Abstract
Widespread ecosystem degradation from noxious substances like industrial waste, toxic dyes, pesticides, and herbicides poses serious environmental risks. For remediation of these hazardous problems, present study introduces an innovative Cu-doped Ce₂Zr₂O₇ nano-photocatalyst, fabricated via a simple, eco-friendly hydrothermal method, designed to degrade toxic textile dye methylene blue. Harnessing Cu doping for pyrochlore Ce2Zr2O7, structure engineering carried out through a hydrothermal synthesis method to achieve superior photocatalytic performance, addressing limitations of rapid charge carrier recombination in existing photocatalysts. Photoluminescence analysis showed that doped pyrochlore slows charge carrier recombination, boosting dye degradation efficiency. UV–Visible analysis demonstrated an impressive 96 % degradation of methylene blue by Cu-doped Ce2Zr2O7 within 50 min, far exceeding the performance of pristine materials. Trapping experiments clarified the charge transfer mechanism, deepening our understanding of the photocatalytic process. These findings highlight the potential for developing innovative, highly efficient photocatalysts for environmental remediation, offering sustainable solutions to combat pollution. This study not only addresses the limitations of existing photocatalysts but also opens new avenues for enhancing photocatalytic performance through strategic material design.
Keywords: Cu doped Ce2Zr2O7, Hydrothermal method, Photocatalytic degradation, Methylene blue, Trapping experiment
Graphical abstract
Highlights
-
•
Cu dopped Ce2Zr2O7 was synthesized via facile hydrothermal approach.
-
•
The PL study indicate lower Cu dopped Ce2Zr2O7 lower recombination rate.
-
•
Visible light source employed toward degradation of methylene blue dye.
-
•
The copper doped Ce2Zr2O7 degrade dye upto 96 % greater than pristine material.
-
•
Photocatalyst exhibited a stable response after 5 cycle.
1. Introduction
The advent of extensive industrialization, coupled with the discharge of effluents in close proximity to water bodies such as rivers and lakes, has precipitated a global crisis of water contamination, imposing deleterious effects on various life forms. Consequently, imperative are ecologically sound and enduring solutions to address these pervasive global issues. Water tainted by chemical pollutants is fraught with an array of challenges, encompassing diverse hues, pharmacologically active compounds, herbicides, personal care products, industrial chemicals, pesticides, and heavy metals, systematically released into the environment. This continuous discharge poses a menacing threat to human health, aquatic ecosystems, and the overall environment [1,2]. A substantial contributor to water pollution is the textile industry, which emerges as a significant source of water pollution. This industry utilizes an extensive spectrum of over ten thousand different pigments and dyes, resulting in an alarming discharge of more than 0.7 million tons of dye waste annually. The persistence of these pollutants is exacerbated by their non-biodegradable nature and resilience to environmental factors such as light, temperature, detergents, and various chemicals. This resilience underscores the gravity of the issue, often leading to the inadvertent neglect of the profound ecological impact of these persistent pollutants on water bodies [3,4]. Efforts to address these challenges necessitate comprehensive and sustainable strategies that transcend conventional approaches, acknowledging the complexity and persistence of the pollutants involved.
Traditionally, diverse methodologies including biodegradation [5], adsorption [6], membrane process [7], coagulation [8], advanced oxidation process (AOP) [9], activated sludge treatment process (ASTP) and photocatalysis [10] have been harnessed for eradication of pernicious pollutants from industrial effluents. Selection of each strategy is contingent upon situational considerations [[11], [12], [13], [14]]. However, it is imperative to acknowledge that each approach possesses its inherent advantages and drawbacks. Commonly employed physical and chemical treatment methods, such as adsorption and coagulation, demand a substantial quantity of chemicals, rendering them environmentally unfavorable [[15], [16], [17], [18], [19], [20], [21]]. Membrane technologies, including membrane bioreactors, though effective, are often characterized by inefficiencies and operational expenses that are prohibitively high. Amid these methods, heterogeneous photocatalysis emerges as a particularly promising strategy for mitigating organic dye contamination. Noteworthy is its superiority over alternative methods, attributed to cost-effectiveness of semiconductor-based photocatalysts and their enhanced capabilities in degradation of organic dyes [22,23]. Economic viability and superior efficacy of heterogeneous photocatalysis position it as a compelling avenue for addressing challenges posed by organic dye pollutants, offering a potential solution that aligns with both environmental and economic considerations.
The pyrochlore Ce2Zr2O7 stands out among various photocatalysts due to its inherent characteristics, including a small optical band gap (approximately 2.5–2.9 eV) and well-positioned valence and conduction bands at 2.20 and −0.280 eV, respectively, versus normal hydrogen electrode (NHE). Notably, it possesses attributes like efficiency, cost-effectiveness, high oxygen vacancy, chemical stability, and ease of preparation, making it a promising candidate for photo-redox reactions [24]. However, practical application of bare Ce2Zr2O7 is constrained by limitations such as a small specific area, poor hydrophilic nature, low catalytic activity, and rapid recombination of photogenerated charge carriers.
To overcome these limitations, modifications to Ce2Zr2O7 have been explored, involving the incorporation of noble metals (e.g., Au, Ag, Pt) [25] and co-catalysts such as MoP [26], FeP [27], RhP [28], Cu3P [29], and Ni2P [30]. These modifications aim to enhance photodegradation outcomes in the reaction system. Moreover, alternative economical materials such as transition metal sulphides and oxides, along with polymeric 2D nanomaterials, have been identified as viable substitutes for noble metals [[31], [32], [33], [34]]. Achieving appropriate band alignment and closely connected surface boundaries between different semiconductors has been demonstrated to impede the recombination process. Consequently, electrons in the conduction band of Ce2Zr2O7 and holes (h+) in the valence band of connected semiconductors exhibit heightened oxidizing and reducing abilities [35,36]. These materials have demonstrated superior performance as photocatalysts, owing to increased specific catalytic active sites and greater available surface area [37]. For the enhancement of the catalytic performance of the catalysts researchers have focused on several techniques such as composite formation, heterostructures, alloying and doping, among these doping of semiconductors via conductive Cu proved to be quite viable and effective, as it resulted in enhanced photocatalytic behavior for organic pollutant breakdown. A study demonstrating effect of doping conducted by Rehman et al. achieved a 66 % photocatalytic efficiency using visible light-driven Cu-doped SrTiO₃ nanomaterials fabricated via the sol-gel technique within 2 h of exposure to visible light [38]. Further highlighting the impact of Cu doping on the efficiency of photocatalysts, Heshan Liyanaarachchi conducted research where Cu doping within TiO₂/g-C₃N₄ for methylene blue removal was done. Study achieved a degradation rate of 4.4 × 10−3 min−1 [39]. Arzu Ekinci also studied the role of copper in dye removal, finding that CuO nanoparticles removed 96.58 % of methylene blue in 70 min [40]. Additionally, Chayet W. et al. used Chlorophyll-Cu/ZnO to remove Rhodamine B, achieving 99.5 % removal of RhB [41]. Hamid Reza Pouretedal et al. synthesized copper-doped ZnS nanoparticles, investigating the effects of different loading catalysts and pH on the photodegradation of methylene blue, resulting in an impressive 87.3 % photocatalytic efficiency [42]. Zheng Jin et al. reported the hydrothermal synthesis of Cu–P25-graphene, achieving 98 % removal of methylene blue [43]. Halil Demir et al. incorporated copper in different concentrations into the lattice of Bi₂S₃, degrading 40 ppm methylene blue dye to 90.7 % [44]. From these investigations it was observed that the copper doping significantly enhanced the photocatalytic efficiency therefore, we have also choose the copper as dopant in the pyrochlore Ce2Zr2O7. These advancements underscore multifaceted strategies employed to tailor photocatalytic materials for enhanced efficiency in degrading organic pollutants. This study builds on these advancements by overcoming limitations, and prevention of rapid recombination of photogenerated charge carriers.
In this study, Ce₂Zr₂O₇ and Cu-doped Ce₂Zr₂O₇ nanomaterials were synthesized using a facile hydrothermal method, targeting the photocatalytic removal of organic contaminants. Previous attempts have shown that practical application of bare Ce₂Zr₂O₇ is limited by its small specific surface area, poor hydrophilicity, low catalytic activity, and rapid recombination of photogenerated charge carriers. This study addresses these challenges by introducing Cu doping to Ce₂Zr₂O₇, thereby enhancing its photocatalytic properties. A comprehensive analysis, employing diffraction, microscopic, and spectroscopic techniques, was conducted to assess phase, elemental composition, shape, and optical characteristics of synthesized materials. Results revealed robust electronic interactions between Ce₂Zr₂O₇ and Cu, creating favorable conditions for reducing recombination of photogenerated electron-hole pairs (e−/h+) and enhancing absorption efficiency of visible light. To evaluate photocatalytic efficiency, a photodegradation test using methylene blue (MB) dye was conducted. Findings indicated that doped Ce₂Zr₂O₇ exhibited an impressive 96 % degradation, significantly surpassing performance of pristine pyrochlore. These results underscore efficacy of doping strategy in elevating photocatalytic performance of Ce₂Zr₂O₇, highlighting its potential for advanced applications in the remediation of organic contaminants. This study not only overcomes existing limitations of Ce₂Zr₂O₇ but also introduces a novel approach to enhancing its practical applicability, marking a significant advancement in the field of photocatalysis.
2. Materials and methods
2.1. Reagents
All the reagents utilized in this work such as Ce(NO3)3.6H2O (Sigma Aldrich, 99.9 %), Cu(NO3)2 (Sigma Adrich, 99.99 %), ZrOCl2.8H2O (Sigma Aldrich, 98 %), KOH (Merck, 99.99 %), and methylene blue (Sigma Aldrich, 75 %) were used without further refinement.
2.2. Fabrication of Cu doped Ce2Zr2O7
A straightforward, highly effective, and economical hydrothermal process was employed to synthesize Cu-doped Ce2Zr2O7 nanomaterials. Synthesis procedure involved preparation of an equimolar (0.05 M) solution of Ce(NO3)3·6H2O and ZrOCl2·8H2O in 50 mL of deionized water (DI), vigorously stirred for 2 h to ensure complete dissolution of precursors. Subsequently, 3.0 M KOH was added to aqueous solution of cerium nitrate and ZrOCl2·8H2O, followed by vigorous stirring on a magnetic hot plate. Next, 5 wt% of Cu(NO3)2·6H2O was introduced into aforementioned mixture and subjected to sonication for 30 min. Resulting reaction solution was then transferred into a hydrothermal reactor and placed in a muffle furnace for 24 h at 180 °C. To remove undesired residues, reaction solution was centrifuged multiple times with ethanol and DI H2O. Resulting precipitate was subsequently dried overnight at 80 °C using a hot air oven. The dried powder underwent annealing at 850 °C for 4 h in a ceramic crucible. For comparative purposes, Ce2Zr2O7 was synthesized using the same procedure, with the only difference being the omission of Cu(NO3)2·6H2O. This approach ensures a controlled comparison between the doped and pristine materials, elucidating the specific impact of copper doping on the properties of Ce2Zr2O7.
2.3. Characterization
Cu-doped Ce2Zr2O7 sample's crystalline nature and phase purity were assessed using a PANalytical X′-Pert Pro X-Ray diffractometer. Cu Kα radiation source with a wavelength (λ) of 1.5406 was employed for this purpose. Scanning electron microscope (Quanta 200-FEG), coupled with an Energy Dispersive Spectroscopy (EDS) instrument, was utilized to analyze surface morphology and composition of material. Core crystalline nature and morphology of prepared material were investigated using a High-resolution Transmission Electron Microscope (HR-TEM), specifically JEM-2100 model from JEOL, Japan. Structural features of fabricated materials were determined through Raman analysis, conducted using a Bruker Multi RAM instrument. Optical properties of synthesized products were examined via an Ultraviolet Diffused Reflectance Spectrum (UV–Vis DRS), measured with a Cary-60 Agilent instrument. BaSO4 served as a standard for this analysis. Photoluminescence (PL) properties of fabricated materials were evaluated using Confocal Micro-PL, employing a laser with a suitable excitation wavelength. A 5-ppm solution was prepared in DMSO solvent, followed by sonication to form a clear solution. The PL study encompassed range of 370–650 nm, with an excitation wavelength, observing an excitation band attributable to absorption of photon energy. These comprehensive analyses provide valuable insights into structural, morphological, and optical characteristics of synthesized Cu-doped Ce2Zr2O7 nanomaterial.
2.4. Photocatalytic evaluation
Degradation efficiency of fabricated material as a photocatalyst was assessed using methylene blue (MB) dye as a probe molecule, employing visible light irradiation from a 200 W Newport Xenon lamp. Experimental procedures were carried out within a custom-made cuboidal photoreactor (45 cm × 45 cm), equipped with a stirring plate, magnetic stir bar, and a visible light source to facilitate photocatalysis. To confirm degradation of MB under visible light irradiation, a photolysis experiment was conducted. In a standard photodegradation experiment, 10 mg of synthesized photocatalysts were introduced into a 100 mL dye solution at a concentration of 20 ppm. Dye and synthesized material were agitated in complete darkness for 30 min to establish adsorption-desorption mechanism. This was monitored using a UV–Vis spectrophotometer. Following adsorption phase, dye solution containing photocatalyst was subjected to visible irradiation, and a predetermined volume of the solution (5 mL) was extracted at regular intervals (10 min). Nano-photocatalysts were separated through centrifugation, and UV–Vis spectrophotometer was utilized to analyze photodegradation of the dye at a specific wavelength (667 nm). The removal efficiency of dye was quantified using following expression:
| (1) |
In this, C0 and Ct, corresponds to the concentration when time is equal to zero and concentration of dye at a time (t) during the reaction, accordingly. Radical trapping tests were performed to examine the different reactive species formed throughout the photocatalytic reaction by the elimination process.
3. Result and discussion
3.1. Structural analysis
The crystalline phases and purity of synthesized Ce2Zr2O7 and Cu-doped Ce2Zr2O7 materials were analyzed through Powder X-ray Diffraction (PXRD). Obtained results revealed formation of a pure cubic phase with an Fd-3m space group for pyrochlore Ce2Zr2O7, perfectly matching JCPDS No. 00-052-1104 standard, as depicted in XRD pattern (Fig. 1a). Diffraction peaks at 2 theta values of 29.19°, 34.12°, 48.48°, 57.6° and 60.0°, corresponded to (222), (400), (440), (622) and (444) planes of pyrochlore Ce2Zr2O7 phase, consistent with previous reports [45]. While copper doping did not alter phase, a noticeable shift in peaks indicated strong interactions between copper and dopant [46]. Effect of doping on crystallite size of pure pyrochlore and Cu doped pyrochlore was measured with Debye Scherer formula are 94 nm and 72 nm. The calculated lattice constant and cell volume were found to be 10.609 Å and 1194 ų, respectively, which increased to 10.633 Å and 1197.4 ų after recycling. Change in lattice constant and cell volume suggest that copper has been incorporated in lattices of cubic structure. Shifting of XRD peaks towards lower angles further confirmed effective doping of copper in materials, as illustrated in Fig. 1b. This thorough XRD analysis provides crucial insights into structural characteristics and incorporation of copper in synthesized materials.
Fig. 1.
(a) XRD diffractogram and (b) enlarged view of fabricated pristine and Cu doped Ce2Zr2O7 material.
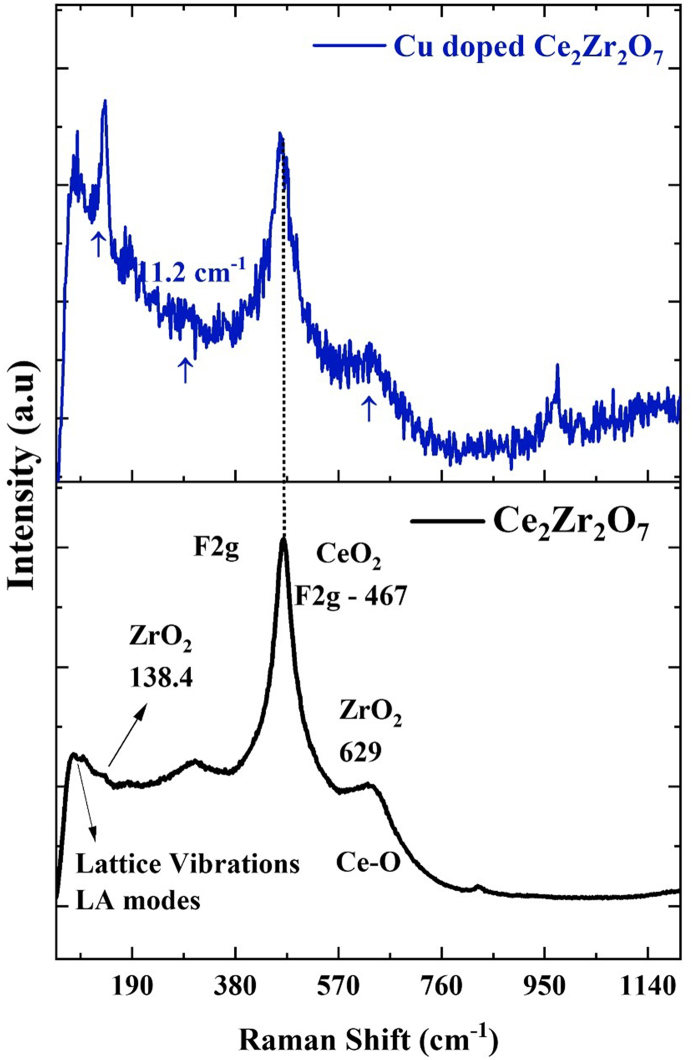
Raman analysis of both undoped and Cu-doped Ce₂Zr₂O₇ samples given in Fig. 2 reveals distinct spectral features that confirm formation of Ce₂Zr₂O₇ structure and effects of Cu doping. In undoped Ce₂Zr₂O₇, prominent peak observed at 467 cm⁻1 corresponds to F₂g mode, characteristic of fluorite structure of Ce₂Zr₂O₇ [47]. Additional bands at 138 cm−¹, 629 cm−¹, and 828 cm−¹ can be assigned to O-Zr-O bending, ZrO2 stretching, and Ce-O stretching vibrations, respectively, further validating presence of Ce₂Zr₂O₇. In Cu-doped Ce₂Zr₂O₇ spectrum, notable shifts and intensity changes are observed. Band appearing near the 100 cm−1 is attribution of lattice vibrations. The main intensity peak shifts from 467 cm⁻1–482 cm⁻1, indicating an 15 cm⁻1 shift, which can be attributed to the incorporation of Cu into Ce₂Zr₂O₇ lattice, causing slight distortions and modifying local environment of lattice vibrations. Moreover, there is an increase in intensity of smaller bands at 138 cm−¹, and 629 cm−¹. Moreover, there is an increase in intensity of smaller bands at 299 cm⁻1, 625 cm⁻1, and 828 cm⁻1. This increase suggests enhanced vibrational modes due to dopant-induced changes in lattice dynamics and improved structural integrity of Cu-doped material. These spectral changes not only confirm successful doping of Cu into Ce₂Zr₂O₇ structure but also demonstrate the altered vibrational properties due to Cu incorporation, supporting enhanced photocatalytic activity observed in doped samples. Thus, Raman analysis effectively corroborates formation and modification of Ce₂Zr₂O₇ with Cu.
Fig. 2.
Raman analysis of Ce2Zr2O7 and Cu doped Ce2Zr2O7.
3.2. Morphological analysis
Surface morphology of pristine Ce2Zr2O7 and Cu-doped Ce2Zr2O7 was examined through SEM analysis, as illustrated in Fig. 3a&b. SEM micrographs depict a rough sheet-like morphology for Ce2Zr2O7 and well-defined porous-shaped particles for Cu-doped Ce2Zr2O7. Distinct morphology of doped material arises from variations in surface energy of its crystalline planes, resulting in differences in preferred geometry due to copper doping. This well-defined morphology with an uneven surface provides more active sites for catalytic processes. Additionally, heterojunction material is advantageous for charge carrier separation, contributing to improved catalytic performance [48]. TEM micrograph of doped material (Fig. 3c) further illustrated nanoparticle morphology with visible spaces, highlighting porous nature of doped material. This porous structure enhances effective surface area of active substance, providing more active sites for adsorption of dye species. Combination of well-defined morphology and porous structure contributes to the enhanced catalytic properties of the Cu-doped Ce2Zr2O7 material.
Fig. 3.
SEM micrograph of (a) Pristine Ce2Zr2O7 (b) Cu doped Ce2Zr2O7 and (c)TEM micrograph of Cu doped Ce2Zr2O7.
3.3. Textural analysis and compositional analysis
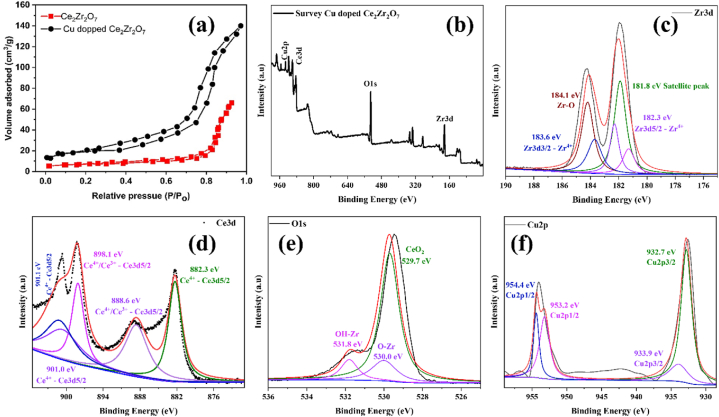
Nitrogen sorption isotherms for developed Ce2Zr2O7 and Cu-doped Ce2Zr2O7 are depicted in Fig. 4a. In high-pressure zone, both materials exhibited an IV-type isotherm with H3-type hysteresis. Desorption process was utilized to assess pore size distribution, revealing a broad dispersion of large pore sizes in all samples. BET analysis indicated that specific surface area of pure Ce2Zr2O7 was 35.51 m2/g, while Cu-doped Ce2Zr2O7 exhibited a higher surface area of 70.21 m2/g, as illustrated in Fig. 4a. This increase in surface area for Cu-doped Ce2Zr2O7 suggests a greater number of active zones on interface, which is expected to enhance photocatalytic activities of these materials. Nitrogen sorption results, combined with BET analysis, provide valuable insights into porous nature and increased surface area, indicating potential for improved photocatalytic performance of Cu-doped Ce2Zr2O7 material [48,49].
Fig. 4.
(a) BET isotherm of the pure and doped material, (b) XPS analysis of Cu doped Ce2Zr2O7 (a) survey, deconvoluted (b) Zr3d spectrum, (c) Ce3d spectrum, (d) O1s spectrum, (e) Cu2p.
X-ray Photoelectron Spectroscopy (XPS) analysis of Cu-doped Ce₂Zr₂O₇ samples reveals detailed insights into chemical states of elements present. Survey spectrum in Fig. 4b displays distinct peaks corresponding to Zr3d, O1s, Ce3d, and dopant Cu2p, indicating successful doping of Cu into Ce₂Zr₂O₇ matrix. In Fig. 4c, high-resolution XPS spectrum of Zr3d is deconvoluted to show peaks at 182.3 eV, attributed to Zr4+ state in Zr3d5/2 spin state [50], and at 183.7 eV, corresponding to Zr3d3/2 spin state also in 4+ state [51]. Additionally, a smaller hump at 186.0 eV is observed, which is associated with Zr–O bond [52]. Fig. 4d presents deconvoluted Ce3d spectrum with peaks at 882.3, 888.6, 890.2, 898.1, 901.0 and 901.1 eV all associated with +3 and + 4 oxidation states [[53], [54], [55]]. O1s spectrum in Fig. 4e is particularly important as it reveals multiple peaks upon deconvolution: 530.0 eV (Zr–O) [56], 529.7 eV (CeO₂) [57], 534.2 eV (Zr–OH) [58], 535.2 eV (C–OH and CeO₂ species). Dopant Cu is confirmed in Fig. 4f through Cu2p spectrum, which is deconvoluted to show peaks at 932.7 eV and 933.9 eV for Cu2p3/2, and at 953.2 eV and 954.4 eV for Cu2p1/2 confirming the +2 oxidation state of copper [[59], [60], [61]]. These detailed peak assignments confirm the presence and chemical states of the respective elements, indicating successful incorporation of Cu into Ce₂Zr₂O₇ structure and providing insight into the local electronic environments within doped samples.
3.4. Optical analysis
UV–Visible spectroscopy was employed to analyze the optical absorbance behavior and band gap of both pure Ce2Zr2O7 and Cu-doped Ce2Zr2O7, as illustrated in Fig. 5a. In comparison to the bare material, the Cu-doped Ce2Zr2O7 nanomaterial exhibited significantly higher absorption, particularly in the longer wavelength range (red shift). This enhanced absorption in the visible light region is attributed to the successful incorporation of copper into Ce2Zr2O7 nanoparticles. This phenomenon not only broadens the absorption spectrum but also mitigates charge-carrier recombination, a crucial factor contributing to improved catalytic activity [62]. The bandgap of all prepared samples was determined from UV–Vis spectra using Tauc relation, as expressed in following equation [63].
| (2) |
Here, , is absorption coefficient, Planck constant, photon frequency, and optical constant, respectively. The optical band gap (Eg) of Ce2Zr2O7 & Cu doped Ce2Zr2O7 are 2.84 eV and 2.38 eV was calculated by plotting (αhυ)2 vs. (Fig. 5b) [64]. The reduction in bandgap in the doped sample indicate that the material can absorb more visible light.
Fig. 5.
(a) UV–Vis. spectra and (b) optical band gap of synthesized material.
The charge carrier migration, oxygen vacancies, and recombination rates induced by photon interactions in the fabricated materials were analyzed through Photoluminescence (PL) spectroscopy at ambient temperature, as illustrated in Fig. 6. A combination of electrons and holes from the valence and conduction bands produces violet emissions. Interstitial vacancies (shallow donors) contribute to the blue transmittance observed in all Cu-doped samples, while deep oxygen vacancies (absent in Ce2Zr2O7) lead to green emission. The presence of oxygen deficiency in the doped sample facilitated the production of reactive species at the catalyst's surface, resulting in improved photocatalytic activity. The recombination of electron-hole pairs significantly influences the photocatalytic behavior of photocatalysts. A decrease in PL intensity indicates a reduction in the recombination rate, resulting in an enhanced photodegradation process. Surprisingly, due to the suppression of electron-hole recombination, Cu-doped samples displayed lower PL excitation intensities than pristine samples. Moreover, the transmittance peak of Cu-doped samples migrated to a longer wavelength than that of Ce2Zr2O7, resulting in a decrease in the energy of the bandgap [65,66]. These PL peaks and the observed blue shift may be attributed to surface oxygen vacancies and interstitial metal ions with Cu dopant nanostructures formed during the growth process. Oxygen vacancies are known to act as excellent electron scavengers, contributing to enhanced photoactivities [67]. Additionally, previous studies by Sriram et al. and Jayaraman et al. provide context for the observed PL characteristics. Sriram et al. reported a high excitation band for Ce2Zr2O7 between 425 and 500 nm, while Jayaraman et al. observed a PL band for Ce2Zr2O7 at 442 nm, excited at 380 nm, attributed to the bandgap of the solid samples [43,44]. These findings underscore the role of PL spectroscopy in elucidating the charge carrier dynamics and defects in the synthesized materials, providing valuable insights into their photocatalytic performance [68].
Fig. 6.
PL spectrum of Ce2Zr2O7 and Cu doped Ce2Zr2O7.
3.5. Photocatalytic activity
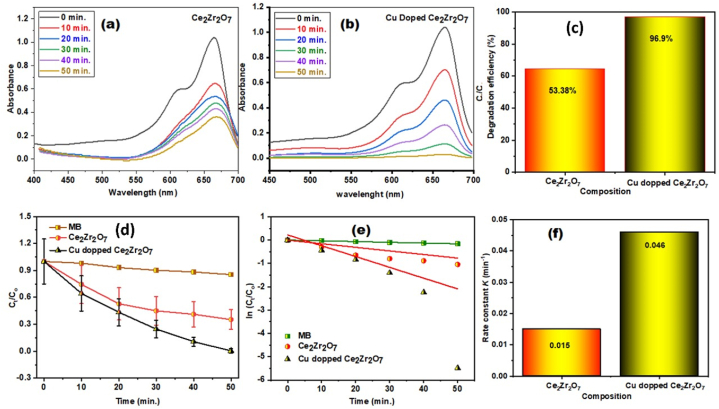
The photocatalytic efficacy of the novel nano-catalyst was determined by the photodegradation of Methylene Blue (MB) in the presence of a light source. MB is a common organic pollutant used in the printing, coloring, and textile industries. In the photodegradation investigation, mixtures of MB dye and fabricated materials were exposed to a visible light source, and the changes in MB concentration were measured at intervals of 10 min for all prepared samples as given in Fig. 7a&b and S1a&b. The photocatalytic degradation of MB with an initial concentration of 30 ppm after 50 min of exposure to pristine Ce2Zr2O7 and Cu-doped Ce2Zr2O7 resulted in degradation percentages of 53 % and 96 %, as shown in Fig. 7c. Furthermore, Cu-doped Ce2Zr2O7 exhibited superior photocatalytic degradation activity compared to pure samples, attributed to the significantly enlarged surface area and a more visible light-sensitive band gap of Cu-doped Ce2Zr2O7, contributing to the efficient elimination of the organic pollutant. Generally, nanomaterials with a higher surface area may possess more active sites for degradation than materials with a smaller surface area [69]. During visible light irradiation, electrons from the generated Cu-doped Ce2Zr2O7 composition more readily react with dye molecules, leading to the destruction of the stable MB organic dye. However, a pseudo-first-order reaction model was utilized to explore its photoactive degradation kinetics and calculated using the following expression 3.
| (3) |
Here, Ct denotes the concentration of dye at any time, Co indicates initial amount, k is rate constant and t is time and plotted graph shown in Fig. 7 d&e. The Cu doped Ce2Zr2O7 has the 0.045 min−1 higher rate constant than that of pristine material 0.015 min−1 as given in Fig. 7f. The increased rate of organic pollutant degradation attributed to the photocatalyst having unique band alignment and large surface area are responsible for adsorption of greater ions. The interfacial charge transfer among the components in the produced doped material may be the explanation for the improved photodegradation efficiency.
Fig. 7.
Degradation of methylene blue with (a) pristine Ce2Zr2O7 and (b) Cu doped Ce2Zr2O7, (c) Graph of Ct/Co vs time, (d) Degradation efficiency concerning composition, (e) Plot of ln(Ct/Co) vs Time and (f) Rate constant with respect to composition. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Effect of photocatalyst quantity and dye concentration
In this study, Fig. 8a&b illustrates photodegradation process of MB dye using various doses of Cu-doped Ce2Zr2O7 catalyst, ranging from 10 to 90 mg. Optimal catalyst weight for achieving maximum degradation efficiency was observed to be 60 mg, as compared to alternative catalyst weights of 10 mg, 25 mg, and 75 mg. Catalyst effects are most effectively achieved with weights of 50 mg or greater, as they effectively inhibit activity. However, photocatalyst's efficiency tends to decrease due to particle-particle interactions, resulting in a more pronounced screening effect as catalyst dosage increases. Consequently, system's capacity to produce reactive oxygen species (ROS) is hindered.
Fig. 8.
Effect of (a) Ce2Zr2O7 and (b) Cu doped Ce2Zr2O7 dosage on the photodegradation of MB dye.
Degradation rate of organic pollutants through photocatalytic process is directly proportional to their initial concentration. In Fig. 9a&b, results are presented for organic pollutant concentrations ranging from 20 to 80 parts per million (ppm). The data illustrates a positive correlation between dye concentration and its degradation efficiency. Specifically, an increase in concentration from 10 to 15 ppm resulted in an observed improvement in degradation efficiency. However, when concentration of Methylene Blue (MB) pollutant exceeded 30 ppm, a decline in catalytic effectiveness of photocatalyst was observed. It has been observed that increased amounts of pollutants can be adsorbed onto interface of photocatalyst. Additionally, absence of reactive oxidative substances such as •OH and O2‾• hinders process of photocatalytic degradation of organic pollutants. Efficiency of photoexcited electron-hole pair in this particular scenario was significantly diminished due to a deficiency in oxidative species, thereby impeding the overall performance of photodegradation.
Fig. 9.
Study of MB dye concentration on photodegradation with (a) Ce2Zr2O7 and (b) Cu doped Ce2Zr2O7.
3.7. The possible degradation mechanism
Irradiation of visible light on pure pyrochlore catalyst resulted in limited photo-induced electron-hole species, leading to poor photodegradation efficiency. However, Cu-doping in Ce2Zr2O7 creates a novel position within the band energy that efficiently traps electrons and prevents the combination of electrons and holes. The photodegradation process is initiated with the formation of electrons (e−) and holes (h+) (eq (1)). The photogenerated electrons react with molecular oxygen (O2) to form superoxide (eq. (2)), and then superoxide reacts with water (H2O) species to form hydroxyl radicals as well as hydroxyl ions (eq. (3)). The hydroxyl radicals react with molecular O2 to form H2O2 (eq. (4)), and decomposition of H2O2 yields hydroxyl radicals, and so on (eq. (5)). The hole species produced in the valence band also react with water species to form hydroxyl radicals (eq. (6)). The hydroxyl radicals are potential species that lead to the photodegradation of MB dye, producing CO2 as well as water (eq. (7)). The photocatalytic mechanism (eqs. (4), (5), (6), (7), (8), (9), (10))) of photodegradation is described as follows:
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
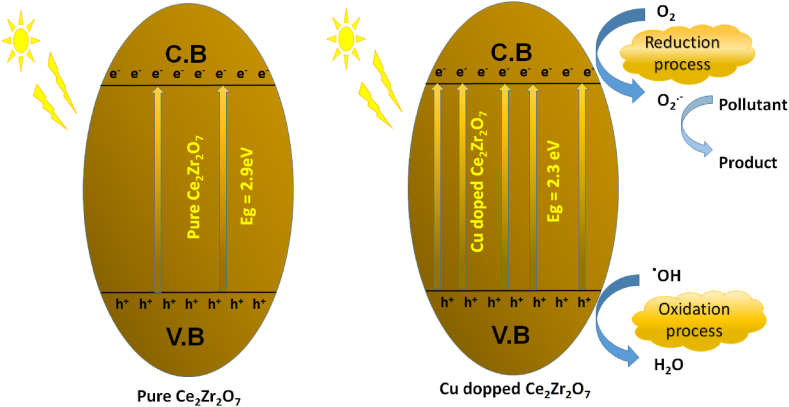
Enhanced efficiency of nano-catalyst due to Cu-doping may be explained as follows: Red-shift of energy band gap towards lower visible range leads to maximal visible harvesting and stimulation of additional electrons to conduction band, contributing to catalyst surface degradation process. Furthermore, as revealed by Photoluminescence (PL), development of new energy levels near bandgap, as well as the presence of defects in crystal such as oxygen deficiency, obstruct photogenerated electron-hole pair combination. This results in additional electrons as well as holes taking part in degradation process. Photocatalytic mechanism of photoinduced Cu-doped Ce2Zr2O7 is shown in Scheme-I.
Scheme I.
Photocatalytic mechanism of photoinduced Ce2Zr2O7 and Cu doped Ce2Zr2O7.
The scavenger analysis conducted in this study provides a nuanced understanding of the intricate mechanisms underlying the photocatalytic activity of Cu-doped Ce2Zr2O7 as shown in Fig. 10a. Employing various scavengers, each designed to selectively quench specific reactive species, allows for a comprehensive exploration of the roles these species play in the degradation process. Firstly, the utilization of parabenzoquinone (PBQ) as a superoxide scavenger implies the active involvement of superoxide radicals (O2•-) in photocatalytic oxidation. Scavenging reaction (4) demonstrates interception of superoxide radicals by PBQ, forming reduced product BQ•- and molecular oxygen. This insight indicates that superoxide radicals play a pivotal role in driving degradation mechanism.
| (11) |
Fig. 10.
(a) scavengers and (b) recyclability test profile of Cu doped Ce2Zr2O7.
Additionally, the inclusion of Na2-EDTA as a hole scavenger suggests participation of photoinduced holes (h+) in degradation pathway. Although specific reactions are not detailed, scavenger analysis likely involves processes where holes are captured by EDTA, impeding their involvement in subsequent steps. Use of dimethyl sulfoxide (DMSO) as a hydroxyl radical scavenger indicates generation of hydroxyl radicals (•OH) during photocatalytic process. Scavenging reaction involves interception of hydroxyl radicals by DMSO, preventing their participation in subsequent reactions. Inclusion of silver nitrate (AgNO3) as an electron scavenger suggests active role of electrons (e−) in overall photodegradation process. Scavenging reaction likely involves reduction of silver ions to metallic silver by captured electrons, illustrating significance of electrons in degradation mechanism. Sustained photocatalytic efficiency observed over five consecutive cycles, as depicted in Fig. 10 (b), underscores stability of Cu-doped Ce2Zr2O7 under visible light illumination. This enduring performance over multiple cycles is crucial for practical applications, indicating material's resilience and effectiveness in repeated photocatalytic processes.
3.8. Post recycle structural analysis
Post-recycle stability analysis of Cu-doped Ce₂Zr₂O₇ photocatalyst reveals that same crystallographic planes, specifically (2 2 2), (4 0 0), (4 4 0) and (6 2 2), were retained at 2θ values of 29.19°, 34.12°, 48.48° and 57.6°, respectively as given in Fig. 11. This retention indicates that fundamental crystal structure remained intact after 5 photocatalytic cycles. However, there was a noticeable decrease in the intensity of (4 0 0) plane, suggesting a reorientation of some crystallites. Additionally (2 2 2) and (4 4 0) planes exhibited widening, along with peak shifted towards lower degree, as well as increased noise in XRD patterns, indicates slight lattice distortions. These distortions likely result from repeated stress and reaction conditions during recycling process. These observations highlight the photocatalyst's structural robustness while also pointing to some degree of degradation, emphasizing need for further optimization to improve long-term stability and performance in practical photocatalytic applications.
Fig. 11.
Pre and Post recycle XRD analysis.
4. Conclusion
This study marks the synthesis and application of Cu-doped Ce₂Zr₂O₇ in photocatalytic removal of methylene blue. By introducing Cu into Ce₂Zr₂O₇, the photocatalytic performance was notably enhanced. Various analytical techniques, including XRD, SEM, UV, and BET analysis, confirmed the phase purity, nano-porous dispersed shape, bandgap (Eg = 2.3 eV), and surface area (70.21 m2/g) of Cu-doped Ce₂Zr₂O₇. Reduction in peak intensity observed in pristine Ce₂Zr₂O₇ with Cu doping, as evidenced by PL analysis confirmed the inhibition of photogenerated charged recombination. The enhanced surface area and the synergistic effects among the components are responsible for the significant improvement in the photocatalytic activity. Cu-doped Ce₂Zr₂O₇ achieved a 96 % degradation efficiency for methylene blue pollutants under visible light within 50 min, outperforming pure photocatalysts. The study explored the effects of dye concentration and catalyst loading on the photodegradation of MB dye. The increased catalytic centers on the expanded surface area significantly improved the composition's effectiveness in removing organic pollutants, leading to a substantial reduction in charge carrier recombination. Superoxide radicals were identified as key agents in the photodegradation process through radical trapping tests. The practical implications of this study are substantial, offering a potential strategy for environmental remediation by developing high-efficiency and stable pyrochlore photocatalysts for the elimination of organic contaminants through photoinduced reactions. This research not only advances the field of environmental science but also provides a promising approach for addressing pollution, highlighting the impact and applicability of Cu-doped Ce₂Zr₂O₇ in environmental cleanup efforts. Future work should focus on scaling up the hydrothermal synthesis process to ensure industrial feasibility, exploring the effectiveness of Cu-doped Ce₂Zr₂O₇ on a broader range of dyes and pollutants, and assessing the long-term stability and reusability of the photocatalyst in continuous environmental remediation processes.
CRediT authorship contribution statement
Razan A. Alshgari: Formal analysis, Data curation, Conceptualization. Muhammad Abdullah: Software, Resources, Formal analysis, Data curation. Syed Imran Abbas Shah: Software, Methodology, Investigation. Abdul Ghafoor Abid: Writing – original draft, Resources, Methodology. Saikh Mohammad: Investigation, Formal analysis, Data curation. Muhammad Fahad Ehsan: Validation, Software, Methodology, Investigation. Muhammad Naeem Ashiq: Writing – original draft, Visualization, Resources, Investigation. Suleyman I. Allakhverdiev: Writing – review & editing, Writing – original draft, Project administration, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was funded by the Researchers Supporting Project Number (RSP2024R265), King Saud University, Riyadh, Saudi Arabia. SIA were supported by the grant from Russian Science Foundation (No: 24-14-00033). Table 1 was obtained within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme No. 122050400128-1).
Table 1.
Comparison between the reported work and the present work.
| Sr. No. | Photocatalyst | % degradation | Time (min) | Dye | References |
|---|---|---|---|---|---|
| 1 | Sn doped K0.51Sb2.67O6.26 | 86 | 60 | Methylene blue | [70] |
| 2 | Ca doped CeO2 | 84 | 80 | Methylene blue | [71] |
| 3 | Ag-doped KTaTeO6 | 76.2 | 180 | Methyl violet | [72] |
| 4 | Pr doped Bi2Sn2O7 | 80 | 240 | Rhodamine B | [73] |
| 5 | Sm2Zr2O7 | 75 | 60 | Congored | [74] |
| 6 | Bi doped Na2Ta2O6 | 40 | 240 | Rhodamine B | [75] |
| 7 | Cu doped Ce2Zr2O7 | 96.9 | 50 | Methylene blue | This work |
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34266.
Contributor Information
Syed Imran Abbas Shah, Email: imran12bukhari@gmail.com.
Suleyman I. Allakhverdiev, Email: suleyman.allakhverdiev@gmail.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Saqib N.U., Shah I., Adnan R. An emerging photocatalyst for wastewater remediation: a mini-review on CaCu3Ti4O12 photocatalysis. Environmental Science and pollution Research. 2022;29:40403–40414. doi: 10.1007/s11356-022-19703-z. [DOI] [PubMed] [Google Scholar]
- 2.Jang Y.J., Simer C., Ohm T. Comparison of zinc oxide nanoparticles and its nano-crystalline particles on the photocatalytic degradation of methylene blue. Mater. Res. Bull. 2006;41:67–77. [Google Scholar]
- 3.Ogugbue C.J., Sawidis T. Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent. Biotechnology Research International. 2011;2011 doi: 10.4061/2011/967925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffri S.B., Ahmad K.S. Neoteric environmental detoxification of organic pollutants and pathogenic microbes via green synthesized ZnO nanoparticles. Environ. Technol. 2019;40:3745–3761. doi: 10.1080/09593330.2018.1488888. [DOI] [PubMed] [Google Scholar]
- 5.Eslami H., Sedighi Khavidak S., Salehi F., Khosravi R., Fallahzadeh R.A., Peirovi R., Sadeghi S. Biodegradation of methylene blue from aqueous solution by bacteria isolated from contaminated soil. Journal of Advances in Environmental Health Research. 2017;5:10–15. [Google Scholar]
- 6.Tan K.B., Vakili M., Horri B.A., Poh P.E., Abdullah A.Z., Salamatinia B. Adsorption of dyes by nanomaterials: recent developments and adsorption mechanisms. Separation and Purification Technology. 2015;150:229–242. [Google Scholar]
- 7.Titchou F.E., Zazou H., Afanga H., El Gaayda J., Akbour R.A., Nidheesh P.V., Hamdani M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chemical Engineering and Processing-Process Intensification. 2021;169 [Google Scholar]
- 8.Kang D., Yu X., Ge M., Xiao F., Xu H. Novel Al-doped carbon nanotubes with adsorption and coagulation promotion for organic pollutant removal. Journal of Environmental Sciences. 2017;54:1–12. doi: 10.1016/j.jes.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Yang Q., Ma Y., Chen F., Yao F., Sun J., Wang S., Yi K., Hou L., Li X., Wang D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019;378 [Google Scholar]
- 10.Sipma J., Osuna B., Collado N., Monclús H., Ferrero G., Comas J., Rodriguez-Roda I. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination. 2010;250:653–659. [Google Scholar]
- 11.Demir H., Şahin Ö., Baytar O., Horoz S. Investigation of the properties of photocatalytically active Cu-doped Bi2S3 nanocomposite catalysts. J. Mater. Sci. Mater. Electron. 2020;31:10347–10354. [Google Scholar]
- 12.Shah A.A., Bhatti M.A., Tahira A., Chandio A.D., Channa I.A., Sahito A.G., Chalangar E., Willander M., Nur O., Ibupoto Z.H. Facile synthesis of copper doped ZnO nanorods for the efficient photo degradation of methylene blue and methyl orange. Ceram. Int. 2020;46:9997–10005. [Google Scholar]
- 13.Baytar O., Sahin O., Kilicvuran H., Horoz S. Synthesis, structural, optical and photocatalytic properties of Fe-alloyed CdZnS nanoparticles. J. Mater. Sci. Mater. Electron. 2018;29:4564–4568. [Google Scholar]
- 14.Jin Z., Duan W., Liu B., Chen X., Yang F., Guo J. Fabrication of efficient visible light activated Cu–P25–graphene ternary composite for photocatalytic degradation of methyl blue. Appl. Surf. Sci. 2015;356:707–718. [Google Scholar]
- 15.Liyanaarachchi H., Thambiliyagodage C., Liyanaarachchi C., Samarakoon U. Efficient photocatalysis of Cu doped TiO2/g-C3N4 for the photodegradation of methylene blue. Arab. J. Chem. 2023;16 [Google Scholar]
- 16.Ekinci A., Kutluay S., Şahin Ö., Baytar O. Green synthesis of copper oxide and manganese oxide nanoparticles from watermelon seed shell extract for enhanced photocatalytic reduction of methylene blue. Int. J. Phytoremediation. 2023;25:789–798. doi: 10.1080/15226514.2022.2109588. [DOI] [PubMed] [Google Scholar]
- 17.Worathitanon C., Jangyubol K., Ruengrung P., Donphai W., Klysubun W., Chanlek N., Prasitchoke P., Chareonpanich M. High performance visible-light responsive Chl-Cu/ZnO catalysts for photodegradation of rhodamine B. Appl. Catal. B Environ. 2019;241:359–366. [Google Scholar]
- 18.Mardani H.R., Forouzani M., Ziari M., Biparva P. Visible light photo-degradation of methylene blue over Fe or Cu promoted ZnO nanoparticles. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;141:27–33. doi: 10.1016/j.saa.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Yu X., Chen H., Ji Q., Chen Y., Wei Y., Zhao N., Yao B. p-Cu2O/n-ZnO heterojunction thin films with enhanced photoelectrochemical properties and photocatalytic activities for norfloxacin. Chemosphere. 2021;267 doi: 10.1016/j.chemosphere.2020.129285. [DOI] [PubMed] [Google Scholar]
- 20.Yu X., Zhang J., Zhang J., Niu J., Zhao J., Wei Y., Yao B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: analysis of degradation pathways and intermediates. Chem. Eng. J. 2019;374:316–327. [Google Scholar]
- 21.Nie J., Yu X., Liu Z., Zhang J., Ma Y., Chen Y., Ji Q., Zhao N., Chang Z. Energy band reconstruction mechanism of Cl-doped Cu2O and photocatalytic degradation pathway for levofloxacin. J. Clean. Prod. 2022;363 [Google Scholar]
- 22.Hitam C.N., Jalil A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020;258 doi: 10.1016/j.jenvman.2019.110050. [DOI] [PubMed] [Google Scholar]
- 23.Yu X., Zhang J., Chen Y., Ji Q., Wei Y., Niu J., Yu Z., Yao B. Ag-Cu2O composite films with enhanced photocatalytic activities for methylene blue degradation: analysis of the mechanism and the degradation pathways. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- 24.Maulidya A., Yulizar Y., Bakri R., Apriandanu D.O.B., Surya R. Synthesis and characterizations of Ce2Zr2O7–TiO2 for increased photocatalytic activity toward degradation of methylene blue. Ceram. Int. 2022;48:29523–29532. [Google Scholar]
- 25.Darabdhara G., Das M. Bimetallic Au-Pd nanoparticles on 2D supported graphitic carbon nitride and reduced graphene oxide sheets: a comparative photocatalytic degradation study of organic pollutants in water. Chemosphere. 2018;197:817–829. doi: 10.1016/j.chemosphere.2018.01.073. [DOI] [PubMed] [Google Scholar]
- 26.Yue Q., Wan Y., Sun Z., Wu X., Yuan Y., Du P. MOP is novel, noble-metal-free cocatalyst for enhanced photocatalytic hydrogen production from water under visible light. J. Mater. Chem. A. 2015;3:16941–16947. [Google Scholar]
- 27.Callejas J.F., McEnaney J.M., Read C.G., Crompton J.C., Biacchi A.J., Popczun E.J., Gordon T.R., Lewis N.S., Schaak R.E. Electrocatalytic and photocatalytic hydrogen production from acidic and neutral-pH aqueous solutions using iron phosphide nanoparticles. ACS Nano. 2014;8:11101–11107. doi: 10.1021/nn5048553. [DOI] [PubMed] [Google Scholar]
- 28.Song L., Li T., Zhang S., Zhang S. Synthesis of rhodium phosphide cocatalyst and remarkably enhanced photocatalytic hydrogen evolution over CdS under visible light radiation. Chem. Eng. J. 2017;314:498–507. [Google Scholar]
- 29.Zou W., Xu L., Pu Y., Cai H., Wei X., Luo Y., Li L., Gao B., Wan H., Dong L. Advantageous interfacial effects of AgPd/g‐C3N4 for photocatalytic hydrogen evolution: electronic structure and H2O Dissociation. Chem.--Eur. J. 2019;25:5058–5064. doi: 10.1002/chem.201806074. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H., Sun S., Jiang P., Xu Z. Graphitic C3N4 modified by Ni2P cocatalyst: an efficient, robust and low cost photocatalyst for visible-light-driven H2 evolution from water. Chem. Eng. J. 2017;315:296–303. [Google Scholar]
- 31.Li K., Huang Z., Zhu S., Luo S., Yan L., Dai Y., Guo Y., Yang Y. Removal of Cr (VI) from water by a biochar-coupled g-C3N4 nanosheets composite and performance of a recycled photocatalyst in single and combined pollution systems. Appl. Catal. B Environ. 2019;243:386–396. [Google Scholar]
- 32.Baytar O., Sahin O., Kilicvuran H., Horoz S. Synthesis, structural, optical and photocatalytic properties of Fe-alloyed CdZnS nanoparticles. J. Mater. Sci. Mater. Electron. 2018;29:4564–4568. [Google Scholar]
- 33.Nie J., Yu X., Liu Z., Wei Y., Zhang J., Zhao N., Yu Z., Yao B. Boosting principles for the photocatalytic performance of Cr-doped Cu2O crystallites and mechanisms of photocatalytic oxidation for levofloxacin. Appl. Surf. Sci. 2022;576 [Google Scholar]
- 34.Nie J., Yu X., Wei Y., Liu Z., Zhang J., Yu Z., Ma Y., Yao B. Interfacial charge transfer effects of α-Fe2O3/Cu2O heterojunction and enhancement mechanism of its photocatalytic oxidation. Process Saf. Environ. Protect. 2023;170:241–258. [Google Scholar]
- 35.Liu X.-H., He Y., Li Z., Cheng A.-H., Song Z., Yu Z.-X., Chai S., Cheng C., He C. Size transformation of Au nanoclusters for enhanced photocatalytic hydrogen generation: interaction behavior at nanocluster/semiconductor interface. Journal of Colloidal and Interface Science. 2023;651:368–375. doi: 10.1016/j.jcis.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Yang F., Yu X., Wang K., Liu Z., Gao Z., Zhang T., Niu J., Zhao J., Yao B. Photocatalytic degradation of methylene blue over BiVO4/BiPO4/rGO heterojunctions and their artificial neural network model. J. Alloys Compd. 2023;960 [Google Scholar]
- 37.Liang M., Borjigin T., Zhang Y., Liu B., Liu H., Guo H. Controlled assemble of hollow heterostructured g-C3N4@ CeO2 with rich oxygen vacancies for enhanced photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019;243:566–575. [Google Scholar]
- 38.Rahman Q.I., Ahmad M., Misra S.K., Lohani M. Efficient degradation of methylene blue dye over highly reactive Cu doped strontium titanate (SrTiO3) nanoparticles photocatalyst under visible light. J. Nanosci. Nanotechnol. 2012;12:7181–7186. doi: 10.1166/jnn.2012.6494. [DOI] [PubMed] [Google Scholar]
- 39.Liyanaarachchi H., Thambiliyagodage C., Liyanaarachchi C., Samarakoon U. Efficient photocatalysis of Cu doped TiO2/g-C3N4 for the photodegradation of methylene blue. Arab. J. Chem. 2023;16 [Google Scholar]
- 40.Ekinci A., Kutluay S., Şahin Ö., Baytar O. Green synthesis of copper oxide and manganese oxide nanoparticles from watermelon seed shell extract for enhanced photocatalytic reduction of methylene blue. Int. J. Phytoremediation. 2023;25:789–798. doi: 10.1080/15226514.2022.2109588. [DOI] [PubMed] [Google Scholar]
- 41.Worathitanon C., Jangyubol K., Ruengrung P., Donphai W., Klysubun W., Chanlek N., Prasitchoke P., Chareonpanich M. High performance visible-light responsive Chl-Cu/ZnO catalysts for photodegradation of rhodamine B. Appl. Catal. B Environ. 2019;241:359–366. [Google Scholar]
- 42.Pouretedal H.R., Norozi A., Keshavarz M.H., Semnani A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard Mater. 2009;162:674–681. doi: 10.1016/j.jhazmat.2008.05.128. [DOI] [PubMed] [Google Scholar]
- 43.Jin Z., Duan W., Liu B., Chen X., Yang F., Guo J. Fabrication of efficient visible light activated Cu–P25–graphene ternary composite for photocatalytic degradation of methyl blue. Appl. Surf. Sci. 2015;356:707–718. [Google Scholar]
- 44.Demir H., Şahin Ö., Baytar O., Horoz S. Investigation of the properties of photocatalytically active Cu-doped Bi2S3 nanocomposite catalysts. J. Mater. Sci. Mater. Electron. 2020;31:10347–10354. [Google Scholar]
- 45.Bolech M., Cordfunke E., Van Genderen A., Van Der Laan R., Janssen F., Van Miltenburg J., Solids C.o. The heat capacity and derived thermodynamic functions of La2Zr2O7 and Ce2Zr2O7 from 4 to 1000 K. J. Phys. Chem. Solid. 1997;58:433–439. [Google Scholar]
- 46.Raoux S., Salinga M., Jordan-Sweet J.L., Kellock A. Effect of Al and Cu doping on the crystallization properties of the phase change materials SbTe and GeSb. J. Appl. Phys. 2007;101 [Google Scholar]
- 47.Mansingh S., Acharya R., Martha S., Parida K. Pyrochlore Ce2Zr2O7 decorated over rGO: a photocatalyst that proves to be efficient towards the reduction of 4-nitrophenol and degradation of ciprofloxacin under visible light. Phys. Chem. Chem. Phys. 2018;20:9872–9885. doi: 10.1039/c8cp00621k. [DOI] [PubMed] [Google Scholar]
- 48.Lei B., Xu D., Wei B., Xie T., Xiao C., Jin W., Xu L. Interfaces, in situ synthesis of α-Fe2O3/Fe3O4 heterojunction photoanode via fast flame annealing for enhanced charge separation and water oxidation. ACS Appl. Mater. Interfaces. 2021;13:4785–4795. doi: 10.1021/acsami.0c19927. [DOI] [PubMed] [Google Scholar]
- 49.Jayaraman V., Mani A., Technology P. Interfacial coupling effect of high surface area Pyrochlore like Ce2Zr2O7 over 2D g-C3N4 sheet photoactive material for efficient removal of organic pollutants. Separation and Purification Technology. 2020;235 [Google Scholar]
- 50.Alvarez M., López T., Odriozola J., Centeno M., Domínguez M., Montes M., Quintana P., Aguilar D., González R. 2, 4-Dichlorophenoxyacetic acid (2, 4-D) photodegradation using an Mn+/ZrO2 photocatalyst: XPS, UV–vis, XRD characterization. Appl. Catal. B Environ. 2007;73:34–41. [Google Scholar]
- 51.Roustila A., Chene J., Séverac C. XPS study of hydrogen and oxygen interactions on the surface of zirconium. J. Alloys Compd. 2003;356:330–335. [Google Scholar]
- 52.Bakradze G., Jeurgens L.P., Mittemeijer E.J. Valence-band and chemical-state analyses of Zr and O in thermally grown thin zirconium-oxide films: an XPS study. J. Phys. Chem. C. 2011;115:19841–19848. [Google Scholar]
- 53.Huang L., Li Y., Xu H., Xu Y., Xia J., Wang K., Li H., Cheng X. Synthesis and characterization of CeO2/gC3N4 composites with enhanced visible-light photocatatalytic activity. Rsc Advances. 2013;3:22269–22279. [Google Scholar]
- 54.Sun Y., Chu Y., Xia X., Wang H., Tan X., Dai Z., Wang L. CeO2 nanowires Inserted into reduced graphene oxide as active Electrocatalyst for oxygen reduction reaction. Indian J. Chem., Sect. A. 2020;58:867–873. [Google Scholar]
- 55.Bera P., Anandan C. XRD and XPS studies of room temperature spontaneous interfacial reaction of CeO2 thin films on Si and Si3 N4 substrates. RSC Adv. 2014;4:62935–62939. [Google Scholar]
- 56.Sinha M., Gupta R., Kiranjot K., Singh A., Modi M.H. Effect of zirconium oxide local structure on soft x-ray optical properties near the oxygen K-edge region. J. Appl. Phys. 2020;128 [Google Scholar]
- 57.Singh P., Srivatsa K., Barvat A., Pal P. X-ray photoelectron spectroscopic studies of CeO2 thin films deposited on Ni-W (100), c-Al2O3 (0001) and Si (100) substrates. Curr. Appl. Phys. 2016;16:1388–1394. [Google Scholar]
- 58.Prabhu S.M., Pawar R.R., Sasaki K., Park C.M. A mechanistic investigation of highly stable nano ZrO2 decorated nitrogen-rich azacytosine tethered graphene oxide-based dendrimer for the removal of arsenite from water. Chem. Eng. J. 2019;370:1474–1484. [Google Scholar]
- 59.Sun M., Xu X., Min S., He J., Li K., Kang L. Controllable preparation of Cu2O/Cu-CuTCPP MOF heterojunction for enhanced electrocatalytic CO2 reduction to C2H4. Appl. Surf. Sci. 2024;659 [Google Scholar]
- 60.Kishi K., Sasanuma M. The interaction of O2 with Cu/Ni (100) and Cu/NiO/Ni (100) surfaces studied by XPS, Journal of electron spectroscopy and related phenomena. Journal of Electron Spectroscopy and Related Phenomenon. 1989;48:421–434. [Google Scholar]
- 61.Wang S., Wang W., Yue L., Cui S., Wang H., Wang C., Chen S. Hierarchical Cu2O nanowires covered by silver nanoparticles-doped carbon layer supported on Cu foam for rapid and efficient water disinfection with lower voltage. Chem. Eng. J. 2020;382 [Google Scholar]
- 62.Gao B., Chen T., Tam D.W., Huang C.-L., Sasmal K., Adroja D.T., Ye F., Cao H., Sala G., Stone M. Experimental signatures of a three-dimensional quantum spin liquid in effective spin-1/2 Ce2Zr2O7 pyrochlore. Nat. Phys. 2019;15:1052–1057. [Google Scholar]
- 63.Munawar T., Nadeem M.S., Mukhtar F., Hasan M., Mahmood K., Arshad M., Hussain A., Ali A., Saif M.S., Iqbal F. Rare earth metal co-doped Zn0·9La0.05M0.05O (M= Yb, Sm, Nd) nanocrystals; energy gap tailoring, structural, photocatalytic and antibacterial studies. Materials Science in Semicondtor Processing. 2021;122 [Google Scholar]
- 64.Manickathai K., Viswanathan S.K., Alagar M. Synthesis and characterization of CdO and CdS nanoparticles. Indian J. Pure Appl. Phys. 2008;46:561–564. [Google Scholar]
- 65.Huang M.-H., Li Y.-B., Li T., Dai X.-C., Hou S., He Y., Xiao G., Xiao F.-X. Self-transformation of ultra-small gold nanoclusters to gold nanocrystals toward boosted photoreduction catalysis. Chem. Commun. 2019;55:10591–10594. doi: 10.1039/c9cc04562g. [DOI] [PubMed] [Google Scholar]
- 66.Dai X.-C., Huang M.-H., Li Y.-B., Li T., Zhang B.-B., He Y., Xiao G., Xiao F.X. Regulating spatial charge transfer over intrinsically ultrathin-carbon-encapsulated photoanodes toward solar water splitting. J. Mater. Chem. A. 2019;7:2741–2753. [Google Scholar]
- 67.Raja K., Ramesh P., Geetha D. Structural, FTIR and photoluminescence studies of Fe doped ZnO nanopowder by co-precipitation method. Spectrochimica acta part A: molecular and biomolecular spectroscopy. 2014;131:183–188. doi: 10.1016/j.saa.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 68.Jayaraman V., Mani A. Interfacial coupling effect of high surface area Pyrochlore like Ce2Zr2O7 over 2D g-C3N4 sheet photoactive material for efficient removal of organic pollutants. Separation and Purification Technology. 2020;235 [Google Scholar]
- 69.Lai Y., Meng M., Yu Y. One-step synthesis, characterizations and mechanistic study of nanosheets-constructed fluffy ZnO and Ag/ZnO spheres used for Rhodamine B photodegradation. Appl. Catal. B Environ. 2010;100:491–501. [Google Scholar]
- 70.Reddy J.R., Veldurthi N.K., Palla S., Ravi G., Guje R., Vithal M. Facile ion‐exchange synthesis of visible light active Sn‐doped defect pyrochlore K0.51Sb2.67O6.26 and study of its photocatalytic activity. J. Chem. Technol. Biotechnol. 2014;89:1833–1841. [Google Scholar]
- 71.Ramasamy V., Mohana V., Rajendran V. Characterization of Ca doped CeO2 quantum dots and their applications in photocatalytic degradation. Open Nano. 2018;3:38–47. [Google Scholar]
- 72.Venkataswamy P., Sudhakar Reddy C., Gundeboina R., Sadanandam G., Veldurthi N.K., Vithal M. Nanostructured KTaTeO6 and Ag-doped KTaTeO6 defect pyrochlores: promising photocatalysts for dye degradation and water splitting. Electron. Mater. Lett. 2018;14:446–460. [Google Scholar]
- 73.Xu W., Zhou G., Fang J., Liu Z., Chen Y., Cen C. Synthesis and characterization of pyrochlore [Bi2Sn2O7] doping with praseodymium by hydrothermal method and its photocatalytic activity study. Int. J. Photoenergy. 2013;2013:1–9. [Google Scholar]
- 74.Wang Q., Cheng X., Li J., Jin H. Hydrothermal synthesis and photocatalytic properties of pyrochlore Sm2Zr2O7 nanoparticles. J. Photochem. Photobiol. Chem. 2016;321:48–54. [Google Scholar]
- 75.Kanhere P., Tang Y., Zheng J., Chen Z. Synthesis, photophysical properties, and photocatalytic applications of Bi doped NaTaO3 and Bi doped Na2Ta2O6 nanoparticles. J. Phys. Chem. Solid. 2013;74:1708–1713. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.