases of pancreatitis secondary to hypercalcemia are reported, 7 which can lead to bias towards the diagnosis. Here we present a case of acute pancreatitis and bilateral renal stones due to hypercalcemia secondary to a parathyroid adenoma.
2. CASE REPORT
2.1. Case history
A 38‐year young male with a history of occasional alcohol consumption and recurrent renal stone formation, was referred to the emergencies services of Chitwan Medical College (CMC) with a provisional diagnosis of acute pancreatitis. This was his second episode of pancreatitis in the past 4 months. He had chief complaints of pain in the epigastrium and left upper quadrant of the abdomen, nausea, and vomiting. At presentation, patient was found to have sinus tachycardia and systemic hypertension [Systolic blood pressure 135–150 mmHg; diastolic blood pressure: 90–100 mmHg]. His pulse was 106 bpm, respiratory rate was 26/min, SpO2 was 96% in room air, and he was afebrile during presentation.
2.2. Methodology
Available laboratory reports from previous hospital showed elevated amylase levels, 286 U/L (range, 20–80 U/L), elevated lipase levels, 1176.0 U/L (range, 13–60 U/L). CT scan which was done outside and reviewed at our center in the department of radiodiagnosis was suggestive of acute pancreatitis (CTSI = 2/10) with bilateral renal stones with moderate left hydronephrosis (Figure 3). No secondary cause for hypertension was identified on evaluation. The patient was admitted to the ICU for further management. In the ICU, he was kept nil per oral for 48 hours, and ringer lactate and dextrose saline was given IV at the rate of 120 mL/h. The patient improved symptomatically with these conservative managements, and gradually solid foods were started Similarly, IV Tramadol 50 mg and IV Ondansetron 4 mg were used as analgesics and antiemetic. He was started on Tab Amlodipine 5 mg following transfer to the Gastroenterology ward. Other work‐up showed an elevated triglyceride level, 187.73 mg/dL (65–170 mg/dL), elevated VLDL, 37.55 mg/dL (range, 13–34 mg/dL), elevated calcium levels, 12.9 mg/dL (range, 9–11 mg/dL), elevated iPTH levels, 105.9 pg/mL (range, 18.5–88 pg/mL), low vitamin D, 11.22 ng/mL (range, deficiency <20 ng/mL) and low phosphorus levels, 2.2 mg/dL (2.5–5 mg/dL). As a part of hypercalcemia evaluation, USG neck revealed features of parathyroid adenoma (Figure 1). Technetium‐99 m Sestamibi scan was performed which was suggestive of a right parathyroid adenoma (Figure 2). Baseline evaluation showed derranged liver pannel tests. The test results are mentioned in Tables 1 and 2. For a possible cause of biliary pancreatitis, USG evlauation and CT discussion did not revealed any biliary pathology. The liver pannel tests remained elevated at day five of the patient admission, suggestive possibly of acute hepatitis.
FIGURE 3.
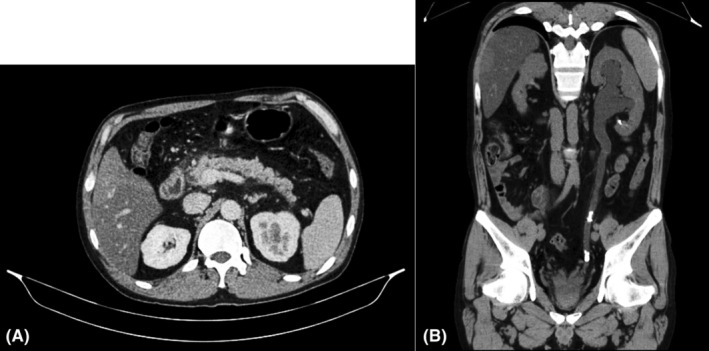
CT scan (Abdomen + Pelvis) suggestive of focal interstitial pancreatitis (A) and left sided ureteric stone (B).
FIGURE 1.
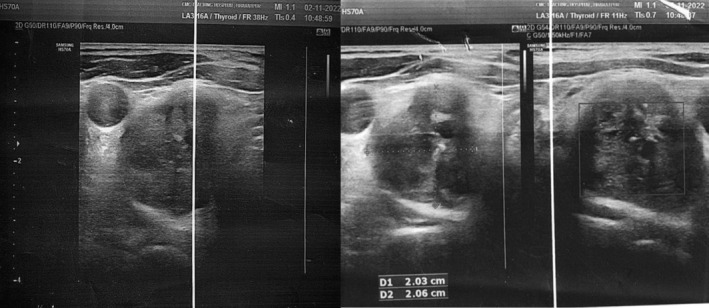
USG of the parathyroid gland showing a well‐defined heterogeneous lesion of 20 × 23 mm with nodular outline showing few areas of calcification noted separately from the right lobe of thyroid lying posteroinferior to it.
FIGURE 2.
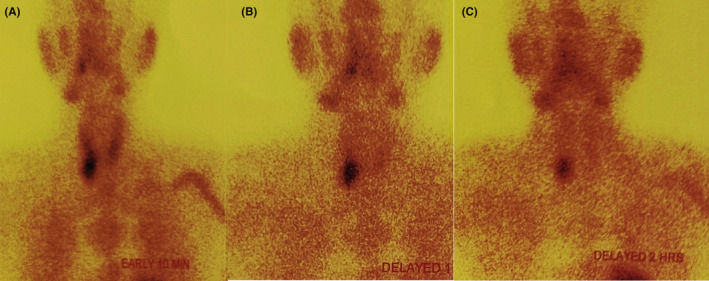
Technitium‐99 m Sestamibi scan showing retention areas near the inferior pole of the right thyroid gland (A) Early 10 min (B) Delayed 1 h (C) Delayed 2 h.
TABLE 1.
Blood Investigation Reports.
| Test | Result | Reference |
|---|---|---|
| Hemoglobin (Hb) | 15.1 | 11.0–16.0 g/dL |
| WBC | 10,500 | 4000‐11,000/mm3 |
| Platelets | 255,000 | 150,000‐400,000/mm3 |
| Urea | 34.0 | 10–50 mg/dL |
| Creatinine | 0.68 | 0.4–1.4 mg/dL |
| Na+/K+ | 139.0/4.3 | (135–155)/ (3.5–5.5) mmol/L |
| LFT | ||
| Bilirubin (total) | 2.65 | <2.0 mg/dL |
| Bilirubin (direct) | 1.16 | <0.4 mg/dL |
| SGOT/SGPT | 123.0/94.0 | (10–50)/ (10–50) U/L |
| Serum ALP | 204.0 | 50.0–136.0 U/L |
| Total Protein | 6.5 | 6.0–8.5 g/dL |
| Serum Albumin | 4.5 | 3.7–5.3 g/dL |
| PT/INR | 1.06 | |
| Serum amylase | 286.0 | 20–80 U/L |
| Lipase | 1176.0 | 13–60 U/L |
| Calcium | 12.9 | 9–11 mg/dL |
| Triglycerides | 187.73 | 65–170 mg/dL |
| LDL/VLDL | 49.97 | <150/ (13–34) mg/dL |
| Phosphorus | 2.2 | Adult: 2.5–5.0 mg/dL |
| Vitamin D (25‐OH), Serum | 11.22 | Deficiency: <20 ng/mL |
| Intact Parathyroid Hormone, Serum | 105.9 | 18.5–88.0 pg/mL |
TABLE 2.
Urine investigation reports.
| Test | Reports | Reference |
|---|---|---|
| Routine test | ||
| Blood (Free Hb, myoglobin, RBCs) | ++ | |
| Ketone | + | |
| Bilirubin/glucose/ascorbic acid | Nil | |
| Microscopic examination | ||
| Pus cells | 6–8 | /HPF |
| Epithelial cells | 2–4 | /HPF |
| RBCs | Plenty | /HPF |
| Urinary calcium | 12.3 | mg/dL |
| Urinary creatinine | 37.77 | mg/dL |
| Urine calcium/creatinine ratio | 0.32 | <0.14 |
3. RESULTS
Urosurgery team was consulted, and the patient was planned for surgical removal of the renal calculus. Department of ENT was also consulted, and the patient was also planned for the surgery for parathyroid adenoma. Parathyroid adenoma removal was done, and the surgery had no complications. Upon subsequent follow ups, the patient had normal calcium and parathyroid levels. He was doing well, and he had no complaints of any features suggestive of the recurrence of the previous diseases.
4. DISCUSSION
In this case report, we present a case of acute pancreatitis and recurrent renal stones due to parathyroid adenoma resulting in hypercalcemia. Hypercalcemia accounts to around 1.5%–8% of the total cases of pancreatitis and most of which is associated with hyperparathyroidism. 7 Hypercalcemia in a setting of recurrent renal stones requires thorough investigation and is strongly suggestive of primary hyperparathyroidism. 8 Cope et al was the pioneer to bring out the concept of pancreatitis due to hyperparathyroidism with his article in the Annals of Surgery in 1957. 9 Mayo clinic in between 1950 and 1975, operated 1153 patients with hyperparathyroidism and reported only 17 individuals (1.5%) to have a coexisting pancreatitis. 10 Prinz et al in a study found only 0.4% of the cases of acute pancreatitis to be caused by hyperparathyroidism. 11 Similarly, Carnaille B et al in 1998 retrospectively evaluated 1224 cases of hyperparathyroidism and found that acute pancreatitis was present in 3.2% cases. 12 The questions thus arise about the relation of hyperparathyroidism and acute pancreatitis and the controversies that follow through. 3 Sitges‐Sara in 1998, has however suggested the causation of pancreatitis due to hypercalcemia, but due to nonparathyroid causes and criticized the reports published by Mayo Clinic to not be true. 13 Amid the controversy, Abdhullah M et al in 2003 reported a case of recurrent pancreatitis with elevated serum calcium and elevated parathyroid hormones leading to the surgical exploration of neck to find a solitary parathyroid adenoma. 13 Despite the debate, it is still recommended to have the parathyroid levels checked in the setting of recurrent pancreatitis. 3 However, calcium stones in the form of calcium oxalate and calcium phosphate contribute the majority (85%) to the cause of renal stones and a history of such may be indicative of hypercalcemic state and may require further evaluation. 8 , 14 Thus, a clinical suspicion arose due to the history (2nd episode of pancreatitis within 4 month) and investigations of the patients which eventually led to the diagnosis of parathyroid adenoma leading to a hypercalcemic state presenting as a combination of acute pancreatitis and bilateral renal calculus (Figure 3).
Acute pancreatitis in a setting of hyperparathyroidism occurs either due to hypercalcemic state leading to de novo activation of trypsinogen to trypsin, leading to auto digestion, or due to the ductal obstruction secondary to formation of ductal calculi, or due to the genetic risk factors that may predispose patients with hyperparathyroidism to acute pancreatitis. 5
Acute pancreatitis is self‐limiting in most cases. Management consists of IV fluids, analgesia, and enteral feeding in those able to tolerate. Treatment focuses on the management of the underlying condition. Parathyroid adenoma causing hypercalcemia should thus be operated for the definitive management of such cases. Without the removal of the PTH secreting adenoma, the symptoms will reappear in the future, and thus any other forms of management will only be symptomatic relief for the time being. USG or Technetium‐99 m Sestamibi scan may be useful in the location of pathology within the parathyroid gland. 15
It is seen that parathyroidectomy seems to prevent the recurrent attacks of pancreatitis and nearly 100% improvement in pancreatitis has been seen following the treatment of hyperparathyroidism but the cases of renal calculus following parathyroidectomy have seen to increase within the immediate years which declines later signifying an overall benefit of the treatment. 16 , 17 Our patient also had systemic hypertension for which no obvious cause was found in the evaluation. Hypertension is reported to be present in 40–60% of patients with primary hyperparathyroidism.
Hypercalcemia secondary to parathyroid adenoma is a relatively uncommon cause of acute pancreatitis. Recurrent renal stones in presence of hypercalcemia should lead to evaluation for parathyroid adenoma in patients with acute pancreatitis.
5. CONCLUSION
This case report highlights the diagnostic challenges and management considerations in a patient presenting with primary hyperparathyroidism secondary to a parathyroid adenoma. Through a comprehensive clinical evaluation, including biochemical tests and imaging modalities such as ultrasound and sestamibi scintigraphy, the diagnosis of parathyroid adenoma was established. Surgical resection of the adenoma led to the normalization of serum calcium and parathyroid hormone levels, thereby reducing the risk of long term complications such as osteoporosis.
This case contributes to the existing literature by providing insights into the clinical presentation, diagnostic approach, and therapeutic interventions for parathyroid adenoma. Further studies are warranted to explore emerging diagnostic modalities and refine treatment strategies for this common endocrine disorder.
AUTHOR CONTRIBUTIONS
Aakash Kumar Pandit: Data curation; formal analysis; investigation; writing – original draft; writing – review and editing. Prajjwal Pokharel: Conceptualization; data curation; formal analysis; resources; writing – original draft; writing – review and editing. Kabin Sapkota: Investigation; methodology; software; writing – review and editing. Sanket Dhakal: Investigation; methodology; resources; writing – original draft; writing – review and editing. Ram Narayan Kurmi: Conceptualization; supervision; validation. Mukesh Kuamr Ranjan: Conceptualization; project administration; supervision; validation; writing – review and editing.
FUNDING INFORMATION
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS STATEMENT
The authors declare that the procedures were followed according to the regulations established by Clinical Research and Ethics Committee and to the Helsinki Declaration of the World Medical Association updated in 2013.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Pandit AK, Pokharel P, Sapkota K, Dhakal S, Kurmi RN, Ranjan MK. Unveiling the hidden culprit: Parathyroid adenoma induced recurrent renal calculus and pancreatitis—A case report. Clin Case Rep. 2024;12:e9248. doi: 10.1002/ccr3.9248
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the principal and the corresponding authors upon reasonable request. Additionally, comprehensive literature sources used for the literature review are cited appropriately, within the manuscript.
REFERENCES
- 1. Ouyang G, Pan G, Liu Q, et al. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990‐2017: a systematic analysis for the global burden of disease study 2017. BMC Med. 2020;18(1):388. doi: 10.1186/s12916-020-01859-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manandhar S, Giri S, Poudel P, Bhandari RS, Lakhey PJ, Vaidya P. Acute biliary pancreatitis: an experience in a tertiary level hospital of Nepal. Indian J Surg. 2013;75(6):449‐453. doi: 10.1007/s12262-012-0533-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee JK, Enns R. Review of idiopathic pancreatitis. World J Gastroenterol. 2007;13(47):6296‐6313. doi: 10.3748/wjg.v13.i47.6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Craven BL, Passman C, Assimos DG. Hypercalcemic states associated with nephrolithiasis. Rev Urol. 2008;10(3):218‐226. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2556488/ [PMC free article] [PubMed] [Google Scholar]
- 5. Khoo TK, Vege SS, Abu‐Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population‐based study. J Clin Endocrinol Metab. 2009;94(6):2115‐2118. doi: 10.1210/jc.2008-1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arya AK, Bhadada SK, Mukherjee S, et al. Frequency & predictors of pancreatitis in symptomatic primary hyperparathyroidism. Indian J Med Res. 2018;148(6):721‐727. doi: 10.4103/ijmr.IJMR_353_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jo IH, Paik C‐N. Acute pancreatitis with hypercalcemia as initial manifestation of multiple myeloma. The Korean J Gastroenterol. 2020;75(4):220‐224. doi: 10.4166/kjg.2020.75.4.220 [DOI] [PubMed] [Google Scholar]
- 8. Ritter A, Vargas‐Poussou R, Mohebbi N, Seeger H. Recurrent nephrolithiasis in a patient with hypercalcemia and Normal to mildly elevated parathyroid hormone. Am J Kidney Dis. 2021;77(6):A13‐A15. doi: 10.1053/j.ajkd.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 9. Cope O, Culver PJ, Mixter CG, Nardi GL. Pancreatitis, a diagnostic clue to hyperparathyroidism. Ann Surg. 1957;145(6):857‐863. doi: 10.1097/00000658-195706000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bess MA, Edis AJ, van Heerden JA. Hyperparathyroidism and pancreatitis. Chance or a causal association? JAMA. 1980;243(3):246‐247. https://jamanetwork.com/journals/jama/article‐abstract/368364 [PubMed] [Google Scholar]
- 11. Prinz RA, Aranha G. The association of primary hyperparathyroidism and pancreatitis. Am Surg. 1985;51(6):325‐329. https://pubmed.ncbi.nlm.nih.gov/3994175/ [PubMed] [Google Scholar]
- 12. Carnaille B, Oudar C, Pattou F, Combemale F, Rocha J, Proye C. Pancreatitis and primary hyperparathyroidism: forty cases. Aust N Z J Surg. 1998;68(2):117‐119. doi: 10.1111/j.1445-2197.1998.tb04719.x [DOI] [PubMed] [Google Scholar]
- 13. Abdullah M. Pancreatitis in Primary Hyperparathyroidism. n.d. http://www.e‐mjm.org/2003/v58n4/Primary_Hyperparathyroidism.pdf [PubMed]
- 14. Coe FL, Worcester EM, Evan AP. Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol. 2016;12(9):519‐533. doi: 10.1038/nrneph.2016.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haber RS, Kim CK, Inabnet WB. Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99m)technetium sestamibi scintigraphy. Clin Endocrinol. 2002;57(2):241‐249. doi: 10.1046/j.1365-2265.2002.01583.x [DOI] [PubMed] [Google Scholar]
- 16. Misgar RA, Mathew V, Pandit K, Chowdhury S. Primary hyperparathyroidism presenting as recurrent acute pancreatitis: a case report and review of literature. Indian J Endocrinol Metabol. 2011;15(1):54‐56. doi: 10.4103/2230-8210.77588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seib CD, Ganesan C, Arnow KD, et al. Kidney stone events following Parathyroidectomy vs nonoperative Management for Primary Hyperparathyroidism. J Clin Endocrinol Metab. 2022;107(7):e2801‐e2811. doi: 10.1210/clinem/dgac193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the principal and the corresponding authors upon reasonable request. Additionally, comprehensive literature sources used for the literature review are cited appropriately, within the manuscript.


