Abstract
Circulating tumor cells (CTCs) have significant potential to become an important tool for monitoring the effects of treatment in solid tumors. The present study reports the occurance of CTCs in cervical cancer (CC) patients during radical chemoradiotherapy (CRT), including brachytherapy (BRT), and during the follow-up period. Patients diagnosed with CC treated with radical CRT were included in the study (n=30). A total of 167 CTC-tests (MetaCell®) were provided at predefined testing time points during the study follow-up (e.g., before CRT, after CRT, every three months of follow-up). In parallel with CTC-testing, SCC-Ag were measured to compare their predictive values during treatment. CTCs were present in 96% (25/26) of patients at the time of diagnosis and in 61% (14/23) after treatment. Patients who relapsed during the 36-month follow-up (n=10) showed an elevation in pre-treatment CTC- numbers, similarly there was a significant increase in pre-treatment SCC-Ag. As next, an increased number of CTCs was observed approximately 12 weeks before relapse was diagnosed by standard imaging modalities (MRI, US, PET-CT) in 3 of 4 patients. In addition to standardized vital cytomorphology of enriched CTCs, quantitative PCR (qPCR) was used to inform the nature of CTCs before treatment. Analysis revealed increased SOX2 and POUSF expression in CTCs in the group of patients with recurrence (P < 0.02). Disease aggressiveness may be related to increased expression of stem cell markers, as found in samples from relapsed patients. CTCs may be an aid to assess tumor burden and disease aggressiveness. An increase in CTCs precedes an increase in SCC-Ag and confirmation of relapse by imaging, as shown in our study.
Keywords: Cervical cancer, circulating tumor cells, brachytherapy, chemoradiotherapy, SCC, CRT, stemness
Introduction
Cervical cancer (CC) is a global public health problem, with a particularly high burden in many low-income and middle-income countries [1]. However, CC is becoming a preventable disease with the development of vaccination against HPV viruses, but the prognosis of advanced stages remains poor [2]. It is essential to introduce innovative and more targeted therapies, precise diagnostics [3] and monitoring of response to treatment into the management of CC. In 2018 cervical cancer was the fourth cause of death worldwide after breast, colorectal, and lung cancer in women. Approximately 570,000 new cases were diagnosed in 2018, and 311,000 cases died [3]. In the aetiology of the disease, the most critical risk factor is HPV infection, especially HPV with a high oncogenic risk [4,5]. CC is diagnosed primarily on symptomatic women (irregular bleeding, postcoital bleeding, discharge, pelvic pain, dysuria or obstipation, etc.) or during regular gynaecological examination using colposcopy/specula examination, oncological cytology, bimanual palpation and imaging methods (vaginal ultrasound, MRI, and CT or PET/CT).
In addition to histological examination of the tumor and modern imaging methods (USG, MRI), only a few laboratory methods are used to help determine the extent of the tumor in the course of the disease. Histologically squamous cell carcinoma (SCC) accounts for 70-80%, and adenocarcinomas account for 20-25% of CC. SCC tumors are more chemo and radiosensitive than adenocarcinomas [6]. The initial stage of the disease is the most decisive prognostic factor. Other negative histopathological prognostic factors are tumor grade (for adenocarcinomas), spreading to lymphatic vessels and lymph nodes, depth of invasion, and primary tumor diameter [7,8]. In squamous CC, squamous cell carcinoma antigen (SCC-Ag) is used as a golden standard marker during concurrent chemoradiotherapy (CRT) [9]. SCC-Ag levels reflect the speed of tumor growth and correlate with regression, progression, or recurrence of the disease [10]. The value of 2.0 ng/ml is the upper limit of the standard setting. The test’s sensitivity is low (53.3%), but the specificity is relatively high (94.3%), which defines SSC-Ag being unsuitable for a diagnostic/screening method [6,10]. Nevertheless, SCC-Ag testing is a non-invasive tool for monitoring the effect of individual treatment in a patient with SCC. Rising levels of SCC-Ag precede the clinical detection of the relapsing disease on average by five months [10].
So far, there is no suitable marker that is readily available to monitor the therapeutic effect in a CC patient. Circulating tumor cells (CTCs) could be used for this purpose according to studies that have confirmed the correlation between CTCs and clinical outcome, for example, in breast cancer [11], ovarian cancer [12] and colorectal cancer [13]. CTCs originate from the tumor and circulate in the bloodstream to set up metastases, possibly. These cells have characteristic properties of the tumor tissue originating (primary tumor, metastases). They can be used in diagnostic periods and in the process of monitoring disease progression. They are relatively rare; one CTC can be detected in approximately 107 blood elements. Increasing CTCs numbers in the blood and elevated tumor markers usually indicate a worse prognosis of the disease [14-18]. CTCs are essential for the development of distant metastases, and their detection has been a long time considered a manifestation of tumor aggressiveness and its ability to metastasize to distant organs. Current evidence suggests that metastatic spread may be an early manifestation of tumor progression and not merely a manifestation of advanced cancer [19].
At present, the selection of a suitable therapy regimen considers the clinical condition of the patient, the assessment of the progression of the disease, and the individual characteristics of the patient tumor. The final therapy is selected according to the specification of the primary tumor [20-22]. Elevated CTC numbers during treatment, regardless of radiographic evaluation (PET, CT), ultimately indicate treatment insufficiency [23]. Similarly, low CTC numbers, especially during treatment, are a favourable indicator of survival time and response to effective therapy [19]. CTCs also reflect tumor biology and aspects in the host environment, as patients with increased CTCs rates are at increased risk of developing thromboembolic disease. Despite significant progress in understanding tumor biology, a more detailed explanation of the mechanisms of tumor spread still needs to be provided [19]. CTCs play a crucial role in tumor spread due to several biological processes, notably epithelial-mesenchymal transformation (EMT), a process in which epithelial cells lose cell-to-cell adhesion mediated by down-regulation of epithelial-associated genes [19,24,25].
The presented study aimed to characterize the relationship between CTCs and squamous cell carcinoma antigen (SCC-Ag) marker in patients with CC. Could CTCs be used during concurrent chemoradiotherapy (cCRT) including brachytherapy (BRT), and subsequent follow-up periods as reliable indicators of disease relapse? Utilizing CTC testing alongside standard imaging and SCC-Ag measurements could provide valuable information for clinicians to make more informed treatment decisions and enable earlier detection of disease progression.
Patients and methods
Study design
In total, 30 CC patients undergoing chemoradiotherapy (CRT), including brachytherapy (BRT), were enrolled in the study in accordance with the Declaration of Helsinki. Ethical committee approval was granted, and informed consent was obtained from each patient. The analysis was comprised of 167 blood samples obtained during regular medical examinations between 2018 and 2021. CTC assessment was performed prior to CRT, during CRT, and/or after CRT and every other three months. The average follow-up period was 36 months. A detailed study protocol is shown in Figure 1. Patient (n=30) characteristics is reported in Table 1. Table S1 reports clinicopathological details of patients and results of regular palpation examination (macrometastasis evaluation), metastasis assessment (imaging), and SCC-Ag values during follow up. Patients underwent CRT and follow-up according to NCCN and ESTRO guidelines [22]. Before the CRT start, every patient underwent histological verification, gynaecological and physical examination, blood count, basic biochemistry, tumor markers CEA, SCC-Ag for squamous cell carcinoma, and Ca19-9 for adenocarcinoma were monitored. Furthermore, a CT or PET/CT examination was performed to complete the staging.
Figure 1.

The protocol outline defines the standard biomarker tests timing (SCC-Ag shown in red: SCC-0, SCC-3, SCC-6, SCC-9, SCC-12) and CTC-tests timing (CTC shown in black: CTC 0 - after diagnosing the disease, CT-AC, CTC-AB in the period of the chemoradiotherapy and brachytherapy, following CTC 3, CTC 6, CTC 9, CTC 12 are sampled during the regular follow up visits).
Table 1.
Patient characteristics reflecting the CTC-status before and after CRT-therapy
| CC study | Number of patients | n=30 | % | CTC positivity - before therapy | % | CTC positivity after CRT therapy | % |
|---|---|---|---|---|---|---|---|
| Average age | 63 (30-87) | ||||||
|
| |||||||
| Tumor stage | T1b2* | 2 | 6% | 0/2 | 0 | 2/2 | 100% |
| T2b | 21 | 70% | 16/16 | 100% | 11/16 | 68% | |
| T3a | 1 | 23% | 6/6 | 100% | 5/6 | 83% | |
| T3b | 6 | ||||||
| Nodal stage | N0 | 19 | 63% | 12/13 | 92% | 9/13 | 69% |
| N1 | 11 | 36% | 9/9 | 100% | 7/9 | 77% | |
| Metastasis | M0 | 30 | 100% | 24/25 | 96% | 20/26 | 76%* |
| M1 | 0 | 0% | x | x | x | x | |
| Histology | Squamous cell carcinoma | 25 | 83% | 24/25 | 96% | 16/22 | 72%* |
| Adenocarcinoma | 4 | 16% | 4/4 | 100% | 4/5 | 80% | |
| Adenosquamous carcinoma | 1 | ||||||
| LVSI | Negative | 2 | 28% | 2/2 | 100% | 1/2 | 50% |
| Positive | 5 | 71% | 4/4 | 100% | 4/4 | 100% | |
| Non-tested | 23 | x | x | x | x | x | |
| Tumor grade | Gx | 7 | x | x | x | x | x |
| G1 | 1 | 47% | 9/9 | 100% | 4/10 | 40%* | |
| G2 | 10 | ||||||
| G3 | 10 | 52% | 11/11 | 100% | 11/12 | 91% | |
| G4 | 2 | ||||||
Asterisk signifies a significant decrease in CTCs positivity after completing CRT.
All patients underwent irradiation to the pelvic area, depending on the extent of the nodal involvement, 8 patients also had irradiation of the paraaortic lymph nodes up to the level of aortic bifurcation, and 5 patients to the upper edge of the L4, and 2 patients also had irradiation of the inguinal lymph nodes. Chemotherapy was administered concomitantly, consisting of 40 mg/m2 of cisplatin once a week in 5 applications. Chemotherapy was combined with external radiotherapy (RT) 45 Gy in 25 fractions (5 weeks in total). The 5 weeks were followed by additional uterovaginal brachytherapy (BRT) in 2 weeks (4 applications, twice a week, 6.5 Gy in one application). The CRT period has taken almost 7 weeks in total. Five patients had no concomitant chemotherapy due to age, comorbidities, and less advanced initial findings. To achieve the highest possible dose in the primary tumor area, uterovaginal BRT was applied from the 5th week or immediately after the end of external irradiation, always twice a week, a total of 4 applications of 6.5 Gy each.
All patients were checked before and during the treatment and quarterly follow-ups. Every health check consists of physical and gynaecological examination, SCC-Ag and CTCs tests, and medical imaging (ultrasound, computed tomography (CT), MRI/PET-CT). The gynaecological (macroscopic) examination was rated as complete response (CR), partial response (PR/stable disease SD), and progressive disease (PD) according to the RECIST criteria. SSC-Ag values were defining risk for 3 patient groups - increasing, decreasing, and stable values. CTC numbers were reported as CTC-positive/negative patients. During the follow-up, the CTC-count dynamics were evaluated (increasing, decreasing, stable CTC-numbers). Finally, the metastasis presence/new occurrence was noted as a part of the follow-up check.
CTC examination
CTCs were enriched from peripheral blood (EDTA/6-8 ml) by a size-based filtration method (MetaCell®, Czech Republic). The enriched cells were incubated for 3-5 days in vitro (37°C, 5% CO2) and evaluated in a two-step manner [23]. A cytomorphologic evaluation of the viable cells by vital fluorescence microscopy (NucBlue®, Celltracker®, Mitotracker®, Thermo Fisher Scientific, USA) was followed by qPCR analysis of RNA isolated from CTC fraction. qPCR analysis included tumor-associated (TA) and chemoresistance-associated (CA) genes. More details on CTC-methodology are described in Supplementary File, including CTCs presentation on Figures S1, S2, S3.
Patient blood samples were classified as CTC-positive by combined microscopic evaluation and molecular analysis. In the cytomorphological analysis, fluorescently stained viable cells were scored according to the following criteria: nucleus size, nuclear membrane irregularity, prominent nucleoli, nucleoli count, cell size, and the presence of 2D and 3D cell sheets, mitochondria network morphology and activity. CTCs were counted after 3 days (the first reading) and 5 days (the second reading) of in vitro culture.
Gene expression profiling
The enriched size-enriched fractions of cells captured on the membrane were lysed in RLT+ β-mercaptoethanol buffer and stored at -20°C for subsequent RNA analysis [26,27]. The qPCR analysed differences between the whole blood leukocyte fraction (white blood cell; WBC) and enriched CTC fractions (after in vitro incubation) obtained before therapy started. The TaqManTM Gene Expression Assays (Thermo Fisher Scientific, USA) were used for gene expression profiling in all samples: Tumor-associated (TA) genes, including ACTB (control), CD68, CD45, CD68, KRT18, KRT19, EpCAM, VIM, HIF1A, POUSF, VEFA, PDL1, SOX2 as well as chemotherapy associated (CA) genes, including MRP1, MRP2, MRP5, MRP7, and ERCC1 were tested. Specifications of qPCR and are shown in Supplementary File. Based on the gene expression analysis, CTC-enriched samples with elevated relative expression levels in two or more TA genes were considered CTC-positive compared to their matched WBC samples.
Statistical analysis
The data were compared by standard tests using GraphPad Prism software vs. 9.1.0 (GraphPad Prism, USA). The evaluation of qPCR data was based on the standard ddCT method (Livak et al. l, 2001). qPCR results were analysed using GenEx Professional software (MultiD, SE), which enabled multifactorial comparisons between the involved groups. Relative RNA levels are shown graphically in clusters (Supplementary File, Figure S8). The differences between the samples were compared by the U Mann-Whitney test (significance level at P < 0.05 if not set automatically by GenEx).
Results
Patient characteristics and therapy outcomes: tumor volume reduction during CRT
Patient (n=30) characteristics is reported in Table 1 and clinicopathological details of regular examinations are shown in Table S1. The median age of the group was 63 years. In the study, 23 patients were postmenopausal (aged 67.2 at diagnosis on average). Of CRT-indicated patients, 14 subjects were treated with lymph node involvement (46.6%), and negative lymph nodes (N0) were reported in 16 of 30 patients (53.3%).
Based on the histological evaluation, CC was classified as squamous invasive carcinoma (SCC) in 25 patients, adenocarcinoma in 4 patients, and adenosquamous carcinoma in 1 patient. Histological examinations reported 52% of tumors as poorly differentiated (grade 3/4; 10+2/23), 43% of the carcinomas were graded as G2, and one carcinoma was well differentiated (G1). All tumors presented very aggressive character based on tumor cells’ proliferation parameters (Ki67). All patients in our cohort completed CRT treatment. The treatment effect was evaluated three months after the CRT was completed. We provided gynaecological examinations for every patient and CT for the majority of the patients.
During follow-up, 10 patients relapsed (30%, n=10/30). One patient moved away after completing CRT. Progression was defined as local relapse of tumor and/or distant metastases. In our group of patients, the most relapsed cases were diagnosed with distant metastases or both. CT or PET/CT proved the relapse of the disease. There were lymph node metastases, liver or lung metastases. The patients who relapsed received additional cisplatin 50 mg/m2 i.v. D1, paclitaxel 135 mg/m2 i.v. D1 and bevacizumab 15 mg/kg i.v. D1 every 21 days until progression or intolerability. They usually had 6 cycles of the combined therapy; then, the treatment was continued with bevacizumab in monotherapy. Two relapsing patients were enrolled into the clinical study testing immunotherapy - atezolizumab, based on their HPV16 positivity.
The effects of CRT were evaluated clinically by bimanual palpation (gynaecological examination). In parallel, blood collection for SCC-Ag, and CTC tests was provided during the CRT and subsequent BRT. The analysis of tumor volume reduction was evaluated immediately following the administration of CRT. Reduction of the tumor mass was assessed as significant if the tumor volume was reduced by more than 50% (assigned as stable disease (SD)/complete response (CR) - responders) and as minimal if the degree of reduction was less than 50% (progressive disease (PD) - non-responders).
The analysis showed that therapy was assessed as responsive in 90% (27/30) of tumors. The effect of BRT evaluated by tumor volume reduction was significant in 46.6% of patients in the tested cohort (14/30). The response rate was slightly higher in squamous cell (SCC) tumors when compared to adenocarcinoma tumors (96% vs. 80%; ns.). Therapy responsiveness for SCC tumors was reported as reaching 75% tumor volume reduction as counted from MRI/US scans. Tumor volume reduction index was significantly indicative of later progressive disease (P=0.0224).
The results of regular follow-up checks are reported as macroscopy (palpation) evaluation (MACRO), resulting in PD, CR/SD listed in Table S1. Ten patients relapsed during the follow up in total.
CTC presence during the follow up
CTCs were detected in 96.5% of patients (25/26) prior to CRT start (CTC 0 time point). During the therapy period, CTCs were tested after CRT (assigned as CTC AC) and after BRT (assigned as CTC AB) (please see Figure 1 for details), too. As next, CTCs were evaluated every 3 months after completing therapy (14 months-follow-up on average includes CTC tests assigned as CTC 3, CTC 6, CTC 9, CTC 12). CTCs were detected in 75% of patients after CRT+BRT therapy (18/24), in 65% of patients 3 months after CRT therapy (13/20), and in 61% of patients after 6 months (14/23).
There was no significant difference comparing CTCs occurrence between the non-responders (n=3) and responders (n=27) group immediately after completing therapy. However, 3 months after completing CRT+BRT, a significant decrease in CTCs-number was observed in 84% (16/19) patients. After CRT/BRT, CTCs were still present in 75% (18/24) of patients (relapsing vs. non-relapsing, 72% vs. 65%; ns.). If patients divided into progressive and non-progressive patients, the CTC-numbers were significantly different comparing these two groups after 3 months of completing therapy (Figure 2).
Figure 2.

Average CTC numbers in different patients’ groups evaluated at two time points. CTC 0 (after diagnosing the disease) and CTC 3 (3 months after completing the CRT therapy).
During the follow-up period (~36 months), 10/30 patients progressed (30%). The blood samples of progressing patients were tested for CTCs and evaluated as positive before and after therapy and then later during the follow-up (15 months). The number of CTCs was increasing during CRT therapy (please see CTC AC, CTC AB data, Figure 3), but decreasing significantly during the post-therapy period (see Figure S4).
Figure 3.
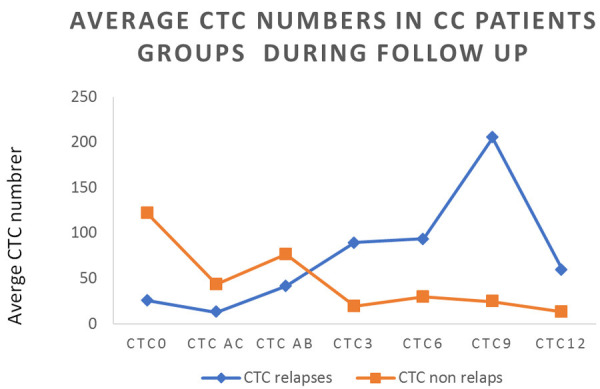
Average CTC-number in the cohort of CC patients is presented for different time points (CTC 0-CTC 12) in relapsing and non-relapsing patients. Significant elevation of CTCs is observed in the period CTC3-CTC6 (3-6 months after completing CRT). In this period, SCC-Ag is elevated only very mild in some of the relapsing patients (see Figure 4).
Colitis episodes might be observed in patients’ post-radiotherapeutically. There were some colitis episodes reported for 26% patients (8/30). Circulating epithelial cells typical for colitis/bowel inflammation were detected in peripheral blood by size-based separation in colitis patients’ samples withdrawn for CTCs analysis. Some of the captured circulating epithelial cells of non-blood origin shared morphological features typical for the intestinal lining. Numbers of circulating epithelial cells in colitis patients are reported in Figures S5, S6.
SCC-Ag concerning CTCs during follow up
The mean baseline value for the tumor marker SCC-Ag at the start of the treatment was 19.33 U/mL (the norm range ≤ 2.5 U/mL). The average number of CTCs at the baseline was 270.23 cells. A significant tumor regression volume during the gynecological examination was confirmed. The regression correlated with the decreasing level of SCC-Ag and CTCs in all patients (later defined as a progressive and non-progressive group). For detailed CTC dynamics, please see Figure 3.
Three months after the end of the treatment, the average value of SCC-Ag (SCC3) was 4.74, and CTC-cells (CTC3) were present in numbers 45.56 (the second reading 52.88). Six months after the end of treatment, the mean SCC (SCC6) value in patients was 1.94, and CTC cells (CTC 6) counted for 69.21 (second reading 51.27).
We saw a decrease in both tumor markers SCC-Ag and CTCs in time manner, which copied the clinical picture of tumor regression in patients. The situation is also consistent with the recommendation that a follow-up examination should be performed ~6 months after the end of treatment, as long as the abscopal effect of radiotherapy is working. At that time, all responders/non-progressing patients had low SCC-Ag tumor marker levels and CTC-numbers (Figure 4). Surprisingly, responders showed higher CTCs numbers at the time of diagnosis compared to non-responders.
Figure 4.
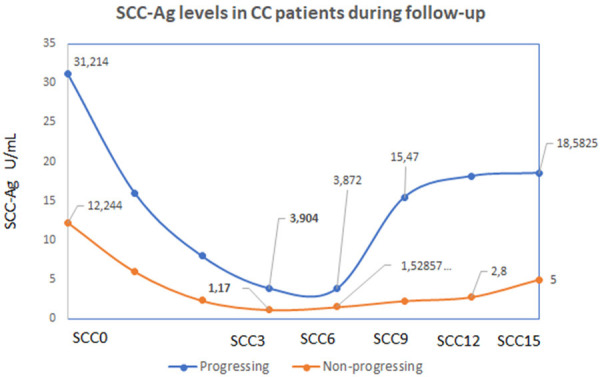
SCC-Ag levels during the follow-up in patients with progressive or non-progressive disease. The graph reports that there is still elevated SCC-Ag in patients with later progression (shown in blue) and that significant increase in SCC-Ag has been documented between SCC6-SCC9 (6 to 9 months) after completing the CRT. This finding is in concordance with observed elevation in CTC-numbers in the period of 6-9 months after the CRT.
Lymphatic nodes involvement in correlation with SCC-Ag and CTCs
During the study, patients without (N0) and with nodal involvement (N1) were compared. At the start of treatment, the SCC-Ag in N0 - patients was significantly lower (8.16 U/mL) than in N1 - group (31.17 U/mL), similarly for CTC numbers in N0 - group (255 CTCs, the second microscopy reading 84.37 CTCs) and CTCs in N1 group (279.61 CTCs-second microscopy reading 449 CTCs). Three months after the CRT, the mean value of SCC-Ag in N0-patients was 1.38 U/mL, with CTC-number reaching 52.38 (the second microscopy reading 44.16), and in N1-patients, the mean SCC-Ag was 10.99 U/mL, CTC cells were counted as 39.44 (the second microscopy reading 72.27). Six months post-treatment, the mean SCC-Ag in N0-patients was 3.36 U/mL, CTC numbers were reaching 108 (from second microscopy reading 45), and in N1-patients, the mean SCC was 1.94 U/mL, CTC cells 69.21 (the second reading 51.72). We report a correlation between the level of SCC-Ag, the clinical stage, and the level of CTC cells. Patients with locally advanced disease have higher levels of SCC-Ag initially. In a subset of patients who relapsed, an elevation of CTCs 3 months before SCC-Ag elevation was detected.
Gene expression analysis of chemoresistance-associated genes and stem-cell like markers in CTCs
The proportion of CTCs expressing elevated levels of chemoresistance-related genes in the CTC fraction was associated with worse treatment outcomes. In patients with relapse, significant increases in stem cell-related genes (SOX2, POUSF) were found in CTCs enriched prior to CRT therapy. There was a significant difference (P ≤ 0.02) in the comparison of DFS rates between the group with increased and decreased expression of SOX2 and POUSF genes.
As an additional, the CTC samples with increased SOX2 expression were divided into subgroups according to the period of DFS (less/more than 12 months). Analysis revealed that enriched CTCs with high SOX2 expression, POUSF and short DFS expressed significantly less VEGF and FLT1 compared to the group with high SOX2 expression and longer DFS (Figures 5, 6). Based on the above, it can be assumed that enriched cells are generally less differentiated in these patient samples. The group with relatively low SOX2 expression showed increased VEGF and FLT1 expression, which could indeed indicate effective anti-VEGF treatment (Figures S7, S8).
Figure 5.

Gene expression profiling in CTC of CC patients. Patients were divided into two groups based on disease free survival (DFS). The gene expression data indicate, that patients with short DFS are expressing significantly more SOX2 and POUSF (stemness markers) (P < 0.02). For this group also reduced expression of VEGF and FLT1 is present.
Figure 6.

Gene expression profiling in CTC of CC patients, comparison based on SOX2. Patients were divided into two groups based on SOX2 expression (high and low levels in comparison to the counted average SOX2 expression) and next after divided based on disease free survival (DFS) period. Gene expression data suggest that patients with high SOX2 expression and long DFS express significantly more VEGF and FLT1 in CTCs (P 0.01). VEFG, FLT1 could be markers of good prognosis despite SOX2 expression.
Discussion
Clinically, we should define at-risk patients with CC at the time of diagnosis and/or after primary treatment, but recently there are no suitable markers for these patients.
The results of the present study clearly show that in the group of patients with progressive disease, the elevation of CTCs was detected between months 3 and 6, which preceded the elevation of SCC-Ag, which increased significantly between months 6 and 9 post-treatment. Based on the results of our study, a follow-up examination 3-6 months after treatment could be defined as another important checkpoint. An increase in CTC could be an indicator to perform follow-up investigations, including imaging, earlier than the standard recommendations.
Although FIGO stage is the most important clinical prognostic marker for CC patients, patients with the same FIGO stage may have different treatment outcomes due to tumor heterogeneity [21-23], suggesting that the FIGO staging system needs to be refined with additional prognostic factors, such as tumor biomarkers of recurrence and metastasis. The goal is to personalize CC treatment as much as possible.
The first study investigating the role of CTCs alone or in combination with SCC-Ag as independent prognostic factors for disease progression in locally advanced CC patients treated with RT was presented. The results demonstrated that CTC and SCC-Ag tests, individually or in combination, were associated with DFS of locally advanced CC patients. In addition, the predictive efficiency was significantly improved when CTCs and SCC-Ag were combined [21].
SCC-Ag is a significant independent prognostic factor during definitive CRT. Persistently elevated values of SCC-Ag predict worse prognosis, as described in BJC in 2018 by Markovina et al. [28].
In a group of 140 patients diagnosed with CC and treated with CRT, a relationship between SCC-Ag and prognosis was demonstrated. Patients were mainly diagnosed with squamous cell CC. Pelvic lymph node positivity was detected in 58.6% during pre/treatment using CT/PET-CT. All patients received concomitant CRT with cisplatin. In patients with elevated pre-treatment SCC-Ag, normalization of these levels on the 27th day after CRT indicates a better prognosis. Post-treatment evaluation of SCC-Ag may allow earlier detection of recurrence, more than a year before clinical symptoms of relapse [28].
SCC-Ag was recently defined as a mediator of radiotherapy resistance. There are two SCC isoforms - SCC1 (SERPINB3) and SCC2 (SERPINB4). SCC1 may protect tumors from the toxic effects of lysosomal cysteine proteases released upon exposure to noxious stimuli. Additionally, SCC1 is directly involved in radiation resistance. Knocking out of SCC1 sensitized cells more than a cisplatin [29].
Therefore, the question arises whether CTCs are a suitable tool for earlier detection of disease relapse than SCC-Ag alone. In a subgroup of patients, we observed an increasing number of CTCs prior to SCC-Ag elevation before relapse became evident in the clinical picture. In addition, patients with nodal involvement had higher baseline CTC values, correlating with tumor burden of disease.
The predictive role of CTCs was shown recently in clinical trial phase III with bevacizumab for recurrent/metastatic disease. There were 452 patients enrolled, diagnosed with advanced CC. Among patients treated with anti-VEGF therapy, higher pre-treatment CTC-numbers were associated with a lower risk of death (HR 0.90; 95% CI, 0.81-0.99) [30]. It can be concluded that a significant reduction in the number of CTCs during treatment may indicate a lower risk of progression (risk of death).
In another retrospective study of Cafforio P. et al., evaluating CTCs in patients after completed RT or CRT for CC, CTC numbers were an independent adverse prognostic factor. Patients with CTCs had a significantly shorter PFS than CTC-negative patients. All stages of the disease were included in this study [31].
Kunpeng et al. expected further value of CTCs in CC in identifying specific molecular targets and discovering resistance mechanisms. The number of CTCs is strongly associated with clinical stage, ongoing treatment, and CTCs isolation methods. The count of CTCs may help to guide treatment selection [32]. In a small prospective study with localized CC receiving definitive CRT, the presence of CTCs correlates with worsened survival [33]. CTCs can indicate the presence of aggressive disease and can be used as a possible tool to prevent overdiagnosis and overtreatment [34].
Further clinical studies should be evaluated to confirm CTCs as significant predictive markers. Interestingly, in our study, a side effect of radiotherapy, post-radiation colitis, was related to the huge presence of circulating epithelial cells (CECs) in the peripheral blood. Detailed microscopic cytopathological examinations revealed a size-enriched mixture of CTCs and CECs on the separation membrane.
CTCs morphology changed during treatment and follow-up. After RT, CTCs were small, with a “hairy” cytoplasm with protrusions. We could also see a change in cell metabolism - a change in mitochondrial activity, identifying less and more active cells.
CTCs might be an auxiliary guide to the tumor burden and the aggressiveness of the disease in the initial diagnosis of the disease. Monitoring of CTCs could help to detect the progression of the disease earlier. Earlier detection of disease relapse may help to improve patients’ prognosis.
Vital CTC cells’ morphology and changes during treatment and monitoring are even more promising because of changed cell metabolic properties and behaviour. In addition to the changes in CTCs, gene expression profiling was provided for the first pre-treatment blood samples tested for CTCs. Significant elevation of stem-cell markers (POUSF, SOX2) has been confirmed for a group of progressing patients.
Based on the recently published data, cancer stem cells (CSCs) may play an essential role in CC, influencing the molecular pathogenesis of CC tumors, their relapse, and their metastatic potential.
A considerable effort is paid to identify signalling pathways involved in the stemness of CC cells. Novel markers for cervical CSCs are being identified and further investigated to obtain diverse therapeutic options to cure CC. There is a need to identify predictive biomarkers of response to newly introduced anticancer drugs. Serial imaging modalities are cost-prohibitive, and no validated serum tumor markers for CC exist.
Another big obstacle is that tumor biopsies are often not readily available to guide second-line therapy for disease progression after treatment (CRT) or targeted therapy (anti-VEGF, anti PD-L1). Overall, the study highlights the potential of CTCs as a tool for monitoring treatment response and predicting disease progression in patients with CC. Further research is needed to validate these finding and optimize the use of CTC monitoring in clinical practice for CC patients.
Conclusion
Monitoring the presence of CTCs concurrently with SCC-Ag during CRT, including gene expression analysis of tumor-associated genes and stemness genes in CTCs, could identify patients at sustained risk of relapse. The combination of imaging methods (e.g., ultrasound, MRI, CT) and CTC-test with SCC-Ag monitoring is beneficial and allows earlier detection of disease relapse. In cases of unresponsive tumors, defined by the volume of the tumor mass and the presence of CTCs with stem cell characteristics, it might be effective to reconsider the initiation of further additional treatment.
However, there are no data on the prolongation of CRT beyond the standard duration. The question remains, if the additionally administered targeted therapy (anti-PDL1, anti-VEGF) would be effective. The clinical utility of CTCs and CTC-related information is limited to palliative indications until the prospective trials focused on the predictive value of CTCs are completed.
Acknowledgements
The work was supported by a research grant provided by Krajská zdravotní a.s., Nr. IGA-KZ-2021-1-9 and by Ministry of Defence, Czech Republic - MO1012.
Disclosure of conflict of interest
None.
Table S1
Supplementary File and Figures S1-S8
References
- 1.Small W Jr, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR, Viswanathan AN, Gaffney DK. Cervical cancer: a global health crisis. Cancer. 2017;123:2404–2412. doi: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 2.Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(Suppl 2):S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Cascardi E, Cazzato G, Daniele A, Silvestris E, Cormio G, Di Vagno G, Malvasi A, Loizzi V, Scacco S, Pinto V, Cicinelli E, Maiorano E, Ingravallo G, Resta L, Minoia C, Dellino M. Association between cervical microbiota and HPV: could this be the key to complete cervical cancer eradication? Biology (Basel) 2022;11:1114. doi: 10.3390/biology11081114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N ESMO Guidelines Committee. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv262. doi: 10.1093/annonc/mdy160. [DOI] [PubMed] [Google Scholar]
- 7.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 8.Bonfrer JMG, Duffy MJ, Radtke M, Segurado O, Torre GC, Van Dalen A, Zwirner M. Tumour markers in gynaecological cancers--EGTM recommendations. European Group on Tumor Markers. Anticancer Res. 1999;19:2807–2810. [PubMed] [Google Scholar]
- 9.Duk JM, de Bruijn HW, Groenier KH, Hollema H, ten Hoor KA, Krans M, Aalders JG. Cancer of the uterine cervix: sensitivity and specificity of serum squamous cell carcinoma antigen determinations. Gynecol Oncol. 1990;39:186–194. doi: 10.1016/0090-8258(90)90430-s. [DOI] [PubMed] [Google Scholar]
- 10.Jeong BK, Choi DH, Huh SJ, Park W, Bae DS, Kim BG. The role of squamous cell carcinoma antigen as a prognostic and predictive factor in carcinoma of uterine cervix. Radiat Oncol J. 2011;29:191–198. doi: 10.3857/roj.2011.29.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wülfing P, Borchard J, Buerger H, Heidl S, Zänker KS, Kiesel L, Brandt B. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12:1715–1720. doi: 10.1158/1078-0432.CCR-05-2087. [DOI] [PubMed] [Google Scholar]
- 12.Romero-Laorden N, Olmos D, Fehm T, Garcia-Donas J, Diaz-Padilla I. Circulating and disseminated tumor cells in ovarian cancer: a systematic review. Gynecol Oncol. 2014;133:632–639. doi: 10.1016/j.ygyno.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 14.Sun YF, Yang XR, Zhou J, Qiu SJ, Fan J, Xu Y. Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J Cancer Res Clin Oncol. 2011;137:1151–1173. doi: 10.1007/s00432-011-0988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2015;7:1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu MC, Janni W, Georgoulias V, Yardley DA, Harbeck N, Wei X, McGovern D, Beck R. First-line doublet chemotherapy for metastatic triple-negative breast cancer: circulating tumor cell analysis of the tnAcity trial. Cancer Manag Res. 2019;11:10427–10433. doi: 10.2147/CMAR.S208712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallwiener M, Riethdorf S, Hartkopf AD, Modugno C, Nees J, Madhavan D, Sprick MR, Schott S, Domschke C, Baccelli I, Schönfisch B, Burwinkel B, Marmé F, Heil J, Sohn C, Pantel K, Trumpp A, Schneeweiss A. Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer. 2014;14:512. doi: 10.1186/1471-2407-14-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson AM, Jansson S, Bendahl PO, Levin Tykjaer Jörgensen C, Loman N, Graffman C, Lundgren L, Aaltonen K, Rydén L. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 2018;20:48. doi: 10.1186/s13058-018-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring A, Nguyen-Sträuli BD, Wicki A, Aceto N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat Rev Cancer. 2023;23:95–111. doi: 10.1038/s41568-022-00536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okazawa-Sakai M, Mabuchi S, Isohashi F, Kawashima A, Yokoi E, Ogawa K, Kimura T. Predictors of distant relapse in patients with FIGO stage IIB-IVA cervical cancer treated with definitive radiotherapy. J Obstet Gynaecol Res. 2017;43:1743–1750. doi: 10.1111/jog.13446. [DOI] [PubMed] [Google Scholar]
- 21.Wen YF, Cheng TT, Chen XL, Huang WJ, Peng HH, Zhou TC, Lin XD, Zeng LS. Elevated circulating tumor cells and squamous cell carcinoma antigen levels predict poor survival for patients with locally advanced cervical cancer treated with radiotherapy. PLoS One. 2018;13:e0204334. doi: 10.1371/journal.pone.0204334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, Köhler C, Landoni F, Lax S, Lindegaard JC, Mahantshetty U, Mathevet P, McCluggage WG, McCormack M, Naik R, Nout R, Pignata S, Ponce J, Querleu D, Raspagliesi F, Rodolakis A, Tamussino K, Wimberger P, Raspollini MR. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol. 2018;127:404–416. doi: 10.1016/j.radonc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, Matulonis UA. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol. 2013;131:352–356. doi: 10.1016/j.ygyno.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Jacob K, Sollier C, Jabado N. Circulating tumor cells: detection, molecular profiling and future prospects. Expert Rev Proteomics. 2007;4:741–756. doi: 10.1586/14789450.4.6.741. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Tirado C, Kale N, Carlini MJ, Shrivastava N, Rodrigues AA, Khalil BD, Bravo-Cordero JJ, Hong Y, Alexander M, Ji J, Behbod F, Sosa MS. NR2F1 is a barrier to dissemination of early-stage breast cancer cells. Cancer Res. 2022;82:2313–2326. doi: 10.1158/0008-5472.CAN-21-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klezl P, Pospisilova E, Kolostova K, Sonsky J, Maly O, Grill R, Pawlak I, Bobek V. Detection of circulating tumor cells in renal cell carcinoma: disease stage correlation and molecular characterization. J Clin Med. 2020;9:1372. doi: 10.3390/jcm9051372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolostova K, Spicka J, Matkowski R, Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res. 2015;7:1203–1213. [PMC free article] [PubMed] [Google Scholar]
- 28.Markovina S, Wang S, Henke LE, Luke CJ, Pak SC, Dewees T, Pfeifer JD, Schwarz JK, Liu W, Chen S, Mutch D, Wang X, Powell MA, Siegel BA, Dehdashti F, Silverman GA, Grigsby PW. Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer. 2018;118:72–78. doi: 10.1038/bjc.2017.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Luke CJ, Pak SC, Shi V, Chen L, Moore J, Andress AP, Jayachandran K, Zhang J, Huang Y, Platik M, Apicelli AA, Schwarz JK, Grigsby PW, Silverman GA, Markovina S. SERPINB3 (SCCA1) inhibits cathepsin L and lysoptosis, protecting cervical cancer cells from chemoradiation. Commun Biol. 2022;5:46. doi: 10.1038/s42003-021-02893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tewari KS, Sill MW, Monk BJ, Penson RT, Moore DH, Lankes HA, Ramondetta LM, Landrum LM, Randall LM, Oaknin A, Leitao MM, Eisenhauer EL, DiSilvestro P, Van Le L, Pearl ML, Burke JJ, Salani R, Richardson DL, Michael HE, Kindelberger DW, Birrer MJ. Circulating tumor cells in advanced cervical cancer: NRG Oncology-Gynecologic Oncology Group Study 240 ( NCT 00803062) Mol Cancer Ther. 2020;19:2363–2370. doi: 10.1158/1535-7163.MCT-20-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cafforio P, Palmirotta R, Lovero D, Cicinelli E, Cormio G, Silvestris E, Porta C, D’Oronzo S. Liquid biopsy in cervical cancer: hopes and pitfalls. Cancers (Basel) 2021;13:3968. doi: 10.3390/cancers13163968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du K, Huang Q, Bu J, Zhou J, Huang Z, Li J. Circulating tumor cells counting act as a potential prognostic factor in cervical cancer. Technol Cancer Res Treat. 2020;19:1533033820957005. doi: 10.1177/1533033820957005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nesbit EA, Driskill E, Robinson P, Murphy K, Andrade J, Showalter TN, Janowski EM, Romano K. The role of circulating tumor cells as potential predictive and prognostic factors in cervical cancers. Int J Radiat Oncol Bio Phys. 2022;114:91. [Google Scholar]
- 34.Lawrence R, Watters M, Davies CR, Pantel K, Lu YJ. Circulating tumour cells for early detection of clinically relevant cancer. Nat Rev Clin Oncol. 2023;20:487–500. doi: 10.1038/s41571-023-00781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


