Abstract
Background
Life-long vitamin K antagonist (VKA) therapy is recommended as a standard of care in antiphospholipid syndrome (APS) patients with thrombosis. Concerns have been raised about the validity of international normalized ratio (INR) measurements in lupus anticoagulant (LA)-positive APS patients because LA may interfere with phospholipid-dependent coagulation tests and could elevate INR measurements.
Objectives
Here, we aimed to determine the interference of antigen-specific monoclonal and isolated patient antibodies with LA activity on INR measurements.
Methods
Pooled normal plasma and control plasma from patients on VKA (without LA) were incubated with monoclonal and isolated patient immunoglobulin G antiprothrombin and anti–beta-2-glycoprotein I antibodies that express LA activity. INR was determined before and after addition using 3 laboratory assays (Owren STA-Hepato Prest, Quick STA-NeoPTimal, and Quick STA-Neoplastine R) and 1 point-of-care test device (CoaguChek Pro II).
Results
Antiprothrombin and anti–beta-2-glycoprotein I antibodies with LA activity interfered with recombinant human thromboplastin reagents (Quick STA-Neoplastine R and CoaguChek Pro II), particularly when added to plasma of VKA-treated controls. This effect was most evident on point-of-care test INR measurements, while the recombinant Quick reagent exhibited a lesser degree of interference. In contrast, tissue-derived thromboplastin reagents (Owren STA-Hepato Prest and Quick STA-NeoPTimal) remained largely unaffected by these antibodies, both in pooled normal plasma and VKA anticoagulated control plasma. Among these reagents, the Owren INR reagent exhibited the lowest sensitivity to the influence of LA antibodies. This observed difference in sensitivity is independent of the plasma dilution factor or the presence of factor V or fibrinogen in Owren reagent.
Conclusion
INR reagents that utilize recombinant human thromboplastin are more sensitive to the presence of monoclonal and patient-derived antibodies with LA activity. Consequently, APS patients positive for LA should be monitored using tissue-derived thromboplastin reagents, given its reduced susceptibility to interference by LA-causing antibodies.
Keywords: anticoagulants, antiphospholipid syndrome, international normalized ratio, lupus coagulation inhibitor, warfarin
Essentials
-
•
Lupus anticoagulant (LA) may interfere with international normalized ratio tests.
-
•
The interference of LA-causing antibodies on international normalized ratio measurements was tested.
-
•
Recombinant human thromboplastin reagents are more sensitive to LA.
-
•
Anticoagulation in LA patients can be monitored safely using tissue-derived thromboplastins.
1. Introduction
Antiphospholipid syndrome (APS) is an acquired autoimmune disorder defined by thrombosis and adverse pregnancy outcome in the presence of persistent antiphospholipid antibodies (aPL) [1]. The current laboratory criteria for the classification of APS include 3 aPL types: anticardiolipin (aCL) autoantibodies, anti–beta-2-glycoprotein I (aβ2GPI) autoantibodies, and lupus anticoagulants (LAs). LAs refer to a heterogeneous group of autoantibodies that interfere with phospholipid-dependent coagulation tests in vitro, causing a prolonged clotting time [2,3]. The LA phenomenon has been attributed to autoantibodies directed to the phospholipid-binding proteins β2GPI or prothrombin [[4], [5], [6]]. Among the 3 aPL populations outlined in the classification criteria for APS, LA exhibits a strong correlation with the occurrence of thrombosis [[7], [8], [9]], while the presence of aCL and aβ2GPI alone demonstrates a weaker association with thrombosis [[9], [10], [11]]. In contrast, combined triple positivity for LA, aCL, and aβ2GPI antibodies has been associated with a substantial risk for both first and recurrent thromboembolisms [12,13].
To reduce the risk of recurrent thrombotic events, APS patients with a history of thrombosis require antithrombotic treatment. While direct oral anticoagulants have become the first-line treatment for most patients with a first thromboembolism, direct oral anticoagulants have proven insufficient in the treatment of thrombotic APS patients [14,15]. Therefore, current guidelines recommend that patients with an APS-related thrombosis should be treated with life-long vitamin K antagonists (VKAs) [16,17]. Nevertheless, the effective use of VKAs is challenging due to their narrow therapeutic window and unpredictable anticoagulant effect [18,19], necessitating frequent laboratory monitoring to ensure optimal dosing and minimize the risk of bleeding. VKA therapy is monitored using the international normalized ratio (INR), a universal normalized scale independent of reagent and method. The INR is calculated from the prothrombin time (PT) and the appropriate international sensitivity index (ISI) of the thromboplastin utilized in the PT test [20]. The optimal intensity of VKA therapy may differ with individual risk factors; however, a target INR of 2.0 to 3.0 is recommended for patients with APS [21,22].
Concerns have been raised about the validity of INR measurements in LA-positive APS patients [23,24]. The presence of LA-causing antibodies may hamper anticoagulant monitoring as they interfere with phospholipid-dependent coagulation tests [3,24,25]. This disruption may result in a prolonged PT and elevated INR measurement [26]. Consequently, INR values may not reflect true anticoagulation intensity, leading to an overestimation of the effect of oral anticoagulation, resulting in a prothrombotic risk. The interference of LA on INR measurements is suggested to be highly dependent on the thromboplastin reagent used due to the variable sensitivity of different thromboplastin reagents to the presence of LA [3,25,27]. Particularly, the impact of these antibodies on point-of-care test (POCT) reagents remains an area of significant concern and clinical relevance [24]. Hence, understanding how LA may affect INR results is crucial for ensuring the accuracy of therapeutic monitoring and clinical decision making. In this study, we aimed to investigate the influence of LA-causing antigen-specific antibodies on INR results measured using different thromboplastin reagents and methods. To our knowledge, this is the first time antigen-specific antibodies with LA activity were investigated in relation to INR, contrary to the patient plasma or total immunoglobulin (Ig)G typically used.
2. Methods
2.1. Reagents
Human prothrombin, bovine factor (F)V, and bovine fibrinogen were obtained from Synapse Research Institute (Maastricht, the Netherlands). Human IgG isotype control purified from pooled normal human serum was obtained from Invitrogen. Monoclonal antiprothrombin (aPT) antibody 28F4 and aβ2GPI 27G7 antibody were prepared as previously described [28]. The monoclonal aPT antibody 3B1 and aβ2GPI antibody 3B7 were produced according to standard procedures [29]. Monoclonal aPT antibodies were directed against F1.2 of prothrombin, whereas monoclonal aβ2GPI antibodies targeted domain I of β2GPI. All monoclonal antibodies expressed LA activity. LA was determined by activated partial thromboplastin time (STA-Staclot LA) and dilute Russell viper venom time (STA-Staclot DRVV Screen and Confirm), both obtained from Diagnostica Stago. INR was determined using the Owren PT reagent STA-Hepato Prest and the Quick PT reagents STA-Neoplastine R and STA-NeoPTimal obtained from Diagnostica Stago. POCT INR was measured using the CoaguChek Pro II purchased from Roche Diagnostics.
2.2. Plasma samples
Left-over citrate anticoagulated plasma samples (3.2% sodium citrate; Beckton Dickinson) of patients from the Thrombosis Service of Meander Medical Centre receiving oral VKAs were selected based on their PT/INR. To study the reliability of the POCT INR analysis based on plasma compared with whole blood (WB), plasma remnants of 10 patients of the Meander Thrombosis Service were collected. These patients treated with VKAs were admitted to the laboratory for regular INR monitoring for capillary WB-POCT INR analysis (measured on the CoaguChek Pro II) as venous sampling as part of periodic validation of the POCT devices, which was approved by informed consent. After venous sampling, the blood was centrifuged at 2100 × g for 10 minutes, and the plasma was stored at −80 °C until further use.
Pooled normal plasma (PNP) was prepared from healthy volunteers, as previously described [30]. In short, blood was collected from healthy controls by venipuncture (3.2% sodium citrate; Beckton Dickinson) after obtaining informed consent. Platelet-poor plasma was obtained from the supernatant fraction after double centrifugation at 2840 × g for 10 minutes at room temperature. Afterward, the plasma was pooled, and aliquots were stored at −80 °C until further use.
2.3. Purification of antigen-specific patient IgG antibodies
The study protocol was approved as non-Medical Research Involving Human Subjects Act research by the medical ethics committee of the Erasmus MC, Rotterdam, the Netherlands (MEC 2021-0131). Fresh apheresis plasma was obtained from APS patients undergoing regular therapeutic plasma exchange for anticoagulant-refractory APS. IgG antibodies were precipitated from the apheresis plasma by gradually adding solid ammonium sulfate to a final concentration of 50%, followed by incubation for at least 1 hour at room temperature and centrifugation at 4200 × g at 4 °C for 20 minutes. The pellet obtained after the centrifugation was resuspended in 0.05 M sodium phosphate buffer (pH 7.0), and the conductivity was adjusted to 28.5 mS/cm using Milli-Q water. IgM antibodies were precipitated by the addition of polyethylene glycol 6000 to a final concentration of 8%, followed by a centrifugation step at 4000 × g at room temperature for 30 minutes. The supernatant was collected and passed through a Protein G Sepharose 4 Fast Flow (Cytiva Life Sciences) affinity chromatography column for IgG isolation. The Protein G column was washed using 0.01 M sodium phosphate buffer (pH 7.0) containing 0.15 M NaCl. Bound IgG antibodies were eluted with 0.1 M glycine (pH 3.0), and the pH of the protein-containing fractions was neutralized immediately using 1.0 M Tris (pH 8.5). The purity of the antibodies was checked with sodium dodecyl sulfate–polyacrylamide gel electrophoresis. G-Trap FPLC columns (G-Biosciences) were packed with immobilized prothrombin- and β2GPI-coupled Sepharose to purify the antigen-specific IgG antibodies from the IgG pool. The total IgG was loaded, and columns were washed with 0.01 M sodium phosphate buffer (pH 7.0) containing 0.15 M NaCl. After washing, bound antibodies were eluted using elution buffer (0.1 M glycine, pH 3.0), and the pH of the protein-containing fractions was neutralized using 1.0 M Tris (pH 8.5) immediately after collection. IgG antibodies bound to the β2GPI-Sepharose column were eluted using a high-salt elution buffer (0.1 M glycine, 1 M NaCl; pH 3.0). Finally, the LA activity of the isolated patient antibodies was tested by incubating PNP with antibody for 10 minutes at 37 °C before LA testing. LA was performed in accordance with the manufacturer’s instructions for dilute Russell viper venom time and activated partial thromboplastin time testing and international guidelines for LA testing [31].
2.4. INR determination
PNP or plasma from anticoagulated patients (without LA) was incubated with aPL for 5 minutes at room temperature before INR determination. Monoclonal and patient-derived IgG aβ2GPI and aPT antibodies with LA activity were tested in this study. In some experiments, prothrombin (1.4 μM), FV (20 nM), fibrinogen (10 μM), or equal volumes of Tris-buffered saline were added to the plasma. The plasma INR was determined on the STA R Max 3 coagulation analyzer (Diagnostica Stago) using 3 commercially available thromboplastin reagents: STA-Hepato Prest, an Owren PT tissue-derived thromboplastin reagent; STA-NeoPTimal, a Quick PT tissue-derived thromboplastin reagent; and STA-Neoplastine R, a Quick PT reagent prepared from human recombinant tissue factor (TF) and phospholipids. The INR on the STA R Max 3 analyzer was calculated automatically using the ISI and mean normal clotting time provided by the manufacturer. The POCT INR was determined and calculated on the CoaguChek Pro II (Roche Diagnostics) using PT test strip containing human recombinant TF. WB or plasma recalcified 1:1 with 17.0 mM calcium chloride was used to determine the POCT INR. The effect of plasma dilution factor on INR interference by LA was determined using the semiautomated STart coagulation analyzer (Diagnostica Stago) and STA-Hepato Prest, STA-Neoplastine R, or STA-NeoPTimal PT reagent. In short, PNP was incubated with LA-causing monoclonal aβ2GPI and aPT antibodies (200 μg/mL) prior to dilution with Owren–Koller buffer to a range of plasma dilution factors. The INR was calculated manually from the PT using the ISI value provided by the manufacturer and the mean normal clotting time, which was determined in-house.
2.5. Statistical analysis
All analyses were performed with GraphPad Prism 9.3.0 software (GraphPad Software). Results were reported as mean and SD. Spearman’s rank correlation coefficient was used to test the correlation between the different INR methods. Bland–Altman plots were used to calculate the degree of agreement between INR methods. Differences between conditions were analyzed with unpaired Student’s t-tests. P values of <.05 were considered statistically significant.
3. Results
3.1. Reliability of the plasma-based POCT INR assay
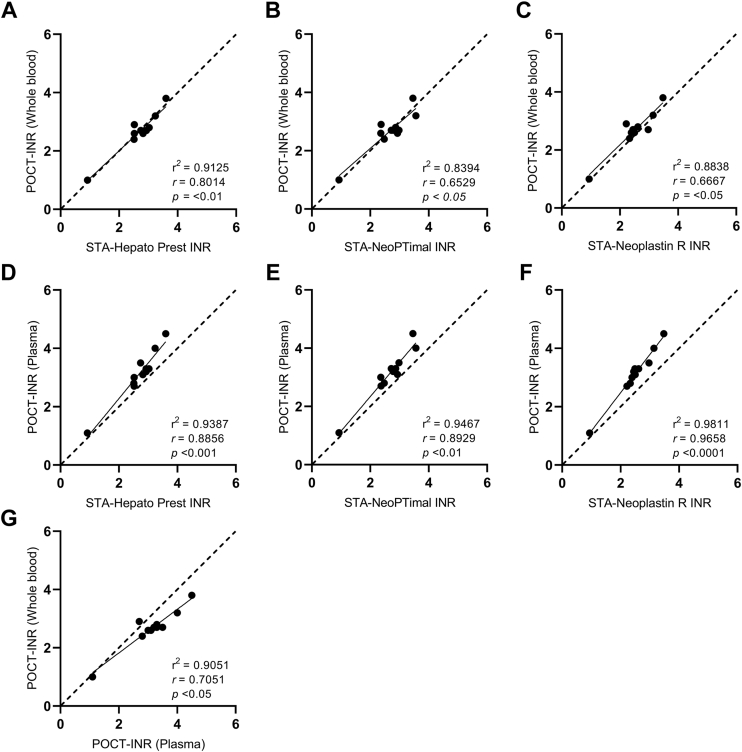
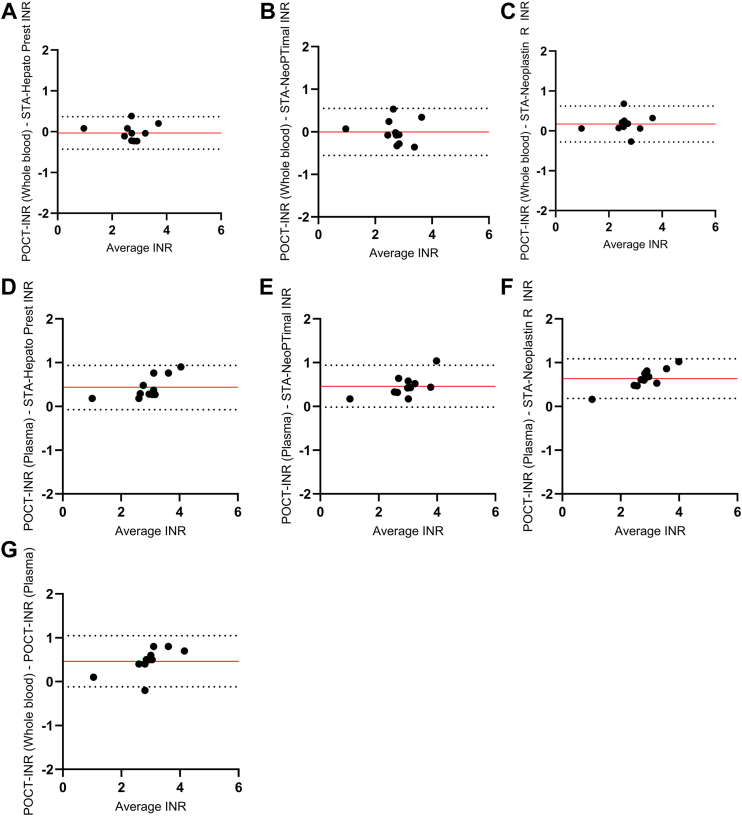
In the pursuit of understanding the effect of LA on INR test results, one important decision was to opt for plasma rather than WB for conducting POCT experiments. This choice was made because experiments with plasma offered significant logistical advantages for the study design. To establish the reliability of the POCT method based on plasma, the agreement and correlation between the plasma-based POCT method and the other INR methods were investigated using plasma and WB from non-APS control patients on VKA therapy. The median INR value was 3.2 (IQR, 2.8-3.5) when measured using the plasma-POCT and 2.7 (IQR, 2.6-2.9) when obtained using the WB-POCT. The median INR values determined on the STA R Max 3 coagulation analyzer were 2.8 (IQR, 2.5-3.0) for the STA-Hepato Prest reagent, 2.5 (IQR, 2.3-3.0) for the STA-Neoplastin R reagent, and 2.8 (IQR, 2.4-3.0) for the STA-NeoPTimal reagent. Overall, plasma-POCT INR values correlated well with the STA-Neoplastin R INR values (Spearman’s rho [ρ], .97; 95% CI, 0.86-0.99), STA-Hepato Prest INR values (ρ = .89; 95% CI, 0.60-0.97), and STA-NeoPTimal INR values (ρ = .89; 95% CI, 0.61-0.97; Figure 1). The correlation between WB-POCT INR values and STA-Neoplastin R INR (ρ = .67; 95% CI, 0.09-0.90), STA-Hepato Prest INR (ρ = .80; 95% CI, 0.37-0.95), and STA-NeoPTimal INR (ρ = .65; 95% CI, 0.06-0.90) values was slightly lower when compared with the plasma-POCT INR measurements (Figure 1). Furthermore, a Bland–Altman analysis was performed to determine the agreement between the POCT method and the laboratory assays (Figure 2). The INR variances between methods remained within the limits of agreement (95% CI) for the majority of the samples. No differences in agreement were observed between the plasma-POCT method and WB-POCT method compared with the 3 laboratory INR assays.
Figure 1.
Spearman correlation analysis to identify the correlation between the international normalized ratio (INR) assays. Whole blood point-of-care test (WB-POCT) vs STA-Hepato Prest (A), WB-POCT vs STA-NeoPTimal (B), WB-POCT vs STA-Neoplastin R (C), plasma-POCT vs STA-Hepato Prest (D), plasma-POCT vs STA-NeoPTimal (E), plasma-POCT vs STA-Neoplastin R (F), and WB-POCT vs plasma-POCT (G). The dashed 45° line represents the perfect concordance. P < .05 was considered statistically significant. r2, Goodness of fit; r, Spearman correlation coefficient.
Figure 2.
Bland–Altman analysis to identify the agreement between the international normalized ratio (INR) assays. Whole blood point-of-care test (WB-POCT) vs STA-Hepato Prest (A), WB-POCT vs STA-NeoPTimal (B), WB-POCT vs STA-Neoplastin R (C), plasma-POCT vs STA-Hepato Prest (D), plasma-POCT vs STA-NeoPTimal (E), plasma-POCT vs STA-Neoplastin R (F), and WB-POCT vs plasma-POCT (G). The solid line indicates mean differences, and the dotted lines represent the upper and lower limits of agreement (95% CI).
3.2. Sensitivity of different INR reagents to monoclonal antibodies with LA activity
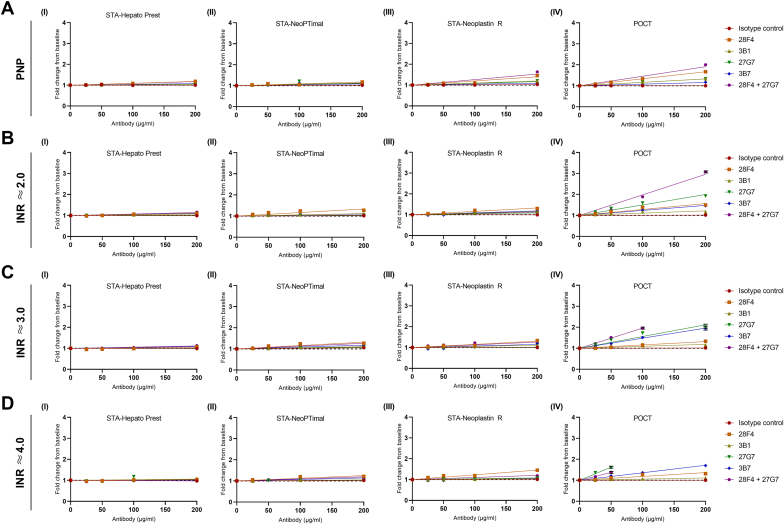
To determine the interference of monoclonal aPL on INR values, PNP was spiked with increasing concentrations of monoclonal aPT and aβ2GPI IgG antibodies with LA activity and measured using tissue-extract thromboplastin reagents (STA-Hepato Prest and STA-NeoPTimal), recombinant human thromboplastin reagent (STA-Neoplastin R), and a POCT INR assay. Monoclonal antibodies with LA activity interfered with recombinant thromboplastin reagents, especially the POCT INR method (Figure 3A). This interference was particularly apparent at high antibody titers. Tissue-extract thromboplastin reagents (STA-Hepato Prest and STA-NeoPTimal) demonstrated minimal interference by LA, even at high antibody titers. This interference was the lowest for STA-Hepato Prest an Owren PT methods. Among these monoclonal aPL, the most pronounced interference was observed with aPT antibodies, while the interference associated with aβ2GPI antibodies was notably less. Interestingly, the interference of the combined aPT and aβ2GPI antibodies exceeded the degree of interference observed in conditions incubated with only aPT antibodies, even though the total concentration of antibodies was the same in both conditions.
Figure 3.
Monoclonal antiphospholipid antibodies with lupus anticoagulant activity interfere with international normalized ratio (INR) measurements. Pooled normal plasma (PNP; A) or pooled vitamin K antagonist-treated plasma with INR values at the low (A; ≈2.0), middle (B; ≈3.0), and upper (C; ≈4.0) parts of the therapeutic range were incubated with monoclonal antiprothrombin antibodies (28F4 and 3B1), monoclonal anti–beta-2-glycoprotein I antibodies (27G7 and 3B7), or both. INR measurements were performed using STA-Hepato Prest (I), STA-NeoPTimal (II), STA-Neoplastin R (III), or point-of-care test (POCT; IV) INR reagents. INR = limit of detection.
To better understand the potential impact of LA on INR measurements in a clinical context, we also evaluated the interference of monoclonal aPL with LA activity on INR values when spiked into pooled plasma from non-APS patients treated with VKAs. Plasma samples, reflecting INRs at the lower (INR ≈ 2.0; Figure 3B), middle (INR ≈ 3.0; Figure 3C), and upper (INR ≈ 4.0; Figure 3D) regions of the therapeutic range, were collected. Similar to the results obtained in PNP, the addition of LA-causing monoclonal aPT or aβ2GPI antibodies had minimal effect on INR values measured using the tissue-extract thromboplastin reagents (STA-Hepato Prest and STA-NeoPTimal). In contrast, recombinant thromboplastin reagents, especially POCT INR values, were more sensitive to interference by LA. Interestingly, the interference of aβ2GPI on POCT INR values was found to increase with increasing INR baseline levels. This phenomenon was not observed with aPT antibodies.
Decreased prothrombin levels in plasma from VKA-treated patients might explain the reduced interference of aPT antibodies when spiked into anticoagulated plasma. To investigate this, we spiked plasma from VKA-treated control patients with aPT antibodies in the presence and absence of plasma concentrations of prothrombin. As expected, the addition of prothrombin lowered the baseline INR value of plasma from VKA-treated patients in all INR methods (Supplementary Figure S1). Interestingly, we observed that the INR was markedly higher when aPT antibodies were added in combination with extra prothrombin compared with the condition without extra prothrombin. This increase was particularly evident when INR values were measured using the POCT INR method (P = .14); however, this difference was not statistically significant (Supplementary Figure S1D). This is likely due to the fact that the limit of detection was reached, suggesting that the actual discrepancy in INR values in the POCT INR assay is even more substantial. In contrast, no difference was observed when the INR was determined using the 2 tissue-extract thromboplastin reagents (P > .99; Supplementary Figure S1A, B).
3.3. Sensitivity of different INR reagents to patient-derived antibodies with LA activity
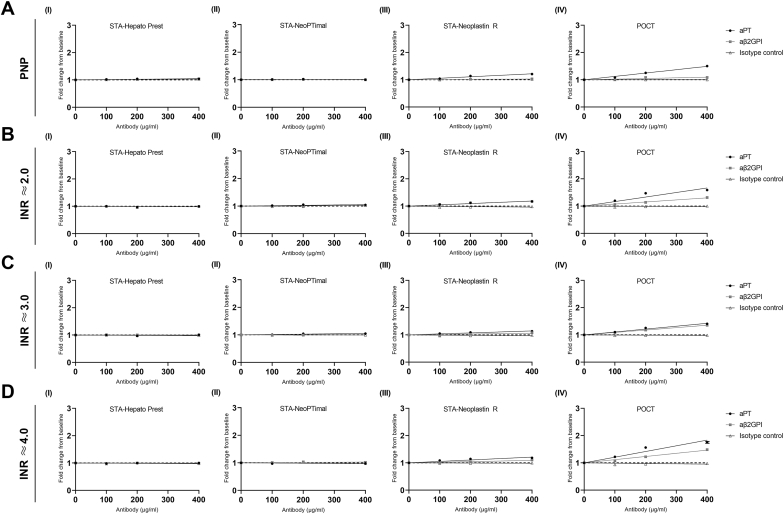
Next, the interference of patient-derived IgG antibodies with LA activity on INR values was determined by spiking PNP and plasma from VKA-treated patients with aPL isolated from apheresis plasma. Similar to the monoclonal antibodies, recombinant thromboplastin reagents (STA-Neoplastin R and POCT) were more sensitive to interference by patient-derived aPT or aβ2GPI antibodies with LA activity compared with tissue-extract thromboplastin reagents (STA-Hepato Prest and STA-NeoPTimal; Figure 4). This interference was particularly evident when INR values were measured using the POCT INR method. Furthermore, the interference by LA antibodies was more pronounced when spiked into plasma from non-APS patients treated with VKAs and increased with higher baseline INR values (Figure 4B–D). The addition of patient-derived LA aPT or aβ2GPI antibodies did not affect the tissue-extract thromboplastin INR reagents, both in PNP and anticoagulated control plasma.
Figure 4.
Patient-derived antiphospholipid antibodies with lupus anticoagulant activity interfere with international normalized ratio (INR) measurements. Pooled normal plasma (PNP; A) or pooled vitamin K antagonist-treated plasma with INR values at the low (A; ≈2.0), middle (B; ≈3.0), and upper (C; ≈4.0) parts of the therapeutic range were incubated with 0 to 400 μg/mL patient-derived antiprothrombin antibodies or anti–beta-2-glycoprotein I (aβ2GPI) antibodies. INR measurements were performed using STA-Hepato Prest (I), STA-NeoPTimal (II), STA-Neoplastin R (III), or point-of-care test (POCT; IV) INR reagents. INR ≈ limit of detection. aPT, antiprothrombin.
3.4. The influence of plasma dilution factor and FV or fibrinogen concentration in the INR reagents on the sensitivity of Owren PT reagents to LA interference
To determine if the decreased sensitivity of the Owren PT reagents (STA-Hepato Prest) to LA is due to differences in plasma dilution factor, PNP was spiked with LA-causing monoclonal aβ2GPI antibody or aPT antibody and diluted using a range of plasma dilution factors. The standard plasma dilution factors for Quick INR and Owren INR methods were 1:6 and 1:40, respectively. Interestingly, for both Quick INR methods (STA-NeoPTimal and STA-Neoplastin R), the interference by LA on INR measurements remained stable over the total range of plasma dilutions (Supplementary Figure S2). Similarly, interference by aPL on the Owren INR values (STA-Hepato Prest) did not increase when the dilution factor of the plasma was lower.
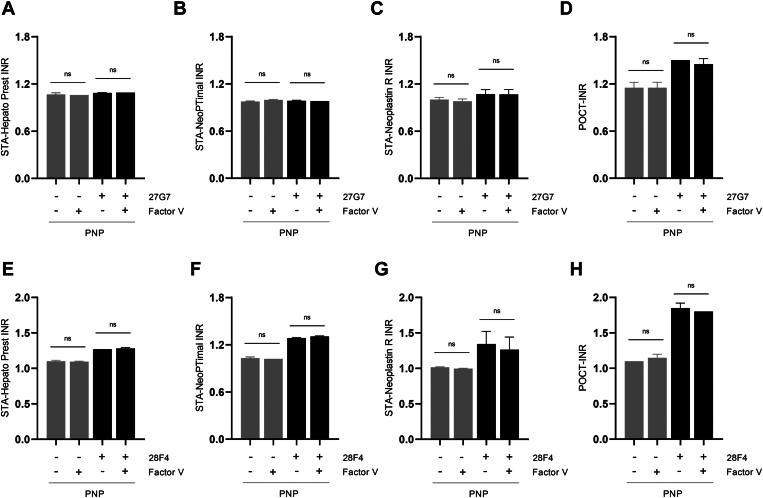
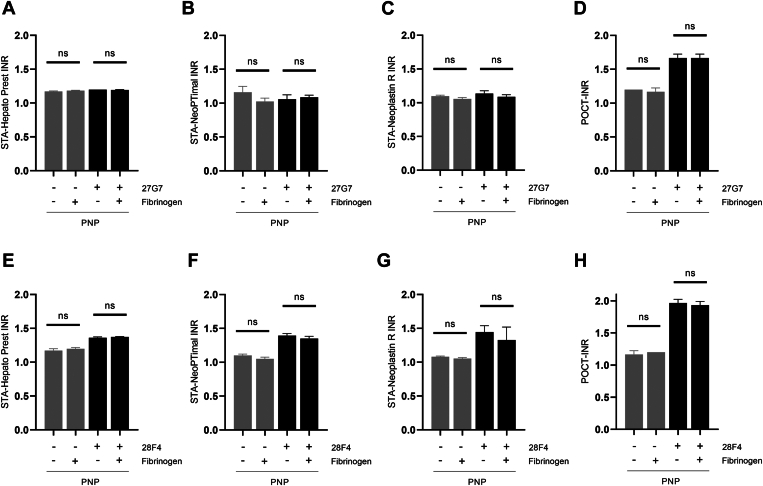
Another factor that could explain the decreased sensitivity of Owren reagents (STA-Hepato Prest) to INR interference by LA is the reagent composition. Owren reagents are enhanced with bovine FV and fibrinogen. To investigate if this difference in reagent composition influences the sensitivity to LA, PNP was spiked with LA-causing monoclonal aβ2GPI or aPT antibodies in the presence or absence of extra FV or fibrinogen (n = 3). The addition of plasma concentrations of FV (20 nM; Figure 5) or fibrinogen (10 μM; Figure 6) did not affect the INR discrepancies observed between Owren and Quick PT reagents when spiked with the aβ2GPI antibody or aPT antibody.
Figure 5.
Interference of international normalized ratio (INR) values by lupus anticoagulants is independent of factor (F)V concentration in the INR reagents. Pooled normal plasma (PNP) was incubated with 200 μg/mL monoclonal anti–beta-2-glycoprotein I antibody (27G7; A–C) or antiprothrombin antibody (28F4; D–F) in the presence or absence of added FV (20 nM). Results are shown as mean ± SD (n = 3). INR measurements were performed using STA-Hepato Prest (A, E), STA-NeoPTimal (B, F), STA-Neoplastin R (C, G), or point-of-care test (POCT; D, H) INR reagents. Ns, not significant.
Figure 6.
Interference of international normalized ratio (INR) values by lupus anticoagulants is independent of fibrinogen concentration in the INR reagents. Pooled normal plasma (PNP) was incubated with 200 μg/mL monoclonal anti–beta-2-glycoprotein I antibody (27G7; A–C) or antiprothrombin antibody (28F4; D–F) in the presence or absence of added fibrinogen (10 μM). Results are shown as mean ± SD (n = 3). INR measurements were performed using STA-Hepato Prest (A, E), STA-NeoPTimal (B, F), STA-Neoplastin R (C, G), or point-of-care test (POCT; D, H) INR reagents. Ns, not significant.
4. Discussion
Life-long VKA therapy is recommended as a standard of care for APS patients with a history of thrombosis [16,17]. The narrow therapeutic window and unpredictable anticoagulant effect make the effective use of VKAs challenging [18,19]. Frequent INR monitoring is necessary to ensure optimal dosing and to minimize the risk of bleeding. However, the validity of INR measurements in LA-positive APS patients has been under debate [25,27,32,33]. LA may hamper anticoagulant monitoring as it interferes with phospholipid-dependent coagulation tests and could elevate INR measurements [3,26]. The impact of LA on INR measurements is thought to be highly dependent on the specific INR method used [3,25,27]. Therefore, we aimed to investigate the interference of monoclonal and patient-derived aPL with LA activity on a POCT INR method and 3 laboratory INR methods (STA-Hepato Prest, STA-NeoPTimal, and STA-Neoplastin R). Our study demonstrated that monoclonal and patient-derived IgG aβ2GPI and aPT antibodies with LA activity interfere with INR reagents utilizing recombinant thromboplastins. This interference was particularly apparent when INR values were measured using the POCT INR methods. Tissue-extract thromboplastin reagents were less sensitive to interference by LA, especially the Owren PT method.
These differences in interference by LA might be explained by differences in the methodology or reagent composition used in these assays. Firstly, the INR results can be influenced by the dilution factor of the plasma. The Owren method demonstrates a relatively high plasma dilution factor compared with Quick and POCT methods [3]. Consequently, the Owren INR assay is reported to have lower sensitivity to interfering substances like LA [3]. Although we confirmed in our study that the interference by LA is lowest in the Owren INR method, we demonstrate that the low sensitivity to LA is not a result of the high plasma dilution factor utilized by the Owren assay. Our results illustrate that the Owren reagent remained insensitive to interference by LA even at lower plasma dilutions.
Secondly, differences in the reagent composition have previously been suggested to cause the difference in sensitivity to LA between Owren INR and POCT or Quick INR [25]. Owren reagents are enhanced with plasma concentration of bovine FV and fibrinogen [34] to preclude clotting time prolongation by deficiencies of FV or fibrinogen. aβ2GPI antibodies cause LA by binding to FV and inhibiting its activation by activated FX [35]. Because of this, Owren INR is suggested to be less sensitive to interference with aβ2GPI antibodies compared with Quick and POCT methods [25]. Furthermore, the addition of high fibrinogen concentrations can significantly shorten the PT in dogs [36]. The fibrinogen added to the Owren reagents might act in a similar way, potentially leading to an underestimation of the antibody effect. To test this hypothesis, we measured INR values using the 2 Quick methods and the POCT INR method in the presence and absence of bovine FV and fibrinogen. Our study demonstrated that the INR values measured by Quick and POCT INR methods remained elevated even when bovine FV or fibrinogen was added, indicating that the decreased interference of aPL in the Owren method is unlikely to be due to the addition of extra FV and fibrinogen to the reagents.
Finally, earlier studies have demonstrated that recombinant thromboplastins are more sensitive to the presence of LA [27]. This is in line with our current work, which demonstrates that INR methods utilizing recombinant thromboplastin display greater sensitivity to LA compared with tissue-extract thromboplastin INR reagents. The reason for the difference in sensitivity to LA remains unknown. However, a possible explanation might be a difference in the phospholipid composition and phospholipid concentration of the reagents [[37], [38], [39]]. Furthermore, the source of TF could also play a role in the increased sensitivity to LA. Previous studies have demonstrated that the activity of natural TF is higher compared with recombinant TF [40,41]. Moreover, recombinant thromboplastins display an increased sensitivity for FVII levels [[42], [43], [44]]. However, the impact of these reported differences on INR interference by LA requires further investigation. Transparency from manufacturers regarding the reagent composition is essential to assess the role of these factors on INR interference by LA.
To better understand the impact of aPL on INR measurements in a clinical context, we also evaluated the interference of LA-causing aPL on INR values in anticoagulated plasma. The interference of aβ2GPI antibodies with LA activity on INR values was greater with increasing INR baseline levels. A similar pattern was not observed for aPT antibodies in this study. We demonstrate that this difference was linked to the reduced concentration of prothrombin in anticoagulated plasma. Specifically, as INR values increase, the concentration of prothrombin in plasma decreases, resulting in a diminishing effect of aPT antibodies.
Furthermore, our study illustrates that spiking with a combination of aPT and aβ2GPI antibodies exceeded the degree of interference observed in conditions with solely aPT or aβ2GPI antibodies. This increased interference can be explained by a synergistic effect. As mentioned before, aβ2GPI-β2GPI complexes bind to FV and inhibit the activation of FV by activated FX, thereby causing LA [35]. In contrast, aPT antibodies cause LA by competing with coagulation factors for binding sites on phospholipids [35,45]. Combining the different mechanisms of action employed by these antibodies further amplifies the interference on INR measurements.
Overall, it is important to acknowledge that only 3 laboratory assays and 1 commercial POCT device were tested in our study. Variations in reagent composition and the thromboplastins utilized among different manufacturers might influence its sensitivity to LA and should be taken into consideration. Furthermore, it is important to note that antibody concentrations, especially the high concentrations used in this study, might exceed the antibody titers found in APS patients. Previous studies reported that monoclonal antibody titers of 12.5 μg/mL to 60 μg/mL reflect the antibody concentrations in patient samples [46,47]. In the current study, we attempted to align with patient antibody titers by using the minimal antibody concentration needed for LA activity. This minimum concentration differed for monoclonal antibodies (50 μg/mL) and isolated patient antibodies (100 μg/mL). Although these concentrations are in line with the previously reported antibody concentrations, the higher concentrations included in this study might exceed the antibody titers found in the majority of APS patients. This should be taken into consideration when interpreting the results.
Another limitation of our study was the choice to use plasma rather than WB for the POCT INR analysis. We opted for plasma due to logistical reasons. Additionally, using plasma offers the advantage that the exact same plasma sample can be utilized for the measurement of both the POCT INR and laboratory INR values, ensuring consistency across measurements. While we did assess the agreement between plasma- and WB-POCT INR methods, it is essential to exercise caution when comparing the results of this study with WB-POCT INR measurements. Finally, we acknowledge that the number of patients used to assess the reliability of our plasma-POCT INR measurements is low, which is a limitation of our study.
In conclusion, our study demonstrates an increased sensitivity of recombinant thromboplastins to the presence of LA. This effect was particularly apparent in the POCT INR method. Consequently, we recommend that APS patients who test positive for LA should be monitored using tissue-extract thromboplastins, given their reduced susceptibility to interference by LA.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from individual participants involved in the study.
Author contributions
J.A.R. conceptualized the study; B.d.L. supervised the study; A.J.G.J. arranged the ethical approval and apheresis plasma. R.G., R.M., and C.B. performed the experiments and analyzed the data; R.G., R.G.M., P.G.d.G., J.C.M.M., B.d.L., and J.A.R. interpreted the data. R.G. wrote the initial version of the manuscript, and R.M., C.B., P.G.d.G., A.J.G.J., J.C.M.M., B.d.L., and J.A.R. provided critical revision of the manuscript. All authors read and approved the final manuscript.
Relationship Disclosure
R.G., C.B., P.G.d.G., and B.d.L. are employees of Synapse Research Institute, part of the Diagnostica Stago group. A.J.G.J. reports speaker’s fees and travel cost payments from 3SBio, Amgen, Sobi, and Novartis; international advisory board member of Novartis; and received research funding from CSL Behring, Principia, Sobi, and Argenx (all not applicable to this study). R.G.M., J.C.M.M., and J.A.R. declare that they have no conflicts of interest.
Footnotes
Handling Editor: Michael Makris
Bas de Laat and Jasper A. Remijn contributed equally to this study.
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102470
Supplementary material
References
- 1.Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Molhoek J.E., de Groot P.G., Urbanus R.T. The lupus anticoagulant paradox. Semin Thromb Hemost. 2018;44:445–452. doi: 10.1055/s-0037-1606190. [DOI] [PubMed] [Google Scholar]
- 3.Della Valle P., Crippa L., Garlando A.M., Pattarini E., Safa O., D'angelo S.V., et al. Interference of lupus anticoagulants in prothrombin time assays: implications for selection of adequate methods to optimize the management of thrombosis in the antiphospholipid-antibody syndrome. Haematologica. 1999;84:1065–1074. [PubMed] [Google Scholar]
- 4.Roubey R., Pratt C.W., Buyon J.P., Winfield J.B. Lupus anticoagulant activity of autoimmune antiphospholipid antibodies is dependent upon beta 2-glycoprotein I. J Clin Invest. 1992;90:1100–1104. doi: 10.1172/JCI115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oostin J.D., Derksen R.H., Entjes H.T.I., Bouma B.N., de Groot P.G. Lupus anticoagulant activity is frequently dependent on the presence of β2-glycoprotein I. Thromb Haemost. 1992;67:499–502. [PubMed] [Google Scholar]
- 6.Bevers E., Galli M., Barbui T., Comfurius P., Zwaal R. Lupus anticoagulant IgG's (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb Haemost. 1991;66:629–632. [PubMed] [Google Scholar]
- 7.Ruffatti A., Del Ross T., Ciprian M., Bertero M.T., Salvatore S., Scarpato S., et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers: a prospective multicentre follow-up study. Ann Rheum Dis. 2011;70:1083–1086. doi: 10.1136/ard.2010.142042. [DOI] [PubMed] [Google Scholar]
- 8.De Groot P., Lutters B., Derksen R., Lisman T., Meijers J., Rosendaal F. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost. 2005;3:1993–1997. doi: 10.1111/j.1538-7836.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 9.Galli M., Luciani D., Bertolini G., Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101:1827–1832. doi: 10.1182/blood-2002-02-0441. [DOI] [PubMed] [Google Scholar]
- 10.Galli M., Luciani D., Bertolini G., Barbui T. Anti–β2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood. 2003;102:2717–2723. doi: 10.1182/blood-2002-11-3334. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D., Lim W., Crowther M., Garcia D. A systematic review of the association between anti-β-2 glycoprotein I antibodies and APS manifestations. Blood Adv. 2021;5:3931–3936. doi: 10.1182/bloodadvances.2021005205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chayoua W., Kelchtermans H., Moore G.W., Musiał J., Wahl D., de Laat B., et al. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. J Thromb Haemost. 2018;16:2016–2023. doi: 10.1111/jth.14261. [DOI] [PubMed] [Google Scholar]
- 13.Yelnik C., Urbanski G., Drumez E., Sobanski V., Maillard H., Lanteri A., et al. Persistent triple antiphospholipid antibody positivity as a strong risk factor of first thrombosis, in a long-term follow-up study of patients without history of thrombosis or obstetrical morbidity. Lupus. 2017;26:163–169. doi: 10.1177/0961203316657433. [DOI] [PubMed] [Google Scholar]
- 14.Pengo V., Hoxha A., Andreoli L., Tincani A., Silvestri E., Prisco D., et al. Trial of Rivaroxaban in AntiPhospholipid Syndrome (TRAPS): two-year outcomes after the study closure. J Thromb Haemost. 2021;19:531–535. doi: 10.1111/jth.15158. [DOI] [PubMed] [Google Scholar]
- 15.Khairani C.D., Bejjani A., Piazza G., Jimenez D., Monreal M., Chatterjee S., et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16–30. doi: 10.1016/j.jacc.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodziewicz M., D’Cruz D.P. An update on the management of antiphospholipid syndrome. Ther Adv Musculoskelet Dis. 2020;12 doi: 10.1177/1759720X20910855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limper M., De Leeuw K., Lely A., Westerink J., Teng Y., Eikenboom J., et al. Diagnosing and treating antiphospholipid syndrome: a consensus paper. Neth J Med. 2019;77:98–108. [PubMed] [Google Scholar]
- 18.Beinema M., Brouwers J.R., Schalekamp T., Wilffert B. Pharmacogenetic differences between warfarin, acenocoumarol and phenprocoumon. Thromb Haemost. 2008;100:1052–1057. [PubMed] [Google Scholar]
- 19.Pedersen F., Hamberg O., Hess K., Ovesen L. The effect of dietary vitamin K on warfarin-induced anticoagulation. J Intern Med. 1991;229:517–520. doi: 10.1111/j.1365-2796.1991.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . World Health Organization; Geneva: 1983. WHO Expert Committee on Biological Standardization, sixty-third report. [PubMed] [Google Scholar]
- 21.Cohen H., Cuadrado M.J., Erkan D., Duarte-Garcia A., Isenberg D.A., Knight J.S., et al. 16th International Congress on antiphospholipid antibodies task force report on antiphospholipid syndrome treatment trends. Lupus. 2020;29:1571–1593. doi: 10.1177/0961203320950461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tektonidou M.G., Andreoli L., Limper M., Amoura Z., Cervera R., Costedoat-Chalumeau N., et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schellings M.W., de Maat M.P., De Lathouder S., Weerkamp F. Prolonged prothrombin time after discontinuing vitamin K antagonist. Clin Chem. 2017;63:1442–1444. doi: 10.1373/clinchem.2016.267245. [DOI] [PubMed] [Google Scholar]
- 24.Cohen H., Efthymiou M., Devreese K.M. Monitoring of anticoagulation in thrombotic antiphospholipid syndrome. J Thromb Haemost. 2021;19:892–908. doi: 10.1111/jth.15217. [DOI] [PubMed] [Google Scholar]
- 25.Noordermeer T., Urbanus R.T., Wong C.Y., Jansma J.J., Wiersma N.M., Zivkovic M., et al. Interference in point-of-care international normalized ratio monitoring in patients with lupus anticoagulant is correlated with anti–β2-glycoprotein I antibody titers. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2022.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efthymiou M., Lawrie A.S., Mackie I., Arachchillage D., Lane P.J., Machin S., et al. Thrombin generation and factor X assays for the assessment of warfarin anticoagulation in thrombotic antiphospholipid syndrome. Thromb Res. 2015;135:1191–1197. doi: 10.1016/j.thromres.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Tripodi A., Chantarangkul V., Clerici M., Negri B., Galli M., Mannucci P.M. Laboratory control of oral anticoagulant treatment by the INR system in patients with the antiphospholipid syndrome and lupus anticoagulant. Results of a collaborative study involving nine commercial thromboplastins. Br J Haematol. 2001;115:672–678. doi: 10.1046/j.1365-2141.2001.03178.x. [DOI] [PubMed] [Google Scholar]
- 28.Arnout J., Wittevrongel C., Vanrusselt M., Hoylaerts M., Vermylen J. Beta-2-glycoprotein I dependent lupus anticoagulants form stable bivalent antibody beta-2-glycoprotein I complexes on phospholipid surfaces. Thromb Haemost. 1998;59:79–86. [PubMed] [Google Scholar]
- 29.Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 30.Bloemen S., Zwaveling S., Douxfils J., Roest M., Kremers R., Mullier F. The anticoagulant effect of dabigatran is reflected in the lag time and time-to-peak, but not in the endogenous thrombin potential or peak, of thrombin generation. Thromb Res. 2018;171:160–166. doi: 10.1016/j.thromres.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Devreese K.M., de Groot P.G., de Laat B., Erkan D., Favaloro E.J., Mackie I., et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2828–2839. doi: 10.1111/jth.15047. [DOI] [PubMed] [Google Scholar]
- 32.Taylor J.R., Richter C., Lindamood C., Liu X., Zumberg M., Fletcher B. Accuracy of CoaguChek XS in patients with antiphospholipid syndrome. Point of Care. 2017;16:161–163. [Google Scholar]
- 33.Jepsen S.Y., Larsen J.B., Christensen T.D., Grove E.L., Maegaard M., Hvas A.M. Warfarin monitoring and interference by lupus anticoagulant in patients with antiphospholipid syndrome. Thromb Res. 2022;211:127–132. doi: 10.1016/j.thromres.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Owren P. Thrombotest a new method for controlling anticoagulant therapy. Lancet. 1959;274:754–758. doi: 10.1016/s0140-6736(59)90857-8. [DOI] [PubMed] [Google Scholar]
- 35.Noordermeer T., Molhoek J.E., Schutgens R.E., Sebastian S.A., Drost-Verhoef S., van Wesel A.C., et al. Anti-β2-glycoprotein I and anti-prothrombin antibodies cause lupus anticoagulant through different mechanisms of action. J Thromb Haemost. 2021;19:1018–1028. doi: 10.1111/jth.15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurata M., Sasayama Y., Yamasaki N., Kitazawa I., Hamada Y., Horii I. Mechanism for shortening PT and APTT in dogs and rats-effect of fibrinogen on PT and APTT. J Toxicol Sci. 2003;28:439–443. doi: 10.2131/jts.28.439. [DOI] [PubMed] [Google Scholar]
- 37.Smith S., Comp P., Morrissey J. Phospholipid composition controls thromboplastin sensitivity to individual clotting factors. J Thromb Haemost. 2006;4:820–827. doi: 10.1111/j.1538-7836.2006.01848.x. [DOI] [PubMed] [Google Scholar]
- 38.Okuda M., Yamamoto Y. Usefulness of synthetic phospholipid in measurement of activated partial thromboplastin time: a new preparation procedure to reduce batch difference. Clin Lab Haematol. 2004;26:215–223. doi: 10.1111/j.1365-2257.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson K., Seddon J. The role of lipids in the detection of lupus anticoagulant by the dilute Russell Viper venom test: are platelets or reagents containing hexagonal HII phases necessary? Br J Haematol. 1994;86:583–589. doi: 10.1111/j.1365-2141.1994.tb04790.x. [DOI] [PubMed] [Google Scholar]
- 40.Krudysz-Amblo J., Jennings M.E., Mann K.G., Butenas S. Carbohydrates and activity of natural and recombinant tissue factor. J Biol Chem. 2010;285:3371–3382. doi: 10.1074/jbc.M109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butenas S. Comparison of natural and recombinant tissue factor proteins: new insights. Biol Chem. 2013;394:819–829. doi: 10.1515/hsz-2012-0350. [DOI] [PubMed] [Google Scholar]
- 42.Remijn J.A., Wildeboer B., van Suijlen J.D., Adriaansen H.J. Recombinant thromboplastins vs tissue-extract thromboplastins in patients on unstable oral anticoagulant therapy. Clin Chem. 2011;57:916–917. doi: 10.1373/clinchem.2010.161364. [DOI] [PubMed] [Google Scholar]
- 43.Remijn J.A., Lucas S., Wildeboer B., van Suijlen J.D., Adriaansen H.J. Strongly increased international normalized ratio with recombinant Neoplastin R® compared with tissue extract Neoplastin Plus® in patients initiating oral anticoagulant therapy: implications for anticoagulation dosage. Clin Chem. 2008;54:1929–1931. doi: 10.1373/clinchem.2008.111336. [DOI] [PubMed] [Google Scholar]
- 44.Bader R., Mannucci P., Tripodi A., Hirsh J., Keller F., Solleder E., et al. Multicentric evaluation of a new PT reagent based on recombinant human tissue factor and synthetic phospholipids. Thromb Haemost. 1994;71:292–299. [PubMed] [Google Scholar]
- 45.Simmelink M.J., Horbach D.A., Derksen R.H., Meijers J.C., Bevers E.M., Willems G.M., et al. Complexes of anti-prothrombin antibodies and prothrombin cause lupus anticoagulant activity by competing with the binding of clotting factors for catalytic phospholipid surfaces. Br J Haematol. 2001;113:621–629. doi: 10.1046/j.1365-2141.2001.02755.x. [DOI] [PubMed] [Google Scholar]
- 46.Le Querrec A., Arnout J., Arnoux D., Borg J., Caron C., Darnige L., et al. Quantification of lupus anticoagulants in clinical samples using anti-β2GP1 and anti-prothrombin monoclonal antibodies. Thromb Haemost. 2001;86:584–589. [PubMed] [Google Scholar]
- 47.Arnout J., Meijer P., Vermylen J. Lupus anticoagulant testing in Europe: an analysis of results from the first European Concerted Action on Thrombophilia (ECAT) survey using plasmas spiked with monoclonal antibodies against human β2-glycoprotein I. Thromb Haemost. 1999;81:929–934. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.