Summary
Bombinin-BO1 (BO1), a bombinin peptide derived from the skin secretion of Bombina orientalis, exhibits broad-spectrum antimicrobial activity. To date, the anticancer effect of BO1 remains unclear. This study confirmed cytotoxicity of BO1 on hepatocellular carcinoma cells by inducing S-phase cycle block and apoptosis. In addition, BO1 was found to be localized in cytoplasm through endocytosis. The combined results of pull down, mass spectrometry, and co-immunoprecipitation suggested that BO1 induced misfolding of CDK1 and degradation by competitively binding HSP90A with Cdc37. It was verified that overexpression of HSP90A in BO1-treated cells significantly inhibited degradation of CDK1. In vivo, BO1 inhibited tumor without being toxic to individuals. This study reveals the anti-tumor mechanism of BO1 in inducing cell-cycle arrest and apoptosis by interfering with HSP90A-Cdc37-CDK1 system. This is the first study to analyze the mechanism of BO1 regulation of tumor cells, providing theoretical basis for BO1 treatment of hepatocellular carcinoma.
Subject areas: Biological sciences, System biology, Cancer
Graphical abstract

Highlights
-
•
BO1 is cytotoxic to liver cancer cells by inducing cycle block and apoptosis
-
•
BO1 localizes in the cytoplasm by endocytosis and targets binding to HSP90A
-
•
BO1 induces CDK1 degradation by disrupting the HSP90A-Cdc37-CDK1 system
-
•
BO1 inhibits tumor growth in vivo without causing toxicity in individuals
Biological sciences; Systems biology; Cancer
Introduction
Hepatocellular carcinoma is one of the prevalent malignant tumors, ranking as the third leading cause of cancer-related mortality. In 2022, it constituted 4.7% of all malignancies and 8.2% of deaths attributed to the disease.1,2,3 The prognosis for hepatocellular carcinoma remains dismal, with merely one-fifth of early stage patients being eligible for surgical intervention due to elevated postoperative complication risks.4 therefore, it poses a substantial menace to human life and well-being. Numerous studies have been conducted on the occurrence and treatment of hepatocellular carcinoma over the past few decades,5,6 but a complete cure for hepatocellular carcinoma is still unknown, so effective treatments and drugs are urgently needed.
Bioactive peptides, characterized by their oligopeptide structure comprising fewer than 20 amino acids, deliver nutritional value, and cf. significant health benefits.7 These bioactive peptides have displayed considerable efficacy across various functions encompassing antitumor, antibacterial, immunomodulatory, antiviral, antiparasitic, and wound healing activities.8,9,10 For example, dermaseptin-PD-1 and dermaseptin-PD-2 were identified in the skin secretion of Pachymedusa dacnicolor, which exhibited significant inhibition against the growth of standard model microorganisms and human neuronal glioblastoma cells with low hemolytic activity.11 Over the past decades, bioactive peptides have attracted significant interest for their selective cytotoxicity toward cancer cells.12 Additionally, these active peptides exhibit high tissue permeability, endowing them with a distinct advantage in the realm of tumor therapy.12 Depending on their mode of cellular entry, active peptides are broadly classified into three primary categories, comprising (i) pore-forming peptides that induce apoptosis or necrosis by binding to negatively charged molecules on the cell membrane; (ii) cell-penetrating peptides, which facilitate the translocation of small molecules across the cell membrane; and (iii) tumor-targeting peptides, capable of internalization by binding to surface receptors on cancer cells.13 Furthermore, the targets of bioactive peptides primarily span signal transduction pathways, cell cycle regulation, and cell death pathways, reflecting their diverse biological functions.14,15,16
Because of the adaptive evolution of toxic species for millions of years, the venom sac is a “treasure bank”, which has millions of biomolecules with high affinity and stability awaiting further development.17 The toad family, with its global distribution, represents a significant reservoir of natural remedies. Several toad-derived products, including venom, skin, and Chanyi (in Chinese), possess substantial medicinal value.18 Bombinins, a class of peptides derived from amphibians, are exclusive to ancient toad species like Bombina and exhibit a wide-ranging antimicrobial activity.19 Recently, bombinins have gained attention for their potential in cancer therapy. Specific bombesins have demonstrated the ability to selectively target and eliminate cancer cells while sparing normal cells. For instance, Bombinin H2 and temporin A exhibit selective cytotoxicity against non-small cell lung carcinoma cells and display minimal hemolytic activity comparable to untreated cells.20 BLP-7 and bombinin H-BO, identified in the skin secretions of Bombina orientalis, exhibit significant antiproliferative effects against human hepatocellular carcinoma cells (HCC) at non-toxic concentrations in vitro.19
Bombinin-BO1 (BO1), a peptide closely related to bombinins with the sequence GIGSAILSAGKSIIKGLAKG LAEHF-NH2, has been isolated from the skin secretions of Bombina orientalis. BO1 has been shown to adopt an α-helical structural configuration and possesses the capacity to inhibit HCC activity.21 This suggests its potential as an agent against hepatocellular carcinoma; however, the impact and mechanism of BO1 on hepatocellular carcinoma have not been investigated in depth. This study endeavors to elucidate the mechanism by which BO1 acts against hepatocellular carcinoma through pull-down assays and LC-MS/MS mass spectrometry to establish a theoretical foundation for hepatocellular carcinoma therapy.
Results
Effect of BO1 on the proliferative activity of HCC
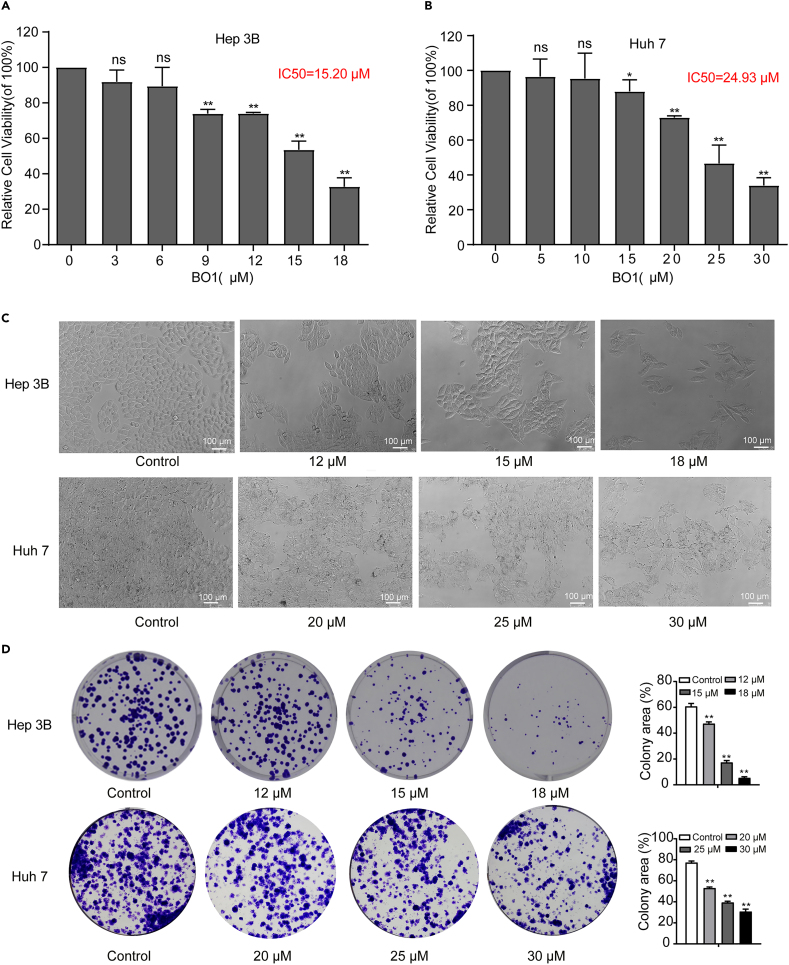
In this study, we analyzed the proliferative activity of BO1-treated HCC Hep 3B, Huh7, and Hep G2 as well as human normal hepatocyte L-O2. The results showed that the proliferative activity of HCC cells treated with 10 μM and 20 μM BO1 was significantly reduced, but there was no significant change in the activity of normal hepatocytes (Figure S1). And then, Hep 3B and Huh7 cells were selected to further analyze the regulatory mechanism of BO1. BO1 significantly inhibited the proliferation of Hep 3B cells (IC50: 15.20 μM) and Huh7 cells (IC50: 24.93 μM) in a concentration-dependent manner (Figures 1A and 1B). Additionally, BO1-treated cells exhibited a wrinkled appearance, and the number of colonies decreased in a concentration-dependent manner (Figures 1C and 1D).
Figure 1.
Effect of BO1 on Hep 3B and Huh7 cells activity
(A) The CCK-8 assay was performed to analyze the proliferative activity of BO1-treated Hep 3B cells after 48 h.
(B) The proliferative activity of BO1-treated Huh7 cells.
(C) Morphology of Hep 3B and Huh7 cells was observed using a phase-contrast microscope, scale bar: 100 μm.
(D) Cell cloning ability of Hep 3B and Huh7 were tested by colony formation assay. Data were analyzed with unpaired two-sided Student’s t tests and presented as mean ± SEM, n = 3. ∗p < 0.05, ∗∗p < 0.01.
BO1 enters cells by endocytosis
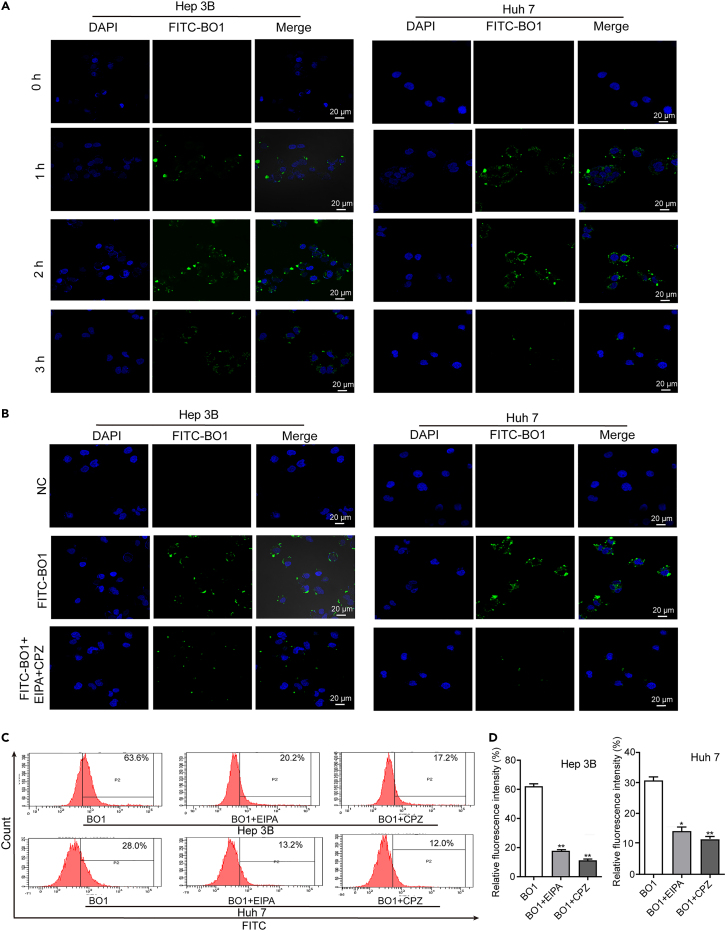
FITC-BO1 was synthesized to investigate BO1’s intracellular localization. Laser confocal microscopy revealed that green fluorescence predominantly localized in the cytoplasm, indicating that BO1 is predominantly expressed in the cytoplasm. Furthermore, the green fluorescence intensity peaked at 2 h after co-incubation with FITC-BO1 and declined over time (Figure 2A). Therefore, cells treated with FITC-BO1 and endocytosis inhibitors for 2 h were chosen for fluorescence intensity analysis. The fluorescence of cells treated with FITC-BO1, ethyliso propylamiloride (EIPA, a micropinocytosis inhibitor), and chlorpromazine hydrochloride (CPZ, a Na+/H+ ion channel-mediated endocytosis inhibitor) was significantly reduced (Figure 2B, suggesting that FITC-BO1 enters cells via endocytosis. This finding was further supported by flow cytometry analysis of cells treated with FITC-BO1 and EIPA/CPZ, which showed that both EIPA and CPZ significantly reduced fluorescence intensity in the cells (Figures 2C and 2D).
Figure 2.
BO1 enters Hep 3B and Huh7 cells via endocytosis
(A) Fluorescence intensity in FITC-BO1 treated Hep 3B and Huh7 cells at different times, scale bar: 20 μm.
(B) Confocal microscopic observation of fluorescence intensity. Hep 3B and Huh7 cells were co-administered with FITC-BO1, EIPA, and CPZ for 2 h, and then the fluorescence in the cells was detected by confocal microscopy, scale bar: 20 μm.
(C and D) Flow cytometry analysis of fluorescence intensity. Hep 3B and Huh7 cells were co-administered with FITC-BO1 and EIPA/CPZ for 2 h, and then the fluorescence in the cells was detected by flow cytometry. Data were analyzed with unpaired two-sided Student’s t tests and presented as mean ± SEM, n = 3. ∗p < 0.05, ∗∗p < 0.01.
HSP90A serves as the target of BO1
To explore the regulatory mechanism of BO1, we synthesized bio-BO1 and employed it to pull down proteins that may interact with BO1 (Figure 3A). LC-MS/MS mass spectrometry analysis identified 662 unique proteins in the bio-BO1 sample (Table S1). Based on protein false discovery rate (FDR) confidence (FDR<0.01) and score SEQUEST HT (> 80), HSP90A emerged as a potential target of BO1 (Figure 3B). To verify the targeted binding of BO1 and HSP90A, western blot was performed for the abundance of HSP90A in samples pulled by bio-BO1. The results showed that HSP90A was significantly detected in the samples extracted with bio-BO1 compared to those extracted with IgG (Figure 3C), which demonstrated that HSP90A is a target of BO1. Furthermore, HSP90A and FITC-BO1 were observed to co-localize in the cytoplasm of Hep 3B and Huh7 cells (Figures 3D and 3E), further substantiating BO1’s capacity to target HSP90A.
Figure 3.
HSP90A serves as the target of BO1
(A) SDS-PAGE analysis of pull-down samples.
(B) Venn plot. Samples were analyzed by LC-MS/MS mass spectrometry and screened for proteins specific to the bio-BO1 pull-down samples in combination with FDR<0.01 and Score Sequest HT > 80.
(C) The abundance of HSP90A in samples was analyzed by western blot. Input: whole protein from cell lysis, NC: sample pulled by IgG, bio-BO1: sample pulled by bio-BO1.
(D and E) The localization of FITC-BO1 and HSP90A in Hep 3B and Huh7 cells was detected by confocal microscopy. green: FITC-BO1, red: HSP90A, yellow: co-localization of FITC-BO1 and HSP90A, scale bar: 20 μm.
BO1 induced CDK1 degradation by inhibiting HSP90A-Cdc37 system
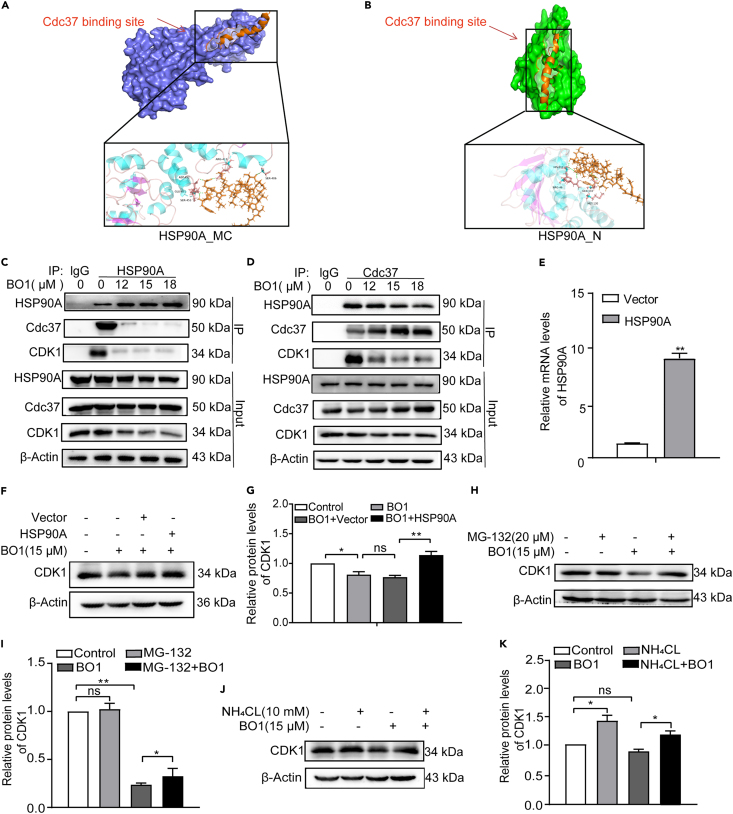
Next, we predicted the binding sites of BO1 on HSP90A through molecular docking. Utilizing Autodock Vina software, we built the BO1 structure using Discovery Studio 2019 software. While the structure of HSP90A had not been constructed, we used HSP90A_N and HSP90A_MC structures for docking analysis. We selected conformations with binding free energies of −15.36 kcal/mol and −8.80 kcal/mol for HSP90A_N and HSP90A_MC, respectively. These docking results indicated that BO1 binding sites on HSP90A_N and HSP90A_MC overlapped with the Cdc37 binding site, implying that BO1 might affect the binding of HSP90A to Cdc37 (Figures 4A and 4B). It is now understood that the HSP90A-Cdc37 system plays a pivotal role in the folding and maturation of CDKs. Previous studies have demonstrated that inhibiting the binding between HSP90A and Cdc37 leads to significant downregulation of CDK2, CDK4, and CDK6. This study extended this observation to CDK1, showing that BO1 could inhibit the interaction between HSP90A, Cdc37, and CDK1 (Figures 4C and 4D). This finding suggests that BO1 interferes with the HSP90A-Cdc37 system to inhibit CDK1. Moreover, overexpression of HSP90A effectively countered BO1-induced CDK1 reduction, further substantiating that BO1 impacts CDK1 by regulating the HSP90A-Cdc37 system (Figures 4E–4G). Notably, the degradation of CDK1 in BO1-treated cells was found to be mediated by the ubiquitin-proteasome system (UPS) and autophagic lysosomal pathway (ALP), as demonstrated by the inhibitory effects of MG-132 (the proteasome inhibitor) and NH4Cl (the autophagy-lysosome inhibitor) (Figures 4H–4K).
Figure 4.
BO1 induces the degradation of CDKs by regulating HSP90A-Cdc37
(A and B) Putative binding site analysis of BO1 on HSP90A_MC or HSP90A_N and the surrounding amino acid residues by molecular docking.
(C and D) Whole-cell lysates from BO1-treated cells were immunoprecipitated and analyzed for the interaction of HSP90A, Cdc37, and CDK1 by immunoblotting.
(E) The expression of HSP90A in cells was determined by RT-qPCR.
(F and G) Cells were pretreated with 20 μM MG-132 for 4 h before treatment with (+) or without (−) 15 μM BO1 for 24 h, and CDK1 was determined by immunoblotting.
(H and I) Overexpression of HSP90A in Hep 3B cells significantly repaired BO1-induced CDK1 reduction.
(J and K) Cells were treated with 10 mM NH4Cl for 4 h before treatment with (+) or without (−) 15 μM BO1 for 24 h, and then CDK1 was determined by immunoblotting. Data were analyzed with unpaired two-sided Student’s t tests or one-way ANOVA, and presented as mean ± SEM, n = 3. ∗p < 0.05, ∗∗p < 0.01.
BO1 induces S-phase arrest in Hep 3B and Huh7 cells
CDKs are integral to ensuring the orderly progression of the cell cycle. This study revealed that BO1-treated Hep 3B and Huh7 cells experienced S-phase arrest. The proportion of cells in the S-phase was significantly elevated in BO1-treated cells (Figures 5A–5D). Furthermore, there were differences in the expression of S-phase cell cycle pathway-related proteins, with a significant reduction in cyclin A2 expression observed in BO1-treated cells (Figures 5E–5H). In addition, BO1-treated L-O2 cell cycle progression was not significantly affected (Figure S2). These findings collectively indicate that BO1 induces an S-phase arrest in Hep 3B and Huh7 cells.
Figure 5.
BO1 induces cell-cycle arrest in Hep 3B and Huh7 cells
(A and B) The cycle distribution of Hep 3B cells treated with BO1 was analyzed by flow cytometry.
(C and D)The cycle distribution of Huh7 cells treated with BO1 was analyzed by flow cytometry.
(E and F) Expression of cell cycle-related proteins in Hep 3B cells treated with different concentrations of BO1 (12, 15, and 18 μM).
(G and H) Expression of cell cycle-related proteins in Huh7 cells treated with different concentrations of BO1 (20, 25, and 30 μM). GAPDH was regarded as the internal reference. Data were analyzed with unpaired two-sided Student’s t tests or one-way ANOVA, and presented as mean ± SEM, n = 3. ∗p < 0.05, ∗∗p < 0.01.
BO1 induces apoptosis in hepatoma Hep 3B and Huh7 cells
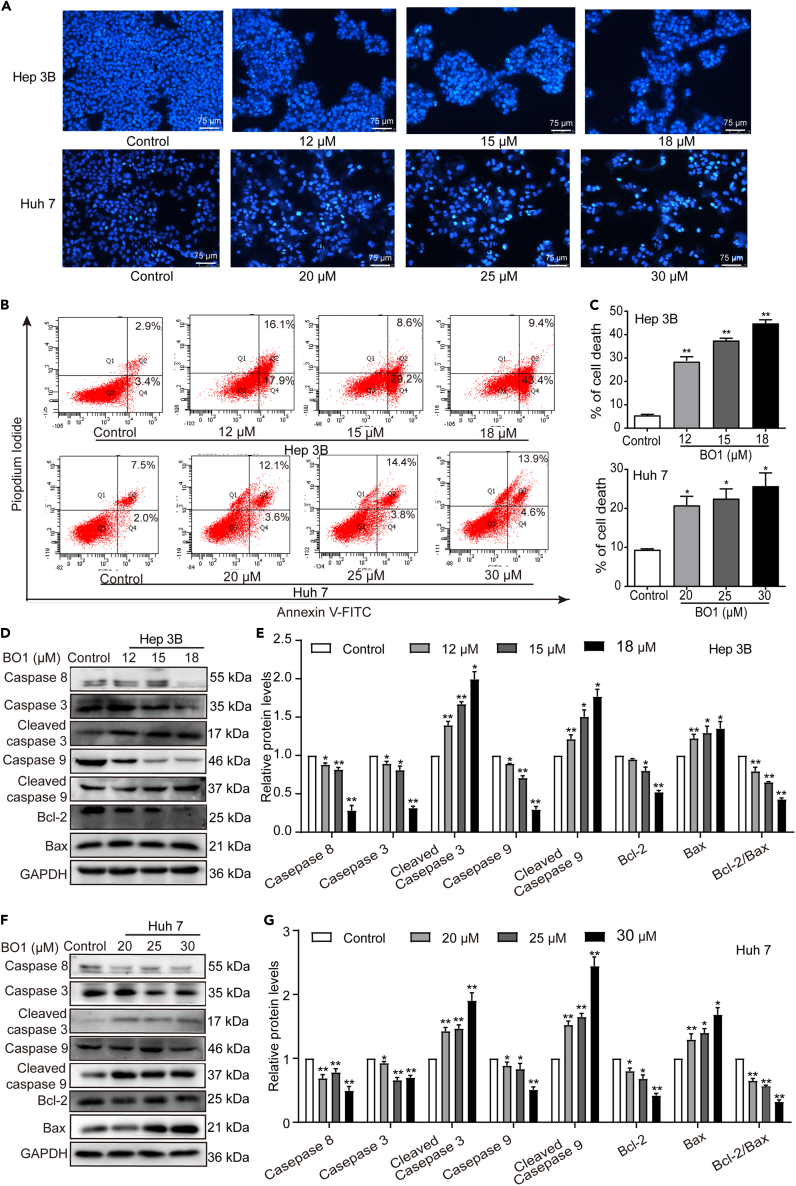
Apoptosis induction represents another pivotal pathway to inhibit tumorigenesis. This study demonstrated that BO1-treated Hep 3B and Huh7 cells displayed increased nuclear fluorescence staining, chromatin condensation, nuclear membrane cleavage, and cytoplasmic atrophy (Figure 6A), whereas no significant changes in nuclear fluorescence staining were found in BO1-treated L-O2 cells (Figure S3). These alterations indicate the pro-apoptotic effect of BO1 on Hep 3B and Huh7 cells. Additionally, the apoptosis rate of BO1-treated cells significantly increased (Figures 6B and 6C). This apoptotic effect was further corroborated by changes in apoptosis-related proteins in BO1-treated Hep 3B and Huh7 cells, including increased cleaved caspase-9 and cleaved caspase-3 levels and decreased Bcl-2/Bax ratio (Figures 6D–6G).
Figure 6.
BO1 induces apoptosis in Hep 3B and Huh7 cells
(A) Hoechst 33258 staining of BO1-treated Hep 3B and Huh7 cells, scale bar: 75 μm.
(B and C) Flow cytometry was applied to assay the apoptosis of BO1-treated Hep 3B and Huh7 cells according to Annexin V-FITC/PI kit.
(D and E) Expression of apoptosis-related proteins in Hep 3B cells treated with different concentrations of BO1 (12, 15, and 18 μM).
(F and G) Expression of apoptosis-related proteins in Huh7 cells treated with different concentrations of BO1 (20, 25, and 30 μM). GAPDH was regarded as the internal reference. Data were analyzed with unpaired two-sided Student’s t tests and presented as mean ± SEM, n = 3. ∗p < 0.05, ∗∗p < 0.01.
BO1 inhibits tumorigenesis in vivo
To investigate the tumor-suppressing potential of BO1 in vivo, we employed Hep 3B cells for subcutaneous tumorigenesis in nude mice. As expected, BO1-treated mice exhibited substantial reductions in tumor volume and weight (Figures 7A–7C). Immunohistochemical analysis of tumor tissues revealed lower Ki-67 expression in BO1-treated mice, indicative of BO1’s inhibitory effect on proliferation in vivo (Figure 7D). Furthermore, the expression of CDK1, cell cycle-related proteins, and apoptosis-related proteins differed significantly in BO1-treated mice (Figures 7E–7H), consistent with in vitro results. Importantly, the body weights of BO1-treated individuals did not significantly differ from those of control mice (Figure 7I). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine (CREA) activities of BO1-treated mice were also comparable to those of control mice (Figures 7J and 7K). Moreover, histopathological examination of major organs (heart, liver, spleen, and kidney) revealed normal findings in BO1-treated mice (Figures 7L–7O). These results collectively demonstrate that BO1 is non-toxic at the administered concentrations in vivo.
Figure 7.
BO1 inhibits tumorigenesis in vivo
(A) Representative images of tumor morphology.
(B) The subcutaneous tumor volume was measured in mice after intraperitoneal injection of BO1.
(C) The mass of the tumor was measured after killing the mouse and removing the tumor.
(D) Immunohistochemical analysis of Ki-67 expression in tumor cells of mice after completion of treatment.
(E and F) Expression of cell cycle-related proteins in tumor cells of mice after completion of treatment.
(G and H) Expression of apoptosis-related proteins in tumor cells of individuals after completion of treatment.
(I) Individual body weights were measured during intraperitoneal injection of BO1.
(J) ALT and AST activity in serum of mice after completion of treatment.
(K) Activity of CREA in serum of mice after completion of treatment.
(L–O) H&E staining was used to analyze the histopathological characteristics of the heart (L), spleen (M), liver (N), and kidney (O). Data were analyzed with unpaired two-sided Student’s t tests or one-way ANOVA, and presented as mean ± SEM, n = 6. ∗p < 0.05, ∗∗p < 0.01.
Discussion
Bombinins are a wide family of antimicrobial peptides from Xenopus skin. The antitumor studies of bombinins have revealed that BO1, bombinin H-BO1, BLP-7, and bombinin H-BO have the ability to inhibit the proliferation of HCC,19,20 and bombinin H2 has selective cytotoxicity against non-small-cell lung carcinoma through the membrane composition of cancer cells.21 However, the mechanisms by which these bombinins inhibit cancer cells have not been reported. In this study, we uncovered that BO1 induces CDK1 degradation by disrupting the HSP90A-Cdc37-CDK1 system via targeted binding to HSP90A, which leads to S-phase arrest and apoptosis and hinders the proliferation of Hep 3B and Huh7 cells (Figure 8). Given the challenging prognosis associated with hepatocellular carcinoma marked by high Hsp90 expression,22 this study provides the foothold for BO1’s development as a potential drug targeting HSP90A for hepatocellular carcinoma.
Figure 8.
Schematic diagram of the mechanism by which BO1 regulates HCC
BO1 enters the cell through endocytosis and competes with Cdc37 for binding to HSP90A in the cytoplasm, thereby inhibiting the formation of the HSP90A-Cdc37-CDK1 complex and inducing the degradation of CDK1 in both the UPS and ALP modes. Decreased levels of CDK1 induced the occurrence of S-phase cycle block and apoptosis in HCC, which, in turn, inhibited cell proliferative activity. preCDK1, immature CDK1 precursor.
It is widely thought that tumorigenesis is intricately linked to the activation and overexpression of tumor-associated proteins. The folding and stabilization of these proteins are heavily reliant on Hsp90’s participation.23,24,25,26 Elevated Hsp90 expression in various tumors contributes to establishing an immunosuppressive tumor microenvironment.27,28 Thus, targeting and inhibiting Hsp90’s expression and activity have emerged as crucial strategies in tumor diagnosis and treatment.29 Cdc37, a co-chaperones of Hsp90, is assumed to be involved in the regulation of CDKs. Cdc37 is to selectively recognize and combine unfolded CDKs, load them onto Hsp90, and form an Hsp90-Cdc37-CDKs complex for further maturation.30,31 CDKs overexpression was an important coordinator of HCC pathogenesis. Among them, CDK1 is involved in regulating the S-G2 and G2-M transitions and apoptosis in HCC.32 In this study, it was demonstrated that BO1 is bound to HSP90A, and this binding inhibits the interaction between HSP90A and Cdc37, which in turn inhibits the maturation of CDK1 and induces HCC cell-cycle arrest and apoptosis. As we know, the normal cell cycle is necessary for cell proliferation. And, induction of proliferation inhibition and apoptosis is considered as the potential pathways for tumorigenesis inhibition by antitumor drugs. Therefore, this study hypothesizes that BO1 reduces tumor volume by inhibiting the proliferation of HCC and inducing apoptosis of HCC. In recent years, several Hsp90 inhibitors have ventured into clinical trials; however, these inhibitors primarily affect Hsp90 by binding to its ATP pocket, inevitably impacting the folding of numerous Hsp90 target proteins and potentially leading to toxic side effects.33,34 In contrast, BO1, being a natural compound, binds to HSP90A more gently than synthetic compounds. Moreover, effective tumor suppressor concentration of BO1 was non-toxic in nude mice, suggesting that BO1 holds potential as a therapeutic HSP90A inhibitor with fewer associated toxicities.
The natural features of bioactive peptides lead to some challenges such as short half-life, faster elimination, toxicity, variable hemolytic character, instability in the in vivo condition, low efficacy and efficiency, sensitivity to enzymatic degradation, kidney damage, and high cost of synthesis.35,36,37,38,39,40 However, due to their designable features, they can be considered as therapeutic agents for humans and can be transformed into more effective and biocompatible drugs.41 The present study demonstrated the anti-hepatocellular carcinoma effect of BO1 only in terms of cellular and animal models. Future studies will further analyze its challenges, e.g., the stability and pharmacokinetics of BO1 in vivo, which may drawback its application in clinical practice. And, its limitations can be overcome via modifications in the structur of BO1 by using it along to the peptide with rigid structures like cyclization, incorporation of non-canonical amino acids (D isomers), C-terminus amidation, and acetylation, or even using the nanotechnology to produce the recombinant peptide without the limitation of the primary one.42,43,44,45
In summary, our results revealed that BO1 inhibits the proliferation of HCC by disrupting the HSP90A-CDc37-CDK1 system, which opens new avenues for understanding the anti-cancer regulatory mechanism of BO1 and highlights its potential application in hepatocellular carcinoma therapy. Based on the results of the current study, future research will focus on translational application of BO1 to clinical practice. We will analyze the stability and pharmacokinetics of BO1 in vivo. In addition, the in vivo delivery of BO1 will be optimized, and efforts will be made to discover a cell-targeted delivery system for BO1 in hepatocellular carcinoma in order to improve its effectiveness in clinical practice. In addition, it may be further explored whether the combination of BO1 with radiotherapy or chemotherapeutic agents can improve the effectiveness of radiotherapy or chemotherapy in clinical practice.
Limitations of the study
Admittedly, this study has some shortcomings. The subcutaneous Hep 3B tumor model does not well mimic the real hepatocellular carcinoma microenvironment, so further validation of in situ tumor models is needed. In addition, the stability and pharmacokinetics of BO1 in vivo are important for its development as a therapeutic agent and need to be analyzed. Moreover, this study only examined the effect of BO1 inhibition in a hepatocellular carcinoma model, and the effects of BO1 on other cancers need to be further investigated.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| GAPDH | proteintech | Cat#10494-1-AP; RRID:AB_2263076 |
| Beta Actin | proteintech | Cat#66009-1-Ig; RRID:AB_2687938 |
| Pro Caspase-8 | Abcam | Cat#ab108333; RRID:AB_10866391 |
| Caspase-9 | proteintech | Cat#10380-1-AP; RRID:AB_2068632 |
| Cleaved Caspase-9 | Abcam | Cat#ab2324; RRID:AB_302981 |
| caspase-3 | proteintech | Cat#19677-1-AP; RRID:AB_10733244 |
| Cleaved Caspase-3 | Abcam | Cat#ab2302; RRID:AB_302962 |
| Bcl-2 | proteintech | Cat#12789-1-AP; RRID:AB_2227948 |
| Bax | proteintech | Cat#50599-2-lg; RRID:AB_2061561 |
| HSP90A | proteintech | Cat#60318-1-Ig; RRID:AB_2881429 |
| Cdc37 | proteintech | Cat#10218-1-AP; RRID:AB_2077735 |
| Cyclin A2 | proteintech | Cat#18202-1-AP; RRID:AB_10597084 |
| CDK1 | proteintech | Cat#19532-1-AP; RRID:AB_10638617 |
| Ki-67 | Abcam | Cat#ab15580; RRID:AB_443209 |
| goat anti-rabbit IgG | proteintech | Cat#66912-1-lg; RRID:AB_2651036 |
| goat anti-mouse IgG | Abcam | Cat#ab150120; RRID:AB_2631447 |
| Chemicals, peptides, and recombinant proteins | ||
| Bombinin-BO1 | GL Biochem Ltd | Cat#HY-1001296 |
| fitc-Bombinin-BO1 | GL Biochem Ltd | Cat#P220311-MX980083 |
| Bombinin-BO1 Biotin-GF-25-NH2 | GL Biochem Ltd | Cat#P220221-HS936919 |
| Amiloride | Solarbio | Cat#IA1570 |
| 5×protein elution buffer | Solarbio | Cat#P1040 |
| DAPI | Solarbio | Cat#C0065 |
| 1% crystal violet | Solarbio | Cat#G1062 |
| protein A/G magnetic beads | MCE | Cat#HY-K0202-1 |
| enhanced chemiluminescence (ECL) | Thermofisher Scientific | Cat#A38556 |
| 4% tissue cell fixative | Solarbio | Cat#P1110 |
| Hoechst 33258 | Solarbio | Cat#C0021 |
| chlorpromazine | Solarbio | Cat#IC0340 |
| RNA Extraction Kit | Aidlab | Cat#RN07 |
| Critical commercial assays | ||
| CCK-8 | MedChemExpress | Cat#HY-K0301 |
| RNA pulldown kit | BersinBio™ | Cat#Bes5102 |
| the DNA Content Quantitation Assay (Cell Cycle) kit | Solarbio | Cat#CA 1510 |
| Annexin V FITC/PI kit | Solarbio | Cat#CA1020 |
| BCA Protein Concentration Assay Kit | Solarbio | Cat#PC0020 |
| Experimental models: Cell lines | ||
| Human Huh7 cells | Wuhan Pricella Biotechnology Co., Ltd | CL-0120 |
| Human Hep 3B cells | Wuhan Pricella Biotechnology Co., Ltd | CL-0102 |
| Experimental models: Organisms/strains | ||
| BALB/c Nude mice | Jiangsu Ji cui yao kang Biotechnology Co., Ltd | N/A |
| Oligonucleotides | ||
| Primer: HSP90A (F): 5′-CATAACGATGATGAGCA GTACGC-3’ (R): 5′-GACCCATA GGTTCACCTGTGT-3′ |
This paper | N/A |
| Software and algorithms | ||
| ImageJ | ImageJ Software46 | https://imagej.nih.gov/ij/ |
| GraphPad Prism 8.0 | GraphPad Prism Software | https://www.graphpad.com/ |
| Adobe Illustrator CC 2019 | Adobe | N/A |
| Other | ||
| RIPA lysis buffer | Solarbio | Cat#KGP702 |
| polyvinylidene difluoride (PVDF) membranes | Millipore | Cat#03010040001 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be sent directly to the primary contact person, qingling jiang (jangqingling@163.com).
Materials availability
No new unique reagents were generated in this study, and all data in this study are commercially available.
Data and code availability
-
•
Data: All data reported in this paper will be shared by the lead contact up on request.
-
•
Code: This paper does not report original code.
-
•
All other requests: Any additional information required to reanalyze the data reported will be shared by the lead contact upon request.
Experimental model and study participant details
Ethics approval and consent to participate
Animal experiments were in accordance with protocols approved by the Animal Care and Experiment Committee of the Binzhou Medical University. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Cell lines
Human Hep 3B cells (Wuhan Pricella Biotechnology Co., Ltd., CL-0102) and Human Huh7 cells (Wuhan Pricella Biotechnology Co., Ltd., CL-0120) were purchased from Procell LIFE Science Technology Co., Ltd (Wuhan, China).
Mice
All animal studies were conducted at the Binzhou Medical University Laboratory Animal Center and approved by the Binzhou Medical University Laboratory Animal Ethics Committee in accordance with the National Guidelines for the Care and Use of Laboratory Animals. Four-week-old BALB/c-Nude male nude mice were purchased from Jiangsu Ji cui yao kang Biotechnology Co., Ltd (Suzhou, China) and acclimatized under standard animal housing conditions for 7 days.
Method details
Cell culture
Human Hep 3B cells and Human Huh7 cells cultured in DMEM (Hyclone, H30022.01) containing 10% fetal bovine serum (FBS, Gibco Invitrogen, 10099141C) and 1% antibiotics (Solarbio, P7630). The cells were cultured in a sterile cell incubator with 5% CO2 at 37°C. Cells in good condition and in the logarithmic phase were used for the experiment.
Chemicals and reagents
BO1 (purity≥98.0% by HPLC and 1H NMR, Figure S4) was obtained from GL Biochem (Shanghai, China). To prepare a stock solution, BO1 was dissolved in dimethyl sulfoxide (DMSO) to achieve a concentration of 4 × 105 μM. This stock solution was subsequently diluted to a concentration of 2 × 105 μM using a gradient of ultrapure water and stored at −20°C. DMEM (high glucose) was acquired from Hyclone (Thermo Scientific Co., Ltd., Logan, Utah, USA). Materials purchased from Proteintech Group (Wuhan, China) included antibodies against GAPDH (proteintech, 10494-1-AP, 1:5000), Beta Actin (proteintech, 66009-1-Ig, 1:1000), goat anti-rabbit IgG (proteintech, 66912-1-lg, 1:10000), Pro Caspase-8 (Abcam, ab108333, 1:10000), Caspase-9 (proteintech, 10380-1-AP, 1:1000), Cleaved Caspase-9 (Abcam, ab2324, 1 ug/mL), caspase-3 (proteintech, 19677-1-AP, 1:2000), cleaved caspase-3 (Abcam, ab2302, 1:100), Bcl-2 (proteintech, 12789-1-AP, 1:1000), Bax (proteintech, 50599-2-lg,1:1000), HSP90A (proteintech, 60318-1-Ig, 1:5000), Cdc37 (proteintech, 10218-1-AP, 1:200), CDK1 (proteintech, 19532-1-Ig, 1:200), Cyclin A2 (proteintech, 18202-1-AP, 1:5000).
Cell counting Kit-8 (CCK-8)
Cells were inoculated into 96-well plates at a cell density of 4×103 per well, and after 24 h of incubation, different doses of BO1 were added, and incubation continued for 48 h. Then, CCK-8 (MedChemExpress, Y-K0301) reagent (10 μL/well) was added to the 96-well plates, and the plates were incubated in a cell culture incubator protected from light for 1–2 h. Finally, the absorbance of the cells was detected by a multiwell plate reader (Infinite 200 PRO, Tecan, Tecan Austria GmbH, Grodlg, Austria) at 450 nm. Three separate and independent experiments were conducted.
Colony formation
Cells were inoculated into 6-well plates and incubated for 24 h. After the initial incubation period, various doses of BO1 were added to the cells and further incubated for 48 h. Following the 48 h incubation with BO1, the medium was replaced with fresh medium, and the cells were incubated for an additional 14 days. After removing the medium, the cells were fixed for 15 min and then treated with 1% crystal violet (Solarbio, G1062) for 15 min. The cells were washed twice with PBS and then observed and photographed.
Cellular localization of BO1
FITC-labeled BO1 (FITC-BO1, GL Biochem Ltd, P220311-MX980083) was obtained from GL Biochem (Shanghai, China). The location of FITC-BO1 was detected with a laser scanning confocal microscope (LSM880, Zeiss, Jena, Germany). Cells were inoculated in confocal dishes (Corning, USA) for 24 h and then treated with FITC-BO1 for different times (1, 2, 3 h). At the end of incubation, the cells were washed 3 times with PBS for 10 min/time. Subsequently, cells were mixed with 4% paraformaldehyde for 30 min and stained with DAPI (Solarbio, C0065, 300 nM). Images were captured at 40× magnification with the laser scanning confocal microscope.
Endocytosis experiment
Cells were inoculated into 6-well plates at a density of 2 × 105 cells per well and incubated for 24 h, and then treated with FITC-BO1 or FITC-BO1 and endocytosis inhibitors (15 μM amiloride or 10 μg/mL chlorpromazine) for 2 h. Amiloride (Solarbio, IA1570) and chlorpromazine (Solarbio, IC0340). After removing the medium, the cells were cleaned 3 times with PBS, and the fluorescence intensity in cells was determined using flow cytometry (BD, NJ, USA). The experiment was repeated thrice.
Biotinylated pull-down mass spectrometry (MS)
Biotin-labeled BO1 (bio-BO1, GL Biochem Ltd, P220221-HS936919) was obtained from GL Biochem (Shanghai, China). The bio-BO1 pulldown experiments were performed using the BersinBio RNA pulldown kit (catalog Bes5102), following the manufacturer’s instructions with some modifications.47 The bio-BO1 and magnetic beads were mixed and incubated at 4°C for 3 h according to the instructions. The bio-BO1-magnetic bead complex was mixed with cell extracts and gently spun at 4°C for 12 h. Collect the magnetic beads, add 1 mL of ice-cold NT2 buffer, mix and wash at 4°C for 5 min. The supernatant was removed and the magnetic beads were repeatedly washed four times. The magnetic beads were collected and eluted with protein elution buffer and DTT (Solarbio, P1040) at 37°C for 2 h. The eluents were analyzed by LC-MS and Western blotting.
Immunofluorescence assay
The FITC-BO1 treated cells were inoculated in a confocal dish (Corning, USA) and mixed with 4% polyformaldehyde for 30 min. Then, the cells were permeabilized with 0.1% Triton X-100 and incubated with PBS containing 5% bovine serum albumin (BSA) for 30 min. Subsequently, the cells were sequentially incubated with an HSP90-specific antibody (proteintech, 60318-1-Ig, 1:100) and fluorescent secondary antibody Alexa Fluor 562-conjugated goat anti-mouse IgG (Abcam, ab150120, 1:200). Finally, cells were stained with DAPI (300 nM) and photographed with a laser scanning confocal microscope (LSM880, Zeiss, Jena, Germany) at 40×magnification.
Molecular docking
The 3D structure of HSP90A (N-terminal domain: HSP90A_N, PDB ID: 4BQG; the middle and C-terminal domain: HSP90A_MC, PDB ID: 3Q6M) was downloaded from the RCSB Protein DataBank (http://www.rcsb.org/pdb, accessed on June 2022). The three-dimensional structure of BO1 was constructed using Discovery Studio 2019 software and then optimized by CHARMM to minimize its energy. Molecular docking was performed with Autodock Vina tools. The prepared protein structures were imported into the software and then hydrogenated and charged using AutoDockTool 1.5.6, followed by docking calculations. The binding free energies of the BO1-HSP90A_N and BO1-HSP90A_MC were estimated based on empirical scoring functions and displayed by Discovery Studio 4.0 Visualizer (BIOVIA, Paris, France).
Immunoprecipitation (IP)
IP assays were performed using protein A/G magnetic beads (MedChemExpress, HY-K0202-1) and antibodies (HSP90A antibody, Cdc37 antibody, and IgG antibody). First, protein A/G magnetic beads (MCE, HY-K0202-1) were conjugated with antibodies for 30 min at room temperature. After the beads were sufficiently washed, proteins from cells or BO1-treated cells were added to the antibody-magnetic bead complexes and spun slowly for 30 min at room temperature. Then, the antibody-magnetic bead complexes were washed well and eluted using 1×SDS-PAGE loading buffer. The bound proteins were denatured by treatment at 95°C for 5 min, and the amount of the proteins in the precipitates was determined by SDS-PAGE.
Hoechst 33258 staining
Cells were inoculated into the 96-well plates at a density of 4×104 cells/well and incubated for 24 h. Then, cells were treated with different doses of BO1 (Human Hep 3B cells: 12, 15, 18 μM, human Huh7 cells: 20, 25, 30 μM) for 48 h. After removing the medium, cells were incubated with 4% tissue fixation solution for 15 min and incubated with Hoechst 33258 (Solarbio, C0021) staining solution for 5 min according to the kit instructions. Then, the cells were lightly washed twice with PBS, and the results were observed under a fluorescence microscope (DMI3000B, Leica, Leica Microsystems CMS GmbH).
Annexin V-FITC/PI staining analysis
Cells were inoculated into the 6-well plates at 2×105 cells/well density and cultivated for 24 h. Then, cells were treated with different doses of BO1 (Human Hep 3B cells: 12, 15, 18 μM, human Huh7 cells: 20, 25, 30 μM) for 48 h. After washing the cells with PBS, apoptosis was analyzed using Annexin V FITC/PI kit (Solarbio, CA1020) and measured by flow cytometry (BD, NJ, USA). The three replicate experiments were conducted independently.
Cell cycle analysis
Cells were inoculated at a density of 5×105 in a 100 mm diameter Petri dish and incubated for 24 h. Then, cells were treated with different doses of BO1 (Human Hep 3B cells: 12, 15, 18 μM, human Huh7 cells: 20, 25, 30 μM) for 48 h. After the above treatment, cell precipitates were collected and fixed in 70% ethanol for 4 h. Then, cells were washed twice with PBS and evaluated according to the DNA Content Quantitation Assay (Cell Cycle) kit (Solarbio, CA 1510). Cell cycle was detected using a flow cytometry (BD, NJ, USA).
Western blot analysis
Proteins were extracted with RIPA lysis buffer (Solarbio, KGP702), and then the concentration was determined by the BCA Protein Concentration Assay Kit (Solarbio, PC0020). After SDS-PAGE, the proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, 03010040001), followed by incubation in nonfat milk for 4 h at room temperature. After incubation of primary and secondary antibodies, the membranes were assayed using an enhanced chemiluminescence (ECL) kit (Thermofisher Scientific, A38556). The spectra were observed by the UVP chemiluminescence imaging system.
Hematoxylin-eosin staining
Soybean-sized tumor blocks were fixed with formaldehyde (Solarbio, P1110) and then embedded in paraffin. H&E staining was performed using 4-μm-thick sections according to standard protocols, and images were taken with a microscope (DMI3000B; Leica Microsystems, Wetzlar, Germany).
Immunohistochemistry (IHC)
4-μm-thick tumor sections were deparaffinized, rehydrated in xylene, and then incubated in 0.01 M citrate buffer (pH 6.0) for 20 min at 90°C for heat-induced antigen repair. Sections were blocked in PBS containing 10% goat serum and 0.3% Triton X-100 for 30 min. Then, the Ki-67 (Abcam, ab15580) antibody was added and incubated overnight at 4°C, followed by incubation with goat secondary antibody for 1 h at room temperature. Detection was performed using a DAB substrate kit (Solarbio, China) and photographed by microscopic observation (DMI3000B; Leica Microsystems, Wetzlar, Germany).
RT-qPCR assay
Total RNA was extracted from the cells using the RNA Extraction Kit (Aidlab, RN07) according to the manufacturer’s instructions. After quality control of the RNA, cDNA was reverse transcribed and synthesized from 1 μg of total RNA using the PrimeScriptTM kit (Takara, RR047A) according to the manufacturer’s instructions. qRT-PCR was performed using SYBR Select Master Mix (Applied Biosystems) and cDNA. The process was carried out on a 7500 Fast Real-Time PCR System (Applied Biosystems). GAPDH was used as the reference gene.
In vivo xenograft model
The mice were randomly divided into two groups (6 animals/group). Human Hep 3B cells were inoculated subcutaneously at a dosage of 2×106 cells/animal. When the tumors reached 100 mm3, mice were injected intraperitoneally with 200 μL of saline or BO1 (4.57 mg/kg) every other day, the mice were weighed, and the tumor volume was measured. After two weeks, the mice were executed. Blood and serum were taken for glutathione (ALT), glutamate aminotransferase (AST), and creatinine (CREA), respectively. Tumors were photographed and weighed, and Ki-67 levels in cells were analyzed by immunohistochemistry. The heart, liver, spleen, and kidney were examined by hematoxylin and eosin (H&E) staining.
Quantification and statistical analysis
All experiments were repeated three times independently. Data were expressed as the mean ± SEM. Differences were calculated by the Student’s t test or one-way ANOVA using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). A p-value <0.05 was statistically significant.
Acknowledgments
The authors would like to thank all the members who made contributions to this research. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service. This work was supported by the Natural Science Foundation of Shandong Province (grant number ZR202103050666), National Natural Science Foundation of China (grant number 82073313), the Yantai School Land Integration Development Project in 2021 (grant number 2021XDRHXMPT15).
Author contributions
Study concept and design, Z.S. and Q.J.; acquisition of data, X.W. and P.T).; analysis and interpretation of data, X.W., H.Y., M.L., H.Q., and X.H.; drafting of the manuscript, X.W. and P.T.) ;critical revision of the manuscript for important intellectual content, X.W., Y.G., and Q.Z.; and administrative, technical, or material support, study supervision, Q.J. and D..
Declaration of interests
The authors declare no competing interests.
Published: June 26, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110382.
Contributor Information
Zhen Shi, Email: zhen_shizhen@163.com.
Defang Li, Email: lidefang@163.com.
Qingling Jiang, Email: jangqingling@163.com.
Supplemental information
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Wang F., Wang N., Zhang M.Y., Wang H.Y., Huang G.L. Diagnostic and prognostic value of protein post-translational modifications in hepatocellular carcinoma. J. Clin. Transl. Hepatol. 2023;11:1192–1200. doi: 10.14218/JCTH.2022.00006S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X., Wang Y., Xia H., Liu S., Huang Z., He R., Yu L., Meng N., Wang H., You J., et al. Roles and molecular mechanisms of biomarkers in hepatocellular carcinoma with microvascular invasion: a review. J. Clin. Transl. Hepatol. 2023;11:1170–1183. doi: 10.14218/JCTH.2022.00013S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Shree Harini K., Ezhilarasan D. Wnt/beta-catenin signaling and its modulators in nonalcoholic fatty liver diseases. Hepatobiliary Pancreat. Dis. Int. 2023;22:333–345. doi: 10.1016/j.hbpd.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y., Wang Z.Z., Han A.Q., Yang M.Y., Zhu L.X., Pan F.M., Wang Y. TuBG1 promotes hepatocellular carcinoma via ATR/P53-apoptosis and cycling pathways. Hepatobiliary Pancreat. Dis. Int. 2024;23:195–209. doi: 10.1016/j.hbpd.2023.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Chalamaiah M., Yu W., Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar-Toala J.E., Hernandez-Mendoza A., Gonzalez-Cordova A.F., Vallejo-Cordoba B., Liceaga A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides. 2019;122 doi: 10.1016/j.peptides.2019.170170. [DOI] [PubMed] [Google Scholar]
- 9.Ashaolu T.J., Le T.D., Suttikhana I., Olatunji O.J., Farag M.A. RETRACTED: Hemp bioactive peptides: Nutrition, functional properties and action mechanisms to maximize their nutraceutical applications and future prospects. Food Chem. 2023;414 doi: 10.1016/j.foodchem.2023.135691. [DOI] [PubMed] [Google Scholar]
- 10.Manzoor M., Singh J., Gani A. Exploration of bioactive peptides from various origin as promising nutraceutical treasures: In vitro, in silico and in vivo studies. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131395. [DOI] [PubMed] [Google Scholar]
- 11.Shi D., Hou X., Wang L., Gao Y., Wu D., Xi X., Zhou M., Kwok H.F., Duan J., Chen T., Shaw C. Two novel dermaseptin-like antimicrobial peptides with anticancer activities from the skin secretion of pachymedusa dacnicolor. Toxins. 2016;8 doi: 10.3390/toxins8050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintal-Bojorquez N., Segura-Campos M.R. Bioactive peptides as therapeutic adjuvants for cancer. Nutr. Cancer. 2021;73:1309–1321. doi: 10.1080/01635581.2020.1813316. [DOI] [PubMed] [Google Scholar]
- 13.Boohaker R.J., Lee M.W., Vishnubhotla P., Perez J.M., Khaled A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012;19:3794–3804. doi: 10.2174/092986712801661004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bidwell G.L., Raucher D. Therapeutic peptides for cancer therapy. Part I - peptide inhibitors of signal transduction cascades. Expet Opin. Drug Deliv. 2009;6:1033–1047. doi: 10.1517/17425240903143745. [DOI] [PubMed] [Google Scholar]
- 15.Chiangjong W., Chutipongtanate S., Hongeng S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review) Int. J. Oncol. 2020;57:678–696. doi: 10.3892/ijo.2020.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raucher D., Moktan S., Massodi I., Bidwell G.L. Therapeutic peptides for cancer therapy. Part II - cell cycle inhibitory peptides and apoptosis-inducing peptides. Expet Opin. Drug Deliv. 2009;6:1049–1064. doi: 10.1517/17425240903158909. [DOI] [PubMed] [Google Scholar]
- 17.Ma R., Wong S.W., Ge L., Shaw C., Siu S.W.I., Kwok H.F. In vitro and md simulation study to explore physicochemical parameters for antibacterial peptide to become potent anticancer peptide. Mol. Ther. Oncolytics. 2020;16:7–19. doi: 10.1016/j.omto.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F.J., Hu J.H., Ren X., Zhou C.M., Liu Q., Zhang Y.Q. Toad venom: A comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch. Pharm. 2021;354 doi: 10.1002/ardp.202100060. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C., Wang Z., Peng X., Liu Y., Lin Y., Zhang Z., Qiu Y., Jin M., Wang R., Kong D. Discovery of two bombinin peptides with antimicrobial and anticancer activities from the skin secretion of Oriental fire-bellied toad, Bombina orientalis. Chem. Biol. Drug Des. 2018;91:50–61. doi: 10.1111/cbdd.13055. [DOI] [PubMed] [Google Scholar]
- 20.Peng X., Zhou C., Hou X., Liu Y., Wang Z., Peng X., Zhang Z., Wang R., Kong D. Molecular characterization and bioactivity evaluation of two novel bombinin peptides from the skin secretion of Oriental fire-bellied toad, Bombina orientalis. Amino Acids. 2018;50:241–253. doi: 10.1007/s00726-017-2509-z. [DOI] [PubMed] [Google Scholar]
- 21.Swithenbank L., Cox P., Harris L.G., Dudley E., Sinclair K., Lewis P., Cappiello F., Morgan C. Temporin A and Bombinin H2 antimicrobial peptides exhibit selective cytotoxicity to lung cancer cells. Sci. Tech. Rep. 2020;2020 doi: 10.1155/2020/3526286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H.Q., Sun L.X., Yu L., Liu J., Sun L.C., Yang Z.H., Shu X., Ran Y.L. HSP90, as a functional target antigen of a mAb 11C9, promotes stemness and tumor progression in hepat- ocellular carcinoma. Stem Cell Res. Ther. 2023;14:273. doi: 10.1186/s13287-023-03453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 24.Mahalingam D., Swords R., Carew J.S., Nawrocki S.T., Bhalla K., Giles F.J. Targeting HSP90 for cancer therapy. Br. J. Cancer. 2009;100:1523–1529. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trepel J., Mollapour M., Giaccone G., Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter S., Buchner J. Molecular chaperones--cellular machines for protein folding. Angew. Chem. Int. Ed. Engl. 2002;41:1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Bagatell R., Whitesell L. Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol. Cancer Therapeut. 2004;3:1021–1030. [PubMed] [Google Scholar]
- 28.Taha E.A., Ono K., Eguchi T. Roles of extracellular hsps as biomarkers in immune surveillance and immune evasion. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S., Xiao H., Cao L. Recent advances in heat shock proteins in cancer diagnosis, prognosis, metabolism and treatment. Biomed. Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.112074. [DOI] [PubMed] [Google Scholar]
- 30.Hallett S.T., Pastok M.W., Morgan R.M.L., Wittner A., Blundell K., Felletar I., Wedge S.R., Prodromou C., Noble M.E.M., Pearl L.H., Endicott J.A. Differential regulation of G1 CDK complexes by the Hsp90-Cdc37 chaperone system. Cell Rep. 2017;21:1386–1398. doi: 10.1016/j.celrep.2017.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray P.J., Jr., Prince T., Cheng J., Stevenson M.A., Calderwood S.K. Targeting the oncogene and kinome chaperone CDC37. Nat. Rev. Cancer. 2008;8:491–495. doi: 10.1038/nrc2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen S., Dean D.C., Yu Z., Duan Z. Role of cyclin-dependent kinases (CDKs) in hepatocellular carcinoma: Therapeutic potential of targeting the CDK signaling pathway. Hepatol. Res. 2019;49:1097–1108. doi: 10.1111/hepr.13353. [DOI] [PubMed] [Google Scholar]
- 33.Prince T.L., Lang B.J., Okusha Y., Eguchi T., Calderwood S.K. Cdc37 as a Co-chaperone to Hsp90. Subcell. Biochem. 2023;101:141–158. doi: 10.1007/978-3-031-14740-1_5. [DOI] [PubMed] [Google Scholar]
- 34.Verba K.A., Wang R.Y., Arakawa A., Liu Y., Shirouzu M., Yokoyama S., Agard D.A. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science. 2016;352:1542–1547. doi: 10.1126/science.aaf5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Son S., Jang M., Lee B., Hong Y.S., Ko S.K., Jang J.H., Ahn J.S. Ulleungdin, a lasso peptide with cancer cell migration inhibitory activity discovered by the genome mining approach. J. Nat. Prod. 2018;81:2205–2211. doi: 10.1021/acs.jnatprod.8b00449. [DOI] [PubMed] [Google Scholar]
- 36.Tan M., Liao C., Liang L., Yi X., Zhou Z., Wei G. Recent advances in recombinase polymerase amplification: Principle, advantages, disadvantages and applications. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1019071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galdiero E., Lombardi L., Falanga A., Libralato G., Guida M., Carotenuto R. Biofilms: Novel strategies based on antimicrobial peptides. Pharmaceutics. 2019;11:322. doi: 10.3390/pharmaceutics11070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riool M., de Breij A., Drijfhout J.W., Nibbering P.H., Zaat S.A.J. Antimicrobial peptides in biomedical device manufacturing. Front. Chem. 2017;5:63. doi: 10.3389/fchem.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koczulla A.R., Bals R. Antimicrobial peptides: current status and therapeutic potential. Drugs. 2003;63:389–406. doi: 10.2165/00003495-200363040-00005. [DOI] [PubMed] [Google Scholar]
- 40.Cole A.M., Ganz T. Human antimicrobial peptides: analysis and application. Biotechniques. 2000;29:822–831. doi: 10.2144/00294rv01. 828, 830-821. [DOI] [PubMed] [Google Scholar]
- 41.Ma R., Kwok H.F. New opportunities and challenges of venom-based and bacteria-derived molecules for anticancer targeted therapy. Semin. Cancer Biol. 2022;80:356–369. doi: 10.1016/j.semcancer.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Wang G. Post-translational modifications of natural antimicrobial peptides and strategies for peptide engineering. Curr. Biotechnol. 2012;1:72–79. doi: 10.2174/2211550111201010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S.K., Song J.W., Gong F., Li S.B., Chang H.Y., Xie H.M., Gao H.W., Tan Y.X., Ji S.P. Design of an alpha-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016;6 doi: 10.1038/srep27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitani H., Naessens J., Kubo M., Nakamura Y., Iraqi F., Gibson J., Yamakawa M. Synthetic nonamer peptides derived from insect defensin mediate the killing of African trypanosomes in axenic culture. Parasitol. Res. 2009;105:217–225. doi: 10.1007/s00436-009-1389-x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J., Liu Y., Shen T., Chen L., Zhang C., Cai K., Liao C., Wang C. Antimicrobial activity of the antibacterial peptide PMAP-36 and its analogues. Microb. Pathog. 2019;136 doi: 10.1016/j.micpath.2019.103712. [DOI] [PubMed] [Google Scholar]
- 46.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y.Z., Lv D.J., Wang C., Song X.L., Xie T., Wang T., Li Z.M., Guo J.D., Fu D.J., Li K.J., et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol. Cancer. 2022;21:12. doi: 10.1186/s12943-021-01480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: All data reported in this paper will be shared by the lead contact up on request.
-
•
Code: This paper does not report original code.
-
•
All other requests: Any additional information required to reanalyze the data reported will be shared by the lead contact upon request.