Abstract
Parkinson's disease (PD) is associated with a reduction in 26/20S proteasome and mitochondrial function and depletion of dopamine. Activation of mitochondrial function with the NAD+ precursor nicotinamide riboside (NR) is a potential therapeutic for PD. However, despite recently started clinical trials, analysis of NR in mammalian animal PD models is lacking and data in simpler PD models is limited. We analyzed the effect of NR in C. elegans and in mouse 26/20S proteasome inhibition models of PD. In C. elegans, NR rescued α-synuclein overexpression induced phenotypes likely by activating the mitochondrial unfolded protein response. However, in a proteasome inhibitor-induced mouse model of PD, NR first partially rescued behavioural dysfunction, but later resulted in decrease in dopamine and its related gene expression in the substantia nigra. Our results suggest that reduction in 26/20S function with long term NR treatment may increase risk for developing reduced nigrostriatal DA function.
Keywords: Nicotinamide riboside, Mitochondrial activation, Proteastasis failure, Parkinson's disease, Dopamine
1. Methods (Extended Methods available at Supplemental Data)
C. elegans growth and maintenance, strains and assays were performed using standard methods. Details are outlined in Extended Methods available at Supplemental Data.
M. musculus maintenance and housing was performed according to national and EU guidelines. Analysis was performed using published standard methods. Details are outlined in Extended Methods.
Statistical analysis Normality of the data was determined with the Shapiro-Wilk test. Comparisons were made with Student's t-test followed by Welch's correction, if the data were normally distributed, or Mann Whitney U test, if the data distribution was not normal. Comparison between more than two groups were performed with (ANOVA) test followed by appropriate post-hoc test using PRISM (GraphPad software). Details are available in Extended Methods.
2. Introduction
In Parkinson's disease (PD), motor symptoms are mainly caused by progressive degeneration of dopamine (DA) axons in the striatum followed by death of DA neurons in the midbrain substantia nigra (SN) [1]. PD motor symptoms first appear then about 50–60 % of SN dopamine neurons and about 70–80 % striatal DA is lost [2,3]. By the end stage patients have lost over 95 % of striatal dopamine with close 100 % loss in the putamen [3] which parallels with 50–100 % loss of DA neurons in the SN depending on SN sub-region [2,4]. End stage patients cannot stand or move as nigrostriatal DA is essential for movement initiation. Current pharmacological treatment focuses on enhancing DA metabolism during the time window between diagnosis and death. Search for new drugs which could better preserve the remaining DA in PD is actively ongoing.
Current evidence suggests that defects in proteasome and mitochondrial function can drive PD [[5], [6], [7]] and that interplay between these two processes exists. Sporadic PD patients make up about 95 % of all PD patients, display failure in proteasome function and the loss of 26S/20S proteasome subunits in the SN [[8], [9], [10]] as summarized [11]. PD is often characterised by an accumulation of ⍺-synuclein (⍺-syn) aggregates which have been suggested to reflect the impairment of proteasome activity and contribute to mitochondrial dysfunction. In PD, mitochondrial dysfunction typically manifests as impaired mitochondrial oxidative capacity, dynamics and quality control PMID: 37951933 [12,13]. Collectively these observations provide a rationale for enhancing mitochondrial function as potential treatment for PD. One of the most promising pharmacological compounds is nicotinamide riboside (NR), an NAD + precursor vitamin B3 [14]. NR is used for the biosynthesis of nicotinamide adenine dinucleotide (NAD+), the central co-enzyme for mitochondrial energy conversion. The maintenance of proper NAD+ homeostasis seems to be important for DA neurons and conversely, disrupted NAD+ metabolism has been reported in PD [[15], [16], [17]]. In preclinical studies addressing Alzheimer's disease (AD) and amyotrophic lateral sclerosis (ALS), NR has shown promise by rescuing NAD+ homeostasis and mitochondrial oxidative capacity, and by decreasing the formation of amyloid-beta and superoxide dismutase −1 protein aggregations likely via activation of mitochondrial unfolded protein response (UPRmt) [18,19]. NR also rescues mitochondrial defects in stem cell-derived DA neurons obtained from heterozygous glucocerebrosidase (GBA) mutant PD patient, which is a rare genetic form of PD, and alleviates climbing deficit in a fly model overexpressing human N370S mutant GBA [17]. NR also improves the survival of DA neurons in C. elegans human ⍺-syn overexpression model [20].
Based on activity in the above model systems, clinical trials with NR have been recently initiated for PD. One clinical Phase I trial with a short-term NR supplementation of 30 days has been concluded and reported a trend for clinical improvement [21]. However, analysis of NR treatment in mammalian animal PD models is at present lacking. Currently a mouse model that phenocopies the progressive etiology of PD is not available [22,23]. Thus, the analysis of new treatment should be performed using available animal models which bona fide model the majority of sporadic PD patients the best.
In this study, we first determine the effect of NR on ⍺-syn overexpression induced phenotypes in transgenic C. elegans strains overexpressing human ⍺-syn in either the body wall muscle cells or in DA neurons. We then analyze the effect of long-term NR pre-treatment (3 months) prior supranigral application of 26S/20S proteasome inhibitor lactacystin (LC) followed by long-term behavioural monitoring of mice for 3 months and analysis of various brain regions at the endpoint. Although precise comparison is difficult, according to current consensus one human year equates to about 9 mouse days. We find that NR treatment attenuates phenotypes in the worm ⍺-syn models. However, in mice the primary endpoint - a rescue of nigrostriatal dopamine levels - was not reached. Instead, we find that after 3 months of delivering proteasome inhibitor above the SN, chronical treatment with NR downregulates DA levels and reduces DA metabolism related gene expression in the SN in LC treated mice. Reduction in 26/20S proteasome function in SN lysates is observed in most sporadic PD patients and is believed to contribute to PD progression [[8], [9], [10]].Our results therefore call for caution and argue for careful monitoring of DA and energy metabolism in long-term clinical trials with NR in PD.
3. Results
3.1. NR supplementation alleviates ⍺-syn overexpression induced phenotypes in C. elegans
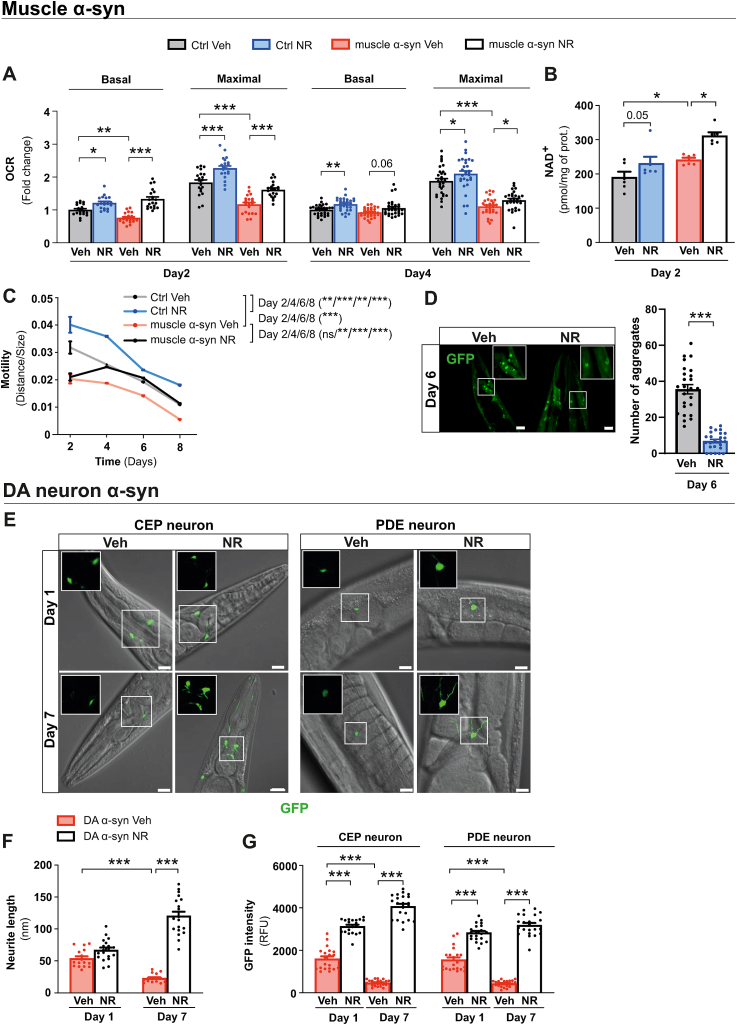
First, we tested a C. elegans model overexpressing human wild-type ⍺-syn in the body wall muscle cells (muscle ⍺-syn) [24]. Animals were supplemented with NR from the L1 larval stage. Basal and maximal respiration rates were significantly decreased in muscle ⍺-syn C. elegans from day 2 of adult age onwards, confirming mitochondrial dysfunction (Fig. 1A). However, the amount of mtDNA was significantly higher in the muscle ⍺-syn strain, suggesting a compensatory increase in mitochondrial content (Fig. S1A). Supplementation with NR resulted in increased NAD+ levels in both strains, confirming that NR was metabolized (Fig. 1B). The muscle ⍺-syn strain also displayed impaired pharyngeal pumping activity during late adult age, on day 6 (Fig. S1B), which was rescued by NR. In fact, NR supplementation improved pharyngeal pumping activity in both control and muscle ⍺-syn overexpressing C. elegans strains at the early and late adult ages (Fig. S1B). Notably, NR supplementation improved both basal and maximal respiration rates at days 2 and day 4 of adult age (Fig. 1A) in muscle ⍺-syn strain, but did not increase the mtDNA level, as in the control strain (Fig. S1A). NR rescued motility defects in muscle ⍺-syn strain from day 4 until day 8 of adult age (Fig. 1C) and diminished the number of ⍺-syn aggregates in body-wall muscle [25] (Fig. 1D). Thus, in C. elegans muscle ⍺-syn models, NR decreases ⍺-syn aggregates and improves motility and mitochondrial function.
Fig. 1.
NR alleviates PD-like phenotypes in ⍺-syn overexpressing C. elegans models.
(A) Basal and maximal mitochondrial respiration after carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (15 μM) injection measured in the control and muscle ⍺-syn strains after vehicle (Veh) or NR supplementation at days 2 and 4 of adult age. Each data point represents the mean of 2 independent experiments in total with 20 wells per group, with each group having 5 to 8 animals per well. (B) Total NAD+ levels in the vehicle and NR supplemented control and muscle ⍺-syn strains at day 2 of adult age measured by modified colorimetric enzymatic method (n = 6 biologically independent samples per group and two independent experiments). (C) Motility (distance travelled normalized to the size of the animals) in the control and muscle ⍺-syn strains with vehicle or NR supplementation at days 4, 6, and 8 of adult age. Each data point represents the mean of 2 independent experiments with 59–99 animals per group. (D) Confocal images (left) and quantification (right) of GFP-positive α-syn aggregates after vehicle or NR supplementation at day 6 of adult age in the C. elegans strain expressing human α-syn fused with GFP in body wall muscle cells. Scale bar, 20 μm. (E) Confocal images of CEP and PDE DA neurons and neurite extensions in the DA ⍺-syn strain after vehicle or NR supplementation at days 1 and 7 of adult age. Inserts show CEP and PDE neurites with 63X magnification. Scale bar, 5 μm. (F) Neurite length in the DA ⍺-syn strain after vehicle or NR supplementation at days 1 and 7 of adult age and (G) quantification of GFP signal intensity in CEP and PDE DA neurons in the DA ⍺-syn strain using ImageJ software (n = 20 to 23 biologically independent samples per group and two independent experiments), analyzed from the confocal images represented in panel (E) using ImageJ software (n = 16 to 19 biologically independent samples per group and two independent experiments).
All data are mean ± SEM *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, not significant. Statistical analysis was performed in GraphPad prism version 9.0.0. Overall differences between conditions were assessed in one-way ANOVA using an uncorrected version of Fisher's LSD test. OCR, oxygen consumption rate; GFP, green fluorescent protein; DA, dopamine; CEP, cephalic; PDE, posterior deirid. See also: Fig. S1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To analyze the effect of NR on DA neurons, we used a strain which expresses GFP with ⍺-syn in DA neurons (DA ⍺-syn) and displays age-dependent DA neuron degeneration [26]. Compromised basal respiration in young DA ⍺-syn animals (Fig. S1C) was corrected by NR in young animals (Fig. S1C). Further, aging-driven DA neurite (Fig. 1E and F) and DA neuron loss (Fig. 1E–G) was protected by NR. NR also maintained normal pharyngeal pumping activity and muscle fitness upon aging in that strain (Figs. S1D and E). Thus, in C. elegans DA ⍺-syn model, NR protects from loss of DA neurites and neurons.
3.2. NR mitigates ⍺-syn overexpression phenotypes via an UPRmt dependent mechanism in C. elegans
In muscle of ⍺-syn strain we observed repressed UPRmt signalling, since the mRNA levels of mitochondrial protease genes ymel-1, lonp-1, and clpP, as well as the positive regulator of UPRmt signalling, ubl-5, were downregulated (Fig. S2A). We found that NR activated UPRmt signalling in the muscle ⍺-syn strain by increasing the expression of lonp-1, clpP, and ubl-5 (Fig. S2A).
Reactive oxygen species (ROS) stimulate UPRmt in C. elegans [27] which confers protection from oxidative stress via atfs-1, the key UPRmt transcription factor, -mediated transcriptional response [28]. NR provides ROS defence in late adult age in C. elegans [29], therefore we determined aging-induced tissue oxidative stress using an anti-8-OH-dG antibody. Upon aging, control and both muscle and DA ⍺-syn C. elegans strains showed an increase in 8-OH-dG signal (Figs. S2B and D). We found that NR decreased the levels of 8-OH-dG in all C. elegans strains especially at the later age (Figs. S2C and E). To understand whether UPRmt is essential for NR-induced improvements in the muscle ⍺-syn strain, we used RNAi to knock down atfs-1. Silencing of atfs-1 abrogated the mitochondrial boosting effect of NR on day 2 of adult age (Fig. S2F). This was accompanied by the loss of NR-induced improvement in motility from day 4 of adulthood onwards (Fig. S2G). Thus, UPRmt mediates the protective capacity of NR in the C. elegans muscle ⍺-syn strain.
3.3. NR supplementation improves NAD+ metabolism in the brain in LC mouse model of PD
Due to above encouraging results and since analysis of NR in PD mammalian animal models is lacking, we subsequently studied NR in a mouse model of PD. We chose the LC PD model for the reasons in detail outlined in Extended Materials and Methods. In short, proteasome function is reduced in the SN in PD patients and supranigral delivery of proteasome inhibitor LC results in well-reproducible PD-like degeneration of the nirgo-striatal dopamine system in mice [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. Mice were preconditioned with vehicle or NR diets for 3 months prior to an induction of Parkinsonism-like features through a unilateral stereotactic injection of LC just above the SN and were analyzed in two experiments - 2 weeks after LC delivery as outlined on (Fig. 3A) and 12 weeks after LC delivery as outlined on (Fig. S3A). We observed that food intake was similar in both vehicle and NR supplemented groups (Fig. S4A) and that NR does not affect body weight during the first 12 weeks. LC injection reduced bodyweight and NR-supplemented mice maintained body weight better for about 2 weeks (Fig. S4B).
Fig. 3.
NR modulates mitochondrial dynamics.
(A) Experimental design for the study in which mice receiving the vehicle diet (Veh) and NR-enriched diet (NR) were analyzed 2 weeks after nigral LC lesion. (B) Complex I [CI], (C) complex II [CII], and (D) Complex IV [CIV] mediated oxygen flux in SN tissues analyzed at the 2-week time point. Veh, unlesioned side (n = 7); NR, unlesioned side (n = 6); Veh, lesioned side (n = 7); NR, lesioned side (n = 6). (E) Representative transmission electron microscopy image and higher magnification inserts of the SN of vehicle and NR supplemented unlesioned and LC-lesioned mice. Mitochondria are highlighted with green colour. Scale bar, 2 μm. Corresponding quantification of (F) mitochondria intensity, mean mitochondrial cross-section (G) number, (H) area, (I) perimeter, and (J) length/width ratio expressed as eccentricity in vehicle and NR supplemented unlesioned and LC-lesioned mice. n = 3/group. Twenty non-overlapping images/sample were taken from each animal. (K-M) Oxygen flux data from (B-D) were normalized to the number of mitochondria from (G).
Data are presented as mean ± SEM. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. Statistical analysis was performed using GraphPad Prism version 8.0.2. Overall differences between conditions were assessed with one-way ANOVA followed by an uncorrected version of Fisher's LSD test. CI, complex I; CII, complex II; CIV, complex IV; Lacta, LC; NR, nicotinamide riboside; SN, SN; Veh, vehicle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, we analyzed NAD+ metabolism in the cerebellum, a brain region not needed for other analyses (see Extended MM for details). NR did not affect NAD+ levels (Fig. S3B), but increased nicotinic acid adenine dinucleotide (NAAD), a biomarker of increased NAD+ metabolism (Fig. S3C) [41]. Similarily, NR levels remained unaltered (Fig. S3D) while the level of nicotinamide mononucleotide (NMN), the phosphorylated form of NR, was increased by NR supplementation (Fig. S3E). Metabolites produced by non-redox NAD+ dependent enzymes, such as sirtuins and poly(ADP)ribose polymerases, nicotinamide (NAM) and ADP-ribose (ADPR), were unaltered (Figs. S3F and G). In contrast NAM clearance pathways products NAM oxide, N-methyl-2-pyridone-5-carboxamide (Me-2-PY), and N-methyl-4-pyridone-5-carboxamide (Me-4-PY) were increased (Figs. S3H–J) while nicotinamide adenine dinucleotide phosphate (NADP), nicotinic acid (NA), and nicotinic acid riboside (NAR) levels were not changed (Figs. S4C–E). Thus, NR was readily metabolized and subsequent elimination of NAM was enhanced (Fig. S3K).
3.4. NR first alleviates behavioural deficits in a LC-induced PD mouse model but later reduces voluntary movement
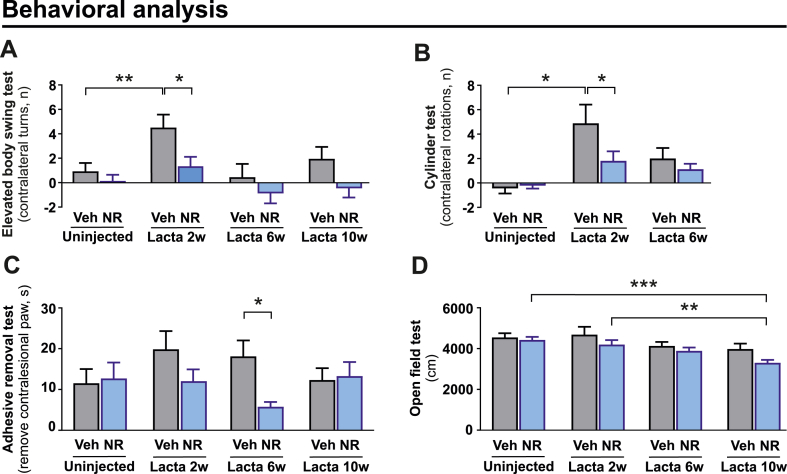
Longitudinal behavioural analysis revealed that NR improved performance at 2 weeks after LC injections (Fig. 2A and B). In adhesive removal test which assesses sensorimotor function NR enhanced performance at 6-week time point (Fig. 2C). However, the initial protection was gradually lost (Fig. 2A–C) and was followed by progressive decline in the total distance travelled at the 10-week time point (Fig. 2D).
Fig. 2.
NR rescues behavioural deficits in LC-induced PD model but causes a mild and gradual decline in motor activity.
(A) Effect of NR on the number of contralateral net turns during the elevated body swing test, measured at baseline (uninjected) and 2, 6, and 10 weeks after LC injection in vehicle and NR supplemented mice. (B) Effect of NR on the number of contralateral rotations during the cylinder test, measured at baseline (uninjected) as well as 2 and 6 weeks after LC injection in vehicle and NR supplemented mice. (C) Time required to remove the adhesive from the contralesional paw (time-to-remove—time-to-contact) during the adhesive removal test, measured at baseline (uninjected) and 2, 6, and 10 weeks after LC injection in vehicle and NR supplemented mice. (D) Effect of NR on the total distance travelled during the open field test, measured at baseline (uninjected) and 2, 6, and 10 weeks after LC injection in vehicle and NR supplemented mice. (A, C, D) Veh (n = 38) and NR (n = 38) before LC injection; Veh (n = 34) and NR (n = 38) after LC injection. (B) Veh (n = 18) and NR (n = 18).
Data are presented as mean ± SEM. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. Statistical analysis was performed in GraphPad prism version 8.0.2. Comparisons between more than two groups were performed with two-way repeated measures ANOVA followed by Sidak's multiple comparison test (A-D). Lacta, LC; NR, nicotinamide riboside; Veh, vehicle.
3.5. NR modulates mitochondrial dynamics but does not affect mitochondrial oxidative capacity in LC-lesioned nigral tissue at the 2-week time-point
Next, we analyzed mitochondrial metabolism in the SN at 2 weeks after LC injections (Fig. 3A), when NR treatment had the clearest positive effect on animal behaviour (Fig. 2A–C). First, we asked whether LC induces mitochondrial dysfunction in the SN as reported in PD [[42], [43], [44]]. We used high-resolution respirometry to study the effect of LC and NR on complex I (CI), complex II (CII), and complex IV (CIV) – mediated mitochondrial respiration. LC resulted in significant drop in mitochondrial oxygen flux in all complexes examined, similar to what is reported in the SN of PD [[42], [43], [44]] (Fig. 3B, C, D). However, NR did not rescue those defects in the SN (Fig. 3B, C, D).
Transmission electron microscopy (TEM) analysis of SN (Fig. 3E) revealed that NR increased intensity of mitochondrial staining, a parameter believed to reflect increased metabolic activity (Freeman et al., 2017) in the LC unlesioned SN side (Fig. 3F). In contrast, LC reduced the same parameter in the lesioned side and this effect was partially rescued by NR (Fig. 3F). NR supplemented unlesioned SN side displayed an increased abundance of mitochondria with smaller mitochondrial area, perimeter and eccentricity (i.e. circularity) as compared to vehicle supplemented unlesioned side (Fig. 3G–J). LC injection reduced the number of mitochondria without a concomitant change in the mitochondrial size or shape (Fig. 3G, H, I). However, NR supplementation promoted a decrease in the number of mitochondria (Fig. 3G) and an increase in mitochondrial eccentricity within the LC-lesioned nigral tissue (Fig. 3J).
Next, we normalized the respirometry results with the mitochondrial number obtained from TEM analysis. We found that NR significantly increased mitochondrial CII- and CIV- mediated oxygen flux per mitochondrion after LC injection (Fig. 3K-M). The disappearance of a LC-induced mitochondrial bioenergetic deficit in lesioned SN upon above normalization suggests that this deficit is linked to a decline in mitochondrial number (Fig. 3K-M).
In conclusion, NR increases the abundance of smaller and metabolically active mitochondria in unlesioned side without affecting mitochondrial oxidative capacity. In lesioned side, LC reduces mitochondrial oxidative capacity by lowering mitochondrial number. In contrast, NR does not influence LC-lesioned nigral tissue mitochondrial oxidative capacity but triggers alterations in mitochondrial dynamics leading to fewer mitochondria with normal shape and higher energetic profile.
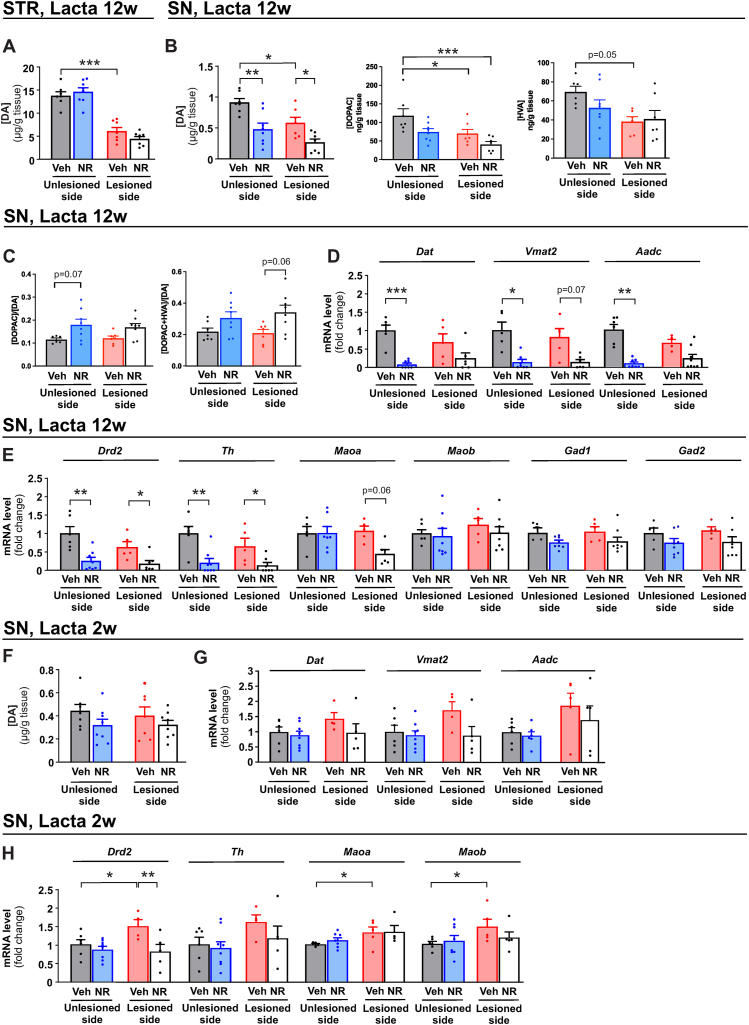
3.6. Long-term NR treatment decreases nigral DA level and DA related gene expression in LC-induced mouse model of PD
In nigrostriatally-lesioned PD models, rescue or restoration of DA levels by the tested drug is the most important primary endpoint. In line with previous results [11,[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]], LC caused about 50 % drop in DA and its metabolite DOPAC level in the SN (Fig. 4B). Surprisingly, NR treatment resulted in further decrease in DA levels in both the unlesioned and LC-lesioned sides of the SN (Fig. 4B). In the striatum, NR did not prevent or exacerbate LC-induced loss of DA (Fig. 4A). Ratio between DA and its metabolites DOPAC and HVA indicated trend towards increased DA turnover (Fig. 4B), which is believed to reflect compensatory response to dopamine system lesion [45,46]. These results prompted us to analyze DA metabolism in a greater detail. First we assessed DA transporter (Dat), vesicular monoamine transporter 2 (Vmat2), and l-DOPA converting enzyme aromatic l-amino acid decarboxylase (Aadc) mRNA levels since encoded proteins could provide clinical endpoint via PET or SPECT imaging [[47], [48], [49], [50]] in the future. We found that Dat, Vmat2 and Aadc mRNA levels were downregulated in the unlesioned side in NR supplemented mice with similar trend at the lesioned side (Fig. 4C). Analysis of a broader spectrum of DA metabolism genes including DA receptor 2 (Drd2), tyrosine hydroxylase (Th), and monoamine oxidases A (Maoa) and B (Maob) also revealed decreased mRNA levels in the SN in both sides (Fig. 4D) while expression of glutamate decarboxylase 1 (Gad1) and glutamate decarboxylase 2 (Gad2), rate limiting enzymes in GABA synthesis, was unaltered (Fig. 4D). Analysis of ventral tegmental area (VTA), the dopaminergic nucleus which connects the left (unlesioned) and the right (lesioned) SN, revealed no difference between control and NR supplemented mice (Fig. S5A). Similarly, analysis of genes involved in unfolded protein response and mitochondrial stress and quality control revealed no changes in the SN (Figs. S5B and C). The only exception was that NR significantly decreased the expression level of Nrf2-target genes Nqo1 and Hmox1 involved in ROS defence in the lesioned side (Fig. S5D). Thus, NR supplementation results in downregulation of DA and DA metabolism related gene expression in both the lesioned and unlesioned sides of the SN upon unilateral LC-induced proteasome inhibition while such changes are not observed in the VTA.
Fig. 4.
Long-term NR treatment decreases DA levels and expression of DA related genes in the SN upon LC injection. Total tissue level of DA in (A) striatum and DA and metabolites DOPAC and HVA in (B) SN 12 weeks after LC injection. Veh, unlesioned side (n = 7); NR, unlesioned side (n = 8); Veh, lesioned side (n = 7); NR, lesioned side (n = 8). (C) Turnover rates of dopamine determined by tissue levels of metabolites DOPAC or DOPAC + HVA over tissue levels of dopamine. Gene expression of (D)Dat, Vmat2, and Aadc and (E)Drd2, Th, Maoa, Maob, Gad1, and Gad2 mRNA in the SN of vehicle and NR supplemented unlesioned and LC-lesioned mice 12 weeks after LC injection. Veh, unlesioned side (n = 6); NR, unlesioned side (n = 8); Veh, lesioned side (n = 6); NR, lesioned side (n = 8). (F) DA tissue level in the SN of vehicle and NR supplemented mice 2 weeks after LC injection. Veh, unlesioned side (n = 7); NR, unlesioned side (n = 8); Veh, lesioned side (n = 7); NR, lesioned side (n = 8). Gene expression of (G) Dat, Vmat2, and Aadc and (H) Drd2, Th, Maoa, and Maob in the SN of vehicle and NR supplemented mice 2 weeks after LC injection. Veh, unlesioned side (n = 6); NR, unlesioned side (n = 8); Veh, lesioned side (n = 5); NR, lesioned side (n = 5).
Data are presented as mean ± SEM. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. Statistical analysis was performed using GraphPad Prism version 8.0.2. Overall differences between conditions were assessed with one-way ANOVA followed by Tukey's post-hoc test for HPLC results or an uncorrected version of Fisher's LSD test or with uncorrected Dunn's test for qPCR results. Aadc, Aromatic l-amino acid decarboxylase; DA, dopamine; Dat, dopamine active transporter; DOPAC, 3,4-dihydroxyphenylacetic acid; Drd2, dopamine receptor 2; HVA, homovanillic acid; Lacta, LC; Maoa monoamine oxidase A; Maob, monoamine oxidase; NR, nicotinamide riboside; SN, SN; STR, striatum; Th, tyrosine hydroxylase; Veh, vehicle; Vmat2, vesicular monoamine transporter 2.
Analysis at 2 weeks after LC injection when NR transiently rescues behavioural deficits and modulates mitochondrial dynamics revealed no significant change in the DA levels in the SN (Fig. 4E). Similarly, mRNA levels of Dat, Vmat2, and Aadc were not changed at that time point (Fig. 4F). However, LC increased Drd2, Maoa, and Maob mRNA levels in lesioned nigral tissue which was not observed in the NR supplemented group (Fig. 4G). NR also had no effect on tissue DA levels or on the expression of DA metabolism related genes in the SN of mice that did not undergo LC injection (Figs. S6A–C).
Thus, despite the alterations in mitochondrial dynamics and the transiently improved behavioural phenotypes NR does not rescue tissue DA levels in the striatum and in the SN at 12 weeks after LC delivery. Instead, LC/NR combination induces bilateral downregulation of tissue DA and many of the DA metabolism related genes expression in the SN.
4. Discussion
The overall success of NR in enhancing mitochondrial function, muscle performance, and metabolic health through short term supplementation in several disease models [51] has led to rapid implementation of NR into clinical trials of various diseases, including PD [21] (ClinicalTrial.gov identifiers: NCT03568968). However, only few supporting studies are performed in worm, fly and cell-line PD models [17,20], while in vivo studies with NR in mammalian PD models are lacking. Our study addresses this important knowledge gap. Our results in model closest to humans - mouse - call for caution in clinical trials with NR in PD and argue for a careful evaluation of dopamine metabolism upon long-term NR treatment.
Our studies using the muscle ⍺-syn C. elegans model revealed that NR partially restores the muscle ⍺-syn overexpression-driven mitochondrial dysfunction and the motility defects via atfs-1-mediated UPRmt activation and confers protection against aging-driven DA neuron loss. These findings are in agreement with previous preclinical studies with NR on AD and ALS [18,19] and in C. elegans ⍺-syn PD model [20]. However, it is challenging to translate findings in C.elegans to mammals.
To model PD in mammals, we used supranigral delivery of the proteasome inhibitor LC in mice (please see Supplementary MM for details). In sporadic PD which account for about 95 % of the PD cases, reduction in 26S/20S proteasome function in the SN is believed to contribute to disease progression [[8], [9], [10]]. Supporting this, pharmacological inhibition of 26S/20S function with supranigral LC delivery results in death of SN DA neurons and emergence of PD-like motor function deficit in rodents [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. Similarly, conditional ablation of Psmc1, a subunit of the 26S proteasome, leads to loss of dopamine neurons in the SN [52].
Previous non-PD related mouse studies have showed NR's capacity to improve mitochondrial function in various tissues [53,54]. We found that NR does not normalize LC-induced mitochondrial respiratory defect in SN at early timepoint after LC application but induces presence of fewer mitochondria with altered morphology with assumedly higher energetic profile. Further studies are needed to understand whether these alterations are translated into higher mitochondrial bioenergetic efficiency. Another important observation is that NR transiently rescues the behavioural deficits in early but not at late timepoints after LC application. Finally, DA metabolism was impaired at the late time point (12 weeks) post LC lesion when also the initial behavioural rescue with NR had disappeared. As observed in previous studies with LC [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]] we noticed a time-dependent reduction in tissue DA levels and critically, the downregulation of expression of the key DA metabolic genes in both the lesioned and unlesioned SN. These findings were paralleled by progressive decrease in spontaneous locomotor activity in the open field test in NR supplemented PD mice. The latter result may reflect reduced nigral DA metabolism driven PD-like reduction in movement or increased body weight observed in NR treated animals. Since spontaneous activity gradually declines in both control and NR treated animals, NR driven increase in spatial memory, so that mice explore less since they remember the open field better as already familiar space despite the loss of DA in the SN, is unlikely.
How does then NR provide functional support at early 2-week time-point in LC PD mouse model? It is likely linked to changes in mitochondrial dynamics and to more energetic mitochondria in the SN and/or to improvements in other brain regions and peripheral tissues, as previously reported for NR [53,54]. Interestingly, DA metabolism deterioration with NR occurs at late 12-week time point after initial mitochondrial changes in mice. Thus NR in conjunction with proteosome dysfunction can in the long run disturb DA metabolism, which is an important consideration for clinical trials [55]. But how does NR/proteasome dysfunction combination influence DA metabolism? One possible mechanism could be the nigral accumulation of NR metabolite, NAM, which was shown to exacerbate DA neuron degeneration in a LC rat model for PD [56]. Unfortunately, due to the small size of SN, its assessment for the measurements of NAD metabolome is currently impossible.
Our results suggest that reduction in 26/20S function in combination with long term NR treatment may increase risk for developing reduced nigrostriatal DA function. Considering that 9 mouse days is believed to be equivalent to one human year, 6 months NR treatment in mice is similar to about 20 human years, of which in our study 10 years were spent with PD-like lesion with NR treatment. We observed that long term NR treatment triggers trend towards increased DA turnover in the SN which has been reported to trigger mitochondrial DNA deletions [57]. NR enhanced mitochondrial function could possibly exacerbate this effect. More research is needed to understand how NR reduces DA levels and DA metabolism related gene expression levels in the SN. Collecting additional safety data, including SPECT and/or PET imaging of dopaminergic markers and longitudinal monitoring of motor scores in individual patients during the long-term NR trial are therefore important. In addition, healthy long-term NR users should be aware of a possibility of potential adverse effect of NR triggered by genetic, environmental or pharmacological reduction in proteasome function.
Study limitations
We recognize that future experiments should also include female mice, extend analysis from mRNA to proteins and deeper characterize changes in the brain dopamine system and mitochondrial function. None of available animal models recapitulate the slow progression of PD observed in humans with concomitant motor and non-motor symptoms [22,23]. Therefore, conclusive assessment of NR safety and efficacy cannot be reached. The only route forward is a careful analysis of the long-term effects of NR on disease symptoms and DA metabolism in available PD animal models and implementation of emerging research results in the ongoing trials in the clinic. We also recognize that our results may not be translated into a situation in which disease has already progressed. We also recognize that we do not know how well LC induced proteasome inhibition in mouse SN models proteasome inhibition in PD patients SN.
Data availability
Has the data associated with your study been deposited into a publicly available repository?
Response – No. This work does not include “omics” or other large datasets. Experimental data is presented and included in article/supp. material/referenced in the article. Raw data is available upon reasonable request.
CRediT authorship contribution statement
Giorgio Turconi: Writing – original draft, Visualization, Validation, Methodology, Investigation. Farhan Alam: Validation, Methodology, Investigation. Tanima SenGupta: Visualization, Validation, Investigation. Sini Pirnes-Karhu: Methodology, Investigation. Soophie Olfat: Methodology, Investigation. Mark S. Schmidt: Investigation. Kärt Mätlik: Methodology, Investigation. Ana Montaño-Rodriguez: Methodology, Investigation. Vladimir Heiskanen: Methodology. Daniel Garton: Formal analysis. Petteri T. Piepponen: Investigation. Charles Brenner: Formal analysis. Carina I. Holmberg: Validation, Funding acquisition, Formal analysis, Conceptualization. Hilde Nilsen: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Eija Pirinen: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Jaan-Olle Andressoo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Charles Brenner reports a relationship with ChromaDex Inc that includes: consulting or advisory and equity or stocks. Charles Brenner reports a relationship with Athena Therapeutics that includes: equity or stocks. Charles Brenner reports a relationship with Juvenis that includes: equity or stocks. Eija Pirinen reports a relationship with ChromaDex Inc that includes: speaking and lecture fees. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Kimmo Haimilahti, Minna Kuusela, Noora Pöllänen, Minna Tuominen, Rita Rinnankoski-Tuikka, and Anita Wagner for their valuable help and technical assistance during the mouse experiments, and we thank Sweta Jha for the assistance with C. elegans experiments. The authors also thank Dr. Kira Holmström and Sonja Koopal for their experimental advice when the project was initiated. The authors also wish to thank Liliya Euro for the development of NAD analysis protocol and for technical advice. We also acknowledge the Chromadex CERP Science Team for the fruitful discussion and the Electron Microscopy Unit of the Institute of Biotechnology, University of Helsinki, for providing laboratory facilities. Finally, the authors thank Dr. Petteri Hirvonen for his assistance with the statistics and Ian Mitchell for language editing. The DA α-syn C.elegans strain was provided by Blakely Lab (FAU Brain Institute) and the UA49 [baInl2; Punc-54::⍺-syn:GFP, rol-6 (su1006)] strain by Nektarios Tavernarakis lab. We thank the funders - The Finnish Parkinson Foundation (G.T.). Research Council of Norway grant no 302483 (HN). Doctoral program Brain and Mind and the Alfred Kordelin Foundation (K.M.). Academy of Finland grant no 297776 and the Sigrid Jusélius Foundation (C.I.H). Academy of Finland grants no 297727 and 350678, the Sigrid Jusélius Foundation, the Helsinki Institute of Life Science (HiLIFE) Fellow grant, the European Research Council (ERC) grant no 724922, Center of Innovative Medicine Young Investigator grant and the Hjärnfonden grant (J.-O.A.). Academy of Finland grant no 286359 (E.P.). Some of the C.elegans strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD01C. elegans0440).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34355.
Contributor Information
Eija Pirinen, Email: Eija.Pirinen@helsinki.fi.
Jaan-Olle Andressoo, Email: Jaan-Olle.Andressoo@helsinki.fi.
Appendix A. Supplementary data
The following is the supplementary data to this article.
References
- 1.DeMaagd G., Philip A. Parkinson's disease and its management: Part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T. 2015;40:504–532. [PMC free article] [PubMed] [Google Scholar]
- 2.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 3.Kish S.J., Shannak K., Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 4.Damier P., Hirsch E.C., Agid Y., Graybiel A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 5.Moehle E.A., Shen K., Dillin A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J. Biol. Chem. 2019;294:5396–5407. doi: 10.1074/jbc.TM117.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olanow C.W., McNaught K.S. Ubiquitin-proteasome system and Parkinson's disease. Mov. Disord. 2006;21:1806–1823. doi: 10.1002/mds.21013. [DOI] [PubMed] [Google Scholar]
- 7.Maiti P., Manna J., Dunbar G.L. Current understanding of the molecular mechanisms in Parkinson's disease: targets for potential treatments. Transl. Neurodegener. 2017;6:28. doi: 10.1186/s40035-017-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukhatwa S., Zeng B.Y., Rose S., Jenner P. A comparison of changes in proteasomal subunit expression in the substantia nigra in Parkinson's disease, multiple system atrophy and progressive supranuclear palsy. Brain Res. 2010;1326:174–183. doi: 10.1016/j.brainres.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 9.McNaught K.S., Belizaire R., Isacson O., Jenner P., Olanow C.W. Altered proteasomal function in sporadic Parkinson's disease. Exp. Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 10.McNaught K.S., Belizaire R., Jenner P., Olanow C.W., Isacson O. Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson's disease. Neurosci. Lett. 2002;326:155–158. doi: 10.1016/s0304-3940(02)00296-3. [DOI] [PubMed] [Google Scholar]
- 11.Bentea E., Verbruggen L., Massie A. The proteasome inhibition model of Parkinson's disease. J. Parkinsons Dis. 2017;7:31–63. doi: 10.3233/JPD-160921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spivey A. Rotenone and paraquat linked to Parkinson's disease: human exposure study supports years of animal studies. Environ. Health Perspect. 2011;119:A259. doi: 10.1289/ehp.119-a259a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner C.M., Kamel F., Ross G.W., Hoppin J.A., Goldman S.M., Korell M., Marras C., Bhudhikanok G.S., Kasten M., Chade A.R., et al. Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bieganowski P., Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. S0092867404004167 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Kam T.I., Mao X., Park H., Chou S.C., Karuppagounder S.S., Umanah G.E., Yun S.P., Brahmachari S., Panicker N., Chen R., et al. Poly(ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson's disease. Science. 2018;362 doi: 10.1126/science.aat8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann S., Loh S.H., Martins L.M. Enhancing NAD(+) salvage metabolism is neuroprotective in a PINK1 model of Parkinson's disease. Biol Open. 2017;6:141–147. doi: 10.1242/bio.022186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schondorf D.C., Ivanyuk D., Baden P., Sanchez-Martinez A., De Cicco S., Yu C., Giunta I., Schwarz L.K., Di Napoli G., Panagiotakopoulou V., et al. The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson's disease. Cell Rep. 2018;23:2976–2988. doi: 10.1016/j.celrep.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Sorrentino V., Romani M., Mouchiroud L., Beck J.S., Zhang H., D'Amico D., Moullan N., Potenza F., Schmid A.W., Rietsch S., et al. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature. 2017;552:187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q., Zhu L., Qiu W., Liu Y., Yang F., Chen W., Xu R. Nicotinamide riboside enhances mitochondrial proteostasis and adult neurogenesis through activation of mitochondrial unfolded protein response signaling in the brain of ALS SOD1(G93A) mice. Int. J. Biol. Sci. 2020;16:284–297. doi: 10.7150/ijbs.38487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SenGupta T., Palikaras K., Esbensen Y.Q., Konstantinidis G., Galindo F.J.N., Achanta K., Kassahun H., Stavgiannoudaki I., Bohr V.A., Akbari M., et al. Base excision repair causes age-dependent accumulation of single-stranded DNA breaks that contribute to Parkinson disease pathology. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brakedal B., Dolle C., Riemer F., Ma Y., Nido G.S., Skeie G.O., Craven A.R., Schwarzlmuller T., Brekke N., Diab J., et al. The NADPARK study: a randomized phase I trial of nicotinamide riboside supplementation in Parkinson's disease. Cell Metabol. 2022;34:396–407 e396. doi: 10.1016/j.cmet.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Airavaara M., Parkkinen I., Konovalova J., Albert K., Chmielarz P., Domanskyi A. Back and to the future: from neurotoxin-induced to human Parkinson's disease models. Current protocols in neuroscience/editorial board, Jacqueline N. Crawley. 2020;91 doi: 10.1002/cpns.88. [et al.] [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Benito M., Granado N., Garcia-Sanz P., Michel A., Dumoulin M., Moratalla R. Modeling Parkinson's disease with the alpha-synuclein protein. Front. Pharmacol. 2020;11:356. doi: 10.3389/fphar.2020.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodhicharla R., Nagarajan A., Winter J., Adenle A., Nazir A., Brady D., Vere K., Richens J., O'Shea P., Bell D.R., de Pomerai D. Effects of alpha-synuclein overexpression in transgenic Caenorhabditis elegans strains. CNS Neurol. Disord.: Drug Targets. 2012;11:965–975. doi: 10.2174/1871527311211080005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamamichi S., Rivas R.N., Knight A.L., Cao S., Caldwell K.A., Caldwell G.A. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson's disease model. Proc. Natl. Acad. Sci. U. S. A. 2008;105:728–733. doi: 10.1073/pnas.0711018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington A.J., Knight A.L., Caldwell G.A., Caldwell K.A. Caenorhabditis elegans as a model system for identifying effectors of alpha-synuclein misfolding and dopaminergic cell death associated with Parkinson's disease. Methods. 2011;53:220–225. doi: 10.1016/j.ymeth.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 27.Runkel E.D., Liu S., Baumeister R., Schulze E. Surveillance-activated defenses block the ROS-induced mitochondrial unfolded protein response. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz A.M., Haynes C.M. UPR(mt)-mediated cytoprotection and organismal aging. Biochim. Biophys. Acta. 2015;1847:1448–1456. doi: 10.1016/j.bbabio.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Canto C., Mottis A., Jo Y.S., Viswanathan M., Schoonjans K., et al. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentea E., Van der Perren A., Van Liefferinge J., El Arfani A., Albertini G., Demuyser T., Merckx E., Michotte Y., Smolders I., Baekelandt V., Massie A. Nigral proteasome inhibition in mice leads to motor and non-motor deficits and increased expression of Ser129 phosphorylated alpha-synuclein. Front. Behav. Neurosci. 2015;9:68. doi: 10.3389/fnbeh.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A., Kopra J., Varendi K., Porokuokka L.L., Panhelainen A., Kuure S., Marshall P., Karalija N., Harma M.A., Vilenius C., et al. GDNF overexpression from the native locus reveals its role in the nigrostriatal dopaminergic system function. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNaught K.S., Bjorklund L.M., Belizaire R., Isacson O., Jenner P., Olanow C.W. Proteasome inhibition causes nigral degeneration with inclusion bodies in rats. Neuroreport. 2002;13:1437–1441. doi: 10.1097/00001756-200208070-00018. [DOI] [PubMed] [Google Scholar]
- 33.Vernon A.C., Johansson S.M., Modo M.M. Non-invasive evaluation of nigrostriatal neuropathology in a proteasome inhibitor rodent model of Parkinson's disease. BMC Neurosci. 2010;11(1) doi: 10.1186/1471-2202-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olfat S., Matlik K., Kopra J.J., Garton D.R., Iivanainen V.H., Bhattacharya D., Jakobsson J., Piepponen T.P., Andressoo J.O. Increased physiological GDNF levels have No effect on dopamine neuron protection and restoration in a proteasome inhibition mouse model of Parkinson's disease. eNeuro. 2023;10 doi: 10.1523/ENEURO.0097-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savolainen M.H., Albert K., Airavaara M., Myohanen T.T. Nigral injection of a proteasomal inhibitor, lactacystin, induces widespread glial cell activation and shows various phenotypes of Parkinson's disease in young and adult mouse. Exp. Brain Res. 2017;235:2189–2202. doi: 10.1007/s00221-017-4962-z. [DOI] [PubMed] [Google Scholar]
- 36.Deneyer L., Albertini G., Bentea E., Massie A. Systemic LPS-induced neuroinflammation increases the susceptibility for proteasome inhibition-induced degeneration of the nigrostriatal pathway. Parkinsonism Relat. Disorders. 2019;68:26–32. doi: 10.1016/j.parkreldis.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Deneyer L., Massie A., Bentea E. Ketamine does not exert protective properties on dopaminergic neurons in the lactacystin mouse model of Parkinson's disease. Front. Behav. Neurosci. 2018;12:219. doi: 10.3389/fnbeh.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du Y., Li X., Yang D., Zhang X., Chen S., Huang K., Le W. Multiple molecular pathways are involved in the neuroprotection of GDNF against proteasome inhibitor induced dopamine neuron degeneration in vivo. Exp Biol Med (Maywood) 2008;233:881–890. doi: 10.3181/0712-RM-329. [DOI] [PubMed] [Google Scholar]
- 39.Du Y., Zhang X., Tao Q., Chen S., Le W. Adeno-associated virus type 2 vector-mediated glial cell line-derived neurotrophic factor gene transfer induces neuroprotection and neuroregeneration in a ubiquitin-proteasome system impairment animal model of Parkinson's disease. Neurodegener. Dis. 2013;11:113–128. doi: 10.1159/000334527. [DOI] [PubMed] [Google Scholar]
- 40.Niu C., Mei J., Pan Q., Fu X. Nigral degeneration with inclusion body formation and behavioral changes in rats after proteasomal inhibition. Stereotact. Funct. Neurosurg. 2009;87:69–81. doi: 10.1159/000202972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trammell S.A., Schmidt M.S., Weidemann B.J., Redpath P., Jaksch F., Dellinger R.W., Li Z., Abel E.D., Migaud M.E., Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016;7 doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perier C., Vila M. Mitochondrial biology and Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J.S., Davis R.L., Sue C.M. Mitochondrial dysfunction in Parkinson's disease: new mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018;18:21. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao X.Y., Yang T., Gu Y., Sun X.H. Mitochondrial dysfunction in Parkinson's disease: from mechanistic insights to therapy. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.885500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zigmond M.J., Hastings T.G., Perez R.G. Increased dopamine turnover after partial loss of dopaminergic neurons: compensation or toxicity? Parkinsonism Relat. Disorders. 2002;8:389–393. doi: 10.1016/s1353-8020(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 46.Pifl C., Hornykiewicz O. Dopamine turnover is upregulated in the caudate/putamen of asymptomatic MPTP-treated rhesus monkeys. Neurochem. Int. 2006;49:519–524. doi: 10.1016/j.neuint.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Brooks D.J. Imaging approaches to Parkinson disease. J. Nucl. Med. 2010;51:596–609. doi: 10.2967/jnumed.108.059998. [DOI] [PubMed] [Google Scholar]
- 48.Strafella A.P., Bohnen N.I., Pavese N., Vaillancourt D.E., van Eimeren T., Politis M., Tessitore A., Ghadery C., Lewis S., Group I.P.-N.S. Imaging markers of progression in Parkinson's disease. Mov Disord Clin Pract. 2018;5:586–596. doi: 10.1002/mdc3.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L., Zhang Q., Li H., Zhang H. SPECT molecular imaging in Parkinson's disease. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/412486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou J., Weng R.H., Chen Z.Y., Wei X.B., Wang R., Chen D., Xia Y., Wang Q. Position emission tomography/single-photon emission tomography neuroimaging for detection of premotor Parkinson's disease. CNS Neurosci. Ther. 2016;22:167–177. doi: 10.1111/cns.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canto C., Menzies K.J., Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metabol. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bedford L., Hay D., Devoy A., Paine S., Powe D.G., Seth R., Gray T., Topham I., Fone K., Rezvani N., et al. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J. Neurosci. 2008;28:8189–8198. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canto C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabol. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. S1550-4131(12)00192-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang E.F., Hou Y., Lautrup S., Jensen M.B., Yang B., SenGupta T., Caponio D., Khezri R., Demarest T.G., Aman Y., et al. NAD(+) augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat. Commun. 2019;10:5284. doi: 10.1038/s41467-019-13172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wheatley K., Stowe R.L., Clarke C.E., Hills R.K., Williams A.C., Gray R. Evaluating drug treatments for Parkinson's disease: how good are the trials? BMJ. 2002;324:1508–1511. doi: 10.1136/bmj.324.7352.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrison I.F., Powell N.M., Dexter D.T. The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson's disease. J. Neurochem. 2019;148:136–156. doi: 10.1111/jnc.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neuhaus J.F., Baris O.R., Hess S., Moser N., Schroder H., Chinta S.J., Andersen J.K., Kloppenburg P., Wiesner R.J. Catecholamine metabolism drives generation of mitochondrial DNA deletions in dopaminergic neurons. Brain. 2014;137:354–365. doi: 10.1093/brain/awt291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Has the data associated with your study been deposited into a publicly available repository?
Response – No. This work does not include “omics” or other large datasets. Experimental data is presented and included in article/supp. material/referenced in the article. Raw data is available upon reasonable request.