Abstract
Progesterone (P4), acting via its nuclear receptor (PR), is critical for pregnancy maintenance by suppressing proinflammatory and contraction-associated protein (CAP)/contractile genes in the myometrium. P4/PR partially exerts these effects by tethering to NF-κB bound to their promoters, thereby decreasing NF-κB transcriptional activity. However, the underlying mechanisms whereby P4/PR interaction blocks proinflammatory and CAP gene expression are not fully understood. Herein, we characterized CCR-NOT transcription complex subunit 1 (CNOT1) as a corepressor that also interacts within the same chromatin complex as PR-B. In mouse myometrium increased expression of CAP genes Oxtr and Cx43 at term coincided with a marked decline in expression and binding of CNOT1 to NF-κB-response elements within the Oxtr and Cx43 promoters. Increased CAP gene expression was accompanied by a pronounced decrease in enrichment of repressive histone marks and increase in enrichment of active histone marks to this genomic region. These changes in histone modification were associated with changes in expression of corresponding histone modifying enzymes. Myometrial tissues from P4-treated 18.5 dpc pregnant mice manifested increased Cnot1 expression at 18.5 dpc, compared to vehicle-treated controls. P4 treatment of PR-expressing hTERT-HM cells enhanced CNOT1 expression and its recruitment to PR bound NF-κB-response elements within the CX43 and OXTR promoters. Furthermore, knockdown of CNOT1 significantly increased expression of contractile genes. These novel findings suggest that decreased expression and DNA-binding of the P4/PR-regulated transcriptional corepressor CNOT1 near term and associated changes in histone modifications at the OXTR and CX43 promoters contribute to the induction of myometrial contractility leading to parturition.
Keywords: progesterone, gene regulation, transcription corepressor, inflammation, pregnancy, myometrium, uterine contraction, myometrial quiescence, NF-κB
Progesterone (P4) acting via its nuclear receptors (progesterone receptor PR-A and PR-B) plays a crucial role in maintaining myometrial quiescence and preventing uterine contractility during pregnancy (1, 2, 3). This is achieved by P4–PR inhibition of inflammatory response pathways and expression of contraction-associated protein (CAP)/contractile genes through various mechanisms. For example, P4 acting via PR maintains myometrial quiescence by increasing expression of the NF-κB inhibitor, IκBα (4, 5), and the mitogen-activated protein kinase inhibitor, mitogen-activated protein kinase phosphatase 1 (MKP-1–dual-specificity phosphatase 1, DUSP1) (6, 7), which together, act to block mediators of inflammatory response pathways. P4 also inhibits activation of the CAP genes, connexin 43 (CX43) and oxytocin receptor (OXTR), by increasing expression of the transcriptional inhibitor ZEB1 (zinc finger E-box-binding homeobox 1), which binds to the promoters of these genes to inhibit their expression (8). Moreover, P4–PR inhibits proinflammatory and CAP gene expression by tethering to NF-κB p65 or to AP-1 (activator protein 1) bound to their response elements within the promoters of these genes to prevent NF-κB or AP-1 activation (5, 9, 10).
To further define mechanisms whereby P4–PR maintains myometrial quiescence, we analyzed the capacity of WT and mutant forms of PR-A and PR-B to repress proinflammatory and CAP genes in telomerase immortalized human myometrial (hTERT-HM) cells. We observed that WT PR-B (PR-BWT) had a greater anti-inflammatory action than PR-AWT (9). Moreover, mutagenesis of amino acids within the P-Box of the PR DNA-binding domain (DBD) (PRmDBD) reduced the anti-inflammatory action of PR-B and completely abolished the anti-inflammatory effect of PR-A (9). Since these P-box mutations did not alter the recruitment of PR-A or PR-B to the NF-κB responsive regions of these genes, we postulated that PR exerts its anti-inflammatory actions by tethering to NF-κB bound to the promoters of proinflammatory and CAP genes and recruiting corepressors via sequences within its DBD (9).
To identify corepressors that interact strongly with PR-BWT, but relatively weakly with PR-AmDBD, we used immunoprecipitation, followed by mass spectrometry analysis of lysates of hTERT-HM cell lines stably expressing PR-BWT or PR-AmDBD. GATAD2B (transcriptional repressor p66-β) was previously identified as a novel corepressor that interacted with the PR-B DBD to mediate P4 suppression of proinflammatory and CAP genes (7). In the same analysis, we identified the CCR4–NOT transcription complex subunit 1 (CNOT1), which is a highly conserved multifunctional assembly of proteins involved in various aspects of gene expression, including transcription and mRNA turnover (11, 12, 13). CNOT1 was originally identified as a negative transcriptional regulator in yeast (14) and acts as a scaffold protein for multiple associated proteins containing deadenylase enzymes (CCR4, CAF1) and NOT subunits. It also is a ligand-dependent repressor of estrogen receptor α (ERα)–mediated transcription (15), indicating the potential role of CNOT1 in regulating P4-mediated gene expression.
In the present study, we investigated the corepressor activity of CNOT1 in P4–PR mediation of myometrial quiescence. Our novel findings revealed that increased P4–PR transcriptional activity in the quiescent mouse myometrium suppressed CAP gene expression. In addition, we found P4-mediated increased expression of CNOT1 and its recruitment to CAP gene promoters. This was associated with an increase in the enrichment of repressive histone marks and decreased binding of active histone marks. Near term, CNOT1 expression and binding to the CAP gene promoters declined, together with increased deposition of active histone marks and enhanced CAP gene expression.
Results
Expression of Cnot1 mRNA and protein declines in pregnant mouse myometrium toward term
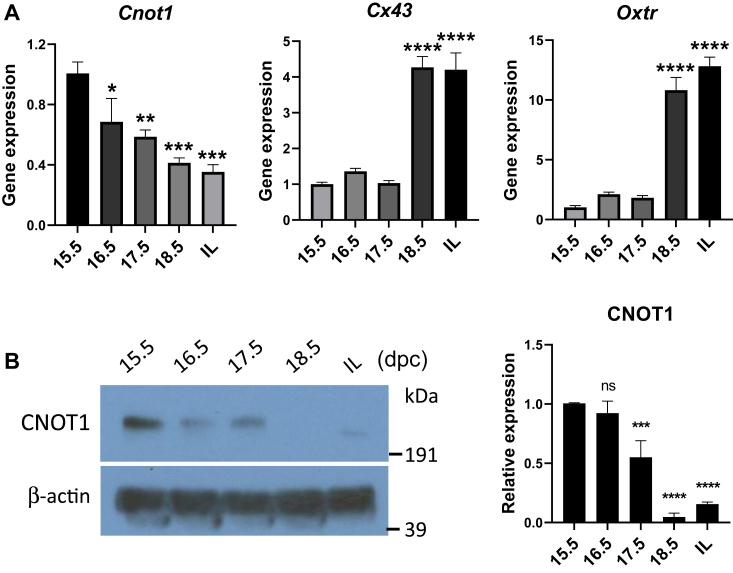
We hypothesized that the anti-inflammatory activity of PR is mediated in part by its capacity to recruit a complex of corepressors to promoters of proinflammatory and CAP genes. We previously identified the transcriptional corepressor CNOT1 as a protein that specifically interacted with the PR-B DBD in hTERT-HM cells treated with P4 + interleukin (IL)-1β (9). To gain insight into the roles of CNOT1 in the regulation of CAP gene expression during pregnancy and parturition, we first analyzed Cnot1 expression in myometrial tissues isolated from timed-pregnant mice at 15.5 to 18.5 days postcoitum (dpc) and in-labor. We observed that Cnot1 mRNA levels significantly declined in pregnant mouse myometrium toward term (Fig. 1A). Decreased mRNA levels of Cnot1 near term were inversely correlated with increased Cx43 and Oxtr gene expression (Fig. 1A).
Figure 1.
Cnot1 expression significantly declines in pregnant mouse myometrium toward term and in-labor.A, mRNA levels of Cnot1, Cx43, and Oxtr were analyzed in myometrial tissues of timed-pregnant mice from three independent gestational series. Myometrial tissues were isolated at 15.5 to 18.5 dpc and in active labor (IL) using RT–qPCR. Data are the mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n = 3 mice. B, whole cell extracts from timed-pregnant mouse myometrium isolated between 15.5 dpc and IL were analyzed for CNOT1 protein expression. Shown on the left is a representative immunoblot from three biological samples. Data are the mean ± SEM. t test was used for statistical analysis. (∗∗∗p < 0.001; ∗∗∗∗p < 0.0001). dpc, days postcoitum; qPCR, quantitative PCR.
Next, we analyzed CNOT1 protein expression by immunoblotting in mouse myometrial extracts isolated at 15.5 to 18.5 dpc and in-labor. Protein expression levels of CNOT1 also declined markedly in pregnant mouse myometrium toward term, compared with 15.5 dpc (Fig. 1B). Thus, the decrease in CNOT1 coregulator expression may contribute to the decline in P4–PR-mediated repression of CAP gene expression in mouse myometrium near term.
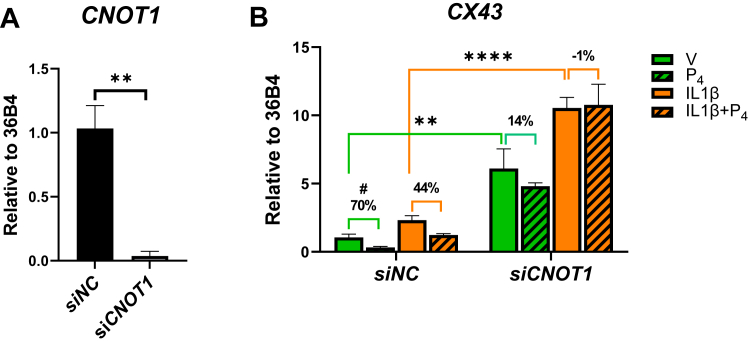
Knockdown of CNOT1 in PR-BWT–expressing hTERT-HM cells increases basal and IL-1β-induced CX43 expression and prevents P4–PR-mediated repression
To determine whether CNOT1 plays a functional role in P4–PR-mediated repressive activity, PR-BWT–expressing hTERT-HM cells were transfected with siRNA for CNOT1 (siCNOT1) or with a nontargeting siRNA control (siNC). These cells were treated ± IL-1β, ± P4, and harvested for mRNA expression analysis using RT–quantitative PCR (qPCR). Knockdown of CNOT1 in hTERT-HM PR-BWT cells completely abolished CNOT1 mRNA expression (Fig. 2A). CNOT1 knockdown significantly increased basal and IL-1β-induced CX43 expression and reduced P4-mediated repression of IL-1β-induced CX43 mRNA expression compared with control cells (Fig. 2B). The effects of CNOT1 knockdown on P4-mediated repression of basal and IL-1β expression levels are shown in Fig. S1. These findings suggest that CNOT1 serves a corepressor of CAP gene expression (Figs. 2B and S1), with the potential to inhibit basal expression of these genes in a P4-independent and/or dependent manner (Fig. 2B).
Figure 2.
CNOT1 regulates CX43 mRNA expression in hTERT-HM cells expressing PR-BWT. hTERT-HM cells expressing PR-BWT were cultured in phenol red–free medium supplemented with 1% charcoal-stripped FBS for 24 h. The cells were transfected with 40 nM siRNAs targeting CNOT1 or scramble siRNA (siNC) control for 48 h and then treated with DMSO vehicle (V), P4 (100 nM), IL-1β (10 ng/ml), or IL-1β + P4 for 16 h before being harvested for mRNA analysis. A, efficiency of CNOT1 mRNA knockdown by the corresponding siRNA was measured by RT–qPCR. B, CX43 mRNA levels were determined by RT–qPCR using acidic ribosomal phosphoprotein P0 (36B4) as an internal control. Data are the mean ± SEM of three replicate determinations for each treatment group in a representative experiment. P4-mediated repression activity was calculated by dividing the levels of mRNA expression in cells treated with vehicle or IL-1β with or without P4. Data are the mean ± SEM. Significant (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001) differences between samples was analyzed by two-way ANOVA. #(p < 0.05) indicates statistical analysis by t test. Percentage indicates repression activity by P4. CNOT1, CCR–NOT transcription complex subunit 1; CX43, connexin 43; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; hTERT-HM, telomerase immortalized human myometrial; IL-1β, interleukin 1β; P4, progesterone.
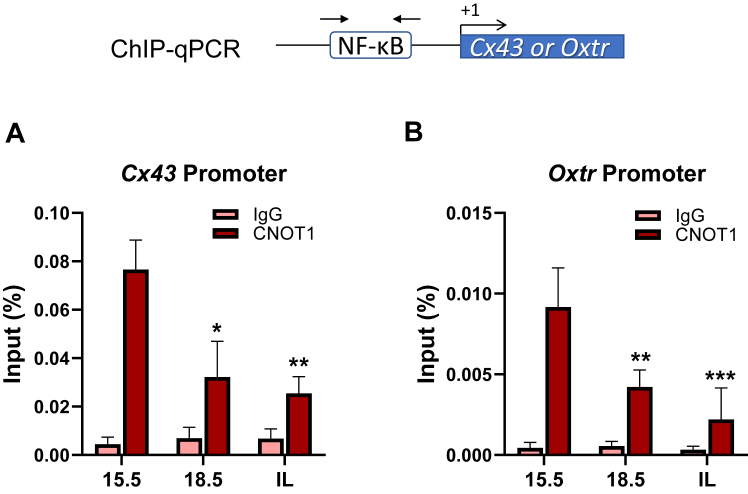
Binding of endogenous CNOT1 to genomic regions containing NF-κB response elements of contractile gene promoters significantly declines in pregnant mouse myometrium toward term
We proposed that P4–PR repression of proinflammatory and CAP gene expression is mediated by PR tethered to NF-κB at NF-κB response elements with recruitment of corepressors to the PR DBD (5, 9). To assess endogenous CNOT1 binding to NF-κB response elements within the Cx43 and Oxtr gene promoters, we performed chromatin immunoprecipitation (ChIP)–qPCR on mouse myometrium isolated at 15.5 and 18.5 dpc and in-labor. Nuclear extracts were prepared from cross-linked myometrial tissues of pregnant mice at each gestational time point and analyzed for binding of CNOT1 to the genomic regions containing NF-κB response elements within the Cx43 and Oxtr gene promoters using ChIP–qPCR. We found that endogenous CNOT1 binding was enriched at the NF-κB responsive regions of Cx43 and Oxtr gene promoters at 15.5 dpc; binding declined markedly at 18.5 dpc and in-labor (Fig. 3, A and B). These results indicate that reduced binding of endogenous CNOT1 to genomic regions containing NF-κB response elements of contractile gene promoters correlates with upregulation of Cx43 and Oxtr expression (Fig. 1A) in pregnant mouse myometrium toward term.
Figure 3.
Upregulation of Cx43 and Oxtr expression in pregnant mouse myometrium toward term is associated with reduced binding of endogenous CNOT1 to genomic regions containing NF-κB response elements.A and B, ChIP–qPCR was used to assess the enrichment of endogenous CNOT1 to genomic regions containing NF-κB response elements within the Cx43 (A) and Oxtr (B) promoters in pregnant mouse myometrium at 15.5 to 18.5 dpc and during labor. Cross-linked myometrial tissues from three tissue samples from three different pregnant mice at each gestational time point were analyzed for binding of CNOT1 by ChIP–qPCR. Data are the mean ± SEM from triplicate samples at each time point. Asterisks represent significant differences compared with 15.5 dpc values (∗p < 0.05; ∗∗p < 0.01). ChIP, chromatin immunoprecipitation; CNOT1, CCR–NOT transcription complex subunit 1; dpc, days postcoitum; qPCR, quantitative PCR.
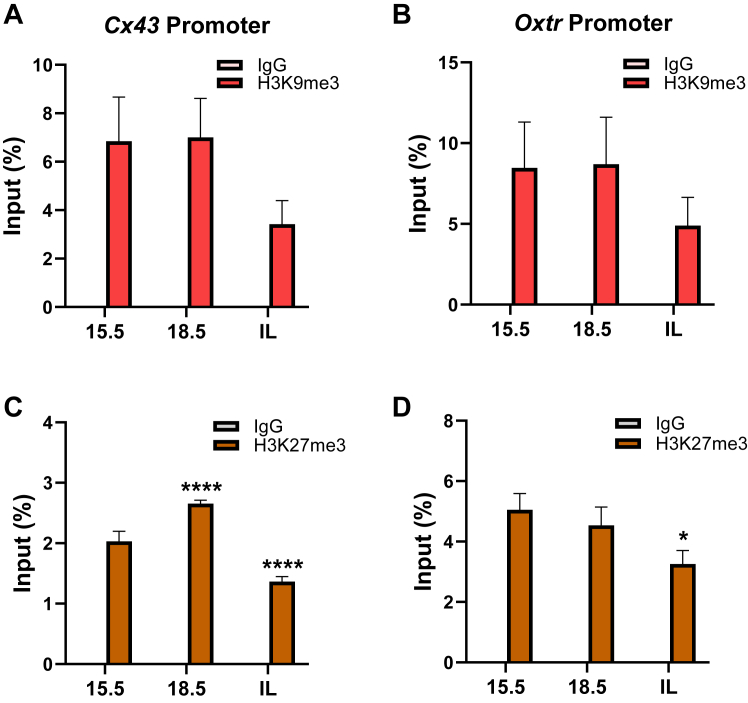
A decline in binding of endogenous corepressor CNOT1 to the NF-κB response elements of Cx43 and Oxtr gene promoters in pregnant mouse myometrium near term is associated with reduced enrichment of the repressive histone mark H3K27me3
The decline in CNOT1 expression and binding to CAP gene promoters near term may release the brakes on contractile gene expression by altering the level of repressive histone marks to the promoter. Indeed, CNOT1 has been found to interact with the EZH2 (enhancer of Zeste 2 polycomb repressive complex 2 subunit) component of polycomb repressive complex 2 (16). EZH2 is a histone lysine N-methyltransferase that catalyzes H3K27 trimethylation, a repressive chromatin mark (17). In addition, it has been reported that the epigenetic repressor human silencing hub (HUSH) complex that mediates trimethylation of H3K9 (H3K9me3) via SETDB1 associates with CNOT1 (15, 18, 19). ChIP–qPCR was therefore used to analyze enrichment of endogenous H3K9me3 and H3K27me3 at NF-κB response elements within the CAP gene promoters in mouse myometrium during late gestation. Enrichment for H3K9me3 (Fig. 4, A and B) and H3K27me3 (Fig. 4, C and D) was evident at the Cx43 and Oxtr gene promoters in quiescent mouse myometrium at 15.5 and 18.5 dpc. Interestingly, binding of H3K27me3 to the Cx43 promoter increased significantly at 18.5 dpc, compared with 15.5 dpc, and then enrichment for the repressive histone mark decreased significantly at both CAP gene promoter regions during the in-labor phase. Collectively, these findings indicate that a decline in binding of CNOT1 corepressor to NF-κB response elements of CAP gene promoters in pregnant mouse myometrium at term is associated with a significantly reduced enrichment of the repressive histone mark H3K27me3 but not H3K9me3.
Figure 4.
Upregulation of CAP gene expression in pregnant mouse myometrium toward term is associated with a significantly decreased enrichment of H3K27me3 at genomic regions containing NF-κB-REs in the Cx43 and Oxtr promoters.A–D, ChIP–qPCR was conducted to assess binding of endogenous H3K9me3 (A and B) and H3K27me3 (C and D) to genomic regions containing NF-κB-REs within the Cx43 (A and C) and Oxtr (B and D) promoters in pregnant mouse myometrium at 15.5 to 18.5 dpc and in-labor. Cross-linked myometrial tissues from three pregnant mice at each gestational time point were analyzed for histone modifications by ChIP–qPCR. Data are the mean ± SEM from triplicate samples at each time point. Asterisks represent significant differences compared with 15.5 dpc values (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). Please note that values for immunoglobulin G controls are too low to be visualized on all graphs. CAP, contraction-associated protein; ChIP, chromatin immunoprecipitation; dpc, days postcoitum; qPCR, quantitative PCR; RE, response element.
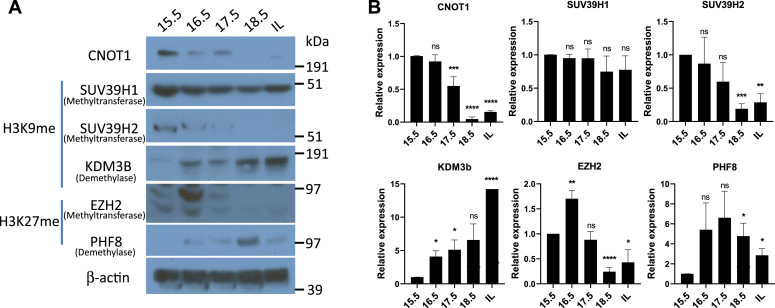
Declined level of repressive histone marks to CAP gene promoter near term correlates with decreased expression levels of corresponding histone-modifying enzymes
Histone marks are known to regulate gene expression by the combinatorial activity of enzymes that write or erase modification of histone residues (20, 21, 22). Expression levels of enzymes that methylate and demethylate H3K9me3 and H3K27me3 were analyzed in 15.5, 16.5, 17.5, 18.5 dpc, and in laboring mouse myometrium. Immunoblotting of whole cell lysates revealed that expression levels of the H3K9 histone methyltransferase SUV39H2, but not SUV39H1, declined toward term and in-labor (Fig. 5, A and B). The decline in SUV39H2 was associated with increased expression level of H3K9me3 demethylase KDM3B near term (Fig. 5, A and B). EZH2, an H3K27me3 methyltransferase, was decreased markedly toward term. This was associated with increased expression of the histone demethylase PHF8 toward term. PHF8 catalyzes demethylation of monomethylated and dimethylated histone K9 and K27. These findings indicate that decreased expression and recruitment of CNOT1 to CAP gene promoters near term and decreased levels of the repressive histone mark, H3K27me3, was associated with decreased expression of H3K27 histone methyltransferases and increased expression of the corresponding histone demethylases. The continuous expression of the H3K9me3 methyltransferase (SUV39H1) supports the observation of no significant change in the repressive mark H3K9me3 enrichment at CAP promoters.
Figure 5.
Histone methyltransferases that catalyze repressive histone marks decline toward term in association with the decrease in Cnot1 in pregnant mouse myometrium, whereas H3K9 and H3K27 demethylases increase.A, whole cell lysates were analyzed for expression levels of the indicated proteins by immunoblotting in timed-pregnant mouse myometrium isolated between 15.5 dpc and in-labor. Panels for CNOT1 and β-actin blots are reused from Fig. 1B. B, the levels of protein expression were analyzed by scanning of immunoblots and normalization to β-actin protein as an internal control. Data show a representative immunoblot from 2 to 3 biological replicates. t Test was used for statistical analysis (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). CNOT1, CCR–NOT transcription complex subunit 1; dpc, days postcoitum.
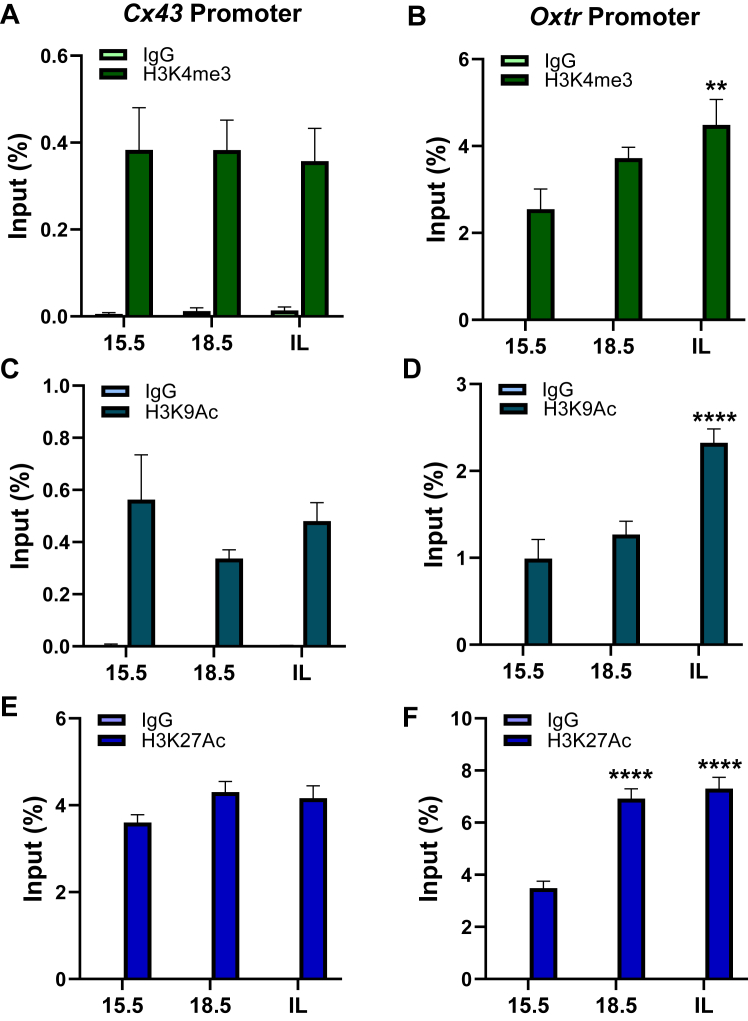
The decline in binding of repressive histone marks at CAP gene promoters is correlated with increased binding of active histone marks and the corresponding histone acetyltransferases toward term in mouse myometrium
Since the enrichment of repressive histone marks to the CAP gene promoters was decreased toward term in mouse myometrium, it was of interest to assess the binding of active histone marks to these genomic regions in association with the increase in CAP gene expression near term. To address this, we used ChIP–qPCR to analyze the enrichment of the active histone marks, H3K4me3 (Fig. 6, A and B), H3K9Ac (Fig. 6, C and D), and H3K27Ac (Fig. 6, E and F) to the Cx43 and Oxtr gene promoters in mouse myometrium during late gestation. ChIP–qPCR revealed that all the active histone marks tested were markedly and significantly enriched at the Oxtr gene promoter at in-labor, compared with 15.5 dpc. By contrast, enrichment of these active histone marks to the Cx43 gene promoter was observed at 15.5 dpc and remained unchanged at 18.5 dpc and in-labor. This suggests differential epigenetic regulation of Cx43 and Oxtr gene expression.
Figure 6.
Upregulation of CAP gene expression in pregnant mouse myometrium toward term is associated with increased enrichment of H3K4me3, H3K9Ac, and H3K27Ac at genomic regions surrounding NF-κB-REs in the Cx43 and Oxtr promoters. ChIP–qPCR was conducted to assess binding of endogenous H3K4me3 (A and B), H3K9Ac (C and D), and H3K27Ac (E and F) to genomic regions containing NF-κB-REs within the Cx43 and Oxtr promoters in pregnant mouse myometrium at 15.5, 18.5 dpc, and in-labor. Cross-linked myometrial tissues from three pregnant mice at each gestational time point were analyzed for histone modifications by ChIP–qPCR. Data are the mean ± SEM from three independent myometrial samples at each time point. Asterisks represent significant differences compared with 15.5 dpc values (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). Please note that values for immunoglobulin G controls are too low to be visualized on the graphs. CAP, contraction-associated protein; ChIP, chromatin immunoprecipitation; dpc, days postcoitum; qPCR, quantitative PCR; RE, response element.
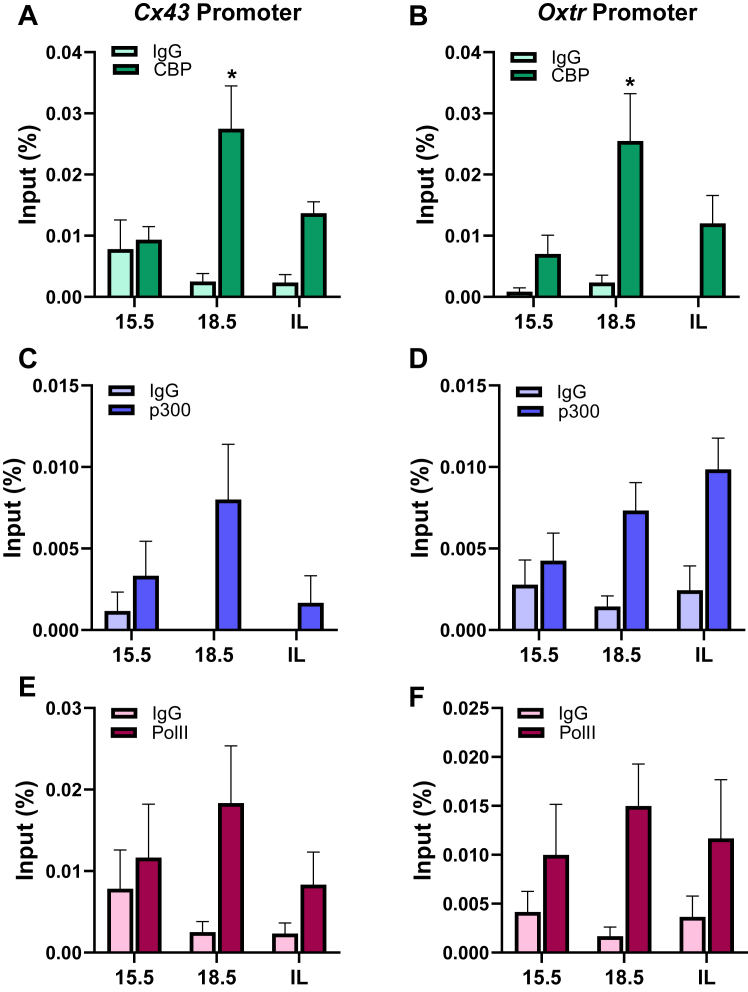
Next, we analyzed the binding of the histone acetyltransferases CBP (Fig. 7, A and B) and p300 (Fig. 7, C and D) as well as RNA polymerase II (Fig. 7, E and F) to Cx43 and Oxtr gene promoters. We observed significant increases in the association of CBP to the Cx43 and Oxtr gene promoters only at 18.5 dpc compared with 15.5 dpc. On the other hand, binding of both p300 and RNA pol II to the CAP gene promoters was enriched at 15.5 dpc and was maintained toward term (Fig. 7, E and F). These findings suggest that distinct histone-modifying enzymes bind and regulate deposition of histone marks at the Cx43 and Oxtr promoters. Indeed, the profound decline in repressive histone marks in parallel with increased enrichment for active histone marks at these CAP gene promoters in mouse myometrium toward term may create an open chromatin state that is permissive for RNA pol II–mediated transcriptional activation.
Figure 7.
Upregulation of CAP gene expression in pregnant mouse myometrium toward term is associated with increased enrichment of CBP and p300 at genomic regions containing NF-κB-REs in the Cx43 and Oxtr promoters. ChIP–qPCR was conducted to assess binding of endogenous CBP (A and B), p300 (C and D), and RNA polymerase II (E and F) to genomic regions containing NF-κB-REs within the Cx43 and Oxtr promoters in pregnant mouse myometrium at 15.5, 18.5 dpc, and in-labor. Cross-linked myometrial tissues from three pregnant mice at each gestational time point were analyzed by ChIP–qPCR for binding of endogenous CBP, p300, and RNA pol II. Data are the mean ± SEM from the three samples at each time point. Asterisks represent significant differences compared with 15.5 dpc values (∗p < 0.05; ∗∗p < 0.01). CAP, contraction-associated protein; ChIP, chromatin immunoprecipitation; dpc, days postcoitum; qPCR, quantitative PCR; RE, response element.
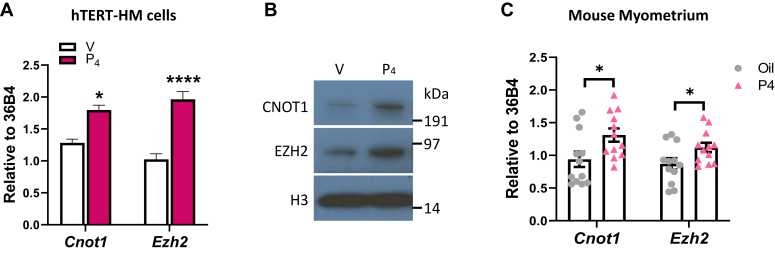
P4 induces CNOT1 and EZH2 expression in PR-B-expressing hTERT-HM cells and in pregnant mouse myometrium
Since expression and binding of the CNOT1 corepressor, together with repressive histone marks at the CAP gene promoters was relatively high at 15.5 dpc and declined in mouse myometrium near term, we postulated that CNOT1 might be regulated by PR, the function of which declines during late gestation (9, 23, 24, 25). In parallel, we assessed effects of P4 on the H3K27 methyltransferase, EZH2, which was previously found to be upregulated by P4 in trophoblasts (26) and in mammary epithelial cells (27). To assess P4–PR regulation of CNOT1 corepressor and of EZH2, hTERT-HM cells, stably expressing PR-B, were treated with P4 or with vehicle. P4 significantly increased mRNA expression of CNOT1 and EZH2 (Fig. 8A) in the human myometrial cells. Protein levels of CNOT1 and EZH2 were similarly increased in these cells by P4 treatment (Fig. 8B). Furthermore, we observed similar upregulation of corepressors by P4 treatment in pregnant mouse myometrium (Fig. 8C). In these studies, timed-pregnant mice were subcutaneously injected with P4 or sesame oil, as vehicle control, from 15.5 to 17.5 dpc, which delays labor by 1 to 2 days (8). Myometrial tissues isolated at 18.5 dpc from P4-treated mice manifested increased expression of Cnot1 and Ezh2, compared with vehicle-treated controls (Fig. 8C).
Figure 8.
CNOT1 and EZH2 expression are induced by P4 in PR-B expressing hTERT-HM cells and in pregnant mouse myometrium. hTERT-HM-PR-BWT cells were cultured in phenol red–free DMEM-F12 without serum for 24 h. The cells were then treated ±100 nM P4 for 16 h in DMEM-F12 without serum. A, RNA from cells were analyzed for CNOT1 and EZH2 mRNA and for 36B4, as an internal control, by RT–qPCR. Data shown are the mean ± SEM from an experiment repeated three times with similar results. Significant (∗p < 0.05; ∗∗∗∗p < 0.0001) differences between samples. B, CNOT1 and EZH2 protein expression was analyzed by immunoblotting of cell lysates from hTERT-HM-PR-BWT cells incubated ±P4 for 16 h using specific antibodies. Immunoblotting was performed twice. Histone H3 was used as a control for loading and transfer. C, pregnant mice were treated with P4 or sesame oil vehicle from 15.5 to 17.5 dpc (1 mg in 0.25 ml sesame oil). Myometrial tissues were harvested at 18.5 dpc and flash frozen for subsequent mRNA analysis. Three technical replicates of three to four biological replicates were tested. CNOT1, CCR–NOT transcription complex subunit 1; dpc, days postcoitum; EZH2, EZH2, enhancer of Zeste 2 polycomb repressive complex 2 subunit; hTERT-HM, telomerase immortalized human myometrial; PR, progesterone receptor; qPCR, quantitative PCR.
CNOT1 colocalizes with endogenous PR and is enriched at the CX43 and OXTR promoters by P4 treatment
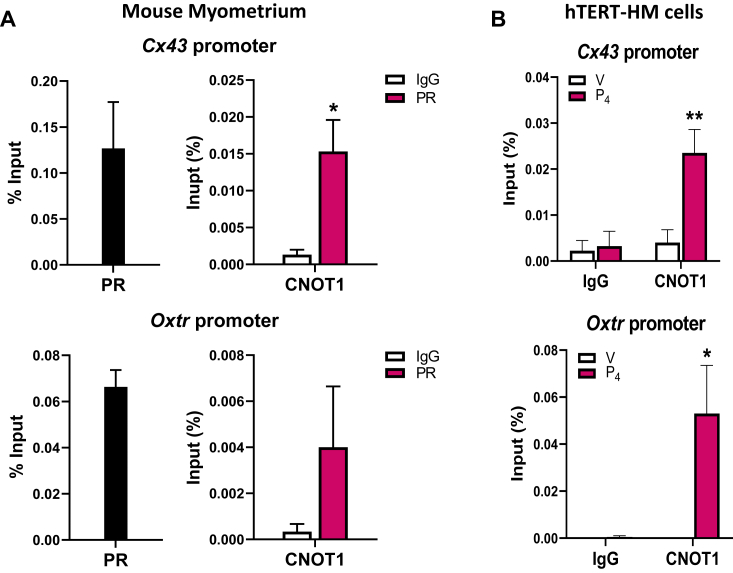
To determine whether corepressor CNOT1 forms a complex with PR at the NF-κB response elements within the 5′-flanking regions of the Cx43 and Oxtr genes, we conducted ChIP–re-ChIP assays. We utilized myometrial tissues from 15.5 to 16.5 dpc pregnant mice because quiescent myometrial tissues from these mice were found to have enriched binding of CNOT1 to CAP gene promoters (Fig. 3). In the first ChIP assays, binding of endogenous PR to the Cx43 and Oxtr promoters was ascertained (Fig. 9A, upper and lower left panels). In the second sequential ChIP assays, we observed that binding of CNOT1 to PR bound to chromatin was significantly increased over the immunoglobulin G negative controls on the Cx43 promoter but not the OxTR promoter (Fig. 9A, upper and lower right panels). Thus, PR colocalizes and forms a complex with CNOT1 in chromatin of quiescent mouse myometrium. Finally, ChIP–qPCR was used to assess the effects of P4 on CNOT1 corepressor binding to NF-κB response elements within the Cx43 and OxTR promoters in hTERT cells stably expressing PR-B. Notably, P4 treatment significantly increased binding of endogenous CNOT1 to the NF-κB response elements of the Cx43 and OxTR promoters (Fig. 9B). These findings suggest a potential dependence of CNOT1 on P4 treatment to bind to NF-κB response elements and regulate CAP gene expression in human myometrial cells. To address this, we knocked down PR-B and CNOT1 corepressor and conducted ChIP–qPCR assay. In the absence of endogenous PRB, the binding of CNOT1 and enrichment of repressive histone marks were maintained at the NF-κB response elements within the Cx43 promoter. Consistent with this observation, Cx43 gene expression was reduced (Fig. S3). Similarly, in the absence of CNOT1, PR-B binding to the Cx43 promoter and repression of Cx43 expression was unaffected (Fig. S3). These data suggest a basal repressive action of CNOT1 that is independent of P4–PR action.
Figure 9.
Endogenous PR binds to the Cx43 and Oxtr promoters in pregnant mouse myometrium and interacts with CNOT1 and EZH2. CNOT1 binding to the Cx43 and Oxtr promoters is enriched in hTERT-HM cells in response to P4 treatment. A, to determine whether endogenous CNOT1 interacts with PR in pregnant mouse myometrium as a complex, ChIP–re-ChIP assays were performed. The first ChIP assay was conducted to assess binding of endogenous PR to an NF-κB-RE in the Cx43 and Oxtr promoters in chromatin of pregnant mouse myometrium at 16.5 dpc. The PR-bound complexes were then eluted and subjected to sequential ChIP assays using antibodies to CNOT1. B, to determine whether P4 regulates recruitment of CNOT1 to NF-κB-REs within the CX43 and OXTR promoters, ChIP assays were performed using hTERT-HM cells expressing PR-BWT. The cells were treated with DMSO (V) or P4 (100 nM) for 16 h. ChIP–qPCR was used to quantify recruitment of CNOT1 to the NF-κB-RE-containing regions of the CX43 and OXTR promoters using specific primers (Table S1). The data are presented as percentage of input and the mean ± SEM of three replicate assays for each treatment group (∗p < 0.05; ∗∗p < 0.01). ChIP, chromatin immunoprecipitation; CNOT1, CCR–NOT transcription complex subunit 1; DMSO, dimethyl sulfoxide; dpc, days postcoitum; hTERT-HM, telomerase immortalized human myometrial; P4, progesterone; PR, progesterone receptor; RE, response element.
Discussion
The mechanisms involved in the myometrial quiescence throughout most of pregnancy and its transition to a coordinately contractile unit at term are highly complex and interrelated. Previous studies have shown that myometrial quiescence is mediated in large part by P4–PR suppression of proinflammatory and CAP gene expression (5, 9). Decreased PR function in the myometrium toward term is known to be caused, in part, by the increased local metabolism of P4 by 20α-hydroxysteroid dehydrogenase (23, 25), the increased interaction of PR with proinflammatory transcription factors (e.g., AP-1, NF-κB) (5), an increased ratio of PR-A to PR-B (24, 28, 29, 30), and increased expression of the truncated PR isoform, PR-C (31). In this study, we identified CNOT1, a scaffold protein of the CCR4–NOT complex (12), as a novel P4-induced PR-B-interacting protein that mediates myometrial quiescence during gestation via repressing CAP gene promoter activity. We suggest that the decrease in Oxtr and Cx43 gene expression in pregnant mouse myometrium toward term or in-labor may be mediated, in part, by the decline in P4–PR induction and recruitment of CNOT1.
The CCR4–NOT complex is a large multifunctional protein complex, primarily known to regulate RNA and protein integrity (32). This complex is comprised of more than nine core subunits, including CNOT1–5 and deadenylase enzymes CCR4 and CAF1. These proteins are localized in both cytoplasm and nucleus, depending on their functional activities. Previous studies showed that the CCR4–NOT complex regulates cytoplasmic mRNA turnover by acting together with polyadenylate binding protein 1 (PABPC1) to induce mRNA deadenylation (32, 33, 34). We observed that increased nuclear CNOT1 plays a functional role in mediating the downregulation of CAP gene transcription during pregnancy. In addition to its role as a transcriptional corepressor in the nucleus, CNOT1 may also maintain myometrial quiescence through its possible role in CAP mRNA degradation within the cytoplasm (Fig. S2). By mass spectrometry analysis, we also previously identified CNOT2 and CNOT3, other components of CCR4–NOT complex, as PR-B-interacting proteins (Table S1 in (9)). CNOT2 was shown to directly interact with CNOT3 and be involved in repression of RNA pol II–directed promoter activity (32, 35). Previous reports indicated that CNOT1 mediated ligand-dependent suppression of ERα and retinoid X receptor target genes via interaction of its LXXLL motif within the ligand-binding domains of ERα and retinoid X receptor (15).
This study found that a decrease in the recruitment of CNOT1 was associated with a marked decrease in the repressive histone mark H3K27me3 at the promoters of CAP gene, whereas enrichment of H3K9me3 remains unchanged (Fig. 4). The expression of histone-modifying enzymes that corresponded to these repressive histone marks was found to be correlated with their enrichment at CAP gene promoters in pregnant mouse myometrium during late pregnancy. Specifically, SUV39H2 was elevated at 15.5 dpc and declined toward term (Fig. 5), whereas KDM3B was upregulated during late gestation (Fig. 5); this was associated with decreased enrichment of H3K9me3 at the CAP gene promoters. H3K9 methyltransferases in mammalian cells include SETDB1, G9a, and the PRDM family (36). It will be of interest to determine whether any of these other H3K9 methyltransferases are expressed in pregnant mouse myometrium and contribute to the suppression of myometrial contractility during late gestation. In addition, EZH2 was found to be an important H3K27 methyltransferase in quiescent mouse myometrium, with its expression elevated at 15.5 dpc and downregulated near term (Fig. 5). However, the inconsistency between enrichment of H3K27me3 and downregulation of EZH2 at Cx43 gene promoter at 18.5 dpc (as shown in Figs. 4C and 5A) suggests the potential for other methyltransferases such as EZH1 to catalyze H3K27me3 at that time point (37, 38). The expression of PHF8 protein, which catalyzes demethylation of monomethylated and dimethylated histone K9 and K27 residues, was increased toward term in the pregnant mouse myometrium (Fig. 5). Previous study has observed H3K27 trimethylation–mediated transcriptional silencing of genes during early gestation in mouse decidual stromal cells. Furthermore, expression of KDM6A, H3K27 demethylase, was increased in the human decidua at term compared with 6 weeks (39). However, we observed that KDM6A expression was slightly decreased from 15.5 dpc toward term in mouse myometrial tissues (unpublished data). Further research is necessary to investigate regulation of H3K27 demethylase in myometrial and decidual tissues.
At 15.5 dpc, the Oxtr gene promoter displayed enrichment for active histone marks (H3K4me3, H3K9Ac, and H3K27Ac), which significantly increased during labor (Fig. 6). In contrast, the enrichment of active histone marks in the Cx43 promoter region remains constant from 15.5 dpc until labor. This suggests that the myometrium is prepared to quickly activate the Cx43 gene to exit the quiescence state (40). Although active histone marks were differentially enriched at Cx43 and Oxtr gene promoters at 15.5 dpc, the histone acetylase CBP was similarly enriched at both gene promoters at 18.5 dpc (Fig. 7). Interestingly, p300 and RNA pol II binding was enriched at the promoters of both Cx43 and Oxtr genes at 15.5 dpc and remained unchanged through term (Fig. 7). These results suggest that the pronounced decline of CNOT1 binding at 18.5 dpc and interplay between repressive and active histone marks may be crucial for RNA pol II induction of both CAP genes during term.
In a previous study, we discovered through mass spectrometry analysis of myometrial proteins that PR-interacting transcriptional corepressors interacted more strongly with PR-BWT than with a mutant form of PR-A, which lost its anti-inflammatory activity (9). These corepressors included C-terminal-binding proteins (CtBP)1 and CtBP2. In addition, ZEB1–2 inhibited transcription of IL-2 and several muscle-specific genes, such as myosin heavy chain and myogenin, by forming a complex with CtBP1 (C-terminal binding protein 1) and CtBP2 (C-terminal-binding protein 2) (41, 42, 43) and HDACs (histone deacetylases) (43). CtBP1–2, in turn, recruited the polycomb repressive complex (PcG–PRC) 2 to suppress gene expression (44). A separate study showed that ZEB1 was upregulated by P4–PR in quiescent pregnant mouse myometrium and bound to the promoters of Cx43 and Oxtr genes to inhibit their expression (8). ZEB1 expression declined near term, which was associated with the decrease in PR function and induction of CAP gene expression (8). These findings suggest that decreased expression of ZEB1 and the associated corepressors may enhance myometrial CAP gene expression near term and contribute to the decline in PR function leading to parturition. In pregnant rat myometrium, P4 repression of Cx43 transcription was mediated by PR binding to AP-1 bound to the Cx43 promoter with subsequent recruitment of the RNA-splicing factors/corepressors, PSF and p54nrb. These putative corepressors declined toward term and in-labor (10, 45). PR also recruited Sin3A and HDAC1, which are PSF–p54nrb interacting proteins, in response to myometrial stretch and an increase in circulating estrogen levels (10).
Our current analysis demonstrates that CNOT1 binding and repressive activity occurs in a PRB-independent manner. As evaluated by ChIP–qPCR, knockdown of PR-B does not impact CNOT1 binding to the Cx43 promoter region. Consequently, the occupancy of CNOT1 results in a significant decline in Cx43 gene expression levels in the myometrial cells (Fig. S3). In accordance with CNOT1 repression independent of PRB, reduced enrichment of repressive histone marks (H3K9me3 and H3K27me3) was observed only in the absence of CNOT1. These findings suggest that CNOT1-mediated gene silencing is reinforced via recruitment of histone methyltransferases to the CAP promoter regions. While knockdown of CNOT1 does not impact PR-B binding to the Cx43 promoter, PR-B alone was not sufficient to silence Cx43 gene expression at basal levels. Unexpectedly, we noted a marked decline in Cx43 gene expression in the absence of PR-B. Based on these observations, we cannot rule out the involvement of another PR-B-independent repressive regulatory mechanism at the chromatin level. Future studies will help to further elucidate the P4–PR regulatory mechanisms and the recruitment of other corepressors, such as ZEB1, to drive CAP gene silencing in the myometrium during pregnancy.
In sum, the present study suggests the following model for the regulation of CAP gene expression in the myometrium during pregnancy (Fig. 10). Throughout most of pregnancy, P4–PR inhibits CAP gene expression by recruiting multiple corepressor complexes. Binding of corepressor CNOT1 to NF-κB response elements in the regulatory regions of CAP genes may occur in a PR-independent manner. CNOT1 binding, in turn, may recruit histone methyltransferases, such as SUV39H2 and EZH2, which catalyze H3K9me3 and H3K27me3, respectively. P4–PR also upregulates EZH2 expression (46) and recruits the transcriptional repressor, ZEB1, to the CAP gene promoters (8). ZEB1, interacting with CtBP1–2, recruits Sin3a and HDACs to further repress CAP and proinflammatory genes (43, 47, 48). Interestingly, both Sin3A and the NuRD–Mi2 complexes contain modules comprised of HDAC1, HDAC2, and the two histone tail-targeting proteins, RbAp46 and RbAp48 (49).
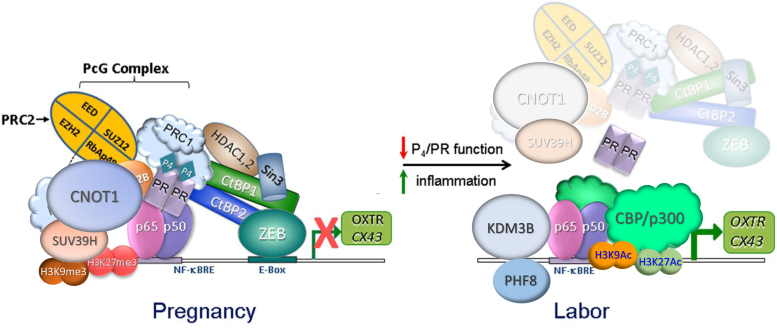
Figure 10.
Proposed model indicates that during pregnancy, P4–PR represses inflammatory and contractile gene expression through binding to NF-κB response elements in the promoters of these genes. CNOT1 in a PR-independent manner binds and recruits the repressive histone modifier PRC2 and SUV39H complexes. The EZH2 component of PRC2 catalyzes trimethylation of H3K27, which recruits the PRC1 repressive complex to reinforce inhibition of contractile gene expression. SUV39H mediates trimethylation of H3K9. P4–PR also increases expression of ZEB1, which interacts with the repressive complex of CtBP1–CtBP2–Sin3A–HDAC 1 and 2. Collectively, these interactions cause a dynamic shift in the abundance of histone methylation marks promoting a closed chromatin configuration, resulting in silencing of contractile gene expression. In the transition to labor, there is a loss of PR function and decreased expression of CNOT1. These changes lead to reduced recruitment of PRC2 and SUV39H complexes and a decrease in the enrichment of the repressive marks, H3K9me3 and H3K27me3. Finally, the associated decrease in the transcriptional repressor ZEB1 and associated factors results in increased binding of CBP and p300 histone acetylases with increased H3K9 and H3K27 acetylation marks. Collectively, these coordinated epigenetic events cause opening of the chromatin structure surrounding contractile and proinflammatory genes, resulting in their increased expression and the initiation of labor. CNOT1, CCR–NOT transcription complex subunit 1; CtBP, C-terminal binding protein; EZH2, enhancer of Zeste 2 polycomb repressive complex 2 subunit; HDAC, histone deacetylase; P4, progesterone; PR, progesterone receptor.
During late gestation, multiple factors including enhanced production of inflammatory signals by the fetal lung (50, 51, 52), increased mechanical stretch (53), and increased circulating estrogen levels (53, 54, 55) contribute to an increased inflammatory response within the myometrium. In addition, local P4 levels decline because of the induction of 20α-hydroxysteroid dehydrogenase (23, 56, 57), leading to reduced expression and/or binding of CNOT1, ZEB1, and GATAD2B. Consequently, there is diminished recruitment of the PcG and NuRD complexes, resulting in decreased enrichment of the repressive histone mark H3K27me3 and increased recruitment of the histone acetyltransferase CBP with increased enrichment of chromatin-associated H3K4me3, H3K9ac, and H3K27ac. These collective changes result in an opening of chromatin structure to enhance RNA pol II–mediated transcription of proinflammatory and CAP genes, leading to the initiation of parturition (Fig. 10). Overall, P4-mediated regulatory networks in the myometrium, in parallel with the unique CNOT1 repressor activity, facilitate inhibition of proinflammatory and CAP gene expression to sustain myometrial quiescence during pregnancy.
Experimental procedures
Timed-pregnant ICR–CD1 mice and collection of murine tissues
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. Timed-pregnant ICR–CD1 mice were bred at UT Southwestern. Uteri were removed at 15.5 to 18.5 dpc and during labor (in-labor) following isoflurane anesthetic inhalation (Baxter Healthcare Corp) and cervical dislocation. The uteri were cleared of all embryonic materials as well as endometrium and decidua. The tissues were further enriched for myometrium by sterile scraping and blotting with a paper towel. The myometrial tissues were then washed in ice-cold PBS, flash-frozen in liquid nitrogen, and stored at −80 °C until analyzed.
P4 treatment studies
Timed-pregnant ICR mice were injected subcutaneously with P4 (Sigma, 1 mg in 0.25 ml sesame oil) or with sesame oil (vehicle) daily at 15.5, 16.5, and 17.5 dpc. Uterine tissues were collected at 18.5 dpc, enriched for myometrium, washed in ice-cold PBS, and flash frozen for subsequent mRNA analysis.
Cell culture conditions and reagents
hTERT-HM cells that were stably transfected with a PR-B-GFP fusion construct, as previously described (9), were maintained in DMEM-F12 (Life Technologies), supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% streptomycin–penicillin solution (Gibco). The cells were cultured in an atmosphere of 95% air/5% CO2 in a 37 °C tissue culture incubator. For all experiments, the cells were seeded in maintenance medium and allowed to reach 70% confluence. Prior to treatment, media were replaced with phenol red–free DMEM-F12 medium (Gibco) without FBS or with charcoal-stripped FBS (1% v/v) (Gibco) for RNAi experiments, and the cells were cultured overnight. The next morning, the cells were incubated in medium containing P4 (100 nM; Sigma), IL-1β (10 ng/ml; Cell Signaling Technology), or with P4 + IL-1β, and cultured for 24 h.
siRNA-mediated RNAi
For siRNA-mediated mRNA knockdown, Silencer Select siRNA oligonucleotides against human CNOT1 (4392420, s22842; ThermoFisher), human PRB (4392422, s10416; ThermoFisher), and negative control oligonucleotides (4390846; ThermoFisher) were transfected using the Lipofectamine RNAiMAX transfection reagent (13778030; ThermoFisher) as per the manufacturer’s recommendations. Briefly, hTERT-HM cells stably expressing WT PR-B were cultured for 24 h in phenol red–free DMEM-F12 medium with 1% charcoal-stripped FBS before transfection with 40 nM of siRNA for CNOT1 or PRB for 48 h. Cells were then treated with vehicle (V), P4, (100 nM) or P4 + IL-1β (10 ng/ml) for 16 h and harvested for subsequent analysis.
RT–qPCR assay
Total RNA was extracted from cell lines and mouse myometrium using an RNeasy Mini kit (Qiagen) as per the manufacturer’s recommendations. Approximately 0.5 μg of RNA was treated with an RNase-free DNAse I (18068-015; ThermoFisher) to digest genomic DNA and reverse transcribed using an iScript RT SuperMix cDNA Synthesis kit (1708841; Bio-Rad). For gene expression analysis, iTaq SYBR Green Supermix (Bio-Rad) and TaqMan PCR master mix (Applied Biosystems) were used in a CFX384TM Real-Time PCR Detection System (Bio-Rad). Relative gene expression was calculated using the comparative cycle threshold (ΔΔCt) method. Acidic ribosomal phosphoprotein P0 (36B4) was used as internal control. Custom-designed primers and TaqMan primers and probes were obtained from Sigma and Applied Biosystems, respectively (Table S1).
Chromatin immunoprecipitation
ChIP assays were performed in the hTERT-HM-PR-BWT cell line treated with or without P4 (100 nM) for 16 h and in pregnant mouse myometrium isolated at 15.5 to 18.5 dpc and during labor. In brief, cells or minced tissues were cross-linked with 1% formaldehyde solution (ThermoFisher) at room temperature for 10 min. Cross-linking was terminated by incubation in a final 0.125 M concentration of glycine. The tissues were homogenized in PBS containing 1× protease inhibitor cocktail (Sigma) with a Dounce homogenizer. The tissue was suspended in Farnham lysis buffer (5 mM Pipes, 85 mM KCl, and 0.5% NP-40), 1× protease inhibitor cocktail (Sigma), and 1 mM PMSF (Sigma) for 10 min to isolate nuclei. The nuclei were incubated with ChIP lysis buffer (50 mM Tris [pH 8.1], 10 mM EDTA, 1% SDS, 1× protease inhibitor cocktail, and 1 mM PMSF) for 10 min and then sonicated to shear the chromatin into 200 to 500 bp fragments. ChIP grade antibodies (Table S2) were used to immunoprecipitate the specific protein–DNA complexes. The immunoprecipitated complexes were washed sequentially with low salt wash buffer (20 mM Tris, pH 8, 0.1% SDS, 1% Triton X-100, 150 mM NaCl, and 2 mM EDTA), high salt wash buffer (20 mM Tris, pH 8, 0.1% SDS, 1% Triton X-100, 500 mM NaCl, and 2 mM EDTA), and LiCl wash buffer (Tris, pH 8, 500 mM LiCl, 1% NP-40, 1% sodium deoxycholate, and 1 mM EDTA), eluted using ChIP elution buffer (1% SDS and 100 mM NaHCO3), and heated at 65 °C overnight to reverse cross-linking. Following treatment with 10 μg RNase A and 20 μg proteinase K, DNA was extracted using a PCR purification kit (28106; Qiagen) and was amplified by qPCR using primers that flanked the NF-κB response elements in the OXTR and CX43 promoters (Table S1).
Sequential ChIP (ChIP–re-ChIP)
ChIP–reChIP assays were performed as previously described (58). Mouse myometrial tissues (100 mg) at 16.5 dpc were processed, as aforementioned, to generate sheared chromatin. The first ChIP assay was performed with PR A/B antibody (Cell Signal Technology). Chromatin immunocomplexes were eluted by incubating in 10 mM DTT/50 μl TE at 37 °C for 30 min. The eluate was diluted 20× with ChIP dilution buffer (16.7 mM Tris–HCl, pH 8.0, 167 mM NaCl, 1.1% Triton, 0.1% SDS, and 1.2 mM EDTA) and subjected to a second round of immunoprecipitation using antibodies against CNOT1 (Proteintech) or EZH2 (Cell Signal Technology) or with immunoglobulin G (Cell Signal Technology) as a negative control. The immunocomplexes were eluted with 1% SDS and 100 mM NaHCO3. Extracted DNA was analyzed to determine interactions of PR with corepressors at the Cx43 and Oxtr promoters by qPCR.
Immunoblotting
Flash-frozen myometrial tissues were homogenized with a Dounce homogenizer in Farnham lysis buffer without NP-40, protease inhibitor cocktail (Sigma), and then incubated with 0.5% NP-40. Isolated nuclei were lysed in 1× radioimmunoprecipitation assay buffer (Cell Signaling Technology) containing 1× protease inhibitor cocktail (Sigma), 1× phosphatase inhibitor (Sigma), and 1 mM PMSF. Protein concentrations were quantified using the Bradford assay (BCA Protein Assay Kit; Pierce). Equivalent amounts of myometrial protein were resolved by SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. Membranes were blocked with 5% nonfat dry milk in 1× PBS containing 0.1% Tween-20 at 4 °C overnight, and incubated with primary antibodies at room temperature. Membranes were incubated for 1 h at room temperature in a blocking buffer containing horseradish peroxidase–conjugated secondary antibody (GE Healthcare). SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Scientific) was used to detect protein signals according to the manufacturer’s protocol.
Statistical analysis
Data are mean with SEM (for error bars) of values from triplicates samples of a representative experiment repeated two to three times with comparable results. t Test, one-way ANOVA, and two-way ANOVA were performed for statistical significance. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Data availability
All data described in this study are contained within the main article and supporting information.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank lab manager Ms Jo Smith for the assistance and other lab members for helpful discussions. This work was supported by the National Institutes of Health grant P01 HD087150 (to C. R. M.), Burroughs Wellcome Preterm Birth grant (grant no.: 1019823; to C. R. M. and M. M.), National Institutes of Health grant R01 DK058110 (to W. L. K.), and funds from the Cecil H. and Ida Green Center for Reproductive Biology Sciences Endowment (to W. L. K.).
Author contributions
C. R. M. and Y.-T. K. conceptualization; Y.-T. K. methodology; M. M., C. R. M., and Y.-T. K. validation; M. M., C. R. M., and Y.-T. K. formal analysis; Y.-T. K. investigation; W. L. K., A. P. M., and A. M. K. resources; Y.-T. K. data curation; C. R. M. and Y.-T. K. writing–original draft; M. M., W. L. K., Y.-T. K., and M. C.-C. writing–review & editing; Y.-T. K. visualization; M. M. and C. R. M. supervision; M. M. and C. R. M. project administration; C. R. M. funding acquisition.
Reviewed by members of the JBC Editorial Board. Edited by Brian D. Strahl
Supporting information
References
- 1.Wu S.P., Wang T., Yao Z.C., Peavey M.C., Li X., Zhou L., et al. Myometrial progesterone receptor determines a transcription program for uterine remodeling and contractions during pregnancy. PNAS Nexus. 2022;1 doi: 10.1093/pnasnexus/pgac155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendelson C.R., Gao L., Montalbano A.P. Multifactorial regulation of myometrial contractility during pregnancy and parturition. Front. Endocrinol. (Lausanne) 2019;10:714. doi: 10.3389/fendo.2019.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renthal N.E., Williams K.C., Montalbano A.P., Chen C.C., Gao L., Mendelson C.R. Molecular regulation of parturition: a myometrial perspective. Cold Spring Harb. Perspect. Med. 2015;5 doi: 10.1101/cshperspect.a023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deroo B.J., Archer T.K. Differential activation of the IkappaBalpha and mouse mammary tumor virus promoters by progesterone and glucocorticoid receptors. J. Steroid Biochem. Mol. Biol. 2002;81:309–317. doi: 10.1016/s0960-0760(02)00072-9. [DOI] [PubMed] [Google Scholar]
- 5.Hardy D.B., Janowski B.A., Corey D.R., Mendelson C.R. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol. Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 6.Chen C.C., Hardy D.B., Mendelson C.R. Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1) J. Biol. Chem. 2011;286:43091–43102. doi: 10.1074/jbc.M111.295865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vicent G.P., Ballare C., Nacht A.S., Clausell J., Subtil-Rodriguez A., Quiles I., et al. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol. Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Renthal N.E., Chen C.C., Williams K.C., Gerard R.D., Prange-Kiel J., Mendelson C.R. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.C., Montalbano A.P., Hussain I., Lee W.R., Mendelson C.R. The transcriptional repressor GATAD2B mediates progesterone receptor suppression of myometrial contractile gene expression. J. Biol. Chem. 2017;292:12560–12576. doi: 10.1074/jbc.M117.791350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie N., Liu L., Li Y., Yu C., Lam S., Shynlova O., et al. Expression and function of myometrial PSF suggest a role in progesterone withdrawal and the initiation of labor. Mol. Endocrinol. 2012;26:1370–1379. doi: 10.1210/me.2012-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalabi Hagkarim N., Grand R.J. The regulatory properties of the Ccr4-not complex. Cells. 2020;9:2379. doi: 10.3390/cells9112379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collart M.A. The Ccr4-Not complex is a key regulator of eukaryotic gene expression. Wiley Interdiscip. Rev. RNA. 2016;7:438–454. doi: 10.1002/wrna.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirai Y.T., Suzuki T., Morita M., Takahashi A., Yamamoto T. Multifunctional roles of the mammalian CCR4-NOT complex in physiological phenomena. Front. Genet. 2014;5:286. doi: 10.3389/fgene.2014.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberholzer U., Collart M.A. In vitro transcription of a TATA-less promoter: negative regulation by the Not1 protein. Biol. Chem. 1999;380:1365–1370. doi: 10.1515/BC.1999.176. [DOI] [PubMed] [Google Scholar]
- 15.Winkler G.S., Mulder K.W., Bardwell V.J., Kalkhoven E., Timmers H.T. Human Ccr4-Not complex is a ligand-dependent repressor of nuclear receptor-mediated transcription. EMBO J. 2006;25:3089–3099. doi: 10.1038/sj.emboj.7601194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliviero G., Brien G.L., Waston A., Streubel G., Jerman E., Andrews D., et al. Dynamic protein interactions of the polycomb repressive complex 2 during differentiation of pluripotent cells. Mol. Cell Proteomics. 2016;15:3450–3460. doi: 10.1074/mcp.M116.062240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirmizis A., Bartley S.M., Kuzmichev A., Margueron R., Reinberg D., Green R., et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–1605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinnadurai G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell. 2002;9:213–224. doi: 10.1016/s1097-2765(02)00443-4. [DOI] [PubMed] [Google Scholar]
- 19.Gong Z., Brackertz M., Renkawitz R. SUMO modification enhances p66-mediated transcriptional repression of the Mi-2/NuRD complex. Mol. Cell Biol. 2006;26:4519–4528. doi: 10.1128/MCB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T., Cooper S., Brockdorff N. The interplay of histone modifications - writers that read. EMBO Rep. 2015;16:1467–1481. doi: 10.15252/embr.201540945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Li B., Carey M., Workman J.L. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Williams K.C., Renthal N.E., Condon J.C., Gerard R.D., Mendelson C.R. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc. Natl. Acad. Sci. U. S. A. 2012;109:7529–7534. doi: 10.1073/pnas.1200650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merlino A.A., Welsh T.N., Tan H., Yi L.J., Cannon V., Mercer B.M., et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J. Clin. Endocrinol. Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 25.Nadeem L., Shynlova O., Matysiak-Zablocki E., Mesiano S., Dong X., Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016;7 doi: 10.1038/ncomms11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv S., Wang N., Lv H., Yang J., Liu J., Li W.P., et al. The attenuation of trophoblast invasion caused by the downregulation of EZH2 is involved in the pathogenesis of human recurrent miscarriage. Mol. Ther. Nucleic Acids. 2019;14:377–387. doi: 10.1016/j.omtn.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal B., Bouras T., Shi W., Vaillant F., Sheridan J.M., Fu N., et al. Global changes in the mammary epigenome are induced by hormonal cues and coordinated by Ezh2. Cell Rep. 2013;3:411–426. doi: 10.1016/j.celrep.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Amini P., Wilson R., Wang J., Tan H., Yi L., Koeblitz W.K., et al. Progesterone and cAMP synergize to inhibit responsiveness of myometrial cells to pro-inflammatory/pro-labor stimuli. Mol. Cell Endocrinol. 2019;479:1–11. doi: 10.1016/j.mce.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Amini P., Michniuk D., Kuo K., Yi L., Skomorovska-Prokvolit Y., Peters G.A., et al. Human parturition involves phosphorylation of progesterone receptor-A at serine-345 in myometrial cells. Endocrinology. 2016;157:4434–4445. doi: 10.1210/en.2016-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peavey M.C., Wu S.P., Li R., Liu J., Emery O.M., Wang T., et al. Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2011643118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condon J.C., Hardy D.B., Kovaric K., Mendelson C.R. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 32.Collart M.A., Panasenko O.O. The Ccr4--not complex. Gene. 2012;492:42–53. doi: 10.1016/j.gene.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Yi H., Park J., Ha M., Lim J., Chang H., Kim V.N. PABP cooperates with the CCR4-NOT complex to promote mRNA deadenylation and block precocious decay. Mol. Cell. 2018;70:1081–1088.e5. doi: 10.1016/j.molcel.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Webster M.W., Chen Y.H., Stowell J.A.W., Alhusaini N., Sweet T., Graveley B.R., et al. mRNA deadenylation is coupled to translation rates by the differential activities of Ccr4-not nucleases. Mol. Cell. 2018;70:1089–1100.e8. doi: 10.1016/j.molcel.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwartjes C.G., Jayne S., van den Berg D.L., Timmers H.T. Repression of promoter activity by CNOT2, a subunit of the transcription regulatory Ccr4-not complex. J. Biol. Chem. 2004;279:10848–10854. doi: 10.1074/jbc.M311747200. [DOI] [PubMed] [Google Scholar]
- 36.Hyun K., Jeon J., Park K., Kim J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017;49:e324. doi: 10.1038/emm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An R., Li Y.Q., Lin Y.L., Xu F., Li M.M., Liu Z. EZH1/2 as targets for cancer therapy. Cancer Gene Ther. 2023;30:221–235. doi: 10.1038/s41417-022-00555-1. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.H., Li Y., Kim H., Eum S., Park K., Lee C.H. The role of EZH1 and EZH2 in development and cancer. BMB Rep. 2022;55:595–601. doi: 10.5483/BMBRep.2022.55.12.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nancy P., Siewiera J., Rizzuto G., Tagliani E., Osokine I., Manandhar P., et al. H3K27me3 dynamics dictate evolving uterine states in pregnancy and parturition. J. Clin. Invest. 2018;128:233–247. doi: 10.1172/JCI95937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shchuka V.M., Abatti L.E., Hou H., Khader N., Dorogin A., Wilson M.D., et al. The pregnant myometrium is epigenetically activated at contractility-driving gene loci prior to the onset of labor in mice. Plos Biol. 2020;18 doi: 10.1371/journal.pbio.3000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postigo A.A., Dean D.C. ZEB represses transcription through interaction with the corepressor CtBP. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6683–6688. doi: 10.1073/pnas.96.12.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siles L., Sanchez-Tillo E., Lim J.W., Darling D.S., Kroll K.L., Postigo A. ZEB1 imposes a temporary stage-dependent inhibition of muscle gene expression and differentiation via CtBP-mediated transcriptional repression. Mol. Cell Biol. 2013;33:1368–1382. doi: 10.1128/MCB.01259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Lee S., Teh C.E., Bunting K., Ma L., Shannon M.F. The transcription repressor, ZEB1, cooperates with CtBP2 and HDAC1 to suppress IL-2 gene activation in T cells. Int. Immunol. 2009;21:227–235. doi: 10.1093/intimm/dxn143. [DOI] [PubMed] [Google Scholar]
- 44.Sewalt R.G., Gunster M.J., van der Vlag J., Satijn D.P., Otte A.P. C-Terminal binding protein is a transcriptional repressor that interacts with a specific class of vertebrate Polycomb proteins. Mol. Cell Biol. 1999;19:777–787. doi: 10.1128/mcb.19.1.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong X., Yu C., Shynlova O., Challis J.R., Rennie P.S., Lye S.J. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1) Mol. Endocrinol. 2009;23:1147–1160. doi: 10.1210/me.2008-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeisig B.B., So C.W.E. Combinatorial tethering: a novel mode to recruit non-canonical PRC1 for normal and malignant GC B cell development. Cancer Cell. 2016;30:185–187. doi: 10.1016/j.ccell.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Postigo A.A., Depp J.L., Taylor J.J., Kroll K.L. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao L.J., Kuppuswamy M., Vijayalingam S., Chinnadurai G. Interaction of ZEB and histone deacetylase with the PLDLS-binding cleft region of monomeric C-terminal binding protein 2. BMC Mol. Biol. 2009;10:89. doi: 10.1186/1471-2199-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleischer T.C., Yun U.J., Ayer D.E. Identification and characterization of three new components of the mSin3A corepressor complex. Mol. Cell Biol. 2003;23:3456–3467. doi: 10.1128/MCB.23.10.3456-3467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Condon J.C., Jeyasuria P., Faust J.M., Mendelson C.R. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montalbano A.P., Hawgood S., Mendelson C.R. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154:483–498. doi: 10.1210/en.2012-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao L., Rabbitt E.H., Condon J.C., Renthal N.E., Johnston J.M., Mitsche M.A., et al. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J. Clin. Invest. 2015;125:2808–2824. doi: 10.1172/JCI78544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell J.A., Shynlova O., Langille B.L., Lye S.J. Mechanical stretch and progesterone differentially regulate activator protein-1 transcription factors in primary rat myometrial smooth muscle cells. Am. J. Physiol. Endocrinol. Metab. 2004;287:E439–E445. doi: 10.1152/ajpendo.00275.2003. [DOI] [PubMed] [Google Scholar]
- 54.Mesiano S., Chan E.C., Fitter J.T., Kwek K., Yeo G., Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J. Clin. Endocrinol. Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- 55.Gao L., Wang G., Liu W.N., Kinser H., Franco H.L., Mendelson C.R. Reciprocal Feedback between miR-181a and E(2)/ERalpha in myometrium enhances inflammation leading to labor. J. Clin. Endocrinol. Metab. 2016;101:3646–3656. doi: 10.1210/jc.2016-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishida M., Choi J.H., Hirabayashi K., Matsuwaki T., Suzuki M., Yamanouchi K., et al. Reproductive phenotypes in mice with targeted disruption of the 20alpha-hydroxysteroid dehydrogenase gene. J. Reprod. Dev. 2007;53:499–508. doi: 10.1262/jrd.18125. [DOI] [PubMed] [Google Scholar]
- 57.Piekorz R.P., Gingras S., Hoffmeyer A., Ihle J.N., Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20alpha-hydroxysteroid dehydrogenase. Mol. Endocrinol. 2005;19:431–440. doi: 10.1210/me.2004-0302. [DOI] [PubMed] [Google Scholar]
- 58.Furlan-Magaril M., Recillas-Targa F. Individual and sequential chromatin immunoprecipitation protocols. Methods Mol. Biol. 2015;1334:205–218. doi: 10.1007/978-1-4939-2877-4_13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data described in this study are contained within the main article and supporting information.