Abstract
The ataxia telangiectasia mutated (ATM) protein kinase is best known as a master regulator of the DNA damage response. However, accumulating evidence has unveiled an equally vital function for ATM in sensing oxidative stress and orchestrating cellular antioxidant defenses to maintain redox homeostasis. ATM can be activated through a non-canonical pathway involving intermolecular disulfide crosslinking of the kinase dimers, distinct from its canonical activation by DNA double-strand breaks. Structural studies have elucidated the conformational changes that allow ATM to switch into an active redox-sensing state upon oxidation. Notably, loss of ATM function results in elevated reactive oxygen species (ROS) levels, altered antioxidant profiles, and mitochondrial dysfunction across multiple cell types and tissues. This oxidative stress arising from ATM deficiency has been implicated as a central driver of the neurodegenerative phenotypes in ataxia-telangiectasia (A-T) patients, potentially through mechanisms involving oxidative DNA damage, PARP hyperactivation, and widespread protein aggregation. Moreover, defective ATM oxidation sensing disrupts transcriptional programs and RNA metabolism, with detrimental impacts on neuronal homeostasis. Significantly, antioxidant therapy can ameliorate cellular and organismal abnormalities in various ATM-deficient models. This review synthesizes recent advances illuminating the multifaceted roles of ATM in preserving redox balance and mitigating oxidative insults, providing a unifying paradigm for understanding the complex pathogenesis of A-T disease.
Keywords: ATM, Ataxia, Mitochondria, Reactive oxygen species, DNA repair
1. Introduction
Oxidative stress, defined as a disturbance in the equilibrium between oxidant production and antioxidant defense mechanisms, represents a fundamental mechanism of cellular injury [1,2]. Reactive oxygen species (ROS), the primary oxidants, can indiscriminately react with and modify cellular macromolecules like DNA, proteins, and lipids, thereby disrupting their structural integrity and biological functions [1,3,4]. If unresolved, excessive ROS accumulation can trigger programmed cell death pathways, including apoptosis initiated by mitochondrial membrane permeabilization, as well as necrosis resulting from the disruption of plasma membrane ion gradients and eventual rupture following lipid peroxidation [1,5,6].
To counteract oxidative insults and maintain redox homeostasis, aerobic organisms have evolved sophisticated antioxidant defense systems comprising non-enzymatic and enzymatic components. Low molecular weight antioxidants such as reduced glutathione (GSH), ascorbic acid (vitamin C), α-tocopherol (vitamin E), uric acid, carotenoids, and ubiquinones constitute the non-enzymatic arm [[7], [8], [9], [10], [11]]. These reductants can directly quench ROS through electron transfer mechanisms or act as cofactors for enzymatic antioxidants. The enzymatic defense machinery includes superoxide dismutases (SODs), which catalyze the dismutation of superoxide radicals to hydrogen peroxide (H2O2) and molecular oxygen [11]. H2O2 is subsequently detoxified by catalases or the GSH-dependent glutathione peroxidase (GPx) family of enzymes. Furthermore, the glutathione system, encompassing GSH, glutathione reductase (GR), and glutathione S-transferases (GSTs), plays a pivotal role in redox cycling and xenobiotic conjugation reactions [12].
In response to oxidative stress, cells mount an adaptive response characterized by the transcriptional upregulation and/or post-translational activation of various antioxidant proteins [13]. Consequently, the expression levels and activities of these enzymes often serve as reliable biomarkers indicative of oxidative insult across diverse pathophysiological contexts. However, when the oxidative burden overwhelms the cellular antioxidant capacity, deleterious oxidative modifications to biomolecules may ensue, compromising organelle function and ultimately triggering cell death cascades [1,13]. Thus, maintaining an optimal redox equilibrium is crucial for cellular homeostasis and survival.
2. Distinct pathways of ATM activation by DNA damage and oxidation
The ataxia telangiectasia mutated (ATM) protein kinase, a master regulator of the DNA damage response (DDR), can be activated through distinct mechanisms upon exposure to DNA double-strand breaks (DSBs) or oxidative stress (Fig. 1) [[14], [15], [16], [17]]. The canonical form of ATM activation is dependent on both DSBs and the MRE11-RAD50-NBS1 (MRN) complex, which recruits the ATM homodimer, induces monomerization of the kinase and promotes interaction of the ATM monomers with DNA ends and with its substrates [[18], [19], [20]]. This process is facilitated by MRN and involves ATM trans-autophosphorylation.
Fig. 1.
ATM's Dual Activation Mechanisms
The ATM kinase can be activated through two distinct pathways, illustrated side-by-side.
(Top) Canonical DNA double-strand break (DSB)-induced activation pathway: In response to DNA damage, the MRN complex senses and binds to DSBs, recruiting and activating the ATM kinase. This causes ATM monomerization from an inactive dimer and initiates phosphorylation of canonical substrates involved in DNA repair and cell cycle control.
(Bottom) Oxidation-induced activation pathway: Oxidative stress, particularly increased hydrogen peroxide (H2O2) levels, leads to the formation of intermolecular disulfide bonds between the monomers of the ATM dimer. This oxidation-dependent activation is mediated by the C2991 residue and results in the phosphorylation of distinct substrates involved in antioxidant responses, mitochondrial homeostasis, and protein aggregation.
In contrast, oxidative stress triggers the formation of an active dimeric ATM species covalently linked via intermolecular disulfide bonds, a process independent of the MRN complex, DNA ends, or ATM autophosphorylation [21,22]. Several disulfide bonds were mapped in this dimer form, including at Cys2991 (C2991) in the PIKK regulatory domain (PRD), which regulates ATM activation by oxidative stress; mutations in this site (e.g. C2991L) cause defects in hydrogen peroxide (H2O2)-mediated ATM activation in vitro and in human cells [23].
Separation-of-function ATM mutants have provided crucial insights into these divergent activation pathways. The oxidation-deficient C2991L mutant remains competent for MRN/DNA-dependent activation yet cannot be activated by oxidants like H2O2. Conversely, the MRN/DNA-binding deficient R2579A/R2580A mutant selectively abrogates activation in response to DSBs while retaining oxidation-induced activation capabilities [22,23].
Remarkably, oxidation-activated ATM exhibits distinct substrate preferences compared to its DSB-activated counterpart. Global phosphoproteomic analyses revealed that while both activation modes converge on some common substrates, oxidation-induced ATM selectively impacts a subset of phosphorylation events largely regulated by the CK2 kinase [17,22,23]. Furthermore, unlike DSB-activated ATM, the oxidized form fails to induce phosphorylation of canonical DSB markers such as γH2AX and KAP1.
These findings uncover an oxidation-sensing role for the ATM kinase, whereby it engages a specific subset of downstream effectors distinct from its canonical DDR functions upon oxidative insults. This redox-regulated ATM signaling axis likely constitutes an important cellular stress response pathway. Elucidating the molecular underpinnings and physiological implications of this oxidation-induced ATM activation may reveal novel strategies for therapeutic intervention in oxidative stress-related pathologies, including the neurodegenerative disorder ataxia-telangiectasia.
3. ATM as a sensor of oxidative stress and regulator of ROS levels
Mounting evidence indicates that the ATM protein itself functions as a critical sensor and regulator of oxidative stress within cells (Fig. 2) [17,22,24]. Numerous studies have reported significant accumulation of ROS, particularly hydrogen peroxide (H2O2), in ATM-deficient models, underscoring ATM's pivotal role in maintaining redox homeostasis [17]. A novel mode of ATM activation occurs through the formation of intermolecular disulfide bonds between the monomers of the ATM dimer in response to oxidants like H2O2 [22,23]. This oxidation-induced activation is dependent on the Cys2991 residue in the PIKK regulatory domain, as mutation of this cysteine (e.g. C2991L) abrogates ATM activation by H2O2 in vitro and in human cells [22,23].
Fig. 2.
Interaction Between ATM and Antioxidant Defense Systems
ATM shows multifaceted roles in maintaining mitochondrial homeostasis through four key mechanisms: regulating mitochondrial DNA (mtDNA) levels by upregulating ribonucleotide reductase (RNR), promoting mitochondrial biogenesis via phosphorylation and nuclear translocation of NRF-1 to induce mitochondrial genes, enhancing cellular antioxidant capacity by inducing glucose-6-phosphate dehydrogenase (G6PD) expression and NADPH production, and orchestrating mitophagy of damaged mitochondria through a signaling cascade involving CHK2, GSNOR, and Beclin-1-mediated autophagosome formation.
In cerebellar and cerebral tissues of ATM knockout mice, altered levels of antioxidants like glutathione (GSH), cysteine, and the redox protein thioredoxin suggest compensatory responses to elevated oxidative stress [11]. Furthermore, decreased catalase activity coupled with increased superoxide dismutase (SOD) activity in these brain regions implies higher H2O2 levels due to impaired scavenging and enhanced generation from superoxide [11]. Similar findings of reduced catalase activity and increased SOD levels have been observed in ATM-deficient lymphoblasts [25], fibroblasts [26,27], and astrocytes [28].
Direct measurements consistently reveal significantly higher intracellular ROS levels, especially H2O2, in various ATM-deficient cell types, including hematopoietic stem cells [29], cerebellar neurons, and specific brain regions like the cerebellum, basal ganglia, hippocampal CA1, and substantia nigra [30]. Importantly, transient ATM depletion or expression of the oxidation-resistant ATM mutant (C2991L) in normal cells can also lead to increased ROS accumulation [31], highlighting ATM's crucial role in regulating redox homeostasis. Mechanistically, ATM promotes the activity and expression of antioxidant enzymes like glucose-6-phosphate dehydrogenase (G6PD) in mitochondria [24]. During oxidative stress, ATM also induces the transcription factor NRF1, which upregulates genes involved in mitochondrial functions [32]. Moreover, A-T patients exhibit slightly lower plasma antioxidant levels compared to healthy controls [33,34], potentially rendering their cells more susceptible to oxidative damage.
Furthermore, ATM regulates the Nrf2-ARE (Nuclear factor erythroid 2-related factor 2 - Antioxidant Response Element) pathway, a crucial defense mechanism against oxidative stress [35]. The Nrf2-ARE pathway is protective in neurodegenerative conditions by reducing oxidative stress and neuroinflammation, making it a potential therapeutic target [35,36]. Nrf2 induces expression of cytoprotective and detoxifying genes, and is essential for the induction of many detoxification enzymes via the ARE enhancer sequence [35]. Nrf2 is regulated by Keap1, which binds and suppresses it. NRF2 can be activated directly by ROS oxidizing Keap1, or indirectly via ATM blocking Nrf2 degradation through the tumor suppressor BRCA1 by increasing its stability [[37], [38], [39]]. Upregulated Nrf2 induces antioxidant genes like HO-1, NQO1, GPx-1 and CAT to bolster the cellular antioxidant defense system [40].
Downstream of oxidative activation, ATM orchestrates a signaling cascade involving the kinase CHK2 and subsequent induction of the denitrosylase GSNOR and autophagy regulator Beclin1 to promote the clearance of damaged mitochondria via mitophagy [41,42]. Furthermore, agents that specifically induce mitochondrial ROS production, such as menadione, are sufficient to engage the oxidation-dependent activation of ATM [24]. Recent findings.
These findings firmly establish ATM as a key sensor and regulator of oxidative stress, with its deficiency leading to ROS accumulation that likely drives various pathological features of A-T. Modulating this redox imbalance through antioxidant therapies could therefore represent a promising strategy for managing this disorder.
4. Mitochondrial aberrations and ATM's role in redox homeostasis
Accumulating evidence positions ATM as a crucial sensor and regulator of mitochondrial homeostasis and cellular redox balance. Cells deficient in ATM exhibit transcriptional changes indicative of mitochondrial dysfunction, including upregulated expression of mitochondrial DNA repair and ROS-scavenging genes [43,44]. Functionally, these cells display impaired mitochondrial respiration, reduced membrane potential, defective mitophagy, and increased mitochondrial mass - phenotypes that are not rescued by oxidation-deficient ATM mutants, directly linking ATM's redox functions to mitochondrial quality control (Fig. 3) [[43], [44], [45]].
Fig. 3.
Interaction Between ATM and Antioxidant Defense Systems
The ATM kinase interacts with and regulates various antioxidant defense systems to maintain cellular redox homeostasis, acting as a central hub that stabilizes the transcription factor NRF2 through inhibition of KEAP1 by BRCA1, leading to the expression of antioxidant enzymes like NQO1, HO-1, GPx, and CAT, while also enhancing mitochondrial function and biogenesis to increase mitochondrial antioxidant capacity via upregulation of NRF1 and G6PD, as well as directly interacting with and activating antioxidant proteins such as peroxiredoxins and thioredoxins through post-translational modifications to boost their enzymatic activity.
A key mechanism by which ATM preserves mitochondrial integrity appears to be through the regulation of mitochondrial DNA (mtDNA) homeostasis. ATM inhibition or depletion decreases the expression of RR (ribonucleotide reductase) subunits, resulting in lower mtDNA levels [46], suggesting a role for ATM in maintaining mtDNA content. Furthermore, ATM has been shown to phosphorylate and activate the transcription factor NRF-1 in response to oxidative stress, promoting its nuclear localization and transcriptional activation of mitochondrial biogenesis genes; expression of a phosphomimetic NRF-1 mutant rescued mitochondrial dysfunction in ATM-deficient neurons [32].
In addition to preserving mitochondrial biogenesis and function, ATM plays a pivotal role in sensing and mitigating mitochondrial oxidative stress. In ATM-deficient cells, mitochondrial dysfunction coincides with elevated mitochondrial ROS levels and aberrant mitophagy, phenotypes that can be rescued by partial depletion of the autophagy regulator Beclin-1 to restore mitophagy [42,44]. Interestingly, ATM localizes to mitochondria and can be activated upon mitochondrial damage, even in the absence of nuclear DNA lesions. Furthermore, activation of ATM by mitochondrial hydrogen peroxide promotes its dimerization and upregulates the expression of glucose-6-phosphate dehydrogenase (G6PD) and the pentose phosphate pathway, thereby increasing NADPH and cellular antioxidant capacity [24]. These findings suggest that ATM senses mitochondrial ROS signals and engages transcriptional programs to restore redox homeostasis.
Recent findings have more elucidated a molecular pathway by which ATM and its downstream effector CHK2 initiate autophagy in response to oxidative stress [47]. Upon exposure to ROS, ATM becomes phosphorylated at S1981, leading to the subsequent phosphorylation of CHK2 at T68. Activated CHK2 then binds and phosphorylates the E3 ubiquitin ligase TRIM32 at S55, enabling TRIM32 to catalyze K63-linked ubiquitination of the autophagy protein ATG7 at K45. This post-translational modification of ATG7 is crucial for initiating the autophagy process [47] and mitophagy since ATG7 is one of the factor for regulating mitochondrial clearance [48].
Moreover, ATM restrains mitochondrial ROS production by regulating the expression of the ROS-producing enzyme NOX4, which is abnormally upregulated in A-T cells and contributes to elevated oxidative DNA damage and replicative defects [49]. Collectively, these studies highlight the multifaceted roles of ATM in preserving mitochondrial function, maintaining redox balance, and mitigating oxidative stress – processes that are critically dysregulated in the absence of ATM and likely contribute to the neurodegenerative pathology observed in ataxia-telangiectasia.
5. Structural insights into ATM activation by oxidative stress vs DNA damage
The ATM kinase, a member of the phosphoinositide 3-kinase-related kinase (PIKK) family, exists as an inactive homodimer with the kinase active sites occluded by the PIKK regulatory domain (PRD) helices in an auto-inhibited “butterfly-like” conformation [[50], [51], [52], [53], [54], [55], [56], [57], [58], [59]]. Recent high-resolution cryo-electron microscopy structures have revealed the molecular architecture of this inactive ATM dimer, which exhibits an extensive dimerization interface mediated by HEAT repeats [50,[54], [55], [56],59]. ATM can be activated via two distinct mechanisms - by oxidative stress or by DNA double-strand break (DSB) sensing.
In the conventional DNA damage response pathway, it was proposed that the Mre11-Rad50-Nbs1 (MRN) complex detects DSBs and directly activates ATM by promoting dissociation of the inactive dimer into monomers, thereby relieving the inhibition imposed by the PRD helices [[60], [61], [62]].
In contrast, oxidative activation of ATM by hydrogen peroxide (H2O2) follows a unique mechanism involving formation of an intermolecular disulfide bond between the Cys2991 residues of the two monomers [23,31]. This disulfide crosslinking stabilizes the ATM dimer but in a dramatically different rotated conformation compared to the inactive state [63]. Accompanying this rotation is the displacement of the inhibitory PRD helices from the substrate binding cleft [63]. Moreover, the kinase N-lobe twists relative to the C-lobe into a catalytically competent active conformation akin to activated mTOR [63]. Interestingly, two distinct conformations were observed for human ATM – a closed symmetrical dimer and an open asymmetrical dimer, with the latter suggesting conformational changes that could facilitate substrate binding [50].
The cryo-EM structure of H2O2-activated ATM bound to a p53 peptide substrate revealed the molecular basis of this redox activation mechanism [63]. A key aspect is that in the basal dimer state, the PRD loop harboring Cys2991 is not ideally positioned for disulfide formation across the dimer interface [63]. However, the conformational changes triggered by oxidation allow the disulfide to form, stabilizing the activated rotated dimeric state [63]. Several known regulatory sites on ATM, including Ser1981, Ser2996, Cys2991, and Lys3016 (the acetylation site), are located in disordered loops in close proximity to the PRD [50,60,64]. Post-translational modifications at these sites after oxidative stress may disrupt the interactions between the PRD and the active site, thereby promoting ATM activation.
Therefore, while both activation pathways involve alleviating the inhibition imposed by the PRD element, oxidative stress promotes disulfide-crosslinking and conformational changes to rotate the dimer into an active state [63]. This contrasts with the MRN/DNA damage pathway proposed to drive dimer dissociation into monomers for activation [[60], [61], [62]]. This oxidation-specific mechanism enables ATM to function as a critical cellular redox sensor [17,23,31].
6. Oxidative stress drives neurodegeneration in A-T
Mitochondria are the major source of cellular ROS, generated as byproducts of oxidative phosphorylation and the electron transport chain [1,65]. Neurons are particularly susceptible to mitochondrial oxidative stress due to their high energy demands and reliance on mitochondrial respiration [1,66]. Impaired mitochondrial function and elevated ROS levels have been implicated in various neurodegenerative disorders, including Parkinson's, Alzheimer's, ALS, and Huntington's disease [67].
Loss of functional ATM kinase has been directly linked to elevated oxidative damage, proposed as an underlying mechanism driving the neuronal degeneration and ataxic phenotypes in A-T patients [66,68,69]. Studies in ATM-deficient mouse models revealed significantly increased oxidative damage to proteins and elevated oxidative stress markers specifically in the brain and cerebellum, but not other organs [70]. This oxidative insult preferentially impacts neural cells, as ATM-null astrocytes and neural stem cells exhibited impaired growth, premature senescence, and earlier death in culture – phenotypes rescued by antioxidant treatment or inhibition of ROS-induced signaling pathways like ERK1/2 and p38 MAPK [71,72].
Accumulation of intracellular ROS in ATM-deficient cells also impairs self-renewal and longevity of hematopoietic stem cells (HSCs) by upregulating p16INK4a, leading to Rb inactivation and p38 MAPK activation. Notably, treatment with antioxidants or p38 inhibition extended the lifespan of ATM-null HSCs [29]. Collectively, these findings implicate elevated oxidative stress as a common pathogenic factor driving the developmental defects and neurodegeneration observed upon ATM loss, with a particular impact on the cerebellum and neuronal compartments.
At the molecular level, cells lacking functional ATM exhibit significantly elevated levels of oxidative DNA lesions like 8-hydroxy-2′-deoxyguanosine (8-OHdG) as well as an increased burden of single-strand breaks (SSBs) compared to normal cells [34,49,73]. The formation of these SSBs is dependent on ROS, as treatment with the antioxidant N-acetylcysteine (NAC) can prevent their accumulation in ATM-depleted human cell lines [73,74]. A downstream consequence of unresolved oxidative DNA damage appears to be widespread protein aggregation. Loss of ATM's oxidation-sensing capability promotes the formation of detergent-resistant protein aggregates in a ROS-dependent manner across multiple human cell types, including brain-derived glioma and neuroblastoma cells [31,74]. Mass spectrometry analysis revealed over 1100 polypeptides significantly enriched in these insoluble aggregates isolated from A-T patient cerebellum samples compared to healthy controls [74].
The accumulation of protein aggregates correlates with signs of poly(ADP-ribose) polymerase (PARP) hyperactivation, as indicated by elevated poly(ADP-ribose) (PAR) levels detected by immunohistochemistry in A-T granule cells [74]. This suggests a model where ROS accumulating in ATM-deficient neurons triggers a pathological cycle of oxidative DNA damage, PARP enzyme hyperactivation due to unrepaired DNA lesions, and ultimately the widespread aggregation of proteins into an insoluble state [31,74]. The cerebellar enrichment of these protein deposits aligns with the cerebellum being the primary region affected by the neurodegenerative process in A-T.
In summary, oxidative stress emerges as a key instigating factor driving the molecular pathogenesis of neurodegeneration in A-T. The ROS burden arising from ATM dysfunction initiates a cascade involving DNA damage, PARP hyperactivation, and finally irreversible protein aggregation, which ultimately disrupts proteostasis and neuronal viability in the cerebellum. The ATM kinase therefore plays a critical role in maintaining mitochondrial redox homeostasis and quality control processes by acting as a mitochondrial ROS sensor to engage antioxidant responses, mitochondrial biogenesis, and clearance programs. Disruption of these protective mechanisms in A-T likely fuels excessive mitochondrial ROS production and oxidative damage that preferentially impacts high energy-demanding neurons, providing a unifying paradigm for the neurodegenerative phenotypes of this disorder.
7. Oxidative stress and transcriptional dysregulation during neurodegeneration in A-T disease
Cells derived from A-T patients and ATM-null mouse models exhibit significantly elevated levels of ROS, altered redox homeostasis, and heightened antioxidant responses compared to controls [11,12,28,30,33,34,[75], [76], [77]], suggesting that loss of ATM function compromises the cellular ability to mitigate oxidative insults. Importantly, ATM itself can be activated through a non-canonical pathway independent of double-strand DNA breaks. This alternative mode of activation involves the formation of intermolecular disulfide bonds between the two monomers of the ATM dimer in response to oxidative stress [23,78]. The source of ROS capable of eliciting this oxidation-dependent ATM activation has been traced to dysfunctional mitochondria [31].
Using separation-of-function ATM mutants, specific variants like R3047X have been identified that abrogate oxidation-dependent activation while retaining DNA damage-induced activation [23,[79], [80], [81]]. Cells lacking ATM oxidation sensing displayed elevated ROS levels, mitochondrial dysfunction, defective ROS-induced checkpoints and autophagy, as well as increased nuclear protein aggregation [31,74]. Notably, oxidation-deficient cells accumulated transcription-dependent single-strand DNA (ssDNA) breaks, a form of genome instability linked to cerebellar neurodegeneration [74,82]. Evidence indicates that ssDNA breaks in ATM-deficient cells arise from persistent RNA-DNA hybrids (R-loops) that induce hyperactivation of PARP1/2, driving protein aggregation [74,83].
Through genome-wide mapping, it has been revealed that loss of ATM activity increases R-loop levels preferentially at promoter regions and GC-rich sequences, coinciding with poly(ADP-ribose) (PAR) accumulation [74,83]. These oxidative genomic lesions correlated with reduced expression of highly transcribed, GC-rich genes in A-T patient cerebellum, many implicated in cerebellar ataxias [83]. Mechanistically, it is proposed that in the absence of ATM's redox functions, elevated ROS triggers transcriptional stress manifested as persistent R-loops. The resulting ssDNA breaks hyper-activate PARP1/2, depleting cellular NAD + pools and promoting aberrant PAR signaling that disrupts transcription [74]. Over time, this oxidative damage accumulates preferentially at highly expressed, GC-rich loci like those encoding calcium signaling proteins (e.g. ITPR1, CA8), progressively disrupting transcriptional programs vital for cerebellar neuron function and survival [[83], [84], [85], [86], [87], [88]].
In addition to its roles in the DNA damage response, ATM also regulates transcriptional processes that may contribute to neurodegeneration when disrupted (Fig. 4). Exposure to ionizing radiation has been shown to induce alternative splicing of pre-mRNAs in an ATM-dependent manner [[89], [90], [91]]. Similar R-loop accumulation has been observed in ATM-deficient systems like mouse testes where it correlates with elevated DNA damage and apoptosis [92]. In plant models, ATM regulates alternative splicing of mitochondrial transcripts like nad2 in response to genotoxic stress [93], indicating an evolutionarily conserved role in coupling mitochondrial function to gene expression programs.
Fig. 4.
Transcriptional Dysregulation in A-T Disease
The transcriptional dysregulation occurs in neurons affected by Ataxia-Telangiectasia (A–T) disease, contrasted with the normal transcriptional processes in healthy neurons.
(Left) In healthy neuron (blue), orderly transcription occurs at a GC-rich promoter, with an active RNA polymerase synthesizing RNA, suggesting that GC-rich genes, ITPR1 and CA8, show normal expression levels. Additionally, functional ATM is shown activating splicing factors, enabling proper RNA splicing.
(Right) In an A-T neuron (orange), transcription is disrupted at a GC-rich promoter, with an R-loop formation. The RNA polymerase II is blocked, unable to transcribe, followed by the reduced expression of ITPR1 and CA8. In the absence of functional ATM, splicing factors remain inactive, leading to impaired splicing. Furthermore, accumulation of PAR chains is observed, suggesting PARP hyperactivation due to unresolved DNA damage. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These findings highlight a previously unappreciated role for ATM in safeguarding transcriptome integrity via oxidation sensing and R-loop resolution. Oxidative stress-induced transcriptional dysregulation, preferentially impacting highly transcribed cerebellar genes encoding essential proteins like calcium signaling factors, likely represents a central pathogenic mechanism underlying the region-specific neurodegeneration in A-T. Therapeutic strategies to ameliorate oxidative damage and restore redox balance may offer novel interventions for this devastating disease.
8. ATM deficiency and the role of antioxidants/reducing agents
Accumulating evidence strongly implicates oxidative stress as a key contributor to the clinical manifestations of A-T, a disorder caused by deficiency in the ATM protein kinase. Treatment with various antioxidants and reducing agents has been shown to ameliorate multiple phenotypic abnormalities associated with ATM deficiency in cellular and animal models (Fig. 5) [17].
Fig. 5.
Therapeutic Interventions in A-T.
This figure illustrates the diverse beneficial effects of antioxidant therapies in various models of ataxia-telangiectasia (A–T). It depicts the antioxidant NAC reducing ROS levels, restoring mitochondrial function, preventing protein aggregation, and alleviating the accumulation of topoisomerase 1-DNA covalent complexes (TOP1cc) in astrocytes. It also indicates that the antioxidant EUK-189 can normalize brain fatty acid levels and extend lifespan in treated mice. Together, these illustrations highlight the potential of antioxidants like NAC and EUK-189 to ameliorate various pathological processes underlying A-T through their diverse mechanisms of action.
Administration of the catalytic antioxidant EUK-189, which has superoxide dismutase and catalase activities, corrected neurobehavioral deficits, normalized brain fatty acid levels, and extended lifespan in ATM knockout mice [94]. Similarly, antioxidant treatment with isoindoline nitroxide rescued impaired Purkinje neuron survival and dendritic differentiation in ATM-null models, underscoring oxidative stress in cerebellar degeneration [95].
NAC, a thiol-containing compound, exhibits multifaceted antioxidant and cytoprotective properties attributed to three main mechanisms [96]. Firstly, the free thiol group in NAC confers disulfide reductant capacity, allowing it to reduce extracellular and intracellular disulfide bonds, which is beneficial in conditions associated with oxidative stress and protein misfolding [97]. Secondly, the sulfhydryl group enables NAC to directly scavenge and neutralize various oxidants, such as hydrogen peroxide, hypochlorous acid, and highly reactive hydroxyl radicals, counteracting oxidative stress and mitigating the deleterious effects of reactive oxygen species [98]. Thirdly, NAC serves as a precursor for the synthesis of the endogenous antioxidant glutathione (GSH), boosting intracellular GSH levels by providing cysteine, the rate-limiting substrate for GSH biosynthesis, thereby enhancing the cellular redox balance and protection against oxidative damage [96].
In ATM-null mice, NAC increased lifespan, reduced ROS, restored mitochondrial membrane potential, and delayed lymphoma development [45]. Additionally, NAC prevented T cell apoptosis [99], premature senescence, and defective T cell development in ATM-deficient cells/mice [29]. Remarkably, NAC relieved widespread protein aggregation, including of CK2β, observed in ATM-deficient lymphoblastoid cells and cells expressing an oxidation-resistant ATM variant [74], suggesting ROS drive this aggregation.
Studies have also revealed ROS-scavenging by NAC can reduce detrimental phenotypes. NAC reduced accumulation of topoisomerase 1-DNA covalent complexes (TOP1cc) in ATM-deficient astrocytes [100], eliminated ROS-dependent single-strand DNA breaks in ATM-depleted human cells [74], and normalized metabolic dysregulation related to the TCA cycle in cells expressing an oxidation-sensing ATM mutant [24]. Moreover, NAC supplementation conferred lifespan extension in ATM-deficient mice and nematode models [101], potentially by alleviating PARP1 hyperactivation driven by unrepaired oxidative lesions.
Collectively, these findings provide compelling evidence that oxidative stress is a central driver of ATM deficiency pathologies. Antioxidant or reducing agent-based therapies thus hold significant therapeutic potential for ameliorating diverse aspects of the complex A-T phenotype.
8.1. Concluding remark
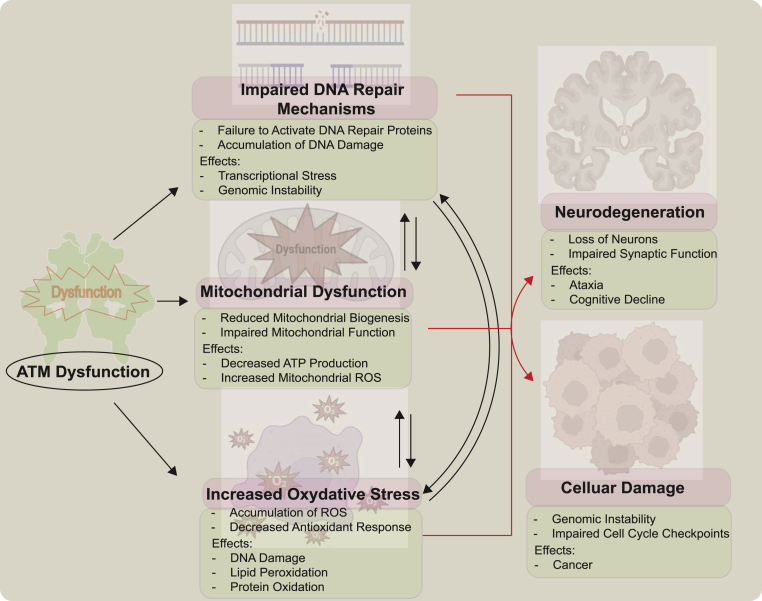
While the link between ATM dysfunction and increased oxidative stress leading to the Ataxia-Telangiectasia phenotype has been established, the precise mechanisms by which ATM maintains redox homeostasis remain incompletely understood (Fig. 6). Several potential mechanisms have been proposed:
Fig. 6.
Consequences of ATM Dysfunction in Redox Homeostasis
Dysfunction of the ATM kinase, which is crucial for maintaining redox homeostasis, leads to a cascade of detrimental cellular events including accumulation of reactive oxygen species (ROS) due to reduced antioxidant responses, oxidative damage to DNA, lipids, and proteins, impaired repair of oxidative DNA lesions resulting in genomic instability, disruption of cell cycle checkpoints and p53 activation causing uncontrolled cell cycle progression and apoptosis, compromised mitochondrial biogenesis and function with reduced ATP production and increased mitochondrial ROS generation exacerbating oxidative stress, ultimately culminating in neuronal damage and degeneration manifesting as the neurodegenerative symptoms characteristic of Ataxia-Telangiectasia (A–T).
Transcriptional Regulation: ATM may modulate redox balance by regulating the transcription of genes involved in antioxidant responses and mitochondrial function. The transcription factors NRF-1 and NRF-2, which are influenced by ATM, play crucial roles in regulating mitochondrial ROS levels and antioxidant gene expression, respectively [102,103]. Additionally, the histone protein H2AX, a target of ATM, is implicated in maintaining mitochondrial homeostasis and ROS regulation [102,103].
RNA Splicing: ATM's involvement in pre-mRNA processing [104] suggests that RNA splicing regulation could contribute to its role in redox homeostasis. Supporting this, cells expressing an oxidation-defective ATM mutant (C2991L) accumulate protein aggregates enriched in factors related to DNA metabolism and gene expression, indicating potential sequestration of proteins involved in ROS homeostasis and mitochondrial function [31].
Protein Aggregation: Aberrant protein aggregation, a common feature in neurodegenerative diseases like Parkinson's and Alzheimer's [105], has been observed in ATM-deficient cells. Notably, this aggregation is dependent on elevated oxidative stress, as treatment with the antioxidant NAC rescues the specific aggregation pattern [31]. Disrupted proteostasis due to protein aggregation may contribute to the A-T pathology.
Despite these insights, several fundamental questions remain unanswered regarding the mechanisms underlying A-T neurodegeneration:
-
1.
What is the role of ROS, given the involvement of the ROS-activated ATM pathway in neurodegeneration [17,23], and how ROS contributes to elevated DNA damage in A-T cells/tissues, potentially via mitochondrial dysregulation [31,32,43,44]. However, this mitochondrial link requires more direct investigation in future studies.
-
2.
What are the precise molecular mechanisms by which loss of ATM function leads to transcriptional changes and neurodegeneration in the cerebellum of A-T patients? The potential contribution of R-loops, which are overabundant in ATM-deficient neurons/cells [74,83,106], to cytoplasmic RNA/DNA hybrid accumulation and innate immunity activation, as R-loops can be excised to the cytoplasm under certain conditions [107].
-
3.
Why are highly expressed genes, particularly those involved in Purkinje cell function and high GC content, preferentially downregulated in A-T patient cerebellum tissues? Recent article shows that there is different patterns of transcripts specific to Purkinje cells in between A-T and control cerebellum [83]. The potential role of DNA sequence context in transcriptional dysregulation could be further investigated.
-
4.
What are the potential therapeutic strategies that could be developed based on the findings related to transcriptional stress, RNA-DNA hybrids, and DNA damage in ATM-deficient cells? Antioxidant treatment or expression of RNA-DNA helicases could reduce the levels of single-stranded breaks in neuron-likes cells [83].
-
5.
What is the role of single-strand DNA breaks observed in ATM-deficient cells, which are linked to cerebellar dysfunction in other DNA repair syndromes [82,108]?
-
6.
What are precise roles of PARP hyperactivation, NAD + depletion, and protein aggregation in driving neurotoxicity and Purkinje cell loss in A-T cerebellum. Parylation levels are higher in cerebellum tissue from humans with A-T compared with control subjects [74].
-
7.
What are the implications of dysregulated calcium signaling, with components of the inositol phosphate pathway like ITPR1 and CA8 being downregulated in A-T cerebellum and mouse models [74,[83], [84], [85], [86], [87], [88],106,109], and whether this is a cause or consequence of neurodegeneration, potentially related to impaired ER-mitochondrial crosstalk [110]?
Elucidating the interplay between these mechanisms and ATM's functions in maintaining redox homeostasis is crucial for developing treatments to slow the progressive neurodegeneration and ataxia in A-T patients.
Author disclosure statement
No competing financial interests exist.
Funding
This research was supported by the National Research Foundation of Korea (NRF), funded by the Korean government (MSIT), South Korea (RS-2024-00339483) and by Chonnam National University, South Korea (2023-0914-01).
CRediT authorship contribution statement
Ji-Hoon Lee: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I thanks Tanya Paull and the members of the Lee laboratory for helpful comments.
Abbreviations
- ATM:
Ataxia-telangiectasia mutated
- ROS:
reactive oxygen species
- PIKKs:
phosphatidylinositol 3-kinase-like kinases
- DSB:
double strand break
- GSH:
reduced glutathione
- NADPH:
Reduced nicotinamide adenine dinucleotide phosphate
- MRN:
Mre11-Rad50-Nbs1
- CL:
C2991L allele of ATM, deficient in oxidative activation (6)
- 2RA:
R2579A/R2580A allele of ATM, deficient in MRN-mediated activation (35)
- HSC:
hematopoietic stem cell
- NAC:
N-acetyl cysteine
- MAPK:
mitogen-activated protein kinase
- G6PD:
glucose-6-phosphate dehydrogenase
- RR:
ribonucleotide reductase
- mtDNA:
mitochondrial DNA
- NRF1:
Nuclear respiratory factor 1
- NRF2:
Nuclear erythroid 2-related factor
Data availability
No data was used for the research described in the article.
References
- 1.Simonian N.A., Coyle J.T. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 2.Dossena S., Marino A. Cellular oxidative stress. Antioxidants. 2021;10:399. doi: 10.3390/antiox10030399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019;20:2407. doi: 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jena N.R. DNA damage by reactive species: mechanisms, mutation and repair. J. Biosci. 2012;37:503–517. doi: 10.1007/s12038-012-9218-2. [DOI] [PubMed] [Google Scholar]
- 5.Vakifahmetoglu-Norberg H., Ouchida A.T., Norberg E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 6.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkman H.N., Rolfo M., Ferraris A.M., Gaetani G.F. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J. Biol. Chem. 1999;274:13908–13914. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- 9.Sies H., Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995;62:1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 10.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic. Biol. Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Kamsler A., Daily D., Hochman A., Stern N., Shiloh Y., Rotman G., Barzilai A. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61:1849–1854. [PubMed] [Google Scholar]
- 12.Barzilai A., Rotman G., Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair. 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 13.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiloh Y., Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013;14:197–210. doi: 10.1038/nrm3546. [DOI] [PubMed] [Google Scholar]
- 15.Ditch S., Paull T.T. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem. Sci. 2011;37:15–22. doi: 10.1016/j.tibs.2011.10.002. S0968-0004(11)00161-7[pii] 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J.H., Paull T.T. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. 1210872[pii]10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.-H., Paull T.T. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat. Rev. Mol. Cell Biol. 2021 doi: 10.1038/s41580-021-00394-2. [DOI] [PubMed] [Google Scholar]
- 18.Paull T.T. Mechanisms of ATM activation. Annu. Rev. Biochem. 2015;84:711–738. doi: 10.1146/annurev-biochem-060614-034335. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.H., Paull T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 20.Uziel T., Lerenthal Y., Moyal L., Andegeko Y., Mittelman L., Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. 330/6003/517[pii]10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.-H., Mand M.R., Kao C.-H., Zhou Y., Ryu S.W., Richards A.L., Coon J.J., Paull T.T. ATM directs DNA damage responses and proteostasis via genetically separable pathways. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aan5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. 330/6003/517[pii]10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Lee J.-H., Paull T.T., Gehrke S., D'Alessandro A., Dou Q., Gladyshev V.N., Schroeder E.A., Steyl S.K., Christian B.E., Shadel G.S. Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aaq0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K.H., Abe S., Yanabe Y., Matsuda I., Yoshida M.C. Superoxide dismutase activity and chromosome damage in cultured chromosome instability syndrome cells. Mutat. Res. 1990;244:251–256. doi: 10.1016/0165-7992(90)90137-9. [DOI] [PubMed] [Google Scholar]
- 26.Vuillaume M., Calvayrac R., Best-Belpomme M., Tarroux P., Hubert M., Decroix Y., Sarasin A. Deficiency in the catalase activity of xeroderma pigmentosum cell and simian virus 40-transformed human cell extracts. Cancer Res. 1986;46:538–544. [PubMed] [Google Scholar]
- 27.Watters D., Kedar P., Spring K., Bjorkman J., Chen P., Gatei M., Birrell G., Garrone B., Srinivasa P., Crane D.I., Lavin M.F. Localization of a portion of extranuclear ATM to peroxisomes. J. Biol. Chem. 1999;274:34277–34282. doi: 10.1074/jbc.274.48.34277. [DOI] [PubMed] [Google Scholar]
- 28.Liu N., Stoica G., Yan M., Scofield V.L., Qiang W., Lynn W.S., Wong P.K.Y. ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Lab. Invest. 2005;85:1471–1480. doi: 10.1038/labinvest.3700354. [DOI] [PubMed] [Google Scholar]
- 29.Ito K., Hirao A., Arai F., Matsuoka S., Takubo K., Hamaguchi I., Nomiyama K., Hosokawa K., Sakurada K., Nakagata N., Ikeda Y., Mak T.W., Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 30.Quick K.L., Dugan L.L. Superoxide stress identifies neurons at risk in a model of ataxia-telangiectasia. Ann. Neurol. 2001;49:627–635. [PubMed] [Google Scholar]
- 31.Lee J.-H., Mand M.R., Kao C.-H., Zhou Y., Ryu S.W., Richards A.L., Coon J.J., Paull T.T. ATM directs DNA damage responses and proteostasis via genetically separable pathways. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aan5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow H.-M., Cheng A., Song X., Swerdel M.R., Hart R.P., Herrup K. ATM is activated by ATP depletion and modulates mitochondrial function through NRF1. J. Cell Biol. 2019;218:909–928. doi: 10.1083/jcb.201806197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichenbach J., Schubert R., Schwan C., Muller K., Bohles H.J., Zielen S. Anti-oxidative capacity in patients with ataxia telangiectasia. Clin. Exp. Immunol. 1999;117:535–539. doi: 10.1046/j.1365-2249.1999.01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichenbach J., Schubert R., Schindler D., Müller K., Böhles H., Zielen S. Elevated oxidative stress in patients with ataxia telangiectasia. Antioxidants Redox Signal. 2002;4:465–469. doi: 10.1089/15230860260196254. [DOI] [PubMed] [Google Scholar]
- 35.Buendia I., Michalska P., Navarro E., Gameiro I., Egea J., León R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Kern J.T., Walker J.R., Johnson J.A., Schultz P.G., Luesch H. A genomic screen for activators of the antioxidant response element. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh K., Mimura J., Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxidants Redox Signal. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 38.Navrkalova V., Kafkova L.R., Divoky V., Pospisilova S. Oxidative stress as a therapeutic perspective for ATM-deficient chronic lymphocytic leukemia patients. Haematologica. 2015;100:994–996. doi: 10.3324/haematol.2015.130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorrini C., Baniasadi P.S., Harris I.S., Silvester J., Inoue S., Snow B., Joshi P.A., Wakeham A., Molyneux S.D., Martin B., Bouwman P., Cescon D.W., Elia A.J., Winterton-Perks Z., Cruickshank J., Brenner D., Tseng A., Musgrave M., Berman H.K., Khokha R., Jonkers J., Mak T.W., Gauthier M.L. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 2013;210:1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q., Liu J., Duan H., Li R., Peng W., Wu C. Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021;34:43–63. doi: 10.1016/j.jare.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirotti C., Rizza S., Giglio P., Poerio N., Allega M.F., Claps G., Pecorari C., Lee J.-H., Benassi B., Barilà D., Robert C., Stamler J.S., Cecconi F., Fraziano M., Paull T.T., Filomeni G. Redox activation of ATM enhances GSNOR translation to sustain mitophagy and tolerance to oxidative stress. EMBO Rep. 2020 doi: 10.15252/embr.202050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Q.-Q., Wang S.-S., Zhang S.-S., Xu H.-D., Li X.-M., Guan Y., Yi F., Zhou T.-T., Jiang B., Bai N., Ma M.-T., Wang Z., Feng Y.-L., Guo W.-D., Wu X., Zhao G.-F., Fan G.-J., Zhang S.-P., Wang C.-G., Cao L.-Y., O'Rourke B.P., Liu S.-H., Wang P.-Y., Han S., Song X.-Y., Cao L. ATM-CHK2-Beclin 1 axis promotes autophagy to maintain ROS homeostasis under oxidative stress. EMBO J. 2020;39 doi: 10.15252/embj.2019103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambrose M., Goldstine J.V., Gatti R.A. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum. Mol. Genet. 2007;16:2154–2164. doi: 10.1093/hmg/ddm166. ddm166[pii]10.1093/hmg/ddm166. [DOI] [PubMed] [Google Scholar]
- 44.Valentin-Vega Y.A., Maclean K.H., Tait-Mulder J., Milasta S., Steeves M., Dorsey F.C., Cleveland J.L., Green D.R., Kastan M.B. Mitochondrial dysfunction in ataxia-telangiectasia. Blood. 2012;119:1490–1500. doi: 10.1182/blood-2011-08-373639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schubert R., Erker L., Barlow C., Yakushiji H., Larson D., Russo A., Mitchell J.B., Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004;13:1793–1802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- 46.Eaton J.S., Lin Z.P., Sartorelli A.C., Bonawitz N.D., Shadel G.S. Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J. Clin. Invest. 2007;117:2723–2734. doi: 10.1172/JCI31604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Lu S., Zheng L., Guo Q., Cao L., Xiao Y., Chen D., Zou Y., Liu X., Deng C., Zhang S., Yang R., Wang Y., Zhang Y., Zhang N., Song X., Xing C., Wang Z., Cao L. ATM-CHK2-TRIM32 axis regulates ATG7 ubiquitination to initiate autophagy under oxidative stress. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.113402. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J., Randall M.S., Loyd M.R., Dorsey F.C., Kundu M., Cleveland J.L., Ney P.A. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood. 2009;114:157–164. doi: 10.1182/blood-2008-04-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyemi U., Redon C.E., Aziz T., Choudhuri R., Maeda D., Parekh P.R., Bonner M.Y., Arbiser J.L., Bonner W.M. NADPH oxidase 4 is a critical mediator in Ataxia telangiectasia disease. Proc. Natl. Acad. Sci. U.S.A. 2015;112:2121–2126. doi: 10.1073/pnas.1418139112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baretić D., Pollard H.K., Fisher D.I., Johnson C.M., Santhanam B., Truman C.M., Kouba T., Fersht A.R., Phillips C., Williams R.L. Structures of closed and open conformations of dimeric human ATM. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stakyte K., Rotheneder M., Lammens K., Bartho J.D., Grädler U., Fuchß T., Pehl U., Alt A., van de Logt E., Hopfner K.P. Molecular basis of human ATM kinase inhibition. Nat. Struct. Mol. Biol. 2021;28:789–798. doi: 10.1038/s41594-021-00654-x. [DOI] [PubMed] [Google Scholar]
- 52.Warren C., Pavletich N.P. Structure of the human ATM kinase and mechanism of Nbs1 binding. Elife. 2022;11 doi: 10.7554/eLife.74218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao J., Liu M., Qi Y., Chaban Y., Gao C., Pan B., Tian Y., Yu Z., Li J., Zhang P., Xu Y. Structural insights into the activation of ATM kinase. Cell Res. 2019;29:683–685. doi: 10.1038/s41422-019-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau W.C.Y., Li Y., Liu Z., Gao Y., Zhang Q., Huen M.S.Y. Structure of the human dimeric ATM kinase. Cell Cycle. 2016;15:1117–1124. doi: 10.1080/15384101.2016.1158362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Chu H., Lv M., Zhang Z., Qiu S., Liu H., Shen X., Wang W., Cai G. Structure of the intact ATM/Tel1 kinase. Nat. Commun. 2016;7 doi: 10.1038/ncomms11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansma M., Linke-Winnebeck C., Eustermann S., Lammens K., Kostrewa D., Stakyte K., Litz C., Kessler B., Hopfner K.-P. Near-complete structure and model of Tel1ATM from chaetomium thermophilum reveals a robust autoinhibited ATP state. Structure. 2020;28:83–95.e5. doi: 10.1016/j.str.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Sawicka M., Wanrooij P.H., Darbari V.C., Tannous E., Hailemariam S., Bose D., Makarova A.V., Burgers P.M., Zhang X. The dimeric architecture of checkpoint kinases Mec1ATR and Tel1ATM reveal a common structural organization. J. Biol. Chem. 2016;291:13436–13447. doi: 10.1074/jbc.M115.708263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xin J., Xu Z., Wang X., Tian Y., Zhang Z., Cai G. Structural basis of allosteric regulation of Tel1/ATM kinase. Cell Res. 2019;29:655–665. doi: 10.1038/s41422-019-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yates L.A., Williams R.M., Hailemariam S., Ayala R., Burgers P., Zhang X. Cryo-EM structure of nucleotide-bound Tel1ATM unravels the molecular basis of inhibition and structural rationale for disease-associated mutations. Structure. 2020;28:96–104.e3. doi: 10.1016/j.str.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 61.Lee J.H., Paull T.T. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 62.Lee J.H., Paull T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 63.Howes A.C., Perisic O., Williams R.L. Structural insights into the activation of ataxia-telangiectasia mutated by oxidative stress. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adi8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Y., Xu Y., Roy K., Price B.D. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol. Cell Biol. 2007;27:8502–8509. doi: 10.1128/MCB.01382-07. MCB.01382-07 [pii] 10.1128/MCB.01382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nolfi-Donegan D., Braganza A., Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020 doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. nature08467 [pii] 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beal M.F. Mitochondria, free radicals, and neurodegeneration. Curr. Opin. Neurobiol. 1996;6:661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 68.Matsuoka S., Ballif B.A., Smogorzewska A., McDonald E.R., Hurov K.E., Luo J., Bakalarski C.E., Zhao Z., Solimini N., Lerenthal Y., Shiloh Y., Gygi S.P., Elledge S.J. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. 316/5828/1160[pii]10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 69.Bensimon A., Schmidt A., Ziv Y., Elkon R., Wang S.Y., Chen D.J., Aebersold R., Shiloh Y. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci. Signal. 2010;3:rs3. doi: 10.1126/scisignal.2001034. 3/151/rs3[pii]10.1126/scisignal.2001034. [DOI] [PubMed] [Google Scholar]
- 70.Barlow C., Dennery P.A., Shigenaga M.K., Smith M.A., Morrow J.D., Roberts L.J., Wynshaw-Boris A., Levine R.L. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J., Wong P.K. Oxidative stress is linked to ERK1/2-p16 signaling-mediated growth defect in ATM-deficient astrocytes. J. Biol. Chem. 2009;284:14396–14404. doi: 10.1074/jbc.M808116200. M808116200[pii]10.1074/jbc.M808116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D'Souza A.D., Parish I.A., Krause D.S., Kaech S.M., Shadel G.S. Reducing mitochondrial ROS improves disease-related pathology in a mouse model of ataxia-telangiectasia. Mol. Ther. 2013;21:42–48. doi: 10.1038/mt.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reliene R., Fischer E., Schiestl R.H. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004;64:5148–5153. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- 74.Lee J.-H., Ryu S.W., Ender N.A., Paull T.T. Poly-ADP-ribosylation drives loss of protein homeostasis in ATM and Mre11 deficiency. Mol. Cell. 2021;81:1515–1533.e5. doi: 10.1016/j.molcel.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pietrucha B., Heropolitanska-Pliszka E., Maciejczyk M., Car H., Sawicka-Powierza J., Motkowski R., Karpinska J., Hryniewicka M., Zalewska A., Pac M., Wolska-Kusnierz B., Bernatowska E., Mikoluc B. Comparison of selected parameters of redox homeostasis in patients with ataxia-telangiectasia and nijmegen breakage syndrome. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/6745840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takao N., Li Y., Yamamoto K. Protective roles for ATM in cellular response to oxidative stress. FEBS Lett. 2000;472:133–136. doi: 10.1016/s0014-5793(00)01422-8. [DOI] [PubMed] [Google Scholar]
- 77.Watters D.J. Oxidative stress in ataxia telangiectasia. Redox Rep. 2003;8:23–29. doi: 10.1179/135100003125001206. [DOI] [PubMed] [Google Scholar]
- 78.Paull T.T., Woolley P.R. A-T neurodegeneration and DNA damage-induced transcriptional stress. DNA Repair. 2024;135 doi: 10.1016/j.dnarep.2024.103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chessa L., Petrinelli P., Antonelli A., Fiorilli M., Elli R., Marcucci L., Federico A., Gandini E. Heterogeneity in ataxia-telangiectasia: classical phenotype associated with intermediate cellular radiosensitivity. Am. J. Med. Genet. 1992;42:741–746. doi: 10.1002/ajmg.1320420524. [DOI] [PubMed] [Google Scholar]
- 80.Gilad S., Chessa L., Khosravi R., Russell P., Galanty Y., Piane M., Gatti R.A., Jorgensen T.J., Shiloh Y., Bar-Shira A. Genotype-phenotype relationships in ataxia-telangiectasia and variants. Am. J. Hum. Genet. 1998;62:551–561. doi: 10.1086/301755. S0002-9297(07)63835-X [pii] 10.1086/301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toyoshima M., Hara T., Zhang H., Yamamoto T., Akaboshi S., Nanba E., Ohno K., Hori N., Sato K., Takeshita K. Ataxia-telangiectasia without immunodeficiency: novel point mutations within and adjacent to the phosphatidylinositol 3-kinase-like domain. Am. J. Med. Genet. 1998;75:141–144. doi: 10.1002/(SICI)1096-8628(19980113)75:2<141::AID-AJMG4>3.0.CO;2-W. [pii] [DOI] [PubMed] [Google Scholar]
- 82.Caldecott K.W. DNA single-strand break repair and human genetic disease. Trends Cell Biol. 2022;32:733–745. doi: 10.1016/j.tcb.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 83.Woolley P.R., Wen X., Conway O.M., Ender N.A., Lee J.-H., Paull T.T. Regulation of transcription patterns, poly(ADP-ribose), and RNA-DNA hybrids by the ATM protein kinase. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J., Kim K., Mo J.-S., Lee Y. Atm deficiency in the DNA polymerase β null cerebellum results in cerebellar ataxia and Itpr1 reduction associated with alteration of cytosine methylation. Nucleic Acids Res. 2020;48:3678–3691. doi: 10.1093/nar/gkaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hara K., Shiga A., Nozaki H., Mitsui J., Takahashi Y., Ishiguro H., Yomono H., Kurisaki H., Goto J., Ikeuchi T., Tsuji S., Nishizawa M., Onodera O. Total deletion and a missense mutation of ITPR1 in Japanese SCA15 families. Neurology. 2008;71:547–551. doi: 10.1212/01.wnl.0000311277.71046.a0. [DOI] [PubMed] [Google Scholar]
- 86.Huang L., Chardon J.W., Carter M.T., Friend K.L., Dudding T.E., Schwartzentruber J., Zou R., Schofield P.W., Douglas S., Bulman D.E., Boycott K.M. Missense mutations in ITPR1 cause autosomal dominant congenital nonprogressive spinocerebellar ataxia. Orphanet J. Rare Dis. 2012;7:67. doi: 10.1186/1750-1172-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimobayashi E., Kapfhammer J.P. Calcium signaling, PKC gamma, IP3R1 and CAR8 link spinocerebellar ataxias and purkinje cell dendritic development. Curr. Neuropharmacol. 2018;16:151–159. doi: 10.2174/1570159X15666170529104000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Türkmen S., Guo G., Garshasbi M., Hoffmann K., Alshalah A.J., Mischung C., Kuss A., Humphrey N., Mundlos S., Robinson P.N. CA8 mutations cause a novel syndrome characterized by ataxia and mild mental retardation with predisposition to quadrupedal gait. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katzenberger R.J., Marengo M.S., Wassarman D.A. ATM and ATR pathways signal alternative splicing of Drosophila TAF1 pre-mRNA in response to DNA damage. Mol. Cell Biol. 2006;26:9256–9267. doi: 10.1128/MCB.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muñoz M.J., Pérez Santangelo M.S., Paronetto M.P., de la Mata M., Pelisch F., Boireau S., Glover-Cutter K., Ben-Dov C., Blaustein M., Lozano J.J., Bird G., Bentley D., Bertrand E., Kornblihtt A.R. DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation. Cell. 2009;137:708–720. doi: 10.1016/j.cell.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 91.Nicholls C.D., Shields M.A., Lee P.W.K., Robbins S.M., Beattie T.L. UV-dependent alternative splicing uncouples p53 activity and PIG3 gene function through rapid proteolytic degradation. J. Biol. Chem. 2004;279:24171–24178. doi: 10.1074/jbc.M401049200. [DOI] [PubMed] [Google Scholar]
- 92.Yeo A.J., Becherel O.J., Luff J.E., Cullen J.K., Wongsurawat T., Jenjaroenpun P., Jenjaroenpoon P., Kuznetsov V.A., McKinnon P.J., Lavin M.F. R-loops in proliferating cells but not in the brain: implications for AOA2 and other autosomal recessive ataxias. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su C., Zhao H., Zhao Y., Ji H., Wang Y., Zhi L., Li X. RUG3 and ATM synergistically regulate the alternative splicing of mitochondrial nad2 and the DNA damage response in Arabidopsis thaliana. Sci. Rep. 2017;7 doi: 10.1038/srep43897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Browne S.E., Roberts L.J., Dennery P.A., Doctrow S.R., Beal M.F., Barlow C., Levine R.L. Treatment with a catalytic antioxidant corrects the neurobehavioral defect in ataxia-telangiectasia mice. Free Radic. Biol. Med. 2004;36:938–942. doi: 10.1016/j.freeradbiomed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Chen P., Peng C., Luff J., Spring K., Watters D., Bottle S., Furuya S., Lavin M.F. Oxidative stress is responsible for deficient survival and dendritogenesis in purkinje neurons from ataxia-telangiectasia mutated mutant mice. J. Neurosci. 2003;23:11453–11460. doi: 10.1523/JNEUROSCI.23-36-11453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pedre B., Barayeu U., Ezeriņa D., Dick T.P. The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021;228 doi: 10.1016/j.pharmthera.2021.107916. [DOI] [PubMed] [Google Scholar]
- 97.Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 2018;52:751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 98.Kalyanaraman B., Nac N.A.C. Knockin’ on Heaven's door: interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022;57 doi: 10.1016/j.redox.2022.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bagley J., Singh G., Iacomini J. Regulation of oxidative stress responses by ataxia-telangiectasia mutated is required for T cell proliferation. J. Immunol. 2007;178:4757–4763. doi: 10.4049/jimmunol.178.8.4757. [DOI] [PubMed] [Google Scholar]
- 100.Alagoz M., Chiang S.C., Sharma A., El-Khamisy S.F. ATM deficiency results in accumulation of DNA-topoisomerase I covalent intermediates in neural cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fang E.F., Kassahun H., Croteau D.L., Scheibye-Knudsen M., Marosi K., Lu H., Shamanna R.A., Kalyanasundaram S., Bollineni R.C., Wilson M.A., Iser W.B., Wollman B.N., Morevati M., Li J., Kerr J.S., Lu Q., Waltz T.B., Tian J., Sinclair D.A., Mattson M.P., Nilsen H., Bohr V.A. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metabol. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weyemi U., Paul B.D., Bhattacharya D., Malla A.P., Boufraqech M., Harraz M.M., Bonner W.M., Snyder S.H. Histone H2AX promotes neuronal health by controlling mitochondrial homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2019;116:7471–7476. doi: 10.1073/pnas.1820245116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weyemi U., Paul B.D., Snowman A.M., Jailwala P., Nussenzweig A., Bonner W.M., Snyder S.H. Histone H2AX deficiency causes neurobehavioral deficits and impaired redox homeostasis. Nat. Commun. 2018;9:1526. doi: 10.1038/s41467-018-03948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tresini M., Warmerdam D.O., Kolovos P., Snijder L., Vrouwe M.G., Demmers J.A., van IjW.F., Grosveld F.G., Medema R.H., Hoeijmakers J.H., Mullenders L.H., Vermeulen W., Marteijn J.A. The core spliceosome as target and effector of non-canonical ATM signalling. Nature. 2015;523:53–58. doi: 10.1038/nature14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ross C.A., Poirier M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10(Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 106.Kwak Y.D., Shaw T.I., Downing S.M., Tewari A., Jin H., Li Y., Dumitrache L.C., Katyal S., Khodakhah K., Russell H.R., McKinnon P.J. Chromatin architecture at susceptible gene loci in cerebellar Purkinje cells characterizes DNA damage-induced neurodegeneration. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg6363. eabg6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crossley M.P., Song C., Bocek M.J., Choi J.-H., Kousorous J., Sathirachinda A., Lin C., Brickner J.R., Bai G., Lans H., Vermeulen W., Abu-Remaileh M., Cimprich K.A. R-loop-derived cytoplasmic RNA-DNA hybrids activate an immune response. Nature. 2023;613:187–194. doi: 10.1038/s41586-022-05545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McKinnon P.J. Genome integrity and disease prevention in the nervous system. Genes Dev. 2017;31:1180–1194. doi: 10.1101/gad.301325.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai J., Demirbas D., Kim J., Jeffries A.M., Tolles A., Park J., Chittenden T.W., Buckley P.G., Yu T.W., Lodato M.A., Lee E.A. ATM-deficiency-induced microglial activation promotes neurodegeneration in ataxia-telangiectasia. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2023.113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeo A.J., Chong K.L., Gatei M., Zou D., Stewart R., Withey S., Wolvetang E., Parton R.G., Brown A.D., Kastan M.B., Coman D., Lavin M.F. Impaired endoplasmic reticulum-mitochondrial signaling in ataxia-telangiectasia. iScience. 2021;24 doi: 10.1016/j.isci.2020.101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.