Summary
During aging and in retinal degenerative diseases, vulnerable retinal pigment epithelial (RPE) cells are subject to mitochondrial dysfunction, creating a need for accessibility to tools which can facilitate assessment of the ocular posterior pole bioenergetics. Here, we present a protocol for quantifying mitochondrial respiration in the posterior eye cup (RPE-choroid-sclera) of young and old mice. We describe steps for eye cup dissection, optimization of tissue size, drug concentrations, and cycle conditions using the XF Cell Mito Stress Test.
Subject areas: Cell Biology, Metabolism, Model Organisms
Graphical abstract

Highlights
-
•
Ex vivo real-time quantification of mitochondrial respiration in the posterior pole
-
•
Assess the mitochondrial kinetics of the posterior pole as a function of age
-
•
Solutions for measuring bioenergetics in a complex three-dimensional tissue
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
During aging and in retinal degenerative diseases, vulnerable retinal pigment epithelial (RPE) cells are subject to mitochondrial dysfunction, creating a need for accessibility to tools which can facilitate assessment of the ocular posterior pole bioenergetics. Here, we present a protocol for quantifying mitochondrial respiration in the posterior eye cup (RPE-choroid-sclera) of young and old mice. We describe steps for eye cup dissection, optimization of tissue size, drug concentrations, and cycle conditions using the XF Cell Mito Stress Test.
Before you begin
The Seahorse Agilent XFe24 analyzer, a tool for measuring cellular bioenergetics, was used throughout the protocol described, to measure oxygen consumption rate (OCR) or mitochondrial respiration, in the mouse ocular posterior pole, a complex tissue composed of the RPE, choroid, and sclera (RPE-ch-sc). This protocol may be adapted to assess mitochondrial respiration of other complex tissues in wild-type and transgenic mice.
In this study our cohort included wild-type C57BL/6J mice (breeding pairs purchased from the Jackson Laboratory) bred and aged. Mice were housed in the animal facility at the Duke Eye Center under ambient temperatures (25°C), in a light-controlled (12-h light / 12-h dark) environment and provided standard mouse chow ad libitum. For the age comparison study, mice from multiple litters were randomly divided into two groups: young mice; aged 2 months and old mice; aged 21 months. Equal numbers of males and females were used in the study.
Institutional permissions
The Duke University Institutional Animal Care and Use Committee (IACUC) approved the study protocol, and all animal experiments were performed in accordance with the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation 1: Assess mouse conditions leading up and prior to use in the study
-
1.
Evaluate animals to be used in the protocol.
-
2.
Ensure proper accessibility to food, water, and light conditions in the animal facility throughout the entire course of the study.
Note: Circadian clock is an important regulator of cell metabolism. It is important to ensure that all the mice to be used in the protocol are exposed to equal light / dark cycles.
Preparation 2: Hydration of the XFe24 sensor cartridge – Day 1
Timing: ∼16 h
XFe24 sensor cartridges are necessary components of the XF assay platform. Each cartridge has probes, the tips of which are spotted with a solid state sensor which detects changes in pH and oxygen concentration during execution of the assay. To acquire the most accurate measurements, the sensor cartridge should be thoroughly hydrated prior to running the assay.
-
3.
The XFe24 sensor cartridge is provided in the XFe24 FluxPak and has three main parts, a white utility plate, a pink hydro booster plate, and a green sensor plate.
-
4.
Separate the white utility plate from the sensor cartridge (green plate) and place the sensor cartridge upside down on the bench.
Note: It is important to place the sensor cartridge upside down to protect the four probes from contacting the surface of the bench.
-
5.
Remove the pink hydro booster plate, located between the white utility plate and the green sensor cartridge.
-
6.
Load 1 mL of XF calibrant solution (stored at ambient room temperature; 68–70°F), in each well of the white utility plate
-
7.
Place the pink booster plate back on top followed by the green sensor cartridge.
-
8.
Incubate the XFe24 sensor cartridge in a non-CO2 incubator at 37°C overnight.
-
9.
Turn on the Seahorse machine to ensure the machine is equilibrated at 37°C in preparation for running assays on day 2.
CRITICAL: Each individual XFe24 sensor cartridge is compatible with a specific XFe24 islet capture microplate. Always double check the expiration date on the flux pack prior to preparation for use. Expired plates will display error messages when inserted in the XFe24 instrument.
CRITICAL: Ensure that the probes are fully hydrated by moving the plate up and down at least twice through the pink hydro booster plate sandwiched between the cartridge and the white utility plate.
CRITICAL: Do not incubate the probes for longer than 48 h to avoid salt build-up from the calibrant solution. Drying or inadequate hydration of the probes can affect the OCR measurements negatively, rendering unreliable results.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Hank’s balanced salt solution (HBSS) | Gibco | 14175-095 |
| RIPA buffer (5×) | Thermo Fisher Scientific | J62524.AD |
| Critical commercial assays | ||
| Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | 23225 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice Young (2 months; n = 4; n = 2 males, n = 2 females) Old (21 months; n = 6; n = 3 males, n = 3 females) |
The Jackson Laboratory | #000664 |
| Software and algorithms | ||
| Seahorse Wave Pro software | Agilent | https://www.agilent.com/en/product/cell-analysis/real-time-cell-metabolic-analysis/xf-software/seahorse-wave-pro-software-2007523 |
| GraphPad Prism v.10.1.2 | GraphPad | NA |
| Other | ||
| Seahorse XFe24 analyzer | Agilent | US00422004 |
| XFe24 FluxPak mini | Agilent | 102342-100 |
| XFe24 Islet Capture microplates | Agilent | 101122-100 |
| Seahorse XF Cell Mito Stress Test Kit | Agilent | 103015-100 |
| Seahorse XF DMEM medium, pH 7.4 | Agilent | 103575-100 |
| Seahorse XF calibrant solution, 500 mL | Agilent | 100840-000 |
| Seahorse XF 1.0 M glucose solution, 50 mL | Agilent | 103577-100 |
| Seahorse XF 200 mM glutamine solution, 50 mL | Agilent | 103579-100 |
| Seahorse XF 100 mM pyruvate solution, 50 mL | Agilent | 103578-100 |
| Sterile disposable biopsy punch | Integra Biosciences | (1 mm) 33-31AA-P/25; (2 mm) 33-31-P/25 |
| Dissecting light microscope | Leica Microsystems | 10447917 |
| Orbital shaker | Pipette | H1001-M |
| Microplate reader | Molecular Devices | 89429-536 |
| Vortex-Genie 2 mixer | Scientific Industries | SKU:SI-0236 |
| Incu-Shaker Mini | Benchmark Scientific | SKU:H1001-M |
Materials and equipment
XF assay medium preparation
Prepare the final XF assay media by supplementing the XF DMEM media with 10 mM glucose, 1 mM pyruvate and 2 mM glutamine. Solutions may be prepared from concentrated stock solutions (Table 1).
Note: XF DMEM media is shipped and maintained at ambient room temperature of 68–70°F. Glucose and pyruvate solutions are shipped on dry ice and stored at 4°C. Glutamine solution is shipped on dry ice and stored at −20°C. All products are stable for one year from the date of manufacture.
Note: Use of DMEM media with three minimal additives (glucose, pyruvate and glutamine) without buffering agents such as HEPES, serum or sodium bicarbonate ensures a reliable baseline measurement of energy metabolism.
Table 1.
Recipe for preparation of the XF assay media
| Reagent | Final concentration (mM) | Stock concentration (mM) | Stock volume (μL) |
|---|---|---|---|
| Glucose | 10 mM | 1000 | 500 |
| Pyruvate | 1 mM | 100 | 500 |
| Glutamine | 2 mM | 200 | 500 |
Note: Stock volumes should all be added to 48.5 mL of XF DMEM media to make the final XF assay media (Total volume = 50 mL).
Step-by-step method details
Part 1: Collection of mouse eye globes - Day 2
Timing: ∼20 min
The following section describes the step by step method to collect mouse eyes with the goal of preserving the structural integrity of the eye as much as possible. Eye collection should be carried out as quickly as possible without compromising tissue quality.
-
1.
Euthanize mice following IACUC guidelines using CO2 gas delivery and monitor until cessation of respiration, at which point discontinue CO2 and apply the secondary method of exsanguination.
-
2.
Collect the eye globes, by first clearing the conjunctiva.
-
3.
Cut the external orbital muscles.
-
4.
Once the eye can be gently rotated 360 degrees in the eye socket, sever the optic nerve head using Vannas scissors.
-
5.
Place enucleated eyes in cold HBSS immediately and on ice for short term storage (up to 2 h).
CRITICAL: Method of euthanasia may affect cell metabolic pathways and should be considered carefully when designing the experiment and analyzing data.1
Note: Euthanasia and eye globe collection should always be done at a fixed time during the day to control for changes in circadian rhythm in mice across experiments. In this case, eye globes were collected at noon.
Note: Forceps used should be sterilized and wiped with an alcohol pad in between handling samples.
Part 2: Isolation and dissection of the posterior pole
Timing: ∼40 min
Due to its small size and spherical shape, dissecting the mouse eye, which averages 3–4 mm in diameter, may be challenging. Below is the step by step procedure for isolation and dissection of the mouse eye to obtain a RPE-choroid-sclera flatmount.
-
6.
In preparation for isolating the posterior eye cup, place the eye on a HBSS moistened filter paper in a Petri dish and under a dissecting microscope, quickly trim the extraocular tissue, muscle, and fat off the sclera using Vannas scissors.
Note: Periodically gently rinse the eye globe with cold HBSS to ensure the outside of the eye is clean and hydrated prior to further dissection.
-
7.
Remove the cornea using Castroviejo corneal scissors.
-
8.
Loosen and tease out the lens and gently separate the neural retina from the RPE, choroid, scleral (RPE-ch-sc) complex. Save the retina for other studies.
CRITICAL: It is important to not let the tissue dry out at any point and keep it moist throughout to avoid curling of the eye cup.
-
9.
Keeping the RPE-ch-sc flat, cut four to five incisions from the periphery of the eye cup towards the optic nerve head using a sharp Vannas scissor, creating a cross-like pattern.
Note: If need be, to ensure a flattened tissue, the rough or jagged edges of the tissue immediately adjacent to the ora serrata may be cut with a sharp razor blade to create smooth edges.
CRITICAL: Take care not to cut through the optic nerve, which would stretch the tissue and make sectioning the RPE-ch-sc into equally spaced sections relatively challenging.
-
10.
Gently place one RPE-ch-sc tissue complex in each well of a prepared Seahorse XFe24 islet capture plate containing XF DMEM Medium.
CRITICAL: Do not add any assay substrates to the medium until the dissection and isolation of all eye globes is completed. Figure 1 illustrates the steps for the isolation of tissue from the mouse posterior eye cup.
Note: Select three wells to serve as blanks with no tissue placed in them. For example, two of the blank wells may be in the first and last well of the plate, chosen as this may also help eliminate edge artifacts that may impact readings, and the third may be placed in the middle. In a 24 well plate, this leaves 21 wells for samples.
Figure 1.
Steps involved in tissue dissection
The steps for posterior eye cup dissection are illustrated. Each step has been described in detail in Steps 1 and 2 of the protocol under ‘Step-by-step method details’.
Part 3: Prepare the islet capture plate
Timing: ∼55 min
The use of the XF24 islet capture plate is useful for securing a three-dimensional tissue, in a 24 – well plate format and assessing its bioenergetics. The plate must be prepared ahead of time for use as outlined below.
-
11.
Once each of the RPE-ch-sc tissue samples have been placed in individual wells of the islet capture plate, remove the XF DMEM medium and replace with 0.5 mL of XF assay medium per well.
-
12.
Using a 1.0 mL pipette tip in one hand and curved iris forceps in the other hand gently move the tissue to the center of each well. Ensure the RPE cell layer is facing upward.
Note: RPE side should be carefully placed facing upwards, to ensure accurate reading by the sensor probe.
-
13.
Using a pair of forceps take an islet mesh screen and position it on top of the tissue.
Note: Ensure, while positioning the screen, that the ring of the islet mesh screen is facing up.
Note: Islet mesh screens are provided in the same pack as the XFe24 islet capture plate. Carefully remove the packing and take the mesh screens out to add to the wells of the plate. Placing an islet mesh screen allows for accurate recording of the OCR, while preventing excessive tissue movement during the assay.
-
14.
An islet mesh screen should be placed in each of the blank wells in the same manner as in the test wells.
-
15.
Incubate the islet capture plate in a non-CO2 incubator at 37°C for 45 min.
CRITICAL: It is important for CO2 levels to equalize between the tissue and the assay medium prior to running the assay for maximum OCR measurement, as CO2 may influence the metabolism readings by the Seahorse.
-
16.
After 45 min remove the XF assay medium and add fresh XF assay medium that has been warmed to 37°C to each well.
Note: A change to fresh pre-warmed media, at this stage, helps to reduce temperature variation during the assay run.
CRITICAL: Confirm there are no air bubbles trapped between the islet mesh screen and the tissue. Air bubbles will interfere with OCR measurements during the assay.
Part 4: Optimize drug concentrations and prepare the XFe24 sensor cartridge for OCR measurement
Timing: ∼55 min
The XF Cell Mito Stress Assay Kit contains three compounds: oligomycin, FCCP [carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone] and rotenone/antimycin A. Oligomycin inhibits ATP synthase (complex V) and impacts electron flow through the electron transport chain, resulting in reduction of mitochondrial respiration or OCR. FCCP is an uncoupling agent that collapses the proton gradient and disrupts the mitochondrial membrane potential. Rotenone and antimycin A are inhibitors of complex I and II of the electron transport chain respectively. An important parameter to optimize in the XF Cell Mito Stress test is the concentration of FCCP as noted below.
-
17.
All three compounds provided in the XF Cell Mito Stress Test kit are in powder form. Prepare solutions of oligomycin, FCCP and rotenone/antimycin A per Table 2.
Note: Prepare stock volumes of oligomycin, FCCP, and rotenone/antimycin A according the manufacturer’s instructions (https://www.agilent.com/cs/library/usermanuals/public/XF_Cell_Mito_Stress_Test_Kit_User_Guide.pdf) and proceed to make the final concentrations (Table 2).
Note: Oligomycin and FCCP are not considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200), while rotenone/antimycin A is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Appropriate pre-cautions must be taken.
-
18.
Remove sensor cartridge from the incubator that was hydrated overnight (see Preparation two) and inject the following drugs in the ports on the green sensor plate: 1.5 μM oligomycin at a final volume of 56 μL in port A, 1.0 μM FCCP at a final volume of 62 μL in port B and 0.5 μM rotenone/antimycin A at a final volume of 69 μL in port C (see step 3 of graphical abstract for the illustration of ports).
Note: If the FCCP concentration needs to be further optimized, which may be the case depending on the source of the tissue (e.g., transgenic mouse model or if the mice have been subjected to stressors such as oxidants or a high fat diet), a range of concentrations (0.125, 0.25, 0.5, 1.0 and 2.0 μM) can be loaded in port B of the different wells (Figure 2; Table 2).
Note: Port D in this case is left blank but may be used to test the effect of any other drug of interest relevant to the experimenter’s study. An empty port will not affect the assay.
-
19.
Place the loaded cartridge in a non-CO2 incubator at 37°C for ∼ 45 min.
CRITICAL: As mentioned earlier, it is important for CO2 levels to equalize between the tissue and the assay medium prior to running the assay for maximum OCR measurement, as CO2 may influence the metabolism readings by the Seahorse.
Table 2.
Instructions for preparing stock solutions of oligomycin, FCCP and rotenone/antimycin A in the FCCP optimization protocol
| Drug | Final concentration (μM) | Stock volume (μL) | Medium volume (μL) |
|---|---|---|---|
| Port A Oligomycin | 1.5 | 450 | 2455 |
| 0.125 | 37.5 | 2962.5 | |
| Port B FCCP | 0.25 | 75 | 2925 |
| 0.5 | 150 | 2850 | |
| 1 | 300 | 2700 | |
| 2 | 600 | 2400 | |
| Port C Rotenone/antimycin A | 0.5 | 300 | 2700 |
Note: Each stock volume should be added to the medium volume. Final volume for each drug will be 3 mL.
Figure 2.
Optimization of FCCP concentrations for oxygen consumption rate (OCR) measurement in the RPE-ch-sc
Normalized measurements of mitochondrial respiration following sequential injections of oligomycin (oligo), FCCP and rotenone/antimycin A (rot/AA) using the XF Cell Mito Stress Test. Normalized values for all mitochondrial respiration parameters including basal respiration, ATP-linked respiration, maximal respiration, spare respiratory capacity, proton leak and non-mitochondrial respiration have been plotted. Data shown are mean ± SEM; n = 6 biological samples/concentration. Values were considered statistically significant at p < 0.05. ns = not significant.
Part 5: Running the XF Cell Mito Stress Test to measure OCR
Timing: ∼3 h 52 min
The XF Cell Mito Stress test measures key parameters of mitochondrial function by directly measuring the oxygen consumption rate (OCR). Efficient OCR measurement in a complex three-dimensional tissue such as the RPE-ch-sc requires optimization of the cycle conditions and must be worked out by the user. Herein we illustrate the optimized method used for OCR measurement in the RPE-ch-sc tissue.
-
20.
Confirm the Seahorse machine has been turned on from the night before to ensure the machine is at 37°C.
-
21.
Open the “Wave” software on the computer connected to the Seahorse instrument.
-
22.
Remove the lid from the XF sensor cartridge plate prepared in Part 4 and discard the pink hydro booster plate.
-
23.
Load the remaining white utility plate and green sensor plate into the XF Seahorse. Allow the cartridge to equilibrate in the machine for 20 min.
CRITICAL: Double check the expiration date on the plate prior to use. Expired plates will display error messages when inserted in the XFe24 instrument. Contact Agilent should you receive an expired plate.
-
24.
Follow prompts from the Seahorse and eject the white utility plate.
-
25.
Remove the lid and load the islet capture plate prepared in Part 3.
-
26.Set up the assay template with the following conditions (Table 3; Figure 3 – Optimization 1):
-
a.Five measurements of basal respiration (3 min mix, 2 min wait, 3 min measure).
-
b.Six measurements after the addition of oligomycin (3 min mix, 2 min wait, 3 min measure).
-
c.Seven measurements after FCCP injection (3 min mix, 2 min wait, 3 min measure).
-
d.Six final measurements following the addition of rotenone/antimycin A (3 min mix, 2 min wait, 3 min measure).
-
a.
-
27.The program will take approximately 3 h 32 min to complete.Note:Table 3 details the cycle parameters tested for maximum measurement of OCR in XF Cell Mito Stress Test.Note: Cycle numbers may be further optimized if needed. We tested alternative cycle numbers and the parameters listed below worked 2nd best (Figure 3 – Optimization 2). The user must optimize their cycle numbers if needed to their tissue of interest. Optimization is recommended when you are measuring OCR in tissue from transgenic mice and / or mice subjected to treatments such as high fat diet or oxidant injury. A user range has been provided in Table 4.
-
a.Three measurements of basal respiration (3 min mix, 2 min wait, 3 min measure).
-
b.Three measurements after the addition of oligomycin (3 min mix, 2 min wait, 3 min measure).
-
c.Three measurements after FCCP (3 min mix, 2 min wait, 3 min measure).
-
d.Three final measurements following rotenone/antimycin A administration (3 min mix, 2 min wait, 3 min measure).
-
a.
Table 3.
Final drug concentrations and cycle parameters used in the XF Cell Mito Stress Test
| Drug | Final concentration (μM) | Number of cycles | Cycle conditions |
|---|---|---|---|
| Port A Oligomycin | 1.5 | 6 cycles | Mix (3); wait (2); measure (3) |
| Port B FCCP | 1 | 7 cycles | Mix (3); wait (2); measure (3) |
| Port C Rotenone/antimycin A | 0.5 | 6 cycles | Mix (3); wait (2); measure (3) |
Figure 3.
Optimization of cycle numbers for measuring oxygen consumption rate (OCR) in RPE-ch-sc
Normalized mitochondrial respiration measurements following sequential injections of oligomycin (oligo), FCCP and rotenone/antimycin A (rot/AA) using XF Cell Mito Stress Test. Top two optimized conditions for mitochondrial respiration assessment are shown. Data shown are mean ± SEM; n = 6 biological replicates.
Table 4.
Optimization of cycle parameters tested for maximum measurement of oxygen consumption rate (OCR) in the XF Cell Mito Stress Test
| Setting | Number of cycles | Cycle conditions |
|---|---|---|
| Basal | 3–5 | Mix (3); wait (2); measure (3) |
| Oligomycin | 3–6 | Mix (3); wait (2); measure (3) |
| FCCP | 3–7 | Mix (3); wait (2); measure (3) |
| Rotenone/antimycin A | 3–6 | Mix (3); wait (2); measure (3) |
Alternative 1: Biopsy punches to optimize RPE-ch-sc tissue size for OCR measurements (alternative step)
Timing: ∼20 min
Tissue size is a critical factor in measuring OCR as excessive metabolic activity may consume the substrates in the individual wells of the plate and confound measurements. The size of the tissue sample should be considered when performing metabolic studies. The user must decide on final size suitable for their studies. In our case we compared 1 mm and 2 mm biopsy punches to the whole eye cup tissue using XF Cell Mito Stress Test as described below:
-
28.
Use a sharp retractable biopsy puncher to obtain 1 mm and 2 mm tissue punches from regions adjacent to the optic nerve head in the RPE-ch-sc flatmount as shown in Figure 4.
Note: The punches should be removed gently using a curved iris forceps without disintegrating the RPE-ch-sc tissue.
Note: Up to five 1 mm punches and three 2 mm punches can be collected/eye.
-
29.
Upon dissection place the 1 mm and 2 mm biopsy punches of the RPE-ch-sc immediately in each well of the prepared Seahorse XFe24 islet capture plate containing XF DMEM Medium.
-
30.
Continue with Parts 3 through 5 listed above.
Figure 4.
Optimization of RPE-ch-sc tissue size for measuring oxygen consumption rate (OCR)
Panel I illustrates samples tested including (A) whole eye cup and (B) 1 mm and 2 mm punches from the posterior pole. White circles indicate regions adjacent to the optic nerve chosen for punch dissection. Normalized oxygen consumption rate (OCR) is shown in Panel II measured following sequential injections of oligomycin (oligo), FCCP and rotenone/antimycin A (rot/AA) using XF Cell Mito stress test. Normalized values for other mitochondrial respiration parameters including basal respiration, ATP-linked respiration, maximal respiration, spare respiratory capacity, proton leak and non-mitochondrial respiration have been plotted. Data shown are Mean ± SEM; n = 6 biological samples of whole eye cup; n = 3 2 mm punches/eye; and n = 5 1 mm punches/eye were evaluated. Values were considered statistically significant at p < 0.05.
Alternative 2: Measuring the effect of age on mitochondrial respiration of RPE-ch-sc
Age plays a critical role in the development or progression of certain retinal diseases which necessitates evaluating and comparing tissue samples from young and aged animals.2 Herein we outline the protocol we followed to compare and assess OCR in tissue samples isolated from young and old mice (Figure 5).
-
31.
Whole eye globes from young C57BL/6J mice (2 months; n = 4; n = 2 males, n = 2 females) and old C57BL/6J mice (21 months; n = 6; n = 3 males, n = 3 females) were isolated as described in Part 1.
-
32.
Flatmounts were prepared as described in Part 2 of protocol or optimize per Alternative 1.
-
33.
The final parameters of oligomycin, FCCP and rotenone/antimycin A and cycle parameters as shown in Table 3 were used to run the XF Cell Mito Stress Test.
-
34.
We continued with Parts 3 through 5 listed above.
Note: Care was taken in the dissection of the ocular tissues from aged animals, which may be more fragile and prone to tears, which would introduce error in the measurements.
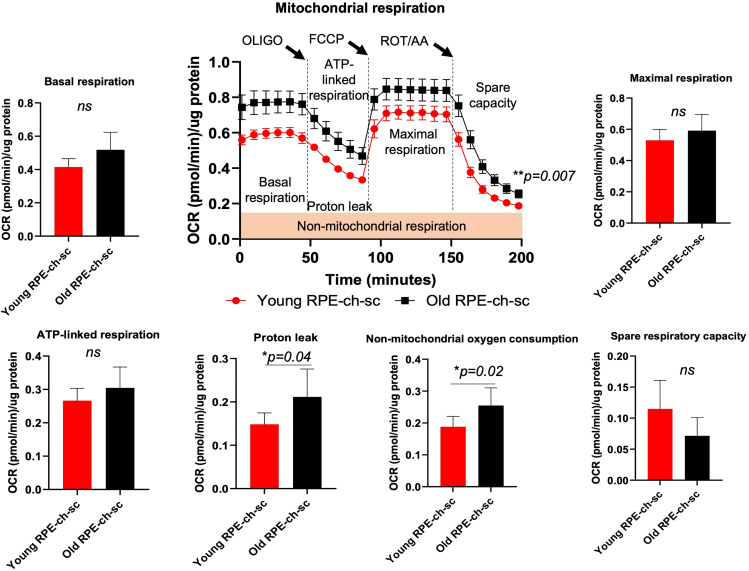
Figure 5.
Oxygen consumption rate (OCR) measurement in RPE-ch-sc whole eye cup from young and old mice
Normalized OCR measurements from C57BL/6J mice; aged 2 (young; n = 4) and 21 (old; n = 6) months using XF Cell Mito Stress Test following sequential injections of oligomycin (oligo), FCCP and rotenone/antimycin A (rot/AA) are shown. Normalized values for other mitochondrial respiration parameters including basal respiration, ATP-linked respiration, maximal respiration, spare respiratory capacity, proton leak and non-mitochondrial respiration have been plotted. Data shown are Mean ± SEM; Values were considered statistically significant at p < 0.05. ns = not significant.
Expected outcomes
The Seahorse Analyzer has accelerated discoveries of cellular energy metabolism by providing a platform that enables extracellular flux measurements of oxygen and pH from relatively small amounts of biologic material. This technology has primarily been used for evaluating the metabolic profile of cell monolayers rather than three dimensional cultures or tissues. As such, it had not been optimized for studying the tissue complex found in the outer ocular posterior pole, composed of the retinal pigment epithelium, choroid and sclera, a region of interest in a variety of retinal degenerative tissues as it is vulnerable and often the primary area affected in disease. We developed a method to quantify mitochondrial respiration of the outer posterior cup (RPE-ch-sc). This method was adapted from previously published methods which used an islet capture plate to record metabolic activity in small, circular sections isolated from the mouse brain.3 We detail the methods for the isolation of the posterior eye cup as shown in Figure 1 and optimization of FCCP concentration and cycle conditions providing the maximum OCR in the RPE-ch-sc as shown in Figures 2 and 3. We found that 1 μM and 2 μM FCCP concentrations provided the best delineated peaks and troughs in the OCR charts (Figure 2). Similarly, conditions proposed and used in ‘optimization 2’ provided clearer responses as seen in the OCR chart, to the oligomycin, FCCP, and rotenone/antimycin drug treatments than ‘optimization 1’ (Figure 3). As alternatives, conditions for measuring mitochondrial respiration in various tissue sizes including 1 mm, 2 mm biopsy punches versus the whole eye cup have also been provided (Figure 4). Though maximum OCR parameters were recorded in the whole RPE-ch-sc tissue as compared to biopsy punches of 1 mm and 2 mm as shown in Figure 4, the methods provided may be adapted as needed and based on tissue size available that fits in the well without folding. Finally, age plays an important role in development and progression of a number of retinal diseases. Mitochondrial respiration parameters may be measured in tissue samples from young and old mice, despite differences in size and developmental stage as shown in Figure 5. OCR assessments significantly changed in the RPE-ch-sc tissue isolated from old versus young mice. While mitochondrial functional parameters of protein leak and non-mitochondrial oxygen consumption increased in RPE-ch-sc with age, no change in basal respiration, ATP-linked respiration, maximal respiration, and spare respiratory capacity were detected (Figure 5). This method may be broadly applied to measure cellular respiration in other complex tissue types including those composed of epithelial / endothelial layers, to shed light on the potential dynamic role of metabolism and metabolic shifts in cellular physiology and in various disease states.
Quantification and statistical analysis
Protein quantification using BCA assay
-
1.
At the completion of the XF Cell Mito Stress Test remove the XF media from the wells of the islet capture plate.
-
2.
Add 100 μL of 1× RIPA buffer to each well, seal the plate using parafilm and place the plate at −80°C, to help maximize protein yield.
Note: Alternatively, should overnight freezing not be possible, protein lysis may be done following a minimum of 4 h RIPA buffer treatment.
-
3.
On the next day, bring the plate to room temperature for 30 min. With a 1 mL pipette, pipette up and down in the wells containing the tissue without removing the mesh, to maximize lysate collection.
-
4.
Collect the supernatant and centrifuge at 10,000 rpm (9391 × g or relative centrifugal force) for 10 min at 4°C.
-
5.
Measure the protein content of the collected supernatant using the Pierce BCA Protein Assay Kit following the manufacturer’s instructions.
Note: Total protein concentration in μg/mL may be used for normalization of OCR values in the Wave software.
Data analysis using WAVE software
The Agilent Seahorse Wave desktop is a software for assay design and data analysis available as a free download from Agilent. The Seahorse XF Cell Mito Stress Test Report Generator is a free software that automatically calculates all the mitochondrial assay parameters from the raw kinetic data.
-
6.
Download the Wave software (https://www.agilent.com/en/products/cell-analysis/software-download-for-wave-desktop) and open the XF Cell Mito Stress Test experiment.
-
7.
Add the values calculated from the BCA protein assay as total protein/μg in the normalization table.
-
8.
Apply the buffer factor and background well correction.
-
9.
Export the raw data in Wave function as Seahorse XF Cell Mito Stress Test Report Generator file. At this point the raw kinetic data from the Seahorse XF Cell Mito Stress Test Report Generator file is exported to Excel or GraphPad for calculation of OCR values (pmol/min)/μg/protein) corresponding to total mitochondrial respiration, basal respiration, ATP-linked respiration, maximal respiration, spare respiratory capacity, proton leak and non-mitochondrial oxygen consumption.
-
10.
Perform statistical analyses.
Note: Statistical methods for analysis in Figures 2, 4, and 5 include two-tailed Student’s t-test when comparing the means between two groups and two-way ANOVA when comparing the means among three groups or more. Values were considered statistically significant at p < 0.05.
Limitations
Agilent Seahorse XF Analyzers have primarily been used for measurements of cell metabolism in adherent monolayers of cells. Recently studies evaluating ex vivo tissues have gained interest, thus expanding Seahorse’s utility to assess cell metabolism in complex tissues.3,4,5,6,7 Tissue complexes or organoids are currently a limited application for Seahorse technology due to a number of factors. First, the XFe24 islet capture plates for tissues are only compatible with the XF Cell Mito Stress test. None of the other kinetic tests including ATP rate assay, Glycolysis Rate Assay and Glycolysis Stress Test work using the islet capture plates, which limits measuring the contributions from cellular glycolysis in our tissue of interest. Second, despite the tissue being secured in place by the islet mesh screen, tissues may still move during the assay, potentially resulting in variability in OCR measurements. Furthermore, placing the islet mesh screen over the tissue while not effecting tissue integrity requires patience and can be a time-consuming process. This additional time may compromise the metabolic integrity of the tissue. Third, care should be taken that while placing the islet mesh screen in the wells, it is placed tight enough to not move during the run but not so tight that it presses down upon the tissue too much. A loosely placed screen may get pulled into the instrument through the probes during the assay run and disrupt the normal function of the instrument. Fourth, the process of protein extraction from the assayed tissue may be challenging, as the islet mesh screen may sit tightly in the well at the end of the run and attempts to lyse the tissue through the screen may tear the tissue and affect sample recovery. There is room to develop additional methods for biochemical assessment of the tissue assayed to improve the normalization of the data.
Troubleshooting
Problem 1
It is important to note that if the goal is to assess mitochondrial respiration from the RPE alone, the presence of the choroid and sclera may influence OCR assessments of the RPE. As shown in Figure 1 preparing RPE-ch-sc mounts is technically challenging and time consuming. This protocol involves the use of a dissecting microscope to better visualize the tissue during the sequential removal of extraocular muscle, the surrounding fat, anterior segment including the cornea and lens, and then the neural retina. This is followed by carefully making radial cuts in the posterior eye cup and keeping the RPE tissue intact and facing up. OCR is then measured from the remaining complex of retinal pigment epithelium, choroid, and sclera.
Potential solution
Isolation of pure populations of RPE or choroidal endothelial cells would be ideal for measuring OCR contributions from the specific cell type. Alternatively, comparing mitochondrial respiration in RPE and choroidal endothelial cells isolated and cultured independently (without passaging) to that of the complex whole RPE-ch-sc may shed light on cell-specific OCR assessments.
Problem 2
Variability in OCR measurements during runs.
Potential solution
Increasing the number of technical replicates in every experiment may help identify potential low read wells and reduce variability in the OCR reads. Variation across experiments can be reduced further by careful calibration of the plate, adhering to identical tissue sample preparation and assay conditions.
Problem 3
Protein extraction at the end of the assay for BCA quantification is a technical constraint as the mesh screen may be tightly stuck in the wells of the islet capture plate. Efforts to remove it may inadvertently lyse or tear the tissue.
Potential solution
Instead of using islet mesh screens, tissue adhesives such as Cell-Tak (Corning) may be adapted to secure the tissue to the bottom of the islet capture plate, though the plates should be carefully inspected to make sure the tissue is intact and its integrity is not affected during the run and not pulled into the ports.
Problem 4
The force of injections during the assay may cause shifts to the position of the tissues in the wells during the run and affect OCR measurement.
Potential solution
After the run is complete each well should be examined carefully under the dissecting microscope. Any sample that has moved may potentially affect final measurements and may be excluded. As mentioned earlier including a higher number of technical replicates may be considered.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Goldis Malek (gmalek@duke.edu).
Technical contact
Questions on the technical execution of the experiments may be directed to Tanu Parmar (tanu.parmar@duke.edu) and/or Goldis Malek (gmalek@duke.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The published article includes all datasets generated or analyzed during this study.
Acknowledgments
This work was supported by several funding agencies: EY035126 (G.M.), EY032751 (G.M.), and P30 EY005722 (Duke Eye Center); Award from LCI Industries (G.M.); and Research to Prevent Blindness, Inc. (RPB) Core grant (Duke Eye Center). Our sincere thanks to Drs. Magali Saint-Geniez and Daisy Shu for valuable scientific conversations.
Author contributions
Conceptualization, T.P. and G.M.; methodology, T.P.; figure preparation, T.P. and V.M.P.; writing – original draft, T.P. and G.M.; writing – review and editing, T.P. and G.M.; supervision and funding acquisition, G.M.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Tanu Parmar, Email: tanu.parmar@duke.edu.
Goldis Malek, Email: gmalek@duke.edu.
References
- 1.Zhu S., Yam M., Wang Y., Linton J.D., Grenell A., Hurley J.B., Du J. Impact of euthanasia, dissection and postmortem delay on metabolic profile in mouse retina and RPE/choroid. Exp. Eye Res. 2018;174:113–120. doi: 10.1016/j.exer.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsantilas K.A., Cleghorn W.M., Bisbach C.M., Whitson J.A., Hass D.T., Robbings B.M., Sadilek M., Linton J.D., Rountree A.M., Valencia A.P., et al. An Analysis of Metabolic Changes in the Retina and Retinal Pigment Epithelium of Aging Mice. Invest. Ophthalmol. Vis. Sci. 2021;62:20. doi: 10.1167/iovs.62.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi G., Mi Y., Yin F. Characterizing brain metabolic function ex vivo with acute mouse slice punches. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millman J.R., Doggett T., Thebeau C., Zhang S., Semenkovich C.F., Rajagopal R. Measurement of Energy Metabolism in Explanted Retinal Tissue Using Extracellular Flux Analysis. J. Vis. Exp. 2019;143 doi: 10.3791/58626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shetty T., Park B., Corson T.W. Measurement of mitochondrial respiration in the murine retina using a Seahorse extracellular flux analyzer. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shosha E., Qin L., Lemtalsi T., Zaidi S.A.H., Rojas M., Xu Z., Caldwell R.W., Caldwell R.B., Fouda A.Y. Investigation of Retinal Metabolic Function in Type 1 Diabetic Akita Mice. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.900640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L., Chaudhari K., Winters A., Sun Y., Liu R., Yang S.H. Characterizing region-specific glucose metabolic profile of the rodent brain using Seahorse XFe96 analyzer. J. Cereb. Blood Flow Metab. 2022;42:1259–1271. doi: 10.1177/0271678X221077341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The published article includes all datasets generated or analyzed during this study.