Abstract
Wasp venom injections from wasp stings can damage several organs, most commonly the kidneys. Despite literature evidence, wasp sting-induced acute kidney injury (AKI) is rare and involves complex pathophysiological processes. While acute tubular necrosis (ATN) is the most prevalent histological result of wasp sting-induced AKI, uncommon combinations of chronic renal lesions have been described, alerting us to the patient's underlying illness. We report a 55-year-old hypertensive patient with unknown renal function who got AKI following multiple wasp stings. His renal function had not improved after continuous hemodialysis and plasma exchange; therefore, a kidney biopsy was performed. The pathology revealed that in addition to ATN, his kidney's distinguishing feature was a mix of chronic interstitial renal disease and chronic glomerulosclerosis. We think that his current renal pathological results were caused by hypertension in addition to wasp venom.
Keywords: Kidney injury, Toxins, Wasp stings, Case report
1. Introduction
Wasp stings are an uncommon illness that presents with a variety of clinical symptoms in the emergency room, including pain at the site of the sting along with skin reddening, localized tissue swelling, high skin temperature, and skin itching. The primary pathogenic mechanisms attributed to wasp stings resulting in either localized or systemic symptoms are allergic reactions and direct toxic consequences. Localized allergies near the stings are one way that allergies might appear, but severe systemic allergic reactions can be fatal [1]. Wasp stings can cause direct toxic effects such as disseminated intravascular coagulation, acute kidney injury (AKI), acute coronary syndrome, rhabdomyolysis, intravascular hemolysis, multi-organ failure, and even death [2].
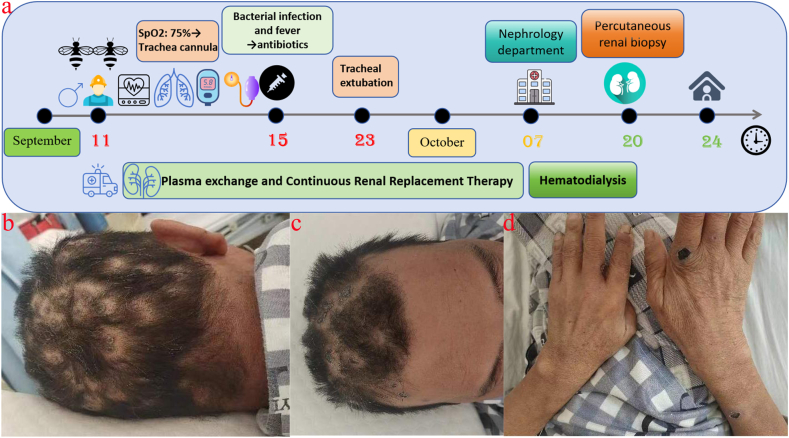
Nitrogen product buildup and electrolyte imbalance are caused by AKI, a brief period of reduced renal function that is followed by renal failure [3]. Direct toxic effects on renal function have been observed in cases of wasp stings [4,5]. Even though wasp stings have been shown to cause AKI in this literature, it remains a rare disease with complex pathophysiological mechanisms. Shock, rhabdomyolysis, hemolysis and direct tubular nephrotoxicity are thought to play an important role in this regard [6]. This article describes a case of multiple wasp stings (Fig. 1a) and gives a review of the signs and therapy of kidney impairment in wasp poisoning.
Fig. 1.
(a) The patient's course of treatment; (b–d) Four weeks following the patient's wasp sting, at which point the stings had turned crusty and black.
2. Case report
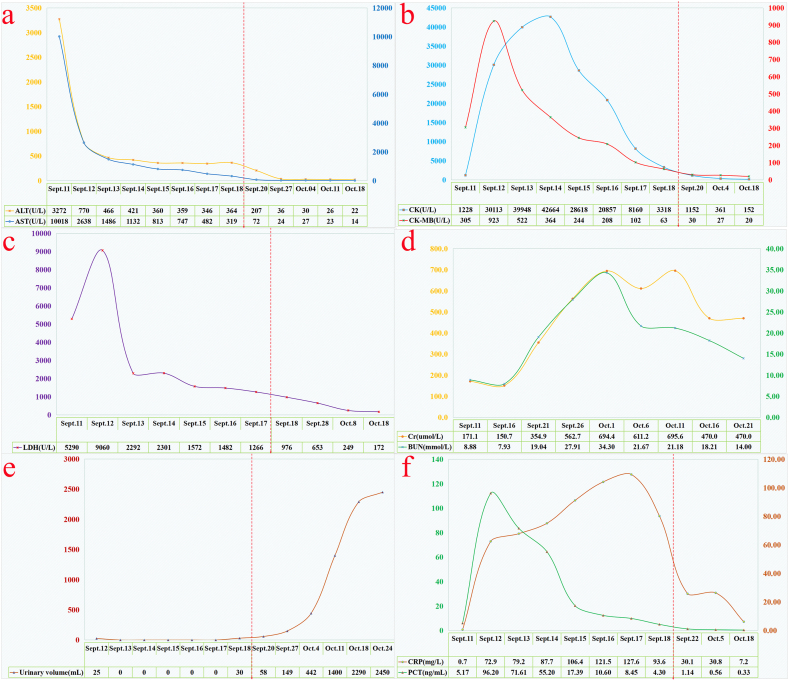
The 55-year-old male patient was unintentionally stung by a wasp swarm while working in the field. He experienced severe pain, panic, tightness in his chest, and dyspnea. His family immediately brought him to the Municipal Hospital's Emergency Centre on September 11, 2023, the day the disease first appeared. Once there, doctors discovered that he had a blood pressure of 80/50 mmHg, a heart rate of 132 beats per minute, a shallow coma with mild consciousness, black spots on his head, limbs, and chest from the wasp stings, and localized swelling at the wasp stings at the backs of his hands with no negative pathological reflex. The emergency room doctors gave the patient extra fluids and dexamethasone to reduce inflammation and swelling right away. As the patient slowly regained awareness, his blood pressure rose to 150/90 mm Hg. It is important to note that the patient has a history of type 2 diabetes mellitus and hypertension spanning more than five years. His blood pressure varies between 150 and 160/100–110 mmHg, and he has not used any antihypertensive drugs. However, he has taken metformin and gliclazide on an irregular basis to regulate his blood sugar. The patient's health worsened quickly, and on the day of admission, the Intensive Care Unit (ICU) was deemed necessary due to the patient's propensity for severe rhabdomyolysis and multiple organ failure, as indicated by the findings of pertinent examinations (Table 1, Fig. 2). The patient was intubated and placed on a ventilator 6 h after being admitted to the ICU due to acute respiratory distress and a decline in oxygen saturation to 75 %. On the first day, the patient's aminotransferases were noticeably raised (Fig. 2a). On the second day, the patient also had elevated levels of creatine kinase (CK), creatine kinase isoenzyme (CK-MB) (Fig. 2b), and lactate dehydrogenase (LDH) (Fig. 2c). Serum creatinine (SCr) and blood urea nitrogen (BUN) levels peaked just two weeks after being admitted to the ICU (Fig. 2d). The patient's urine output was greatly decreased or not present at all (Fig. 2e), but he started receiving continuous plasma exchange (PE) to get rid of toxins and continuous renal replacement therapy (CRRT) as soon as he was admitted to the ICU. Furthermore, the patient's hematology revealed dynamic changes, including gradual reductions in hemoglobin and platelets that peaked on the fourth day of the patient's admission to the ICU (Table 1). As a result, the patient required an erythrocyte and platelet transfusion. The patient's body temperature increased to 38.5 °C on the fifth day, and the inflammatory indicators C-reactive protein (CRP) was also noticeably elevated (Fig. 2f). An anti-infective treatment consisting of piperacillin sodium/tazobactam sodium was administered to cover more Gram-positive and Gram-negative organisms. The patient's body temperature was maintained at 37–38 °C following the administration. On the seventh day, the patient started coughing up thick, yellow sputum. A culture of the mucus showed that it contained Stenotrophomonas melophilia and Acinetobacter baumannii, so levofloxacin was added to treat the infection. Following four weeks of ICU treatment, the patient's wasp stings developed a charred scab (Fig. 1b–d). Additionally, the patient's blood count, liver function, inflammation indexes, coagulation function, and other indexes were gradually improved (Table 1, Fig. 2). Urine output also gradually improved, but the patient's renal function remained severely compromised, and it was advised that the patient be moved to the nephrology department for additional care.
Table 1.
Progression of biochemical parameters of organ injury.
| Test item | Sept, 11th, 2023 | Sept, 14th, 2023 | Sept, 17th, 2023 | Oct 3rd, 2023 | Normal value |
|---|---|---|---|---|---|
| Blood test | |||||
| WBC ( × 109/L) | 19.9↑ | 16.9↑ | 13.4↑ | 12.0↑ | 4.1–11.0 |
| RBC ( × 1012/L) | 4.44 | 2.96↓ | 2.48↓ | 3.30↓ | 4.10–5.80 |
| Hb (g/L) | 146 | 68↓ | 81↓ | 94↓ | 114∼154 |
| PLT ( × 109/L) | 245 | 16↓ | 70↓ | 171 | 150∼407 |
| Biochemical indexes | |||||
| TBIL (umol/L) | 84.7↑ | 76.6↑ | 42.8↑ | 30.7↑ | ≤23.0 |
| DBIL (umol/L) | 56.8↑ | 58.1↑ | 33.9↑ | 22.1↑ | ≤8.0 |
| IBIL (umol/L) | 27.9↑ | 18.5↑ | 8.9 | 8.6 | 0∼17.1 |
| ALP (U/L) | 211↑ | 170↑ | 154↑ | 140↑ | 45∼125 |
| UA (umol/L) | 497↑ | 98↓ | 76↓ | 319 | 142∼416 |
| TP (g/L) | 53↓ | 56↓ | 60↓ | 68 | 65∼85 |
| ALB (g/L) | 33↓ | 36↓ | 36↓ | 38↓ | 40∼55 |
| GLB (g/L) | 30 | 20 | 24 | 27 | 20∼40 |
| PAB (mg/L) | 224 | 201 | 88↓ | 163↓ | 180∼390 |
| FBG (mmol/L) | 22.0↑ | 6.2↑ | 4.4 | 5.3 | 3.9–6.1 |
| TnI (ng/mL) | 4.800↑ | 0.480↑ | 0.055↑ | 0.010 | 0.010–0.023 |
| MYO (ng/mL) | >1200↑ | >1200↑ | >1200↑ | 386↑ | <118 |
| Coagulation function | |||||
| TT (s) | 20 | 18 | 17 | 16 | 14∼21 |
| APTT (s) | 41.2↑ | 48.1↑ | 34.9↑ | 28.4 | 23.3–32.5 |
| PT (s) | 31.0↑ | 12.1 | 12.1 | 11.8 | 9.8–12.1 |
| FIB (g/L) | 2.1 | 5.3↑ | 5.9↑ | 4.2↑ | 1.8–3.5 |
| D-D (mg/L) | 9.20↑ | 5.10↑ | 2.31↑ | 0.52 | 0∼0.55 |
Note: WBC, White blood cell count; RBC, Red blood cell count; Hb, Hemoglobin; PLT, Platelet count; TBIL, Total bilirubin; DBIL, Direct bilirubin; IBIL, Indirect bilirubin; ALP, Alkaline phosphatase; UA, Uric acid; TP, Total protein; ALB, Albumin; GLB, Globulin; PAB, Prealbumin; FBG, Fasting blood glucose; TnI, Troponin I; MYO, Myoglobin; TT, Thrombin time; APTT, Activated partial thromboplastin time; PT, Prothrombin time; FIB, Fibrinogen; D-D, D-Dimer.
Fig. 2.
Changes in Aspartate aminotransferase and alanine aminotransferase (a); creatine kinase and creatine kinase isoenzyme (b); lactate dehydrogenase (c); serum creatinine and blood urea nitrogen (d); urine output (e); C-reactive protein and procalcitonin (f) during the patients' admission.
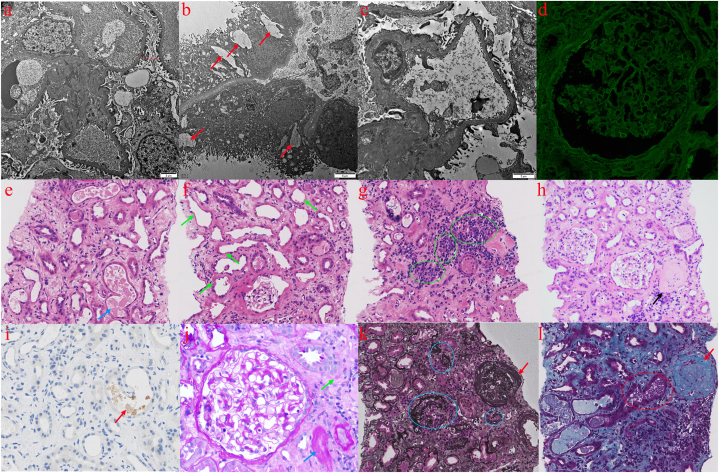
On October 20, 2023, the patient had an ultrasound-guided percutaneous nephrectomy biopsy to identify the precise kidney lesions. Pathological observations: The ultrastructure (Fig. 3a–c) showed that the glomerular tethering zone, intra-basement membrane, subendothelial basement membrane of capillary collaterals, and subepithelial did not exhibit any clear deposits of electron-dense material. In the renal interstitial, there was a modest infiltration of inflammatory cells with collagen fiber growth and vacuolar degeneration of renal tubular epithelial cells. The renal interstitial capillaries' lumen was seen to include erythrocytes. Immunofluorescence (Fig. 3d): IgG (−); IgA (−); IgM (±); C3 (−); C1q (−); Fib (−); ALB (−); kappa (−); lambda (−); PLA2R (−); THSD7A (−). Histology analysis (Fig. 3e-l): 14 glomeruli, including 7 glomerulosclerosis, were seen in 1 strip of cortical renal tissue when observed under light microscopy with HE staining. The glomerular mesangial area showed mild proliferation of the mesangial cells and the stroma; the wall of the glomerular capsules of several glomeruli was observed to be thickened. Multiple renal tubular atrophy, glomerulonephritis tubular lumens seen as eosinophilic debris, polarized light-negative basophilic material seen in individual tubular lumens, some tubular lumens dilated with loss of bristle margins, and detached tubular epithelial cells with exposed basement membranes are all observed. Multifocal inflammatory cells were infiltrated into the renal interstitium, which also showed moderate-to-heavy fibrosis. The tiny arteriole hyaline degeneration occurs in the renal interstitial. The peritubular capillary lumen displays infiltration of inflammatory cells. Tubular myoglobin immunohistochemical staining in the lumen of renal tubules was positive; renal interstitial inflammatory lesions and tubular necrosis were observed in PAS staining; and no diplomatic hemoglobin accumulation was observed in any section of the glomeruli in Masson or PASM staining. Light microscopy, immunofluorescence microscopy, and electron microscopy were used to make the diagnosis. They showed that the kidneys had been damaged by wasp stings venom, with acute rhabdomyolysis and acute tubular necrosis, along with glomerulosclerosis (7/14 glomeruli), and chronic tubulo-interstitial disease of moderate to severity. The patient tested negative for all renal tissue-associated antibodies (Table 2). The patient's urine output recovered to normal five weeks after the disease started, and his internal environment stabilized. He was then temporarily discharged from hemodialysis and given instructions to follow up with the nephrology department for a long time and to take medication to control his blood pressure and blood sugar.
Fig. 3.
The renal pathological findings of the patient. Electron microscopy revealed segmental hyperplasia of mesangial cells and stroma in the glomerular mesangial area, about 60 % of cases of podocyte peduncle fusion, tubular epithelial cells' vacuolar degeneration (red arrow), a small number of inflammatory cells infiltration in the kidneys' interstitium, and hyperplasia of collagenous fibers (a–c); Immunofluorescence of renal tissues revealed IgG(−) (d); HE staining showed partial glomerulosclerosis (black arrows), the mild proliferation of mesangial cells and stroma in the glomerular mesangial zone, thickening of some glomerular capsule walls, vacuolation of tubular epithelial cells and degeneration of granules, massive bare basement membrane formation, renal tubular epithelial cell cytoplasm and brush border barely visible (green arrow), fragmentation of eosinophilic red in some glomerulonephric tubule lumens (blue arrow), multifocal inflammatory cell infiltration of the renal mesangium (green circle), and moderate-to-severe fibrosis (e-h, 200 × ); Immunohistochemical staining of tubular myoglobin in the lumen of renal tubules was positive, with positive Myoglobin-associated granules indicated by red arrows (i, 400 × ); PAS staining showed renal tubular necrosis (blue arrows) and renal interstitial inflammatory lesions (green arrows) (j, 400 × ); PASM (k, 200 × ) and Masson (l, 200 × ) staining of glomerular sites did not show complexophilic hemoglobin deposition, the red arrows indicate sclerotic glomeruli, the blue circles show atrophied tubules and surrounding dark grey fibrotic areas, the purple granular tubular patterns in the official lumen may be seen in the red circles, and the myoglobin histochemistry suggests positive. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Blood and urine related tests in this patient with wasp stings.
| Test item | Normal value | Test item | Normal value | ||
|---|---|---|---|---|---|
| urine tests | Serum antibody | ||||
| Protein | ++ | (−) | Anti-Human Globulin Test | +++ | (−) |
| 24h-urinary proteins | 2.187↑ | 0.050–0.100 | PLA2R antibody (RU/mL) | <5.0 | <14.0 |
| Glucose | ++++ | (−) | Anti-GBM IgG (AU/mL) | <2.0 | <16.0 |
| Red blood cell (/μL) | 779↑ | 0∼17 | MPO IgG (AU/mL) | 1.68 | <16.0 |
| White blood cell (/μL) | 7 | 0∼28 | PR3-ANCA IgG (AU/mL) | <2.0 | <16.0 |
| Urinary cylinder | (−) | (−) | ANA | (−) | (−) |
| PH | 8 | 5∼8 | Anti-dsDNA antibody | (−) | (−) |
Note: PLA2R, Anti-phospholipase A2 receptor; GBM, Glomerular basement membrane; MPO, Myeloperoxidase; PR3, Proteinase 3; ANCA, Anti-neutrophil cytoplasmic antibodies; ANA, Antinuclear antibody; dsDNA, Double-stranded deoxyribonucleic acid; +, Positive; (−), Negative.
3. Discussion
The International Society of Nephrology established the "0 by 25″ campaign, which seeks to end untreated acute kidney injury-related mortality by the year 2025 [7]. Considering this, we ought to focus on the poorly understood origins of acute renal damage. Nevertheless, the exact mechanism by which wasp stings result in kidney damage remains unclear. Wasp stings cause massive intoxication, which sets off a cascade of immunological reactions that cause rhabdomyolysis, intravascular hemolysis, hypotension, and direct cytotoxic effects on the renal tubules [8]. The kidneys are especially vulnerable to toxins after stinging because they are greatly dilated excretory organs [9].
Enzymes (phospholipases and hyaluronidase), amines (histamine, serotonin, and several catecholamines), peptides (apamin, and wasp kinins), and other substances make up the complex composition of wasp venom [10,11]. Numerous investigations have demonstrated that hemolytic toxins result in hemolysis, rhabdomyolysis, and coagulation abnormalities. The main mechanism causing AKI is believed to be myoglobin's tubular blockage and toxicity, which are secondary to tubular necrosis [9,11]. Hemoglobin, which produces free radicals and causes lipid peroxidation and cellular damage, is the mechanism by which myoglobin directly damages renal tubular epithelial cells. Myoglobin is catabolically metabolized in the proximal tubule and easily filtered through the glomerular basement membrane [12]. The integrity of renal tubular cell membranes can be damaged by phospholipases and mast cells, which can cause intracellular components to extrude [13]. The components of wasp venom that including hyaluronidase, phospholipase A2, and apamin can produce systemic vasodilatation, which can result in an elevated heart rate and severe hypotension. This, in turn, can lower renal blood flow and promote the development of AKI. Furthermore, wasp venom causes the body to generate mediators like prostaglandins, bradykinin, histamine, and 5-hydroxytryptamine, which lowers blood pressure [14]. The development of AKI is caused by vasoconstriction of small renal inlet and outlet arterioles, a decrease in glomerular and tubular blood flow, and activation of the renin-angiotensin-aldosterone system due to inadequate renal perfusion, enhanced adrenergic nerve stimulation, and catecholamine secretion [11,15]. Wasp venom either directly or indirectly caused tubular poisoning in the patient in this investigation, resulting in acute tubular necrosis (ATN). A severe allergic reaction, low blood pressure resulting in inadequate renal perfusion, elevated creatine kinase, and renal pathology indicating positive tubular myoglobin immunohistochemical staining in the tubule lumen are all observed in the patient. These findings are consistent with ATN brought on by rhabdomyolysis.
Curiously, though, the patient's pathology revealed tubulointerstitial and glomerular lesions, and there are no reports of wasp stings linked to glomeruli. However they have been linked to acute interstitial nephritis, wasp stings are relatively uncommon and are thought to be caused by immune-mediated tubulointerstitial injury or blockage of the tubules by macromolecules like myoglobin and hemoglobin [16]. Furthermore, the patient's pathology indicated a mix of acute and chronic renal lesions; however, the kidney biopsy was done just one month after the wasp stings, and the patient's negative for antibodies against any renal tissue compelled us to take his underlying condition into account. The patient's urine routine revealed a large increase in urinary protein at the beginning of wasp stings (Table 2), and he had a five-year history of hypertension that was typically uncontrolled. It became evident that the patient's uncontrolled hypertension, rather than the wasp stings, was the cause of his chronic glomerulosclerosis and chronic interstitial lesions. It is interesting that until around 50 % of renal function is lost [17], Scr concentrations remain unchanged, and large rises in serum creatinine are typically not seen until 48–72 hours following the initial renal injury [18]. Therefore, hypertension nephropathy may be the cause of the patient's initial test's rise in BUN and Scr. Also, most people who get wasp stings have a good outcome after renal replacement therapy. The recovery time for renal function is 1–8 weeks, with an average of 5.5 weeks [19]. This means that hypertensive nephropathy may be another reason why serum creatinine doesn't drop much even though the patient's urine output has returned to normal after a long period of dialysis treatment. Therefore, chronically unmanaged or uncontrolled hypertension was most likely the cause of the chronic glomerulopathy. It is evident that these consequences may worsen renal damage already present in cases of ATN and AKI that occur after wasp stings. This study focuses on a particular clinical phenomenon, the reporting of which has contributed to our understanding of kidney disease and its complex causative factors. It also highlights the significance of managing chronic diseases, which can simplify the diagnosis of the condition in the event of unexpected external factors like insect bites. This study focuses on a particular clinical phenomenon, the reporting of which has contributed to our understanding of kidney disease and its complex causative factors. It also warns of the significance of managing chronic diseases, which can simplify the diagnosis of the condition in the event of unexpected external factors like insect bites. Nevertheless, this study is a report that relies on a solitary case; hence, its conclusions may lack generalizability. There is a possibility that chronic kidney disease is caused by inadequate management or insufficient control of a patient's chronic condition, which, although reasonable, is not supported by direct evidence.
AKI from wasp stings is typically treatable, just like from other sources of AKI. Consequently, to reverse renal impairment and enhance patient prognosis, greater illness awareness as well as early detection and care in individuals at risk are crucial. Hemodialysis treatment should be taken into consideration for patients with wasp stings who exhibit symptoms of gross hematuria or serum LDH of more than 463.5 U/L upon hospital admission, as these conditions significantly enhance the risk of AKI [20]. After wasp stings, blood purification-which mostly consists of plasma exchange, intermittent hemodialysis, and continuous respiratory support-is now thought to be the most effective treatment for AKl. PE can eliminate not just small and medium-sized pollutants and waste products from the body, but also large molecules like proteins, antigens, and antibodies, inflammatory mediators, and immunocomplexes. Additionally, it can restore the body's deficient levels of albumin and coagulation factors. It has the benefit of eliminating inflammatory mediators, secondary harmful compounds, and wasp venom constituents [21]. Blood purification therapy should be started as soon as possible when the clinical manifestations are severe and AKI is accompanied by severe hemolysis, rhabdomyolysis, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome (MODS). CRRT is preferred and can be combined with PE at the same time to remove wasp venom elements, inflammatory mediators, myoglobin, cytokines, and complement components [22,23]. Thus, early application of hemodialysis procedures in critically ill wasp-stung patients can enhance patient survival and considerably improve recovery of the patient's systemic organ function, as demonstrated by the patient described in this paper, whose LDH surpassed 5000 U/L on the first test.
4. Conclusions
To summarize, this case presents a patient with chronic glomerulopathy who experienced wasp stings and subsequently developed AKI. The patient's renal state is most likely a result of long-term mismanagement or inadequate control of hypertension, although there was no previous definitive diagnosis of hypertensive nephropathy. The occurrence of ATN and AKI after being stung by wasps is frequently observed in clinical settings. It is evident that these problems can have more severe repercussions for individuals with pre-existing renal impairment. In addition to early renal biopsy in patients who have not recovered renal function, consideration must be given to the association between the patient's currently present underlying condition and renal function when AKI arises.
Funding
This work was supported by the Fujian Province Natural Science Fund Project (2022J01409, 2021J02053, 2023J011159, 2022J01996), the Fujian Province Medical Innovation Foundation (2022CXA001, 2021CXB001, 2022CXB002), the Special Research Foundation of Fujian Provincial Department of Finance (No. 2023-830, 2021-848, 2021-917, 2022-840), and National famous and old Chinese medicine experts (Xuemei Zhang, Xiaohua Yan) inheritance studio construction project.
Ethics approval and consent to participate
All procedures were performed in accordance to the tenets of the Declaration of Helsinki and the study was approved by the Ethics Committee of Fujian Provincial Hospital, Fuzhou, China. The participant provided written informed consent.
Consent for publication
Written informed consent for publication this study was obtained from the patient. A copy of those written consents is available for review by the Editor-in-Chief of this journal.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Jian-hui Zhang: Writing – original draft, Data curation. Jing Zou: Writing – original draft, Data curation. Dan-dan Ruan: Data curation. Qian Chen: Writing – original draft. Min Wu: Formal analysis. Hong-ping Yu: Formal analysis. Qiu-yan Wu: Formal analysis. Fan Lin: Supervision, Funding acquisition. Jie-wei Luo: Supervision, Funding acquisition. Li Zhang: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Contributor Information
Fan Lin, Email: linfan@fjmu.edu.cn.
Jie-wei Luo, Email: docluo0421@aliyun.com.
Li Zhang, Email: lilydoctor9902@163.com.
Abbreviations
- AKI
Acute kidney injury
- ICU
Intensive Care Unit
- CK
Creatine kinase
- CK-MB
Creatine kinase isoenzyme
- LDH
lactate dehydrogenase
- Scr
Serum creatinine
- BUN
Blood urea nitrogen
- PE
Plasma exchange
- CRRT
Continuous renal replacement therapy
- CRP
C-reactive protein
- ATN
Acute tubular necrosis
- MODS
Multiple organ dysfunction syndrome
References
- 1.Sahiner U.M., Durham S.R. Hymenoptera venom allergy: how does venom immunotherapy prevent anaphylaxis from bee and wasp stings? Front. Immunol. 2019;10:1959. doi: 10.3389/fimmu.2019.01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen T.N., Jeng M.J., Chen N.Y., Yang C.C. Outcomes of wasp and bee stings in Taiwan. Clin. Toxicol. 2023;61(3):181–185. doi: 10.1080/15563650.2023.2173075. [DOI] [PubMed] [Google Scholar]
- 3.Kellum J.A., Romagnani P., Ashuntantang G., Ronco C., Zarbock A., Anders H.J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021;7(1):52. doi: 10.1038/s41572-021-00284-z. [DOI] [PubMed] [Google Scholar]
- 4.Ruwanpathirana P., Priyankara D. Clinical manifestations of wasp stings: a case report and a review of literature. Trop. Med. Health. 2022;50(1):82. doi: 10.1186/s41182-022-00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan H., Gao Z., Chen G., Peng C., Sun Y., Jiang B., Zhou H., Cheng Y., Hu F., Zhang Q. An integrative proteomics metabolomics based strategy reveals the mechanisms involved in wasp sting induced acute kidney injury. Toxicon. 2022;205:1–10. doi: 10.1016/j.toxicon.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Xie C., Xu S., Ding F., Xie M., Lv J., Yao J., Pan D., Sun Q., Liu C., Chen T., et al. Clinical features of severe wasp sting patients with dominantly toxic reaction: analysis of 1091 cases. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta R.L., Cerda J., Burdmann E.A., Tonelli M., Garcia-Garcia G., Jha V., Susantitaphong P., Rocco M., Vanholder R., Sever M.S., et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y.O., Yoon S.A., Kim K.J., Lee B.O., Kim B.S., Chang Y.S., Bang B.K. Severe rhabdomyolysis and acute renal failure due to multiple wasp stings. Nephrol. Dial. Transplant. 2003;18(6):1235. doi: 10.1093/ndt/gfg106. [DOI] [PubMed] [Google Scholar]
- 9.Yu F., Wang L., Yuan H., Gao Z., He L., Hu F. Wasp venom-induced acute kidney injury: current progress and prospects. Ren. Fail. 2023;45(2) doi: 10.1080/0886022X.2023.2259230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo L., Kamau P.M., Lai R. Bioactive peptides and proteins from wasp venoms. Biomolecules. 2022;12(4) doi: 10.3390/biom12040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno M., Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins. 2015;7(4):1126–1150. doi: 10.3390/toxins7041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong J., Yuan H., Gao Z., Hu F. Wasp venom and acute kidney injury: the mechanisms and therapeutic role of renal replacement therapy. Toxicon. 2019;163:1–7. doi: 10.1016/j.toxicon.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Sharma M., Das H.J., Barman A.K., Mahanta P.J. Acute kidney injury due to multiple wasp stings. Saudi J Kidney Dis Transpl. 2017;28(1):196–198. doi: 10.4103/1319-2442.198279. [DOI] [PubMed] [Google Scholar]
- 14.Mingomataj E.C., Bakiri A.H., Ibranji A., Sturm G.J. Unusual reactions to hymenoptera stings: what should we keep in mind? Clin. Rev. Allergy Immunol. 2014;47(1):91–99. doi: 10.1007/s12016-014-8434-y. [DOI] [PubMed] [Google Scholar]
- 15.dos Reis M.A., Costa R.S., Coimbra T.M., Teixeira V.P. Acute renal failure in experimental envenomation with Africanized bee venom. Ren. Fail. 1998;20(1):39–51. doi: 10.3109/08860229809045088. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R., Meleg-Smith S., Batuman V. Acute tubulointerstitial nephritis after wasp stings. Am. J. Kidney Dis. 2001;38(6):E33. doi: 10.1053/ajkd.2001.29289. [DOI] [PubMed] [Google Scholar]
- 17.Martensson J., Martling C.R., Bell M. Novel biomarkers of acute kidney injury and failure: clinical applicability. Br. J. Anaesth. 2012;109(6):843–850. doi: 10.1093/bja/aes357. [DOI] [PubMed] [Google Scholar]
- 18.Coca S.G., Yalavarthy R., Concato J., Parikh C.R. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73(9):1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 19.Vikrant S., Parashar A. Acute kidney injury due to multiple Hymenoptera stings-a clinicopathological study. Clin Kidney J. 2017;10(4):532–538. doi: 10.1093/ckj/sfx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M., Prince S., Tang Y., Zhong X., Chen S., Li G., Wang L., Wang W. Macroscopic hematuria in wasp sting patients: a retrospective study. Ren. Fail. 2021;43(1):500–509. doi: 10.1080/0886022X.2021.1896547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Yang Y., Tang Y., Zhao Y., Cao Y., Su B., Fu P. Recovery from AKI following multiple wasp stings: a case series. Clin. J. Am. Soc. Nephrol. 2013;8(11):1850–1856. doi: 10.2215/CJN.12081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhanapriya J., Dineshkumar T., Sakthirajan R., Shankar P., Gopalakrishnan N., Balasubramaniyan T. Wasp sting-induced acute kidney injury. Clin Kidney J. 2016;9(2):201–204. doi: 10.1093/ckj/sfw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Si X., Li J., Bi X., Wu L., Wu X. Clinical evaluation of high-volume hemofiltration with hemoperfusion followed by intermittent hemodialysis in the treatment of acute wasp stings complicated by multiple organ dysfunction syndrome. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.