Abstract
Indole acetic acid (IAA) is one of the prime communicator playing a chief role in the interaction between host plant and endophytes. IAA produced by the endophytes primarily contributes to plant growth and development. Here, we optimized IAA production by an endophytic fungus Diaporthe terebinthifolli GG3F6 isolated from the asymptomatic rhizome of Glycyrrhiza glabra employing response surface methodology (RSM) and exploring its effect on the host plant biology. The methodology revealed 1.1 fold increases in IAA accumulation. The maximum IAA (121.20 μg/mL) was achieved using tryptophan substrate (1 mg/mL) in Potato dextrose broth (48 g/L) adjusted to pH 12 and incubated at 35 °C for 7 days. The significantly low p-value (p < 0.0001) of the experiment propounded that the model best fits the experimental data, and the independent variables have considerable effects on the production of IAA. Morphologically, the in-vitro grown G. glabra plants showed enhanced root and shoot growth when co-cultivated with the isolated endophytic fungal strain (GG3F6) relative to the control plants. Also, the enhanced accumulation of total phenolic (10.7 %) and flavonoid (10.2 %) in the endophyte treated plants was observed. The optimization of IAA production by an endophytic fungus using (RSM) has not been reported so far. Interestingly, 2.1 fold increase in glycyrrhizin content was recorded in GG3F6 treated in-vitro host plants as compared to the control plants. This suggested a potential use of D. terebinthifolli as a biostimulator for plant and enhanced accumulation of glycyrrhizin. The study highlights the dynamic host-endophyte interaction for exploitation in agricultural and pharmaceutical applications.
Keywords: Biostimulator, Diaporthe, Response surface methodology, Box bekhen design, Plant-microbe interaction, Glycyrrhizin, Indole acetic acid
Highlights
-
•
IAA is a master player of plant and endophyte interactions.
-
•
Endophyte increases IAA accumulation and in response increases glycyrrhizin content by 2.1 fold under in-vitro conditions.
-
•
The vital host-endophyte interaction evoked enhanced IAA production and accumulation of its bioactive secondary metabolite.
1. Introduction
Endophytes are the microorganisms asymptomatically associated with plants that benefit the host in multitude facets including the vast endogenous pool of secondary metabolites, and getting food and shelter in reward [1]. The host plant gains an advantage in various aspects encompassing growth, defense, stress, and physiology of the plant [2]. During plant-microbe interactions, phytohormones of microbial origin directly influence the physiology of the plant as in root colonization strategies adopted by the microorganisms [[3], [4], [5]]. Studies have shown plant-associated endophytes being involved in auxin production which has been demonstrated to regulate multifaceted functions in the plant [6,7]. Particularly, fungal endophytes play a crucial role in promoting plant growth and aiding in various physiological processes. They release plant growth hormones like cytokines, indole acetic acid, and gibberellins, which help in enhancing plant growth, specifically under stress conditions viz. biotic and abiotic [[8], [9], [10], [11], [12], [13]]. Additionally, they are known for their ability to improve plant promotion and contribute to processes like nitrogen fixation and phosphate solubilization [14]. In the realm of biological control, these fungi are gaining attention for their potential to enhance plant productivity. To colonize plant tissues and extract nutrients from the environment, endophytic fungi produce enzymes, which have significant applications across various industries such as food, biofuel, pharmaceuticals, and environmental remediation [15]. In recent years, there has been a growing interest in using endophytic fungi for environmental remediation, particularly in eliminating harmful pollutants like hydrocarbons, polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), radionuclides, and metals [16].
The symbiotic relationship between fungal endophytes and host plants is mutually beneficial, as both partners remain unaffected, and the advantages are dependent on the interaction between them [17,18]. Specifically, Indole Acetic Acid (IAA) has been elucidated to play a key role in the cross-talk between plant and fungal signaling during the ectomycorrhizal establishment [19,20]. The IAA has been demonstrated to be significantly effective in influencing the physiology of the plant influencing photosynthesis, growth, gravitropism, biosynthesis of phenylpropanoids, pigments, phytoalexins and pathogenicity-related proteins [[21], [22], [23], [24]] thereby helping plant to grow and adapt to habitat stress [[23], [24], [25], [26], [27]]. It was observed that IAA is involved in the establishment of biotrophy in endophytic fungus Piriformospora indica–barley symbiosis and represented as a compatibility factor in this system [20]. Interestingly, the yeast isolate Candida tropicalis was able to produce IAA and was showed to improve grain quality by 85 % [28]. Also, an endophyte Williopsis saturnus in maize roots could produce IAA and was reported to enhance the growth of maize plants [29]. Hence, IAA synthesis is the first plant growth-promoting trait being screened to qualify as plant growth promoting fungi (PGPF) [3,30]. The global demand for IAA metabolite is estimated to reach USD 36 million by 2028, growing at a CAGR of 5.5 % [31], therefore the production of cost-effective plant growth-promoting agents has been the focus of study for several researchers.
Microbial communities integrate an exceptional plant specific micro-ecosystem. Among all the known pathways to synthesize IAA which involve shikimate, the frequently adopted route is the use of l-tryptophan for the production of IAA [3]. Studies have suggested the indole-3-pyruvic acid (IPA) pathway is predominantly utilized for IAA biosynthesis in fungi stimulating growth in hairy root cultures and producing a myriad of secondary metabolites in various plants [32,33]. Extensive studies have shown the involvement of IAA in signaling pathways in both IAA producing as well as non producing endophytes [34,35]. Several nutritional and physical parameters like pH, temperature, incubation period, medium concentration, and tryptophan concentration influence the production of IAA [36,37]. The optimization of these factors can be achieved by a variety of conventional techniques like one factor at a time (OFAT) [[38], [39], [40]]. However, conventional optimization methodologies, for the production of novel and known bioactives from microorganisms encounter major disadvantages like being time-consuming, extravagant for multiple variables, and having insufficient knowledge about interactive effects among the variables resulting in inadequate data leading to confusion and lack of predictive ability [38] or incomplete effects of the variables on the responses [41,42]. Statistical optimization using multivariate statistic techniques was incorporated to overcome these shortcomings [43]. One of the most significant multivariate techniques used in optimization is response surface methodology (RSM) which describes the behavior of a dataset with the objective of making statistical previsions, applicable to responses of interest under the influence of several variables. RSM simultaneously optimizes the levels of variables to attain the best system performance [39]. Recent publications endorse RSM to be better than the Artificial Neural Network (ANN) technique in fermentation media optimization [44,45]. Literature, however, cautions of certain limitations. For example while employing RSM, the levels of variables must be appropriately selected and care should be taken in deciding the statistically significant model to get the best optimization results. Further, if the responses of variables are independent then the effect of the variables being investigated will affect the overall result and will require redesigning the experiment and or achievable targets [46]. To rule out any such uncertainties, the RSM can be complemented with other optimization techniques such as ANN, fuzzy logic, search algorithm, and genetic algorithm. Recent reports have suggested the RSM approach for optimal production of phytohormones by the endophytes by optimizing a response that is influenced by several independent variables relating to hormone production. For instance, the production of IAA by fungus Pichia fermentans was formulated and optimized using statistical approach wherein IAA production was significantly enhanced by nine folds [47]. The optimized medium for Rhodosporidiobolus fuvialis using the one-factor-at-a-time (OFAT) resulted in a 3.3-fold improvement in IAA production and a 3.6-fold reduction in cost compared with those obtained with a non-optimized medium [48]. RSM based optimization of growth conditions of Herbaspirillum seropedicae could be achieved for its application as agricultural inoculants for plant growth promotion [49]. Similarly, RSM aided medium optimization of IAA by Saccharothrix texasensis, isolated from the organic wastes [50], and IAA production by the basidiomycetous yeast Rhodosporidiobolus uvialis DMKU-CP293 for weed control (weed biocontrol) [51] were successfully demonstrated. Relatively few studies have focused on the endophyte mediated production and regulation of IAA on the growth and development of medicinal plants particularly, in Glycyrrhiza species. The roots of the Glycyrrhiza plant (Licorice) are the source of pharmaceutically valued metabolites [7,[52], [53], [54]] of high demand resulting in its threatened status [55]. Phytohormone aided alterations in the phytochemical profile triggers a chemically controlled network of related gene circuits and the underlying molecular regulation mechanism of the targeted secondary metabolite(s). Recent literature has demonstrated enhanced accumulation of glycyrrhizic acid in the roots of G. uralensis subjected to exogenous auxins and gibberellins treatments [56] along with growth promotion when grown in a mutualistic symbiosis with Ascomycetes [57]. This is the foundation of our previous work; to explore the endophytic diversity and identify the endophytes involved in glycyrrhizin hyper-accumulation [58]. The current study reports an IAA producing fungal endophyte, Diaporthe terebinthifolii GG3F6 associated with G. glabra and aimed to optimize the factors to enhance the endophyte-driven IAA production using RSM. Further, the study demonstrated the effect of the isolated endophyte and the endophyte extracted IAA on the plant morphology and glycyrrhizin accumulation under the in-vitro conditions.
2. Materials and methods
2.1. Source of microorganism
The endophytic fungus D. terebinthifolii (GG3F6) was previously isolated from G. glabra [58] and submitted to the National Fungal Culture Collection of India under Voucher No. NFCCI 3711. Previous acquisition of the ITS1-5.8S-ITS2 ribosomal gene sequence identified it to be D. terebinthifolii (KU168142). In our previous study, the functional characterization of fungal endophytes associated with G. glabra was carried out wherein IAA was quantified for isolated endophytes [58]. In the present study, the endophyte D. terebinthifolii GG3F6 producing IAA in a significant quantity (195 μg/ml) along with extracellular enzymes; amylase and lipase and showing antimycotic activity was selected for the IAA optimization using RSM methodology. After obtaining the culture from the in-house repository, the culture was subsequently sub-cultured and maintained on PDA plates.
2.2. Quantitative analysis for IAA production
The selected endophyte was revalidated for IAA production. Estimation of IAA was carried out using the previously described protocol [58]. Briefly, 1 ml supernatant of 7 days old fungal culture supplemented with 1 mg/ml of tryptophan was centrifuged (Eppendorf, Centrifuge 5430R) at 6797 g for 5 min at room temperature and mixed with 2 ml of Salkowaski's reagent. The tube was then incubated at room temperature in the dark for 15 min. The cherry red colouration in the tube indicated the production of IAA from the fungus, which was quantified by measuring absorbance at 530 nm (Eppendorf, BioSpectrometer 22331). The amount of IAA produced by the fungus was calculated by comparing it with the standard curve [59]. A Standard curve was plotted by measuring the absorbance of known standards of IAA (0, 5, 10, 20, 50, and 100 μg/ml). The equation of IAA was obtained by plotting absorbance at 530 nm (y) versus the concentration (x, μg/ml) of IAA as y = 0.0069x + 0.0037 (R2 = 0.9991). The experiment was carried out in duplicates.
Time course IAA production in comparison to biomass was also evaluated as described previously [60]. Briefly, the culture was grown for 3, 6, 9, 12, and 15 days in a PDB medium supplemented with 1 mg/ml of tryptophan. After each time interval, the wet weight (grams) and absorbance at 530 nm were measured. The plot showing the calculated IAA and wet weight was used to set up the Box-Behnken design (BBD).
2.3. Box-Behnken design for response surface methodology (RSM)
The Box-Behnken design (BBD) of RSM (Design-Expert 8.0.6 software) was employed to optimize the most significant five factors (initial pH, temperature, tryptophan concentration, medium strength and incubation period) for enhancing IAA production from D. terenbinthifollii. These factors were selected based on single-factor experiments and available literature [27]. A set of 45 experiments was obtained using the BBD design at two levels (−1, 1) viz. low and high limits for the significant five factors as shown in Table 1, Table 2. The fungal IAA produced was quantified spectro-photometrically as described above and the average concentration of IAA was calculated and recorded as actual response. Further, the fungal IAA was reconfirmed using the Ultra High Performance Liquid Chromatography (UPLC) method.
Table 1.
Different factors and their levels used in the optimization of IAA production by GG3F6 using central composite design.
| Independent Variables | Low Level | High Level |
|---|---|---|
| Tryptophan (g/l) | 0 | 2 |
| Temperature (°C) | 25 | 45 |
| pH ([H+]) | 4 | 12 |
| Potato dextrose broth (g/l) | 6 | 48 |
| Incubation period (dpi) | 3 | 11 |
Table 2.
Experimental conditions of Box-Behnken design (Design-Expert 8.0.6 software) for optimization of significant five factors viz. initial pH, temperature (°C), tryptophan concentration (mg/mL), medium strength (g/L) and incubation period (days) with the actual response i.e. Indole acetic acid (μg/mL) produced by GG3F6.
| Run Code | A: Tryptophan | B: Temperature | C: pH | D: Potato dextrose broth | E: Incubation Peroid | R: Indole acetic acid |
|---|---|---|---|---|---|---|
| 1 | 1.00 | 35.00 | 12.00 | 6.00 | 7.00 | 28.128 |
| 2 | 1.00 | 45.00 | 8.00 | 27.00 | 11.00 | 42.5829 |
| 3 | 1.00 | 35.00 | 12.00 | 27.00 | 11.00 | 61.1137 |
| 4 | 2.00 | 35.00 | 8.00 | 48.00 | 7.00 | 105.237 |
| 5 | 1.00 | 35.00 | 8.00 | 27.00 | 7.00 | 43.2938 |
| 6 | 1.00 | 35.00 | 4.00 | 6.00 | 7.00 | 28.8389 |
| 7 | 2.00 | 35.00 | 8.00 | 27.00 | 3.00 | 88.0806 |
| 8 | 2.00 | 45.00 | 8.00 | 27.00 | 7.00 | 113.578 |
| 9 | 0.00 | 35.00 | 8.00 | 27.00 | 3.00 | 36.8957 |
| 10 | 1.00 | 35.00 | 8.00 | 48.00 | 11.00 | 60.4028 |
| 11 | 1.00 | 25.00 | 8.00 | 27.00 | 3.00 | 42.6303 |
| 12 | 1.00 | 35.00 | 12.00 | 27.00 | 3.00 | 62.2512 |
| 13 | 1.00 | 45.00 | 12.00 | 27.00 | 7.00 | 66.6588 |
| 14 | 1.00 | 45.00 | 8.00 | 6.00 | 7.00 | 35.0474 |
| 15 | 1.00 | 45.00 | 8.00 | 48.00 | 7.00 | 66.1374 |
| 16 | 1.00 | 35.00 | 8.00 | 6.00 | 3.00 | 31.6825 |
| 17 | 0.00 | 35.00 | 12.00 | 27.00 | 7.00 | 49.9763 |
| 18 | 0.00 | 35.00 | 8.00 | 48.00 | 7.00 | 49.455 |
| 19 | 1.00 | 35.00 | 12.00 | 48.00 | 7.00 | 121.209 |
| 20 | 1.00 | 35.00 | 4.00 | 48.00 | 7.00 | 55.3318 |
| 21 | 1.00 | 35.00 | 8.00 | 48.00 | 3.00 | 60.2607 |
| 22 | 0.00 | 35.00 | 8.00 | 6.00 | 7.00 | 22.4882 |
| 23 | 1.00 | 25.00 | 8.00 | 6.00 | 7.00 | 41.7773 |
| 24 | 2.00 | 35.00 | 12.00 | 27.00 | 7.00 | 110.924 |
| 25 | 2.00 | 25.00 | 8.00 | 27.00 | 7.00 | 97.3223 |
| 26 | 0.00 | 45.00 | 8.00 | 27.00 | 7.00 | 39.6919 |
| 27 | 1.00 | 35.00 | 8.00 | 27.00 | 7.00 | 44.2417 |
| 28 | 2.00 | 35.00 | 4.00 | 27.00 | 7.00 | 52.2512 |
| 29 | 1.00 | 35.00 | 8.00 | 27.00 | 7.00 | 44.6682 |
| 30 | 1.00 | 35.00 | 8.00 | 27.00 | 7.00 | 46.9431 |
| 31 | 0.00 | 25.00 | 8.00 | 27.00 | 7.00 | 33.9573 |
| 32 | 1.00 | 25.00 | 4.00 | 27.00 | 7.00 | 37.1327 |
| 33 | 1.00 | 25.00 | 12.00 | 27.00 | 7.00 | 64.2891 |
| 34 | 0.00 | 35.00 | 8.00 | 27.00 | 11.00 | 36.327 |
| 35 | 1.00 | 35.00 | 4.00 | 27.00 | 11.00 | 33.7678 |
| 36 | 2.00 | 35.00 | 8.00 | 6.00 | 7.00 | 30.8294 |
| 37 | 1.00 | 35.00 | 8.00 | 6.00 | 11.00 | 27.7962 |
| 38 | 0.00 | 35.00 | 4.00 | 27.00 | 7.00 | 29.1706 |
| 39 | 1.00 | 25.00 | 8.00 | 27.00 | 11.00 | 43.7204 |
| 40 | 1.00 | 45.00 | 4.00 | 27.00 | 7.00 | 36.1374 |
| 41 | 1.00 | 25.00 | 8.00 | 48.00 | 7.00 | 61.5877 |
| 42 | 1.00 | 35.00 | 4.00 | 27.00 | 3.00 | 35.7109 |
| 43 | 1.00 | 45.00 | 8.00 | 27.00 | 3.00 | 39.6919 |
| 44 | 1.00 | 35.00 | 8.00 | 27.00 | 7.00 | 45.9479 |
| 45 | 2.00 | 35.00 | 8.00 | 27.00 | 11.00 | 106.043 |
2.4. Confirmation of IAA produced by fungus using UPLC
The IAA was extracted from the endophytic fungus GG3F6 essentially following the published protocol [61]. The fungus was inoculated into 250 ml of potato dextrose broth supplemented with 1 mg/ml tryptophan and incubated at 28 °C for 15 days at 150 rpm. Un-inoculated growth medium was used as negative control (blank). The supernatant was harvested by centrifugation (Eppendorf, Centrifuge 5430R) at 6797 g for 5 min at room temperature. The IAA was extracted by acidifying the supernatant to 2.5–3.0 pH with 1 N HCl followed by extraction twice with ethyl acetate at double the volume of the supernatant. The extract was then dried using rota-vapour at 50 °C and was finally dissolved in methanol (1 ml) for quantification using UPLC. Stock solution (1 mg/ml) of Indole acetic acid (Sigma-Aldrich, Budapest, Hungary) was prepared in methanol and filtered through a 0.45 μm membrane filter (Millipore). The concentration utilized for the preparation of six point calibration curve for IAA ranged between 31.25 and 1000 μg/ml (1/2 dilution pattern). The calibration equation of IAA was obtained by plotting HPLC peak area (y) versus the concentration (x, μg/ml) of calibrators as y = 0.62321x+106.972251 (R2 = 0.9978). The equation showed excellent linearity over the range. The analyses were performed using in Thermo Ultimate 3000 UPLC (Thermo, USA) system equipped with Dionex RS quaternary pumps, an autosampler, and a thermostat column compartment. The chromatographic separation was performed on a Purospher RP-18e column (250 × 4.6 mm) (Merck, Germany), which was kept at a constant temperature of 300 °C. Mobile phases A and B consisted of 0.1 % formic acid in water and acetonitrile, respectively. The measurement consisted of 20 min gradient program starting at 20 % B during the injection of the sample, whilst the flow rate was set at 1.5 mL/min. Subsequently, the solvent B 60 % in 5.0 min, B 70 % in 12.0 min, B 90 % in 14.0 min, B 90 % in 16.0 min, B 20 % in 18.0 min and 20 % in 2.0 min was followed. The injection volume was 2 μL and the wavelength was 254 nm.
2.5. Co-culture of plant and endophyte
The effect of the endophyte on the host plant was investigated initially by co-culturing the selected endophyte (GG3F6) with the in-vitro regenerated G. glabra plants and subsequently by inoculating the in-vitro plants with the IAA extracted from the fungus. The fungus was grown under optimized conditions obtained from the RSM data and fungal spores (5.8 × 108/ml) were co-cultured with 3-month-old plants in the optimized liquid medium under tissue culture conditions for 7 days for any pathogenic response. The co-cultivation with the fungus was continued for 10 days for recording the growth parameters, and assessment of phenolic and flavonoid contents following the published protocol [58]. Further, to detect the presence of fungus in the in-vitro grown plants microscopic analysis was carried out by modifying the previously described protocol [60]. Specifically, the plants (treated and control) were washed with tap water, immersed in 5 % (w/v) Sodium hypochlorite for 15 min, and thoroughly rinsed with sterile water. The tissues were cut into thin segments using sterile razor blade (Laser® Ultra), rinsed three times with deionized water and placed in 10 % (w/v) KOH for 15 min followed by 1 N HCl treatment. After a rinse in sterile water, the segments were stained with 0.05 % (w/v) trypan blue at 60 °C for 1 h, and mounted on sterile glass slides with lactophenol cotton blue dye. The slides were examined by light microscopy (Leica DM750).
For the subsequent treatment of purified IAA (50 μm) extracted from the fungus, the experiment was designed following the previously used protocol [62] using in-vitro regenerated G. glabra plants harvested post 16 h and 18 h.
2.6. Quantification of glycyrrhizin
The extraction procedure employed was essentially similar to a previously published protocol [62,63] using LC-MS/MS on Purospher RP-8 (125 × 2 mm, 5 μl) column with 0.1 % (v/v) formic acid in water (A) and acetonitrile (B) in gradient mode. The gradient program was as follows: 0–3 min, 10 % B; 3.2–5 min, 90 % B; 5.2–7 min, 10 % B. The analysis was carried out in a selected reaction monitoring mode using precursor/product ion transition at m/z 821.3/351.1 for glycyrrhizin. Data were obtained and processed using Chromeleon and LC quant software.
3. Results
3.1. IAA produced by the indigenous endophytic culture
Before the co-cultivation experiments of the selected fungus with the host plant, the fungal IAA was assessed and it was observed that the selected fungal culture was producing a lower (110 μg/ml) amount of IAA than reported earlier [58]. The variation in the quantity of the IAA after subsequent sub-culturing prompted us to optimize the conditions for the optimum production of IAA by the fungal endophyte following RSM.
3.2. Optimization of IAA production by response surface methodology analysis
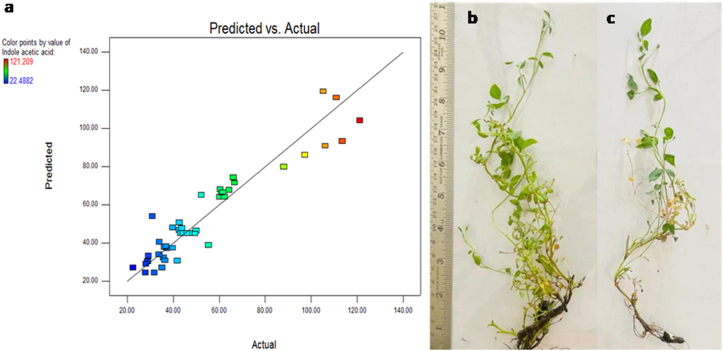
Two culture parameters, days and biomass, were individually studied for the optimization of IAA from the fungal culture. The temporal investigation from the 3rd day to the 15th day showed D. terebinthifolli GG3F6 produced maximum IAA (110.27 μg/ml) at the 9th days post inoculation (dpi) followed by a steep decline (40.26 μg/ml) till 15 dpi, while biomass was found to be maximum (1.52 g) at 12th dpi (Fig. 1 a). Further, Box-Behnken design (BBD) was used to optimize the most significant five factors (initial pH, temperature, tryptophan concentration, medium strength and incubation period) for optimizing IAA production in D. terenbinthifollii. As can be seen in Table 2 the RSM run code 19 showed a maximum IAA value (121.209 μg/mL) in the design (Table 2 and Fig. 2 a - g) where the optimized conditions for substrate (tryptophan (A)) was 1 mg/mL, at the incubation temperature (B) of 35 °C, pH (C) of 12 grown in 48 g/L of Potato dextrose broth medium (D) for of 7 days (E). The lowest IAA yield (22.488 μg/mL) was obtained when no tryptophan was given at 35 °C at pH 8, in 6 g/L of Potato dextrose broth after 7 days. The calculated p-value was found to be very low (p < 0.0001). Further, the effects of two components were examined keeping the other components constant. The interactions between each pair of variables and maximum IAA production by fungal endophyte GG3F6 are represented in the contour plots (Fig. 3 a).
Fig. 1.
(a) Time course accumulation of biomass and Indole Acetic Acid (IAA) produced by the endophyte over a period of two weeks; chromatogram of (b) Standard IAA; (c) GG3F6 endophyte producing IAA at the retention time of 8.860; and (d) Overlay of standard and sample chromatograms.
Fig. 2.
Three-dimensional response surfaces (a, c, e and g) showing the effects of Tryptophan (substrate) in relation to temperature (a); pH (c); Potato Dextrose Broth (e); and Incubation period (g) and their respective contour plots b, d, f and h.
Fig. 3.
(a) Experimental validation of the model for IAA production showing the relationship between predicted and actual values. Co-culture of Glycyrrhiza glabra plants with fungal endophyte GG3F6 under in-vitro conditions following the RSM optimized conditions. Effect of endophytes on treated plant (b); control plant (c). The control blank (plug having PDA) and the endophyte treatment (equal sized plug having endophyte) were placed on the tissue culture media in proximity with the explants.
After experimentally validating the 34 solutions (out of 71 in Table S1) which predicted IAA levels higher than the run code 19 (121 μg/ml) as given by the RSM aided optimization, none of them showed IAA levels comparable to 121 μg/ml. The code 19 conditions were further used for the IAA production by the fungus. The co-cultivation of the plant with fungus and the extracted IAA from fungus under the same conditions enhanced glycyrrhizin conditions under in-vitro conditions.
3.3. Effect of co-culture
The endophytic fungal strain GG3F6 co-cultivated with the in-vitro grown G. glabra plants showed enhanced plant growth (4.4 ± 0.8 fwg) as compared to the control plants (1.5 ± 0.8 fwg) (Fig. 3 b and c) post ten days. The fungus influenced plants showed enhanced total phenolic (10.7 %) and flavonoid (10.2 %) contents in comparison to the control plants (Fig. 4 c). The treated plants investigated for glycyrrhizin content (0.19 ppm) showed 2.1 fold increase as compared to the control plants (0.14 ppm) (Fig. 4 c).
Fig. 4.
Microscopic observation of root segments of in-vitro grown plants for endophytic fungal colonization (a) control Glycyrrhiza glabra roots without Diaporthe terebinthifolli and (b) treated roots showing presence of hyphal mycelia and spores (portion highlighted red). Bar graph illustrating the quantification of total phenolic content (TPC) measured in gallic acid equivalents per gram of dry weight (GAE/g of DW), total flavonoid content (TFC) measured in quercetin equivalents per gram of dry weight (QE/g of DW), and glycyrrhizin accumulation measured in parts per million (PPM) in G. glabra plants cultivated under in-vitro conditions. The experiment includes two treatments: (c) inoculated with the fungal endophyte GG3F6 compared to control plants, and (d) treated with purified indole-3-acetic acid (IAA) of microbial origin. The data were compared and analyzed using two-way ANOVA in GraphPad Prism 8.0 software. Values are expressed as means ± SD representing three independent biological samples, each with three technical replicates. Differences were scored as statistical significance at ***P < 0.0001, **P < 0.001 and *P < 0.05. Asterisks indicate difference in expression levels of respective promoters in treated and control plants.
To confirm the successful colonization of the fungus to the in-vitro grown plants, we first examined the roots and shoots of both the plants; treated as well as control. In the shoot segments of both the treated and control plants, no colonization was observed. However, in the treated root segments, hyphal mycelia were observed, confirming the colonization of the endophytic fungus in the root tissues compared to control roots (Fig. 4 a and b).
Similar results were obtained when in-vitro plants were treated with purified IAA of microbial origin. The glycyrrhizin content was enhanced (92.6 ppm) after 16 h of incubation as compared to control (87.3 ppm) and 18 h (77.3 ppm) treatments (Fig. 4 d).
4. Discussion
Endophyte-host interactions are based on mutual exploitation [64,65] primarily being protective and/or growth-promoting in nature [66,67]. Some endophytes possess the ability to produce phyto-hormones assigned as PGPRs [3] for better adaptability and growth of the host plant. Literature confirms several reports where exogenous IAA treatment was seen to influence growth-promoting activities in Zea mays, Solanum lycopersicum and Sophora flavescens, stress tolerance and nutrient uptake in Triticum spp [25,68,69]. The present study explores the conditions for the optimum production of IAA by the endophyte isolated from G. glabra, a medicinal plant of immense medicinal importance. The reported endophyte was isolated on water agar medium from surface-sterilized, asymptomatic G. glabra rhizome collected from the experimental farm of the Institute. The endophyte was found to possess antagonistic activity against fungal phytopathogens and produced amylase and lipase hydrolytic enzymes [58], and was a rich source of IAA (195 μg/ml). Literature establishes that the IAA secreted by the microbial resources, in the auxin pool of the plant, enhanced the nutrient uptake [70], elevated the photosynthesis process [71,72], and assisted the host in various growth promoting processes [3] and yield [73]. It is estimated that by 2028, the global demand for the IAA metabolite will reach USD 36 million [31].
Assimilating the results from our previous study and literature reports, the present research exploited the identified endophyte as a potential IAA producer for plant growth promotion and sustainable cultivation. In the present study, RSM was employed for the optimization of various parameters and conditions for the optimum production of IAA by the isolated endophytic fungus D. terebinthifolii GG3F6. The Box Behnken Design was adopted to estimate five variables (initial pH, temperature, tryptophan concentration, medium strength and incubation period) and their interactive effects on overall IAA yield. The significantly low p-value (p < 0.0001) of the experiment propounded that the model best fits the experimental data, and the independent variables have considerable effects on the production of IAA. Further, the F- & P- values (at p < 0.05) and predicted R2 value were in agreement with the adjusted R2 value (Table 3) indicating the model is statistically significant which was also corroborated by the F-value (9.83) of the independent variables. Furthermore, the high degree of similarity between experimental and predicted values reflected the accuracy and validity of the design of the experiment (Fig. 4). Maximum IAA production was achieved at 35 °C with 48 g/L PDB having pH 12, while the other components (substrate and time) were used at the lowest levels (1 g/L substrate for 7 days of incubation period). Hence, the RSM statistical model was successful in determining the conditions for the maximum production of IAA and the independent variables used in the study. The RSM-aided experimentation resulted in a 1.09 fold increase in IAA production.
Table 3.
Analysis of variance for the experimental results of Box–Behnken methodology for quadratic model.
| Source | Sum of Squares | Df | Mean Square | F Value | p-value Prob > F |
|---|---|---|---|---|---|
| Model | 25904.31 | 20 | 1295.22 | 9.83 | <0.0001 |
| A-Tryptophan | 561.25 | 1 | 561.25 | 4.26 | 0.0500 |
| B-Temperature | 136.43 | 1 | 136.43 | 1.04 | 0.3189 |
| C-pH | 208.86 | 1 | 208.86 | 1.59 | 0.2200 |
| D-Potato Dextrose Broth | 242.83 | 1 | 242.83 | 1.84 | 0.1871 |
| E-Incubation Period | 3.59 | 1 | 3.59 | 0.027 | 0.8702 |
| AB | 27.67 | 1 | 27.67 | 0.21 | 0.6508 |
| AC | 358.48 | 1 | 358.48 | 2.72 | 0.1120 |
| AD | 562.66 | 1 | 562.66 | 4.27 | 0.0497 |
| AE | 85.85 | 1 | 85.85 | 0.65 | 0.4274 |
| BC | 2.83 | 1 | 2.83 | 0.021 | 0.8847 |
| BD | 31.81 | 1 | 31.81 | 0.24 | 0.6276 |
| BE | 0.81 | 1 | 0.81 | 6.157E-003 | 0.9381 |
| CD | 1108.48 | 1 | 1108.48 | 8.42 | 0.0078 |
| CE | 0.16 | 1 | 0.16 | 1.232E-003 | 0.9723 |
| DE | 4.06 | 1 | 4.06 | 0.031 | 0.8621 |
| A2 | 1945.87 | 1 | 1945.87 | 14.78 | 0.0008 |
| B2 | 115.94 | 1 | 115.94 | 0.88 | 0.3574 |
| C2 | 178.75 | 1 | 178.75 | 1.36 | 0.2555 |
| D2 | 5.17 | 1 | 5.17 | 0.039 | 0.8446 |
| E2 | 3.34 | 1 | 3.34 | 0.025 | 0.8749 |
| Lack of fit | 3152.45 | 20 | 157.62 | 76.25 | 0.0004 |
| Residual | 3160.71 | 24 | 131.70 | – | – |
| Pure Error | 8.27 | 4 | 2.07 | – | – |
| Cor Total | 29065.02 | 44 | – | – | – |
The optimization studies reflected the maximum amount of IAA that could be produced at the higher range of pH akin to studies in Aspergillus tamari, white rot fungus Pleurotus ostreatus and Trichoderma harzianum were reported to produce maximum levels of IAA at higher pH values compared to lower values [[74], [75], [76]]. One probable reason for this could be alteration in the conformational structure accounting for the denaturation of related enzymes thus altering the metabolite pool [77,78]. The other independent variables (temperature and PDB concentration) employed for experimentation were also found to be higher. The optimum temperature observed at 35 °C was supported by research indicating that Trichoderma spp. may exhibit enhanced IAA production at elevated temperatures, particularly within the range of 30 °C–35 °C [79]. This is the first report of IAA optimization using RSM by a fungal endophyte displaying host-endophyte interaction from G. glabra. Further, in this study the accumulated IAA produced by the isolated fungus was purified, confirmed and quantified using UPLC (Fig. 1 b - d) for further confirmation of its effect on plant.
The assessment of the endophyte-plant co-culture on the morphology of the host plant manifested longer and bushy roots, taller plants and higher fresh weight. Glycyrrhizin, a commercially important bioactive secondary metabolite of the host was seen to be enhanced in-planta. We observed around 2.1 fold increase in glycyrrhizin content in the endophyte treated plants compared to the control (Fig. 4 c). Earlier reports have also established exogenous auxin treatment significantly promoted the accumulation of glycyrrhizin in G. uralensis and G. glabra [56,57,62]. Literature confirms the crucial role of endophytic-IAA-mediated plant growth promoting and regulating activities [5,58,60]. Research shows that the interaction between the fungus and the host plant involves the exchange of chemical crosstalk mediated by three key players IAA, phenols and flavonoids [80]. Plant-fungal interaction studies have demonstrated restricted symbiotic interaction abilities of both partners on depletion of flavonoids and or IAA [80,81]. Here, the administration of the purified IAA extracted from the endophytic fungi to the plant manifested similar effects as the plant-fungi co-culture, implying IAA plays a pivotal role in the enhanced accumulation of glycyrrhizin, phenols and flavonoids in the plant thereby suggesting the exploitation of endophyte in plant defense and resilience to climate change besides being an excellent stimulator for plant growth.
5. Conclusion
In conclusion, the dynamic host plant-endophytic interaction evoked enhanced IAA production manifested in increased plant growth and enhanced accumulation of bioactive secondary metabolite glycyrrhizin-an anticancer compound highly valued in Pharmaceutical Industry. The study also highlighted the role of IAA in establishing the symbiotic partnership. This study lays the foundation for future routing in endophytes to be utilized as bio-stimulators for enhanced resilience for sustainable agriculture with commercial implications.
Funding statement
Authors acknowledge the Council for Scientific and Industrial Research grant (CSIR-MLP0048) for funding the study. PA acknowledges CSIR Fellowship; RT acknowledges CSIR for Senior Research Fellowship and Academy of Scientific and Innovative Research, CSIR-HRDC Campus, Sector-19, Ghaziabad, UP., India for PhD registration. All the authors acknowledge director, CSIR-IIIM for providing all the necessary facilities. The manuscript bears the institutional manuscript no. CSIR-IIIM/IPR/00615.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Palak Arora: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. Rubeena Tabassum: Methodology, Dr. Ajai P. Gupta: Methodology, Ms. Saajan Kumar: Methodology. Suphla Gupta: Writing – review & editing, Supervision, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization, Mr.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34356.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Schulz B., Boyle C. The endophytic continuum. Mycol. Res. 2005;109(6):661–686. doi: 10.1017/s095375620500273x. [DOI] [PubMed] [Google Scholar]
- 2.Sieber T. In: The Hidden Half. Waisel Y., Eshel A., Kafkafi U., editors. Dekker; New York: 2002. Fungal root endophytes. [Google Scholar]
- 3.Keswani C., Singh S.P., Cueto L., García-Estrada C., Mezaache-Aichour S., Glare T.R., Borriss R., Singh S.P., Blázquez M.A., Sansinenea E. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020;104(20):8549–8565. doi: 10.1007/s00253-020-10890-8. [DOI] [PubMed] [Google Scholar]
- 4.Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007;31(4):425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 5.Fu S.-F., Wei J.-Y., Chen H.-W., Liu Y.-Y., Lu H.-Y., Chou J.-Y. Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015;10(8) doi: 10.1080/15592324.2015.1048052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tudzynski B., Sharon A. Industrial Applications. Springer; 2002. Biosynthesis, biological role and application of fungal phytohormones; pp. 183–211. [Google Scholar]
- 7.Yang R., Yuan B.-C., Ma Y.-S., Zhou S., Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017;55(1):5–18. doi: 10.1080/13880209.2016.1225775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain A., Hamayun M., Rahman H., Iqbal A., Shah M., Irshad M., Qasim M., Islam B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere. 2018;211:653–663. doi: 10.1016/j.chemosphere.2018.07.197. [DOI] [PubMed] [Google Scholar]
- 9.Bilal L., Asaf S., Hamayun M., Gul H., Iqbal A., Ullah I., Lee I.J., Hussain A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis. 2018;76:117–127. [Google Scholar]
- 10.Ikram M., Ali N., Jan G., Jan F.G., Rahman I.U., Iqbal A., Hamayun M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0208150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehmood A., Hussain A., Irshad M., Hamayun M., Iqbal A., Rahman H., Tawab A., Ahmad A., Ayaz S. Cinnamic acid as an inhibitor of growth, flavonoids exudation and endophytic fungus colonization in maize root. Plant Physiol. Biochem. 2019;135:61–68. doi: 10.1016/j.plaphy.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Hamayun M., Hussain A., Khan S.A., Kim H.Y., Khan A.L., Waqas M., Irshad M., Iqbal A., Rehman G., Jan S., Lee I.J. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017;(8):686. doi: 10.3389/fmicb.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain A., Shah S.T., Rahman H., Irshad M., Iqbal A. Effect of IAA on in vitro growth and colonization of Nostoc in plant roots. Front. Plant Sci. 2015;(6):46. doi: 10.3389/fpls.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehmood A., Hussain A., Irshad M., Hamayun M., Iqbal A., Khan N. In-vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis. 2019;77(3):225–235. [Google Scholar]
- 15.Taha A.S., Abo-Elgat W.A., Fares Y.G., Salem M.Z. Isolated essential oils as antifungal compounds for organic materials. Biomass Convers. Biorefin. 2024;14(3):3853–3873. [Google Scholar]
- 16.Khan N.A., Asaf S., Ahmad W., Jan R., Bilal S., Khan I., Khan A.L., Kim K.M., Al-Harrasi A. Diversity, lifestyle, genomics, and their functional role of Cochliobolus, Bipolaris, and Curvularia species in environmental remediation and plant growth promotion under biotic and abiotic stressors. J. Fungi. 2023;9(2):254. doi: 10.3390/jof9020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook D.E., Mesarich C.H., Thomma B.P. Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 2015;53:541–563. doi: 10.1146/annurev-phyto-080614-120114. [DOI] [PubMed] [Google Scholar]
- 18.Lo Presti L., Lanver D., Schweizer G., Tanaka S., Liang L., Tollot M., Zuccaro A., Reissmann S., Kahmann R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015;(66):513–545. doi: 10.1146/annurev-arplant-043014-114623. [DOI] [PubMed] [Google Scholar]
- 19.Felten J., Martin F., Legué V. Signaling and Communication in Plant Symbiosis. Springer; 2012. Signalling in ectomycorrhizal symbiosis; pp. 123–142. [Google Scholar]
- 20.Hilbert M., Voll L.M., Ding Y., Hofmann J., Sharma M., Zuccaro A. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol. 2012;196(2):520–534. doi: 10.1111/j.1469-8137.2012.04275.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsavkelova E., Klimova S.Y., Cherdyntseva T., Netrusov A. Microbial producers of plant growth stimulators and their practical use: a review. Appl. Biochem. Microbiol. 2006;42(2):117–126. [PubMed] [Google Scholar]
- 22.Beneventi M.A., da Silva O.B., de Sá M.E.L., Firmino A.A.P., de Amorim R.M.S., Albuquerque É.V.S., da Silva M.C.M., da Silva J.P., Campos MdA., Lopes M.J.C. Transcription profile of soybean-root-knot nematode interaction reveals a key role of phythormones in the resistance reaction. BMC Genom. 2013;14(1):1–17. doi: 10.1186/1471-2164-14-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shani E., Salehin M., Zhang Y., Sanchez S.E., Doherty C., Wang R., Mangado C.C., Song L., Tal I., Pisanty O. Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr. Biol. 2017;27(3):437–444. doi: 10.1016/j.cub.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie L., Chen F., Du H., Zhang X., Wang X., Yao G., Xu B. Graphene oxide and indole-3-acetic acid cotreatment regulates the root growth of Brassica napus L. via multiple phytohormone pathways. BMC Plant Biol. 2020;20(1):1–12. doi: 10.1186/s12870-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheynier V., Comte G., Davies K.M., Lattanzio V., Martens S. Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Maheshwari D.K., Dheeman S., Agarwal M. Bacterial Metabolites in Sustainable Agroecosystem. Springer; 2015. Phytohormone-producing PGPR for sustainable agriculture; pp. 159–182. [Google Scholar]
- 27.Baliyan N., Dhiman S., Dheeman S., Kumar S., Arora N.K., Maheshwari D.K. Optimization of gibberellic acid production in endophytic Bacillus cereus using response surface methodology and its use as plant growth regulator in chickpea. J. Plant Growth Regul. 2021;47(7):1–11. [Google Scholar]
- 28.Mukherjee S., Sen S.K. Exploration of novel rhizospheric yeast isolate as fertilizing soil inoculant for improvement of maize cultivation. J. Sci. Food Agric. 2015;95(7):1491–1499. doi: 10.1002/jsfa.6848. [DOI] [PubMed] [Google Scholar]
- 29.Fu S.F., Wei J.Y., Chen H.W., Liu Y.Y., Lu H.Y., Chou J.Y. Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015;10(8) doi: 10.1080/15592324.2015.1048052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etesami H., Alikhani H.A., Hosseini H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX. 2015;2:72–78. doi: 10.1016/j.mex.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MarketWhatch . 2022. Global Indole-3-Acetic Acid (IAA) Market Research Report. [Google Scholar]
- 32.Sardar P., Kempken F. Characterization of indole-3-pyruvic acid pathway-mediated biosynthesis of auxin in Neurospora crassa. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0192293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharon A., Elad Y., Barakat R., Tudzynski P. Botrytis: Biology, Pathology and Control. Springer; 2007. Phytohormones in botrytis-plant interactions; pp. 163–179. [Google Scholar]
- 34.Kanwar M.K., Yu J., Zhou J. Phytomelatonin: recent advances and future prospects. J. Pineal Res. 2018;65(4) doi: 10.1111/jpi.12526. [DOI] [PubMed] [Google Scholar]
- 35.Tiwari P., Bajpai M., Singh L.K., Mishra S., Yadav A.N. Agriculturally Important Fungi for Sustainable Agriculture. Springer; 2020. Phytohormones producing fungal communities: metabolic engineering for abiotic stress tolerance in crops; pp. 171–197. [Google Scholar]
- 36.Sridevi M., Mallaiah K.V. Bioproduction of indole acetic acid by Rhizobium strains isolated from root nodules of green manure crop, Sesbania sesban (L.) Merr. J. Biotechnol. 2007;5(3):178–182. [Google Scholar]
- 37.Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. Soil Sci. Plant Nutr. 2013;13(3):638–649. [Google Scholar]
- 38.Latha S., Sivaranjani G., Dhanasekaran D. Response surface methodology: a non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017;43(5):567–582. doi: 10.1080/1040841X.2016.1271308. [DOI] [PubMed] [Google Scholar]
- 39.Bezerra M.A., Ricardo E.S., Eliane P.O., Leonardo S.V., Luciane A.E. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Nor N.M., Mohd S.M., Teck C.L., Hooi L.F., Raha A.R., Joo S.T., Rosfarizan M. Comparative analyses on medium optimization using one-factor-at-a-time, response surface methodology, and artificial neural network for lysine–methionine biosynthesis by Pediococcus pentosaceus RF-1. Biotechnol. Biotechnol. Equip. 2017;31(5):935–947. [Google Scholar]
- 41.Lundstedt T., Seifert E., Abramo L., Thelin B., Nyström Å., Pettersen J., Bergman R. Experimental design and optimization. Chemometr. Intell. Lab. Syst. 1998;42(1–2):3–40. [Google Scholar]
- 42.Aydar A.Y. Utilization of response surface methodology in optimization of extraction of plant materials. Statistical approaches with emphasis on design of experiments applied to. chemical processes. 2018:157–169. [Google Scholar]
- 43.Izenman A.J. Modern multivariate statistical techniques. Regression, classification and manifold learning. 2008;10:978–980. [Google Scholar]
- 44.Desai K.M., Shrikant A.S., Parag S.S., S S.L., Rekha S.S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: case study of fermentative production of scleroglucan. Biochem. Eng. J. 2008;41(3):266–273. [Google Scholar]
- 45.Jha A.K., Nandan S. Comparison of response surface methodology (RSM) and artificial neural network (ANN) modelling for supercritical fluid extraction of phytochemicals from Terminalia chebula pulp and optimization using RSM coupled with desirability function (DF) and genetic algorithm (GA) and ANN with GA. Ind. Crops Prod. 2021;170 [Google Scholar]
- 46.Naik L., Mann B., Bajaj R., Sangwan R.B., Sharma R. Process optimization for the production of bio-functional whey protein hydrolysates: adopting response surface methodology. Int. J. Pept. Res. Therapeut. 2013;19(3):231–237. [Google Scholar]
- 47.Giri R., Sharma R.K. Fungal pretreatment of lignocellulosic biomass for the production of plant hormone by Pichia fermentans under submerged conditions. Bioresour. Bioprocess. 2020;(7):1. 1. [Google Scholar]
- 48.Bunsangiam S., Thongpae N., Limtong S., Srisuk N. Large scale production of indole-3-acetic acid and evaluation of the inhibitory effect of indole-3-acetic acid on weed growth. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-92305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheidt W., dos Santos Pedroza I.C.P., Fontana J., da Cruz Meleiro L.A., de Barros Soares L.H., Reis V.M. Optimization of culture medium and growth conditions of the plant growth-promoting bacterium Herbaspirillum seropedicae BR11417 for its use as an agricultural inoculant using response surface methodology (RSM) Plant Soil. 2020;451(1):75–87. [Google Scholar]
- 50.Benadjila A., Zamoum M., Aouar L., Zitouni A., Goudjal Y. Optimization of cultural conditions using response surface methodology and modeling of indole-3-acetic acid production by Saccharothrix texasensis MB15. Biocatal. Agric. Biotechnol. 2022;39 [Google Scholar]
- 51.Bunsangiam S., Sakpuntoon V., Srisuk N., Ohashi T., Fujiyama K., Limtong S. Biosynthetic pathway of indole-3-acetic acid in basidiomycetous yeast Rhodosporidiobolus fluvialis. MYCOBIOLOGY. 2019;47(3):292–300. doi: 10.1080/12298093.2019.1638672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park H.-Y., Park S.-H., Yoon H.-K., Han M.J., Kim D.-H. Anti-allergic activity of 18β-glycyrrhetinic acid-3-O-β-D-glucuronide. Arch Pharm. Res. (Seoul) 2004;27(1):57–60. doi: 10.1007/BF02980047. [DOI] [PubMed] [Google Scholar]
- 53.van de Sand L., Bormann M., Alt M., Schipper L., Heilingloh C.S., Steinmann E., Todt D., Dittmer U., Elsner C., Witzke O. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13(4):609. doi: 10.3390/v13040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng S.L., Khaw K.-Y., Ong Y.S., Goh H.P., Kifli N., Teh S.P., Ming L.C., Kotra V., Goh B.H. Licorice: a potential herb in overcoming SARS-CoV-2 infections. J. Evid-Based Integr. Méd. 2021;26 doi: 10.1177/2515690X21996662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ved D., Goraya G. Demand and supply of medicinal plants in India. NMPB, New Delhi & FRLHT, Bangalore, India. 2007;18(85):210–252. [Google Scholar]
- 56.Li G., Nikolic D., Van Breemen R.B. Identification and chemical standardization of licorice raw materials and dietary supplements using UHPLC-MS/MS. J. Agric. Food Chem. 2016;64(42):8062–8070. doi: 10.1021/acs.jafc.6b02954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He C., Cui J., Chen X., Wang W., Hou J. Effects of enhancement of liquorice plants with dark septate endophytes on the root growth, glycyrrhizic acid and glycyrrhizin accumulation amended with organic residues. Curr. Plant Biol. 2020;23 [Google Scholar]
- 58.Arora P., Wani Z.A., Ahmad T., Sultan P., Gupta S., Riyaz-Ul-Hassan S. Community structure, spatial distribution, diversity and functional characterization of culturable endophytic fungi associated with Glycyrrhiza glabra L. Fungal Biol. 2019;123(5):373–383. doi: 10.1016/j.funbio.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Gordon S.A., Weber R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26(1):192. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wani Z.A., Mirza D.N., Arora P., Riyaz-Ul-Hassan S. Molecular phylogeny, diversity, community structure, and plant growth promoting properties of fungal endophytes associated with the corms of saffron plant: an insight into the microbiome of Crocus sativus Linn. Fungal Biol. 2016;120(12):1509–1524. doi: 10.1016/j.funbio.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Jasim B., Jimtha John C., Shimil V., Jyothis M., Radhakrishnan E. Studies on the factors modulating indole-3-acetic acid production in endophytic bacterial isolates from Piper nigrum and molecular analysis of ipdc gene. J. Appl. Microbiol. 2014;117(3):786–799. doi: 10.1111/jam.12569. [DOI] [PubMed] [Google Scholar]
- 62.Manzoor M.M., Goyal P., Gupta A.P., Khan S., Jaswal P., Misra P., Pandotra P., Ahuja A., Vishwakarma R.A., Gupta S. Chemical and real-time based analysis revealed active gene machinery of glycyrrhizin biosynthesis and its accumulation in the aerial tissues of in-vitro regenerated Glycyrrhiza glabra L. Plant Growth Regul. 2020;92(2):263–271. [Google Scholar]
- 63.Khan S., Pandotra P., Manzoor M.M., Kushwaha M., Sharma R., Jain S., Ahuja A., Amancha V., Bhushan S., Guru S.K. Terpenoid and flavonoid spectrum of in-vitro cultures of Glycyrrhiza glabra revealed high chemical heterogeneity: platform to understand biosynthesis. Plant Cell Tissue Organ. Cult. 2016;124(3):507–516. doi: 10.1007/s11240-015-0910-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao C., Onyino J., Gao X. Current advances in the functional diversity and mechanisms underlying endophyte–plant interactions. Microorganisms. 2024;12(4):779. doi: 10.3390/microorganisms12040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia M., Chen L., Xin H.L., Zheng C.J., Rahman K., Han T., Qin L.P. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol. 2016;7:906. doi: 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baron N.C., Rigobelo E.C. Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology. 2022;13(1):39–55. doi: 10.1080/21501203.2021.1945699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atugala D., Deshappriya N. Effect of endophytic fungi on plant growth and blast disease incidence of two traditional rice varieties. J. Natl. Sci. Found. 2015;43(2) [Google Scholar]
- 68.Turbat A., Rakk D., Vigneshwari A., Kocsubé S., Thu H., Szepesi Á., Bakacsy L., D. Škrbić B., Jigjiddorj E.-A., Vágvölgyi C. Characterization of the plant growth-promoting activities of endophytic fungi isolated from Sophora flavescens. Microorganisms. 2020;8(5):683. doi: 10.3390/microorganisms8050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikram M., Ali N., Jan G., Jan F.G., Rahman I.U., Iqbal A., Hamayun M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0208150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahemad M., Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J. King Saud Univ. Sci. 2014;26:1–20. [Google Scholar]
- 71.Duca D., Lorv J., Patten C.L., Rose D., Glick B.R. Indole-3-acetic acid in plant–microbe interactions. Anton Leeuw. Int. J. G. 2014;106:85–125. doi: 10.1007/s10482-013-0095-y. [DOI] [PubMed] [Google Scholar]
- 72.Tivendale N.D., Ross J.J., Cohen J.D. The shifting paradigms of auxin biosynthesis. Trends Plant Sci. 2014;19:44–51. doi: 10.1016/j.tplants.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Keswani C., Singh S.P., Cueto L., García-Estrada C., Mezaache-Aichour S., Glare T.R., Borriss R., Singh S.P., Blázquez M.A., Sansinenea E. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020;104:8549–8565. doi: 10.1007/s00253-020-10890-8. [DOI] [PubMed] [Google Scholar]
- 74.Bose P., Gowrie S.U., Chathurdevi G. Optimization of culture conditions for growth and production of bioactive metabolites by endophytic fungus-Aspergillus tamarii. Int. J. Pharm. Biol. Sci. 2019;9:469–478. [Google Scholar]
- 75.Bose A., Shah D., Keharia H. Production of indole-3-acetic-acid (IAA) by the white rot fungus Pleurotus ostreatus under submerged condition of Jatropha seedcake. Mycology. 2013;4(2):103–111. [Google Scholar]
- 76.Napitupulu T.P., Kanti A., Sudiana I.M. Evaluation of the environmental factors modulating indole-3-acetic acid (IAA) production by Trichoderma harzianum InaCC F88. InIOP conference series: earth and environmental science. IOP Publishing. 2019;308(1) [Google Scholar]
- 77.Kishore D., Kundu S., Kayastha A.M. Thermal, chemical and pH induced denaturation of a multimeric β-galactosidase reveals multiple unfolding pathways. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teo H.L., Wahab R.A., Zainal-Abidin M.H., Mark-Lee W.F., Huyop F., Susanti E., Mahat N.A., Azman A.R. Statistically assisted optimisation for the simultaneous production of Trichoderma harzianum and Aspergillus fumigatus cellulolytic enzymes. Biomass Convers. Biorefin. 2024:1–7. [Google Scholar]
- 79.Nieto-Jacobo M.F., Steyaert J.M., Salazar-Badillo F.B., Nguyen D.V., Rostás M., Braithwaite M., De Souza J.T., Jimenez-Bremont J.F., Ohkura M., Stewart A., Mendoza-Mendoza A. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017;8:102. doi: 10.3389/fpls.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehmood A., Hussain A., Irshad M., Khan N., Hamayun M., Ismail Afridi S.G., Lee I.J. IAA and flavonoids modulates the association between maize roots and phytostimulant endophytic Aspergillus fumigatus greenish. J. Plant Interact. 2018;13(1):532–542. [Google Scholar]
- 81.Kumar S., Abedin M.M., Singh A.K., Das S. Role of phenolic compounds in plant-defensive mechanisms. Plant phenolics in sustainable agriculture. 2020;1:517–532. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.