Abstract
Leukemia is a malignant tumor of the hematologic system. Studies have shown that cernuumolide J (TMJ-105), an extract of Carpesium cernuum, has anti-cancer effects, but the underlying mechanism is unclear. In this study, we investigated the effect of TMJ-105 on the proliferation of human leukemia HEL cells and its molecular mechanism. MTT analysis showed TMJ-105 had revealed that it shows significant IC50 in HEL cells at lower doses (1.79 ± 0.29 μmol/L) than in K562 cells (3.89 ± 0.80 μmol/L), and the suppression of HEL cell proliferation was time- and concentration-dependent. Meanwhile, TMJ-105 induced G2/M phase blockage, leading to DNA damage in HEL cells. TMJ-105 promoted HEL cells to release of reactive oxygen species (ROS) and changed mitochondrial membrane potential (MMP). Furthermore, TMJ-105 induced apoptosis by upregulating the cleaved-caspase9 and cleaved-caspase3 protein expression, while caspase pan inhibitor (Z-VAD-FMK) blocked the inhibition effect. Finally, TMJ-105 downregulated the phosphorylation of JAK2, STAT3 and Erk, and activated the phosphorylation of JNK and p38. Collectively, these results demonstrated that TMJ-105 inhibited proliferation of leukemia cells and the underlying mechanism via the JAK2/STAT3 axis and MAPKs signaling pathway. Based on these results, the present study suggested the sesquiterpene lactone TMJ-105 is a new chemotherapeutic agent for the treatment of leukemia.
Keywords: Carpesium cernuum, TMJ-105, Leukemia, Apoptosis, JAK2/STAT3, MAPKs
1. Introduction
Cancer is a dominant public health issue worldwide and one of the leading causes of death [1,2]. Since 1975, the overall incidence of cancer has increased in children and adolescents at a rate of 1% per year. However, the types of cancer and their distribution vary. Leukemia is the most common cancer in children, accounting for approximate 28% [1]. Leukemia is a heterogeneous group of blood cancers caused by abnormal proliferation of developing leukocytes [3]. In addition to classical chemotherapy and allogeneic stem cell transplantation, molecular targeting is also being used for treating leukemia. Nevertheless, the current strategies of leukemia treatment are limited by drug resistance, side effects and high expense. Therefore, it is urgent to find new low-cost alternatives, such as anticancer activities compounds of natural origin [3,4].
Dysfunction of normal cell death pathways could cause many diseases, including cancer. The Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), and mitogen-activated protein kinases (MAPKs) are highly conserved pathways, which have been shown to play a vital role in carcinogenesis and regulate a variety of biological functions, such as cell growth, apoptosis and metastasis [[5], [6], [7], [8]]. Phosphorylated STAT3, activated by JAK2, is translocated to the nucleus, and interacting with MAPKs signaling pathways, which are involved in the proliferation of cancer cells. Inhibition of the JAK2/STAT3 pathway could arrest the cell cycle of cancer and mediate the mitochondrial apoptosis pathway to induce apoptosis of cancer cells. Moreover, MAPKs including Erk, JNK and p38, are evolutionarily conserved enzymes in intracellular regulatory targets. Studies have shown that activation of the MAPKs pathway triggers the apoptosis of cancer cells through the mitochondrial apoptosis pathway and induces G2/M arrest [[9], [10], [11]]. Dysregulation of the cell cycle causes uncontrolled proliferation of cells, which is a prerequisite for cancer formation [12,13]. The cell cycle mechanism plays a vital role in regulating the progression from apoptosis to mitosis to growth [14]. Apoptosis is the genetically controlled process of autonomous and orderly cell death, which maintains the stability of the intracellular environment. Therefore, controlling the cell cycle and restoring the programmed death function of cancer cells are an important way to control cancer occurrence and development.
Traditional Chinese medicine (TCM) has been identified as a promising avenue for anti-cancer drug discovery and integrative oncology [15,16]. Previous studies shown that TCM have the potential to boost the efficiency and reduce the adverse consequences of chemotherapies, as well as induce apoptosis in cancerous cells [[17], [18], [19]]. Carpesium cernuum (Fig. 1A) is a TCM for analgesia, detoxification and anti-inflammation. Previous studies have evidenced that the main components of isolated extracts from Carpesium cernuum are sesquiterpenoids, which have exhibited anticancer activities [[20], [21], [22]]. It has been reported that incaspitolide A extracted from Carpesium cernuum has excellent anti-leukemia activity [22,23]. Studies have shown that cernuumolide J (TMJ-105, Fig. 1B) is an analogue of incaspitolide A, which has anti-cancer activity against breast cancer, non-small cell lung cancer, colorectal cancer, hepatocellular carcinoma and leukemia [20,21], but the underlying mechanism of its against leukemia is unclear. In this study we demonstrated that TMJ-105 has excellent activity against leukemia HEL cells. TMJ-105 induced HEL cell cycle arrest and apoptosis via inhibiting JAK2/STAT3 and activating MAPKs signaling pathway, and could be a potential anti-tumor candidate in leukemia.
Fig. 1.
TMJ-105 inhibited the proliferation of HEL cells. (A) Carpesium cernuum. (B) Chemical structure of TMJ-105. (C) The IC50 values of TMJ-105 in human leukemia cell lines HEL and K562 at 72 h. (D) HEL cells were treated with different concentrations of TMJ-105 (0.5, 1, 2, 4 and 8 μmol/L, and DMSO as control) for 72 h, and the inhibition rate was measured using MTT assay. (E) The effects of TMJ-105 on HEL cells proliferation was detected by the MTT assay. (F) Cell morphology of HEL cells after treatment with different concentrations of TMJ-105 for 24 and 48 h (Magnification × 400, Scale bar: 50 μm). Data were denoted by means ± SD (n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group).
2. Materials and methods
2.1. Cell culture
Human leukemia cell lines HEL and K562 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured with 1640 RPMI medium containing 5% fetal bovine serum (FBS) in a humidified incubator containing 5% CO2 at 37 °C.
2.2. Cell viability assay
Cells (7 × 104) were inoculated in 96-well culture plates. After 4 h, cells were treated with different concentrations of TMJ-105 for the indicated times. Then, MTT assay was used to detect the absorbance. The absorbance value at 570 nm was analyzed on a multiwell spectrophotometer. The IC50 was assessed from growth curves.
2.3. Flow cytometry analysis and cell morphology analysis
Cells (3 × 105) were seeded in 6-well culture plates. After 4 h, they were treated with TMJ-105 for 12 h and 24 h. Cells were collected and washed twice with pre-cooled PBS. For cell cycle analysis, cells were re-suspended with 500 μL of 70% ethanol and fixed overnight at −20 °C. After washing, 500 μL of dye solution (0.1 mg/mL RNase, 50 μg/mL PI and 0.05% Triton100) was added and stained for 15 min at room temperature away from light, and analyzed by flow cytometry. For analysis of apoptosis, cells were resuspended with 80 μL of 1 × binding buffer, and added 1.5 μL FITC-Annexin V and PI respectively, incubated for 15 min at room temperature, and then analyzed by flow cytometry. Cell morphology was observed and photographed with an inverted microscope.
2.4. Hoechst 33258 staining
Cells (3 × 105) were seeded in 6-well culture plates. After 4 h, they were treated with TMJ-105 for 24 h. Cells were collected, washed twice with PBS, dyed with Hoechst 33258 (Beyotime, Jiangsu, China) for 15 min, and finally observed under fluorescence microscopy.
2.5. Detection of mitochondrial membrane potential (MMP)
Cells (3 × 105) were cultured in a 6-well culture plates. JC-1 was used as a probe to determine the variations of MMP after treatment with TMJ-105 for 24 h. Cells were collected, which were re-suspended with 0.5 mL of 1640 RPMI medium and incubated with 0.5 mL of JC-1 staining solution for 20 min at 37 °C. Then, the cells were washed three times with 1 × JC-1 staining buffer. Lastly, they were re-suspended with 0.5 mL of 1 × JC-1 staining buffer, analyzed and photographed with a fluorescence microscope.
2.6. Detection of reactive oxygen species (ROS)
Cells (3 × 105) were cultured in a 6-well culture plates. DCFH-DA was used as the probe to explore the level of ROS after 12 h treatment with TMJ-105. The cells were harvested, and the probe was loaded at 37 °C in dark room for 20 min, followed by washing and observation and imaging by fluorescence microscope.
2.7. Western blot assay
Cells (9 × 105) were inoculated in 60-mm dishes, treated with TMJ-105 for 12 h. Cells were collected and lysed on ice for 30 min after mixing with protein lysis buffer. Protein samples (50 μg) were isolated with SDS-PAGE and shifted to PVDF membranes by wet transfer. The membranes were sealed with 3% BSA for 1 h at room temperature, and the corresponding primary antibodies were incubated overnight at 4 °C. Primary antibodies were Chk2 (ab109413), c-Myc (ab32072), Cyclin B1 (ab32053), cyclin-dependent kinase 1 (CDK1, ab133327), extracellular signal-regulated kinase (Erk, ab184699), p-Erk (ab32538), p38 (ab47363), p-p38 (ab178867), JAK2 (ab108596), STAT3 (ab119352) and p-STAT3 (ab76315) (Abcam, UK); p53 (2527S), p21 (2947S), p-CDC2 (45395), Caspase3 (9662S), Caspase9 (7237S), poly (ADP-ribose) polymerase (PARP, 9542S), c-Jun NH2 terminal kinase (JNK, 9252T), and p-JNK (4668T) were purchased from Cell Signaling Technology (CST, USA); p-JAK2 (381556) and GAPDH (AF7021) were purchased from ZEN-BIO (Chengdu, China) and Affinity Biosciences (USA) respectively. Next, the membranes were washed with TBS and incubated with secondary antibody Anti-rabbit IgG (H + L) (DyLight™ 800 4X PEG Conjugate) (5151S, CST, USA) for 1.5 h at room temperature in the dark. Lastly, the membranes were scanned and quantified using the Odyssey Platform (LI-COR Biosciences, Lincoln, NE, USA). GAPDH was taken as a control.
2.8. Statistical analysis
All experiments were at least three independent replications. The data were denoted by means ± standard error and p-values (*p < 0.05, **p < 0.01, ***p < 0.001) represent statistical significance. All statistical analyses were performed by two-tailed Student t-test or two-way ANOVA with Tukey’s post-hoc test, using GraphPad Prism 9.0.
3. Result
3.1. TMJ-105 inhibited the proliferation of HEL cells
To study the anti-leukemia activity of TMJ-105 (Fig. 1A and B), MTT assay was used to detect its effect on cells viability. The results showed that it inhibited the proliferation of human leukemia cells HEL (Fig. 1D) and K562 (S1 A), and the IC50 values were 1.79 ± 0.29 μmol/L and 3.89 ± 0.80 μmol/L, respectively (Fig. 1C). Meanwhile, the results of cell growth curve showed that TMJ-105 significantly reduced the viability of HEL (Fig. 1E) and K562 (S1 B) cells in a concentration- and time-dependent manner. It is noteworthy that TMJ-105 showed better anti-leukemia activity against HEL cells than K562 cells. Therefore, we selected HEL cells for further studies. In addition, we have observed that TMJ-105 induced significant morphological changes in HEL cells (Fig. 1F). TMJ-105 caused HEL cells condensation and debris in a dose- and time-dependent manner. In short, the datas suggested that TMJ-105 effectively suppressed the proliferation of leukemia cells.
3.2. TMJ-105 induced G2/M phase arrest in HEL cells
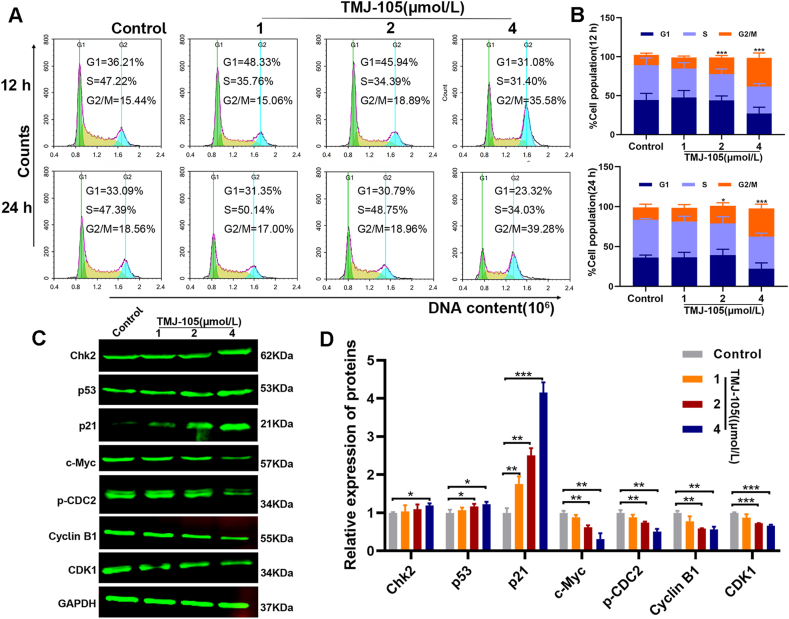
To evaluate whether TMJ-105 affects the cell cycle of HEL cells, the content of cellular DNA after TMJ-105 treatment was analyzed by flow cytometry. The results showed that DNA was accumulated in the G2/M phase of the cells after TMJ-105 treatment, with concentration- and time-dependence (Fig. 2A and B). In particular, the accumulation of DNA content was more remarkable at 4 μmol/L treatments of TMJ-105 after 24 h. It was implied that TMJ-105 induces DNA damage and arrests G2/M phase. Moreover, it also induced G2/M phase arrest in K562 cells at 24 h (S2). To understand the underlying molecular mechanism of TMJ-105 induced G2/M phase arrest in HEL cells, we detected changes in cell cycle regulatory factors by Western blot. The findings showed that Chk2, p53, and p21 were evidently up-regulated; and c-Myc, p-CDC2, Cyclin B1, and CDK1 were significantly down-regulated after TMJ-105 treatment (Fig. 2C and D). These results suggested that suppression of cell proliferation by TMJ-105 is associated with cell cycle arrest.
Fig. 2.
TMJ-105 dose- and time-dependent induced G2/M phase arrest in HEL cells. (A) HEL cells were treated with various concentrations of TMJ-105 (1, 2 and 4 μmol/L, and DMSO as control) for 12 and 24 h. Cell cycle phase distribution was detected by flow cytometry. (B) Quantification of cell cycle distribution in G1, S, and G2 phases. (C) HEL cells were treated with various concentrations of TMJ-105 for 12 h. Cell cycle regulatory factors were determined by Western blot. (D) Quantification of cell cycle regulatory factors. Data were denoted by means ± SD (n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group).
3.3. TMJ-105 promoted the apoptosis of HEL cells
To further explore whether TMJ-105 caused cell death through apoptosis, we investigated apoptosis by flow cytometry after treating HEL cells with TMJ-105. The findings displayed that TMJ-105 promoted apoptosis in a concentration- and time-dependent manner (Fig. 3A and B). The apoptosis rate exceeded 30% after 24 h in TMJ-105 (4 μmol/L) (25.34% for early apoptosis and 5.88% for late apoptosis). Moreover, it also induced apoptosis of K562 cells (S3). Simultaneously, we also observed the morphological characteristics of apoptotic cells under the fluorescence microscope. After 24 h of treatment with different concentrations of TMJ-105, the Hoechst 33258 dyeing displayed that DNA damage occurred in the nucleus. As the concentration increases, the nuclei became denser and showed more bright blue fluorescence (Fig. 3C and D).
Fig. 3.
TMJ-105 dose- and time-dependent induced apoptosis in HEL cells. (A) HEL cells were treated with various concentrations of TMJ-105 (1, 2 and 4 μmol/L, DMSO as control) for 12 and 24 h The apoptosis of HEL cells were analyzed with the Annexin V-FITC/PI double staining assay. (B) Quantification of the apoptosis Percentage. (C) HEL cells were treated with TMJ-105 for 24 h. DNA damage was detected by Hoechst 33258 staining (the white arrow represents apoptotic cells) (Magnification × 200, Scale bar: 100 μm). (D) Quantification of fluorescence levels indicated the extent of DNA damage. Data were denoted by means ± SD (n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group).
3.4. TMJ-105 downregulated MMP and caused the release of ROS
To explore whether mitochondria of HEL cells were affected by TMJ-105. We assayed the variation of MMP by JC-1 staining after treatment with TMJ-105 for 24 h. The results revealed that TMJ-105 excited green fluorescence and increased the positive percentage with increasing concentration (Fig. 4A–D). At the same time, we also determined the level of ROS after TMJ-105 treatment for 12 h. The results demonstrated that TMJ-105 triggered the release of ROS in a dose-dependent manner (Fig. 4E and F). These studies suggested that TMJ-105 caused mitochondrial damage.
Fig. 4.
The variation of MMP and ROS after treatment in HEL cells. (A) HEL cells were treated with various concentrations of TMJ-105 (1, 2 and 4 μmol/L, DMSO as control) for 24 h and then stained with JC-1 staining buffer. Mitochondrial membrane potential was assessed by fluorescence microscopy (Magnification × 200; Scale bar: 100 μm). (B) Quantification of the JC-1 aggregates. (C) Quantification of the JC-1 monomers. (D) Quantification of the JC-1 ratio of monomers/aggregate. (E) HEL cells were treated with TMJ-105 for 12 h and then incubated with DCFH-DA probe. ROS level was determined by fluorescence microscopy (Magnification × 100; Scale bar: 50 μm). (F) Quantification of DCF fluorescence levels. Data were denoted by means ± SD (n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group).
3.5. TMJ-105 promoted HEL cell apoptosis via activating caspase
The above experimental results suggested that TMJ-105 induced apoptosis by disrupting mitochondria. The activation of caspase is a hallmark of apoptosis, therefore we detected caspase-related proteins. Western blot results revealed that the expression levels of Cleaved-Caspase3, Cleaved-Caspase9 and Cleaved-PARP were upregulated after TMJ-105 treatment for 12 h (Fig. 5A and B). Next, we assayed whether the inhibition of caspases affected the apoptosis induced by TMJ-105 on HEL cells. We found that Z-VAD-FMK (Z-VAD, 50 μmol/L), an inhibitor of pan-caspase, which remarkably decreased the ability of TMJ-105 to exert apoptotic effects on HEL cells (Fig. 5C and D). Moreover, Cleaved-Caspase3 and Cleaved-PARP were also significantly downregulated (Fig. 5E and F). In summary, TMJ-105 induced apoptosis in HEL cells by activating caspases.
Fig. 5.
TMJ-105 promoted HEL cell apoptosis via activating caspase. (A) HEL cells were treated with various concentrations of TMJ-105 (1, 2 and 4 μmol/L, DMSO as control) for 12 h. The expression levels of Caspase9, Cleaved-Caspase9, Caspase3, Cleaved-Caspase3, PARP, and Cleaved-PARP were determined by Western blot. (B) Quantification of Caspase-related proteins. (C) Z-VAD-FMK (Z-VAD, 50 μmol/L) reversed the apoptosis effect of TMJ-105 (4 μmol/L) on HEL cells, after treatment with TMJ-105 and/or Z-VAD at 24 h. (D) Quantification of apoptosis after treatment with TMJ-105 (4 μmol/L) and/or Z-VAD (50 μmol/L). (E) TMJ-105 (4 μmol/L) upregulated the protein expression of Cleaved-Caspase3 and Cleaved-PARP in HEL cells, which were blocked by Z-VAD (50 μmol/L), after treatment with TMJ-105 and/or Z-VAD at 12 h. (F) Quantification of Caspase3, Cleaved-Caspase3, PARP and Cleaved-PARP. Data were denoted by means ± SD (n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group).
3.6. TMJ-105 mediated cycle and apoptosis by JAK2/STAT3 and MAPKs pathways
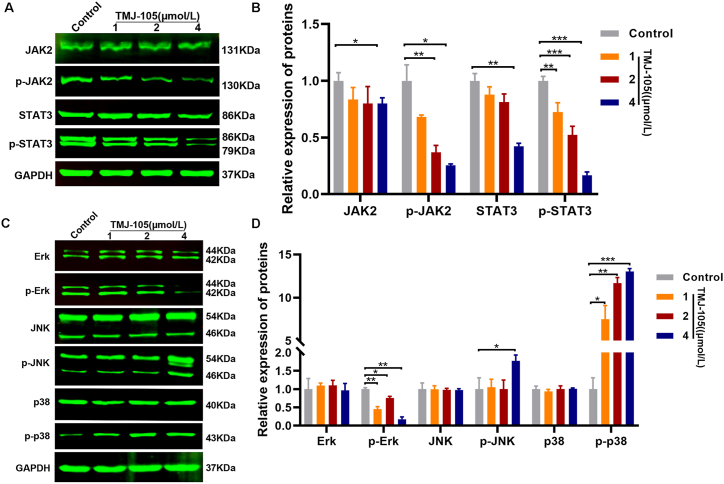
Previous studies have shown that abnormal stimulation of JAK2/STAT3 and MAPKs signals are closely related to the survival and apoptosis of cancer cells [24]. Therefore, we utilized Western blot to detect the relevant proteins. The results showed that TMJ-105 notably downregulated the expression of p-JAK2, p-STAT3 and p-Erk (Fig. 6A–D). Meanwhile, TMJ-105 significantly upregulated the expression of p-JNK and p-p38 (Fig. 6C and D). These suggested that TMJ-105 increased apoptosis and cycle arrest in HEL cells by inactivating JAK2 and STAT3, and activating JNK and p38.
Fig. 6.
The effect of TMJ-105 on JAK2/STAT3 and MAPK pathway. (A) HEL cells were treated with various concentrations of TMJ-105 (1, 2 and 4 μmol/L, DMSO as control) for 12 h. The expression levels of JAK2, p-JAK2, STAT3 and p-STAT3 were determined by Western blot. (B) Quantification of JAK2, p-JAK2, STAT3 and p-STAT3. (C) The Western blot of pathway consists of three major subfamilies: ERK, P38 and JNK. (D) Quantification of Erk, p-Erk, p38, p-p38, JNK and p-JNK. Data were denoted by means ± SD (n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group).
4. Discussion
Active phytochemicals found in herbs have shown the potential to inhibit cancer cell growth and induce apoptosis, making them a promising research field for preclinical and clinical research [25]. For instance, the compound curcumin, found in turmeric, has been shown to possess anti-inflammatory and anticancer properties. Increasing evidence suggests that curcumin affects a variety of cellular and molecular pathways, including apoptosis, MAPK, p53 and microRNA [26,27]. In addition, flavonoids or alkaloids exhibit anticancer activity through a variety of molecular signal transduction pathways or cell cycle disturbance [25]. In the present study, we found that TMJ-105 showed excellent anti-leukemia activity, a compound isolated from Carpesium cernuum. Interestingly, TMJ-105 induced G2/M phase arrest and apoptosis in HEL cells. These effects are closely associated with the activation of caspase family proteins, reduction of MMP, stimulation of ROS release, inhibition of JAK2/STAT3 and activation MAPKs signal pathways.
The occurrence of tumors is attributed to the combined effects of genetic factors, environmental factors and gene mutations, which destroy the homeostasis between cell proliferation and death, leading to the uncontrolled growth of tumor cells. Therefore, apoptosis and cycle retardation are usually used as two indicators of anti-tumor evaluation in the study [28,29]. We confirmed that TMJ-105 has the activity of suppressing the proliferation of HEL and K562 cells. The MTT results revealed that TMJ-105 inhibited HEL and K562 in a concentration- and time-dependent manner, IC50 of 1.79 ± 0.29 μmol/L and 3.89 ± 0.80 μmol/L at 72 h, respectively (Fig. 1C and D; Supplementary Figure1 A). More importantly, flow cytometry analysis demonstrated that TMJ-105 induced apoptosis and G2/M phase blockade in HEL cells. Those suggested that TMJ-105 affected the initiation and development of HEL cells.
Caspase could be activated by the mitochondrial or intrinsic pathway, which is related to the deprivation of MMP [27,30]. Previous studies have shown that activating the caspase cascade plays an essential in the mechanism of apoptosis [31,32]. Our investigation indicated that TMJ-105 induced apoptosis by decreasing MMP, activating Caspase9 and Caspase3. We further used the caspase pan inhibitor (Z-VAD-FMK) to attenuate the apoptosis of HEL cells induced by TMJ-105, and concomitantly decreased the expression of Cleaved-caspase3 and Cleaved-PARP. In the meantime, any intrinsic pathway triggered may cause cell cycle arrest [33]. Our findings demonstrated that TMJ-105 induced G2/M phase arrest by up-regulating Chk2, p21 and p53, while there was a down-regulation of c-Myc, p-CDC2, Cyclin B1, and CDK1. These results demonstrated that TMJ-105 induced apoptosis and cycle arrest in HEL cells through intrinsic pathways.
JAK2/STAT3 and MAPKs signaling pathways are extensively studied in tumors. In recent years, many studies have suggested that targeted inhibition of the JAK2/STAT3 and activation of MAPKs pathway suppress proliferation and promote apoptosis in cancer cells [[34], [35], [36], [37], [38]]. In the process of apoptosis, the increase of intracellular ROS level is directly related to apoptosis and causes mitochondrial DNA strand breaks and DNA degradation [39,40]. It has been demonstrated that increasing the phosphorylation levels of JNK and p38, and decreasing phosphorylated levels of JAK2 and STAT3 could effectively reduce MMP and increase ROS content in cells, then inducing apoptosis [35,39,41,42]. Our study indicated that TMJ-105 notably up-regulated the expression of p-JNK and p-p38, and remarkably down-regulated the expression of p-STAT3, p-JAK2 and p-Erk in HEL cells. Moreover, TMJ-105 caused the decrease of MMP and the release of ROS. The results suggested that TMJ-105 mediated apoptosis and cell cycle by inhibiting the JAK2/STAT3 and MAPKs singnal pathways.
However, this study does have some limitations. First, although it was found that TMJ-105 regulates JAK2/STAT3 and MAPK signaling pathways, its specific mechanism has not been further studied. Second, TMJ-105 has demonstrated anti-leukemia activity in vitro but has not been evaluated for anti-leukemia efficacy in vivo. Therefore, additional comprehensive studies are warranted to validate and elucidate these aspects.
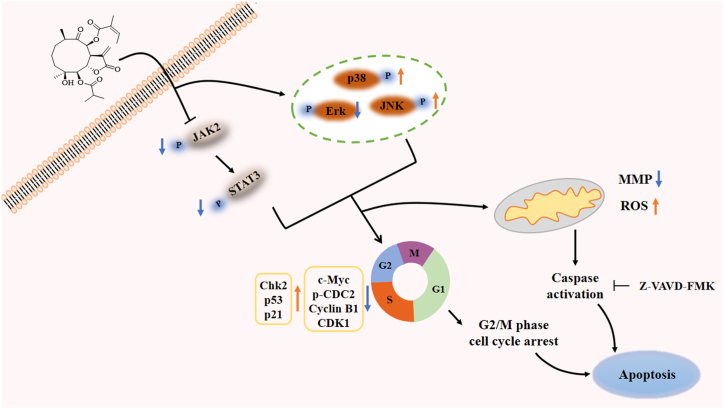
To sum up, our study demonstrated that TMJ-105 extracted from Carpesium cernuum is a potent inhibitor of leukemia cells that mediated apoptosis and G2/M-phase blockade in HEL cells through blocking JAK2/STAT3 and MAPKs activation. Collectively, these results demonstrated that TMJ-105 affected multiple signaling events associated with tumorigenesis, suggesting that TMJ-105 is a new chemotherapeutic agent for the treatment of leukemia (Fig. 7). In future, we will detect the anti-leukemia effect and mechanism in vivo.
Fig. 7.
Schematic representation of TMJ-105 effects on HEL cells.
Ethics statement
Review and approval by an ethics committee was not needed for this study, because there is no human or animal experimentation.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
CRediT authorship contribution statement
Xuenai Wei: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jingrui Song: Resources. Qing Rao: Resources. Yubing Huang: Resources. Qin Liu: Resources. Jialei Song: Conceptualization. Wei liang: Conceptualization. Shuhui Feng: Resources. Chen Yan: Resources, Conceptualization. Yanmei Li: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant Numbers: 82360035, 81960546, 81872772 and U1812403), the Science and Social Development of Anshun City (ASKS(2021)19).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34115.
Contributor Information
Chen Yan, Email: nazi3647@sina.com.
Yanmei Li, Email: liyanmei518@hotmail.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., et al. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. http://dx.dio.org/10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Laversanne M., Weiderpass E., et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. http://dx.dio.org/10.1002/cncr.33587 [DOI] [PubMed] [Google Scholar]
- 3.Chennamadhavuni A., Lyengar V., Mukkamalla S.K.R., et al. Leukemia. StatPearls Publishing; Treasure Island (FL) with ineligible companies: 2023. [PubMed] [Google Scholar]
- 4.Yuan Y., Long H., Zhou Z., et al. PI3K-AKT-targeting breast cancer treatments: natural products and synthetic compounds. Biomolecules. 2023;13:93. doi: 10.3390/biom13010093. http://dx.dio.org/10.3390/biom13010093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B., Ning K., Sun M.L., et al. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in oa: a systematic review. Cell Commun. Signal. 2023;21:67. doi: 10.1186/s12964-023-01094-4. http://dx.dio.org/10.1186/s12964-023-01094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang B., Lang X., Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1023177. http://dx.dio.org/10.3389/fonc.2022.1023177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye C., Ruan X., Zhao Y., et al. BP-1-102 exerts antitumor effects on t-cell acute lymphoblastic leukemia cells by suppressing the JAK2/STAT3/c-Myc signaling pathway. Exp. Ther. Med. 2023;25:191. doi: 10.3892/etm.2023.11890. http://dx.dio.org/10.3892/etm.2023.11890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang X., Ju J. Matrine inhibits ovarian cancer cell viability and promotes apoptosis by regulating the ERK/JNK signaling pathway via p38MAPK. Oncol. Rep. 2021;45:82. doi: 10.3892/or.2021.8033. http://dx.dio.org/10.3892/or.2021.8033 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Qin X., Chen X., Guo L., et al. Hinokiflavone induces apoptosis, cell cycle arrest and autophagy in chronic myeloid leukemia cells through MAPK/NF-κB signaling pathway. BMC Complement Med Ther. 2022;22:100. doi: 10.1186/s12906-022-03580-7. http://dx.dio.org/10.1186/s12906-022-03580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak A.W., Lee J.Y., Lee S.O., et al. Echinatin induces reactive oxygen species-mediated apoptosis via JNK/p38 MAPK signaling pathway in colorectal cancer cells. Phytother Res. 2023;37:563–577. doi: 10.1002/ptr.7634. http://dx.dio.org/10.1002/ptr.7634 [DOI] [PubMed] [Google Scholar]
- 11.Kwak A.W., Lee M.J., Lee M.H., et al. The 3-deoxysappanchalcone induces ros-mediated apoptosis and cell cycle arrest via JNK/p38 MAPK signaling pathway in human esophageal cancer cells. Phytomedicine. 2021;86 doi: 10.1016/j.phymed.2021.153564. http://dx.dio.org/10.1016/j.phymed.2021.153564 [DOI] [PubMed] [Google Scholar]
- 12.Oropeza E., Seker S., Carrel S., et al. Molecular portraits of cell cycle checkpoint kinases in cancer evolution, progression, and treatment responsiveness. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf2860. http://dx.dio.org/10.1126/sciadv.adf2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sofi S., Mehraj U., Qayoom H., et al. Targeting cyclin-dependent kinase 1 (CDK1) in cancer: molecular docking and dynamic simulations of potential CDK1 inhibitors. Med. Oncol. 2022;39:133. doi: 10.1007/s12032-022-01748-2. http://dx.dio.org/10.1007/s12032-022-01748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvaraj C. Therapeutic targets in cancer treatment: cell cycle proteins. Adv. Protein. Chem. Struct. Biol. 2023;135:313–342. doi: 10.1016/bs.apcsb.2023.02.003. http://dx.dio.org/10.1016/bs.apcsb.2023.02.003 [DOI] [PubMed] [Google Scholar]
- 15.Sucher N.J. The application of Chinese medicine to novel drug discovery. Expet Opin. Drug Discov. 2013;8:21–34. doi: 10.1517/17460441.2013.739602. http://dx.dio.org/10.1517/17460441.2013.739602 [DOI] [PubMed] [Google Scholar]
- 16.Lam C.S., Peng L.W., Yang L.S., et al. Examining patterns of traditional Chinese medicine use in pediatric oncology: a systematic review, meta-analysis and data-mining study. J Integr Med. 2022;20:402–415. doi: 10.1016/j.joim.2022.06.003. http://dx.dio.org/10.1016/j.joim.2022.06.003 [DOI] [PubMed] [Google Scholar]
- 17.Wu T., Yang X., Zeng X., et al. Traditional Chinese medicinal herbs in the treatment of patients with esophageal cancer: a systematic review. Gastroenterol. Clin. N. Am. 2009;38:153–167. doi: 10.1016/j.gtc.2009.01.006. http://dx.dio.org/10.1016/j.gtc.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 18.Ying J., Zhang M., Qiu X., et al. The potential of herb medicines in the treatment of esophageal cancer. Biomed. Pharmacother. 2018;103:381–390. doi: 10.1016/j.biopha.2018.04.088. http://dx.dio.org/10.1016/j.biopha.2018.04.088 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y.S., Shen Q., Li J. Traditional Chinese medicine targeting apoptotic mechanisms for esophageal cancer therapy. Acta Pharmacol. Sin. 2016;37:295–302. doi: 10.1038/aps.2015.116. http://dx.dio.org/10.1038/aps.2015.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q.X., Yang Y.X., Zhang J.P., et al. Isolation, structure elucidation, and absolute configuration of highly oxygenated germacranolides from carpesium cernuum. J. Nat. Prod. 2016;79:2479–2486. doi: 10.1021/acs.jnatprod.6b00315. http://dx.dio.org/10.1021/acs.jnatprod.6b00315 [DOI] [PubMed] [Google Scholar]
- 21.Zhu N.L., Tang C., Xu C., et al. Cytotoxic germacrane-type sesquiterpene lactones from the whole plant of carpesium lipskyi. J. Nat. Prod. 2019;82:919–927. doi: 10.1021/acs.jnatprod.8b01004. http://dx.dio.org/10.1021/acs.jnatprod.8b01004 [DOI] [PubMed] [Google Scholar]
- 22.Yan C., Long Q., Zhang Y.D., et al. Germacranolide sesquiterpenes from carpesium cernuum and their anti-leukemia activity. Chin. J. Nat. Med. 2021;19:528–535. doi: 10.1016/S1875-5364(21)60052-3. http://dx.dio.org/10.1016/s1875-5364(21)60052-3 [DOI] [PubMed] [Google Scholar]
- 23.Chen X., Song J., Yuan D., et al. Incaspitolide a extracted from carpesium cernuum induces apoptosis in vitro via the PI3K/AKT pathway in benign prostatic hyperplasia. Biosci. Rep. 2021;41 doi: 10.1042/BSR20210477. http://dx.dio.org/10.1042/bsr20210477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan C., Chen G., Jing C., et al. Eriocitrin, a dietary flavonoid suppressed cell proliferation, induced apoptosis through modulation of JAK2/STAT3 and JNK/p38 MAPK signaling pathway in MCF-7 cells. J. Biochem. Mol. Toxicol. 2022;36 doi: 10.1002/jbt.22943. http://dx.dio.org/10.1002/jbt.22943 [DOI] [PubMed] [Google Scholar]
- 25.Tayeb B.A., Kusuma I.Y., Osman A.A.M., et al. Herbal compounds as promising therapeutic agents in precision medicine strategies for cancer: a systematic review. J Integr Med. 2024;22:137–162. doi: 10.1016/j.joim.2024.02.001. http://dx.dio.org/10.1016/j.joim.2024.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Zoi V., Galani V., Lianos G.D., et al. The role of curcumin in cancer treatment. Biomedicines. 2021;9 doi: 10.3390/biomedicines9091086. http://dx.dio.org/10.3390/biomedicines9091086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao P., Song H., Gao F., et al. A novel derivative of curcumol, HCL-23, inhibits the malignant phenotype of triple-negative breast cancer and induces apoptosis and HO-1-dependent ferroptosis. Molecules. 2023;28:3389. doi: 10.3390/molecules28083389. http://dx.dio.org/10.3390/molecules28083389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs Y. The therapeutic promise of apoptosis. Science. 2019;363:1050–1051. doi: 10.1126/science.aaw3607. http://dx.dio.org/10.1126/science.aaw3607 [DOI] [PubMed] [Google Scholar]
- 29.Talib W.H. Melatonin and cancer hallmarks. Molecules. 2018;23:518. doi: 10.3390/molecules23030518. http://dx.dio.org/10.3390/molecules23030518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S., Zhao X., Hao J., et al. The role of ATF6 in Cr(VI)-induced apoptosis in DF-1 cells. J. Hazard Mater. 2021;410 doi: 10.1016/j.jhazmat.2020.124607. http://dx.dio.org/10.1016/j.jhazmat.2020.124607 [DOI] [PubMed] [Google Scholar]
- 31.Ding J., Lu B., Wang J., et al. Long non-coding RNA Loc554202 induces apoptosis in colorectal cancer cells via the caspase cleavage cascades. J. Exp. Clin. Cancer Res. 2015;34:100. doi: 10.1186/s13046-015-0217-7. http://dx.dio.org/10.1186/s13046-015-0217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasenjäger A., Gillissen B., Müller A., et al. Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells. Oncogene. 2004;23:4523–4535. doi: 10.1038/sj.onc.1207594. http://dx.dio.org/10.1038/sj.onc.1207594 [DOI] [PubMed] [Google Scholar]
- 33.Chao H.X., Poovey C.E., Privette A.A., et al. Orchestration of DNA damage checkpoint dynamics across the human cell cycle. Cell Syst. 2017;5:445–459.e5. doi: 10.1016/j.cels.2017.09.015. http://dx.dio.org/10.1016/j.cels.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Zhang J.Q., Zhang T., et al. Calycosin induces gastric cancer cell apoptosis via the ROS-mediated MAPK/STAT3/NF-κB pathway. OncoTargets Ther. 2021;14:2505–2517. doi: 10.2147/OTT.S292388. http://dx.dio.org/10.2147/ott.S292388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Chen Z. Downregulation of miR-19a inhibits the proliferation and promotes the apoptosis of osteosarcoma cells by regulating the JAK2/STAT3 pathway. Oncol. Lett. 2020;20:173. doi: 10.3892/ol.2020.12033. http://dx.dio.org/10.3892/ol.2020.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang W., Sun H., Hu T., et al. Blocking the short isoform of augmenter of liver regeneration inhibits proliferation of human multiple myeloma U266 cells via the MAPK/STAT3/cell cycle signaling pathway. Oncol. Lett. 2021;21:197. doi: 10.3892/ol.2021.12458. http://dx.dio.org/10.3892/ol.2021.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwak A.W., Kim W.K., Lee S.O., et al. Licochalcone b induces ros-dependent apoptosis in oxaliplatin-resistant colorectal cancer cells via p38/JNK MAPK signaling. Antioxidants. 2023;12:656. doi: 10.3390/antiox12030656. http://dx.dio.org/10.3390/antiox12030656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Q., Li L., Miao C., et al. Osteopontin promotes hepatocellular carcinoma progression through inducing JAK2/STAT3/NOX1-mediated ROS production. Cell Death Dis. 2022;13:341. doi: 10.1038/s41419-022-04806-9. http://dx.dio.org/10.1038/s41419-022-04806-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akhtar S., Achkar I.W., Siveen K.S., et al. Sanguinarine induces apoptosis pathway in multiple myeloma cell lines via inhibition of the JAK2/STAT3 signaling. Front. Oncol. 2019;9:285. doi: 10.3389/fonc.2019.00285. http://dx.dio.org/10.3389/fonc.2019.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivas U.S., Tan B.W.Q., Vellayappan B.A., et al. ROS and the DNA damage response in cancer. Redox Biol. 2019;25 doi: 10.1016/j.redox.2018.101084. http://dx.dio.org/10.1016/j.redox.2018.101084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y., Zhang Q., Zhou J., et al. Down-regulation of SOX18 inhibits laryngeal carcinoma cell proliferation, migration, and invasion through JAK2/STAT3 signaling. Biosci. Rep. 2019;39 doi: 10.1042/BSR20182480. http://dx.dio.org/10.1042/bsr20182480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X., Zhao Z., Zeng C., et al. HNGF6A inhibits oxidative stress-induced MC3T3-E1 cell apoptosis and osteoblast phenotype inhibition by targeting Circ_0001843/miR-214 pathway. Calcif. Tissue Int. 2020;106:518–532. doi: 10.1007/s00223-020-00660-z. http://dx.dio.org/10.1007/s00223-020-00660-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.