Abstract
This study aimed to evaluate the impact of different pre-transplant local treatments on the survival of liver transplantation (LTx) recipients with BCLC Stage A Hepatocellular Carcinoma (HCC). We analyzed data from the Taiwan Cancer Registry and National Health Insurance Research Databases spanning 2012 to 2018. Employing propensity score matching, patients were categorized into three groups: those receiving local treatments (180 patients), hepatectomy (179 patients), and combined treatments (180 patients). The primary outcomes were overall mortality and HCC-specific death, assessed using time-varying Cox regression models and Kaplan-Meier survival analysis. During a median follow-up period of 3.92 years, all-cause mortality rates were observed as 74.44% for local treatments, 42.46% for hepatectomy, and 65.00% for combined treatments. HCC-specific mortality rates followed a similar pattern at 65.00%, 39.11%, and 59.44%, respectively. Adjusted hazard ratios demonstrated significantly elevated mortality risks associated with local and combined treatments compared to hepatectomy. Notably, the 2-year overall and HCC-specific survival rates were highest in the hepatectomy group, surpassing those observed in both the combined treatment and local treatment groups. The findings of our study highlight that for patients with BCLC Stage A HCC, undergoing hepatectomy prior to LTx is associated with superior survival outcomes compared to solely local treatments. This underscores the importance of considering hepatectomy as a vital component of the treatment strategy in this patient population.
Keywords: Hepatocellular carcinoma, BCLC stage A, liver transplantation, hepatectomy, local regional therapy
Introduction
Hepatocellular carcinoma (HCC) ranks as the fourth leading cause of cancer-related deaths globally [1,2]. Its main risk factors encompass viral hepatitis, alcohol-related liver disease (ALD), and nonalcoholic liver diseases. HCC’s complexity stems from its neoplastic pathology and the cirrhotic liver environment caused by chronic inflammation. Notably, HCC is the primary cause of mortality in patients with cirrhosis [3]. Clinically, HCC is stratified using various staging systems, such as the American Joint Committee on Cancer (AJCC) TMN stage [4], the Barcelona Clinic Liver Cancer (BCLC) classification [5], and the Okuda stage [6]. Among these, the BCLC staging system, which encapsulates both tumor burden and liver cirrhosis condition, is renowned for its clinical pertinence and comprehensive scope. Patients in BCLC stage 0 have a five-year survival rate of up to 80%, whereas those in BCLC stage A have a survival rate between 50-60% [7]. The heterogeneity within BCLC stage A, characterized by larger tumor sizes and multiple nodules, necessitates variable treatment approaches. Curative interventions like hepatectomy and radiofrequency ablation (RFA) are the mainstay treatments for early-stage HCC according to BCLC guidelines [8]. Moreover, alternative therapeutic modalities include transcatheter arterial chemoembolization (TACE), radioembolization, and percutaneous ethanol injection (PEI).
While hepatectomy and RFA for HCC offer superior overall survival outcomes in patients with well-preserved liver function, intrahepatic tumor recurrence post-treatment remains a significant risk factor for cancer mortality in these patients [9]. Liver transplantation (LTx), however, has shown to yield superior five-year survival rates compared to other curative therapies [10], particularly in patients adhering to the Milan and UCSF criteria. In such cases, five-year survival rates exceed 70%, with recurrence rates ranging between 10% and 15% [11,12]. This improved outcome is primarily due to the complete removal of the cirrhotic liver, a potential site for future tumor development. Nonetheless, the fraction of BCLC stage A HCC patients undergoing LTx is relatively low compared to those receiving RFA or hepatectomy. This trend is attributed to the elevated surgical mortality risks and the scarcity of donor organs associated with LTx. Therefore, other curative interventions like hepatectomy or RFA are frequently considered before LTx in the treatment hierarchy.
The efficacy of radiofrequency ablation (RFA) versus hepatectomy for early-stage hepatocellular carcinoma (HCC) remains debated. Some studies find no significant difference in survival outcomes between these modalities [13,14], while more recent evidence points to superior disease-free survival and a higher five-year overall survival rate associated with surgical intervention compared to RFA [15-18]. Additionally, when contrasting with other local treatments like transcatheter arterial chemoembolization (TACE), hepatectomy consistently demonstrates more favorable results [19]. Notably, these studies have predominantly concentrated on the immediate outcomes of overall and disease-free survival, with scant data regarding the influence of initial treatment choice for BCLC stage A HCC on survival following salvage LTx. Our study, a comprehensive nationwide population-based analysis, aims to address this gap. Employing propensity score matching, we seek to ascertain the most effective initial treatment strategy for BCLC stage A HCC patients who may subsequently require LTx.
Patients and methods
Study cohort
This cohort study utilized patient data extracted from the Taiwan Cancer Registry Database (TCRD) and the National Health Insurance Research Database (NHIRD), spanning the period from January 1, 2012, to December 31, 2018. The study population consisted of individuals diagnosed with HCC who underwent LTx. The index date in this study was defined as the date of LTx, and the follow-up period extended until December 31, 2020. The TCRD, managed by the Collaboration Center of Health Information Application, provided comprehensive information on cancer patients, including clinical stage, treatment modalities, chemotherapy regimens, pathology, and surgical procedures [20-22]. The study protocols received approval from the Institutional Review Board of Tzu-Chi Medical Foundation (IRB109-015-B).
Inclusion and exclusion criteria
To ensure study eligibility, specific criteria were applied to the patient selection process. Inclusion criteria encompassed patients aged 18 years or older, with a confirmed diagnosis of HCC and classified as BCLC stage A, indicating their suitability for LTx. Exclusion criteria comprised patients with a prior history of other cancers, distant metastasis, missing sex data, age below 18, ambiguous staging, or non-hepatocellular carcinoma cases. Individuals presenting severe liver dysfunction, heart disease, renal failure, or other significant comorbidities that contraindicate LTx were excluded from the study. Moreover, patients affected by severe immune system disorders, or organ failure were also excluded from participation.
A comprehensive comparative study is warranted to determine the optimal pre-transplant local treatment strategy for LTx recipients diagnosed with BCLC Stage A HCC and its impact on overall survival. The “local treatments” group includes interventions such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE), radiotherapy (RT), and percutaneous ethanol injection (PEI). We will ensure this definition is consistently used throughout the manuscript. This study aims to evaluate the effectiveness of distinct pre-transplant treatment strategies for patients undergoing liver transplantation (LTx). The treatment groups are: (1) patients receiving local treatments such as RFA, TACE, RT, or PEI; (2) patients undergoing hepatectomy; and (3) patients receiving a combination of local treatments and hepatectomy. Furthermore, we ensured that all enrolled patients attended regular outpatient follow-up visits at least every three months throughout the study period.
Currently, the optimal management strategy before LTx for BCLC Stage A HCC remains uncertain, leading to ambiguity regarding the treatment approach associated with the most favorable overall survival outcomes. Thus, conducting a comprehensive comparative study will significantly contribute to enhancing our understanding of the most effective treatment approach in this specific patient population.
Propensity score matching
In order to account for potential confounding factors when comparing the survival outcomes among the pre-transplant local treatment groups in LTx recipients with BCLC Stage A HCC, a propensity score matching (PSM) method was employed. Patient matching was based on variables including age, sex, income levels, LTx centers, urbanization, Charlson Comorbidity Index (CCI) scores, and other comorbidities (such as diabetes mellitus, hypertension, hyperlipidemia, chronic kidney disease, cardiovascular diseases, chronic obstructive pulmonary disease, and alcohol liver disease), as indicated in Table 1. The matching process resulted in a 1:1 ratio for each group, utilizing the greedy matching method with a caliper of 0.1 [23]. Comorbidities were identified by employing ICD-9-CM or ICD-10-CM codes for the primary diagnoses recorded in inpatient records or outpatient visits occurring at least twice within a one-year period. Continuous variables were presented as means ± standard deviations, where applicable.
Table 1.
Characteristics of liver transplant recipients with BCLC stage A hepatocellular carcinoma (HCC) and different pre-transplant local treatments (after propensity score matching)
| Local treatments (RFA, TACE, RT, or PEI) | Hepatectomy | Local treatments and hepatectomy | P | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N=180 | N=179 | N=180 | |||||
|
|
|
|
|||||
| N | % | N | % | N | % | ||
| Age (mean ± SD) | 61.89 ± 12.03 | 60.45 ± 13.22 | 60.44 ± 12.59 | 0.4534 | |||
| Age, Median (IQR, Q1, Q3) | 62.65 (54.30, 70.54) | 62.96 (52.47, 69.67) | 60.44 (52.11, 69.97) | 0.7271 | |||
| Age group, years | 0.8039 | ||||||
| ≤55 | 48 | 26.67% | 55 | 30.73% | 56 | 31.11% | |
| 56-65 | 55 | 30.56% | 50 | 27.93% | 53 | 29.44% | |
| 66-75 | 57 | 31.67% | 60 | 33.52% | 51 | 28.33% | |
| >75 | 20 | 11.11% | 14 | 7.82% | 20 | 11.11% | |
| Sex | 0.4493 | ||||||
| Female | 46 | 25.56% | 53 | 29.61% | 43 | 23.89% | |
| Male | 134 | 74.44% | 126 | 70.39% | 137 | 76.11% | |
| Income levels (NTD) | 0.9653 | ||||||
| Low income | 0 | 0.00% | 2 | 1.12% | 1 | 0.56% | |
| Financially dependent | 47 | 26.11% | 43 | 24.02% | 49 | 27.22% | |
| ≤20,000 | 56 | 31.11% | 56 | 31.28% | 49 | 27.22% | |
| 20,001-30,000 | 10 | 5.56% | 11 | 6.15% | 12 | 6.67% | |
| 30,001-45,000 | 12 | 6.67% | 13 | 7.26% | 15 | 8.33% | |
| >45,000 | 55 | 30.56% | 54 | 30.17% | 54 | 30.00% | |
| Liver Transplant Centers | 0.6384 | ||||||
| Liver Transplants in Taiwan | 119 | 66.11% | 113 | 63.13% | 114 | 63.33% | |
| Liver Transplants Outside Taiwan | 61 | 33.89% | 66 | 36.87% | 66 | 36.67% | |
| Urbanization | 0.7447 | ||||||
| Rural | 53 | 29.44% | 52 | 29.05% | 47 | 26.11% | |
| Urban | 127 | 70.56% | 127 | 70.95% | 133 | 73.89% | |
| CCI Scores | 0.3956 | ||||||
| 0 | 5 | 2.78% | 10 | 5.59% | 9 | 5.00% | |
| ≥1 | 175 | 97.22% | 169 | 94.41% | 171 | 95.00% | |
| CCI Scores | |||||||
| Congestive Heart Failure | 8 | 0.0444 | 6 | 0.0335 | 11 | 0.0611 | 0.4566 |
| Dementia | 3 | 1.67% | 0 | 0.00% | 4 | 2.22% | 0.1539 |
| Chronic Pulmonary Disease | 34 | 18.89% | 35 | 19.55% | 21 | 11.67% | 0.0843 |
| Rheumatic Disease | 2 | 1.11% | 3 | 1.68% | 0 | 0.00% | 0.2415 |
| DM with complications | 13 | 7.22% | 16 | 8.94% | 13 | 7.22% | 0.1460 |
| Hemiplegia and Paraplegia | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0.9999 |
| Renal Disease | 11 | 6.11% | 9 | 5.03% | 15 | 8.33% | 0.4317 |
| AIDS | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0.9999 |
| Other comorbidities | |||||||
| DM | 47 | 26.11% | 45 | 25.14% | 53 | 29.44% | 0.7859 |
| Hypertension | 102 | 56.67% | 90 | 50.28% | 95 | 52.78% | 0.5014 |
| Hyperlipidemia | 34 | 18.89% | 34 | 18.99% | 38 | 21.11% | 0.7648 |
| Cardiovascular diseases | 62 | 34.44% | 55 | 30.73% | 60 | 33.33% | 0.7452 |
| COPD | 38 | 21.11% | 45 | 25.14% | 46 | 25.56% | 0.6996 |
| Alcohol liver disease | 17 | 9.44% | 13 | 7.26% | 17 | 9.44% | 0.8520 |
| Outcomes | |||||||
| All-cause Death | 134 | 74.44% | 76 | 42.46% | 117 | 65.00% | <0.0001 |
| HCC Death | 117 | 65.00% | 70 | 39.11% | 107 | 59.44% | <0.0001 |
Abbreviations: HCC, Hepatocellular Carcinoma; RFA, Radiofrequency Ablation; TACE, Transcatheter Arterial Chemoembolization; RT, Radiotherapy; PEI, Percutaneous Ethanol Injection; N, Number; IQR, Interquartile Range; NTD, New Taiwan Dollars; DM, Diabetes Mellitus; COPD, Chronic Obstructive Pulmonary Disease; CCI, Charlson Comorbidity Index; AIDS, Acquired Immunodeficiency Syndrome; SD, Standard Deviation.
Outcome measures
The primary objective of this study was to assess and compare the overall mortality rate among LTx recipients with BCLC Stage A HCC across distinct pre-transplant local treatment groups. Tumor recurrence or metastasis represents a prominent cause of mortality following LTx in HCC patients. Although LTx is an effective treatment for primary liver cancer, it does not completely eradicate the presence of existing or potential metastatic lesions. Hence, the risk of tumor recurrence or metastasis persists even after the transplantation procedure. As a result, our investigation also places emphasis on a secondary outcome of interest, specifically HCC-specific death, which specifically examines mortality attributed to HCC. This comprehensive evaluation endeavors to provide valuable insights into the overall survival outcomes and the specific impact of HCC on post-transplantation mortality.
Statistical analysis
We assessed the association between survival outcomes and distinct pre-transplant local treatment groups among LTx recipients with BCLC Stage A HCC. To account for potential confounding factors, such as age, sex, income levels, urbanization, CCI scores, and other comorbidities, we employed time-varying Cox regression models. In our analysis, we incorporated a time-varying covariate that captured the interval between HCC diagnosis and LTx [24,25]. This covariate provided valuable insights into disease progression and treatment timing, both of which could significantly impact the primary outcome of interest, namely all-cause mortality. By dividing the follow-up time into intervals and assessing covariate values at specific time points, our Cox regression models estimated hazard ratios (HR) or coefficients associated with these time-varying covariates. These estimates enabled us to quantify the effects of these covariates on the risk of all-cause mortality. Incorporating these time-varying covariates and coefficients allowed us to comprehensively account for their dynamic nature and their influence on the outcome over time, thus enhancing our understanding of the factors contributing to all-cause mortality in the context of LTx for HCC patients.
Furthermore, we conducted an additional analysis to estimate the risk of mortality within the pre-transplant local treatment groups. To achieve this, we employed the Kaplan-Meier method to estimate mortality rates, and the stratified log-rank test was used to compare mortality curves across the groups. All statistical analyses were performed using SAS version 9.4.
Results
The present study initially included 1,990 patients with HCC who underwent LTx before PSM. Following 1:1 PSM, a total of 719 patients were retained across the three different pre-transplant local treatment groups. Specifically, these groups consisted of 180 patients who received local treatments (such as RFA, TACE, RT, or PEI), 179 patients who underwent hepatectomy, and 180 individuals who underwent both local treatments and hepatectomy prior to LTx. After PSM, an equal number of patients were included in each group, and baseline characteristics such as age, sex, income levels, urbanization, LTx centers, CCI scores, and other comorbidities were balanced between the groups (Table 1).
During the median follow-up of 3.92 years, crude all-cause mortality rates were 74.44% for patients who received local treatments, 42.46% for those who underwent hepatectomy, and 65.00% for those who received both local treatments and hepatectomy before LTx (P<0.0001), respectively. Similarly, the HCC-specific mortality rates were 65.00% for patients who received local treatments, 39.11% for those who underwent hepatectomy, and 59.44% for individuals who underwent both local treatments and hepatectomy before LTx (P<0.0001), respectively (Table 1).
The results of the Time-Varying Cox Proportional Model analysis examining the association between different pre-transplant local treatments and the risk of all-cause death and HCC-specific death in LTx recipients with BCLC Stage A HCC are summarized in Table 2. The aHRs for all-cause mortality were 3.67 (95% CI: 2.63, 5.14) for local treatments plus hepatectomy, and 6.53 (95% CI: 3.93, 10.83) for local treatments, compared to patients who received hepatectomy before LTx for BCLC Stage A HCC. Similarly, the aHRs for HCC-specific death were 4.39 (95% CI: 3.06, 6.29) for local treatments plus hepatectomy, and 7.14 (95% CI: 4.18, 12.18) for local treatments, compared to patients who received hepatectomy before LTx for BCLC Stage A HCC. These findings suggest that local treatments and combined local treatments with hepatectomy are associated with significantly increased risks of all-cause mortality and HCC-specific death compared to hepatectomy in pre-LTx recipients with BCLC Stage A HCC.
Table 2.
Time-varying cox proportional model analysis of all-cause death and HCC-specific death in liver transplant recipients with BCLC stage A hepatocellular carcinoma (HCC) and different pre-transplant local treatments
| HR (95% CI) | P | aHR* (95% CI) | P | |
|---|---|---|---|---|
| All-Cause Death | ||||
| Hepatectomy (Ref.) | 1.00 | - | 1.00 | - |
| Local treatments (RFA, TACE, RT, or PEI) | 3.08 (2.32, 4.1) | <0.0001 | 6.53 (3.93, 10.83) | <0.0001 |
| Local treatments and hepatectomy | 2.37 (1.77, 3.18) | <0.0001 | 3.67 (2.63, 5.14) | <0.0001 |
| HCC-Specific Death | ||||
| Hepatectomy (Ref.) | 1.00 | - | 1.00 | - |
| Local treatments (RFA, TACE, RT, or PEI) | 3.37 (2.46, 4.61) | <0.0001 | 7.14 (4.18, 12.18) | <0.0001 |
| Local treatments and hepatectomy | 2.82 (2.06, 3.87) | <0.0001 | 4.39 (3.06, 6.29) | <0.0001 |
Abbreviations: HCC, Hepatocellular Carcinoma; HR, Hazard Ratio; aHR, Adjusted Hazard Ratio; CI, Confidence Interval; RFA, Radiofrequency Ablation; TACE, Transcatheter Arterial Chemoembolization; RT, Radiotherapy; PEI, Percutaneous Ethanol Injection; Ref., Reference group.
All covariates in Table 1 were adjusted using a time-varying Cox proportional model.
The time-varying covariate in this study is the interval between the diagnosis of HCC and liver transplant. The index date in the study refers to the date of liver transplant.
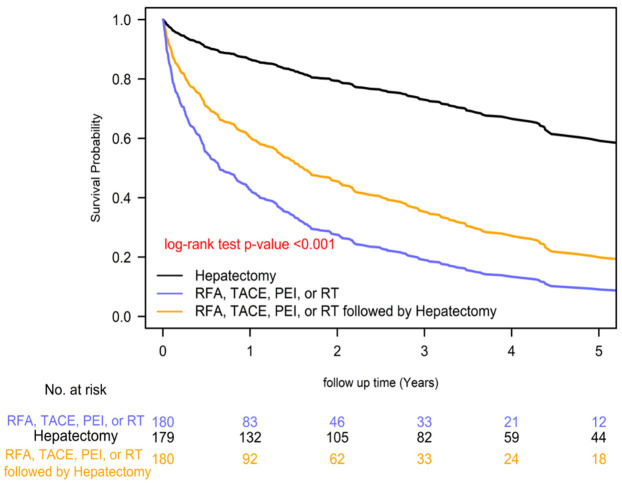
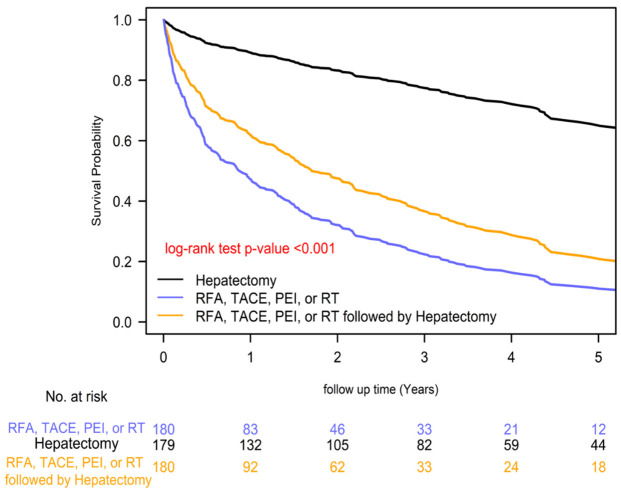
The 2-year overall survival rates differed significantly among the distinct pre-transplant local treatment groups, with rates of 84.88% for hepatectomy, 57.91% for local treatments plus hepatectomy, and 38.30% for local treatments prior treatments groups (P=0.0001; Figure 1). Similarly, the 2-year HCC-specific survival rates also varied significantly among the groups, with rates of 85.90% for hepatectomy, 59.11% for local treatments plus hepatectomy, and 39.09% for local treatments prior treatments groups (P=0.0001; Figure 2). These results suggest that pre-transplant hepatectomy is associated with improved survival compared to other treatments. Among the different treatment groups prior LTx, the order of superior survival was as follows: hepatectomy, combined local treatments and hepatectomy, and local treatments.
Figure 1.
Kaplan-Meier overall survival curves of liver transplant recipients with BCLC stage A hepatocellular carcinoma (HCC) and different pre-transplant local treatments.
Figure 2.
Kaplan-Meier HCC-specific survival curves of liver transplant recipients with BCLC stage A hepatocellular carcinoma (HCC) and different pre-transplant local treatments.
Discussion
Our study reveals that pre-transplant hepatectomy, when employed as the initial intervention for BCLC stage A HCC, confers enhanced outcomes in reducing both all-cause and HCC-specific mortality in subsequent LTx scenarios. This superiority holds when compared to either local treatments alone or a combined regimen of local treatments and hepatectomy, as detailed in Table 2 and illustrated in Figures 1 and 2.
While LTx demonstrates a lower recurrence rate compared to hepatectomy for HCC [26], it is challenged by organ scarcity, heightened surgical mortality, and the risk of patients being removed from the waiting list. In light of these challenges, salvage LTx has emerged as a viable alternative [27]. Various studies have evaluated primary versus salvage LTx. Despite variability in results from several meta-analyses, the general consensus is that salvage LTx is a safe and feasible option [28,29].
The selection between RFA and hepatectomy for early-stage HCC remains contentious. Feng et al. reported that RFA is not inferior to hepatectomy for small HCCs [13]. Conversely, a Korean study indicated similar overall survival rates for both treatments, but better disease-free survival (DFS) with hepatectomy [30]. Recent evidence increasingly favors surgical intervention, showing not only better progression-free survival but also enhanced overall survival outcomes compared to ablation therapy [15-18]. A randomized control trial suggested that repeat hepatectomy might offer improved local disease control and long-term survival relative to RFA, particularly in patients with larger HCCs or elevated AFP levels [31]. Additionally, another study highlighted that for patients with microvascular invasion (MIV), hepatectomy is preferred over RFA even for tumors smaller than 3 cm [32].
These findings highlight the critical role of pre-salvage transplantation strategy in managing HCC patients eligible for LTx. Given the biological heterogeneity of HCC, some tumors at BCLC stage A may exhibit a more aggressive nature and harbor occult metastasis, potentially escaping complete eradication by local treatments [33,34]. This incomplete tumor removal can lead to increased HCC-related mortality following LTx. HCC tumors, particularly smaller ones, may demonstrate a micro-metastasis pattern with invasion into the portal vein branches [33,34]. In this context, hepatectomy, and specifically anatomic liver resection, offers a more effective means of removing potential tumor spread along these branches compared to RFA [30]. RFA, especially in larger tumors requiring multiple ablations, often struggles to achieve clear margins, increasing the likelihood of residual viable tumor cells [18,35]. This observation is particularly pertinent in our cohort, where patients initially diagnosed with early-stage HCC and treated with hepatectomy or other local treatments eventually required LTx. This progression suggests a predisposition towards more aggressive HCC variants in this group.
In identifying high-risk HCC patients, factors such as larger tumor diameters, multiple nodules, or elevated AFP levels are pivotal [36]. Our study demonstrates that for BCLC stage A HCC patients, hepatectomy as the initial treatment with curative intent or as a bridge therapy prior to LTx yields superior survival outcomes compared to local treatments. Particularly for BCLC stage A patients at elevated risk of recurrence, hepatectomy emerges as a logical first-line therapy, especially when subsequent LTx is contemplated.
Consistent with other studies, our research confirms a stable overall survival rate for patients undergoing hepatectomy [29]. However, the survival rate for our local treatment group was markedly lower (Table 2; Figures 1 and 2). A meta-analysis indicated that patients receiving locoregional therapy followed by salvage LTx exhibited a less favorable overall survival rate than those undergoing primary LTx [37]. In our study, the precise pre-transplantation tumor status remains unspecified since the BCLC stage A stage was based on the index date. Consequently, it is plausible that tumors in patients of the local treatment group were in more advanced stages at the time of LTx.
This study has several limitations. First, the data were sourced from the Taiwan Cancer Registry Database, which lacks detailed tumor characteristics such as precise size, number, and specific locations. Moreover, the exact tumor status of patients before LTx is not available. Second, the retrospective design introduces inherent biases. To address potential confounding factors, we employed PSM based on variables such as age, sex, income levels, LTx centers, urbanization, CCI scores, and various comorbidities, including diabetes mellitus, hypertension, hyperlipidemia, chronic kidney disease, cardiovascular diseases, chronic obstructive pulmonary disease, and alcohol liver disease, which reflect lifestyle factors. Additionally, we ensured that all enrolled patients had regular outpatient follow-up visits at least every three months during the study period. This regular follow-up schedule demonstrated good adherence to treatments and helped minimize the impact of non-compliance on the study outcomes. Despite these efforts, the retrospective nature may still influence the findings. Future prospective studies are needed to provide a more detailed comparison of treatment outcomes.
Conclusion
Our study represents a pioneering effort to assess the optimal local treatments preceding LTx, and it uncovers that hepatectomy, when utilized as the initial treatment for BCLC stage A patients who later undergo LTx, leads to enhanced post-transplantation survival outcomes compared to those receiving other local treatments. This finding is particularly salient for patients categorized as BCLC stage A yet potentially earmarked for future LTx. In such scenarios, hepatectomy should be prioritized as the primary treatment with curative intent or as an effective bridging therapy. This approach could significantly influence clinical decision-making and patient management strategies in hepatocellular carcinoma care.
Acknowledgements
We thank Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital for supporting Szu-Yuan Wu’s work (Grant Numbers: 10908, 10909, 11001, 11002, 11003, 11006).
Disclosure of conflict of interest
None.
Abbreviations
- HCC
Hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- LTx
Liver transplant
- DTLT
deceased donor Liver transplant
- LDLT
living donor Liver transplant
- RFA
radiofrequency ablation
- TACE
trans-arterial chemoembolization
- BCLC
Barcelona classification of liver Cancer
- AJCC
American Joint Committee on Cancer
References
- 1.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Lee CW, Tsai HI, Yu MC, Wang CC, Lee WC, Yeh TS, Yeh CN, Lin CY, Kuo T, Chen HY. A proposal for T1 subclassification in hepatocellular carcinoma: reappraisal of the AJCC 8th edition. Hepatol Int. 2022;16:1353–1367. doi: 10.1007/s12072-022-10422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. In: Seminars in liver disease: 1999: © 1999 by Thieme Medical Publishers, Inc.; 1999. pp. 329–338. [DOI] [PubMed] [Google Scholar]
- 6.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Tsilimigras DI, Bagante F, Sahara K, Moris D, Hyer JM, Wu L, Ratti F, Marques HP, Soubrane O, Paredes AZ, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Prognosis after resection of Barcelona Clinic Liver Cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26:3693–3700. doi: 10.1245/s10434-019-07580-9. [DOI] [PubMed] [Google Scholar]
- 8.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, Brunello F, Pinna AD, Giorgio A, Giulini SM, De Sio I, Torzilli G, Fornari F, Capussotti L, Guglielmi A, Piscaglia F, Aldrighetti L, Caturelli E, Calise F, Nuzzo G, Rapaccini GL, Giuliante F. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl 2):S44–57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 12.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 13.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao CY, Hu RH, Ho CM, Wu YM, Lee PH, Ho MC. Surgical resection versus radiofrequency ablation for Barcelona Clinic Liver Cancer very early stage hepatocellular carcinoma: long-term results of a single-center study. Am J Surg. 2020;220:958–964. doi: 10.1016/j.amjsurg.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Chong CC, Lee KF, Chu CM, Chan AW, Yu SC, Lai PB. Laparoscopic hepatectomy (with or without robotic assistance) versus radiofrequency ablation as a minimally invasive treatment for very early-stage or early-stage hepatocellular carcinoma. Dig Surg. 2020;37:65–71. doi: 10.1159/000497112. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4:7252. doi: 10.1038/srep07252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 19.Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, Kubo S, Izumi N, Kadoya M, Sakamoto M, Nakashima O, Matsuyama Y, Kokudo T, Hasegawa K, Yamashita T, Kashiwabara K, Takayama T, Kokudo N, Kudo M Liver Cancer Study Group of Japan. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg. 2020;272:145–154. doi: 10.1097/SLA.0000000000003192. [DOI] [PubMed] [Google Scholar]
- 20.Lin WC, Ding YF, Hsu HL, Chang JH, Yuan KS, Wu ATH, Chow JM, Chang CL, Chen SU, Wu SY. Value and application of trimodality therapy or definitive concurrent chemoradiotherapy in thoracic esophageal squamous cell carcinoma. Cancer. 2017;123:3904–3915. doi: 10.1002/cncr.30823. [DOI] [PubMed] [Google Scholar]
- 21.Yen YC, Chang JH, Lin WC, Chiou JF, Chang YC, Chang CL, Hsu HL, Chow JM, Yuan KS, Wu ATH, Wu SY. Effectiveness of esophagectomy in patients with thoracic esophageal squamous cell carcinoma receiving definitive radiotherapy or concurrent chemoradiotherapy through intensity-modulated radiation therapy techniques. Cancer. 2017;123:2043–2053. doi: 10.1002/cncr.30565. [DOI] [PubMed] [Google Scholar]
- 22.Chang CL, Tsai HC, Lin WC, Chang JH, Hsu HL, Chow JM, Yuan KS, Wu ATH, Wu SY. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol. 2017;125:73–79. doi: 10.1016/j.radonc.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6:121. doi: 10.21037/atm.2018.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S, Shetterly S, Powers D, Raebel MA, Tsai TT, Ho PM, Magid D. Extension of Kaplan-Meier methods in observational studies with time-varying treatment. Value Health. 2012;15:167–174. doi: 10.1016/j.jval.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menahem B, Lubrano J, Duvoux C, Mulliri A, Alves A, Costentin C, Mallat A, Launoy G, Laurent A. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: an attempt to perform an ideal meta-analysis. Liver Transpl. 2017;23:836–844. doi: 10.1002/lt.24758. [DOI] [PubMed] [Google Scholar]
- 27.Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899–906. doi: 10.1053/he.2000.5763. [DOI] [PubMed] [Google Scholar]
- 28.Yadav DK, Chen W, Bai X, Singh A, Li G, Ma T, Yu X, Xiao Z, Huang B, Liang T. Salvage liver transplant versus primary liver transplant for patients with hepatocellular carcinoma. Ann Transplant. 2018;23:524–545. doi: 10.12659/AOT.908623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrini GP, Esposito G, Olivieri T, Magistri P, Ballarin R, Di Sandro S, Di Benedetto F. Salvage versus primary liver transplantation for hepatocellular carcinoma: a twenty-year experience meta-analysis. Cancers (Basel) 2022;14:3465. doi: 10.3390/cancers14143465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HW, Lee JM, Yoon JH, Kim YJ, Park JW, Park SJ, Kim SH, Yi NJ, Suh KS. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018;94:74–82. doi: 10.4174/astr.2018.94.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 2020;6:255–263. doi: 10.1001/jamaoncol.2019.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai S, Yang P, Xie Z, Li J, Lei Z, Xia Y, Qian G, Zhang B, Pawlik TM, Lau WY, Shen F. Preoperative estimated risk of microvascular invasion is associated with prognostic differences following liver resection versus radiofrequency ablation for early hepatitis B virus-related hepatocellular carcinoma. Ann Surg Oncol. 2021;28:8174–8185. doi: 10.1245/s10434-021-09901-3. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]
- 34.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman AL, Wu SS, Obaid AK, French SW, Lois J, McMonigle M, Ramos HC, Sher LS, Lopez RR. Histologic evaluation and treatment outcome after sequential radiofrequency ablation and hepatic resection for primary and metastatic tumors. Am Surg. 2002;68:1038–1043. [PubMed] [Google Scholar]
- 36.Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 37.Murali AR, Patil S, Phillips KT, Voigt MD. Locoregional therapy with curative intent versus primary liver transplant for hepatocellular carcinoma: systematic review and meta-analysis. Transplantation. 2017;101:e249–e257. doi: 10.1097/TP.0000000000001730. [DOI] [PubMed] [Google Scholar]