Abstract
Introduction
Spondylodiscitis (SD) is an infection of the intervertebral disc with involvement of the adjacent vertebral bodies. Diagnostic tests with CT-guided biopsy only provide a positive yield in 14%–48% of cases. Percutaneous endoscopic debridement and drainage (PEDD) has recently shown promise in the treatment of spondylodiscitis.
Research question
The purpose of this study is to determine differences in pathogen identification and clinical outcomes for PEDD versus CT-guided needle biopsy in SD patients.
Materials and methods
We conducted a systematic review of the literature using PRISMA guidelines to determine differences in positive microbiology results, perioperative complications, pain control, and long-term clinical outcomes for PEDD vs. CT-guided needle biopsy in SD patients.
Results
1078 studies were evaluated, 87 of which underwent full review. 15 studies met the inclusion and exclusion criteria, including 7 PEDD, 7 CT-guided biopsy, and 1 CT-guided biopsy vs. PEDD article, for a total of 192 PEDD patients and 604 CT-guided biopsy patients. We found 36.59% of CT-guided biopsy patients had positive microbiology results, compared to 84.38% of PEDD patients. No major perioperative complications occurred as a result of the PEDD procedure. Of the five PEDD studies that reported pain outcomes, greater than 80% of patients experienced relief after intervention.
Discussion and conclusion
These results suggest that PEDD may improve pathogen identification while simultaneously reducing pain compared to CT-guided needle biopsy in SD. Although current treatment guidelines recommend CT-guided biopsy, in patients with severe back pain and suspected SD, PEDD can be considered an alternative intervention.
Keywords: Spondylodiscitis, Vertebral osteomyelitis, Percutaneous endoscopic debridement and drainage, PEDD, CT-Guided needle biopsy
Highlights
-
•
We performed a systematic review of PEDD vs. CT-guided biopsy in spondylodiscitis.
-
•
PEDD may improve pathogen identification compared to CT-guided biopsy in SD.
-
•
A majority of patients who underwent PEDD experienced pain relief.
-
•
No major perioperative complications occurred because of the PEDD procedure.
1. Introduction
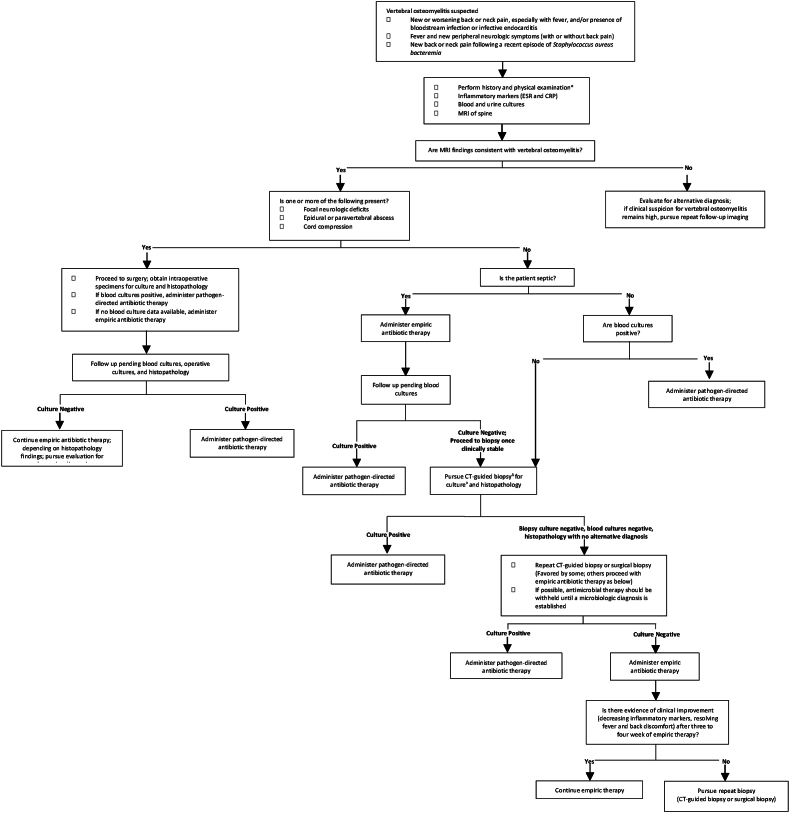
Spondylodiscitis (SD) is a microorganism infection of the intervertebral disc with subsequent involvement of the neighboring vertebral bodies (Sobottke et al., 2008). While the incidence is rare, with 5.4 cases per 100,000 people, the resulting pain substantially decreases patient quality of life (Mylona et al., 2009). Most patients present with severe, chronic back pain, but the clinical presentation and symptoms vary wildly (Mylona et al., 2009). The current standard of care requires infection confirmation through laboratory tests with elevated levels of white blood cells (WBC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR)(Waheed et al., 2019). Unfortunately, due to the limited positive yield from blood cultures (Stangenberg et al., 2021; Aagaard et al., 2013), so the gold standard of SD diagnosis is MRI imaging (92% sensitivity and 96% specificity) (Herren et al., 2017) with evidence of disc destruction, inflammation, and extent of infection/abscess (if present) being cited as hallmarks of the condition. The treatment algorithm for SD (Fig. 1) depends on the clinical presentation and symptoms of the patient, but typically requires targeted or empiric antibiotic treatment with or without surgical intervention.
Fig. 1.
Current treatment guidelines for patients with suspected spondylodiscitis. Adapted from UpToDate Copyright © 2022 UpToDate, Inc. and its affiliates and/or licensors. All rights reserved.
For non-septic patients without neurologic deficits, CT-guided biopsy is the current standard of care for pathogen identification (Peel and McDonald). However, this diagnostic test in SD patients only provides positive yield in 14%–48% of cases (McNamara et al., 2017; Garg et al., 2014). When the initial biopsy fails to identify causative pathogens, repeat CT-guided biopsies are recommended (Peel and McDonald). Not only does this current practice delay antibiotic treatment, but patients also continue to suffer with severe back pain, reducing their quality of life. Additionally, a recent study showed that only 14% of patients who underwent repeat CT-guided needle biopsy received positive microbiology results following the repeat procedure (Czuczman et al., 2018). For those with intractable pain and minimal aversion to surgery, surgeons may recommend an open biopsy. While this has an increased pathogen identification rate of 76%, there is a concurrent exposure to additional risk with this invasive procedure (McNamara et al., 2017; Czuczman et al., 2018; Kasalak et al., 2018a). Indeed, for at-risk patients such as the elderly or immunocompromised this option may incur too much risk. The implementation of a minimally invasive procedure similar to CT-guided biopsy that improves pathogen identification in SD could reduce risk relative to open surgery, minimize delay in antibiotic treatment, and ultimately improve patient outcomes.
Percutaneous endoscopic debridement and drainage (PEDD), a minimally invasive procedure adapted from percutaneous endoscopic discectomy in the 1980s, has recently shown promise in the treatment of spondylodiscitis. Since its inception, Yang et al. and others have demonstrated its efficacy in the diagnosis and treatment of pyogenic spondylitis (Yang et al., 2008). Ultimately, however, surgeons often reserve this approach for more serious infections in which paraspinal abscesses or recurrent postoperative infections are identified (Wang et al., 2018).
In this study, we conduct a systematic review of the literature, as well as comparison of positive microbiology results and clinical outcomes in CT-guided needle biopsy versus PEDD to determine whether this procedure could be implemented to allow for higher yield of early targeted antibiotic therapy for spondylodiscitis. We also aimed to assess patient-reported pain outcomes to evaluate symptomatic treatment in PEDD, despite patients being subjected to a surgical procedure.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to query PubMed, Web of Science, SCOPUS, and SpringerLink. The search was performed using the key words (“spondylodiscitis” OR “discitis”) AND (“CT” OR “image guided” OR “percutaneous endoscopy” OR “percutaneous endoscopic debridement” OR “PEDD”) AND (“pathogen” OR “bacteria” OR “causative”). There were a total of 1078 returns through our search. Following removal of duplicate studies, a total of 951 unique results were identified. From this group, all case reports, animal studies, editorials, letters, poster presentations, abstracts, editorials, letters, and non-English results were excluded. Abstract and title review were completed for the remaining 410 papers and led to the removal of another 323 papers. Complete paper review was completed on 87 papers. From this cohort the following inclusion criteria were applied: 1) paper provides percentage of patients with positive pathogen identification following initial intervention; 2) paper reports the types of pathogens identified. In addition, the following exclusion criteria were applied: 1) demographic data from subgroups of interest were unextractable; 2) convoluted data from repeat interventions; 3) empiric antibiotics used within seven days of intervention; 4) lack of data demonstrating that pathogen positivity did not differ between those on or off antibiotics. AL and AC served as the final arbiters for the papers included in this analysis. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
2.1. Quality and potential bias assessment quality
The included studies were evaluated based on the Oxford Centre for Evidence-Based Medicine (OCEBM) from a scale of 1 (highest level of evidence) to 5 (lowest level of evidence).
2.2. Data collection and statistical analysis
The following variables were extracted: year of publication, journal of publication, patient number, age, gender, antibiotic use prior to intervention, symptoms at presentation, CRP, spinal infection location, percent positive pathogen identification, pathogen species, VAS score, and patient outcomes.
3. Results
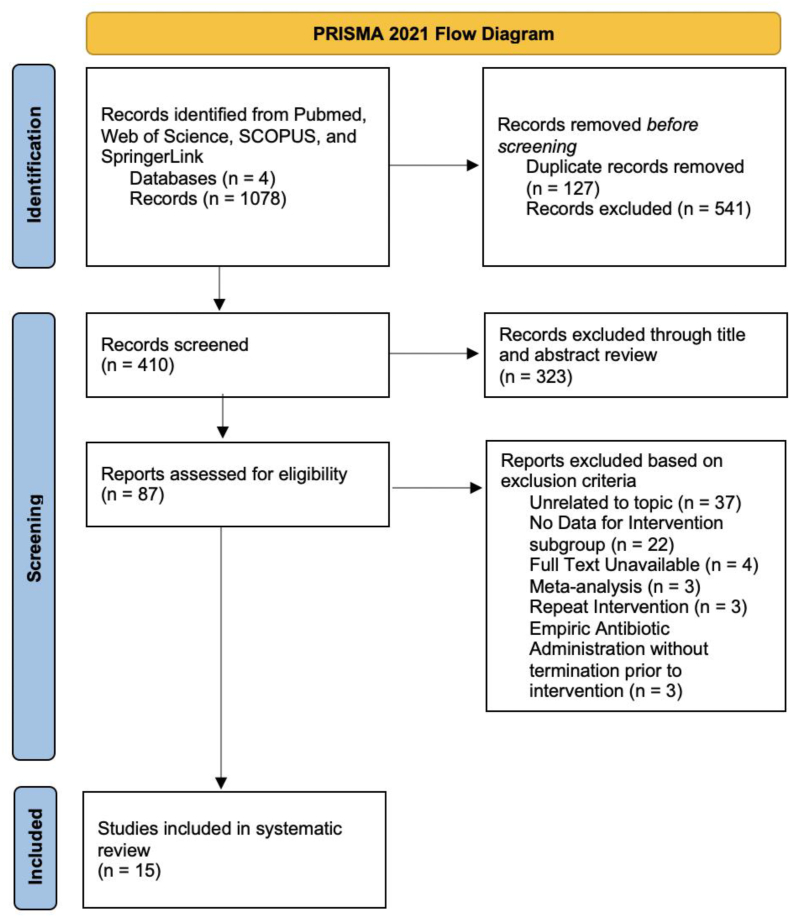
The search produced 1078 unique results from which 15 studies were ultimately included in the study – 7 articles on PEDD, 7 articles on CT-guided biopsy, and 1 directly comparing CT-guided biopsy and PEDD (resulting in 8 total papers for both PEDD and CT-guided biopsy) (Fig. 2).
Fig. 2.
PRISMA Diagram delineating study selection process, resulting in 15 studies in the present analysis.
3.1. Demographic data
Demographic data for all studies is included in Table 1. Eight articles that reported positive pathogen yield using PEDD were included in this analysis, ranging from the years 2007–2019 and including 192 patients (68.8% male). All studies were classified as OCEBM Level 3 and included data about patient age and symptoms at presentation. In all studies, the only clinical symptom reported at time of presentation was back pain. C-reactive protein (CRP) values were given in five papers.
Table 1.
Demographic data.
| Demographic Data | |||||||
|---|---|---|---|---|---|---|---|
| Study, year | Evidence Level | Number of Patients | Sex (M/F) | Age | Symptoms at Presentation | C-reactive protein (CRP) | |
|
Percutaneous Endoscopic Debridement and Drainage (PEDD) |
Yang et al., 2007 | III | 15 | 9/6 | 64.2 (Range 27–88) | Back pain | 78.8 ± 68.8 mg/L |
| Yang et al., 2008 | III | 20 | 12/8 | 62.9 ± 14.9 | Back pain | 52.1 ± 26.5 mg/L | |
| Fu et al., 2010 | III | 6 | 4/2 | 62 (Range 32–88) | Back pain | 103.6 ± 63.5 mg/dL | |
| Yang et al., 2014 | III | 32 | 23/9 | 57.4 (Range 38–88) | Back pain | 89.1 ± 72 mg/L | |
| Yang et al., 2014 | III | 21 | 14/7 | 56.5 (Range 39–87) | Back pain | Not reported | |
| Wang et al., 2016 | III | 41 | 29/12 | 55.2 (Range 28–88) | Back pain | 67.1 ± 68.5 mg/L | |
| Yang et al., 2019 | III | 20 | 14/6 | 60 (Range 38–72) | Back pain | Not reported | |
|
Fu et al., 2019 |
III |
37 |
27/10 |
56.5 ± 14.4 |
Back pain |
Not reported |
|
|
CT-Guided Needle Biopsy |
Yang et al., 2008 | III | 32 | 17/15 | 63.0 ± 14.6 | Back pain | 56.8 ± 20.8 mg/L |
| Garg et al., 2014 | III | 84 | Not reported | Not reported | Not reported | Not reported | |
| Spira et al., 2016 | III | 34 | 21/13 | 57.5 (Range 7–80) | Not reported | Not reported | |
| Kasalak et al., 2018 | III | 64 | 34/30 | 61.7 ± 16.5 (Range 16–91) | Not reported | Not reported | |
| Braun et al., 2019 | III | 40 | Not reported | 65.1 (Range 10–89) | Not reported | 51.2 ± 62.9 mg/L | |
| Afshari et al., 2020 | III | 5 | 3/2 | 5.29 ± 4.7 (Range 1.2–14) | Fever Back pain Antalgic gait |
62 ± 64 mg/L | |
| Husseini et al., 2020 | III | 241 | 158/83 | 59 (Range 4–99) | Fever Back pain Diaphoresis Chills |
Not reported | |
|
Wong et al., 2021 |
III |
104 |
65/39 |
57.7 ± 17.9 |
Fever Sepsis |
61.8 ng/mL (Range 12–76) |
|
| Total (PEDD) | 8 studies | III - 8 studies (100%) | 157 | 132/60 (68.8% male) | -- | Number of studies | -- |
| Back pain – 8 (100%) | |||||||
| Total (CT-Guided Needle Biopsy) | 8 studies | III – 8 studies (100%) | 604 | 298/182 (62.1% male) | -- | Number of studies | -- |
| Back pain – 3 (75%) | |||||||
| Fever – 3 (75%) | |||||||
| Sepsis – 1 (25%) | |||||||
| Diaphoresis – 1 (25%) | |||||||
| Chills – 1 (25%) | |||||||
| Antalgic gait – 1 (25%) | |||||||
Eight studies were identified that calculated positive pathogen yield following CT-guided biopsy, ranging from 2008 to 2020. These studies were classified as OCEBM Level 3.604 patients (62.1% male of 480 patients who reported sex) participated in these studies. Age data was reported in six studies, and four studies included symptoms at presentation. The most to least common clinical symptoms were back pain (n = 3, 75%), fever (n = 3, 75%), sepsis (n = 1, 25%), diaphoresis (n = 1, 25%), chills (n = 1, 25%), and antalgic gait (n = 1, 25%). Four studies also included data about CRP values.
3.2. Microbiology results
Of the 192 patients who underwent PEDD for suspected spondylodiscitis, 162 patients (84.38%) received positive microbiology results following the procedure (Table 2). All patients had infection sites in the lumbar region. 164 specific pathogens were reported in these studies. The most common pathogens were Staphylococcus species (43.90%), followed by Streptococcus species (18.29%) and gram-negative bacilli (14.02%) (Table 3). Staphylococcus aureus was the most identified microorganism in this group (37.20%).
Table 2.
Infection site, pathogen identification.
| Infection Site, Pathogen Identification | ||||
|---|---|---|---|---|
| Procedure | Study, Year | Site of Infection by Region (N, %) | Pathogen Identification after procedure (X/Y) | Positive Microbiology Results |
|
Percutaneous Endoscopic Debridement and Drainage (PEDD) |
Yang et al., 2007 | Lumbar – 15 (100%) | 13/15 | 86.67% |
| Yang et al., 2008 | Lumbar – 20 (100%) | 18/20 | 90.00% | |
| Fu et al., 2010 | Lumbar – 6 (100%) | 5/6 | 83.33% | |
| Yang et al., 2014 | Lumbar – 32 (100%) | 28/32 | 87.50% | |
| Yang et al., 2014 | Lumbar – 21 (100%) | 19/21 | 90.48% | |
| Wang et al., 2016 | Lumbar – 41 (100%) | 32/41 | 78.05% | |
| Yang et al., 2019 | Lumbar – 20 (100%) | 17/20 | 85.00% | |
|
Fu et al., 2019 |
Lumbar – 37 (100%) |
30/37 |
81.08% |
|
|
CT-Guided Needle Biopsy |
Yang et al., 2008 | Lumbar – 32 (100%) | 15/32 | 46.88% |
| Garg et al., 2014 | Cervical – 1 (1.2%) | 16/84 | 19.05% | |
| Thoracic – 37 (44%) | ||||
| Lumbosacral – 46 (54.8%) | ||||
| Spira et al., 2016 | Thoracic – 11 (32.4%) Lumbar – 23 (67.4%) |
10/34 | 29.41% | |
| Kasalak et al., 2018 | Cervical – 5 (7.8%) Thoracic – 18 (28.1%) Thoracolumbar – 3 (4.7%) Lumbar – 27 (42.2%) Lumbosacral – 11 (17.2%) |
20/64 | 31.25% | |
| Braun et al., 2019 | Not reported | 11/40 | 27.50% | |
| Afshari et al., 2020 | Thoracic – 1 (20%) Lumbar – 3 (60%) Lumbosacral – 1 (20%) |
1/5 | 20.00% | |
| Husseini et al., 2020 | Thoracic – 69 (29%) Lumbar – 172 (71%) |
95/241 | 39.42% | |
|
Wong et al., 2021 |
Cervical – 5 (4.8%) Thoracic – 34 (32.7%) Lumbar – 65 (62.5%) |
53/104 |
50.96% |
|
| Total (PEDD) | 8 studies | Lumbar – 192 (100%) | 162/192 | 84.38% |
| Total (CT) | 8 studies |
Cervical – 11 (1.95%) Thoracic – 170 (30.14%) Thoracolumbar – 3 (0.53%) Lumbar – 322 (57.09%) Lumbosacral – 58 (10.28%) |
221/604 | 36.59% |
Table 3.
Types of pathogens identified.
| Types of Pathogens Identified | |||
|---|---|---|---|
| Percutaneous Endoscopic Debridement and Drainage (PEDD) |
CT-Guided Biopsy |
||
| Study | Types of Pathogens Identified (N, %) | Study | Types of Pathogens Identified (N, %) |
| Yang et al., 2007 | Oxacillin-resistant Staphylococcus aureus – 3 (23.1%) Oxacillin-sensitive Staphylococcus aureus – 3 (23.1%) Mycobacterium tuberculosis – 2 (15.4%) Viridans Streptococci – 1 (7.7%) Prevotella species – 1 (7.7%) Enterococcus faecalis – 1 (7.7%) Pseudomonas aeruginosa – 1 (7.7%) Candida albicans – 1 (7.7%) |
Yang et al., 2008 | Oxacillin-resistant Staphylococcus aureus – 4 (22.22%) Oxacillin-sensitive Staphylococcus aureus – 4 (22.22%) Tuberculosis – 1 (5.56%) Streptococcus viridans – 1 (5.56%) Candida albicans – 2 (11.11%) Propionibacterium acnes – 2 (11.11%) Pseudomonas aeruginosa – 1 (5.56%) Proteus mirabilis – 1 (5.56%) Enterococcus faecalis – 1 (5.56%) Escherichia coli – 1 (5.56%) |
| Yang et al., 2008 | Oxacillin-resistant Staphylococcus aureus – 5 (27.78%) Oxacillin-sensitive Staphylococcus aureus – 4 (22.22%) Mycobacterium tuberculosis – 3 (16.67%) Viridans streptococci – 2 (11.11%) Candida albicans – 1 (5.56%) Pseudomonas aeruginosa – 1 (5.56%) Prevotella species – 1 (5.56%) Enterococcus faecalis – 1 (5.56%) |
Garg et al., 2014 |
Staphylococcus aureus – 8 (50%) Coagulase-negative Staphylococcus – 3 (18.8%) Escherichia coli – 1 (6.3%) Enterococcus faecium – 1 (6.3%) Proteus mirabilis – 1 (6.3%) Candida – 1 (6.3%) Mycobacterium tuberculosis – 1 (6.3%) |
| Fu et al., 2010 | Candida albicans – 2 (40%) Mycobacterium tuberculosis – 1 (20%)Staphylococcus aureus – 1 - (20%) Enterococcus faecalis – 1 (20%) |
Spira et al., 2016 | Enterobacteria – 2 (20%) Staphylococci – 4 (40%, 1 methicillin-resistant) Aggregatibacter aphrophilus – 1 (10%) Mycobacterium tuberculosis – 2 (20%) Escherichia coli – 1 (10%) |
| Yang et al., 2014 | Oxacillin-sensitive Staphylococcus aureus – 9 (30%) Oxacillin-resistant Staphylococcus aureus – 8 (26.7%) Haemophilus influenzae – 3 (10%) Viridans Streptococci – 2 (6.7%) Pseudomonas aeruginosa – 2 (6.7%) Prevotella species – 1 (3.3%) Mycobacterium tuberculosis – 1 (3.3%) Enterococcus faecalis – 1 (3.3%) Escherichia coli – 1 (3.3%) Klebsiella pneumoniae – 1 (3.3%) Candida albicans – 1 (3.3%) |
Kasalak et al., 2018 | Propionibacterium acnes – 7 (35%)Staphylococcus aureus – 3 (15%) Enterococcus faecalis – 2 (10%) Candida albicans – 1 (5%) Candida krusei – 1 (5%) Escherichia coli and Bacteroides species – 1 (5%) Staphylococcus saccharolyticus – 1 (5%) Streptococcus agalactiae – 1 (5%) Streptococcus salivarius, Streptococcus species, and Neisseria species – 1 (5%) Streptococcus species – 1 (5%) Streptococcus sanguinis – 1 (5%) |
| Yang et al., 2014 | Oxacillin-resistant Staphylococcus aureus – 6 (31.6%) Oxacillin-sensitive Staphylococcus aureus – 4 (21.1%) Pseudomonas aeruginosa – 2 (10.5%) Viridans Streptococci – 2 (10.5%) Candida albicans – 1 (5.3%) Enterococcus faecalis – 1 (5.3%) Mycobacterium tuberculosis – 1 (5.3%) Haemophilus influenzae – 1 (5.3%) Escherichia coli – 1 (5.3%) |
Braun et al., 2019 | Escherichia coli – 3 (27.3%) Staphylococcus species – 6 (54.5%) Streptococcus species – 2 (18.2%) |
| Wang et al., 2016 | Mycobacterium tuberculosis – 6 (18.8%) Oxacillin-resistant Staphylococcus aureus – 5 (15.6%) Oxacillin-sensitive Staphylococcus aureus – 4 (12.5%) Escherichia coli – 3 (9.4%) Enterococcus faecalis – 3 (9.4%) Candida albicans – 2 (6.3%) Gram-positive bacilli – 2 (6.3%) Viridans Streptococci – 2 (6.3%) Streptococcus bovis – 1 (3.1%) Prevotella species – 1 (3.1%) Peptostreptococcus micros – 1 (3.1%) Pseudomonas aeruginosa – 1 (3.1%) Klebsiella pneumoniae – 1 (3.1%) |
Afshari et al., 2020 | Staphylococcus aureus – 1 (100%) |
| Yang et al., 2019 | Oxacillin-sensitive Staphylococcus aureus – 5 (29.5%) Oxacillin-resistant Staphylococcus aureus – 4 (23.5%) Mycobacterium tuberculosis – 4 (23.5%) Pseudomonas aeruginosa – 2 (11.8%) Viridans Streptococci – 1 (5.9%) Escherichia coli – 1 (5.9%) |
Husseini et al., 2020 |
Staphylococcus aureus – 31 (32.6%) Streptococcal species – 11 (11.6%) Fungal species (Candida albicans, Candida parpsilosis, Aspergillus, Scedosporium) – 9 (9.5%) Escherichia coli – 6 (6.3%) Coagulase-negative Staphylococci species, (Staph epidermidis, hominis, lugdunensis) – 3 (3.2%) Enterococcus – 4 (4.2%) Klebsiella species – 4 (4.2%) Mycobacterium species (tuberculosis, abscessus) – 4 (4.2%) Salmonella species (including typhi) – 4 (4.2%) Enterobacter species – 3 (3.2%) Propionibacterium acnes – 3 (3.2%) Pseudomonas aeruginosa – 3 (3.2%) Other bacteria (Brucella, Bacteroides, Bifidobacterium, Citrobacter, Corynebacterium, Lactobacilius, Peptostreptococcus, Prevotella) – 10 (10.5%) |
|
Fu et al., 2019 |
Methicillin-sensitive Staphylococcus – 6 (20%) Enterococcus species – 6 (20%) Methicillin-resistant Staphylococcus aureus – 5 (16.7%) Streptococcus species – 4 (13.3%) Mycobacterium tuberculosis – 4 (13.3%) Gram-positive bacilli – 2 (6.7%) Pseudomonas aeruginosa – 1 (3.3%) Klebsiella pneumoniae – 1 (3.3%) Fungus – 1 (3.3%) |
Wong et al., 2021 |
Staphylococcus aureus – 11 (20.8%) Mycobacterium tuberculosis – 10 (18.9%) Escherichia coli – 8 (15.1%) |
| Total |
Staphylococcus species – 72 (43.90%) Streptococcus species – 30 (18.29%) Gram-negative bacilli – 23 (14.02%) Acid-fast bacteria – 22 (13.41%) Fungi – 9 (5.49%) Gram-positive bacilli – 4 (2.44%) Gram-negative coccobacilli – 4 (2.44%) |
Total |
Staphylococcus species – 79 (39.90%) Gram-negative bacilli – 39 (19.70%) Streptococcus species – 26 (13.13%) Acid-fast bacteria – 18 (9.09%) Fungi – 14 (7.07%) Gram-positive bacilli – 11 (5.56%) Gram-negative coccobacilli – 1 (0.56%) Other – 10 (5.05%) |
Comparatively, 221 out of 604 patients (36.59%) who underwent CT-guided needle biopsy had positive microbiology results following the procedure (Table 2). The most common infection sites were lumbar (57.19%), thoracic (30.20%), and lumbosacral (10.30%). Similar to the PEDD studies, 198 specific microorganisms were reported. These pathogens were also primarily Staphylococcus species (39.90%), gram-negative bacilli (19.70%), and Streptococcus species (13.13%), with Staphylococcus aureus being the most common (33.33%) (Table 3).
3.3. Clinical outcomes
Regarding surgical complications, four PEDD studies noted transient paresthesias in the affected lumbar segment for a minority of patients (Yang et al., 2014a, 2014b; Wang et al., 2016; Fu et al., 2019)–(Yang et al., 2014a, 2014b; Wang et al., 2016; Fu et al., 2019). All other studies reported no surgery-related complications or side effects from the PEDD procedure.
In the long-term clinical outcomes of PEDD studies, a subset of patients required subsequent open surgery following the procedure. In six studies, this arose due to an inability to identify the causative pathogen, leading to disease progression and osteolytic destruction of the vertebral body, ultimately causing spinal instability or kyphotic deformity (Yang et al., 2007, 2008, 2014a, 2014b, 2019; Fu et al., 2019)–(Yang et al., 2007, 2008, 2014a, 2014b, 2019; Fu et al., 2019). Additionally, many cases involved patients initially presenting severe multilevel disease and paraspinal abscesses that were not adequately controlled with targeted antibiotics despite positive microbiology results (Yang et al., 2008, 2014b, 2019; Fu et al., 2010, 2019). Apart from one study by Yang et al., (2014) where a patient underwent instrumented fusion after controlling the spinal infection (Yang et al., 2014a), all other instances requiring open surgery post-PEDD procedure fell into these categories.
Few CT-guided biopsy studies discussing long-term clinical outcomes (Yang et al., 2008; Kasalak et al., 2018b; Afshari et al., 2020), as these aspects were often beyond their intended scope. Yang et al., 2008 directly compared outcomes between PEDD and CT-guided biopsy patients, finding that among 32 CT-guided biopsy cases, 18 eventually required surgery due to the failure to identify causative pathogens, leading to progressive infection and spinal instability (Yang et al., 2008). This contrasted with 5 out of 20 PEDD patients who underwent surgery. Kasalak et al., 2018 reported adverse outcomes in 13 out of 64 patients, such as vertebral collapse, need for spinal surgery, or paraplegia from epidural abscess development (Kasalak et al., 2018b). However, they observed no significant outcome difference between patients with positive versus negative microbiology results. Afshari et al., 2020 noted successful resolution of symptoms in all patients treated with intravenous antibiotics (Afshari et al., 2020). All other CT-guided biopsy studies included in our review did not focus on reporting long-term clinical outcomes, as it wasn't their primary study objective.
3.4. Patient-reported pain reduction after PEDD
Seven out of eight PEDD studies reported pain measurements for a total of 155 patients. Two studies used VAS pain scores pre-vs. postoperatively, both reporting >50% pain reduction following PEDD (Table 4). The remaining five articles reported on the percentage of patients experiencing back pain relief postoperatively. In all five studies, over 80% of patients experienced back pain relief, ranging from immediately to one month postoperatively (Table 5).
Table 4.
PEDD studies with VAS Pain Scores.
| PEDD Studies with VAS Scores | |||||
|---|---|---|---|---|---|
| Author, Year | Time to Follow-up Visit | Number of Patients | Pre-treatment VAS Score (Mean ± SD) | Post-treatment VAS Score (Mean ± SD) | Reduction in VAS Score (Mean ± SD) |
| Wang et al., 2016 | 1 month | 41 | 6.7 ± 1.0 | 2.5 ± 0.7 | 4.2 ± 1.2 |
| Yang et al., 2019 | 1 day | 20 | 7.6 ± 0.8 | 3.2 ± 0.8 | 4.4 ± 1.1 |
*These are the only studies that report VAS scores for pre-vs. postoperative pain.
Table 5.
PEDD studies with patient-reported pain relief.
| PEDD Studies with Patient-Reported Pain Relief | |||
|---|---|---|---|
| Author, Year | Number of Patients | Patients experiencing back pain relief (%) | Reported time of pain relief |
| Yang et al., 2007 | 15 | 13 (86.7%) | Immediate |
| Yang et al., 2008 | 20 | 18 (90%) | Immediate |
| Fu et al., 2010 | 6 | 5 (83.3%) | One week |
| Yang et al., 2014 | 32 | 27 (84.4%) | 1 month |
| Yang et al., 2014 | 21 | 18 (85.7%) | One week |
*These are the only studies that report pre-vs. postoperative pain relief.
4. Discussion
Given an aging population that presents with increased morbidity, the incidence of spondylodiscitis along with its associated poor outcomes will likely increase, placing a heightened burden on the healthcare system.3,5 Thus, significant improvement in the diagnosis and treatment of SD is essential. Patients with SD present with nonspecific symptoms, but the most common and debilitating of these symptoms is progressive back pain (Mylona et al., 2009).
The current standard of care employed in the treatment of a non-septic SD patient with negative blood work is a CT-guided biopsy for pathogen identification and ultimately targeted long-term antibiotics (Peel and McDonald). However, we found causative pathogens were only identified in 36.59% of cases using CT-guided biopsy. For patients with negative biopsy results, the current treatment algorithm for SD recommends repeat biopsies until the pathogen is identified or the condition progresses. During this time, many patients will continue to experience chronic back pain. Our data suggests that PEDD may improve positive microbiology results and could serve as a viable alternative to CT-guided biopsy for pathogen identification. Moreover, the included studies also demonstrated that PEDD can directly reduce patient pain as at least 80% and up to 90% of patients reported pain relief. Together, these results suggest that PEDD may help address the most debilitating symptom of SD while reducing time to targeted therapeutic administration. Interestingly, we found that all 192 SD patients that underwent PEDD had infections in the lumbar region. This is in stark contrast to CT-guided biopsy, which was implemented with greater versatility in 57.09% of lumbar spine cases, 30.14% of thoracic cases, 10.28% of lumbosacral cases, and 1.95% of cervical cases. This aligns with the associated difficulties and surgeon hesitancy of conducting the PEDD procedure within the cervical or thoracic spine. We also note limited data on pain outcomes and presentation with CT-guided biopsy for SD patients, with only a single paper providing post-intervention pain data. In this study, 0 of the 32 patients reported in the Yang et al., 2008 study who underwent CT-guided biopsy experienced immediate back pain relief (Yang et al., 2008).
Our data indicates that PEDD could be considered as an intervention in the diagnosis and treatment of SD. Importantly, implementation of PEDD earlier in the disease course of SD may not only decrease time to target antibiotic administration via improvements in pathogen identification, but also decrease patient pain. It is important to note that due to the paucity of literature that directly compares these two interventions, we do not know if the improvement in pathogen identification is a result of increased microbiological capture due to increased infection severity or a result of true differences in the performance of these interventions for pathogen identification. Indeed, four out of the five PEDD studies with CRP data reported values that were greater than CRP levels in all the CT-guided biopsy studies. However, due to lack of individual patient data, we cannot determine if these differences were statistically significant across studies. In this analysis, only one study – a single-institution retrospective cohort study by Yang et al., 2008) – directly compared these interventions in the treatment of SD (Yang et al., 2008). Although these authors reported that PEDD increased pathogen identification and significantly reduced pain in SD patients compared to CT-guided biopsy,36 we are unable to conclusively determine whether PEDD can provide better long-term clinical outcomes without more supporting data. There is also a lack of data investigating procedure-associated risks and later complications (e.g. local kyphotic changes) in using PEDD as an alternative to CT-guided needle biopsy. Ultimately, these unanswered questions require prospective clinical trials to evaluate differences in infection severity, pain reduction, latency to antibiotic administration, perioperative complications, and long-term health outcomes within a standardized treatment protocol and data analysis.
Moreover, further research is required to elucidate how components of the PEDD procedure impact outcomes. To explain, Fu et al., 2010 implemented PEDD without the use of irrigation and used the Hemovac negative pressure pump until drainage stopped or was <10 mL/day for three consecutive days (Fu et al., 2010). This contrasts with Yang et al., 2008, where a Hemovac negative pressure was used following normal saline irrigation (Yang et al., 2008). In the 2014 papers, Yang et al. reports usage of 10 L of 3.5% betadine solution for irrigation in addition to the negative pressure Hemovac (Yang et al., 2014a, 2014b). The percentage of patients reporting pain relief remained relatively similar, ranging from 83.3% to 90%, regardless of the surgical procedure implemented (Yang et al., 2008, 2014a, 2014b; Fu et al., 2010). However, we do not know how these differences impacted long-term outcomes, reinfection rates, latency to antibiotic administration or perioperative complications. Ultimately, further investigation is required.
4.1. Limitations
As most of the data reported in this current study are from numerous institutions with various providers that may follow slightly different treatment algorithms, a direct comparison of these two interventions is not possible. Indeed, while the current treatment algorithm recommends targeted antibiotic administration for the causative microorganism, this is not always possible or implemented. Two studies were excluded from this present study after full-text review because patients were administered empiric antibiotics prior to biopsy and no information was provided regarding time of antibiotic arrest prior to intervention. We, however, elected to include two CT-guided biopsy studies with pre-antibiotic administration – Spira et al., 2016 and Braun et al., 2019 – as antibiotics were held for at least seven days prior to biopsy (Spira et al., 2016; Braun et al., 2019). However, multiple studies have demonstrated that prior antibiotic administration does not impact pathogen positivity in SD (Sobottke et al., 2008; Mylona et al., 2009; Waheed et al., 2019; Titlic and Josipovic-Jelic, 2008; Zimmerli, 2010; Priest and Peacock, 2005; Hsieh et al., 2004; Ryang and Akbar, 2020)–(Sobottke et al., 2008; Mylona et al., 2009; Waheed et al., 2019; Titlic and Josipovic-Jelic, 2008; Zimmerli, 2010; Priest and Peacock, 2005; Hsieh et al., 2004; Ryang and Akbar, 2020)–(Sobottke et al., 2008; Mylona et al., 2009; Waheed et al., 2019; Titlic and Josipovic-Jelic, 2008; Zimmerli, 2010; Priest and Peacock, 2005; Hsieh et al., 2004; Ryang and Akbar, 2020). To understand how exclusion of these studies would impact our results, we performed a post hoc analysis excluding these studies and found that pathogen positivity in CT-guided biopsy increased from 221/604 (36.59%) to 200/530 (37.74%) (Supplementary Table 1). This suggests that antibiotic cessation seven days before intervention may not impact pathogen identification. Lastly, as mentioned in the methods section, studies that provided statistical analysis demonstrating that the administration of antibiotics did not impact positive yield were included in this study. Accordingly, we included data from three CT-guided biopsy studies – Garg et al., 2014, Kasalak et al., 2018, and Wong et al., 2021 (Garg et al., 2014; Kasalak et al., 2018b; Wong et al., 2021). To assess whether the inclusion of studies altered our data we reperformed our analysis without these papers. Under these circumstances, pathogen identification increased from 221/604 (36.59%) to 132/352 (37.50%) (Supplementary Table 1). In total, this further suggests that, even when the treatment algorithm is not followed and patients receive broad-spectrum antibiotics prior to intervention, PEDD is superior to CT-guided biopsy for pathogen identification. Lastly, to entirely eliminate the potential confounding variable of empiric antibiotic administration prior to intervention, the following studies were excluded: Garg et al., 2014, Spira et al., 2016, Kasalak et al., 2018, Braun et al., 2019, and Wong et al., 2021 (Garg et al., 2014; Kasalak et al., 2018b; Spira et al., 2016; Braun et al., 2019; Wong et al., 2021). Upon analysis, we found that pathogen identification changed from 221/604 (36.59%) to 111/278 (39.93%). Compared to the microbiology results of the included PEDD studies (162/192 positive results, 84.38%), in which no prior antibiotics were administered, this still suggests that PEDD should be investigated as an alternative to CT-guided needle biopsy.
Another important limitation to this study is the lack of individual patient data. Many studies did not include demographic data for each patient. Thus, it was not possible to conduct statistical analyses to compare or correlate data on the patient populations, microbiology results, infection sites, and VAS scores or pain reduction.
5. Conclusion
Our data suggest that CT-guided biopsy resulted in positive yield in 221 out of 604 (36.59%, range, 19.05%–50.96%) patients while PEDD provided definitive diagnosis in 222 out of 271 (84.38%, range, 78.05%–90.48%) patients. Although long-term clinical outcomes were not consistently reported for CT-guided biopsy studies, in PEDD patients, subsequent open surgery was largely associated with failure to identify causative pathogens. Regarding pain outcomes eight PEDD studies reported data on pain relief following intervention. Two studies reported a greater than 50% reduction in mean VAS score following PEDD. The remaining five studies reported that over 80% of patients experienced back pain relief secondary to PEDD intervention, ranging from immediate to one month postoperatively. These results demonstrate the multifunctionality of the PEDD procedure in both the diagnosis of SD and management of patient symptoms.
Although our data suggest that PEDD may be considered a viable and potentially more effective alternative to the current standard of care, further prospective studies are required to assess complications secondary to these interventions, clinical outcomes, pain reduction, and latency of antibiotic administration in SD patients undergoing PEDD vs. CT-guided needle biopsy. Implementing PEDD for pathogen identification in non-septic suspected SD patients with negative blood cultures who do not have urgent neurological concerns may increase positive pathogen identification and reduce pain when compared to CT-guided biopsy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof F Kandziora
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bas.2024.102854.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aagaard T., Roed C., Dragsted C., Skinhøj P. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006-2011. Scand. J. Infect. Dis. 2013;45:417–424. doi: 10.3109/00365548.2012.753160. [DOI] [PubMed] [Google Scholar]
- Afshari F.T., Rodrigues D., Bhat M., Solanki G.A., Walsh A.R., Lo W.B. Paediatric spondylodiscitis: a 10-year single institution experience in management and clinical outcomes. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2020;36:1049–1054. doi: 10.1007/s00381-019-04470-z. [DOI] [PubMed] [Google Scholar]
- Braun A., Germann T., Wünnemann F., Weber M.-A., Schiltenwolf M., Akbar M., et al. Impact of MRI, CT, and clinical characteristics on microbial pathogen detection using CT-guided biopsy for suspected spondylodiscitis. J. Clin. Med. 2019;9:32. doi: 10.3390/jcm9010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuczman G.J., Marrero D.E., Huang A.J., Mandell J.C., Ghazikhanian V., Simeone F.J. Diagnostic yield of repeat CT-guided biopsy for suspected infectious spondylodiscitis. Skeletal Radiol. 2018;47:1403–1410. doi: 10.1007/s00256-018-2972-y. [DOI] [PubMed] [Google Scholar]
- Fu T.-S., Yang S.-C., Tsai T.-T., Chen L.-H., Lai P.-L., Niu C.-C., et al. Percutaneous endoscopic debridement and drainage in immunocompromised patients with complicated infectious spondylitis. Minim Invasive Ther Allied Technol MITAT Off J Soc Minim Invasive Ther. 2010;19:42–47. doi: 10.3109/13645700903384450. [DOI] [PubMed] [Google Scholar]
- Fu T.-S., Wang Y.-C., Lin T.-Y., Chang C.-W., Wong C.-B., Su J.-Y. Comparison of percutaneous endoscopic surgery and traditional anterior open surgery for treating lumbar infectious spondylitis. J. Clin. Med. 2019;8:1356. doi: 10.3390/jcm8091356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V., Kosmas C., Young P.C., Togaru U.K., Robbin M.R. Computed tomography-guided percutaneous biopsy for vertebral osteomyelitis: a department's experience. Neurosurg. Focus. 2014;37:E10. doi: 10.3171/2014.6.FOCUS14134. [DOI] [PubMed] [Google Scholar]
- Herren C., Jung N., Pishnamaz M., Breuninger M., Siewe J., Sobottke R. Spondylodiscitis: diagnosis and treatment options. Dtsch Ärztebl Int. 2017;114:875–882. doi: 10.3238/arztebl.2017.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P.C., Wienecke R.J., O’Shaughnessy B.A., Koski T.R., Ondra S.L. Surgical strategies for vertebral osteomyelitis and epidural abscess. Neurosurg. Focus. 2004;17:E4. doi: 10.3171/foc.2004.17.6.4. [DOI] [PubMed] [Google Scholar]
- Kasalak Ö., Adams H.J.A., Jutte P.C., Overbosch J., Dierckx R.A.J.O., Wouthuyzen-Bakker M., et al. Culture yield of repeat percutaneous image-guided biopsy after a negative initial biopsy in suspected spondylodiscitis: a systematic review. Skeletal Radiol. 2018;47:1327–1335. doi: 10.1007/s00256-018-3006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasalak Ö., Wouthuyzen-Bakker M., Adams H.J.A., Overbosch J., Dierckx R.A.J.O., Jutte P.C., et al. CT-guided biopsy in suspected spondylodiscitis: microbiological yield, impact on antimicrobial treatment, and relationship with outcome. Skeletal Radiol. 2018;47:1383–1391. doi: 10.1007/s00256-018-2944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara A.L., Dickerson E.C., Gomez-Hassan D.M., Cinti S.K., Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 2017;38:2021–2027. doi: 10.3174/ajnr.A5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylona E., Samarkos M., Kakalou E., Fanourgiakis P., Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin. Arthritis Rheum. 2009;39:10–17. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Peel T., McDonald M. Vertebral osteomyelitis and discitis in adults n.d. https://medilib.ir/uptodate/show/7663
- Priest D.H., Peacock J.E. Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South. Med. J. 2005;98:854–862. doi: 10.1097/01.smj.0000168666.98129.33. [DOI] [PubMed] [Google Scholar]
- Ryang Y.-M., Akbar M. [Pyogenic spondylodiscitis: symptoms, diagnostics and therapeutic strategies] Orthopä. 2020;49:691–701. doi: 10.1007/s00132-020-03945-1. [DOI] [PubMed] [Google Scholar]
- Sobottke R., Seifert H., Fätkenheuer G., Schmidt M., Goßmann A., Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Ärztebl Int. 2008;105:181–187. doi: 10.3238/arztebl.2008.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira D., Germann T., Lehner B., Hemmer S., Akbar M., Jesser J., et al. CT-guided biopsy in suspected spondylodiscitis--the association of paravertebral inflammation with microbial pathogen detection. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangenberg M., Mende K.C., Mohme M., Krätzig T., Viezens L., Both A., et al. Influence of microbiological diagnosis on the clinical course of spondylodiscitis. Infection. 2021;49:1017–1027. doi: 10.1007/s15010-021-01642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titlic M., Josipovic-Jelic Z. Spondylodiscitis. Bratisl. Lek. Listy. 2008;109:345–347. [PubMed] [Google Scholar]
- Waheed G., Soliman M.A.R., Ali A.M., Aly M.H. Spontaneous spondylodiscitis: review, incidence, management, and clinical outcome in 44 patients. Neurosurg. Focus. 2019;46:E10. doi: 10.3171/2018.10.FOCUS18463. [DOI] [PubMed] [Google Scholar]
- Wang Y.-C., Wong C.-B., Wang I.-C., Fu T.-S., Chen L.-H., Chen W.-J. Exposure of prebiopsy antibiotics influence bacteriological diagnosis and clinical outcomes in patients with infectious spondylitis. Medicine (Baltim.) 2016;95 doi: 10.1097/MD.0000000000003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhou S., Bian Z., Li M., Jiang W., Hou C., et al. Unilateral percutaneous endoscopic debridement and drainage for lumbar infectious spondylitis. J. Orthop. Surg. 2018;13:306. doi: 10.1186/s13018-018-1009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H., Tarr G.P., Rajpal K., Sweetman L., Doyle A. The impact of antibiotic pre-treatment on diagnostic yield of CT-guided biopsy for spondylodiscitis: a multi-centre retrospective study and meta-analysis. J. Med. Imag. Radiat. Oncol. 2021;65:146–151. doi: 10.1111/1754-9485.13118. [DOI] [PubMed] [Google Scholar]
- Yang S.-C., Fu T.-S., Chen L.-H., Niu C.-C., Lai P.-L., Chen W.-J. Percutaneous endoscopic discectomy and drainage for infectious spondylitis. Int. Orthop. 2007;31:367–373. doi: 10.1007/s00264-006-0188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-C., Fu T.-S., Chen L.-H., Chen W.-J., Tu Y.-K. Identifying pathogens of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin. Orthop. 2008;466:3086–3092. doi: 10.1007/s11999-008-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-C., Chen W.-J., Chen H.-S., Kao Y.-H., Yu S.-W., Tu Y.-K. Extended indications of percutaneous endoscopic lavage and drainage for the treatment of lumbar infectious spondylitis. Eur. Spine. J. Off. Publ. Eur Spine Soc Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2014;23:846–853. doi: 10.1007/s00586-013-3157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-C., Fu T.-S., Chen H.-S., Kao Y.-H., Yu S.-W., Tu Y.-K. Minimally invasive endoscopic treatment for lumbar infectious spondylitis: a retrospective study in a tertiary referral center. BMC Muscoskel. Disord. 2014;15:105. doi: 10.1186/1471-2474-15-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-C., Chiu Y.-C., Chen H.-S., Kao Y.-H., Jou I.-M., Tu Y.-K. Percutaneous endoscopic debridement and drainage for the treatment of instrumented lumbar spine infection. J. Orthop. Surg. Hong Kong. 2019;27 doi: 10.1177/2309499019863356. [DOI] [PubMed] [Google Scholar]
- Zimmerli W. Clinical practice. Vertebral osteomyelitis. N. Engl. J. Med. 2010;362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.