Abstract
Anaplastic thyroid cancer (ATC) is a clinically aggressive form of undifferentiated thyroid cancer with limited treatment options. Tumor-associated macrophages (TAMs) constitute over 50% of ATC-infiltrating cells, and their presence is associated with a poor prognosis. We have previously shown that paracrine signals released by ATC cells induced pro-tumor M2-like polarization of human monocytes. However, which soluble factors derived from ATC cells drive monocyte activation, are largely unknown. In this study we investigated the participation of transforming growth factor β1 (TGFβ1) on the phenotype of macrophage activation induced by ATC cell-derived conditioned media (CM). THP-1 cells exposed to CM derived from ATC cells and recombinant human TGFβ1 induced M2-like macrophage polarization, showing high CD163 and Dectin1 expression. Moreover, we showed that TGFβ1 induced the messenger RNA (mRNA) and protein expression of the transcription factors SNAIL and SLUG. Accordingly, increased TGFβ1 secretion from ATC cells was confirmed by enzyme-linked immunosorbent assay (ELISA). Addition of SB431542, a TGFβ receptor inhibitor, significantly decreased the Dectin1, CD163, SNAIL and SLUG expression stimulated by ATC cell-derived CM. We validated the clinical significance of the expression of TGFβ ligands, their receptors, as well as SNAIL and SLUG in human ATC by analyzing public microarray datasets. We found that the expression of the main TGFβ ligands, TGFβ1 and TGFβ3, along with their receptors, TGFR1 and TGFR2, as well as SLUG, was significantly higher in human ATC tissue samples than in normal thyroid tissues. Our findings indicate that ATC cell-secreted TGFβ1 may play a key role in M2-like macrophage polarization of human monocytes and in the up-regulation of SNAIL and SLUG transcription factors. Thus, ours results uncovered a novel mechanism involved in the activation of TAMs by soluble factors released by ATC cells, which suggest potential therapeutic targets for ATC.
Keywords: Anaplastic thyroid cancer, TGFβ1, tumor-associated macrophages, M2-like macrophage polarization
Introduction
Thyroid cancer (TC) is one of the most common malignant tumor of the endocrine system [1]. Anaplastic thyroid cancer (ATC), although rare, is the most malignant pathological type because of its aggressive behavior and resistance to traditional treatments [2-4]. Until recently, ATC patients had a median survival of only three to five months after diagnosis [2]. The approval of the combination therapy of a BRAF inhibitor, dabrafenib, with the MEK inhibitor, trametinib, has improved the overall survival of patients with BRAF-mutated ATCs [5]. However, resistance is inevitable and therefore, the development of new therapeutic strategies for these patients is urgently needed.
Recent studies highlighted the important role of the tumor microenvironment (TME) in the progression, invasion and metastasis in many different types of tumors [6,7]. A variety of immune cells are found in the TME, with macrophages being one of its main immune cellular components [8]. Due to the remarkable plasticity, they can acquire distinct phenotypes and polarize into a continuum spectrum of functional states from pro-inflammatory/anti-tumor, M1-like macrophages, to anti-inflammatory/pro-tumor, M2-like macrophages [9]. Macrophages in the TME, or tumor-associated macrophages (TAMs), as a results of local signals secreted by tumor cells, are mostly polarized towards an M2-like phenotype and can promote the malignant progression of many tumors [10]. Interestingly, TAMs largely infiltrate the TME of ATC [11-14]. Consistently, increase in TAMs density correlates with poor prognosis in ATC [11]. Therefore, targeting TAMs may offer opportunities for the development of novel combination therapies for ATC patients.
We previously provided valuable insights into the process by which soluble factors derived by ATC cells induce pro-tumor M2-like polarization of macrophages, both in vitro and in vivo [15,16]. Thus, we observed that treatment of THP-1 cells with conditioned media (CM) produced by ATC cells induced an increase in the expression of M2 markers without affecting the levels of M1 markers. Once activated by ATC cell derived-CM, macrophages adopted a more pro-tumor phenotype [15]. More recently it was demonstrated by us and by others the recruitment of M2-like macrophages into ATC-cell-derived xenograft tumors, recapitulating the human disease [16,17]. However, the soluble factors secreted by ATC cells responsible for macrophage activation remain largely unknown.
Transforming growth factor β (TGFβ) is a pleiotropic cytokine frequently present in tumors [18]. Furthermore, it has been demonstrated that TGFβ affects macrophage activation. Thus, macrophages stimulated by TGFβ induce the expression and secretion of classical M2-like markers, including interleukin (IL)-10, while decreasing M1-like macrophage markers, such as IL-12 [19,20]. Therefore, we explored its participation in the macrophage activation phenotype induced by soluble factors released by ATC cells and investigated the underlying molecular mechanism. We found for the first time that, similar to ATC cell-derived CM, TGFβ1 treatment induced macrophage polarization toward an M2-like phenotype, and TGFβ receptor blockade greatly inhibited the promoted M2-like polarization by ATC cell-derived CM. Furthermore, the expression of the transcription factors SNAIL and SLUG was positively regulated by TGFβ produced by the ATC cells, that could suggest their potential contribution in the activation of human monocytes. Together, our results suggest that TGFβ plays a key role in macrophage activation in ATC and in the regulation of SNAIL and SLUG expression.
Materials and methods
Cell cultures
The THP1 cell line (human monocytes) was obtained from American Type Culture Collection (ATCC) and confirmed as mycoplasma free. THP-1 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco) as previously described [15]. Human ATC cell line 8505C was obtained from the European Collection of Authenticated Cell Cultures while the ATC-derived C643 cell line was obtained from the University of Colorado Cancer Center Cell Bank. Human ATC cells were authenticated by short tandem repeat (STR) profiling analysis by the Science Cordoba Agency (CEPROCOR, Córdoba, Argentina), as previously described [15,21]. ATC cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) as previously reported [15,16,21]. All cell lines were cultured in medium supplemented with 10% fetal bovine serum (FBS, Hyclone), penicillin/streptomycin and L-glutamine (Gibco).
Conditioned media (CM) harvesting and TGFβ1 interference
CM preparation from ATC cell lines were performed as previously described [15,21]. Briefly, ATC cells (8505C and C643) were seeded (1×106 cells) on 100 mm tissue culture plates in DMEM 10% FBS. 48 hours (h) later, the media were replaced with 5% FBS. After additional 48 h, CM were collected and clarified by centrifugation (2000 rpm for 10 minutes). CM from ATC cells were called 8505C CM or C643 CM. The THP-1 cells (1×106 cells/well) were cultured in complete media containing 5% FBS (THP-1 control), treated with 100% CM of 8505C CM or recombinant human TGFβ1 (#240-B-010, R&D Systems) for different periods of time, and a variety of parameters were analyzed. To explore the role of TGFβ produced by ATC cells on macrophage polarization, THP-1 cells were pre-incubated with the TGFβ receptor, SB431542 (20 µM, #1614, Tocris) for 1 h before the 8505C cell-derived CM treatment.
In vitro cell proliferation assay
Cell proliferation assay was performed as previously described [15,21]. Briefly, 2×106 THP-1 cells/well were incubated with ATC cell-derived CM, recombinant human TGFβ1 or DMEM-FBS 5% in six-well plates each with duplicates. The number of viable cells was counted at 24 h and 48 h after treatment using a cell counter (Countless II, ThermoFisher Scientific).
RNA isolation and quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA purification, complementary DNA (cDNA) synthesis, and quantitative PCR (qPCR) were performed as previously described [15,21]. The primers used are listed in Table S1
Flow cytometry
Cells were harvested, washed with phosphate-buffered saline (PBS) and resuspended in 1% FBS in PBS. Cells were then incubated with the Phycoerythrin (PE) anti-human CD369 (Dectin1, #355403, BioLegend) fluorochrome-conjugated monoclonal antibody for 20 minutes at 4°C and used at a 1:50 dilution. To discriminate dead cells in our samples, a viability stain was performed (LIVE/DEAD™ Fixable dead cell stain kits, #L10119, Thermofisher). It was diluted 1/1000 in PBS (Gibco), and cells were incubated in 30 μL of this dilution for 15 minutes at room temperature. Finally, the samples were acquired using the BD FACS Canto II flow cytometer (BD Biosciences) and the data were processed using the FlowJo software [16].
Determination of human TGFβ1 by enzyme-linked immunosorbent assay (ELISA)
Culture supernatants were harvested, clarified by centrifugation, and frozen for the subsequent determination of human TGFβ1 concentration by ELISA according to the manufacturer’s instructions (#ab100647, Abcam).
Immunofluorescence staining
Human tumor xenografts were established by subcutaneous injection of 8505C cells into NOD/SCID mice. Experiments were conducted as previously described [16] in full accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, NIH). The experimental protocols were approved by the Institutional Animal Care and Use Committee. Tumors were harvested, embedded in CryoplastR (Biopack), rapidly frozen and cut into 5 µm thick sections using a Cryostat 0620E (Thermo Scientific Shandon, USA). Sections were rehydrated in sodium citrate-Tris buffer 10 mM and then blocked for 60 minutes with 10% goat serum-Tris buffer. After blocking, slides were incubated overnight at 4°C with Fluorescein Isothiocyanate (FITC)-labeled anti-mouse F4/80 (1:100 dilution, #11-4801-81 eBioscience, Thermo Fisher Scientific) or anti-SMAD3 (1:100 dilution, #9523, Cell Signaling) in blocking buffer, followed by incubation with the secondary antibody conjugated with Alexa Fluor 555 (#A-31572, Invitrogen). Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Slices were mounted with FluorSave (Merck Millipore, Burlington, MA, USA). Images were collected with a Leica microscope (DMi8) [16].
Western blot analysis
Whole-cell lysates were prepared as previously described [15,21,22]. The protein samples (20-30 µg) were analyzed by Western blot as described previously [15,21]. Antibodies used according to the manufacturers’ manuals include anti-phosphorylated Stat3 (Tyr705, #9131), total Stat3 (#4904), vimentin (#5741), SMAD3 (#9523), SNAIL (#3895) and SLUG (#9585), all from Cell Signaling and used at a 1:1000 dilution. Anti βActin (#sc-47778) and GAPDH (#sc-25778) from Santa Cruz Biotechnology and used at a 1:200 dilution. Band intensities were quantified by using NIH IMAGE software (ImageJ 1.50i; NIH).
Gene expression analysis from public datasets
Microarray datasets, generated on the Affymetrix GPL570 platform containing ATCs (GSE29265, GSE33630, GSE76039 and GSE65144) and normal thyroid tissue (GSE3467, GSE3678, GSE6004, GSE29265, GSE33630, GSE53157, GSE35570, GSE60542), were downloaded from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (http://www.nibi.nih.gov/geo/) and processed in R version 3.6.3 (https://www.r-project.org/). Data were normalized using the GeneChip Robust Multiarray Averaging method, and a matrix of 171 normal tissues and 52 ATCs was generated. Genes were annotated using hgu133plus2.db package version 3.2.3 [16].
Statistical analysis
Analysis of intergroup differences was conducted by one-way analysis of variance (ANOVA), followed by the Turkey’s test. Comparisons between two groups were carried out using unpaired Student’s t-test or non-parametric Mann-Whitney U-test. Statistical analysis was conducted using GraphPad Prism 8.0.1 software (GraphPad Software, San Diego, CA, USA). Values of P<0.05 were considered to be statistically significant.
Results
Our previous studies have shown that paracrine signals derived from ATC cells induce pro-tumor M2-like polarization of human monocytes [15]. It remains to identify which soluble ATC cell-derived mediators are involved in the macrophage activation phenotype. TGFβ is one of the main cytokine secreted by tumor cells [18]. In addition, it has been previously described that TGFβ signaling induced a M2-like macrophage polarization [19]. Since TGFβ1 is the most relevant member of the family [18], we examined the contribution of TGFβ1 on macrophage activation induced by ATC cell-derived CM.
Firstly, we investigated the involvement of TGFβ1 on macrophage activation phenotype. THP-1 cells were exposed to CM derived from 8505C cells and recombinant human TGFβ1 protein, and the effects on cell morphology were analyzed. Consistent with our previous findings [15], THP-1 cells increased their adherence and changed their morphology after treatment with 8505C cell-derived CM for 18 h (Figure 1A-II), compared with THP-1 cell controls (Figure 1A-I). Contrarily, in THP-1 cells after treatment with 20 ng/ml TGFβ1, the extent of the morphological changes was less pronounced (Figure 1A-III). We previously reported the impact of ATC cell-derived soluble factors in decreasing human monocytes proliferation [15]. Thus, we examined the effect of TGFβ1 on human monocytes proliferation by cell counting. We found that treatment with 12.5 ng/ml TGFβ1 significantly reduced THP-1 cell number at 24 h and 48 h (Figure 1B), mimicking the impact of 8505C cell-derived CM on human monocytes proliferation (Figure 1B). Next, we investigated whether TGFβ1 influence the expression of M2 macrophage markers. As previously reported [15], THP-1 cells exposed to 8505C cell-derived CM expressed high messenger RNA (mRNA) levels of classic M2 macrophage markers, including C-C motif chemokine ligand 13 (CCL13), Dectin1 and CD163, compared to THP-1 controls (Figure 1C). In line with these observations, TGFβ1 treatment increased Dectin1 and CD163 mRNA expression (Figure 1C). Flow cytometry assays further confirmed that Dectin1 protein levels were increased in monocytes incubated for 24 h with 20 ng/ml TGFβ1 (Figure 1D). Figure 1E shows the quantitative data. In contrast to what was observed after treatment with 8505C cell-derived CM [15], mRNA levels of CCL13 were significantly decreased in TGFβ1 treated human monocytes (Figure 1C). Our observations were further confirmed through dose response curves of TGFβ1 by qPCR assays. THP-1 cells were stimulated with a range of concentrations of TGFβ1 (1-15 ng/mL) for 24 h (Figure S1A-C). Consistent with our observations, we found that the use of different concentrations of TGFβ1 also caused a reduction in CCL13 mRNA levels (Figure S1A) while inducing an increment in Dectin1 and CD163 mRNA levels (Figure S1B and S1C). Altogether, our data are in agreement with previous observations [19], confirming that TGFβ1 regulates the expression of macrophage markers in human monocytes. The presence of TGFβ signaling in TAMs from ATC was further validated in 8505C xenograft tumors by immunofluorescence assays. Similar to our previous findings [16], we evidenced extensive macrophage infiltration in xenografts using the F4/80 antibody (a marker of mouse macrophages). Additionally, here we showed that some macrophages were positive only for F4/80 or for the TGFβ signaling mediator, SMAD3, while others showed co-expression of both (Figure S1D, white arrows). Accordingly, western blot revealed a significant increased abundance of SMAD3 in THP-1 cells treated for 18 h and 24 h with CM derived from 8505C cells, compared with THP-1 cell controls [Figure S1E, compare lane 1 (18 h) and lane 3 (24 h) (THP-1 control) with lanes 2 (18 h) and 4 (24 h) (8505C cell-derived CM)]. We next ascertained whether ATC cells secreted TGFβ1 into the media. In line with our findings, we found that 8505C and C643 cells secreted high amounts of TGFβ1 (3251 pg/mL and 2752 pg/mL, respectively) into the media as compared with DMEM containing 5% FBS and DMEM alone (1350 pg/mL and 10 pg/mL, respectively), under the same experimental conditions (Figure 1F).
Figure 1.
The effect of transforming growth factor β1 (TGFβ1) on the phenotype of THP-1 cells. (A-I-A-III) Soluble factors derived from anaplastic thyroid cancer (ATC) cells induced morphological changes in THP-1 cells. Monocytes were treated for 18 hours (h) with 8505C-derived conditioned media (CM) and TGFβ1 20 ng/ml. Representative bright-field imaging of THP-1 cells control (A-I) or treated with 8505C-derived CM (A-II) and TGFβ1 20 ng/ml (A-III). (B) Proliferation of THP-1 cells incubated with 8505C-derived CM and TGFβ1 12.5 ng/ml for 24 h and 48 h, estimated by cell counting. (C) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) assay to detect human C-C motif chemokine ligand 13 (hCCL13), hDectin1 and CD163 in THP-1 cells grown under normal conditions (control) and treated with 8505C-derived CM and TGFβ1 20 ng/ml for 24 h. (D, E) Flow cytometry analysis showed significant induction of hDectin1 in THP-1 cells treated with 8505C-derived CM and TGFβ1 20 ng/ml for 24 h compared to THP-1 control (E). Representative histograms and quantification (D) are shown. (F) Concentrations of TGFβ1 in Dulbecco’s modified Eagle’s medium (DMEM) alone, DMEM FBS 5% (control) and in the CM of 8505C and C643 cells, determined by enzyme-linked immunosorbent assay (ELISA) as described in Methods and Materials. Data are expressed as mean ± SEM. *P<0.05, **P<0.005, ***P<0.0005, ****P<0.0001.
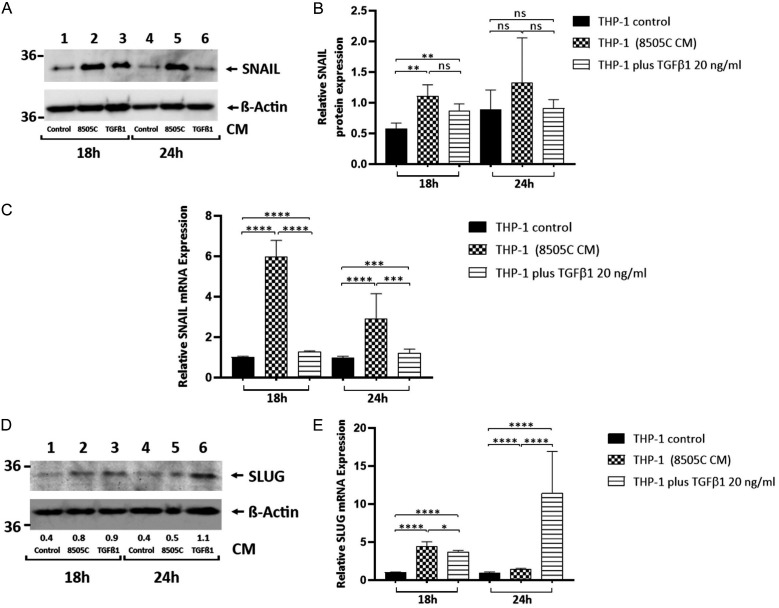
Previous studies reported that TGFβ1 induces M2-like macrophage polarization through up-regulation of the transcription factor SNAIL1 (SNAIL) [19]. Since we found that ATC cells secrete plenty amounts of TGFβ1 (Figure 1F), we next investigated whether 8505C cell-Secreted TGFβ1 regulates SNAIL expression in human monocytes. THP-1 cells were treated for 18 h and 24 h with 8505C cell-derived CM or TGFβ1. Western blot revealed a significant increased abundance of SNAIL in THP-1 cells treated for 18 h with CM derived from 8505C cells and TGFβ1 20 ng/ml, compared with THP-1 cell controls [Figure 2A, compare lane 1 (18 h) (THP-1 control) with lanes 2 (18 h) (8505C cell-derived CM) and 3 (18 h) (TGFβ1 20 ng/ml)], with Figure 2B showing a quantitative comparison of the band intensities from Figure 2A. We further determined the expression of SNAIL mRNA, and in agreement with the changes in the protein levels, we observed that the treatment of human monocytes for 18 h and 24 h with 8505C cell-derived CM and TGFβ1 20 ng/ml induced a significant increase in the expression of SNAIL mRNA compared with THP-1 cell controls (Figure 2C). Furthermore, we examined the expression of another TGFβ target gene, SNAIL2 (SLUG) [23]. Interestingly, by Western blot (Figure 2D) and RT-qPCR (Figure 2E) assays we found a significant increase in SLUG abundance in THP-1 cells treated with CM derived from 8505C cells, as well as 20 ng/ml TGFβ1, compared to THP-1 cell controls. Since SNAIL and SLUG belong to the transcriptional factors that drives the epithelial-mesenchymal transition (EMT) process, we determined whether TGFβ1 impact the expression of vimentin, another EMT marker, in human monocytes. Contrary to the increment in vimentin levels observed in THP-1 cells after treatment with CM derived from 8505C cells [15] [Figure S2A, compare lanes 1 (24 h) and 4 (48 h) (THP-1 control) with lanes 2 (24 h) and 5 (48 h) (8505C cell-derived CM)], we did not observe any changes in the expression levels of this protein in THP-1 cells treated for 24 h and 48 h with TGFβ1 20 ng/ml [Figure S2A, compare lanes 1 (24 h) and 4 (48 h) (THP-1 control) with lanes 3 (24 h) and 6 (48 h) (TGFβ1 20 ng/ml)]. We previously reported an increase in STAT3 phosphorylation in human monocytes exposed to 8505C cell-derived CM [15]. Therefore, we explore the possible participation of TGFβ1 in these effects. However, we did not find any significant changes in phosphorylation of STAT3 in THP-1 cells treated with 20 ng/ml TGFβ1 for 18 h and 24 h (Figure S2B) as well as stimulated with different concentrations of TGFβ1 (1-10 ng/mL) for 24 h (Figure S2C).
Figure 2.
SNAIL and SLUG are up-regulated in THP-1 cell activation induced by cancer cell-secreted TGFβ1. (A, B) Immunoblot analysis of SNAIL in THP-1 cells grown under normal conditions (control) or exposed to CM derived from 8505C cells and TGFβ1 20 ng/ml for 18 h and 24 h (A). The figure shows a representative Western blot of 4 independent experiments. Quantification of relative expression of SNAIL protein in human monocytes after using βActin as loading control (B). (C) Expression levels of SNAIL messenger RNA (mRNA) levels by RT-qPCR in control and THP-1 cells treated for 12 h and 24 h with 8505C-derived CM and TGFβ1 20 ng/ml. (D, E) Immunoblot for SLUG from THP-1 cells treated with CM derived from 8505C cells and TGFβ1 20 ng/ml for 18 h and 24 h. The same blot was stripped and re-blotted using anti-βActin antibody. The figure shows a representative Western blot of 4 independent experiments (D). Expression levels of SLUG mRNA levels by RT-qPCR in control and THP-1 cells treated for 18 h and 24 h with 8505C-derived CM and TGFβ1 20 ng/ml. Data are expressed as mean ± SEM. **P<0.005, ***P<0.0005, ****P<0.0001. ns, not significant.
Next and to confirm the contribution of TGFβ1 on the macrophage activation phenotype induced by 8505C cell-derived CM, we used TGFβ receptor inhibitor (SB431542). THP-1 cells were pre-incubated with SB431542 or vehicle [dimethyl sulfoxide (DMSO)] for 1 h before stimulation with ATC cell-derived CM. The expression of M2 macrophage markers were determined by qPCR. Consistent with our previous observations, the qPCR results confirmed the up-regulation of CCL13, Dectin1, and CD163 expression in THP-1 cells incubated for 18 h and 24 h with 8505C cell-derived CM plus DMSO (Figure 3A-C). Interestingly, a combination treatment of 8505C cell-derived CM and SB431542 significantly reduced the mRNA expression levels of Dectin1 and CD163, in THP-1 cells (Figure 3B, 3C). Instead, mRNA levels of CCL13 were significantly increased in SB431542 treated human monocytes for 18 h and 24 h (Figure 3A). In addition, we observed a significantly reduced in the mRNA expression levels of SNAIL (Figure 3D) and SLUG (Figure 3E) in human monocytes incubated for 18 h and 24 h with 8505C cell-derived CM plus SB431542 compared to THP-1 cell incubated with 8505C cell-derived CM plus DMSO. Collectively, our results support the idea that TGFβ in the ATC cell-derived CM is one of the critical signals for M2-like polarization of THP-1 cells.
Figure 3.
TGFβ receptor inhibition affects 8505C-derived CM induced macrophage polarization as well as expression of SNAIL and SLUG. (A-C) THP-1 cells pre-incubated with SB431542 (20 µM) or dimethyl sulfoxide (DMSO) as control for 1 h, were treated with 8505C-derived CM for 18 h and 24 h. The expression of hCCL13 (A), hDectin1 (B) and hCD163 (C) were analyzed by RT-qPCR. (D, E) RT-qPCR of SNAIL (D) and SLUG (E) expression in THP-1 cells pre-treated with SB431542 (20 µM) before 8505C-derived CM treatment for 18 h and 24 h. Data are expressed as mean ± SEM. *P<0.05, **P<0.005, ***P<0.0005, ****P<0.0001. ns, not significant.
We next studied the clinical significance of our findings by analyzing public datasets. We compared the expression levels of the main TGFβ ligands, its receptors and the transcription factors SNAIL and SLUG between 171 normal thyroid tissues and 52 ATCs. As shown in Figure 4A, ATCs exhibited a significantly higher expression of TGFβ1 and TGFβ3 compared with normal thyroids. Besides, ATC expressed high levels of TGFBR1 and TGFBR2 but low levels of TGFBR3 compared with normal thyroids (Figure 4B). Furthermore, ATC exhibited a significantly higher expression of SLUG but lower expression of SNAIL compared with normal thyroids (Figure 4C). Interestingly, the expression of SNAIL (Figure 4D) and SLUG (Figure 4E) were positive correlated with TGFβ1, while CD68 (Figure 4F) and CD163 (Figure 4G), both macrophage markers, were positive correlated with SLUG expression.
Figure 4.
TGFβs, TGFβ receptors (TGFβRs), SNAIL and SLUG in ATC. (A-C) Boxplots showing TGFβ1-3 (A), TGFβ receptors, TGFβR1-3 (B), SNAIL and SLUG (C) gene expression in normal thyroids (NT) and ATC samples derived from Gene Expression Omnibus (GEO) datasets (GSE3467, GSE3678, GSE6004, GSE29265, GSE33630, GSE53157, GSE35570, GSE60542, GSE29265, GSE33630, GSE76039 and GSE65144). Statistical significance by Mann Whitney test; ****P<0.0001. ns, not significant. (D-G) Correlation between expression of TGFβ1 and SNAIL (D) and SLUG (E). Correlation between expression of SLUG and macrophages markers (CD68 and CD163).
Discussion
In previous studies we found that soluble factors secreted by ATC cells induce a tumor-promoting M2-like phenotype in macrophages in vitro. Consistent with these observations, a large number of M2-like macrophages infiltrate ATC-cell-induced xenograft tumors [15-17]. However, the key signals released by ATC cells responsible for TAMs reprogramming remain largely unidentified. In the present study, we found that similarly to THP-1 cells treated with ATC cell-derived CM, monocytes incubated with TGFβ1 showed a decrease in cell proliferation and an increase in the expression of classic M2 markers including Dectin1 and CD163. Moreover, both 8505C cell-derived CM and TGFβ1 upregulated the expression of the transcription factors SNAIL and SLUG during macrophage differentiation. In accordance, inhibitors of the TGFβ receptor partially inhibited macrophage polarization as well as mRNA expression of SNAIL and SLUG induced by treatment with ATC cell-derived CM. Interestingly, high concentrations of TGFβ1 in ATC cell-derived CM were further confirmed by ELISA assays. Altogether in the present study, we have identified TGFβ1 as one of the key regulators of macrophage polarization towards the M2-like phenotype, as well as of the expression of the transcription factors SNAIL and SLUG. Conversely, the IL-6/STAT3 signaling pathway was not implicated in the M2-like macrophage polarization induced by TGFβ1 secreted by anaplastic thyroid cancer cells (Figure 5).
Figure 5.
Schematic model showing the contribution of ATC cell-secreted TGFβ1 on macrophage activation. ATC cells, among other soluble factors, produce and secrete high levels of TGFβ1, which activates its signaling pathway including up-regulation of SMAD2/SMAD3 transcription factors. Activation of the TGFβ1 signaling pathway in turn increases the expression of the transcription factors SNAIL and SLUG in THP-1 cells. The up-regulation of SNAIL and SLUG would induce M2-like macrophage polarization of human monocytes triggered by TGFβ1, thereby contributing to the progression of thyroid cancer. The activation of macrophages induced by ATC cell-secreted TGFβ1 would be independent of the IL-6/STAT3 signaling pathway activation induced by ATC cell-derived CM. Created with BioRender.
Our results were consistent with those of several previous studies that reported the involvement of TGFβ in the M2-like macrophage polarization. Gong et al demonstrated that knocking out TGFβRII in mouse macrophages resulted in their inability to upregulate M2-like polarized genes [24]. Furthermore, Zhang et al showed that TGFβ treated THP-1 macrophages increased the expression of markers of the M2-like phenotype, while M1 markers were downregulated [19]. A recent study demonstrated that TGFβ1 secreted by triple-negative breast cancer cells induced M2-like polarization of human monocytes in vitro [25]. In another work, it was described that bone-marrow-derived macrophages transfected with TGFβ1 siRNA suppressed M2-like macrophage polarization but this trend was reversed after the treatment with TGFβ1 [26]. Collectively, these results, along with those reported here, clearly establish the role of TGFβ in macrophage differentiation towards an M2-like phenotype.
Our results demonstrated that after exposure to TGFβ1, THP-1 cells increased the mRNA and protein levels of SNAIL and SLUG, detected with qRT-PCR and Western Blot assays, respectively. Accordingly, the inhibitor of TGFβ1 receptor resulted in decreased expression of both proteins induced by ATC cell-derived CM. In agreement with our findings, Zhang et al demonstrated that TGFβ promotes M2-like phenotype by increasing the expression of SNAIL [19]. SNAIL (SNAIL1) and SLUG (SNAIL2) are the main epithelial-to-mesenchymal transition (EMT)-inducing transcription factors. However, they also have other cellular functions. In addition to the regulation of cell movements, they also play an important role in cell proliferation and immune regulation. The induction of SNAIL and SLUG expression in ATC cell-derived TGFβ1 activated macrophages could suggest their role as possible regulators of the M2-like phenotype. However, their contribution to TGFβ-mediated macrophage activation remains to be elucidated. Moreover, we do not exclude the participation of other transcription factors to these effects. The key regulators involved in the activation of macrophages mediated by TGFβ-derived from ATC cells will be studied in future research. On the other hand, EMT is a hallmark of metastasis. EMT provides tumor cells with great plasticity so that they acquire mesenchymal characteristics, helping them in the metastatic process and therefore in tumor progression. Although we did not evidence an increase in the expression of vimentin (another marker of EMT), we do not rule out the possibility that the increased expression of SNAIL and SLUG in macrophages activated by ATC cell-derived TGFβ1 could represent a marker of those macrophages with a greater capacity for migration and motility to the tumor in ATC. Future research are required to validate this hypothesis.
Although TGFβ1 is a key mediator, it is not the only soluble factor that can explain macrophage polarization induced by ATC cell-derived CM. In this sense and contrary to what was observed after treatment with ATC cell-derived CM, TGFβ1 stimulation decreased CCL13 expression in THP-1 cells, which is also an M2-like phenotype marker. Accordingly, TGFβ blockade caused increased expression of this chemokine in human monocytes incubated with ATC cell-derived CM. In line with our findings, it was previously documented that TGFβ downregulated the expression of CCL13 mRNA in human airway smooth muscle cells [27]. The reason why ATC cell-derived TGFβ1 negatively regulates CCL13 expression in human monocytes remains unknown. Given the dual functions of inhibiting and promoting cancer of TGFβ [28], it is not surprising to observe this negative regulation between this multifunctioning cytokine and CCL13 in THP-1 cells, at least, at the time points tested. We will need further studies to explore the mechanisms as well as the meaning of down-regulation of CCL13 expression by TGFβ1 in human monocytes in ATC.
We have previously found that ATC cell-derived CM drives macrophage activation through modulation of STAT3 signaling pathway [15]. For that reason, we explored potential regulation of p-STAT3 in human monocytes by TGFβ1. Contrary to what happened with ATC cell-derived CM, THP-1 cells treated with different concentrations of TGFβ1 did not show any changes in the phosphorylation of pSTAT3 (Figure S2B). Therefore, in addition to TGFβ1, other ATC cell-derived paracrine signals must be involved in macrophage polarization and activation. In this regard, Mazzoni et al showed that prostaglandin E2 (PGE2) produced by different thyroid tumor cells and senescent cells was also capable of inducing M2-like macrophage polarization of primary monocytes [29]. It is unknown whether PGE2 is also playing a role in THP-1 polarization induced by ATC cell-derived CM. Future studies will be performed to identify other local cues, in addition to TGFβ1, released by ATC cells that also participate in macrophage differentiation.
In conclusion, our observations identified TGFβ1 secreted by ATC cells as a critical paracrine signal involved in the activation of macrophages. Thus, TGFβ1 inhibition might represent a new therapeutic strategy for ATC by modulating the phenotype and activity of TAMs. This has specific relevance in ATC, in which TAMs constitute more than 50% of tumor mass, and for which no effective therapeutic options are available.
Acknowledgements
We thank M. P. Abadie, M. P. Crespo, A. Romero, G. Furlán, and N. Maldonado for their excellent technical assistance. The present research has been supported by research grants from the Intramural Research Program at the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 112-20210100535CO), Fondo para la Investigación Científica y Tecnológica-Agencia Nacional de Promoción Científica y Técnica (FONCyT, PICT 2018-01052) and Secretaría de Ciencia y Técnica (Universidad Nacional de Córdoba). LF was supported by funds from the Short Term Scientist Exchange Program (STSEP) of the Center for Global Health (CGH), National Cancer Institute.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini A, Torregrossa L, Basolo F, Vitti P, Elisei R. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13:644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 3.Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov YE, Randolph G, Rosenthal MS, Sawka AM, Shah M, Shaha A, Smallridge R, Wong-Clark CK. 2021 American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2021;31:337–386. doi: 10.1089/thy.2020.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, Deschamps F, Lamartina L, Lacroix L, Dupuy C, Baudin E, Do Cao C, Hadoux J. Anaplastic thyroid carcinoma: an update. Cancers (Basel) 2022;14:1061. doi: 10.3390/cancers14041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018;36:7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronte V, Murray PJ. Understanding local macrophage phenotypes in disease: modulating macrophage function to treat cancer. Nat Med. 2015;21:117–119. doi: 10.1038/nm.3794. [DOI] [PubMed] [Google Scholar]
- 11.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M, Dupuy C. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One. 2011;6:e22567. doi: 10.1371/journal.pone.0022567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, Park YJ. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015;49:318–324. doi: 10.4132/jptm.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126:1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stempin CC, Geysels RC, Park S, Palacios LM, Volpini X, Motran CC, Acosta Rodriguez EV, Nicola JP, Cheng SY, Pellizas CG, Fozzatti L. Secreted factors by anaplastic thyroid cancer cells induce tumor-promoting M2-like macrophage polarization through a TIM3-dependent mechanism. Cancers (Basel) 2021;13:4821. doi: 10.3390/cancers13194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacios LM, Peyret V, Viano ME, Geysels RC, Chocobar YA, Volpini X, Pellizas CG, Nicola JP, Motran CC, Rodriguez-Galan MC, Fozzatti L. TIM3 expression in anaplastic-thyroid-cancer-infiltrating macrophages: an emerging immunotherapeutic target. Biology (Basel) 2022;11:1609. doi: 10.3390/biology11111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzoni M, Mauro G, Minoli L, Cleris L, Anania MC, Di Marco T, Minna E, Pagliardini S, Rizzetti MG, Manenti G, Borrello MG, Scanziani E, Greco A. Senescent thyrocytes, similarly to thyroid tumor cells, elicit M2-like macrophage polarization in vivo. Biology (Basel) 2021;10:985. doi: 10.3390/biology10100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, Wang H, Fang R, Bu X, Cai S, Du J. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7:52294–52306. doi: 10.18632/oncotarget.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerneur C, Cano CE, Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. 2022;13:1026954. doi: 10.3389/fimmu.2022.1026954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fozzatti L, Alamino VA, Park S, Giusiano L, Volpini X, Zhao L, Stempin CC, Donadio AC, Cheng SY, Pellizas CG. Interplay of fibroblasts with anaplastic tumor cells promotes follicular thyroid cancer progression. Sci Rep. 2019;9:8028. doi: 10.1038/s41598-019-44361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fozzatti L, Lu C, Kim DW, Park JW, Astapova I, Gavrilova O, Willingham MC, Hollenberg AN, Cheng SY. Resistance to thyroid hormone is modulated in vivo by the nuclear receptor corepressor (NCOR1) Proc Natl Acad Sci U S A. 2011;108:17462–17467. doi: 10.1073/pnas.1107474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trelford CB, Dagnino L, Di Guglielmo GM. Transforming growth factor-beta in tumour development. Front Mol Biosci. 2022;9:991612. doi: 10.3389/fmolb.2022.991612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFbeta signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Han X, Lin Z, Wang C, Fu Z, Sun Q, Li C. G6PD activation in TNBC cells induces macrophage recruitment and M2 polarization to promote tumor progression. Cell Mol Life Sci. 2023;80:165. doi: 10.1007/s00018-023-04810-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Lyu N, Wang Q, Yang M, Kimchi ET, Cheng K, Joshi T, Tukuli AR, Staveley-O’Carroll KF, Li G. A novel role of TGFBI in macrophage polarization and macrophage-induced pancreatic cancer growth and therapeutic resistance. Cancer Lett. 2023;578:216457. doi: 10.1016/j.canlet.2023.216457. [DOI] [PubMed] [Google Scholar]
- 27.Matsukura S, Odaka M, Kurokawa M, Kuga H, Homma T, Takeuchi H, Notomi K, Kokubu F, Kawaguchi M, Schleimer RP, Johnson MW, Adachi M. Transforming growth factor-beta stimulates the expression of eotaxin/CC chemokine ligand 11 and its promoter activity through binding site for nuclear factor-kappabeta in airway smooth muscle cells. Clin Exp Allergy. 2010;40:763–771. doi: 10.1111/j.1365-2222.2010.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tie Y, Tang F, Peng D, Zhang Y, Shi H. TGF-beta signal transduction: biology, function and therapy for diseases. Mol Biomed. 2022;3:45. doi: 10.1186/s43556-022-00109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzoni M, Mauro G, Erreni M, Romeo P, Minna E, Vizioli MG, Belgiovine C, Rizzetti MG, Pagliardini S, Avigni R, Anania MC, Allavena P, Borrello MG, Greco A. Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism. J Exp Clin Cancer Res. 2019;38:208. doi: 10.1186/s13046-019-1198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.