Abstract
To investigate the roles of human T-cell leukemia virus type 1 (HTLV-1) envelope (Env) proteins gp46 and gp21 in the early steps of infection, the effects of the 23 synthetic peptides covering the entire Env proteins on transmission of cell-free HTLV-1 were examined by PCR and by the plaque assay using a pseudotype of vesicular stomatis virus (VSV) bearing the Env of HTLV-1 [VSV(HTLV-1)]. The synthetic peptide corresponding to amino acids 400 to 429 of the gp21 Env protein (gp21 peptide 400-429, Cys-Arg-Phe-Pro-Asn-Ile-Thr-Asn-Ser-His-Val-Pro-Ile-Leu-Gln-Glu-Arg-Pro-Pro-Leu-Glu-Asn-Arg-Val-Leu-Thr-Gly-Trp-Gly-Leu) strongly inhibited infection of cell-free HTLV-1. By using the mutant peptide, Asn407, Ser408, and Leu413, -419, -424, and -429 were confirmed to be important amino acids for neutralizing activity of the gp21 peptide 400-429. Addition of this peptide before or during adsorption of HTLV-1 at 4°C did not affect its entry. However, HTLV-1 infection was inhibited about 60% when the gp21 peptide 400-429 was added even 30 min after adsorption of HTLV-1 to cells, indicating that the amino acid sequence 400 to 429 on the gp21 Env protein plays an important role at the postbinding step of HTLV-1 infection. In contrast, a monoclonal antibody reported to recognize the gp46 191-196 peptide inhibited the infection of HTLV-1 at the binding step.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia (ATL) (14, 29) and HTLV-1-associated myelopathy/tropical spastic paraparesis (11, 19, 26). The glycoproteins encoded by the env gene of HTLV-1 are essential for interaction with an unidentified receptor on the surface of target cells and play a crucial role in the infection process (41). The HTLV-1 Env proteins are initially synthesized in infected cells as a precursor protein (Pr61), which is subsequently glycosylated and cleaved in the Golgi apparatus into two mature products: the extracellular surface glycoprotein (gp46) and the transmembrane glycoprotein (gp21), which spans the lipid bilayers (5, 17). These two glycoproteins are linked with each other through noncovalent interactions and are anchored to the surface of infected cells or of virions via the gp21 protein (27). The Env glycoproteins govern the entry of the virus into target cells by mediating specific attachment to a cellular receptor, which is followed by fusion between viral and cellular membranes. In addition, fusion between Env-expressing cells and receptor-bearing cells leads to the formation of multinucleated giant cells (syncytia) (16, 25).
Recently, we reported that the syncytium formation induced by HTLV-1-producing cells is inhibited by the Env synthetic peptides corresponding to amino acids 197 to 216 of gp46 and amino acids 400 to 429 of gp21 (33), suggesting that these regions are necessary for Env functions of HTLV-1, such as adsorption or penetration of HTLV-1 or cell fusion induced by HTLV-1. With regard to gp46, anti-gp46 rat monoclonal antibody (MAb) (LAT-27) was also reported to recognize the gp46 peptide 191-196 and to inhibit the syncytium formation and transmission of cell-free HTLV-1 (12, 40). However, it still remains to be determined how these two Env synthetic peptides and LAT-27 MAb interfere with the life cycle of HTLV-1.
Although infection of cells with cell-free HTLV-1 is quite inefficient compared with that with other retroviruses even in vitro (3, 8, 9), we reported that cell-free HTLV-1 prepared from S+L-cat cells is highly transmissible and developed a new assay system to detect infection with cell-free HTLV-1 using PCR (12, 13). HTLV-1-specific PCR bands are detectable 1 day after infection with cell-free HTLV-1, and their formation is inhibited by the treatment of virus with neutralizing antibodies. Compared to the syncytium formation assay, this PCR assay system is thought to be useful for examining the early steps in HTLV-1 infection such as adsorption or penetration of HTLV-1, the reverse transcription of HTLV-1 RNA, integration of HTLV-1 DNA into cellular DNA, or the late steps of HTLV-1 infection. In this study, we used synthetic peptides covering the Env proteins gp46 and gp21 and LAT-27 MAb to identify Env regions which play a role in HTLV-1 infection.
We used MOLT-4 clone 8 (38) human T cells and 8C feline kidney cells (10) as indicator cells to be infected with cell-free HTLV-1. The HTLV-1-producing cells were c77 (15), a subclone line of 8C feline kidney cells that had been cocultivated with lethally irradiated ATL-2M HTLV-1-producing cells. MOLT-4 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). 8C and c77 cells were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% FCS. All cells were maintained at 37°C in a humidified, 5% CO2 atmosphere.
Cell-free HTLV-1 was prepared as described before (12). Namely, after incubation of c77 cells (3 × 105 cells per ml) for 2 days, medium was replaced by fresh medium and the cells were cultured for another 24 h. The culture supernatant was harvested and centrifuged at low speed and then passed through 0.22-μm-pore-size cellulose acetate syringe filters (Gelman Science, Ann Arbor, Mich.). The virus sample was stored at −80°C until use. The cell-free HTLV-1 was inoculated into MOLT-4 cells (5 × 105) for 1 h at 37°C. The cells were then washed three times with phosphate-buffered saline, and fresh medium was added. After incubation for 20 h, the cells were washed and lysed with 10 mM Tris-HCl (pH 8.3) containing 1 mM EDTA, 0.45% Nonidet P-40 (Sigma, St. Louis, Mo.), 0.45% Tween 20 (Sigma), and 0.2 mg of proteinase K (Sigma) per ml. The cell lysates were incubated for 2 h at 52°C and heated for 10 min at 96°C to inactivate proteinase K. To detect formation of HTLV-1 DNA after infection, PCR was performed with pX outer primers (pXO 7302-7326, 5′-CCCACTTCCCAGGGTTTGGACAGAG-3′; pXO 7504-7481, 3′-CTGTAGAGCTGAGCCGATAACGCG-5′) in a Perkin-Elmer cycler under the following conditions: 35 cycles of 93°C for 1 min, 67°C for 45 s, and 72°C for 1 min and one cycle of 72°C for 5 min. As an internal control the human β-globin gene primers KM29 and KM38 (5′-GGTTGGCCAATCTACTCCCAGG-3′ and 5′-TGGTCTCCTTAAACCTGTCTTG-3′, respectively) (36) were used. DNA amplification was performed under the following conditions: 30 cycles of 93°C for 1 min, 60°C for 45 s, and 72°C for 1 min and one cycle of 72°C for 5 min. PCR products were visualized by electrophoresis through 3% agarose gels containing 0.5 μg of ethidium bromide per ml.
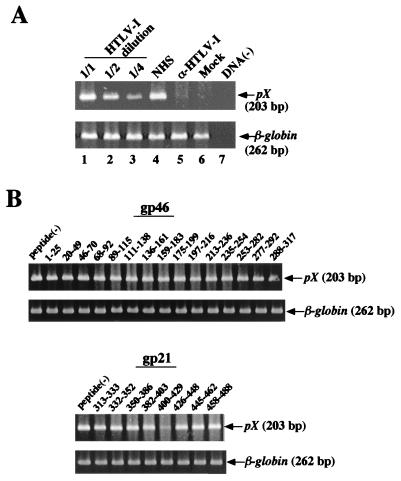
MOLT-4 cells were infected with twofold-diluted cell-free HTLV-1, and cell lysates were prepared 20 h later. Formation of HTLV-1 DNA in MOLT-4 cells was detected by PCR (Fig. 1A). The expected pX DNA fragments (203 bp) were amplified, and the relative intensities determined by calculation after densitometry were 100 (lane 1), 55 (lane 2), and 22 (lane 3), which were well correlated with dilution of inoculate HTLV-1. Cell-free HTLV-1 infection was neutralized by human serum seropositive for HTLV-1 (lane 5) but not by human serum seronegative for HTLV-1 (lane 4), indicating the specificity of infection. Thus, we used these PCR conditions to detect HTLV-1 DNA after transmission of cell-free HTLV-1 to MOLT-4 cells.
FIG. 1.
Detection of reverse-transcribed HTLV-1 RNA by PCR and effects of the HTLV-1 Env synthetic peptides on transmission of cell-free HTLV-1. (A) MOLT-4 cells were infected with serially diluted cell-free HTLV-1, lysed after incubation for 20 h, and examined by PCR amplification using the pXO 7302-7326 and pXO 7504-7481 primers (lanes 1 to 3). MOLT-4 cells were infected with cell-free HTLV-1 treated with HTLV-1-seronegative human serum (normal human serum [NHS]) or with HTLV-1-seropositive human serum (α-HTLV-1) for 1 h at 37°C. Lane 6, mock-infected control. PCR amplification was performed without template DNA (lane 7). (B) MOLT-4 cells were infected with HTLV-1 for 1 h in the presence of the Env gp46 or gp21 synthetic peptides at 10 μM. Amino acid positions of Env (the initiation codon of Met is 1) are shown above each lane. The amino acid sequences of the peptides are listed in Table 1. β-Globin DNA was amplified as a control to confirm the efficiency of the amplification of each sample.
To determine which region in the HTLV-1 Env proteins plays an important role in infection of cell-free HTLV-1, effects of the 23 peptides (Table 1) covering the whole Env protein at 10 μM on HTLV-1 infection were examined by the PCR assay system as described above (Fig. 1B). The peptide corresponding to amino acids 400 to 429 of the gp21 (gp21 peptide 400-429) strongly inhibited synthesis. None of the gp46 peptides inhibited cell-free HTLV-1 infection. In the case of the syncytium assay, we reported that the gp46 peptide 197-216 inhibits the formation of syncytia (33). We confirmed that this gp46 peptide 197-216 at a concentration of 10 μM inhibited syncytium formation by 60%. The gp46 peptide 197-216 at concentrations up to 100 μM did not have much of an inhibitory effect on cell-free HTLV-1 infection (data not shown).
TABLE 1.
Amino acid sequences of HTLV-1 Env synthetic peptides
| Peptide | Sequence |
|---|---|
| gp46 | |
| 1-25 | MGKFLATLIL FFQFCPLIFG DYSPS |
| 20-49 | GDYSPSCCTL TIGVSSYHSK PCNPAQPVCS |
| 46-70 | PVCSWTLDLL ALSADQALQP PCPNL |
| 68-92 | PNLVSYSSYH ATYSLYLFPH WTKKP |
| 89-115 | TKKPNRNGGG YYSASYSDPC SLKCPYL |
| 111-138 | KCPYLGCQSW TCPYTGAVSS PYWKFQHD |
| 136-161 | QHDVNFTQEV SRLNINLHFS KCGFPF |
| 159-183 | FPFSLLVDAP GYDPIWFLNT EPSQL |
| 175-199 | FLNTEPSQLP PTAPPLLPHS NLDHI |
| 197-216 | DHILEPSIPW KSKLLTLVQL |
| 213-236 | LVQLTLQSTN YTCIVCIDRA SLST |
| 235-254 | STWHVLYSPN VSVPSSSSTP |
| 253-282 | TPLLYPSLAL PAPHLTLPFN WTHCFDPQIQ |
| 277-292 | FDPQIQAIVS SPCHNS |
| 288-317 | PCHNSLILPP FSLSPVPTLG SRSRRAVPVA |
| gp21 | |
| 313-333 | AVPVAVWLVS ALAMGAGVAG G |
| 332-352 | GGITGSMSLA SGKSLLHEVD K |
| 350-386 | VDKDISQLTQ AIVKNHKNLL KIAQYAAQNR RGLDLLF |
| 382-403 | LDLLFWEQGG LCKALQEQCR FP |
| 400-429 | CRFPNITNSH VPILQERPPL ENRVLTGWGL |
| 426-448 | GWGLNWDLGL SQWAREALQT GIT |
| 445-462 | TGITLVALLL LVILAGPC |
| 458-488 | LAGPCILRQL RHLPSRVRYP HYSLIKPESS L |
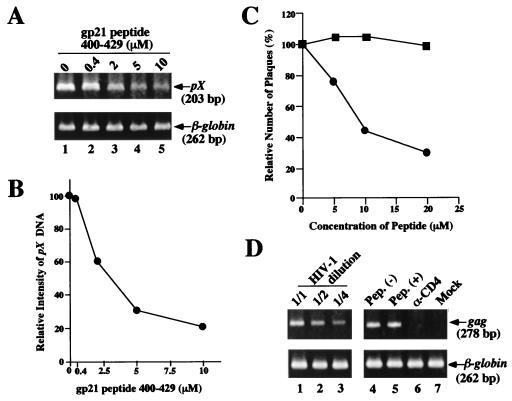
We next examined the dose dependency of the inhibitory effect of the gp21 peptide 400-429. This peptide reduced the infectivity of cell-free HTLV-1 in a dose-dependent manner (Fig. 2A). The approximate peptide concentration giving 50% inhibition of the formation of HTLV-1 DNA was 2.5 μM (Fig. 2B). The inhibitory activity of the gp21 peptide 400-429 against the formation of HTLV-1 DNA was detected up to 96 h after incubation. The half-life of the inhibitory activity of the gp21 peptide 400-429 incubated at 37°C in RPMI 1640 medium supplemented with 10% FCS was calculated to be 72 h (data not shown).
FIG. 2.
Dose-dependent and specific inhibition of cell-free HTLV-1 infection by the gp21 peptide 400-429. (A) MOLT-4 cells were infected with HTLV-1 in the presence of the gp21 peptide 400-429 at 0 to 10 μM. Formation of HTLV-1 DNA in the MOLT-4 cells was detected by PCR as described for Fig. 1. (B) Relative intensities of pX DNA were determined by densitometry: band intensities of pX DNA were divided by those of β-globin bands. (C) Inhibitory effect of the gp21 peptide 400-429 on plating of the VSV(HTLV-1) pseudotype. The VSV pseudotype infection was carried out in the presence of the gp21 peptide 400-429 (circles) or the gp21 peptide 458-488 (squares). About 100 plaques were formed in the absence of the peptides. Each datum point is the mean for two independent experiments. (D) Effect of the gp21 peptide 400-429 on transmission of cell-free HIV-1. MOLT-4 cells were infected with serially diluted cell-free HIV-1 (IIIB strain) (lanes 1 to 3), lysed 20 h later, and examined by PCR using the primers specific for the HIV-1 gag region. Cell-free HIV-1 was inoculated into MOLT-4 cells in the absence (lane 4) or presence (lane 5) of the gp21 peptide (Pep.) 400-429 (10 μM). Lane 6, MOLT-4 cells treated with neutralizing MAb against CD4 receptor (α-CD4) prior to infection. Lane 7, mock-infected control. β-Globin DNA was amplified as a control.
We also examined the inhibitory effect of the gp21 peptide 400-429 on cell-free HTLV-1 infection by the vesicular stomatitis virus [VSV(HTLV-1)] pseudotype assay. The VSV(HTLV-1) pseudotype was incubated with goat anti-VSV serum before inoculation into cells. The 8C cells were infected with the VSV pseudotype in the presence of the Env peptide for 1 h at 37°C and overlaid with EMEM containing 1% agarose, and plaques were counted 24 h later. As shown in Fig. 2C, the plating of VSV(HTLV-1) was apparently inhibited by the gp21 peptide 400-429 but not by the gp21 peptide 458-488, corresponding to the region of the cytoplasmic tail of gp21, used as a control. Again, no inhibition by the gp46 peptide 197-216 was observed at concentrations up to 20 μM (data not shown). The inhibitory effects of the gp21 peptide 400-429 at 10 μM on the plating of VSV(HTLV-1) and on transmission of cell-free HTLV-1 detected by the PCR assay were similar.
To confirm the specificity of the gp21 peptide 400-429, we examined the effect of this peptide on transmission of cell-free human immunodeficiency virus type 1 (HIV-1) (IIIB strain) to MOLT-4 cells (Fig. 2D). The formation of HIV-1 DNA in MOLT-4 cells was detected by PCR using primers specific for the HIV-1 gag region. The virus preparation was treated with RNase-free DNase I (10 U/ml) (Boehringer Mannheim Corporation, Indianapolis, Ind.) for 30 min at 37°C to remove contaminating viral DNA or host cellular DNA. DNase I-treated IIIB was inoculated into MOLT-4 cells (5 × 105) for 2 h at 37°C in the presence of 10 μM gp21 peptide 400-429. After incubation for 20 h, the cell lysates were prepared and PCR was performed with the HIV-1 gag-specific primers SK38 and SK39 (5′-AAGGGGAAGTGACATAGCAG-3′ and 3′-GGACCAACAAGGTTTCTGTC-5′, respectively) (30) in a Perkin-Elmer cycler under the following conditions: 1 cycle of 95°C for 9 min; 30 cycles of 94°C for 1 min, 60°C for 45 s, and 72°C for 1 min; and one cycle of 72°C for 5 min. The relative intensities of amplified DNA were determined to be 100 (lane 1), 47 (lane 2), and 22 (lane 3), which were correlated with the dilution of inoculated virus, indicating that PCR was performed within the linear range. The synthesis of gag DNA was not inhibited by the gp21 peptide 400-429 at 10 μM (lane 5). The neutralizing MAb against CD4 (Nu-Th/i; 5 μg/ml) (Nichirei Co., Tokyo, Japan), which is a primary receptor for HIV-1, blocked entry of HIV-1 into MOLT-4 cells (lane 6). Thus, the inhibitory effect of the gp21 peptide 400-429 is specific for HTLV-1. We also examined the cell toxicity of the gp21 peptide 400-429. For this, MOLT-4 cells were cultured in the presence of the gp21 peptide 400-429 at 50 μM for up to 4 days. There was little effect of the gp21 peptide 400-429 on the cell growth and the viability determined by the trypan blue dye exclusion assay, indicating that the anti-HTLV-1 activity of the gp21 peptide 400-429 did not appear to be related to cytotoxic or cytostatic effect.
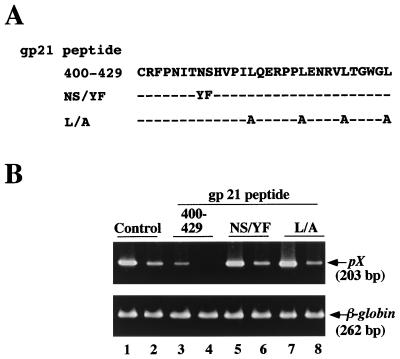
We next tried to determine a functional amino acid necessary for inhibitory activity of the gp21 peptide 400-429 (Fig. 3). Because previous mutagenesis experiments had shown that the Asn407-Tyr and Ser408-Phe mutations in gp21 affected both cell-to-cell fusion and cell-to-cell transmission of HTLV-1 (32), we synthesized a mutant peptide, in which Asn407 and Ser408 had been replaced by Tyr407 and Phe408, respectively, in the gp21 peptide 400-429 (gp21 peptide NS/YF; Fig. 3A). Furthermore, we changed all leucines in the gp21 peptide 400-429 to alanines (Leu413-Ala, Leu419-Ala, Leu424-Ala, and Leu429-Ala) (gp21 peptide L/A; Fig. 3A). As for Leu419, it had been shown to be important for cell-to-cell fusion and cell-to-cell transmission of HTLV-1 (32). Using these two mutant peptides, we examined the effects of mutations on the transmission of cell-free HTLV-1 to MOLT-4 cells (Fig. 3B). In contrast to marked inhibition of infection by the gp21 peptide 400-429 (10 μM) (lanes 3 and 4), no inhibition was detected upon addition of the gp21 peptide NS/YF (10 μM) (lanes 5 and 6) or the gp21 peptide L/A (10 μM) (lanes 7 and 8) compared to the no-peptide control (lanes 1 and 2). This finding suggested that Asn407, Ser408, and leucines in the gp21 peptide 400-429 play important roles in inhibition of transmission of cell-free HTLV-1.
FIG. 3.
Effects of mutations in the gp21 peptide 400-429 on inhibitory activity. (A) Amino acid sequences of the mutant peptides. (B) Effects of the mutant peptides on the transmission of cell-free HTLV-1. MOLT-4 cells were infected with either undiluted (odd-number lanes) or diluted (even-number lanes) cell-free HTLV-1 in the presence of the peptides at 10 μM. Cell lysates were examined by PCR as described for Fig. 1. β-Globin DNA was amplified as a control.
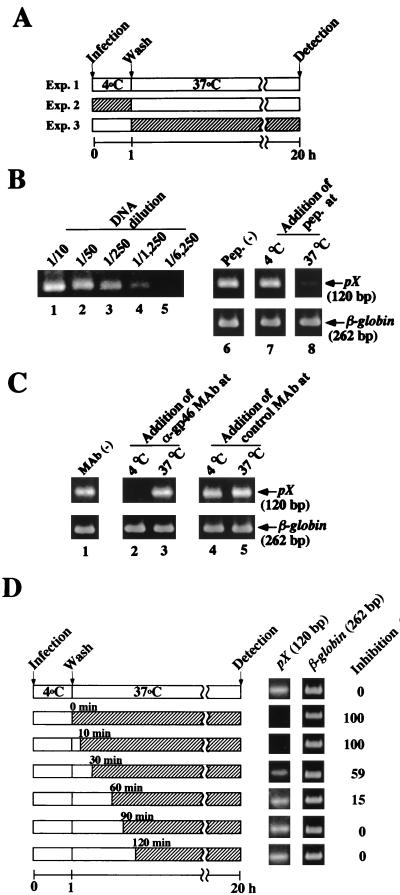
In general, retroviruses will bind to the cell surface efficiently at 4°C (binding step), but the postbinding step such as fusion between virus and cell membranes, or penetration of viral core into host cells, appears to be dependent on temperature (4, 28, 39). Therefore, we examined which step, binding to cells or postbinding to cells, was inhibited by the gp21 peptide 400-429 (Fig. 4A). For this, MOLT-4 cells were incubated with cell-free HTLV-1 for 1 h at 4°C (binding step), and the cells were washed to remove unbound viruses. Then, the cell suspension was incubated at 37°C in a CO2 incubator for 20 h to allow viruses to enter cells (postbinding step). Then, the cell lysates were prepared, and formation of HTLV-1 DNA was detected by nested PCR to amplify the pX region because amplified DNA was hardly detected when the cells had been infected with HTLV-1 at 4°C. Indeed, it had already been reported that HTLV-1 binds to cells inefficiently at 4°C (21). The conditions for the first PCR were the same as those described above. Serially fivefold-diluted first PCR products were then subjected to nested PCR using pX inner primers (pXI 7341-7360, 5′-ACCCAGTCTACGTGTTTGGA-3′; pXI 7460-7441, 3′-TGATCTGATGCTCTGGACAG-5′). The PCR cycling conditions were 20 cycles of 93°C for 1 min, 60°C for 45 s, and 72°C for 1 min and one cycle of 72°C for 5 min. PCR products were detected as described above.
FIG. 4.
Effects of the gp21 peptide 400-429 and a neutralizing MAb against gp46 on the early stage of HTLV-1 infection. (A) Schematic representation of the experiments (Exp.) investigating HTLV-1 infection at the binding step or the postbinding step. Precooled MOLT-4 cells were inoculated with cell-free HTLV-1 at 4°C for 1 h (Exp. 2, binding step), washed, and resuspended in fresh culture medium. Then, the incubation temperature of the cells was shifted to 37°C for 20 h (Exp. 3, postbinding step). The reverse-transcribed HTLV-1 RNA in MOLT-4 cells was detected by nested PCR. (B) Inhibition of the postbinding step of HTLV-1 infection by the gp21 peptide 400-429. To detect the formation of HTLV-1 DNA, serially diluted first PCR products were used as templates for nested PCR amplification using the pXI 7341-7360 and pXI 7460-7441 primers (lanes 1 to 4). The temperatures at the time of addition of the gp21 peptide (pep.) 400-429 are indicated in panel A as hatched bars. Fiftyfold-diluted first PCR products were used as templates for nested PCR. The 120 bp of pX DNA in lanes 6 to 8 corresponds to Exp. 1 to 3 in panel A, respectively. (C) Inhibition of transmission of cell-free HTLV-1 at the binding step by anti-gp46 neutralizing MAb LAT-27. HTLV-1 was incubated with LAT-27 MAb (30 μg/ml) or control MAb against mouse recombinant IL-2 (30 μg/ml) for 1 h at 37°C prior to infection. Then, experiments were done as described above. Lane 1, no-MAb control. MAbs were added to MOLT-4 cells either at 4°C (lanes 2 and 4) or at 37°C (lanes 3 and 5). β-Globin DNA was amplified as a control. (D) Effect of the timing of the addition of the gp21 peptide 400-429 after adsorption of HTLV-1 to MOLT-4 cells on the transmission of cell-free HTLV-1. After adsorption of HTLV-1 to MOLT-4 cells at 4°C, the gp21 peptide 400-429 was added at the indicated times and the cells were cultured for 20 h in the presence of the peptide. Formation of HTLV-1 DNA in MOLT-4 cells was detected by nested PCR (120 bp of pX). β-Globin DNA was amplified as a control. Relative intensities of pX DNA bands were calculated by densitometry. Inhibition by the no-peptide control was assigned a value of 0%.
We confirmed that PCR was performed within the linear range (Fig. 4B, lanes 1 to 5) because correlation between DNA dilution and the intensities of HTLV-1 DNA was observed. When the gp21 peptide 400-429 was added 1 h before or during adsorption at 4°C (Fig. 4A, Exp. 2), no inhibition of the synthesis of HTLV-1 DNA was observed (Fig. 4B, lane 7), compared to the no-peptide control (Fig. 4B, lane 6). However, synthesis was inhibited when the peptide was added just before the postbinding step (Fig. 4B, lane 8), i.e., the peptide was present during incubation at 37°C for 20 h (Fig. 4A, Exp. 3). These findings suggested that the gp21 peptide 400-429 did not affect the binding step but inhibited the postbinding step of HTLV-1 infection.
The viral gp46 Env protein will recognize the specific cell surface receptor at the binding step. We examined whether rat MAb (LAT-27), which was reported to neutralize HTLV-1 infection and recognize the gp46 amino acids 191 to 196 (Leu-Pro-His-Ser-Asn-Leu) (40), blocked the binding step of HTLV-1 infection under our assay conditions. The purified rat MAb (LAT-27) and rat control MAb against recombinant mouse interleukin 2 (IL-2) were kindly provided by Y. Tanaka (Kitasato University, Kanagawa, Japan). Plating of HTLV-1, which had been preincubated with LAT-27 MAb (30 μg/ml), to MOLT-4 cells at 4°C (Fig. 4A, Exp. 2) was inhibited (Fig. 4C, lane 2). In contrast, HTLV-1 DNA was clearly detected when the LAT-27 MAb was added after adsorption of HTLV-1 to the cells but just before the shift of the incubation temperature to 37°C (Fig. 4A, Exp. 3) from 4°C (Fig. 4C, lane 3). Addition of the control rat MAb against recombinant mouse IL-2 did not inhibit the formation of HTLV-1 DNA (Fig. 4C, lanes 4 and 5).
To confirm that the gp21 peptide 400-429 inhibited the postbinding step of HTLV-1 infection, it was added at various time points after adsorption of virus to the cells at 4°C. As shown in Fig. 4D, 60% inhibition of the formation of HTLV-1 DNA was observed when the gp21 peptide 400-429 was added even 30 min after adsorption of HTLV-1 to the cells. These results strongly suggested that the gp21 peptide 400-429 inhibits the postbinding step of HTLV-1 infection.
In this study, we examined effects of synthetic peptides covering HTLV-1 Env proteins and anti-gp46 neutralizing MAb (LAT-27) on transmission of cell-free HTLV-1. We found that a synthetic peptide corresponding to amino acids 400 to 429 of the gp21 transmembrane protein (peptide 400-429) strongly inhibited infection by cell-free HTLV-1. We reported previously that this peptide inhibits syncytium formation induced by cocultivation with HTLV-1-producing cells (33), suggesting that the amino acid sequence 400 to 429 on the gp21 transmembrane protein is important for Env functions not only in cell-to-cell infection but also virus-to-cell infection (Table 2).
TABLE 2.
Inhibition of syncytium formation and infection of cell-free HTLV-1 by HTLV-1 Env synthetic peptides
Inhibition of syncytium formation induced by cocultivation of HTLV-1-negative MOLT-4 cells with HTLV-1-positive C91/PL cells. The cells were cultured for 2 days at 37°C in the presence of the peptides at 10 μM, and syncytia were counted. The inhibitory effect of each peptide was calculated in comparison with numbers of syncytia in the control coculture without a peptide.
MOLT-4 cells were infected with cell-free HTLV-1 in the presence of the peptides at 10 μM, and the formation of reverse-transcribed HTLV-1 RNA was detected by PCR. The intensities of PCR bands were determined by densitometry, and the inhibitory effect of each peptide was calculated in comparison with the no-peptide control.
8C cells were infected with the VSV(HTLV-1) pseudotype in the presence of the peptides at 10 μM, and plaques were counted 24 h later. The inhibitory effect of each peptide was calculated in comparison with the no-peptide control.
We also reported that LAT-27 MAb, which recognizes the gp46 amino acid sequence 191 to 196, inhibits transmission of cell-free HTLV-1 (12). In the present study, we showed that LAT-27 MAb inhibited the binding step of HTLV-1 infection in the PCR assay (Fig. 4). The LAT-27 MAb was also reported to inhibit the formation of syncytia and transformation of normal T lymphocytes by HTLV-1 in vitro (40). Therefore, LAT-27 MAb inhibits both the cell-to-cell and the virus-to-cell HTLV-1 infections.
In contrast to LAT-27 MAb, the gp46 peptide 197-216 hardly affected the entry of cell-free HTLV-1 into MOLT-4 cells (Fig. 1B), although it inhibited syncytium formation upon cocultivation (Table 2). Syncytium formation due to cell-to-cell fusion has been considered to be a strong predictor of virion-to-cell infection and has frequently been used to estimate the efficiency of virus entry. However, our findings suggested that inhibition of syncytium formation is not always associated with the inhibition of cell-free HTLV-1 infection. More recently, we identified the 71-kDa heat shock cognate protein (HSC70) as a component binding to the gp46 peptide 197-216 and showed that anti-HSC70 MAb blocks syncytium formation (35). It remains to be elucidated how HSC70 is involved in the cell-to-cell and virus-to-cell HTLV-1 infection.
The mechanism of action of the gp21 peptide 400-429 is likely the inhibition of the interaction between the HTLV-1 Env protein and the cellular receptor. More recently, we reported that a nonprotein component derived from MOLT-4 cells, possibly carbohydrate-containing lipids, interacts with the gp21 peptide 400-429 (34). This nonprotein component also binds to the mature gp21 protein purified from culture fluids of HTLV-1-producing cells and inhibits syncytium formation induced by cocultivation with HTLV-1-producing cells. By using 125I-labelled gp21, MOLT-4 cells have been shown to have 1,433 high-affinity binding sites per cell and 19,220 low-affinity sites per cell, with a Kd of 102 pM and 36.3 nM, respectively, for gp21 protein (34). We also reported the nature of a nonprotein component which binds to 125I-labelled gp21 even in the presence of large amounts of unlabelled gp21 (34). The concentration of the gp21 peptide 400-429 required for the inhibition of HTLV-1 infection is much higher than the Kd of the gp21-binding component. This might be due to the nature of the nonprotein component. The interaction between HTLV-1 Env protein and the nonprotein component on the cell surface may be necessary for both syncytium formation and transmission of cell-free HTLV-1.
We recently reported that human Clq, which is a component of the complement system, binds to the gp21 peptide 400-429 in the binding assay using HTLV-1 Env synthetic peptides (18). We also demonstrated that human Clq binds to the HTLV-1 virion and inhibits its infectivity (18). Since the inhibitory effect of the gp21 peptide 400-429 was observed at a postbinding step of HTLV-1 infection (Fig. 4), the amino acid sequence corresponding to the gp21 400-429 might play an important role as a binding site for the human Clq to inhibit the infection of cell-free HTLV-1, especially at the postbinding step.
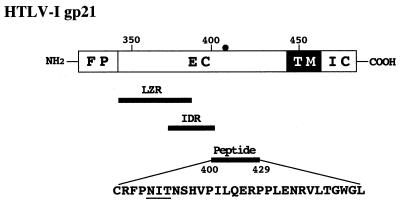
It has been shown that gp21 is responsible for the fusion between virions and target cell membranes (7, 32). Several regions of the transmembrane protein of retroviruses, including HTLV-1 (5), have been reported to be involved in the conformational rearrangements required for the fusion process (37) (Fig. 5). Namely, three motifs are highly conserved in the retroviral ectodomain of transmembrane glycoproteins: (i) an amino-terminal hydrophobic stretch, which has characteristics of a fusion peptide and is probably directly involved in the membrane fusion process (17, 43), (ii) a leucine zipper-like region which exhibits an amphipathic alpha-helical secondary structure capable of self-association as a coiled coil (6), and (iii) a sequence described as an immunodominant region in retroviruses, which contains cysteine residues thought to form an intramolecular disulfide bridge (2, 37).
FIG. 5.
Schematic representation of the HTLV-1 Env glycoprotein gp21 and the amino acid sequence of the gp21 peptide 400-429. The fusion peptide domain (FP), extracellular domain (EC), transmembrane domain (TM), and intracellular domain (IC) are indicated. The positions of the leucine zipper-like region (LZR) (amino acids 340 to 392) and the immunodominant region (IDR) (amino acids 377 to 402) are also indicated. The N-glycosylation site of HTLV-1 Env gp21 (at position 404) (closed circle) is underlined.
In the case of HIV-1, the gp41 transmembrane Env protein plays an important role in fusion between virus and cell membranes (23). It has been proposed that the assembly of viral Env glycoprotein oligomers is required for the viral fusion reaction (22). On the basis of the recently determined high-resolution crystal structure of the HIV-1 gp41 core structures (1, 42), the two domains of gp41 are considered to be necessary for formation of alpha-helical oligomers. One of these domains was predicted to contain a leucine zipper-like region in the NH2-terminal region of the gp41 molecule. The other domain is located in the COOH-terminal end of the gp41 ectodomain. A synthetic peptide (DP178) corresponding to the COOH-terminal ectodomain sequence (36 amino acid residues 643 to 678 of the HIV-1LAI isolate) inhibits both virus-mediated cell-cell fusion and infection of cell-free virus (44). The inhibitory effect of the DP178 peptide on the membrane fusion process has been explained by the finding that it binds to the leucine zipper-like region in gp41 and prevents formation of the heteromeric coiled-coil structure of gp41 (22, 24, 31).
Both the HTLV-1 gp21 peptide 400-429 and the HIV-1 DP178 peptide are located between the immunodominant regions and the transmembrane domains in the ectodomain of the Env transmembrane glycoprotein (Fig. 5). However, no significant amino acid homology between these peptides was observed, and HIV-1 infection was not inhibited by the gp21 peptide 400-429 (Fig. 2D). Moreover, when the gp21 peptide 400-429 was incubated with the synthetic peptides including the leucine zipper-like region (gp21 peptides 313-333, 332-352, 350-386, and 382-403) before cocultivation of HTLV-1-positive cells with HTLV-1-negative MOLT-4 cells, the inhibitory effect of the gp21 peptide 400-429 on the formation of syncytia was not abrogated (data not shown). Thus, the action of the gp21 peptide 400-429 might differ from that of the DP178 peptide in the course of virus infection, especially at the postbinding step.
More recently, the crystal structure of the gp21 ectodomain was reported to have been determined (20). The molecular surface of gp21 was shown to be buried between the trimeric coiled coil of gp21 (residues 338 to 387) and the COOH-terminal segment (residues 390 to 421), forming the structure specific for gp21 (20). The amino acid region from 390 to 421 at the surface area of the gp21 molecule might be involved in interaction of gp21 with a nonprotein component on the surface of target cells. The gp21 peptide 400-429 may be useful for development of therapeutic agents against HTLV-1 and as a tool to study fusion between the viral membrane and the target cell membrane.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Science and Culture and the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Chan D C, Fass D, Berger J M, Kim P S. Core structure from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 2.Cianciolo G J, Copeland T D, Oroszlan S, Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985;230:453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- 3.Clapham P, Nagy K, Cheingsong-Popov R, Exley M, Weiss R A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983;222:1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- 4.Dales S, Hanafusa H. Penetration and intracellular release of the genomes of avian RNA tumor viruses. Virology. 1972;50:440–458. doi: 10.1016/0042-6822(72)90396-0. [DOI] [PubMed] [Google Scholar]
- 5.Delamare L, Rosenberg A R, Pique C, Pham D, Callebaut I, Dokhelar M-C. The HTLV-1 envelope glycoproteins: structure and functions. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:S85–S91. doi: 10.1097/00042560-199600001-00015. [DOI] [PubMed] [Google Scholar]
- 6.Delwart E L, Mosialos G, Gilmore T. Retroviral envelope glycoproteins contain a “leucine zipper”-like repeat. AIDS Res Hum Retroviruses. 1990;6:703–706. doi: 10.1089/aid.1990.6.703. [DOI] [PubMed] [Google Scholar]
- 7.Denesvre C, Sonigo P, Corbin A, Ellerbrok H, Sitbon M. Influence of transmembrane domains on the fusogenic abilities of human and murine leukemia retrovirus envelopes. J Virol. 1995;69:4149–4157. doi: 10.1128/jvi.69.7.4149-4157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derse D, Mikovits J, Polianova M, Felver B K, Ruscetti F. Virions released from cells transfected with a molecular clone of human T-cell leukemia virus type 1 give rise to primary and secondary infections of T cells. J Virol. 1995;69:1907–1912. doi: 10.1128/jvi.69.3.1907-1912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan N, Gavalchin J, Paul B, Wells K H, Lane M J, Poies B J. Infection of peripheral blood mononuclear cells and cell lines by cell-free human T-cell lymphoma/leukemia virus type I. J Clin Microbiol. 1992;30:905–910. doi: 10.1128/jcm.30.4.905-910.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischinger P J, Peebles T, Nomura S, Haapala D K. Isolation of RD114-like oncornavirus from a cat cell line. J Virol. 1973;11:978–985. doi: 10.1128/jvi.11.6.978-985.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gessain A, Barin, Vernent J C, Gout O, Maurs L, Calender A, de-The G. Antibodies to human T-lymohotropic virus type I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 12.Haraguchi Y, Yang D-W, Handa A, Shimizu N, Tanaka N Y, Hoshino H. Detection of neutralizing antibodies against human T-cell leukemia virus type I using a cell-free infection system and polymerase chain reaction. Int J Cancer. 1994;59:416–421. doi: 10.1002/ijc.2910590321. [DOI] [PubMed] [Google Scholar]
- 13.Haraguchi Y, Jinno A, Takeuchi Y, Hoshino H. Different properties of human T-cell leukemia virus type I prepared from 8C cat cells. Leukemia. 1997;11:47–49. [PubMed] [Google Scholar]
- 14.Hinuma Y, Nagata K, Nanaoka M, Matsumoti T, Kinoshita K, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino H, Esumi H, Miwa M, Shinoyama M, Minato K, Tobinai K, Hirose M, Watanabe S, Inada N, Kinoshita K, Kamihara S, Icimaru M, Sugimura T. Establishment and characterization of 10 cell lines derived from patients with adult T-cell leukemia. Proc Natl Acad Sci USA. 1983;80:6061–6065. doi: 10.1073/pnas.80.19.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino H, Shimoyama M, Miwa M, Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult-T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci USA. 1983;80:7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter E, Swanstorm U R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda F, Haraguchi Y, Jinno A, Iino Y, Morishita Y, Shiraki H, Hoshino H. Human complement Clq inhibits the infectivity of cell-free HTLV-1. J Immunol. 1998;161:5712–5719. [PubMed] [Google Scholar]
- 19.Jacobson S, Raine C S, Mingiolio E S, McFarlin D E. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature (London) 1988;331:540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- 20.Kobe B, Center R J, Kemp B E, Poumbourios P. Crystal structure of human T cell leukemia virus type 1 gp21 ectodomain crystallized as a maltose-binding protein chimera reveals structural evolution of retroviral transmembrane proteins. Proc Natl Acad Sci USA. 1999;96:4319–4324. doi: 10.1073/pnas.96.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krichbaum-Stenger K, Poiesz B J, Keller P, Ehrlich G, Gavalchin J, Davis B H, Moore J L. Specific adsorption of HTLV-1 to various target human and animal cells. Blood. 1987;70:1303–1311. [PubMed] [Google Scholar]
- 22.Matthews T J, Wild C, Chen C-H, Bolognesi D P, Greenberg M L. Structural rearrangements in the transmembrane glycoprotein after receptor binding. Immunol Rev. 1994;140:93–104. doi: 10.1111/j.1600-065x.1994.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 23.Moore J P, Jameson B A, Weiss R A, Sattentau Q J. The HIV-cell fusion reaction. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press; 1993. pp. 233–289. [Google Scholar]
- 24.Munoz-Barroso I, Durell S, Sakaguchi K, Apella E, Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy K, Clapham P, Cheingsons-Popov R, Weiss R. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patient’s sera. Int J Cancer. 1983;32:321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- 26.Osame M, Usuku K, Izumo S, Ijuchi N, Amitani H, Igata A, Matsumoto M, Tata M. HTLV-1 associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 27.Pique C, Pham D, Tursz T, Dokhelar M-C. Human T-cell leukemia virus type 1 envelope protein maturation process: requirements for syncytium formation. J Virol. 1992;66:906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piraino F. The mechanism of genetic resistance of chick embryo cells to infection by Rous sarcoma virus-Bryan strain (BS-RSV) Virology. 1967;32:700–707. doi: 10.1016/0042-6822(67)90046-3. [DOI] [PubMed] [Google Scholar]
- 29.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiros E, Garcia F, Maroto M C, Bernal M C, Cabezas T, Piedrola G. Human immunodeficiency virus type-1 can be detected in monocytes by polymerase chain reaction. J Med Microbiol. 1995;42:411–414. doi: 10.1099/00222615-42-6-411. [DOI] [PubMed] [Google Scholar]
- 31.Rimsky L T, Shugars D C, Matthews T J. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J Virol. 1998;72:986–993. doi: 10.1128/jvi.72.2.986-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg A R, Delamarre L, Pique C, Pham D, Dokhelar M-C. The ectodomain of the human T-cell leukemia virus type I TM glycoprotein is involved in postfusion events. J Virol. 1997;71:7180–7186. doi: 10.1128/jvi.71.10.7180-7186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagara Y, Inoue Y, Shiraki H, Jinno A, Hoshino H, Maeda Y. Identification and mapping of functional domains on human T-cell lymphotropic virus type I envelope proteins by using synthetic peptides. J Virol. 1996;70:1564–1569. doi: 10.1128/jvi.70.3.1564-1569.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda Y. Trypsin-sensitive and resistant components in human T-cell membranes required for syncytium formation by human T-cell lymphotropic virus type I-bearing cells. J Virol. 1997;71:601–607. doi: 10.1128/jvi.71.1.601-607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda Y. 71-kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J Virol. 1998;72:535–541. doi: 10.1128/jvi.72.1.535-541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saiki R, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullins K B, Erlich H A. Primer-directed enzyme amplification of DNA with thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 37.Schulz T F, Jameson B A, Lopalco L, Siccardi A G, Weiss R A, Moore J P. Conserved structural features in the interaction between retroviral surface and transmembrane glycoproteins? AIDS Res Hum Retroviruses. 1992;8:1571–1580. doi: 10.1089/aid.1992.8.1571. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava B I, Minowada J. Terminal deoxynucleotidyl transferase activity in a cell line (Molt-4) derived from the peripheral blood of a patient with acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1978;51:529–535. doi: 10.1016/0006-291x(73)91346-6. [DOI] [PubMed] [Google Scholar]
- 39.Steck F T, Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966;29:642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Zeng L, Shiraki H, Shida H, Tozawa H. Identification of a neutralization epitope on the gp46 antigen of human T cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J Immunol. 1991;147:354–360. [PubMed] [Google Scholar]
- 41.Weiss R A, Clapham P, Nagy K, Hoshino H. Envelope properties of human T-cell leukemia viruses. Curr Top Microbiol Immunol. 1985;115:235–246. doi: 10.1007/978-3-642-70113-9_15. [DOI] [PubMed] [Google Scholar]
- 42.Weissenhorn W, Desse A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–428. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 43.White J M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- 44.Wild C T, Shugars D C, Greenwell T K, McDanal C B, Matthews T J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]