Summary
Ovarian cancer is a highly lethal gynecological malignancy, emphasizing the need for effective treatment strategies. This study investigated the synergistic effects of quercetin and paclitaxel on ovarian cancer. Using SKOV3 and A2780 cell lines, we found that the combined treatment significantly enhanced cell apoptosis and inhibited invasion and migration compared to individual treatments. Then, we identified 32 common targets between quercetin/paclitaxel and ovarian cancer, with 29 genes showing differential expression between normal ovarian tissue and ovarian tumor tissue. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed that quercetin and paclitaxel modulated cancer-related pathways in ovarian cancer treatment. Mechanistic analysis further discovered that the synergistic effect was mediated by downregulating ERBB2 and BIRC5 and upregulating CASP3 expression. This study provides strong evidence that quercetin enhances the effectiveness of paclitaxel in treating ovarian cancer.
Subject areas: Therapy, Cell biology, Cancer
AB_2064048Graphical abstract

Highlights
-
•
Quercetin significantly enhances paclitaxel sensitivity in ovarian cancer cells
-
•
Key targets identified between quercetin/paclitaxel treatment and ovarian cancer
-
•
Quercetin/paclitaxel treatment affects cancer-related pathways in ovarian cancer
-
•
Quercetin shows dose- and time-dependent inhibitory effects on ovarian cancer cells
Therapy; Cell biology; Cancer
Introduction
Ovarian cancer frequently manifests in an advanced stage and continues to be the primary cause of gynecological cancer mortality.1 In the United States, approximately 19,680 new cases of ovarian cancer have been reported in 2024, leading to an estimated 12,740 mortalities.1,2 Despite the initial response observed in patients with advanced ovarian cancer following cytoreductive surgery, relapse occurs in 70%–85% of cases within 12–24 months, leading to progression into the platinum-resistant subtype.3 Despite several studies elucidating the various mechanisms responsible for platinum resistance in ovarian cancer, the intricate mechanisms involved remain elusive.
Quercetin, an organic compound with substantial healing capabilities and low toxicity, is a vital factor in cancer prevention and treatment.4,5,6 A previous study demonstrated the remarkable antitumor properties of quercetin, including its ability to induce mitochondrial-mediated apoptosis and impede metastatic ovarian cancer cell proliferation.7,8 The findings by Ren8 suggested that quercetin can impede SKOV3 cell proliferation in ovarian cancer, hinder cell-cycle progression from G0/G1 to G2/M, and trigger cell apoptosis in vitro. Furthermore, in a human ovarian cancer xenograft model, the concurrent administration of quercetin and radiation significantly suppressed cancer growth by inhibiting DNA double-strand break repair and augmenting radiosensitivity.9 Consequently, to enhance the radiosensitivity of patients with ovarian cancer, combination therapies are necessary. Paclitaxel, a naturally derived anticancer compound with low toxicity, is classified into taxane-based chemotherapeutics. Its ability to stabilize microtubules establishes it as a fundamental treatment alongside platinum-based therapy across several malignancies, including ovarian cancer.10,11 Paclitaxel prevents cell division by stabilizing β-tubulin heterodimers within microtubules and blocking depolymerization, thereby inhibiting the G2/M phase of the cell cycle, leading to cell death and stopping replication.12,13 An in vitro study demonstrated that paclitaxel dose-dependently inhibits endothelial cell proliferation, motility, invasiveness, and cord formation on Matrigel.14 However, resistance in cancer cells frequently limits paclitaxel’s efficacy. Ampelopsin and paclitaxel combination sensitizes paclitaxel-resistant ovarian cancer cells, underscoring natural compounds’ role in boosting chemotherapy effectiveness.15 Our previous study indicated that quercetin and cisplatin synergistically inhibit cervical cancer cell growth, thereby enhancing cisplatin’s antitumor effects by decreasing cell proliferation, migration, and invasion, and increasing apoptosis, possibly through MMP2, ezrin, METTL3, and P-Gp downregulation.16 Although the study by Maciejczyk17 showed that quercetin inhibited proliferation and enhances the sensitivity of ovarian cancer cells to cisplatin and paclitaxel, the synergistic effect of quercetin and paclitaxel in patients with ovarian cancer remains incompletely characterized.
Considering these findings, we aimed to establish that quercetin and paclitaxel combination therapy presents a promising approach for treating patients with ovarian cancer. We here aimed to evaluate the potential synergistic effect of combined quercetin and paclitaxel treatment on various aspects of ovarian cancer cell growth, invasion, migration, and apoptosis. We specifically examined the underlying mechanisms of this combination therapy in the A2780 and SKOV3 cell lines, which are associated with ovarian cancer. Our findings offer new insights into the mechanistic basis of this therapeutic approach for patients with ovarian cancer.
Results
Quercetin and paclitaxel combination demonstrated enhanced cytotoxic effects in A2780 and SKOV3 cell lines
Cell Counting Kit-8 (CCK-8) assays were employed to evaluate the reduction in cell viability in A2780 and SKOV3 cells caused by quercetin and paclitaxel, aiming to investigate their cytotoxic effects. Both quercetin and paclitaxel showed dose-dependent and time-dependent inhibitions of cell viability in both cell lines, as indicated by the CCK-8 assay results. The IC50 values for quercetin and paclitaxel in A2780 and SKOV3 cells are displayed in Figures 1A–1D. We analyzed the survival rate of A2780 and SKOV3 cells after exposing them to different quercetin and paclitaxel concentrations to evaluate the possible combined effects of quercetin and paclitaxel. To determine the combination index (CI) values, the CompuSyn software and CCK-8 assays were used; all drug combinations indicated synergistic effects (CI < 1) (Table 1). Notably, the combination of 40 μM quercetin and 3 nM paclitaxel exhibited a significant effect on cell viability for A2780 cells (Figure 1E), with a CI value of 0.62. The optimal synergistic effect for SKOV3 cells (Figure 1F) was achieved through the combination of 15 μM quercetin and 6 nM paclitaxel, resulting in a CI value of 0.57. Consequently, subsequent experiments employed the aforementioned concentrations of each drug. Further, we assessed cell viability 24 h after treatment; treatments had no significant effect on cell viability, supporting the specificity of migration and invasion effects (Figure S1).
Figure 1.
The effects of quercetin and paclitaxel on the viability of ovarian cancer cells
(A and B) A2780 and SKOV3 were exposed to quercetin with various concentrations (0, 25, 50, 100, and 200 μM) for 24 h and 48 h, respectively.
(C and D) A2780 and SKOV3 were exposed to paclitaxel with various concentrations (0, 2, 4, 8, and 16 nM in A2780 and 0, 2, 10, 50, and 250 nM in SKOV3) for 24 h and 48 h, respectively.
(E) A2780 was exposed to combination of various concentrations of quercetin (0, 20, 40, and 80 μM) and 3 nM paclitaxel for 48 h.
(F) SKOV3 was exposed to combination of various concentrations of quercetin (0, 7.5, 15, and 30 μM) and 6 nM paclitaxel for 48 h. Data are represented as mean ± SD (n = 3). ∗ indicates p < 0.05 vs. the control group, determined by one-way ANOVA followed by the least significant difference (LSD) post hoc test for multiple comparisons.
Table 1.
Combined index data on combination treatment of quercetin and paclitaxel in ovarian cancer cells
| Paclitaxel (nM) | Cell | Quercetin (μM) | CI value |
|---|---|---|---|
| Paclitaxel (6) | SKOV3 | 7.5 | 0.52 |
| 15 | 0.57 | ||
| 30 | 0.72 | ||
| Paclitaxel (3) | A2780 | 20 | 0.73 |
| 40 | 0.62 | ||
| 80 | 0.91 |
A2780 and SKOV3 cell apoptosis was enhanced by quercetin combined with paclitaxel
Flow cytometry was employed for examining the effects of quercetin on paclitaxel-induced apoptosis of ovarian cancer, using Annexin-V/PI double-staining analysis. Compared with the control group, the presence of quercetin increased the rate of cell apoptosis in A2780 and SKOV3 cells (Figure 2). Furthermore, compared with the control or single-drug groups, quercetin combined with paclitaxel exhibited an accelerated apoptosis rate in A2780 cells (Figures 2A and 2C) and SKOV3 cells (Figures 2B and 2D). These findings suggested that quercetin can enhance the paclitaxel-mediated apoptosis of ovarian cancer cells.
Figure 2.
Quercetin elevated the effect of paclitaxel on the apoptosis of ovarian cancer cells
A2780 (A) and SKOV3 (B) cells were treated with control (complete culture medium), quercetin (40 μM for A2780 and 15 μM for SKOV3), paclitaxel (3 nM for A2780 and 6 nM for SKOV3), or the cotreatment of quercetin and paclitaxel. The data of A2780 (C) and SKOV3 (D) are represented as mean ± SD (n = 3). ∗∗ indicates p < 0.01, ∗∗∗ indicates p < 0.001, determined by one-way ANOVA followed by the LSD post hoc test for multiple comparisons.
Effects of paclitaxel on A2780 and SKOV3 cell migration and invasion were improved by quercetin
Separate transwell chambers were employed for evaluating cell migration. The migration of A2780 and SKOV3 cells was effectively hindered by paclitaxel and quercetin, respectively (p < 0.05; Figure 3). Furthermore, the combination groups demonstrated a further decrease in migration compared with the paclitaxel group in A2780 cells (Figure 3C). To assess ovarian cancer cell invasion, Matrigel-containing transwell chambers were used. A2780 or SKOV3 cells were exposed to either a single substance or a combination of drugs and subsequently placed in the transwell chamber with the matrix gel for 24 h. Of note, quercetin or paclitaxel alone exhibited a marked suppression of cell invasion in A2780 and SKOV3 cells, respectively (p < 0.05; Figure 4). Moreover, compared with quercetin alone, the invasion ability of A2780 and SKOV3 cells exhibited a notable decrease when quercetin and paclitaxel were present (p < 0.05; Figure 4). Moreover, compared with the paclitaxel group, the combination groups demonstrated a further decrease in the invasion ability of SKOV3 cells (Figure 4). This finding indicates a synergistic effect on diminishing the invasiveness and mobility of ovarian cancer cells.
Figure 3.
Quercetin enhanced the effect of paclitaxel on the migration of ovarian cancer cells
A2780 (A) and SKOV3 (B) cells were treated with control (complete culture medium), quercetin (40 μM for A2780 and 15 μM for SKOV3), paclitaxel (3 nM for A2780 and 6 nM for SKOV3) or the cotreatment of quercetin and paclitaxel. The data of A2780 (C) and SKOV3 (D) are represented as mean ± SD (n = 3). ∗ indicates p < 0.05, ∗∗ indicates p < 0.01; ∗∗∗ indicates p < 0.001, ∗∗∗∗ indicates p < 0.0001, determined by one-way ANOVA followed by the LSD post hoc test for multiple comparisons.
Figure 4.
Quercetin enhanced the effect of paclitaxel on the invasion of ovarian cancer cells
A2780 (A) and SKOV3 (B) cells were treated with control (complete culture medium), quercetin (40 μM for A2780 and 15 μM for SKOV3), paclitaxel (3 nM for A2780 and 6 nM for SKOV3), or the cotreatment of quercetin and paclitaxel. The data of A2780 (C) and SKOV3 (D) are represented as mean ± SD (n = 3). ∗ indicates p < 0.05, ∗∗ indicates p < 0.01, ∗∗∗ indicates p < 0.001, ∗∗∗∗ indicates p < 0.0001, determined by one-way ANOVA followed by the LSD post hoc test for multiple comparisons.
Differential response of IOSE-80 cells to quercetin and paclitaxel treatment compared with ovarian cancer cells
Compared with the paclitaxel group, the viability of IOSE-80 cells was not significantly influenced by the combination therapy (Figure 5A). No significant difference in the apoptosis levels of IOSE-80 cells was noted between the paclitaxel and combination therapy groups at A2780 drug concentrations (Figure 5B). Specifically, combination therapy significantly reduced IOSE-80 cell migration compared with paclitaxel alone under A2780 drug concentrations. Nevertheless, no notable disparity in IOSE-80 cell migration was observed between the paclitaxel and combination therapy groups under SKOV3 drug concentrations (Figure 5C). Conversely, under A2780 treatment concentrations, the IOSE-80 cell invasion did not significantly differ between the paclitaxel and combination therapy groups. However, combination therapy significantly reduced the IOSE-80 cell invasion compared with paclitaxel alone under SKOV3 drug concentrations (Figure 5D). Our findings revealed that the effects of quercetin and paclitaxel on the growth of normal cells are considerably lower than those in the ovarian cancer cell lines A2780 and SKOV3. Further, the inhibitory effects of quercetin-paclitaxel cotreatment on IOSE-80 cell apoptosis, migration, and invasion may depend on the specific drug concentration.
Figure 5.
The effects of quercetin and paclitaxel on the IOSE-80 cells
(A) The effects of quercetin and paclitaxel on the viability of IOSE-80 cells.
(B) The effects of quercetin and paclitaxel on the apoptosis of IOSE-80 cells.
(C) The effects of quercetin and paclitaxel on the migration of IOSE-80 cells.
(D) Quercetin enhanced the effect of paclitaxel on the invasion of IOSE-80 cells. ISOE-A2780 means IOSE-80 cells treated with A2780 drug concentration; ISOE-SKOV3 means IOSE-80 cells treated with SKOV3 drug concentration. The data of IOSE-80 with A2780 drug concentration and SKOV3 drug concentration are represented as mean ± SD (n = 3). ∗ indicates p < 0.05, ∗∗ indicates p < 0.01, ∗∗∗ indicates p < 0.001, ∗∗∗∗ indicates p < 0.0001, determined by one-way ANOVA followed by the LSD post hoc test for multiple comparisons.
Synergistic inhibition of tumor growth by quercetin and paclitaxel in vivo
To ascertain the potential synergistic effects of quercetin and paclitaxel in BALB/c nude mice, the mice were allocated into distinct treatment groups upon reaching a tumor size of approximately 60–100 mm3. To determine whether quercetin and paclitaxel synergistically act in BALB/c nude mice, the groups received either saline, quercetin, and paclitaxel or a combination of the two drugs. Notably, the cotreatment group exhibited a significant deceleration in tumor growth compared with the other three groups (Figure 6).
Figure 6.
In vivo anticancer activity of quercetin or paclitaxel alone or combination against the SKOV3 cancer-bearing mice
Tumor gross picture (A); body weight curves (B); cancer growth curves (C); comparison of tumor weight (D); and volumes (E) after treatment. Data are represented as mean ± SD (n = 4), ∗ indicates p < 0.05, ∗∗ indicates p < 0.01, ∗∗∗ indicates p < 0.001, determined by one-way ANOVA followed by the LSD post hoc test for multiple comparisons.
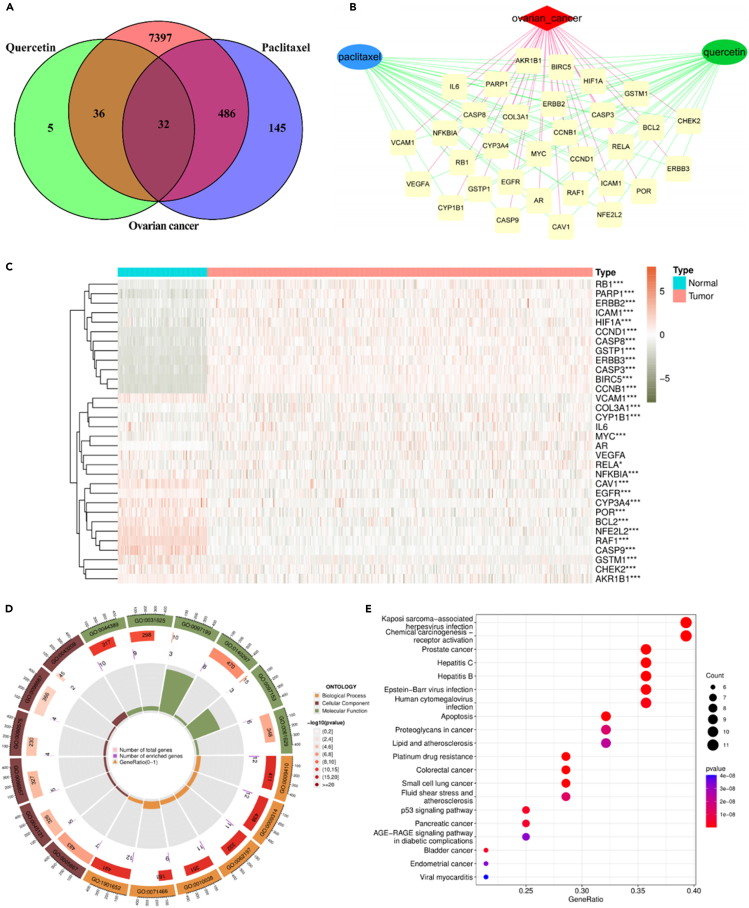
Candidate genes associated with quercetin/paclitaxel and ovarian cancer
The discovery of 73 quercetin-associated genes via the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP), along with the identification of 663 paclitaxel-related genes from SuperTarget, Kyoto Encyclopedia of Genes and Genomes (KEGG), Comparative Toxicogenomics Database (CTD), and DrugBank databases after an extensive search, filtering, and deduplication procedure, offers significant understanding into the molecular mechanisms contributing to the observed synergistic effect. Furthermore, the identification of 7,951 ovarian cancer-associated genes was accomplished through an extensive search of the Therapeutic Target Database (TTD), KEGG, Online Mendelian Inheritance in Man (OMIM), and GeneCards databases (Table S1). To investigate the mechanism behind the combined impact of quercetin and paclitaxel on ovarian cancer treatment, a thorough compilation of 32 genes that interact with each other was performed and identified as potential candidate genes (Figure 7A; Table S2). To construct a network of quercetin/paclitaxel and ovarian cancer candidate target genes, the Cytoscape software 3.8.0 was employed (Figure 7B). Subsequently, 29 genes exhibited differential expression between ovarian cancer tissues and paracancer tissues (Figure 7C; Table S3).
Figure 7.
Exploring the common targets in quercetin, paclitaxel, and ovarian cancer
(A) Venn map of intersection targets in quercetin, paclitaxel, and ovarian cancer. The orange circle and blue circle indicate the targets of quercetin and paclitaxel, respectively. The purple circle indicates the targets of ovarian cancer.
(B) Disease-drugs network created by Cytoscape 3.8.0. The red diamond node represents ovarian cancer; the blue ellipse and green ellipse represent quercetin and paclitaxel, respectively. The yellow round nodes represent common targets.
(C) The expression changes of common targets in ovarian samples coloring the samples groups. Red stands for tumor sample from TCGA (n = 379); blue stands for normal sample from GTEx (n = 112). ∗ indicates p < 0.05; ∗∗ indicates p < 0.01, ∗∗∗ indicates p < 0.001, ∗∗∗∗ indicates p < 0.0001 vs. normal sample from GTEx, determined by Mann-Whitney U test.
(D) Circular plot for GO enrichment analysis of 29 DEGs. The first circle represents GO entries, and three kinds of GO ontologies are displayed in different colors; the second circle represents the number of total genes involved in this item, and the color is associated with the p value; the third circle represents the number of DEGs enriched in GO entries; and the last circle represents gene ratio.
(E) KEGG pathway enrichment analysis of 29 DEGs. The horizontal axis represents the gene ratio, and the vertical axis represents the KEGG pathway. The size of each sphere represents the number of genes overlapped with each KEGG pathway, and the color of each sphere represents the significance level of enrichment, as shown in the color bar.
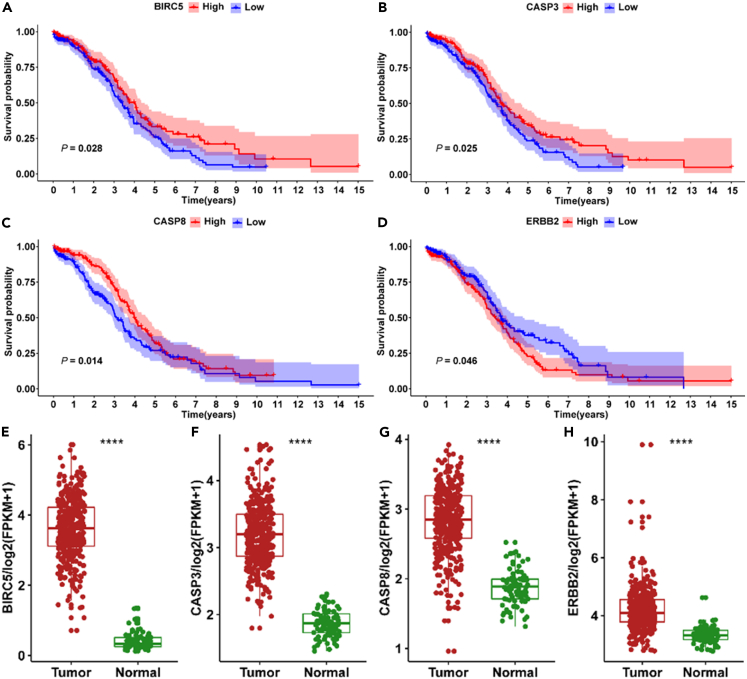
To demonstrate the biological mechanism underlying the synergistic effect of quercetin and paclitaxel in ovarian cancer treatment, Gene Ontology (GO) and KEGG analysis of 29 differentially expressed genes (DEGs) was performed using “clusterProfiler.” The results of the analysis are shown in Figures 7D and 7E, which revealed significant enrichments in the cellular components of chromosomal regions, RNA polymerase II transcription regulator complexes, and membrane rafts. The molecular function was significantly enriched in cysteine-type endopeptidase activity involved in the apoptotic process, cysteine-type endopeptidase activity involved in the apoptotic signaling pathway, and DNA-binding transcription factor. The biological process was significantly enriched in response to metal ion, cellular response to xenobiotic stimulus, and response to radiation (Table 2). The KEGG enrichment analysis revealed the potential signaling pathway through which the synergistic antitumor effect of quercetin and paclitaxel was exerted in ovarian cancer. The top 20 signaling pathways including platinum drug resistance, apoptosis, p53 signaling pathway, and proteoglycans in cancer are presented in Figure 7E. As shown in Figures 8A–8C, the Kaplan-Meier curves show that patients with ovarian cancer with increased BIRC5, CASP3, and CASP8 mRNA levels experienced extended overall survival duration, suggesting positive survival results. Conversely, high ERBB2 expression predicted unfavorable overall survival in ovarian cancer (Figure 8D). Ovarian cancer tissues showed a significant increase in BIRC5, CASP3, CASP8, and ERBB2 mRNA levels compared with paracancer tissues (p < 0.0001; Figures 8E–8H). Subsequently, BIRC5, CASP3, and ERBB2 were selected for further investigation.
Table 2.
Information on GO entries enriched by 29 differentially expressed genes
| Category | ID | Item | Adjusted p value | Gene symbols |
|---|---|---|---|---|
| Cellular component | GO:0098687 | chromosomal region | 0.033421 | CCNB1/BIRC5/PARP1/CHEK2 |
| Cellular component | GO:0090575 | RNA polymerase II transcription regulator complex | 0.009505 | NFE2L2/RB1/HIF1A/MYC |
| Cellular component | GO:0045121 | membrane raft | 0.003898 | CASP3/CASP8/CAV1/ICAM1/EGFR |
| Cellular component | GO:0098857 | membrane microdomain | 0.003898 | CASP3/CASP8/CAV1/ICAM1/EGFR |
| Cellular component | GO:0005667 | transcription regulator complex | 0.000563 | CCND1/PARP1/NFE2L2/RB1/HIF1A/MYC/RELA |
| Molecular function | GO:0097153 | cysteine-type endopeptidase activity involved in apoptotic process | 5.43E-05 | CASP3/CASP9/CASP8 |
| Molecular function | GO:0097199 | cysteine-type endopeptidase activity involved in apoptotic signaling pathway | 1.96E-05 | CASP3/CASP9/CASP8 |
| Molecular function | GO:0140297 | DNA-binding transcription factor binding | 1.96E-05 | PARP1/NFE2L2/BCL2/RB1/HIF1A/NFKBIA/MYC/RELA |
| Molecular function | GO:0031625 | ubiquitin protein ligase binding | 4.38E-08 | ERBB3/CASP8/BCL2/RB1/HIF1A/EGFR/CHEK2/NFKBIA/RELA |
| Molecular function | GO:0044389 | ubiquitin-like protein ligase binding | 5.13E-09 | CCNB1/ERBB3/CASP8/BCL2/RB1/HIF1A/EGFR/CHEK2/NFKBIA/RELA |
| Biological process | GO:0010038 | response to metal ion | 1.03E-09 | CASP3/CASP9/CCND1/PARP1/NFE2L2/CASP8/BCL2/CAV1/HIF1A/VCAM1/EGFR |
| Biological process | GO:0071466 | cellular response to xenobiotic stimulus | 1.03E-09 | GSTP1/NFE2L2/CYP3A4/RB1/POR/GSTM1/CHEK2/CYP1B1/MYC |
| Biological process | GO:0009314 | response to radiation | 7.88E-10 | CASP3/CASP9/CCND1/PARP1/BCL2/HIF1A/VCAM1/EGFR/CHEK2/MYC/COL3A1/RELA |
| Biological process | GO:0062197 | cellular response to chemical stress | 7.88E-10 | CASP3/PARP1/NFE2L2/BCL2/CAV1/HIF1A/AKR1B1/EGFR/CYP1B1/MYC/RELA |
| Biological process | GO:0009410 | response to xenobiotic stimulus | 7.74E-10 | CASP3/CCND1/GSTP1/NFE2L2/BCL2/CYP3A4/RB1/POR/GSTM1/CHEK2/CYP1B1/MYC |
Figure 8.
The sequencing data analysis of 379 ovarian cancers and 112 nontumorous tissues, and corresponding clinical data
(A–D) Kaplan-Meier analysis of overall survival based on tissue expressing the four genes including (A) BIRC5, (B) CASP3, (C) CASP8, and (D) ERBB2 in TCGA database, determined by log rank test.
(E–H) Comparative analysis of substantial expression of the four genes between ovarian cancers tissues and normal ovarian tissues. ∗∗∗∗ indicates p < 0.0001 vs. normal ovarian tissues, determined by Wilcoxon test.
Quercetin increased the effects of paclitaxel on BIRC5, CASP3, and ERBB2 protein expression levels in A2780 and SKOV3 cells
To clarify the contribution of quercetin combined with paclitaxel in ovarian cancer, we evaluated BIRC5, CASP3, and ERBB2 protein expression levels in A2780 and SKOV3 cells. The cotreatment group exhibited notably reduced BIRC5 and ERBB2 expressions compared with the control and paclitaxel groups (Figure 9). Conversely, the cotreatment group displayed a significant increase in CASP3 levels (p < 0.05; Figures 9 and S2). To investigate the roles of ERBB2, BIRC5, and CASP3 in ovarian cancer cells, cell lines with ERBB2 and BIRC5 knockdown and CASP3 overexpression were separately established in SKOV3 cells (Figure S3A). The use of both quercetin and paclitaxel in treatment resulted in a marked decrease in cell viability in cell lines with ERBB2 knockdown, BIRC5 knockdown, and CASP3 overexpression, compared with the control cells (Figure S3). Furthermore, compared with the control cells, the movement of cells was diminished in cells where ERBB2 and BIRC5 were suppressed. However, the inhibitory effects of cell migration and invasion ability were not statistically different between control cells and CASP3 overexpression cell lines when treated with the quercetin and paclitaxel combination (Figures S4 and S5). The results suggested that quercetin could increase paclitaxel toxicity by reducing BIRC5 and ERBB2 protein levels while increasing CASP3. The findings of the present study offered compelling evidence for substantiating the crucial role of quercetin synergized with paclitaxel in ovarian cancer treatment (Figure 10).
Figure 9.
Quercetin downregulates the expression levels of BIRC5 and ERBB2 and upregulates CASP3 proteins in A2780 and SKOV3
(A) Immunoblot of BIRC5, cleaved-CASP3, CASP3, GAPDH, ERBB2, and Vinculin in A2780 and SKOV3 cells. GAPDH and Vinculin are used as a loading control.
(B–G) Densitometry for quantitation of relative differences in band intensity of BIRC5, CASP3 normalized to GAPDH, and ERBB2 normalized to Vinculin in (A). Data are represented as mean ± SD (n = 3). ∗ indicates p < 0.05, ∗∗ indicates p < 0.01, ∗∗∗ indicates p < 0.001, ∗∗∗∗ indicates p < 0.0001, determined by one-way ANOVA followed by the LSD post hoc test for multiple comparisons.
Figure 10.
Schematic illustration depicting the effect and mechanism of the synergistic effect of quercetin and paclitaxel on ovarian cancer
The combination of quercetin and paclitaxel can promote cell apoptosis and inhibit cell invasion and migration, and the synergistic effect is completed through downregulated ERBB2 and BIRC5 expression and upregulated CASP3 expression in ovarian cancer cells.
Discussion
By targeting key genes in a synergistic or additive manner, the combination of anticancer drugs has been shown to improve efficacy compared with monotherapy. The findings of this study confirm that quercetin and paclitaxel combination therapy effectively boosts cell apoptosis while inhibiting cell proliferation, migration, and invasion in ovarian cancer cells. Following a comprehensive screening of multiple databases including the TCMSP, SuperTarget, KEGG, CTD, DrugBank, TTD, DisGeNET, OMIM, GeneCards, The Cancer Genome Atlas (TCGA), and Genotype-Tissue Expression (GTEx), 32 genes were identified as potential candidates associated with quercetin/paclitaxel and ovarian cancer. Notably, survival analysis revealed that CASP3, CASP8, BIRC5, and ERBB2 were the key target genes. The proposed mechanism of action involves CASP3 upregulation and BIRC5 and ERBB2 downregulation.
In patients with ovarian cancer, metastasis is a primary contributor to treatment failure and unfavorable prognosis. Our findings align with the expanding literature that advocates for the therapeutic benefits of drug amalgamation, specifically quercetin and paclitaxel combination therapy, which surpasses the monotherapy approach. Of note, quercetin has been demonstrated to specifically enhance the responsiveness of cancer cells to radiotherapy and chemotherapy.18 Quercetin reduces ERBB2 expression in colon cancer, potentially downregulating ERBB2/AKT signaling and sensitizing ovarian tumors to paclitaxel owing to its role in chemoresistance.19 Similarly, dihydromyricetin, another natural flavonoid, increases paclitaxel’s chemosensitivity by targeting the antiapoptotic protein BIRC5.20 The study by Li et al. indicated that a quercetin-paclitaxel combination elevates cleaved-CASP3 levels, suggesting enhanced apoptosis as a synergistic mechanism.21 Moreover, previous studies reported the combined inhibitory effect of quercetin and cisplatin on cervical cancer cells.16,22 The present study revealed a decrease in the invasiveness and metastatic capacity of ovarian cancer cells upon quercetin and paclitaxel coadministration. As a result of its anticancer properties, quercetin, a traditional Chinese medicine, holds promise as a therapeutic agent for gynecological malignancies.
The survivin protein, a member of the inhibitor of apoptosis family, is produced by the BIRC5 gene and is recognized for its ability to hinder apoptosis and stimulate cell growth.23 In the last two decades, several studies showed the significant presence of BIRC5 in most cancer cells.16 A previous study reported that patients with ovarian cancer exhibiting positive BIRC5 expression were at a greater risk of chemoresistance and higher mortality rates than those with negative BIRC5 expression.24 Additionally, the study by Chen25 demonstrated that surviving short hairpin RNA-mediated silencing can effectively suppress BIRC5 expression, promote apoptosis, and augment the sensitivity of ovarian cancer cells to paclitaxel. In our study, we observed an elevation in the BIRC5 expression level in ovarian cancer, which is consistent with previously published findings. Nevertheless, the relationship between BIRC5 and the clinical results in patients with ovarian cancer remains uncertain. The study by Ferrandina26 revealed that cytoplasmic and nuclear BIRC5 status does not significantly affect time to progression or overall survival curves in patients with ovarian cancer. Conversely, multiple studies have demonstrated that positive BIRC5 expression in ovarian cancer is linked to unfavorable clinical outcomes.25,27 However, decreased BIRC5 levels are associated with unfavorable outcomes in breast and gastric cancer.28 Additionally, decreased BIRC5 expression in patients with prostate cancer following prostatectomy is linked to less favorable outcomes.29 Furthermore, in patients with ovarian carcinoma with prechemotherapy effusions, higher BIRC5 nuclear expression is correlated with improved progression-free and overall survival.30 Moreover, our study indicated that BIRC5 expression is associated with favorable survival in ovarian cancer. BIRC5 depletion enhances the sensitivity of OVCAR3 ovarian cancer cells to paclitaxel by a factor of 15, while having no impact on carboplatin resistance.25 BIRC5 has been implicated in conferring chemoresistance to both platinum-based25 and taxane-based chemotherapy31 in ovarian cancer. A comparative analysis was conducted to examine BIRC5 expression in patients with ovarian cancer who received either platinum/cyclophosphamide or taxane/platinum treatment. The findings indicated that a high nuclear BIRC5 expression level, combined with TP53 accumulation in tumor cells, was linked to a reduced likelihood of recurrence and mortality in patients treated with taxane/platinum.32 When quercetin and paclitaxel were administered in combination, our findings indicated that BIRC5 knockdown effectively decreased cell viability, migration, and invasion in SKOV3 ovarian cancer cells. These investigations imply that the involvement of BIRC5 in cancer is complex, particularly in the context of ovarian cancer.
ERBB2 (HER2) is responsible for a component of the receptor tyrosine kinase group of epidermal growth factor receptors, playing a vital role in organizing the complex ERBB signaling system that controls cellular growth and specialization. Yu33 discovered that MAPK1/MAPK3 signaling pathway inhibition through ERBB2 expression suppression can potentially impede ovarian cancer cell proliferation, invasion, and migration. The incidence of ERBB2 overexpression in ovarian carcinomas ranges from 19% to 44%34 and is associated with advanced stages, poorly differentiated tumors, chemoresistance, and reduced survival rates.35 Considering the relatively frequent ERBB2 overexpression/amplification occurrence, several anti-ERBB2 agents have been explored as potential treatments for ovarian cancer.36,37 Our results indicated that ovarian cancer exhibits ERBB2 overexpression, which is linked to an unfavorable prognosis. This strengthens the existing evidence supporting ERBB2 as a viable target for molecular therapy in ovarian cancer.
Furthermore, CASP3, a well-known apoptotic executor, is triggered by initiator CASP8 or CASP9 and subsequently cuts multiple functionally vital proteins inside the cell, ultimately resulting in apoptosis.38 This mechanism has been used in various anticancer therapies, including cytotoxic drugs, radiotherapy, and immunotherapy to induce tumor cell death. Thus, CASP3 activation has been widely regarded as a surrogate indicator of cancer treatment effectiveness. Recent evidence has demonstrated that CASP3 and CASP8 exhibit multifaceted involvement in cancer. The nonapoptotic functions of caspases can be exploited by cancer cells to facilitate cell survival, proliferation, migration, and metastasis.39 For instance, CASP3 exerts its influence on cell proliferation by modulating cell-cycle checkpoints and cleaving cell-cycle regulators, such as p27Kip1.40 Additionally, CASP3 functions as a discerning agent of extracellular stress, thereby dictating cellular fate in response to stimuli, such as ultraviolet radiation.41 CASP8 expression is paradoxically upregulated in colorectal, cervical, and renal cancers compared with normal tissues, as indicated by a previous study.42 This upregulation leads to the promotion of angiogenesis, inflammation, and resistance to therapy through the sustained nuclear factor κB activation, as supported by two separate studies.43,44 Our study noted that treatment with quercetin and paclitaxel combination significantly reduced cell viability in CASP3 overexpression cell lines compared with control cells. These findings imply that the tumor-suppressive functions of CASP3 and CASP8 are not absolute but rather contingent on the specific context. In ovarian cancer, the heightened expression of these caspases may still be associated with a more favorable prognosis. Our observations suggested that the cotreatment group exhibited CASP3 upregulation and BIRC5 and ERBB2 protein level downregulation compared with the control and paclitaxel groups. Therefore, the involvement of ERBB2, BIRC5, and CASP3 in the process of quercetin-paclitaxel synergy-induced apoptosis in ovarian cancer cells appears highly likely.
In conclusion, the results of this study showed that quercetin and paclitaxel combination therapy has a synergistic effect on ovarian cancer cells, thereby leading to increased cell death and reduced cell movement and invasion, compared with using paclitaxel alone. Cotreatment results in the expression of genes, including ERBB2 and BIRC5 being downregulated and CASP3 being upregulated, associated with enhanced chemosensitivity by ovarian cancer cells.
Limitations of the study
In this article, we demonstrate how quercetin synergized with paclitaxel in ovarian cancer cells. However, the observed low apoptosis rate in SKOV3 cells following combination therapy suggests a potential need to optimize the assay or evaluate the apoptotic pathways more thoroughly. Future studies exploring the in vivo mechanism of this cotreatment on tumor cell inhibition are warranted. Moreover, to examine the potential negative effects of quercetin on human participants, additional investigations are required. Additionally, to elucidate the signaling pathways implicated in the antitumor properties of quercetin in ovarian cancer, further exploration of the underlying mechanisms is necessary.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Cleaved-CASP3 | Cell Signaling Technology | Cat# 9661; RRID: AB_2341188 |
| Anti-CASP3 | Cell Signaling Technology | Cat# 14220; RRID: AB_2798429 |
| Anti-ERBB2 | Cell Signaling Technology | Cat# 4290; RRID: AB_10557104 |
| Anti-BIRC5 | Proteintech | Cat# 10508-1-AP; RRID: AB_2064048 |
| Anti-GAPDH | Proteintech | Cat# 60004-1-Ig; RRID: AB_2107436 |
| Anti-vinculin | Affinity Biosciences | Cat# AF5122; RRID: AB_2837608 |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM medium | Gibco | #11965092 |
| RPMI 1640 medium | Invitrogen | #C11875500BT |
| FBS | Gibco | #10099141 |
| quercetin | Sigma | #Q4951 |
| paclitaxel | MedChemExpress | #HY-B0015 |
| Critical commercial assays | ||
| Cell Counting Kit-8 | Meilunbio | MA0218 |
| FITC Annexin-V Apoptosis Detection Kit I | BD Biosciences | #556547 |
| Software and algorithms | ||
| CompuSyn | ComboSyn, Inc. | https://www.combosyn.com/ |
| GraphPad Prism | GraphPad | 6.0 |
| R | R Foundation for Statistical Computing | 4.2.1 |
| Cytoscape | NIGMS | 3.8.0 |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Other | ||
| Transcriptomic data of ovarian samples | TCGA | https://portal.gdc.cancer.gov/ |
| Transcriptomic data of normal human ovarian tissues | GTEx | https://www.gtexportal.org/ |
| Quercetin-related target genes | TCMSP | https://tcmspw.com/ |
| paclitaxel-related target genes | SuperTarget | https://insilico.charite.de/supertarget/ |
| KEGG | https://www.genome.jp/kegg/ | |
| CTD | https://ctdbase.org/ | |
| DrugBank | https://go.drugbank.com/ | |
| Ovarian cancer- related target genes | TTD | https://db.idrblab.org/ttd/ |
| KEGG | https://www.genome.jp/kegg/ | |
| DisGeNET | https://www.disgenet.org/ | |
| OMIM | https://www.omim.org/ | |
| GeneCards | https://www.genecards.org/ | |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Xueqiong Zhu (wzzxq@wzhealth.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Data: This paper analyzes existing, publicly available data. These accession websites for the datasets are listed in the key resources table. Original western blot images have been included as supplemental information figure.
-
•
Code: This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental models and study participant details
Cell lines
For our experiments, we grew IOSE-80, A2780 and SKOV3 cells in DMEM (Gibco, Grand Island, NY, USA) and RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) with the addition of 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA). Cells were subsequently incubated at 37°C in a 5% CO2 incubator.
Animals
Six-week-old female Balb/c nude mice were maintained under specific pathogen-free conditions with a 12 h light-dark cycle. The Animal Care and Use Committee of Wenzhou Medical University in China approved all animal care practices and research (number wydw2024-0050).
Method details
Reagents
To produce a 200 mM stock solution, quercetin (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) and subsequently stored at −20°C. In a complete medium, a quercetin solution with a 200 μM concentration was prepared and used in cell viability assays for A2780 and SKOV3 cells, with various concentrations (0, 25, 50, 100, and 200 μM). To produce a 5 mM stock solution, paclitaxel (MedChemExpress, Monmouth Junction, NJ, USA) was dissolved in DMSO. A complete medium was used to prepare a working concentration of 5 μM paclitaxel, which was subsequently applied at varying concentrations (0, 2, 4, 8, and 16 nM) in an A2780 cell viability assay. In a separate SKOV3 cell viability assay, various paclitaxel concentrations (0, 2, 10, 50, and 250 nM) were employed. Cell Signaling Technology (Danvers, MA, USA) provided the antibodies for Cleaved-CASP3 (#9661), CASP3 (#14220), and ERBB2 (#4290), whereas Proteintech (Rosemount, IL, USA) supplied the antibodies against BIRC5 (#10508-1-AP) and GAPDH (#60004-1-Ig). Affinity Biosciences (Jiangsu, China) provided the antibody against vinculin (#AF5122).
Cell viability assay and drug combination analysis
SKOV3 and IOSE-80 cells were plated at a density of 5 × 103 per well, and A2780 were plated at a density of 3 × 103 cells per well in 96-well dishes. All cells were left to attach overnight. Subsequently, the original medium was replaced with a complete medium containing varying quercetin or paclitaxel concentrations for 24 and 48 h, respectively. Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay from Meilunbio, China. To determine the percentage of viable cells, the absorbance at 450 nm was measured using a Microplate Reader (Bio-Tek Instruments, Winooski, VT, USA). In the cell viability assay, we selected the IC25-30 of quercetin on A2780 and SKOV3 cells to combine with paclitaxel. CompuSyn software by the Chou-Talalay method was used to evaluate the combination effect between quercetin and paclitaxel,45 with combination indexes (CI) of <1 indicating synergistic effects. Finally, the experiments had the following four groups: control, quercetin, paclitaxel, and cotreatment groups.
Flow cytometry analysis cell apoptosis measurement
The FITC Annexin-V Apoptosis Detection Kit I (BD Biosciences, Franklin Lakes, NJ, USA) was used to determine the cell apoptosis percentage. The appropriate number of cells was placed in six-well plates and left to grow for 24 h. Subsequently, they were exposed to different treatments, including complete culture medium, quercetin, paclitaxel, or a quercetin and paclitaxel combination for 48 h. Next, cells were mixed with prechilled 1 × binding buffer and then stained with FITC and PI for 15 min at room temperature without any light. The samples obtained were examined using CytoFLEX flow cytometry (Beckman Coulter, Fullerton, CA, USA) to ascertain the proportion of cells displaying FITC+/PI− and FITC+/PI + traits, which indicate early and late apoptosis, respectively.
Transwell invasion and migration assay
Different treatments were applied to the IOSE-80, A2780 and SKOV3 cell lines, including complete culture medium, quercetin, paclitaxel, or a quercetin and paclitaxel combination, for 24 h. Following the treatment, cells were placed in the upper chamber of a transwell with an 8 μm pore size. The densities of cells used for migration and invasion assays were as follows: for A2780, 1.5 × 105/well for migration and 2.5 × 105/well for invasion; for SKOV3, 3 × 104/well for both migration and invasion; and IOSE-80, 8 × 104/well for migration and 1.6× 105/well for invasion. The upper chamber was either uncoated or precoated with Matrigel. Afterward, 100 μL of the medium was introduced into the upper chamber, whereas 600 μL of the culture medium containing 10% FBS was placed in the lower chamber to promote cell migration. After incubating for 24 h at 37°C in a 5% CO2 atmosphere, transwell chambers were treated with 4% paraformaldehyde for fixation and subsequently stained with 0.1% crystal violet. The cells on the top side of the transwell membrane were eliminated using a cotton swab, whereas those that had moved to the bottom side were observed and recorded using microscopy. Counting cells in five randomly selected fields determined the mean number of cells that successfully crossed the membrane.
Establishment of a database of drug target genes and disease-related genes
Quercetin-related target genes were identified using the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://tcmspw.com/).46 Furthermore, the gene targets of paclitaxel were explored by analyzing SuperTarget (https://insilico.charite.de/supertarget/),47 Kyoto Encyclopedia of Genes and Genomes (KEGG, https//www.genome.jp/kegg/),48 Comparative Toxicogenomics Database (CTD, https://ctdbase.org/),49 and DrugBank (https://go.drugbank.com/).50 Ovarian cancer-associated target genes were identified by searching various databases, including the Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/),51 KEGG, DisGeNET (https://www.disgenet.org/),52 Online Mendelian Inheritance in Man (OMIM, https://www.omim.org/), and GeneCards database (https://www.genecards.org/).53 To screen for cancer-associated targets, a search was performed using the keyword “ovarian cancer”. Duplicate targets were eliminated to identify all targets linked to ovarian cancer.
Integration of candidate targets of drugs for disease
Potential targets for ovarian cancer treatment were discovered by analyzing the overlap between quercetin/paclitaxel-associated targets and ovarian cancer-associated targets. The identification process involved the use of the “Venn diagram” R package and the visualization of the candidate targets using the Cytoscape software.
Screening prognostic-related genes on the basis of public database
To ascertain which genes demonstrated variable expression levels between ovarian cancer and normal ovarian tissues, differential expression analysis was subsequently performed. To obtain the necessary RNA-seq and clinical profiles for The Cancer Genome Atlas (TCGA)-ovarian samples (https://portal.gdc.cancer.gov/), the Data Transfer Tool (GDC Apps) was used. Overall, 379 patients with ovarian cancer with RNA-seq profiles were screened within the TCGA ovarian cohort. Owing to the absence of normal tissue samples in the TCGA ovarian cohort, we augmented our sample size by procuring 112 RNA-seq profiles of normal human ovarian tissues from the Genotype–Tissue Expression (GTEx) dataset (https://www.gtexportal.org/).54 In both TCGA and GTEx datasets, the expression profiles underwent log2 transformation (fragments per kilobase of transcript per million mapped reads +1). After integrating transcriptomic data from both datasets, batch effects were removed using the normalizeBetweenArray function of the R software’s “LIMMA” package. In a stepwise manner, we extracted the gene expression data of common genes associated with quercetin/paclitaxel and ovarian cancer from the merged transcriptomic data. To detect differentially expressed genes (DEGs) between ovarian cancer and normal tissue samples, the LIMMA software package was employed, with a significance threshold of p < 0.05. Afterward, the R packages “enrichplot” and “clusterProfiler”55 were used to perform Gene Ontology (GO) functional analysis and KEGG pathway enrichment analysis. Regarding functionality, the DEGs were categorized into three primary domains by GO, encompassing cellular component (CC), molecular function (MF), and biological process (BP). Using KEGG pathway enrichment analysis, the potential biological mechanisms of DEGs were identified. To narrow our investigation to DEGs with potential clinical significance, prognostic data from patients with ovarian cancer was incorporated. Eventually, prognostic-related DEGs were identified using Kaplan–Meier curves.
Western blot analysis
Following lysis with RIPA buffer containing 1% phenylmethanesulfonyl fluoride (Boster Biological Technology), the resulting cell lysates were subjected to separation on SDS–PAGE gels and subsequently transferred onto PVDF membranes. Next, the membranes were obstructed using 5% milk for 1.5 h at ambient temperature. Subsequently, primary antibodies were applied and left overnight at 4°C for immunoblotting. The primary antibodies used included BIRC5 (diluted 1:1,000), cleaved-CASP3 (diluted 1:1,000), CASP3 (diluted 1:1,000), ERBB2 (diluted 1:1,000), GAPDH (diluted 1:5,000), and vinculin (diluted 1:2,000). Afterward, the membranes were incubated with secondary antibodies that were linked to affinipure goat anti-Rabbit IgG or goat anti-mouse IgG at a concentration of 1:5,000 and subsequently observed using imaging systems.
Mouse studies
To validate the in vitro findings, xenograft studies were conducted. SKOV3 ovarian cancer cells (1.5 × 106 cells/100 μL) were subcutaneously injected into the rear flank of a 6-week-old female Balb/c nude mice. After tumors grew to a size of 50 mm3, mice were randomly divided into four groups (n = four mice per group) and administered the following treatments: saline (control), quercetin (50 mg/kg, administered intraperitoneally daily), paclitaxel (15 mg/kg, administered intraperitoneally every 3 days), or a combination of both. Tumor volume and body weight were measured twice weekly. After 15 days, mice were euthanized, and tumors were excised and weighed.
Quantification and statistical analysis
The “survival” package was used to perform Kaplan–Meier curves with the log rank test. To analyze differences between two groups, two-tailed Student’s t-tests were performed. For multiple groups, one-way analysis of variance (ANOVA) was used when data met assumptions of normality and equal variances, followed by post hoc comparisons using the Least Significant Difference (LSD) method. For analysis involving two independent variables, two-way ANOVA was performed, followed by post hoc comparisons using the Dunnett’s method. For non-normally distributed data or when variances were unequal, appropriate non-parametric tests such as the Mann–Whitney U-test (for two groups) or the Kruskal-Wallis test (for more than two groups) were employed. Figures were generated using R version 4.2.1 and Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA). Data are represented as mean ± SD. p-values of <0.05 were considered statistically significant.
Acknowledgments
This work was supported by Zhejiang Key Laboratory of Traditional Chinese Medicine for Diagnosis and Treatment of Gynecological Cancers (2022-11). The graphical abstract and Figure 8 were created with BioRender.com.
Author contributions
Conceptualization, H.J. and X.Z.; methodology, W.X., C.C., and Z.Z.; software, H.J.; validation, T.L., J.W., and Y.D.; formal analysis, H.J., H.W., and Z.Z.; investigation, Z.Z., C.C., W.X., T.L., Y.D., J.W., and X.Z.; resources, H.J.; data curation, H.J. and C.C.; writing – original draft preparation, H.J., Z.Z., and C.C.; writing – review and editing, H.J., Z.Z., C.C., W.X., T.L., Y.D., J.W., H.W., and X.Z.; visualization, H.J., Z.Z., H.W., and T.L.; supervision, X.Z.; project administration, H.J. and X.Z.; funding acquisition, X.Z.. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: July 1, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110434.
Supplemental information
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA. Cancer J. Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 3.Wong-Brown M.W., van der Westhuizen A., Bowden N.A. Targeting DNA Repair in Ovarian Cancer Treatment Resistance. Clin. Oncol. 2020;32:518–526. doi: 10.1016/j.clon.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Hussain Y., Mirzaei S., Ashrafizadeh M., Zarrabi A., Hushmandi K., Khan H., Daglia M. Quercetin and Its Nano-Scale Delivery Systems in Prostate Cancer Therapy: Paving the Way for Cancer Elimination and Reversing Chemoresistance. Cancers. 2021;13 doi: 10.3390/cancers13071602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vafadar A., Shabaninejad Z., Movahedpour A., Fallahi F., Taghavipour M., Ghasemi Y., Akbari M., Shafiee A., Hajighadimi S., Moradizarmehri S., et al. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10:32. doi: 10.1186/s13578-020-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan H., Ullah H., Martorell M., Valdes S.E., Belwal T., Tejada S., Sureda A., Kamal M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021;69:200–211. doi: 10.1016/j.semcancer.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Teekaraman D., Elayapillai S.P., Viswanathan M.P., Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019;300:91–100. doi: 10.1016/j.cbi.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Ren M.X., Deng X.H., Ai F., Yuan G.Y., Song H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015;10:579–583. doi: 10.3892/etm.2015.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong C., Yang Z., Zhang L., Wang Y., Gong W., Liu Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. OncoTargets Ther. 2018;11:17–27. doi: 10.2147/OTT.S147316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsibulak I., Zeimet A.G., Marth C. Hopes and failures in front-line ovarian cancer therapy. Crit. Rev. Oncol. Hematol. 2019;143:14–19. doi: 10.1016/j.critrevonc.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Renneberg R. Biotech History: Yew trees, paclitaxel synthesis and fungi. Biotechnol. J. 2007;2:1207–1209. doi: 10.1002/biot.200790106. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D., Yang R., Wang S., Dong Z. Paclitaxel: new uses for an old drug. Drug Des. Devel. Ther. 2014;8:279–284. doi: 10.2147/DDDT.S56801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiff P.B., Fant J., Horwitz S.B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 14.Belotti D., Vergani V., Drudis T., Borsotti P., Pitelli M.R., Viale G., Giavazzi R., Taraboletti G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin. Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]
- 15.Asnaashari S., Amjad E., Sokouti B. Synergistic effects of flavonoids and paclitaxel in cancer treatment: a systematic review. Cancer Cell Int. 2023;23:211. doi: 10.1186/s12935-023-03052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W., Xie S., Chen X., Pan S., Qian H., Zhu X. Effects of Quercetin on the Efficacy of Various Chemotherapeutic Drugs in Cervical Cancer Cells. Drug Des. Devel. Ther. 2021;15:577–588. doi: 10.2147/DDDT.S291865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maciejczyk A., Surowiak P. Quercetin inhibits proliferation and increases sensitivity of ovarian cancer cells to cisplatin and paclitaxel. Ginekol. Pol. 2013;84:590–595. doi: 10.17772/gp/1609. [DOI] [PubMed] [Google Scholar]
- 18.Pozsgai E., Bellyei S., Cseh A., Boronkai A., Racz B., Szabo A., Sumegi B., Hocsak E. Quercetin increases the efficacy of glioblastoma treatment compared to standard chemoradiotherapy by the suppression of PI-3-kinase-Akt pathway. Nutr. Cancer. 2013;65:1059–1066. doi: 10.1080/01635581.2013.810291. [DOI] [PubMed] [Google Scholar]
- 19.Kim W.K., Bang M.H., Kim E.S., Kang N.E., Jung K.C., Cho H.J., Park J.H. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT-29 human colon cancer cells. J. Nutr. Biochem. 2005;16:155–162. doi: 10.1016/j.jnutbio.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y., Wang S., Chan H.F., Lu H., Lin Z., He C., Chen M. Dihydromyricetin Induces Apoptosis and Reverses Drug Resistance in Ovarian Cancer Cells by p53-mediated Downregulation of Survivin. Sci. Rep. 2017;7 doi: 10.1038/srep46060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Huang J., Yu C., Xiang L., Li L., Shi D., Lin F. Quercetin Enhanced Paclitaxel Therapeutic Effects Towards PC-3 Prostate Cancer Through ER Stress Induction and ROS Production. OncoTargets Ther. 2020;13:513–523. doi: 10.2147/OTT.S228453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji H., Li K., Xu W., Li R., Xie S., Zhu X. Prediction of the Mechanisms by Which Quercetin Enhances Cisplatin Action in Cervical Cancer: A Network Pharmacology Study and Experimental Validation. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.780387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan S., Ferguson Bennit H., Asuncion Valenzuela M.M., Turay D., Diaz Osterman C.J., Moyron R.B., Esebanmen G.E., Ashok A., Wall N.R. Localization and upregulation of survivin in cancer health disparities: a clinical perspective. Biologics. 2015;9:57–67. doi: 10.2147/BTT.S83864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaffaroni N., Pennati M., Colella G., Perego P., Supino R., Gatti L., Pilotti S., Zunino F., Daidone M.G. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell. Mol. Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L., Liang L., Yan X., Liu N., Gong L., Pan S., Lin F., Zhang Q., Zhao H., Zheng F. Survivin status affects prognosis and chemosensitivity in epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2013;23:256–263. doi: 10.1097/IGC.0b013e31827ad2b8. [DOI] [PubMed] [Google Scholar]
- 26.Ferrandina G., Legge F., Martinelli E., Ranelletti F.O., Zannoni G.F., Lauriola L., Gessi M., Gallotta V., Scambia G. Survivin expression in ovarian cancer and its correlation with clinico-pathological, surgical and apoptosis-related parameters. Br. J. Cancer. 2005;92:271–277. doi: 10.1038/sj.bjc.6602332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian X., Xi X., Li L. Nuclear survivin is associated with malignant potential in epithelial ovarian carcinoma. Appl. Immunohistochem. Mol. Morphol. 2011;19:126–132. doi: 10.1097/PAI.0b013e3181e30dcd. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy S.M., O'Driscoll L., Purcell R., Fitz-Simons N., McDermott E.W., Hill A.D., O'Higgins N.J., Parkinson M., Linehan R., Clynes M. Prognostic importance of survivin in breast cancer. Br. J. Cancer. 2003;88:1077–1083. doi: 10.1038/sj.bjc.6600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Büscheck F., Sulimankhil M., Melling N., Hoflmayer D., Hube-Magg C., Simon R., Gobel C., Hinsch A., Weidemann S., Izbicki J.R., et al. Loss of cytoplasmic survivin expression is an independent predictor of poor prognosis in radically operated prostate cancer patients. Cancer Med. 2020;9:1409–1418. doi: 10.1002/cam4.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinberg L., Florenes V.A., Silins I., Haug K., Trope C.G., Nesland J.M., Davidson B. Nuclear expression of survivin is associated with improved survival in metastatic ovarian carcinoma. Cancer. 2007;109:228–238. doi: 10.1002/cncr.22426. [DOI] [PubMed] [Google Scholar]
- 31.Du J., Li B., Fang Y., Liu Y., Wang Y., Li J., Zhou W., Wang X. Overexpression of Class III beta-tubulin, Sox2, and nuclear Survivin is predictive of taxane resistance in patients with stage III ovarian epithelial cancer. BMC Cancer. 2015;15:536. doi: 10.1186/s12885-015-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felisiak-Golabek A., Rembiszewska A., Rzepecka I.K., Szafron L., Madry R., Murawska M., Napiorkowski T., Sobiczewski P., Osuch B., Kupryjanczyk J., Polish Ovarian Cancer Study G. Nuclear survivin expression is a positive prognostic factor in taxane-platinum-treated ovarian cancer patients. J. Ovarian Res. 2011;4:20. doi: 10.1186/1757-2215-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu T.T., Wang C.Y., Tong R. ERBB2 gene expression silencing involved in ovarian cancer cell migration and invasion through mediating MAPK1/MAPK3 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:5267–5280. doi: 10.26355/eurrev_202005_21309. [DOI] [PubMed] [Google Scholar]
- 34.Kupryjańczyk J., Madry R., Plisiecka-Halasa J., Bar J., Kraszewska E., Ziolkowska I., Timorek A., Stelmachow J., Emerich J., Jedryka M., et al. TP53 status determines clinical significance of ERBB2 expression in ovarian cancer. Br. J. Cancer. 2004;91:1916–1923. doi: 10.1038/sj.bjc.6602238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrano-Olvera A., Duenas-Gonzalez A., Gallardo-Rincon D., Candelaria M., De la Garza-Salazar J. Prognostic, predictive and therapeutic implications of HER2 in invasive epithelial ovarian cancer. Cancer Treat Rev. 2006;32:180–190. doi: 10.1016/j.ctrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Lheureux S., Krieger S., Weber B., Pautier P., Fabbro M., Selle F., Bourgeois H., Petit T., Lortholary A., Plantade A., et al. Expected benefits of topotecan combined with lapatinib in recurrent ovarian cancer according to biological profile: a phase 2 trial. Int. J. Gynecol. Cancer. 2012;22:1483–1488. doi: 10.1097/IGC.0b013e31826d1438. [DOI] [PubMed] [Google Scholar]
- 37.Bookman M.A., Darcy K.M., Clarke-Pearson D., Boothby R.A., Horowitz I.R. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003;21:283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 38.Galluzzi L., Lopez-Soto A., Kumar S., Kroemer G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity. 2016;44:221–231. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Dabrowska C., Li M., Fan Y. Apoptotic Caspases in Promoting Cancer: Implications from Their Roles in Development and Tissue Homeostasis. Adv. Exp. Med. Biol. 2016;930:89–112. doi: 10.1007/978-3-319-39406-0_4. [DOI] [PubMed] [Google Scholar]
- 40.Eymin B., Sordet O., Droin N., Munsch B., Haugg M., Van de Craen M., Vandenabeele P., Solary E. Caspase-induced proteolysis of the cyclin-dependent kinase inhibitor p27Kip1 mediates its anti-apoptotic activity. Oncogene. 1999;18:4839–4847. doi: 10.1038/sj.onc.1202860. [DOI] [PubMed] [Google Scholar]
- 41.Khalil H., Peltzer N., Walicki J., Yang J.Y., Dubuis G., Gardiol N., Held W., Bigliardi P., Marsland B., Liaudet L., Widmann C. Caspase-3 protects stressed organs against cell death. Mol. Cell Biol. 2012;32:4523–4533. doi: 10.1128/MCB.00774-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stupack D.G. Caspase-8 as a therapeutic target in cancer. Cancer Lett. 2013;332:133–140. doi: 10.1016/j.canlet.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contadini C., Ferri A., Di Martile M., Cirotti C., Del Bufalo D., De Nicola F., Pallocca M., Fanciulli M., Sacco F., Donninelli G., et al. Caspase-8 as a novel mediator linking Src kinase signaling to enhanced glioblastoma malignancy. Cell Death Differ. 2023;30:417–428. doi: 10.1038/s41418-022-01093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fianco G., Mongiardi M.P., Levi A., De Luca T., Desideri M., Trisciuoglio D., Del Bufalo D., Cina I., Di Benedetto A., Mottolese M., et al. Caspase-8 contributes to angiogenesis and chemotherapy resistance in glioblastoma. Elife. 2017;6 doi: 10.7554/eLife.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 46.Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hecker N., Ahmed J., von Eichborn J., Dunkel M., Macha K., Eckert A., Gilson M.K., Bourne P.E., Preissner R. SuperTarget goes quantitative: update on drug-target interactions. Nucleic Acids Res. 2012;40:D1113–D1117. doi: 10.1093/nar/gkr912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wixon J., Kell D. The Kyoto encyclopedia of genes and genomes--KEGG. Yeast. 2000;17:48–55. doi: 10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis A.P., Grondin C.J., Johnson R.J., Sciaky D., King B.L., McMorran R., Wiegers J., Wiegers T.C., Mattingly C.J. The Comparative Toxicogenomics Database: update 2017. Nucleic Acids Res. 2017;45:D972–D978. doi: 10.1093/nar/gkw838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z., et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y., Zhang Y., Lian X., Li F., Wang C., Zhu F., Qiu Y., Chen Y. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022;50:D1398–D1407. doi: 10.1093/nar/gkab953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piñero J., Bravo A., Queralt-Rosinach N., Gutierrez-Sacristan A., Deu-Pons J., Centeno E., Garcia-Garcia J., Sanz F., Furlong L.I. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45:D833–D839. doi: 10.1093/nar/gkw943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Safran M., Solomon I., Shmueli O., Lapidot M., Shen-Orr S., Adato A., Ben-Dor U., Esterman N., Rosen N., Peter I., et al. GeneCards 2002: towards a complete, object-oriented, human gene compendium. Bioinformatics. 2002;18:1542–1543. doi: 10.1093/bioinformatics/18.11.1542. [DOI] [PubMed] [Google Scholar]
- 54.Consortium G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: This paper analyzes existing, publicly available data. These accession websites for the datasets are listed in the key resources table. Original western blot images have been included as supplemental information figure.
-
•
Code: This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.