Abstract
Steroid 5α-reductase1/2 (SRD5A1/2) is an androgen protein that resembles the plant DET2 and is responsible for the conversion of testosterone to the more active dihydrotestosterone (DHT). Both proteins share functional homology and have a common ancestor. In mammals, the SRD5A1/2 can reduce a wide range of steroid substrates, such as testosterone, progesterone, and aldosterone, by reducing the Δ4 in ring A. The plant DET2 catalyzes the conversion of campesterol (CR) to campestanol (CN) by reducing the Δ5 in ring B during brassinosteroid (BRs) biosynthesis. We compared the evolution, structural, and functional homology of the SRD5A family and tried to identify the origin and functional diversification of duplicated genes in eukaryotes. We presented a detailed phylogeny that includes representative species from all eukaryotic groups. Our study indicated that protist is a common ancestor for all the subgroups of the SRD5A family. However, not all protists possess SRD5A1/2/DET2 or other homologs, suggesting that some protists may have lost these gene families during evolution. Lineage-specific duplication leads Caenorhabditis elegance to have three SRD5A1/2 genes but none was found in Drosophila melanogaster. We also identified a new subclass, DET2-like (DET2L), in plants that closely resemble SRD5A1/2/DET2. The hypothesized reductase DET2L showed no phenotypic enhancement when expressed in Arabidopsisdet2 mutants, suggesting its possible role in the reduction of polyprenol or other substrates.
Keywords: Androgen, Steroid 5α -reductase, DET2, Brassinosteroids, DET2L
1. Introduction
In humans, the steroid 5α-reductase (SRD5A) family has been studied extensively because of their vital role in numerous diseases such as pseudohermaphroditism in males [[1], [2], [3]], prostate cancer [4], hepatic dysfunction, and male pattern alopecia [5,6]. The membrane-embedded enzyme carries out a vital step of conversion of testosterone, a principal androgen, into the more potent dihydrotestosterone (DHT), which is essential for the normal development of male external genitalia and prostate [7]. The isoenzymes, SRD5A1 and SRD5A2, resemble the plant steroid 5α-reductase, de-etoilated2 (DET2) enzyme, which converts campesterol (CR) to campestanol (CN), a critical step in brassinosteroids (BRs) biosynthesis (Fig. 1) [8,9]. BRs are a group of polyhydroxylated steroid hormones crucial for normal cell growth and plant development [10].
Fig. 1.
Biosynthesis of different steroids through reductase reactions in animals and plants. The structures were drawn using ChemDraw Ultra 7.0 software.
In animals, the SRD5A was first identified, purified, and studied in rate liver tissue [[11], [12], [13]]. These enzymes were able to convert the Δ4 group of C-19 and C-21 steroids into 5α-stereoisomers in mammals by carrying out a reducing step. Due to the hydrogenation ability of the Δ4 group, these enzymes were originally called Δ4-5α-hydrogenase [[14], [15], [16]]. Currently, these enzymes are known by many names, such as 5α-reductase, testosterone 5α-reductase, Δ4-3-ketosteroids-5α-reductase, and 3-oxo-5α-reductase-4-dehydrogenase [17]. Later discoveries were made to demonstrate the existence of steroid-reductase activity in liver slices of non-mammalian species of frog, trout, chicken, and newt [18,19].
In the 1960s, a series of biochemical studies were performed to test the hypothesis of the existence of multiple SRD5A enzymes [20]. Later, Wilson (1975) observed a comparatively high level of DHT formation in fibroblasts grown from genital skin compared to nongenital skin [21]. SRD5A activity was directly studied in fibroblast grown from different tissues of individuals with pseudohermaphroditism and other sex development disorders through a series of substrate specificity studies [22]. These early experiments suggested the existence of at least two SRD5A isoenzymes. Following these findings, the hypothesis of the existence of multiple SRD5A isoenzymes was supported by the subsequent experiment [23,24].
The comparative study between human and rat SRD5A showed different sensitivities to SRD5A inhibitor, 4-azasteroids, suggesting the existence of two SRD5A isozymes [25]. The two isoenzymes SRD5A1 and SRD5A2 were finally identified using the expression cloning technique. These two isozymes are pH dependent and exhibit different activity in their respective tissues from the liver and prostate. The SRD5A1 is found in various tissues like the liver, kidneys, adipose tissue, and brain and favors neutral or alkaline conditions ranging from 7.0 to 8.5 [26,27]. In contrast, SRD5A2, primarily expressed in the male reproductive system, such as the prostate, exhibits a pH-optimum of around 5.0 [24]. However, in Chinese hamster ovary cell lysates, the SRD5A2 exhibits high activity at pH 5.0 but has a higher substrate affinity at neutral pH [27]. The SRD5A2 pH-optimum is also dependent on substrate concentration. In rate prostate and epididymis homogenates, the observed pH-optimum is 5.0 at high substrate concentration, while the pH-optimum is 5.5 at low substrate concentration [28]. The acidic pH-optimum of SRD5A2 appears to be an intrinsic characteristic as residue mutation or substitution with less acidic properties compromises the enzymatic activity [[29], [30], [31]]. The third isozyme, SRD5A3, was identified in hormone-refractory prostate cancer cells (HRPC) [32]. Further studies showed that the SRD5A3 has a surprising role in N-linked protein glycosylation (Fig. 1) [33,34].
The plant DET2 was first identified by studying dark-drown mutant plants with de-etiolated seedling phenotype [8]. The DET2 enzyme is involved in an essential step in BRs biosynthesis by catalyzing CR to CN (Fig. 1) [9]. BRs are steroid hormones first purified from Brassica napus pollens [35]. The most active BR is brassinolide (BL), which shares structure similarities with animal reproductive hormones such as estrogens, progestins, and androgens, as well as adrenal cortex hormones such as glucocorticoids. Like animal hormones, BRs directly impact the activity of different plant metabolic pathways involved in cell division, cell elongation and differentiation, vascular differentiation, morphogenesis, reproduction, and stress responses [36,37]. BRs are perceived by the extracellular domain of a membrane-based receptor-like kinases (RLKs) receptor, brassinosteroid insensitive1 (BRI1). Following BRs perception, BRI1 directly associates with co-receptor brassinosteroid insensitive 1-associated kinase1 (BAK1) via transphosphorylation [[38], [39], [40]]. The BAK1 association with BRI1 activates another two components of the BR signaling pathway, the BR signaling kinases (BSKs) and the constitutive differential growth1 (CDG1), that phosphorylate the bri1-suppressor1 phosphatase (BSU1) [41,42]. The BSU1 inhibits BIN2 (GSK3/Shaggy-like kinase brassinosteroid insensitive2), which causes the dissociation of two important transcription factors, BES1 (bri1-EMS suppressor1) and BZR1 (brassinozole resistant1) from 14-3-3 proteins. These transcription factors regulate the expression of several other BR-response genes, which ultimately leads to the control of different plant processes, including plant growth and development [[43], [44], [45], [46], [47]]. Besides BRI1, another two RLKs, BRL1 and BRL3 also directly bind BRs while functioning in vascular differentiation [48]. To date, more than 70 BL-related phytosteroids have been identified in plants [49]. Despite their strong effects on plant growth and regulation, it took more than ten years since its discovery for BRs to be widely considered as essential plant growth regulatory hormones. The previous studies have shown that the BRs biosynthesis mutants, such as det2, cpd, and dwf4, exhibit extreme phenotypes such as dwarfism, reduced male fertility, leaf senescence, delayed flowing time, rounded leaves, and de-etiolation in the dark [9,[50], [51], [52]].
The SRD5A family is highly conserved across land plants and vertebrates. This review summarizes the crucial diverse role of the SRD5A family in plants and animals. We aim to discuss SRD5A family evolution and classification in eukaryotes, its crystal structure, vital functional roles, and related diseases in plants and animals.
2. Phylogenetic analysis of SRD5A family
In vertebrates, the SRD5A family initially consisted of three genes: SRD5A1, SRD5A2, and SRD5A3. The SRD5A1 and SRD5A2 are the functional homologs of plant DET2 [53], while the SRD5A3 is the ortholog of PPRD1 and PPRD2 in plants, and DFG10 in yeast [54]. Later, two more members with diverse functional characteristics were added to the SRD5A family. These members include trans-2-enoyl-CoA reductase (TECR) and TECR-like (TECRL), also known as GSPN2 and GSPN2-like [17,55].
Phylogenetic analysis is regarded as one of the most useful ways to provide a historical narrative for explaining the similarities and differences among different genes, gene families, or species [56]. In order to identify the origin of the SRD5A family and better understand its classification and functional diversity, we conducted a detailed phylogenetic analysis and included representative species from plants, animals, fungi, and protists. For this purpose, plant representative species that were considered come from rhodophyta (Porphyra umbilicalis: Pum), chlorophyta (Chlamydomonas reinhardtii: Cre, Micromonas pusilla: Mipus, Volvox carteri: Vocar), moss (Physcomitrella patens: Pp), fern (Selaginella moellendorffii: Smoe), gymnosperm (Picea abies: Pab, Thuja plicata: Thupl), monocot (Amborella trichopoda: AmT, Oryza sativa: Os, Zea mays: GRMZM, Zostera marina: Zosma), and different groups of dicot (Aquilegia coerulea: Aqcoe, Beta vulgaris: Bvulg, Solanum lycopersicum: Solyc, Lotus japonicus: Lj, Vigna unguiculata: Vigun, Theobroma cacao: Thecc, Eucalyptus grandis: Eucgr, Populus trichocarpa: Potri, Gossypium hirsutum: Gohir, Arabidopsis thaliana: AT, Brassica rapa: Brara). Similarly, animal representative species that were considered come from porifera (Amphimedon queenlandia: Amq), nematode (Caenorhabditis elegans: Cre), arthropod (Drosophila melanogaster: Dpm), echinoderm (Strongylocentrotus purpuratus: Slp), actinopterygii (Danio rerio: Dnr), amphibia (xenopus tropicalis: Xnp), aves (Gallus gallus: Glg), and mammals (Mus musculus: Msm, Homo sapian: Hs). In addition, amoebozoa (Entamoeba histolytica: Enh), alveolata (Eimeria brunetti: Emb), and fungi (Rhizopus delemar: Rhd, Ustilago maydis: Usm, Saccharomyces cerevisiae: Sacer) were also included (Fig. 2). To identify and retrieve the homolog sequences from the databases, A. thaliana DET2, PPRD1/2, and CER10 protein sequences were used as queries against database phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) to retrieve their homologs. Similarly, human SRD5A1/2, SRD5A3, and TECR protein sequences were used as queries against NCBI (https://www.ncbi.nlm.nih.gov/) and ensemble (https://www.ncbi.nlm.nih.gov/) databases. Congenie database (http://congenie.org/) was used to retrieve P. abies sequences.
Fig. 2.
Phylogenetic analysis of SRD5A family in eukaryotes. Animal SRD5A1/2 and plant DET2 protein sequences were used to Blast against the databases to retrieve the SRD5A1/2/DET2 homologs in eukaryotes, including fungi and protists. All the retrieved sequences were aligned with MAFFT, and the tree was inferred by the maximum likelihood (ML) method with 1000 bootstraps using IQ-TREE software. Green branch colors represent plant species, red branch colors represent animal species, and purple node colors represent fungi. Different taxa colors denote different major subclasses. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Previous phylogenetic-based analysis divided the steroid 5α-reductase family into three major subclasses. The SRD5A1/2/DET2 subclass containing the animal SRD5A1, SRD5A2, and plant DET2 grouped together on the sister branches, forming one major clade. Similarly, the SRD5A3/PPRD subclass includes the animal SRD5A3, plants PPRD1 and PPRD2, and fungi DFG10. The third TECR/CER subclass includes animal TECR and TECRL and plant CER10 in one major clade (Fig. 2) [17].
In addition, we identified a new subclass of the SRD5A family in plants. This new subclass could not classify together with any of the three known subclasses but showed a close resemblance of 42 % in sequence similarity to the plant DET2 and 43 % to human SRD5A2 making it the fourth clade in the SRD5A family (Fig. 2; Table 1). This subclass clustered on the separate branch between the SRD5A1/2/DET2 and SRD5A3/PPRD subclasses making an independent clade while displaying close resemblance to both groups (Fig. 2). In Arabidopsis, this gene is referred as hypothetical 3-oxo-5-alpha-steroid 4-dehydrogenase family protein (Accession number: AT5G16010). This hypothetical 5-alpha-steroid gene has not been studied previously nor subjected to phylogenetic analysis and showed close sequence similarity to DET2, and is thus referred to as DET2-like (DET2L) (Table 1). DET2L subclass was only plant-specific, and no gene related to this subclass was found in animals. In addition, not all the protists possess the SRD5A1/2/DET2, SRD5A3/PPRD, or the TECR/CER subfamily members. This suggests that some protists either lost these gene families during evolution or gained them through horizontal gene transfer from other eukaryotes, as horizontal gene transfer is a common mechanism of gene transfer between eukaryotes, protists, and bacteria [57]. Some lineage-specific duplication events resulted in two or more genes. For example, the C. elegans have three copies of SRD5A1/2, including Cre.F19H6.4, Cre.ZK1251.3, and Cre.F42F12.3, while other animal species have only one copy (Fig. 2). On the contrary, the D. melanogaster does not harbor any SRD5A1/2.
Table 1.
Number of amino acids, location, and function of different Arabidopsis reductases and their sequence similarities with DET2 and SRD5A2. The online tool, EMBL-EBI (https://www.ebi.ac.uk/Tools/psa/), was used to determine the similarity between different proteins.
| Gene | Function | Amino acids | Chromosome | Similarity % Plant DET2 | Similarity % Homosapnian SRD5A2 |
|---|---|---|---|---|---|
| DET2 | Campesterol reduction | 262 | 2 | – | 50 |
| DET2L (AT5G16010) | Unknown | 268 | 5 | 42 | 43 |
| PPRD1 | Polyprenol reduction | 320 | 1 | 35 | 27.5 |
| PPRD2 | Polyprenol reduction | 343 | 2 | 34 | 27.5 |
| CER10 | trans-2,3-enoyl-CoA reduction | 310 | 3 | 29 | 28 |
Gene duplication is a common phenomenon in plants. Whole genome duplication (WGD) is considered the driver of genome diversification in evolution [58]. The repeated duplication events have led plants to carry multiple copies of the DET2 family. For instance, the P. patens have two copies of DET2 and two of CET10. Similarly, the P. abies from gymnosperm have multiple copy numbers of DET2L. One of the most recently duplicated genes in the SRD5A family is PPRD1 (AT1G72590), which is only found in A. thaliana. The remaining genes from all three subfamilies are found in all eukaryotic classes. The new subfamily DET2L first originated in moss (P. patens), suggesting an early duplication event occurred in these families. This indicates that the gene duplication that led to the emergence of DET2L happened early in the evolutionary history of land plants, specifically within the lineage of non-vascular plants like mosses [59] (Fig. 2). From their expansion pattern, it can also be suggested that the diversification of SRD5A1/2/DET2, SRD5A3/PPRD, and TECR/CER subfamilies likely established in the protists. However, some protists appear to lost some of these families over the course of evolution. Besides these, another three uncharacterized hypothetical steroid 5α-reductase with remote similarities were also found in A. thaliana, which include the truncated PPRD3 (AT3G43840), AT1G73650, and AT2G46890. The homolog of AT1G73650 has already been reported in Glycine max previously [60]. Because of their remote similarities, none of these genes were included in the phylogeny.
3. Homology of SRD5A family
Members of the subclass SRD5A1/2/DET2 are highly conserved in their reductase domain and thus share a high degree of functional homology. The functional homology between mammalian SRD5A1/2 and plant DET2 has already been shown. The DET2 protein shares 38 % and 42 % sequence identity with the mammalian SRD5A1 and SRD5A2, respectively, and they all share the same hydropathy characteristics [53]. The similarity index of DET2 with mammalian SRD5A1 and SRD5A2 was 48 % and 50 %, respectively (Table 2). The study proved that both genes can substitute for each other and show the same properties in plants and mammals [53]. The DET2 from fern S. moellendorffii also shows high sequence conservation with the DET2 of A. thaliana and can rescue the dwarf phenotype of det2 mutant when expressed in A. thaliana [61]. Another study reported the existence of two DET2 isoenzymes in tomato (Lycopersicon esculentum) that showed high activity on different substrates, including testosterone and progesterone. However, these isoenzymes were less active on plant-derived campestenone when expressed in COS-7 mammalian cells [62]. Similarly, the fungus ustilago mydis gene, UDH1, encodes a protein with high similarity with plant DET2 and mammalian SRD5A1/2. The UDH1 expressed in det2 mutants completely rescued the dwarf mutant phenotype to the wild-type [63]. Although the UDH1 differs from the SRD5A1/2 and DET2 by an additional six domains, it still complements the reductase function in plants. Steroid reductase activity has also been reported in bacteria, such as Treponema denticola. T. denticola is an obligate pathogen found inside the human oral cavity [64]. The study showed that T. denticola encodes a functional steroid reductase protein resembling the mammalian SRD5A1/2, which can reduce different substrates such as progesterone, testosterone, 4-androstenedione, and corticosterone [65]. The acquisition of SRD5A1/2 by T. denticola could have occurred through horizontal gene transfer events. During evolution, a group of bacteria could have acquired this gene from the unicellular protists. Horizontal gene transfer is a common and important phenomenon in bacteria and protists for their survival and environmental adaptation [57,66]. A recent study demonstrated cross-kingdom horizontal gene transfer between plants and their microbiome, where a DET2 of bacterial origin can completely rescue the det2 mutant plants [67].
Table 2.
Number of amino acids, location, and function of different human steroid reductases and their sequence similarities with SRD5A2 and DET2. The online tool, EMBL-EBI (https://www.ebi.ac.uk/Tools/psa/), was used to determine the similarity between different proteins.
| Gene | Function | Amino acids | Chromosome | Similarity % Homosapnian SRD5A2 | Similarity % Plant DET2 |
|---|---|---|---|---|---|
| SRD5A1 | Androgen reduction | 259 | 5p15 | 59 | 48 |
| SRD5A2 | Androgen reduction | 254 | 2p23 | – | 50 |
| SRD5A3 | Polyprenol reduction | 318 | 4q12 | 26 | 32 |
| TECR | trans-2,3-enoyl-CoA reduction | 308 | 19p13 | 26 | 31 |
| TECRL | Unknown | 363 | 4q13 | 25 | 29 |
Since the phylogenetic analysis showed that the newly identified DET2L group of the SRD5A family is closer to the SRD5A1/2/DET2 subclass, it is possible that it could partially complement the det2 mutant due to its weak nature. The phenotypic complementation assay showed that DET2L under the 35S promoter could not complement the det2 mutant phenotype, suggesting that this gene may play a role in polyprenol reduction or other physiological processes (Fig. 3).
Fig. 3.
Phenotypes of transgenic plants expressing DET2 and DET2L in det2 mutants. The DET2 gene was amplified from cDNA synthesized from mRNA extracted from A. thaliana (0-Columbia) with DET2-SacI-F: GAGCTCATGGAAGAAATCGCCGATAAAACCT and DET2-BamHI-R: GGATCCGTACACAAAAGGAATAACAGCTTTAC forward and reversed primers, while DET2L was amplified with DET2L-SacI-F: GAGCTCATGGAAATGGTGACAAGTTTTGTATATCC DET2L-BamHI-R: GGATCCGAATACAAAGGGAATGAGAGCC forward and reversed primers. Amplified genes were cloned it into pCHF3 expression vector and expressed in det2 deficient plants under 35S promoter. The phenotypic complementation assay showed that newly identified DET2L could not complement the mutant plants.
4. Molecular characteristics
The SRD5A1 and SRD5A2 isozymes are composed of 259 and 254 amino acids encoded by SRD5A-1 and SRD5A-2 genes with five exons and four introns, respectively [68,69]. In both genes, the intron positions separating the exons are identical but located on different chromosomes. The human SRD5A-1 is located on chromosome 5p15, whereas the SRD5A-2 is located on chromosome 2p23 (Table 2) [16,70]. Both genes have polymorphisms in their sequences. However, only a few polymorphisms are reported to have an impact on the enzymatic activity of these isozymes. The number of single nucleotide polymorphisms (SNPs) reported for SRD5A-1 and SRD5A-2 are more than 850 and 550, respectively [15]. According to the Human Gene Mutation Database (HGMD, http://www.hgmd.cf.ac.uk), in humans, there are around 124 mutations in SRD5A-2 alone, causing the enzyme deficiency, ultimately leading to different abnormalities. Most of these mutations are missense mutations (n = 86) followed by splicing mutations (n = 10), small deletion (n = 14), small insertion (n = 6), gross deletion (n = 4), small indels (n = 3), and regulatory mutations (n = 1). The first ever SRD5A-2 mutation was reported in individuals from Papua New Guinea, causing the entire gene deletion from affected individuals, and most of these mutations are predominantly localized in exons 1 and 4 [16,68,71].
The SRD5A3 is encoded by SRD5A-3 and is composed of 318 amino acids located on chromosome 4q12 (Table 2). There are over 300 identified SNPs in SRD5A-3 with unclear clinical significance [15]. In SRD5A-3, there are around 16 reported mutations, which include missense mutations (n = 9), small deletion (n = 3), small insertion (n = 2), small indels (n = 1), and complex rearrangements (n = 1). In mice, the loss of function mutation is lethal to mouse embryos, while in humans, it leads to neurological diseases and early visual impairments [33]. To date, TECR and TECRL are the least studied members in the group. TECR and TECRL polypeptides comprise 308 and 363 amino acids, respectively. The TECR is located on chromosome 19p13, while TECRL is located on chromosome 4q13 (https://genome.ucsc.edu/). The TECR has only one missense mutation, while TECRL has two reported mutations, one missense and one splicing (HGMD, http://www.hgmd.cf.ac.uk).
The DET2 encodes a 262 amino acid polypeptide located on chromosome 2 with two exons separated by one intron, which shares a significant amount of sequence similarity with human SRD5A1 and SRD5A2 (Table 1). DET2 carries a missense mutation, where the Gly-204 is replaced by Ala-204, which leads to severe defects in plant light-dependent development. The BR-treated seedlings exhibit a rescued phenotype of det2 mutant, leading to normal plant development. This suggests that residue Glu-204 is essential for steroid reductase activity [8,9,53]. In other plant species, such as Pharbitis nil (Japanese morning glory), L. esculentum (tomato), and Pisum sativum (pea), the presence of det2 mutant has also been reported [62,72,73]. The uncharacterized DET2L also encodes a hypothetical 3-oxo-5α-steroid 4-dehydrogenase family protein positioned on chromosome 5. The polypeptide is 268 amino acids long and is believed to play a role in lipid metabolic processes similar to PPRD1 and PPRD2 (https://www.arabidopsis.org/). Similar in function to human SRD5A3 and yeast DFG10 [74], the genes PPRD1 and PPRD2 are located on chromosomes 1 and 2, respectively. Both genes are redundant in nature and encode polyprenol reductase, with PPRD1 comprising of 320 amino acids and PPRD2 comprising 343 amino acids. PPDR1 can complement the PPDR2 knock-out mutants that display male sterility and produce non-viable plants in Arabidopsis [54]. The CER10 gene is located on chromosome 3, and the polypeptide is 310 amino acids long (https://www.arabidopsis.org/) (Table 1). Deletion/rearrangement cer10 mutants exhibit aerial organ size reduction and display other severe morphological abnormalities [75].
5. Conserved crystal structure of HsSRD5A2
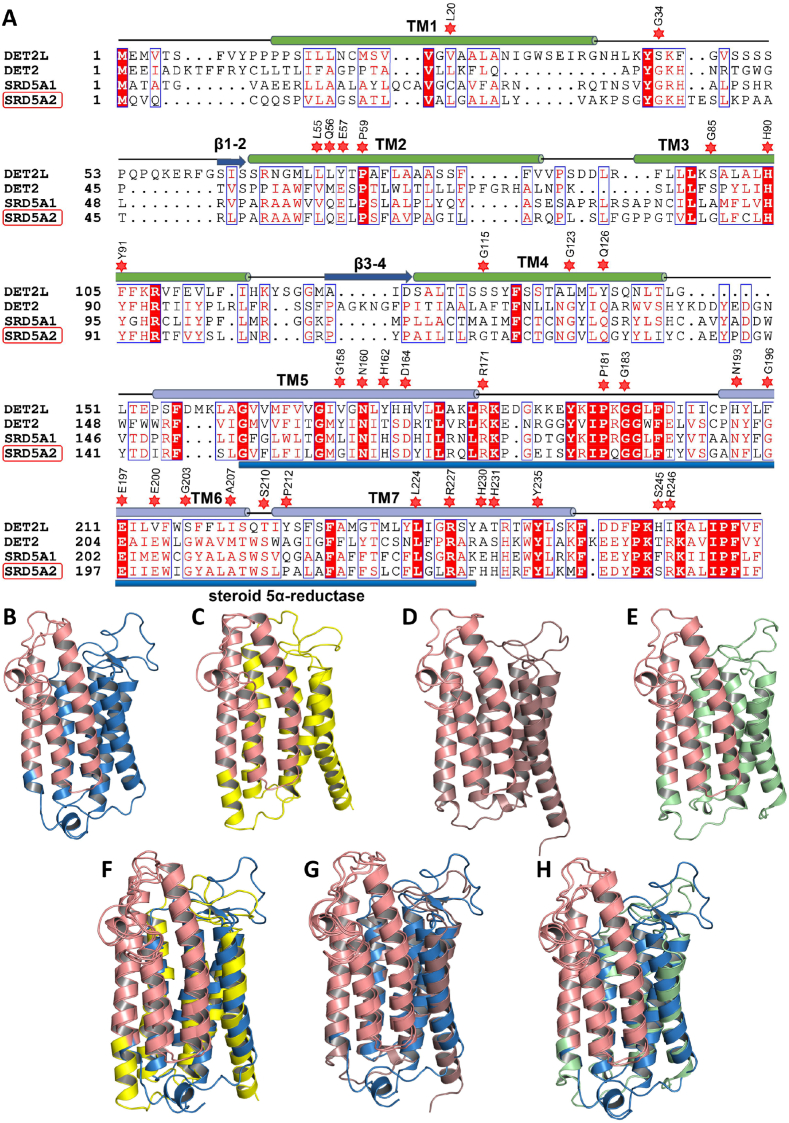
Protein crystal structures are essential as they offer insights into mechanisms of biological activities and facilitate drug development to manipulate or inhibit protein function. Until recently, the crystal structure of any member of the SRD5A family was unknown, and the only closest crystal structure available was of a bacterial membrane-embedded sterol reductase, MaSR1, without any steroid substrate [76]. MaSR1 crystal structure consists of ten transmembrane segments (TM1–10). TM6-10 makes the catalytic domain with two interconnected pockets, one accommodating the NADPH. Compared with the steroid 5β-reductase (AKR1D1) structure, the MaSR1 structure revealed that the junction of the two pockets provides the foundation for NADPH and sterol substrate interaction [76]. AKR1D1 is a steroid 5β-reductase enzyme that reduces Δ4-3-ketosteroids to 5β-dihydrosteroids during bile acid biosynthesis [77]. The first report of the crystal structure of the SRD5A family came from human SRD5A2 (HsSRD5A2). The study revealed that the main HsSRD5A2 peptide consists of seven transmembrane segments (TM1-7) rather than 10-TMs of MaSR1. TM1-4 are responsible for the hydrophobic substrate-binding cavity, while TM5-7 coordinate the cofactor NADPH through an extensive network of hydrogen bonds (Fig. 4A) [29]. The HsSRD5A2 structure conservation was further confirmed by consecutive PbSRD5A crystal structure studies in Proteobacteria bacterium with similar findings [78]. Through mapping the disease-causing missense mutations of SRD5A2, these studies suggested molecular mechanisms for their pathological effects. For example, the HsSRD5A2 G56, E57, and Y91 located in the catalytic pocket are involved in reaction catalysis and finasteride inhibition [29,78], while E57Q and Y91D mutations have been previously shown to reduce enzymatic activity significantly [30,79]. Similarly, N160, D164, R171, N193, E197, R227, H231, and Y235 are essential for NADPH binding (Fig. 4A) [78]. R171S and R227Q likely disrupt the hydrogen bond with NADPH, thus diminishing SRD5A2 activity [29]. R227Q and R246Q are associated with genotype-phenotype correlations. Patients with the R227Q variant exhibit a masculine phenotype with a lower incidence of cryptorchidism, whereas R246Q patients display lower masculinity and a higher incidence of cryptorchidism [80]. Another two compound heterozygous mutations p.Q6X and p.H232R, were identified recently that were contributing to autosomal recessive Disorder of Sex Development (46, XY DSD) [81].
Fig. 4.
Domain organization and crystal structure conservation of DET2L, DET2, SRD5A1, and SRD5A2. (A) Protein sequence alignments of DET2L, DET2, SRD5A1, and SRD5A2. Different transmembranes (TMs) are labeled above the sequences. The blue bar below the sequences represents the steroid 5α-reductase domain. The position of disease-related 34 missense mutations are shown above the sequences. (B–E) Crystal structures of DET2L (B), DET2 (C), SRD5A1 (D), and SRD5A2 (E). (F–H) Aligned structures of DET2L with DET2 (F), SRD5A1 (G), and SRD5A2 (H). The blue, yellow, brown, and pale green colors represent DET2L, DET2, SRD5A1, and SRD5A2, respectively, while the salmon color represents their steroid reductase domains. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The comparison of SRD5A1, SRD5A2, DET2, and DET2L reductase domains shows the same conservation in the steroid 5α-reductase domain. DET2 and DET2L also consist of seven TMs, among which the TM5-7 constitute the steroid 5α-reductase domain, making it a crucial NADPH-dependent regulatory hotspot for substrate reduction (Fig. 4A) [29]. Dali searches (http://ekhidna2.biocenter.helsinki.fi/dali/) performed by using DET2L structure (AlphaFold protein structure database code: Q9LFS3) indicated that all the DET2L TMs could be superimposed with TMs of PbSRD5A (AlphaFold protein structure database code: A0A3A0FWV3) and HsSRD5A2 (AlphaFold protein structure database code: P31213) and overall predicted crystal structure and reductase domain of DET2L can be perfectly aligned with the DET2 and SRD5A2 (Fig. 4B–H). Given the level of sequence conservation, it is likely that DET2L may also exhibit some steroid substrate reduction properties similar to that of SRD5A2 and DET2.
6. Biological functions of SRD5A family
The SRD5A1/2/DET2 subclass is widely distributed across animal and plant kingdoms. Within the SRD5A family, SRD5A1, SRD5A2, and, to a lesser extent, SRD5A3 have been studied extensively. The recently added members, TECR and TECRL, also show a closer resemblance in sequences similarity to other SRD5A family members but not in function [17,55]. In vertebrates, the co-localized pattern of these enzymes in various tissues might suggest some similarity in their biological function. The SRD5A1 and SRD5A2 catalyze the Δ4 bond in ring A of a substrate using cofactor NADPH [16]. These substrates are the steroids “3-keto, C19, and C21”. The 3-keto represents the double bond between oxygen and carbon at carbon atoms 4 and 5. This reaction yields an irreversible, stereospecific double bond breakage between C4 and C5 followed by hydrogenation of C5 and insertion of proton at position C4 with the help of NADPH (Fig. 1). Different reduction substrates for SRD5A1/2 are testosterone, progesterone, aldosterone, cortisol, deoxycorticosterone, and androstenedione [15]. Cholesterol is the common precursor for biosynthesis of these steroid substrates. These steroids are produced in the adrenal cortex, the gonads, and the placenta. In addition, some of these hormones are also produced in the brain, known as neurosteroids [82]. For instance, progesterone is commonly regarded as a pregnancy hormone in females [83]. In the brain, the progesterone is first converted to 5α-dihydroprogesterone by the action of SRD5A1/2. This is followed by the synthesis of allopregnanolone from 5α-dihydroprogesterone, catalyzed by 3α-hydroxysteroid dehydrogenase (3α-HSD). The allopregnanolone binds the γ-amino-butyric type A (GABAA) receptor, regulating anxiety and neurosteroid-mediated effects [[84], [85], [86]]. Similarly, cortisol is released from the adrenal gland, which is mostly known for its physiological role associated with behavioral processes, glucose metabolism, energy regulation, and stress responses [87]. Metabolism of cortisol involves the synthesis of 5α-dihydrocortisol from cortisol catalyzed by SRD5A1/2. 5α-dihydrocortisol is subsequently converted into 5α-tetrahydrocortisol (5α-THF) by 3α-HSD [85,88]. Androstenedione is another steroid hormone that plays a role in “castration-resistant” prostate cancer and is catalyzed by SRD5A1/2 to produce 5α-androstenedione, which is either reduced to 5α-dihydrotestosterone or to androsterone [84,85,89]. A recent study reported that epitestosterone, a stereoisomer of the active androgen testosterone, elevates the androgen receptor transactivation through 5α-reduction by SRD5A1 and SRD5A2. This evidence indicates that steroid 5α-reductases play a role in regulating the modulatory effect of epitestosterone on androgen receptor signaling [90].
The dominant androgen in vertebrates is DHT, associated with prostate gland growth and differentiation, male external genitalia development, and secondary characteristics in males [16]. The deficiency in DHT leads to several human diseases, such as prostate cancer, pseudohermaphroditism, alopecia, acne, and hirsutism [71,91,92]. To regulate the SRD5A transcription, both testosterone and DHT can bind the androgen receptor (AR), but DHT can bind the AR with a much greater affinity (2–5 times) than testosterone. In addition, the potency rate of DHT is also ten times higher than testosterone [26,93]. Recently, DHT has been reported to increase bladder cancer risk by interacting with AR in vivo and in vitro [94,95]. Previous studies have shown that mutation in SRD5A2 leads to abnormal embryogenesis and results in defects in male external genitalia and prostate cancer. However, another study revealed that SRD5A2 deficient pseudohermaphrodite males still show virilization, which was explained by the existence of SRD5A1 activity [68]. There is reported evidence of SRD5A enzyme secretion into the sperm, which highlights its potential role in sperm physiopathology and related varicocele infertility [96]. Similarly, an enhanced SRD5A activity has been reported in females with polycystic ovary syndrome [97,98]. SRD5A2 also plays a key role in sex assignment during embryogenesis. The amphibian Rana clamitans eggs produce 98 % male offspring if exposed to DHT until metamorphosis [99]. SRD5A2 deficiency (46, XY DSD) and DHT deficiency or chronic exposure to SRD5A inhibitor results in undervirilization. It shifts the balance in favor of the female phenotype, suggesting that SRD5A2 reduction reaction is necessary for DHT formation, which leads to the normal development of male reproductive organs [17,100,101]. Two SRD5A-2 genetic variants have also been found to be associated with sex differences in the disease characteristics of hepatitis B-infected patients [102]. Moreover, the human SRD5A-2 promoter shows repressed activity in human prostate cell lines when treated with Phyllostachys pubescens leaf extract. The study also showed that the rats treated with P. pubescens leaves extract showed reduced prostate weight, while the SRD5A2, testosterone, dihydrotestosterone, and prostate-specific antigen serum levels were significantly reduced [103]. During the recent COVID-19 outbreak, it was found that SARS-CoV-2 and other coronaviruses utilize the transmembrane serine protease 2, which is expressed through the androgen receptor signaling, for the entry into the host system [104,105]. As a result, androgen signaling has been associated directly with the COVID-19 disease severity, especially in men. Therefore, the SRD5A inhibitors, including finasteride and dutasteride, which mainly target the SRD5A2 but also SRD5A1, have been suggested to hold the potential for treating COVID-19 [106,107]. Another novel type of SRD5A inhibitor, the dehydroepiandrosterone derivatives, could also inhibit the proliferation of lymph node carcinoma of the prostate (LNCaP) cells during prostate cancer, showing therapeutic potential to treat metastatic prostate cancer [108].
Similarly, besides the roles in sex assignment and sex-related diseases, SRD5A1/2 is also directly associated with facial sebum production [109,110]. The root extract of Asparagus racemosus controls sebum production by down-regulating the SRD5A-1 and SRD5A-2 mRNA levels, thus promoting the antioxidant effects [111]. Similarly, the increased conversion of testosterone to DHT by SRD5A2 also leads to a hair loss condition called androgenetic alopecia (AGA) [112]. Alternative to finasteride, the tubtim chumphae rice extract compounds significantly downregulated the expression of SRD5A-1, SRD5A-2, and SRD5A-3 without causing any adverse effects, which could potentially be used as an anti-hair loss product [113]. Moreover, the banana flower extract and arabica coffee pulp extracts have been analyzed to prevent AGA by stimulating hair cell growth by downregulating SRD5A-1, SRD5A-2, SRD5A-3, and DHT [114,115]. A computational approach showed that bioactive metabolites (zhankuic acid, sterenin M, and melleolide K) from edible mushrooms have SRD5A2 inhibitory potential, which could also be used as an alternative to finasteride to treat AGA [116].
The SRD5A3 was first identified to play a role in DHT production and maintenance of androgen-androgen receptor-pathway activation in HRPC cells [32,117]. Later, an in vitro expression assay in human embryonic kidney 293 (HEK-293) cells showed that hamster and human SRD5A3 were unable to reduce testosterone, progesterone, androstenedione, and corticosterone [118]. In human abdominal adipose tissues, SRD5A3 was the most highly expressed isoenzyme, followed by SRD5A1 [119]. In addition, high expression levels of SRD5A3 have also been reported in prostate cancer, endometrial cancer, and human fetal liver [118,120,121]. In breast cancer tissues, the high expression level of SRD5A3 is linked to poorer prognosis [122]. Interestingly, a rare congenital neurodevelopmental disorder develops due to mutations in the SRD5A-3 gene. The patient exhibits mental retardation, ophthalmologic, facial dysmorphia, and cerebellar defects [33,123,124]. This could be explained by SRD5A3 implication in N-linked protein glycosylation by the polyprenol reductase activity [33]. The N-glycosylation is required for the correct protein folding and trafficking in the endoplasmic reticulum membrane, where a 14-sugar glycan is added to a selected asparagine residue. SRD5A3/interferon regulatory factor2 (IRF2) complex promotes malignant growth and glycolysis in glioma cells, while the si-SRD5A3-1 or si-SRD5A3-2 suppresses the growth and proliferation of glioma cells [125].
The TECR enzyme, on the other hand, is known to play a role in the synthesis of very long-chain fatty acid (VLCFA) and sphingosine degradation. The TECR reduces the trans-2-enoyl-CoA into an elongated acyl-CoA with the help of the cofactor NADPH [126]. In ER, this elongation is mediated by the TECR interaction with 3-hydroxyacyl-CoA dehydratase (HACD1/2) in substrate transfer in VLCFA [127]. Mutation in the TECR gene results in a rare autosomal syndrome of nonsyndromic mental retardation [128]. Moreover, TECR is also involved in the protective role of blood-brain barrier (BBB) integrity against transcytosis to maintain BBB homeostasis [129].
The plant DET2 is a member of the SRD5A family. The DET2 is homolog in function to the mammalian SRD5A1 and SRD5A2 involved in BRs biosynthesis. It has been shown that human SRD5A1 and SRD5A2 can rescue the det2 mutant to a completely normal phenotype. Similarly, the plant DET2 expressed in HEK-293 cells showed reductase activity to several animal steroid substrates [53], suggesting that the plant and animal SRD5A can be substituted. However, unlike the human SRD5A1 and SRD5A2, which catalyze the Δ4 in ring A, the DET2 reduces the Δ5 in ring B (Fig. 1) [53]. The DET2 catalyzes a second step in BRs biosynthesis by converting (24R)-ergost-4-en-3-one (4-en-3-one) into (24R)-5α-ergost-3-one (3-one) [130,131]. In 2002, a new secondary pathway was discovered in the det2 mutant by an early C-22 oxidation, revealing that the defective mutant was unable to convert 22-OH-4-en-one to 22-OH-3-one, which is a vital step in BR biosynthesis [132]. In contrast to human SRD5A1 and SRD5A2, the DET2 shows no inhibition to finasteride (4-azasteroide) [53], suggesting the existence of a DET2-like gene. Given its vital role in BR biosynthesis, DET2 has been studied in numerous plant species. For instance, enhanced resistance to oxidative stress has been reported in the A. thaliana det2 mutant [133]. In cotton (Gossypium hirsutum L.), the DhDET2 and BRs play a crucial role in the initiation and elongation of cotton fiber cells [134]. Similarly, DET2 has also been implicated in improved CS levels, enhanced cambium cell division, and xylem differentiation in poplar and rose gum (Eucalyptus grandis) [135,136]. Recently, DET2 has also been associated with the regulation of grape berries ripeness and water stress response in cabernet sauvignon (Vitis vinifera L.) by down-regulating the expression of VvDET2, suggesting its role in modulation of grape berry maturation and adaptation to water stress in cabernet sauvignon [137]. Another recent study reported that PyDET2e plays an important role in the rapid growth period of Populus yunnanensis, giving a new insight into the crucial role of steroid hormones in plants [138]. BRs defective det2-1 mutant, together with bin-2-1, display more sensitivity to salt stress than the wild-type Columbia [139]. In some plant species, more than one copy of DET2 has been reported [60,62]. Since the det2 is a relatively strong mutant but not null, there is a possibility of a second gene complementing the det2 phenotype in Arabidopsis.
In plants, the SRD5A3 has two orthologues, the PPRRD1 and PPRD2, which play a prominent role in plant protein glycosylation. They encode a polyprenol reductase that converts polyprenol to dolichol, an active cofactor required for protein glycosylation [54]. The glycosylation is required for protein functioning as it plays essential role in protein folding, quality control, and a variety of biological recognition events [140]. The pprd2 mutant plants display an abnormal male gametophyte and sporophyte development [54].
Similar to animal TECR, the plant enoyl-CoA reductase (CER, CER10, GLASSY HAIR6) is also involved in the biosynthesis of VLCFA, which are required for lipid storage and sphingolipid and cuticular wax formation [75,141]. The cer10 mutants exhibit severe reduction in cell size, abnormal organ morphology, and stem glossiness. Most likely, the reduction in VLCFA content of sphingolipids contributes to the morphological changes [75]. In Arabidopsis, the VLCFA synthesis is regulated by bax inhibitor-1 (BI-1) and CER10 complex, together with other interacting enzymes, during oxidative stress responses [142]. CER10 also plays a crucial role in Arabidopsis osmotolerance by regulating VLCFA metabolism involved in wax loading and endocytic membrane trafficking [143].
7. Conclusion and perspective
This study illustrates the origin and functional conservation of the SRD5A family in all eukaryotes. We developed a detailed phylogenetic analysis of early-originated species along with fungi, animal, and plant kingdoms and tried to identify the origin and functional diversification of SRD5A family members. The reductase family traces back to a common ancestor, followed by diversification into different subgroups. The highly conserved biochemical function in animal and plant kingdoms implies the importance of their physiological roles. Although not all the steroid-5α reductases would reduce common substrates, the overlapping role of SRD5A1/2/DET2 could help to understand the dynamics of steroid-related diseases in plants and animals such as prostate cancer, prostate hyperplasia, dwarfism, and male infertility. It could help treat these conditions in animals and improve stress-tolerant crop production in plants. Following duplication, most commonly, the duplicated genes are eliminated from the genome, but in some cases, small mutations accumulate and are fixed, causing the functional divergence between the duplicated genes [144,145]. Duplication events have been observed in nematodes, where three orthologs of SRD5A1/2 were found but none in Drosophila. The det2 mutant displays a comparatively weaker phenotype than the other BRs biosynthetic pathway mutants, such as cpd and dwf4 [49,51,146,147]. This could result from another potential gene partially complementing the det2 mutant. Based on our phylogenetic study, we found that both DET2 and DET2L are found in the moss and are the closest. Thus, the DET2L could partially complement the mutant phenotype and other physiological functions. However, the Over-expression (Ox) of DET2L in det2 BRs-deficient Arabidopsis could not enhance the dwarf phenotype, suggesting that this gene has already evolved and diverted into a new function gene and plays some other role in other substrates reduction. To further investigate the potential reductase function of DET2L in Arabidopsis, a double knockout mutant of det2 and DET2L would be required to check whether it further affects the phenotype.
Fundings
This work was supported by Fundamental Research Fund for Central Universities to K.A. (GK202304041) and G.W. (GK202001010); Chinese Natural Foundation of Science to G.W. (g32070325, 31270324, and 31741014).
Data availability statement
All datasets generated for these findings are available in the main text, further inquiries can be directed to the corresponding author.
CRediT authorship contribution statement
Khawar Ali: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Funding acquisition, Conceptualization. Wenjuan Li: Writing – review & editing, Methodology, Investigation, Formal analysis. Guang Wu: Writing – review & editing, Validation, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no conflict of interests and that they have no known competing financial interests that could have appeared to influence the work reported in this manuscript.
Contributor Information
Khawar Ali, Email: aali@snnu.edu.cn.
Guang Wu, Email: gwu3@snnu.edu.cn.
References
- 1.Imperato-McGinley J., Guerrero L., Gautier T., Peterson R.E. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186:1213–1215. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- 2.Mendonca B.B., Inacio M., Costa E.M., Arnhold I.J., Silva F.A., Nicolau W., Bloise W., Russel D.W., Wilson J.D. Male pseudohermaphroditism due to steroid 5alpha-reductase 2 deficiency. Diagnosis, psychological evaluation, and management, Medicine (Baltimore) 1996;75:64–76. doi: 10.1097/00005792-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Wilson J.D., Griffin J.E., Russell D.W. Steroid 5 alpha-reductase 2 deficiency. Endocr. Rev. 1993;14:577–593. doi: 10.1210/edrv-14-5-577. [DOI] [PubMed] [Google Scholar]
- 4.J.M. Kokontis, S. Liao Molecular action of androgen in the normal and neoplastic prostate, in: G. Litwack (Ed.) Vitamins & Hormones, Academic Press1998, pp. 219-307. [DOI] [PubMed]
- 5.Clayton P.T., Mills K.A., Johnson A.W., Barabino A., Marazzi M.G. Delta 4-3-oxosteroid 5 beta-reductase deficiency: failure of ursodeoxycholic acid treatment and response to chenodeoxycholic acid plus cholic acid. Gut. 1996;38:623–628. doi: 10.1136/gut.38.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998;317:865–869. doi: 10.1136/bmj.317.7162.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson J.D. Recent studies on the mechanism of action of testosterone. N. Engl. J. Med. 1972;287:1284–1291. doi: 10.1056/NEJM197212212872508. [DOI] [PubMed] [Google Scholar]
- 8.Chory J., Nagpal P., Peto C.A. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 1991;3:445–459. doi: 10.1105/tpc.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Nagpal P., Vitart V., McMorris T.C., Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 10.Bishop G.J., Koncz C. Brassinosteroids and plant steroid hormone signaling. Plant Cell. 2002;14:S97–S110. doi: 10.1105/tpc.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomkins G.M. The enzymatic reduction of delta 4-3-ketosteroids. J. Biol. Chem. 1957;225:13–24. [PubMed] [Google Scholar]
- 12.Samuels L.T., Sweat M.L., Levedahl B.H., Pottner M.M., Helmreich M.L. Metabolism of testosterone by livers of different species of animals. J. Biol. Chem. 1950;183:231–239. [Google Scholar]
- 13.Okuda A., Okuda K. Purification and characterization of delta 4-3-ketosteroid 5 beta-reductase. J. Biol. Chem. 1984;259:7519–7524. [PubMed] [Google Scholar]
- 14.Dorfman R.I., Forchielli E. Separation of delta 4-5 alpha-hydrogenases from rat liver homogenates. J. Biol. Chem. 1956;223:443–448. [PubMed] [Google Scholar]
- 15.Azzouni F., Godoy A., Li Y., Mohler J. The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Advances in Urology. 2012;2012:1–18. doi: 10.1155/2012/530121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell D.W., Wilson J.D. Steroid 5α-reductase: two genes/two enzymes. Annu. Rev. Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 17.Langlois V.S., Zhang D., Cooke G.M., Trudeau V.L. Evolution of steroid-5α-reductases and comparison of their function with 5β-reductase. Gen. Comp. Endocrinol. 2010;166:489–497. doi: 10.1016/j.ygcen.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Lisboa B.P., Breuer H. [Studies on the metabolism of steroid hormones in vertebrates. VI. Reductive metabolism of testosterone and related C19-steroids in liver preparations from the trout, triton, frog and fowl] Gen. Comp. Endocrinol. 1966;6:114–124. doi: 10.1016/0016-6480(66)90113-4. [DOI] [PubMed] [Google Scholar]
- 19.Lisboa B.P., Breuer H., Witschi E. [Studies on the metabolism of steroid hormones in vertebrates. IX. The metabolism of (4- 14 C)testosterone in the liver of the African water frog (Xenopus laevis)] Hoppe-Seyler's Zeitschrift fur physiologische Chemie. 1972;353:1907–1914. [PubMed] [Google Scholar]
- 20.McGuire J.S., Jr., Tomkins G.M. The heterogeneity of Δ4-3-ketosteroid reductases (5α) J. Biol. Chem. 1960;235:1634–1638. [Google Scholar]
- 21.Wilson J.D. Dihydrotestosterone formation in cultured human fibroblasts. Comparison of cells from normal subjects and patients with familial incomplete male pseudohermaphroditism, Type 2. J. Biol. Chem. 1975;250:3498–3504. [PubMed] [Google Scholar]
- 22.Moore R.J., Griffin J.E., Wilson J.D. Diminished 5alpha-reductase activity in extracts of fibroblasts cultured from patients with familial incomplete male pseudohermaphroditism, type 2. J. Biol. Chem. 1975;250:7168–7172. [PubMed] [Google Scholar]
- 23.Bruchovsky N., Rennie P.S., Batzold F.H., Goldenberg S.L., Fletcher T., McLoughlin M.G. Kinetic parameters of 5 alpha-reductase activity in stroma and epithelium of normal, hyperplastic, and carcinomatous human prostates. J. Clin. Endocrinol. Metabol. 1988;67:806–816. doi: 10.1210/jcem-67-4-806. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins E.P., Andersson S., Imperato-McGinley J., Wilson J.D., Russell D.W. Genetic and pharmacological evidence for more than one human steroid 5 alpha-reductase. J. Clin. Invest. 1992;89:293–300. doi: 10.1172/JCI115574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson S., Russell D.W. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc. Natl. Acad. Sci. U.S.A. 1990;87:3640–3644. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normington K., Russell D.W. Tissue distribution and kinetic characteristics of rat steroid 5 alpha-reductase isozymes. Evidence for distinct physiological functions. J. Biol. Chem. 1992;267:19548–19554. [PubMed] [Google Scholar]
- 27.Thigpen A.E., Cala K.M., Russell D.W. Characterization of Chinese hamster ovary cell lines expressing human steroid 5 alpha-reductase isozymes. J. Biol. Chem. 1993;268:17404–17412. [PubMed] [Google Scholar]
- 28.Span P.N., Smals A.G., Sweep C.G., Benraad T.J. Rat steroid 5 alpha-reductase kinetic characteristics: extreme pH-dependency of the type II isozyme in prostate and epididymis homogenates. J. Steroid Biochem. Mol. Biol. 1995;54:185–192. doi: 10.1016/0960-0760(95)00125-j. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Q., Wang L., Supekar S., Shen T., Liu H., Ye F., Huang J., Fan H., Wei Z., Zhang C. Structure of human steroid 5α-reductase 2 with the anti-androgen drug finasteride. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wigley W.C., Prihoda J.S., Mowszowicz I., Mendonca B.B., New M.I., Wilson J.D., Russell D.W. Natural mutagenesis study of the human steroid 5 alpha-reductase 2 isozyme. Biochemistry. 1994;33:1265–1270. doi: 10.1021/bi00171a029. [DOI] [PubMed] [Google Scholar]
- 31.Batista R.L., Mendonca B.B. The molecular basis of 5α-reductase type 2 deficiency. Sex. Dev. 2022;16:171–183. doi: 10.1159/000525119. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K., Furihata M., Tsunoda T., Ashida S., Takata R., Obara W., Yoshioka H., Daigo Y., Nasu Y., Kumon H., Konaka H., Namiki M., Tozawa K., Kohri K., Tanji N., Yokoyama M., Shimazui T., Akaza H., Mizutani Y., Miki T., Fujioka T., Shuin T., Nakamura Y., Nakagawa H. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67:5117–5125. doi: 10.1158/0008-5472.CAN-06-4040. [DOI] [PubMed] [Google Scholar]
- 33.Cantagrel V., Lefeber D.J., Ng B.G., Guan Z., Silhavy J.L., Bielas S.L., Lehle L., Hombauer H., Adamowicz M., Swiezewska E., De Brouwer A.P., Blümel P., Sykut-Cegielska J., Houliston S., Swistun D., Ali B.R., Dobyns W.B., Babovic-Vuksanovic D., van Bokhoven H., Wevers R.A., Raetz C.R.H., Freeze H.H., Morava É., Al-Gazali L., Gleeson J.G. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:203–217. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stiles A.R., Russell D.W. SRD5A3: a surprising role in glycosylation. Cell. 2010;142:196–198. doi: 10.1016/j.cell.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grove M.D., Spencer G.F., Rohwedder W.K., Mandava N., Worley J.F., Warthen J.D., Steffens G.L., Flippen-Anderson J.L., Cook J.C. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979;281:216–217. [Google Scholar]
- 36.Clouse S.D. Brassinosteroids. Arabidopsis Book. 2011;9 doi: 10.1199/tab.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Z., Li J. Brassinosteroids regulate root growth, development, and symbiosis. Mol. Plant. 2016;9:86–100. doi: 10.1016/j.molp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Nam K.H. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science. 2002;295:1299–1301. doi: 10.1126/science.1065769. [DOI] [PubMed] [Google Scholar]
- 39.Wang X., Goshe M.B., Soderblom E.J., Phinney B.S., Kuchar J.A., Li J., Asami T., Yoshida S., Huber S.C., Clouse S.D. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell. 2005;17:1685–1703. doi: 10.1105/tpc.105.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Kota U., He K., Blackburn K., Li J., Goshe M.B., Huber S.C., Clouse S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Mora-García S., Vert G., Yin Y., Caño-Delgado A., Cheong H., Chory J. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 2004;18:448–460. doi: 10.1101/gad.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim T.-W., Guan S., Burlingame A.L., Wang Z.-Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol cell. 2011;43:561–571. doi: 10.1016/j.molcel.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J., Nam K.H., Vafeados D., Chory J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 2001;127:14–22. doi: 10.1104/pp.127.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He J.X., Gendron J.M., Yang Y., Li J., Wang Z.Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10185–10190. doi: 10.1073/pnas.152342599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z.Y., Nakano T., Gendron J., He J., Chen M., Vafeados D., Yang Y., Fujioka S., Yoshida S., Asami T., Chory J. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell. 2002;2:505–513. doi: 10.1016/s1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 46.Yin Y., Wang Z.Y., Mora-Garcia S., Li J., Yoshida S., Asami T., Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z.-Y., Bai M.-Y., Oh E., Zhu J.-Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 2012;46:701–724. doi: 10.1146/annurev-genet-102209-163450. [DOI] [PubMed] [Google Scholar]
- 48.Cano-Delgado A. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development. 2004;131:5341–5351. doi: 10.1242/dev.01403. [DOI] [PubMed] [Google Scholar]
- 49.Zhao B., Li J. Regulation of brassinosteroid biosynthesis and InactivationF. J. Integr. Plant Biol. 2012;54:746–759. doi: 10.1111/j.1744-7909.2012.01168.x. [DOI] [PubMed] [Google Scholar]
- 50.Clouse S.D., Sasse J.M. BRASSINOSTEROIDS: essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- 51.Choe S., Dilkes B.P., Fujioka S., Takatsuto S., Sakurai A., Feldmann K.A. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szekeres M., Németh K., Koncz-Kálmán Z., Mathur J., Kauschmann A., Altmann T., Rédei G.P., Nagy F., Schell J., Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- 53.Li J., Biswas M.G., Chao A., Russell D.W., Chory J. Conservation of function between mammalian and plant steroid 5alpha-reductases. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3554–3559. doi: 10.1073/pnas.94.8.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jozwiak A., Gutkowska M., Gawarecka K., Surmacz L., Buczkowska A., Lichocka M., Nowakowska J., Swiezewska E. POLYPRENOL REDUCTASE2 deficiency is lethal in Arabidopsis due to male sterility. Plant Cell. 2015;27:3336–3353. doi: 10.1105/tpc.15.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scaglione A., Montemiglio L.C., Parisi G., Asteriti I.A., Bruni R., Cerutti G., Testi C., Savino C., Mancia F., Lavia P., Vallone B. Subcellular localization of the five members of the human steroid 5α-reductase family. Biochimie Open. 2017;4:99–106. doi: 10.1016/j.biopen.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiley E.O. Why trees are important. Evolution: Education and Outreach. 2010;3:499–505. [Google Scholar]
- 57.Eme L., Gentekaki E., Curtis B., Archibald J.M., Roger A.J. Lateral gene transfer in the adaptation of the anaerobic parasite blastocystis to the gut. Curr. Biol. : CB. 2017;27:807–820. doi: 10.1016/j.cub.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Clark J.W., Donoghue P.C.J. Whole-genome duplication and plant macroevolution. Trends Plant Sci. 2018;23:933–945. doi: 10.1016/j.tplants.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Chater C.C., Caine R.S., Tomek M., Wallace S., Kamisugi Y., Cuming A.C., Lang D., MacAlister C.A., Casson S., Bergmann D.C., Decker E.L., Frank W., Gray J.E., Fleming A., Reski R., Beerling D.J. Origin and function of stomata in the moss Physcomitrella patens. Nat. Plants. 2016;2 doi: 10.1038/nplants.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huo W., Li B., Kuang J., He P., Xu Z., Wang J. Functional characterization of the steroid reductase genes GmDET2a and GmDET2b form Glycine max. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu W., Zheng B., Bai Q., Wu L., Liu Y., Wu G. Functional study of the brassinosteroid biosynthetic genes from Selagnella moellendorfii in Arabidopsis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosati F., Bardazzi I., De Blasi P., Simi L., Scarpi D., Guarna A., Serio M., Racchi M.L., Danza G. 5alpha-Reductase activity in Lycopersicon esculentum: cloning and functional characterization of LeDET2 and evidence of the presence of two isoenzymes. J. Steroid Biochem. Mol. Biol. 2005;96:287–299. doi: 10.1016/j.jsbmb.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 63.Basse C.W., Kerschbamer C., Brustmann M., Altmann T., Kahmann R. Evidence for a Ustilago maydis steroid 5alpha-reductase by functional expression in Arabidopsis det2-1 mutants. Plant Physiol. 2002;129:717–732. doi: 10.1104/pp.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dashper S.G., Seers C.A., Tan K.H., Reynolds E.C. Virulence factors of the oral spirochete Treponema denticola. J. Dent. Res. 2011;90:691–703. doi: 10.1177/0022034510385242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark D.T., Soory M. Steroid 5alpha-reductase activity of Treponema denticola. Oral Microbiol. Immunol. 2007;22:326–332. doi: 10.1111/j.1399-302X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 66.Sun D. Pull in and push out: mechanisms of horizontal gene transfer in bacteria. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haimlich S., Fridman Y., Khandal H., Savaldi-Goldstein S., Levy A. Widespread horizontal gene transfer between plants and their microbiota. bioRxiv. 2022 doi: 10.1093/ismeco/ycae073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thigpen A.E., Davis D.L., Gautier T., Imperato-McGinley J., Russell D.W. The molecular basis of steroid 5α-reductase deficiency in a large Dominican kindred. N. Engl. J. Med. 1992;327:1216–1219. doi: 10.1056/NEJM199210223271706. [DOI] [PubMed] [Google Scholar]
- 69.Thigpen A.E., Davis D.L., Milatovich A., Mendonca B.B., Imperato-McGinley J., Griffin J.E., Francke U., Wilson J.D., Russell D.W. Molecular genetics of steroid 5 alpha-reductase 2 deficiency. J. Clin. Invest. 1992;90:799–809. doi: 10.1172/JCI115954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jenkins E.P., Hsieh C.-L., Milatovich A., Normington K., Berman D.M., Francke U., Russell D.W. Characterization and chromosomal mapping of a human steroid 5α-reductase gene and pseudogene and mapping of the mouse homologue. Genomics. 1991;11:1102–1112. doi: 10.1016/0888-7543(91)90038-g. [DOI] [PubMed] [Google Scholar]
- 71.Andersson S., Berman D.M., Jenkins E.P., Russell D.W. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nomura T., Jager C.E., Kitasaka Y., Takeuchi K., Fukami M., Yoneyama K., Matsushita Y., Nyunoya H., Takatsuto S., Fujioka S., Smith J.J., Kerckhoffs L.H., Reid J.B., Yokota T. Brassinosteroid deficiency due to truncated steroid 5alpha-reductase causes dwarfism in the lk mutant of pea. Plant Physiol. 2004;135:2220–2229. doi: 10.1104/pp.104.043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki Y., Saso K., Fujioka S., Yoshida S., Nitasaka E., Nagata S., Nagasawa H., Takatsuto S., Yamaguchi I. A dwarf mutant strain of Pharbitis nil, Uzukobito (kobito), has defective brassinosteroid biosynthesis. Plant J. : for cell and molecular biology. 2003;36:401–410. doi: 10.1046/j.1365-313x.2003.01887.x. [DOI] [PubMed] [Google Scholar]
- 74.Mösch H.U., Fink G.R. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng H., Rowland O., Kunst L. Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell. 2005;17:1467–1481. doi: 10.1105/tpc.104.030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X., Roberti R., Blobel G. Structure of an integral membrane sterol reductase from Methylomicrobium alcaliphilum. Nature. 2015;517:104–107. doi: 10.1038/nature13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Costanzo L., Drury J.E., Penning T.M., Christianson D.W. Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J. Biol. Chem. 2008;283:16830–16839. doi: 10.1074/jbc.M801778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han Y., Zhuang Q., Sun B., Lv W., Wang S., Xiao Q., Pang B., Zhou Y., Wang F., Chi P., Wang Q., Li Z., Zhu L., Li F., Deng D., Chiang Y.-C., Li Z., Ren R. Crystal structure of steroid reductase SRD5A reveals conserved steroid reduction mechanism. Nat. Commun. 2021;12:449. doi: 10.1038/s41467-020-20675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vilchis F., Valdez E., Ramos L., García R., Gómez R., Chávez B. Novel compound heterozygous mutations in the SRD5A2 gene from 46,XY infants with ambiguous external genitalia. J. Hum. Genet. 2008;53:401–406. doi: 10.1007/s10038-008-0274-2. [DOI] [PubMed] [Google Scholar]
- 80.Seo J., Shin S., Kim S.-w., Kim S.J., Lee M., Song K., Suh J., Lee S.-T., Lee Y.S., Chae H.W., Kim H.-S., Choi J.R., Han S., Kwon A. The genotype-phenotype correlation in human 5α-reductase type 2 deficiency: classified and analyzed from a SRD5A2 structural perspective. Int. J. Mol. Sci. 2023;24:3297. doi: 10.3390/ijms24043297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L., Zhang J., Li Q., Qiao L., Li P., Cui Y., Li S., Hao S., Wu T., Liu L., Yin J., Hu P., Dou X., Li S., Yang H. Mutational analysis of compound heterozygous mutation p.Q6X/p.H232R in SRD5A2 causing 46,XY disorder of sex development. Ital. J. Pediatr. 2022;48 doi: 10.1186/s13052-022-01243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tateiwa H., Evers A.S. Neurosteroids and their potential as a safer class of general anesthetics. J. Anesth. 2024;38:261–274. doi: 10.1007/s00540-023-03291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goletiani N.V., Keith D.R., Gorsky S.J. Progesterone: review of safety for clinical studies. Exp. Clin. Psychopharmacol. 2007;15:427–444. doi: 10.1037/1064-1297.15.5.427. [DOI] [PubMed] [Google Scholar]
- 84.Porcu P., Barron A.M., Frye C.A., Walf A.A., Yang S.Y., He X.Y., Morrow A.L., Panzica G.C., Melcangi R.C. Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational Research. J. Neuroendocrinol. 2016;28 doi: 10.1111/jne.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schiffer L., Barnard L., Baranowski E.S., Gilligan L.C., Taylor A.E., Arlt W., Shackleton C.H.L., Storbeck K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J. Steroid Biochem. Mol. Biol. 2019;194 doi: 10.1016/j.jsbmb.2019.105439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edwards D.P. Regulation of signal transduction pathways by estrogen and progesterone. Annu. Rev. Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 87.Vanessa Wandja K., Mercy M., Modeste W.-N. In: Cortisol - between Physiology and Pathology. Diana Loreta P., editor. IntechOpen; Rijeka: 2023. Biological effects of cortisol. Ch. 1. [Google Scholar]
- 88.Podgorski R., Suminska M., Rachel M., Fichna M., Fichna P., Mazur A. Alteration in glucocorticoids secretion and metabolism in patients affected by cystic fibrosis. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharifi N. The 5alpha-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer. J. Invest. Med. : the official publication of the American Federation for Clinical Research. 2012;60:504–507. doi: 10.231/JIM.0b013e31823874a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schiffer L., Arlt W., Storbeck K.H. 5alpha-reduction of epitestosterone is catalysed by human SRD5A1 and SRD5A2 and increases androgen receptor transactivation. J. Steroid Biochem. Mol. Biol. 2024;241 doi: 10.1016/j.jsbmb.2024.106516. [DOI] [PubMed] [Google Scholar]
- 91.Cilotti A., Danza G., Serio M. Clinical application of 5α-reductase inhibitors. J. Endocrinol. Invest. 2001;24:199–203. doi: 10.1007/BF03343844. [DOI] [PubMed] [Google Scholar]
- 92.Thomas L.N., Douglas R.C., Rittmaster R.S., Too C.K. Overexpression of 5 alpha-reductase type 1 increases sensitivity of prostate cancer cells to low concentrations of testosterone. Prostate. 2009;69:595–602. doi: 10.1002/pros.20911. [DOI] [PubMed] [Google Scholar]
- 93.Saartok T., Dahlberg E., Gustafsson J.A. Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984;114:2100–2106. doi: 10.1210/endo-114-6-2100. [DOI] [PubMed] [Google Scholar]
- 94.Seok J., Kwak H.J., Kwak Y., Lee M., Park K.S., Kim A., Cho S.-G. Anti-oncogenic effects of dutasteride, a dual 5-alpha reductase inhibitor and a drug for benign prostate hyperplasia, in bladder cancer. J. Transl. Med. 2023;21 doi: 10.1186/s12967-023-03972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen J., Chou F., Yeh S., Ou Z., Shyr C., Huang C., Xiang Z., Sun Y., Messing E., Zu X., Chang C. Androgen dihydrotestosterone (DHT) promotes the bladder cancer nuclear AR-negative cell invasion via a newly identified membrane androgen receptor (mAR-SLC39A9)-mediated Gαi protein/MAPK/MMP9 intracellular signaling. Oncogene. 2020;39:574–586. doi: 10.1038/s41388-019-0964-6. [DOI] [PubMed] [Google Scholar]
- 96.Aquila S., Montanaro D., Guido C., Santoro M., Perrotta I., Gervasi S., De Amicis F., Lanzino M. Human sperm molecular anatomy: the enzyme 5α-reductase (SRD5A) is present in the sperm and may be involved in the varicocele-related infertility. Histochem. Cell Biol. 2015;144:67–76. doi: 10.1007/s00418-015-1320-8. [DOI] [PubMed] [Google Scholar]
- 97.Wu C., Wei K., Jiang Z. 5α-reductase activity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2017;15 doi: 10.1186/s12958-017-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Graupp M., Wehr E., Schweighofer N., Pieber T.R., Obermayer-Pietsch B. Association of genetic variants in the two isoforms of 5α-reductase, SRD5A1 and SRD5A2, in lean patients with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;157:175–179. doi: 10.1016/j.ejogrb.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Coady K., Murphy M., Villeneuve D., Hecker M., Jones P., Carr J., Solomon K., Smith E., Van Der Kraak G., Kendall R., Giesy J. Effects of atrazine on metamorphosis, growth, and gonadal development in the green frog (Rana clamitans) J. Toxicol. Environ. Health, Part A. 2004;67:941–957. doi: 10.1080/15287390490443722. [DOI] [PubMed] [Google Scholar]
- 100.Byers H.M., Mohnach L.H., Fechner P.Y., Chen M., Thomas I.H., Ramsdell L.A., Shnorhavorian M., McCauley E.A., Amies Oelschlager A.-M.E., Park J.M., Sandberg D.E., Adam M.P., Keegan C.E. Unexpected ethical dilemmas in sex assignment in 46,XY DSD due to 5-alpha reductase type 2 deficiency. Am. J. Med. Genet. Part C: Seminars in Medical Genetics. 2017;175:260–267. doi: 10.1002/ajmg.c.31560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramos L., Vilchis F., Chávez B., Mares L. Mutational analysis of SRD5A2: from gene to functional kinetics in individuals with steroid 5α-reductase 2 deficiency. J. Steroid Biochem. Mol. Biol. 2020;200 doi: 10.1016/j.jsbmb.2020.105691. [DOI] [PubMed] [Google Scholar]
- 102.Duan H., Wang X., Qi W., Shi J., Han L., Wang G., Xu Y., Liu J., Wang J. Two genetic variants in the SRD5A2 gene are found to be associated with sex differences in the disease characteristics of patients with chronic hepatitis B virus infection. Biol. Sex Differ. 2023:14. doi: 10.1186/s13293-023-00553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song K.H., Seo C.S., Yang W.K., Gu H.O., Kim K.J., Kim S.H. Extracts of Phyllostachys pubescens leaves represses human steroid 5-alpha reductase type 2 promoter activity in BHP-1 cells and ameliorates testosterone-induced benign prostatic hyperplasia in rat model. Nutrients. 2021;13 doi: 10.3390/nu13030884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhowmick N.A., Oft J., Dorff T., Pal S., Agarwal N., Figlin R.A., Posadas E.M., Freedland S.J., Gong J. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr. Relat. Cancer. 2020;27:R281–r292. doi: 10.1530/ERC-20-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020;10:779–782. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wambier C.G., Goren A., Vaño-Galván S., Ramos P.M., Ossimetha A., Nau G., Herrera S., McCoy J. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev. Res. 2020;81:771–776. doi: 10.1002/ddr.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Samuel R.M., Majd H., Richter M.N., Ghazizadeh Z., Zekavat S.M., Navickas A., Ramirez J.T., Asgharian H., Simoneau C.R., Bonser L.R., Koh K.D., Garcia-Knight M., Tassetto M., Sunshine S., Farahvashi S., Kalantari A., Liu W., Andino R., Zhao H., Natarajan P., Erle D.J., Ott M., Goodarzi H., Fattahi F. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27:876–889.e812. doi: 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cabeza M., Menes L.A., Fuentes E., Bahena I., Heuze Y. Novel type 1 5a-reductase inhibitors with antiproliferative potential on LNCaP cells. Orient. J. Chem. 2023;39:523–532. [Google Scholar]
- 109.Shamloul G., Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol. Ther. 2021;34 doi: 10.1111/dth.14695. [DOI] [PubMed] [Google Scholar]
- 110.Endly D.C., Miller R.A. Oily skin: a review of treatment options. The Journal of clinical and aesthetic dermatology. 2017;10:49–55. [PMC free article] [PubMed] [Google Scholar]
- 111.Ruksiriwanich W., Khantham C., Linsaenkart P., Chaitep T., Jantrawut P., Chittasupho C., Rachtanapun P., Jantanasakulwong K., Phimolsiripol Y., Sommano S.R., Arjin C., Berrada H., Barba F.J., Sringarm K. In vitro and in vivo regulation of SRD5A mRNA expression of supercritical carbon dioxide extract from Asparagus racemosus willd. Root as anti-sebum and pore-minimizing active ingredients. Molecules. 2022;27:1535. doi: 10.3390/molecules27051535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ceruti J.M., Leiros G.J., Balana M.E. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell. Endocrinol. 2018;465:122–133. doi: 10.1016/j.mce.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 113.Khantham C., Yooin W., Sringarm K., Sommano S.R., Jiranusornkul S., Carmona F.D., Nimlamool W., Jantrawut P., Rachtanapun P., Ruksiriwanich W. Effects on steroid 5-alpha reductase gene expression of Thai rice bran extracts and molecular dynamics study on SRD5A2. Biology. 2021;10:319. doi: 10.3390/biology10040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liang C.-H., Lin Y.-H., Lin Y.-K., Chiang C.-F. Hair growth-promotion effects and antioxidant activity of the banana flower extract HappyAngel®: double-blind, placebo-controlled trial. Food Sci. Hum. Wellness. 2023;12:1917–1923. [Google Scholar]
- 115.Muangsanguan A., Linsaenkart P., Chaitep T., Sangta J., Sommano S.R., Sringarm K., Arjin C., Rachtanapun P., Jantanasakulwong K., Phimolsiripol Y., Castagnini J.M., Ruksiriwanich W. Hair growth promotion and anti-hair loss effects of by-products arabica coffee pulp extracts using supercritical fluid extraction. Foods. 2023;12:4116. doi: 10.3390/foods12224116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tiwari A., Kumar S., Choudhir G., Singh G., Gangwar U., Sharma V., Srivastava R.K., Sharma S. Bioactive metabolites of edible mushrooms efficacious against androgenic alopecia: targeting SRD5A2 using computational approach. J. Herb. Med. 2022;36 [Google Scholar]
- 117.Uemura M., Tamura K., Chung S., Honma S., Okuyama A., Nakamura Y., Nakagawa H. Novel 5α-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99:81–86. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chávez B., Ramos L., García-Becerra R., Vilchis F. Hamster SRD5A3 lacks steroid 5α-reductase activity in vitro. Steroids. 2015;94:41–50. doi: 10.1016/j.steroids.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 119.Fouad Mansour M., Pelletier M., Tchernof A. Characterization of 5α-reductase activity and isoenzymes in human abdominal adipose tissues. J. Steroid Biochem. Mol. Biol. 2016;161:45–53. doi: 10.1016/j.jsbmb.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z.H., Zhang Y.Z., Wang Y.S., Ma X.X. Identification of novel cell glycolysis related gene signature predicting survival in patients with endometrial cancer. Cancer Cell Int. 2019;19:296. doi: 10.1186/s12935-019-1001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Shaughnessy P.J., Monteiro A., Bhattacharya S., Fraser M.J., Fowler P.A. Steroidogenic enzyme expression in the human fetal liver and potential role in the endocrinology of pregnancy. Mol. Hum. Reprod. 2013;19:177–187. doi: 10.1093/molehr/gas059. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y.P., Na W.T., Dai X.Q., Li R.F., Wang J.X., Gao T., Zhang W.B., Xiang C. Over-expression of SRD5A3 and its prognostic significance in breast cancer. World J. Surg. Oncol. 2021;19:260. doi: 10.1186/s12957-021-02377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rieger M., Turk M., Kraus C., Uebe S., Ekici A.B., Krumbiegel M., Huchzermeyer C., Reis A., Thiel C. SRD5A3-CDG: twins with an intragenic tandem duplication. Eur. J. Med. Genet. 2022;65 doi: 10.1016/j.ejmg.2022.104492. [DOI] [PubMed] [Google Scholar]
- 124.Ben Ayed I., Ouarda W., Frikha F., Kammoun F., Souissi A., Ben Said M., Bouzid A., Elloumi I., Hamdani T.M., Gharbi N., Baklouti N., Guirat M., Mejdoub F., Kharrat N., Boujelbene I., Abdelhedi F., Belguith N., Keskes L., Gibriel A.A., Kamoun H., Triki C., Alimi A.M., Masmoudi S. SRD5A3-CDG: 3D structure modeling, clinical spectrum, and computer-based dysmorphic facial recognition. Am. J. Med. Genet. 2021;185:1081–1090. doi: 10.1002/ajmg.a.62065. [DOI] [PubMed] [Google Scholar]
- 125.Dong T., Yue H. IRF2/SRD5A3 promotes malignant growth and glycolysis of glioma cells by regulating ERK/CREB pathway. J Biol Reg and Homeo Agents. 2023;37:3879–3888. [Google Scholar]
- 126.Moon Y.A., Horton J.D. Identification of two mammalian reductases involved in the two-carbon fatty acyl elongation cascade. J. Biol. Chem. 2003;278:7335–7343. doi: 10.1074/jbc.M211684200. [DOI] [PubMed] [Google Scholar]
- 127.Zhou Y., Lv R., Ye R.D., Ren R., Yu L. The 3-hydroxyacyl-CoA dehydratase 1/2 form complex with trans-2-enoyl-CoA reductase involved in substrates transfer in very long chain fatty acid elongation. Biochem. Biophys. Res. Commun. 2024;704 doi: 10.1016/j.bbrc.2024.149588. [DOI] [PubMed] [Google Scholar]
- 128.Abe K., Ohno Y., Sassa T., Taguchi R., Caliskan M., Ober C., Kihara A. Mutation for nonsyndromic mental retardation in the trans-2-enoyl-CoA reductase TER gene involved in fatty acid elongation impairs the enzyme activity and stability, leading to change in sphingolipid profile. J. Biol. Chem. 2013;288:36741–36749. doi: 10.1074/jbc.M113.493221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang J., Xu J., Zang G., Zhang T., Wu Q., Zhang H., Chen Y., Wang Y., Qin W., Zhao S., Qin E., Qiu J., Zhang X., Wen L., Wang Y., Wang G. trans-2-Enoyl-CoA reductase tecr-driven lipid metabolism in endothelial cells protects against transcytosis to maintain blood-brain barrier homeostasis. Research. 2022;2022 doi: 10.34133/2022/9839368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fujioka S., Li J., Choi Y.H., Seto H., Takatsuto S., Noguchi T., Watanabe T., Kuriyama H., Yokota T., Chory J., Sakurai A. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Noguchi T., Fujioka S., Takatsuto S., Sakurai A., Yoshida S., Li J., Chory J. Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-En-3-one to (24R)-24-methyl-5alpha-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol. 1999;120:833–840. doi: 10.1104/pp.120.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fujioka S., Takatsuto S., Yoshida S. An early C-22 oxidation branch in the brassinosteroid biosynthetic pathway. Plant Physiology. 2002;130:930–939. doi: 10.1104/pp.008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao S., Xu Q., Cao Y., Qian K., An K., Zhu Y., Binzeng H., Zhao H., Kuai B. Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol. Plantarum. 2005;123:57–66. [Google Scholar]
- 134.Luo M., Xiao Y., Li X., Lu X., Deng W., Li D., Hou L., Hu M., Li Y., Pei Y. GhDET2, a steroid 5alpha-reductase, plays an important role in cotton fiber cell initiation and elongation. Plant J. : for cell and molecular biology. 2007;51:419–430. doi: 10.1111/j.1365-313X.2007.03144.x. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y., Hao Y., Guo Y., Shou H., Du J. PagDET2 promotes cambium cell division and xylem differentiation in poplar stem. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.923530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou F., Hu B., Li J., Yan H., Liu Q., Zeng B., Fan C. Exogenous applications of brassinosteroids promote secondary xylem differentiation in Eucalyptus grandis. PeerJ. 2024;12 doi: 10.7717/peerj.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cui J., Zeng G., Gao F., Jiang Y., Wang Y., Li D., Wang X., Xi Z. Cloning, characterization and expression analysis of a brassinosteroids biosynthetic gene VvDET2 in Cabernet Sauvignon (Vitis vinifera L.) Plant Cell Tissue Organ Cult. 2023;154:43–54. [Google Scholar]
- 138.Qiao Z., Li J., Zhang X., Guo H., He C., Zong D. Genome-wide identification, expression analysis, and subcellular localization of DET2 gene family in Populus yunnanensis. Genes. 2024;15 doi: 10.3390/genes15020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zeng H., Tang Q., Hua X. Arabidopsis brassinosteroid mutants det2-1 and bin2-1 display altered salt tolerance. J. Plant Growth Regul. 2009;29:44–52. [Google Scholar]
- 140.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kohlwein S.D., Eder S., Oh C.S., Martin C.E., Gable K., Bacikova D., Dunn T. Tsc13p is required for fatty acid elongation and localizes to a novel structure at the nuclear-vacuolar interface in Saccharomyces cerevisiae. Mol. Cell Biol. 2001;21:109–125. doi: 10.1128/MCB.21.1.109-125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nagano M., Kakuta C., Fukao Y., Fujiwara M., Uchimiya H., Kawai-Yamada M. Arabidopsis Bax inhibitor-1 interacts with enzymes related to very-long-chain fatty acid synthesis. J. Plant Res. 2019;132:131–143. doi: 10.1007/s10265-018-01081-8. [DOI] [PubMed] [Google Scholar]
- 143.Fukuda N., Oshima Y., Ariga H., Kajino T., Koyama T., Yaguchi Y., Tanaka K., Yotsui I., Sakata Y., Taji T. ECERIFERUM 10 encoding an enoyl-CoA reductase plays a crucial role in osmotolerance and cuticular wax loading in Arabidopsis. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.898317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Innan H., Kondrashov F. The evolution of gene duplications: classifying and distinguishing between models. Nat. Rev. Genet. 2010;11:97–108. doi: 10.1038/nrg2689. [DOI] [PubMed] [Google Scholar]
- 145.Moore R.C., Purugganan M.D. The evolutionary dynamics of plant duplicate genes. Curr. Opin. Plant Biol. 2005;8:122–128. doi: 10.1016/j.pbi.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 146.Azpiroz R., Wu Y., LoCascio J.C., Feldmann K.A. An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ohnishi T., Godza B., Watanabe B., Fujioka S., Hategan L., Ide K., Shibata K., Yokota T., Szekeres M., Mizutani M. CYP90A1/CPD, a brassinosteroid biosynthetic cytochrome P450 of Arabidopsis, catalyzes C-3 oxidation. J. Biol. Chem. 2012;287:31551–31560. doi: 10.1074/jbc.M112.392720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for these findings are available in the main text, further inquiries can be directed to the corresponding author.