Abstract
Background
Methicillin‐resistant Staphylococcus aureus (MRSA) infection after surgery is usually rare, but incidence can be up to 33% in certain types of surgery. Postoperative MRSA infection can occur as surgical site infections (SSI), chest infections, or bloodstream infections (bacteraemia). The incidence of MRSA SSIs varies from 1% to 33% depending upon the type of surgery performed and the carrier status of the individuals concerned. The optimal antibiotic regimen for the treatment of MRSA in surgical wounds is not known.
Objectives
To compare the benefits and harms of various antibiotic treatments in people with established surgical site infections (SSIs) caused by MRSA .
Search methods
In February 2013 we searched the following databases: The Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL); Database of Abstracts of Reviews of Effects (DARE); NHS Economic Evaluation Database; Health Technology Assessment (HTA) Database; Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; and EBSCO CINAHL.
Selection criteria
We included only randomised controlled trials (RCTs) comparing one antibiotic regimen with another antibiotic regimen for the treatment of SSIs due to MRSA. All RCTs irrespective of language, publication status, publication year, or sample size were included in the analysis.
Data collection and analysis
Two review authors independently decided on inclusion and exclusion of trials, and extracted data. We planned to calculate the risk ratio (RR) with 95% confidence intervals (CI) for comparing the binary outcomes between the groups and mean difference (MD) with 95% CI for comparing the continuous outcomes. We planned to perform the meta‐analysis using both a fixed‐effect and a random‐effects model. We performed intention‐to‐treat analysis whenever possible.
Main results
We included one trial involving 59 people hospitalised because of MRSA SSIs. Thirty participants were randomised to linezolid (600 mg either intravenously or orally every 12 hours for seven to 14 days) and 29 to vancomycin (1 g intravenously every 12 hours for seven to 14 days). The type of surgical procedures that were performed were not reported. The trial reported one outcome, which was the eradication of MRSA. The proportion of people in whom MRSA was eradicated was statistically significantly higher in the linezolid group than in the vancomycin group (RR 1.80; 95% CI 1.20 to 2.68).
Authors' conclusions
There is currently no evidence to recommend any specific antibiotic in the treatment of MRSA SSIs. Linezolid is superior to vancomycin in the eradication of MRSA SSIs on the basis of evidence from one small trial that was at high risk of bias, but the overall clinical implications of using linezolid instead of vancomycin are not known. Further well‐designed randomised clinical trials are necessary in this area.
Keywords: Humans; Methicillin‐Resistant Staphylococcus aureus; Acetamides; Acetamides/therapeutic use; Administration, Oral; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Injections, Intravenous; Linezolid; Oxazolidinones; Oxazolidinones/therapeutic use; Randomized Controlled Trials as Topic; Staphylococcal Infections; Staphylococcal Infections/drug therapy; Staphylococcal Infections/microbiology; Surgical Wound Infection; Surgical Wound Infection/drug therapy; Surgical Wound Infection/microbiology; Vancomycin; Vancomycin/therapeutic use
Plain language summary
Antibiotic therapy for the treatment of methicillin‐resistant Staphylococcus aureus (MRSA) infections in people with surgical wounds
Some people having surgery develop wound infections. These are usually caused by bacteria. Most of these wound infections heal naturally, or after treatment with routine antibiotics . However, some bacteria are resistant to routine antibiotics, for example, methicillin‐resistant Staphylococcus aureus (MRSA). MRSA infection after surgery is rare, but can occur in wounds (surgical site infections, or SSIs), the chest, or bloodstream (bacteraemia). MRSA SSIs occur in 1% to 33% of people having surgery, depending on the type of surgery concerned; they can be life‐threatening and cause extended hospital stays.
We do not know what is the best antibiotic treatment for a person who has an MRSA SSI. We aimed to resolve this uncertainty by performing a thorough search of the medical literature for studies that compared different antibiotic treatments for MRSA SSIs. We included only randomised controlled trials, as, if conducted properly, these provide the best information. We did not limit our search for trials according to language or year of publication. Two review authors independently identified the trials and extracted information.
We identified only one trial that compared different antibiotic treatments for MRSA SSIs. This trial had 59 participants who were hospitalised because of their MRSA SSIs. Thirty participants in this trial received an antibiotic called linezolid, which can be taken in tablet form or given as injections through the vein (intravenously). The remaining participants received another antibiotic called vancomycin, which can be only given intravenously. The type(s) of surgical procedures that the participants had were not specified. MRSA was eradicated in more people who received linezolid than vancomycin. It would be helpful if this finding were confirmed by other studies. This trial did not report on other features of eradicating MRSA with these antibiotics, such as:
1. whether the wound healed quickly; 2. length of hospital stay; 3. quality of life, and 4. whether the benefits of treatment outweighed any unwanted side‐effects of the medicine.
Overall, the quality of available evidence was poor. Currently, we cannot recommend any particular antibiotic for treating MRSA SSIs. Linezolid appears to be better than vancomycin for eradication of MRSA SSIs according to low‐quality evidence from this one small trial, but the wider implications of this treatment are not known. Further well‐designed randomised clinical trials are necessary to identify the best antibiotic treatment for MRSA SSIs.
Summary of findings
Summary of findings for the main comparison. Antibiotic therapy for the treatment of methicillin‐resistant Staphylococcus aureus (MRSA) infections in surgical wounds.
| Antibiotic therapy for the treatment of methicillin‐resistant Staphylococcus aureus (MRSA) infections in surgical wounds | |||||
| Patient or population: people with MRSA infection in surgical wounds Settings: secondary or tertiary care Intervention: linezolid Comparison: vancomycin | |||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Vancomycin | Linezolid | ||||
| Eradication of MRSA | 483 per 1000 | 869 per 1000 (579 to 1000) | RR 1.8 (1.2 to 2.68) | 59 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| *The basis for the assumed risk was the control group risk in the study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | |||||
1 This trial was of high risk of bias 2 This was the only trial, so we could not assess inconsistency 3 There was only trial, so we were unable to assess reporting bias
Background
Description of the condition
Methicillin‐resistant Staphylococcus aureus (MRSA), that is isolates of the bacterium Staphylococcus aureus that are not susceptible to the antibiotic methicillin, were first discovered in 1961 (Barber 1961; Jevons 1961; Knox 1961), and outbreaks of MRSA have been reported since the 1970s (Klimek 1976; O'Toole 1970). MRSA infection is associated with significant mortality and morbidity. In the European Union member states plus Norway and Iceland, MRSA infections cause an estimated one million extra hospital stays and cost an estimated EUR 600 million annually (ECDC 2009a), while in the USA, they cause an estimated 125,000 hospitalisations annually (Kuehnert 2005). Although there has been a decrease in the incidence of MRSA in some countries, such as the USA (Kallen 2010), probably as a result of measures to combat MRSA infections (ECDC 2009b), there has also been an increase in incidence of infections in Nordic countries (Skov 2005). Methicillin (meticillin is the International Nonproprietary Name but we have used 'methicillin' in this review in line with the way we speak) resistance is a marker of resistance to some beta‐lactam antibiotics (i.e. the penicillin and cephalosporin group of antibiotics, which are some of the most commonly‐used antibiotics for patients) (Otter 2011). In addition to beta‐lactam antibiotics, MRSA may be resistant to many other commonly‐used antibiotics such as erythromycin, clindamycin, gentamycin, ciprofloxacin, and fusidic acid (Otter 2011). So, even though methicillin is not commonly used at present, methicillin resistance is an indicator of resistance to a wide range of antibiotics. Currently, there are concerns that farm animals, which do not exhibit the symptoms and signs of MRSA infection and with which farmers are in regular contact, may become reservoirs of methicillin‐resistant organisms and a source of a major epidemic of MRSA in the community in developed countries (Wulf 2008).
MRSA infection after surgery is usually rare, but incidence can be up to 33% in certain types of surgery (Sanjay 2010). Postoperative MRSA infection can occur as surgical site infections (SSI), chest infections, or bloodstream infections (bacteraemia) (Fraser 2010; Reddy 2007; Sanjay 2010). The incidence of MRSA SSIs varies from 1% to 33% depending upon the type of surgery performed and the carrier status of the individuals concerned (Harbarth 2008; Reddy 2007; Ridgeway 2005; Sanjay 2010; Shukla 2009). Emergency surgery, contaminated surgery, immunosuppression, and presence of co‐morbidities such as diabetes in patients are all associated with a higher incidence of MRSA SSIs (Harbarth 2008). The role of MRSA SSIs in development of MRSA bacteraemia is not known, however, it would be logical to expect deep SSIs and organ SSIs to have the potential to cause it. Rates of MRSA bacteraemia vary between types of surgery, and, while it is usually rare, infection rates can reach 5% in some types of surgery (Sanjay 2010). MRSA bacteraemia is associated with a 30‐day mortality of about 15% to 38% (Lamagni 2011; Lewis 2011; Kang 2012; Wang 2010), and a one‐year mortality of about 55% (Kaye 2008),compared to a 6% 30‐day mortality in methicillin‐sensitive Staphylococcus aureus bacteraemia (Kang 2012), and a 31% 30‐day mortality in bacteraemia due to other organisms (Melzer 2013).
Surgery that involves indwelling implants and prostheses is prone to the development of biofilms (layers of micro‐organisms) that can increase antimicrobial resistance (Donlan 2002). Indwelling drains and catheters can also predispose individuals to infections. This means that people undergoing surgery with indwelling implants, prostheses, drains, and catheters are more prone to infection with Staphylococcus aureus, which has a very high capacity to develop biofilms, although the methicillin resistant form itself is not associated with a higher capacity to form biofilms (Sanchez 2013). There is currently no evidence that operations involving prostheses are more prone to MRSA infection than operations not involving prostheses.
MRSA SSIs have been shown to be associated with increased mortality in people undergoing cardiac surgery (Reddy 2007): those with MRSA SSIs have an in‐hospital mortality rate of 12.9% compared to a rate of 3% in those without infection. People who developed MRSA SSIs stayed longer in hospitals than those who did not develop them (Chemaly 2010; Shukla 2009).
Horan et al published the Centers for Disease Control and Prevention's (CDC) criteria for SSI (Horan 1992), which have been summarised in Table 2.
1. CDC criteria for surgical site infection.
| Name | Location | Criteria | Time limit |
| Superficial incisional surgical site infection | Skin and subcutaneous tissue only | At least one of the following:
|
30 days |
| Deep incisional surgical site infection | Deep soft tissues (e.g. fascial and muscle layers) of the incision | At least one of the following:
|
30 days, if no implant; 1 year, if implant or prosthesis |
| Organ space surgical site infection | Any part of the anatomy (e.g. organs or spaces), other than the incision, opened or manipulated during the operative procedure | At least one of the following:
|
30 days, if no implant; 1 year, if implant or prosthesis |
Abbreviation
SSI = surgical site infection
Description of the intervention
The Oxford English Dictionary defines an antibiotic substance as, "One of a class of substances produced by living organisms that is capable of destroying, or inhibiting the growth of, micro‐organisms, and is especially used for therapeutic purposes. Synthetic organic compounds that have similar properties are also called antibiotics" (OED 2011). A variety of antibiotics, including beta‐lactams (penicillin derivatives, cephalosporins), glycopeptide antibiotics (e.g. vancomycin, teicoplanin), clindamycin, trimethoprim‐sulfamethoxazole (TMP‐SMX), tetracyclines (doxycycline or minocycline), linezolid, daptomycin, telavancin, rifampicin, gentamycin, and fluoroquinolone, can be used for the treatment of MRSA, since, despite the resistance of MRSA to some antibiotics, it is sensitive to one or more of the above antibiotics (Liu 2011). Different antibiotics are administered in different ways, with the common routes being oral, intravenous, and topical (surface) administration (Liu 2011). Antibiotics can be given as a single agent or in combinations, preoperatively, during operations, postoperatively, or in a combination of the above (Liu 2011).
How the intervention might work
The mechanisms of action vary for different types of antibiotics, but in general terms they either destroy MRSA or prevent the bacteria from dividing (i.e. reproducing).
Why it is important to do this review
A systematic review that addressed the use of different microbial treatments for the treatment of people colonised with MRSA either nasally, or at extra‐nasal sites, concluded that there was insufficient evidence to support use of topical or systemic antimicrobial therapy for eradicating nasal or extra‐nasal MRSA (Loeb 2003).Thus far, there has been no systematic review comparing antibiotics for the treatment of post‐operative MRSA infection. Information to inform clinical guidelines for surgical centres that treat people with MRSA is needed.
Objectives
To compare the benefits and harms of various antibiotic treatments in people with established surgical site infections (SSIs) caused by MRSA.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials (RCTs), irrespective of their use of blinding, language of publication, publication status, date of publication, study setting, or sample sizes. We planned to include cluster‐randomised clinical trials if the effect estimate was available after adjusting for clustering effect. We excluded quasi‐randomised studies because of the risk of selection bias in these trials.
Types of participants
Any person having undergone a surgical procedure (irrespective of age, or whether their surgery involved implants or prostheses, or whether they had impaired immunity or any other co‐morbidities or disease manifestations, such as cancer, diabetes, or jaundice, that could affect wound healing) with established MRSA‐associated postoperative SSIs. All types of surgery were eligible, so surgical wounds could be on any part of the body (for example: the head, trunk, and the limbs). People with non‐surgical wounds will be covered in another review (Gurusamy 2013a).
Types of interventions
We considered comparisons of different antibiotic treatments (and regimens) and comparisons of the same antibiotic with different regimens. We planned to include a combination of antibiotics as a complex intervention, that is, consider the combination as a package. A complex intervention is one that can be divided into one or more individual components, but where the component that causes the treatment effect is not known.
Co‐interventions were allowed where they were performed equally across trial groups. We considered antibiotic administration prior to development of SSI as a co‐intervention, but, provided that participants in all arms of a trial received the same antibiotic prior to development of SSIs, trials addressing prophylactic antibiotic administration were included in the review. We included only trials in which the trial groups differed systematically only in the antibiotic regimens used.
We excluded trials where antibiotics used as prophylaxis for the prevention of MRSA SSI in people undergoing surgery (but not as treatment for patients who developed post‐operative MRSA) as these interventions are covered in a different review (Gurusamy 2013b).
Types of outcome measures
Primary outcomes
All cause mortality.
Other serious adverse events: defined as any event that is life‐threatening; requires inpatient hospitalisation; results in a persistent or significant disability; or any important medical event, which might jeopardise the patient or requires intervention to prevent it (ICH‐GCP 1996) (at maximal follow‐up) e.g. rates of bacteraemia, wound dehiscence.
Quality of life (as defined by trial authors).
Secondary outcomes
Total length of hospital stay.
Use of healthcare resources (e.g. hospital visits).
Eradication of MRSA (at least three consecutive wound or discharge samples negative for MRSA separated by a time interval of at least two days between samples, or as defined by trial authors) at maximal follow‐up.
Time to complete wound healing (as defined by trial authors).
Search methods for identification of studies
Electronic searches
In February 2013 we searched the following electronic databases to identify reports of relevant randomised clinical trials
The Cochrane Wounds Group Specialised Register (searched 28 February 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 1);
The Database of Abstracts of Reviews of Effects (DARE) (2013, Issue 1);
NHS Economic Evaluation Database (2013, Issue 1);
Health Technology Assessment (HTA) Database (2013, Issue 1);
Ovid MEDLINE (1948 to February Week 3 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, 28 February 2013);
Ovid EMBASE (1974 to 2013 Week 08);
EBSCO CINAHL (1982 to 21 February 2013)
We used the following search strategy in The Cochrane Central Register of Controlled Trials (CENTRAL): #1 MeSH descriptor Methicillin Resistance explode all trees #2 MeSH descriptor Staphylococcal Infections explode all trees #3 MeSH descriptor Staphylococcus aureus explode all trees #4 (#2 OR #3) #5 (#1 AND #4) #6 MeSH descriptor Methicillin‐Resistant Staphylococcus aureus explode all trees #7 (methicillin NEXT resistan*) or (meticillin NEXT resistan*) or MRSA #8 (#5 OR #6 OR #7) #9 MeSH descriptor Wound Infection explode all trees #10 MeSH descriptor Sepsis explode all trees #11 MeSH descriptor Soft Tissue Infections explode all trees #12 MeSH descriptor Surgical Wound Dehiscence explode all trees #13 surg* NEAR/5 infect* #14 surg* NEAR/5 wound* #15 surg* NEAR/5 site* #16 surg* NEAR/5 incision* #17 surg* NEAR/5 dehisc* #18 wound* NEAR/5 dehisc* #19 (deep NEXT infection*) or "deep sepsis" or (infected NEXT collection*) #20 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 MeSH descriptor Anti‐Bacterial Agents explode all trees #22 MeSH descriptor Cephalosporins explode all trees #23 MeSH descriptor Tetracycline explode all trees #24 MeSH descriptor Penicillins explode all trees #25 antibiotic* or penicillin* or beta‐lactam* or cephalosporin* or clindamycin or trimethoprim* or tetracycline* or doxycycline or minocycline or linezolid or vancomycin or daptomycin or telavancin or rifampicin or gentamycin or gentamicin or fluoroquinolone #26 (#21 OR #22 OR #23 OR #24 OR #25) #27 (#8 AND #20 AND #26)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). We did not restrict studies with respect to language, date of publication or study setting.
We searched the metaRegister of Controlled Trials (mRCT) (http://www.controlled‐trials.com/mrct) and the ICTRP (International Clinical Trials Registry Platform) portal maintained by World Health Organization (http://apps.who.int/trialsearch/) on 11 December 2012. The meta‐register includes the ISRCTN Register and the NIH ClinicalTrials.gov Register amongst others. The ICRTP portal includes these trial registers, along with trial registry data from a number of countries.
Searching other resources
We searched the references of included trials to identify further relevant trials. We also contacted experts in MRSA infection to identify further relevant trials.
Data collection and analysis
We performed the systematic review following the instructions in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011a).
Selection of studies
Two review authors (KG or CT and RK) independently identified trials for inclusion by screening the titles and abstracts. We obtained full texts for references selected by at least one of the reviewers. We made the final selection based on full texts. We have listed excluded studies with reasons for their exclusion (Characteristics of excluded studies). We resolved any differences through discussion.
Data extraction and management
Two review authors (KG and RK) independently extracted the following data from trial reports:
Year, and language, of publication.
Country in which the trial was performed.
Year(s) of conduct of the trial.
Inclusion and exclusion criteria.
Sample sizes.
Type(s) of surgery.
Details of antibiotic treatment including dose(s), route(s), frequency, and duration(s).
Outcomes (as described above).
Risk of bias (as described below).
We sought further information from trial authors when sufficient information was not available in the report. In future, if multiple reports exist for a trial, we will examine all the reports for information (there were no multiple reports for the included trial). If there is any doubt about whether the trials share the same participants ‐ completely or partially (by identifying common authors and centres) ‐ we plan to contact the trial authors to check whether the trial report has been duplicated, and to seek clarification for any unclear or missing information. We planned to resolve any differences in opinion through discussion with PW and BRD.
Assessment of risk of bias in included studies
We followed the guidance in the Cochrane Handbook for Systematic Reviews of Intervention to assess risk of bias in included studies (Higgins 2011b). According to empirical evidence (Kjaergard 2001; Moher 1998; Schulz 1995; Wood 2008), the risk of bias of the trials was assessed on the following domains:
Sequence generation
Low risk of bias: the method used was either adequate (e.g. computer‐generated random numbers, table of random numbers), or unlikely to introduce confounding.
Uncertain risk of bias: there was insufficient information to assess whether the method used was likely to introduce confounding.
High risk of bias: the method used was improper and likely to introduce confounding (e.g. quasi‐randomised studies). Such studies were excluded.
Allocation concealment
Low risk of bias: the method used was unlikely to induce bias on the final observed effect (e.g. central allocation).
Uncertain risk of bias: there is insufficient information to assess whether the method used was likely to induce bias on the estimate of effect.
High risk of bias: the method used was likely to induce bias on the final observed effect (e.g. open random allocation schedule).
Blinding of participants and personnel
Low risk of bias: blinding was performed adequately in the participants and personnel, or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data were unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether the missing data mechanism, in combination with the method used to handle missing data, was likely to induce bias on the estimate of effect.
High risk of bias: the crude estimate of effects (e.g. complete case estimate) was clearly biased due to the underlying reasons for missing data, and the methods used to handle missing data were unsatisfactory.
Selective outcome reporting
Low risk of bias: the trial protocol was available and all of the trial's pre‐specified outcomes of interest in the review have been reported, or if the trial protocol was not available, the primary outcomes in this review were reported.
Uncertain risk of bias: there was insufficient information to assess whether the magnitude and direction of the observed effect was related to selective outcome reporting.
High risk of bias: not all of the trial's pre‐specified primary outcomes were reported.
We consider trials that are classified as being at low risk of bias in all the above domains to be low bias‐risk trials. All other remaining trials will be considered as being high bias‐risk trials.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). Risk ratio calculations do not include trials in which no events occurred in either group, whereas risk difference calculations do. We planned to report the risk difference where the results obtained by using this association measure were different from risk ratio in terms of interpretation of information or arriving at conclusions. For continuous variables, we planned to calculate the mean difference (MD) with 95% CI for outcomes that are measured or can be converted to the same units (such as length of hospital stay), and standardised mean difference (SMD) with 95% CI for outcomes (such as quality of life), where different assessment scales might have been used. For time‐to‐event outcomes such as survival at maximal follow‐up, we planned to calculate the hazard ratio (HR) with 95% CI.
Unit of analysis issues
The unit of analysis were individual people undergoing surgical procedures.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). We imputed missing data for binary outcomes using various scenarios such as best‐best, best‐worst, worst‐best, worst‐worst scenarios (Gurusamy 2009). In the best‐best scenario, the outcomes of people with missing data in both groups were assumed to be good. In the best‐worst scenario, the outcomes of people with missing data in intervention group were assumed to be good while the outcomes were assumed to be bad for those with missing data in the control group. The worst‐best scenario was the opposite of the best‐worst scenario, i.e. the outcomes of people with missing data in the intervention group were assumed to be bad while the outcomes were assumed to be good for those in the control group with missing data. In the worst‐worst scenario, the outcomes of people with missing data in both groups were assumed to be bad.
For continuous outcomes, we planned to use available‐case analysis. We planned to impute the standard deviation from P values according to the instructions in the Cochrane Handbook for Systematic Reviews of Intervention, and planned to use the median for meta‐analysis when the mean was not available (Higgins 2011c). If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation would decrease the weight of the study for calculation of mean differences, and bias the effect estimate to no effect in case of standardised mean difference (Higgins 2011c).
For time‐to‐event outcomes, if the hazard ratio and 95% confidence intervals were not reported, we planned to obtain the logarithm of hazard ratios (ln(HR)) and the standard error (SE) of ln(HR) according to the methods described by Parmar 1998.
Assessment of heterogeneity
We planned to explore heterogeneity by means of the Chi2 test, with significance set at P value 0.10, and measure the quantity of heterogeneity by I2 (Higgins 2002). We also planned to use overlapping of confidence intervals on the forest plot to determine heterogeneity (Schunemann 2009).
Thresholds for the interpretation of I2 can be misleading. A rough guide to interpretation follows (Deeks 2011):
0 to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: may represent considerable heterogeneity.
The decision to classify the overlapping I2 levels will be based on the overlapping of confidence intervals, and whether the heterogeneity was in the direction of effect or just in the magnitude of effect.
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias in the presence of at least 10 trials (Egger 1997; Macaskill 2001). We planned to perform the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry. Selective reporting was also be considered as evidence of reporting bias.
Data synthesis
We planned to perform meta‐analysis only if there was sufficient clinical homogeneity in terms of both participants included in the trials and of antibiotics used (i.e. similar class of antibiotics). We planned to perform meta‐analyses using the RevMan 5 software package (RevMan 2011), and to follow the recommendations of The Cochrane Handbook for Systematic Reviews of Intervention (Deeks 2011). We planned to use both a random‐effects model (DerSimonian 1986), and a fixed‐effect model in meta‐analyses (DeMets 1987). Should there be discrepancy between the two models, we planned to report both results; otherwise we planned to report the results of the fixed‐effect model. We planned to use the generic inverse variance method to combine hazard ratios for time‐to‐event outcomes.
Summary of findings
We have presented the 'Summary of findings' table for all the primary and secondary outcomes that were reported (Table 1; Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses:
Different antibiotics.
Operations involving prosthetic implants and indwelling devices.
Different types of surgery.
People with co‐morbidities versus those without serious co‐morbidities.
We planned to use the 'test for subgroup differences' available via RevMan output to identify the differences between subgroups.
Sensitivity analysis
We planned to perform a sensitivity analysis by imputing data for binary outcomes using various scenarios such as best‐best, best‐worst, worst‐best, worst‐worst scenarios as described previously (Gurusamy 2009). We also planned to perform a sensitivity analysis by testing the effect of removing trials at unclear or high risk of bias and trials in which the mean and the standard deviation were imputed from the analysis.
Results
Description of studies
Results of the search
We identified a total of 489 unique references through electronic searches. We excluded 484 clearly irrelevant references through reading titles and abstracts. We retrieved five references in full for further assessment. We did not identify any additional references to trials by scanning reference lists of included trials. We did not identify any new trials through other searches. We excluded four references because of the reasons specified in the Characteristics of excluded studies. One trial met the inclusion criteria and was included in this review (Weigelt 2004). Since this was the only trial that met the inclusion criteria, no meta‐analysis was performed. The reference flow chart is shown in Figure 1.
1.

Study flow diagram
Included studies
We included one trial involving 59 people hospitalised because of MRSA SSIs, randomised to either linezolid (600 mg either intravenously or orally every 12 hours for seven to 14 days) (30 people) or to vancomycin (1 g intravenously every 12 hours for seven to 14 days) (29 people) (Weigelt 2004). The average age of participants, the proportion of women and the type(s) of surgical procedures was not reported (Weigelt 2004). The only outcome reported was eradication of MRSA, which the trial authors defined as previously‐infected participants being culture‐negative for MRSA one week after the end of treatment. Further details of this trial are shown in Characteristics of included studies.
Excluded studies
We excluded four studies. Two trials were excluded because there were no data on people who underwent surgery (Lucasti 2008; Van Laethem 1988); one study involved animals (Jacqueline 2011); and in the fourth trial all MRSA episodes were treated with no randomisation in the choice of antibiotics (Philpott 2003).
Risk of bias in included studies
The only trial included in this review was at of high risk of bias. The risk of bias in the individual domains are shown in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods used for generation of random sequence and allocation concealment were not reported (Weigelt 2004).
Blinding
Blinding of participants, personnel and outcome assessors was not reported (Weigelt 2004).
Incomplete outcome data
Although there were post‐randomisation drop‐outs, imputations of outcomes under different scenarios did not alter the conclusions, so there was no attrition bias (Weigelt 2004).
Selective reporting
Many of the important outcomes that would normally have been measured in such a trial were not reported, so there was selective reporting bias (Weigelt 2004).
Other potential sources of bias
There was no other bias in this trial (Weigelt 2004).
Effects of interventions
See: Table 1
The only outcome (of interest for this review) reported in the trial was eradication of MRSA (Weigelt 2004). The proportion of people in whom MRSA was eradicated was significantly higher in the linezolid group than in the vancomycin group (RR 1.80; 95% CI 1.20 to 2.68) (Analysis 1.1). The interpretation did not change by calculating the risk difference. Since this was the only trial included in this review, questions about heterogeneity and fixed‐effect model versus random‐effects model were not relevant.
1.1. Analysis.
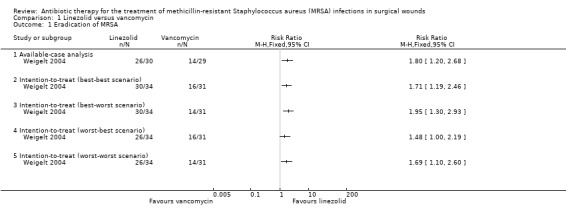
Comparison 1 Linezolid versus vancomycin, Outcome 1 Eradication of MRSA.
Subgroup analysis
Subgroup analysis was not performed because of the inclusion of only one trial.
Sensitivity analysis
Six participants were excluded from the report: four belonged to the linezolid group and two to the vancomycin group. We performed a sensitivity analysis by imputing the outcomes under various scenarios described in the Dealing with missing data and Sensitivity analysis sections. The sensitivity analysis did not change the results (Analysis 1.1). The worst‐best scenario, in which the effect estimate was (RR 1.48; 95% CI 1.00 to 2.19), deserves a specific mention: although the confidence interval included 1, this was because of rounding. The actual lower and upper confidence intervals before rounding to two decimals were 1.00463849 and 2.1850555. The P value before rounding to two decimal places was 0.0473. So, even though it appears that there was no statistically significant difference between the two groups on the forest plot, the eradication of MRSA was significantly higher in the linezolid group than in the vancomycin group.
Reporting bias
We did not explore reporting bias because of the presence of only one trial in this review.
Discussion
Summary of main results
We included only one trial, with 59 participants, in this review. This trial was at high risk of bias, and found that the eradication of MRSA was significantly better with linezolid than with vancomycin (RR 1.80; 95% CI 1.20 to 2.68). MRSA was eradicated in approximately 87% of people after seven to 14 days treatment with linezolid compared with an eradication in approximately 49% of people after seven to 14 days treatment with vancomycin. This trial did not report on the clinical impact of the increase in the proportion eradicated with linezolid compared with vancomycin, or whether there were any serious adverse events related to the antibiotic treatment. Another review that investigated the safety of linezolid reported that people with neutropenia, Gram‐positive infections, and foot infections who received treatment with linezolid showed similar serious adverse events to those treated with other antibiotics such as vancomycin, dalbavancin, and teicoplanin (Vinh 2009). This review reported that serious antibiotic‐related adverse events occurred in fewer than 1% of the people taking linezolid (Vinh 2009); these serious adverse events included transient ischaemic attack (mini stroke), hypertension, severe vomiting, elevated liver enzymes, and thrombocytopenia (low blood platelet levels). The authors of the review concluded that linezolid was generally safe and well tolerated (Vinh 2009). It is must also be noted that linezolid was administered either intravenously or orally, although the authors did not state the reasons that governed the choice of intravenous or oral preparations for individual participants. Previous research has shown that oral linezolid is well tolerated, and that bioavailability of linezolid in oral and intravenous forms is similar (Keel 2011). This appears to be an advantage of linezolid compared to vancomycin, which is administered intravenously. However, since complications, such as thrombocytopaenia and peripheral neuropathy, can occur with prolonged treatment, linezolid is not recommended for treatment that is longer than four weeks (Vinh 2009). However, further information on serious adverse events in surgical patients and further clinical information is necessary before we can recommend linezolid rather than vancomycin for treatment of MRSA wound infections.
The incidence of MRSA SSIs varies from 1% to 33% depending upon the type of surgery performed and the carrier status of the individuals concerned (Harbarth 2008; Reddy 2007; Ridgeway 2005; Sanjay 2010; Shukla 2009). Considering the disease burden posed by MRSA SSIs, it is very surprising that we were able to identify only one trial that compared different antibiotic regimens in people with MRSA SSIs. One possible reason for the paucity of trials in this field may be that researchers usually make use of evidence from antibiotic comparisons for treatment of spontaneous skin and subcutaneous infections such as abscesses. However, the pathophysiology of spontaneous skin and subcutaneous infections is different from that of SSI. Spontaneous MRSA abscesses are likely to develop in people with other local or systemic illnesses that predispose them to such abscesses. While such systemic illnesses may predispose people to develop SSI (Watanabe 2008), the primary cause of SSIs is surgery. Another possible reason for the paucity of randomised clinical trials, is the use of evidence from observational studies. RCTs are currently considered to be the best design for evaluating interventions, and are clearly necessary in this field.
Overall completeness and applicability of evidence
This review included only one trial, in which the type of people undergoing surgery was not stated clearly, so we are unable to generalise the findings to all people undergoing surgery.
Quality of the evidence
As shown in the Table 1, the quality of evidence was very low. There are no insurmountable barriers to obtaining very high quality evidence in this area, unlike some other surgical comparisons where it is impossible, or unethical, to blind people.
Potential biases in the review process
We have performed a thorough search of the literature without any language or time of publication restrictions. In spite of this, we will not be able to identify any trials that were conducted, but not reported, in the pre‐mandatory trial registration era. We included only trials in which MRSA infection and surgical treatment was mentioned. It is highly likely that several thousand trials have been conducted that investigated antibiotic treatments for all surgical site infections without assessing or reporting the methicillin‐resistance status of the organisms. Given the nature of the topic, one has to be pragmatic and accept that such trials are unlikely to be identified electronically, and that contacting the authors of every trial that has assessed antibiotic treatment for SSIs in order to obtain information about whether the trialists measured the MRSA status of the organisms, and to obtain the information in way that it could be used for this systematic review, would be very resource intensive. The reliability of information obtained in such a manner would also be a major concern.
Agreements and disagreements with other studies or reviews
This is the first systematic review on this topic. We agree with the trial author that linezolid is superior to vancomycin in the eradication of MRSA SSI (Weigelt 2004), although this conclusion is based on evidence from one small trial of high risk of bias and the overall clinical implications of using linezolid instead of vancomycin are not known.
Authors' conclusions
Implications for practice.
Currently, there is no evidence to recommend any specific antibiotic in the treatment of methicillin‐resistant Staphylococcus aureus (MRSA) surgical site infections (SSIs). Linezolid appears to be superior to vancomycin in the eradication of MRSA SSIs on the basis of evidence from one small trial at high risk of bias, but the overall clinical implications of using linezolid instead of vancomycin is not known.
Implications for research.
Further well‐designed randomised clinical trials are necessary. Such trials should include mortality, morbidity, serious antibiotic‐related adverse events, quality of life, and resource use as the basic outcomes.
Acknowledgements
The authors would like to acknowledge the contribution of the Wounds Group Editors (Susan O'Meara) and peer referees (Catriona McDaid, Gill Worthy, Patricia Danielsen, Roy Buffery) and copy‐editor (Elizabeth Royle).
Appendices
Appendix 1. Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL Search Strategies
Ovid MEDLINE
1 Methicillin Resistance/ (8026) 2 exp Staphylococcal Infections/ (23428) 3 exp Staphylococcus aureus/ (30286) 4 2 or 3 (39399) 5 1 and 4 (7604) 6 exp Methicillin‐Resistant Staphylococcus aureus/ (5190) 7 (methicillin‐resistan* or meticillin‐resistan* or MRSA).tw. (15592) 8 or/5‐7 (17196) 9 exp Wound Infection/ (14971) 10 exp Sepsis/ (51757) 11 exp Soft Tissue Infections/ (1929) 12 exp Surgical Wound Dehiscence/ (2990) 13 (surg* adj5 infect*).tw. (11123) 14 (surg* adj5 wound*).tw. (5735) 15 (surg* adj5 site*).tw. (8208) 16 (surg* adj5 incision*).tw. (4303) 17 (surg* adj5 dehisc*).tw. (395) 18 (wound* adj5 dehisc*).tw. (1856) 19 (deep infection* or deep sepsis or infected collection).tw. (1695) 20 or/9‐19 (91225) 21 exp Anti‐Bacterial Agents/ (215537) 22 exp Penicillins/ (16714) 23 exp Cephalosporins/ (12581) 24 exp Tetracycline/ (4066) 25 (antibiotic* or penicillin* or beta‐lactam* or cephalosporin* or clindamycin or trimethoprim* or tetracycline* or doxycycline or minocycline or linezolid or vancomycin or daptomycin or telavancin or rifampicin or gentamycin or gentamicin or fluoroquinolone).tw. (156631) 26 or/21‐25 (283068) 27 8 and 20 and 26 (1883) 28 randomized controlled trial.pt. (243797) 29 controlled clinical trial.pt. (39777) 30 randomized.ab. (198478) 31 placebo.ab. (92392) 32 clinical trials as topic.sh. (80116) 33 randomly.ab. (136484) 34 trial.ti. (73737) 35 or/28‐34 (550390) 36 (animals not (humans and animals)).sh. (1629396) 37 35 not 36 (500869) 38 27 and 37 (117)
Ovid EMBASE
1 exp penicillin resistance/ (6486) 2 exp Staphylococcus infection/ (20926) 3 exp Staphylococcus aureus/ (71187) 4 2 or 3 (79541) 5 1 and 4 (3433) 6 exp methicillin resistant Staphylococcus aureus/ (24269) 7 (methicillin‐resistan* or meticillin‐resistan* or MRSA).tw. (23553) 8 or/5‐7 (31101) 9 exp wound infection/ (19415) 10 exp sepsis/ (108581) 11 exp soft tissue infection/ (5213) 12 exp wound dehiscence/ (6928) 13 (surg* adj5 infect*).tw. (16679) 14 (surg* adj5 wound*).tw. (8175) 15 (surg* adj5 site*).tw. (12421) 16 (surg* adj5 incision*).tw. (6567) 17 (surg* adj5 dehisc*).tw. (551) 18 (wound* adj5 dehisc*).tw. (2660) 19 (deep infection* or deep sepsis or infected collection).tw. (2255) 20 or/9‐19 (168503) 21 exp antibiotic agent/ (544500) 22 exp penicillin derivative/ (117164) 23 exp cephalosporin derivative/ (92762) 24 exp tetracycline derivative/ (65922) 25 (antibiotic* or penicillin* or beta‐lactam* or cephalosporin* or clindamycin or trimethoprim* or tetracycline* or doxycycline or minocycline or linezolid or vancomycin or daptomycin or telavancin or rifampicin or gentamycin or gentamicin or fluoroquinolone).tw. (232206) 26 or/21‐25 (615812) 27 8 and 20 and 26 (5216) 28 Randomized controlled trials/ (26835) 29 Single‐Blind Method/ (15611) 30 Double‐Blind Method/ (86092) 31 Crossover Procedure/ (31945) 32 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. (944695) 33 (doubl$ adj blind$).ti,ab. (90521) 34 (singl$ adj blind$).ti,ab. (9694) 35 or/28‐34 (979655) 36 animal/ (725595) 37 human/ (8657232) 38 36 not 37 (485192) 39 35 not 38 (947254) 40 27 and 39 (354)
EBSCO CINAHL
S28 S8 and S20 and S27 S27 S21 or S22 or S23 or S24 or S25 or S26 S26 AB (antibiotic* or penicillin* or beta‐lactam* or cephalosporin* or clindamycin or trimethoprim* or tetracycline* or doxycycline or minocycline or linezolid or vancomycin or daptomycin or telavancin or rifampicin or gentamycin or gentamicin or fluoroquinolone) S25 TI (antibiotic* or penicillin* or beta‐lactam* or cephalosporin* or clindamycin or trimethoprim* or tetracycline* or doxycycline or minocycline or linezolid or vancomycin or daptomycin or telavancin or rifampicin or gentamycin or gentamicin or fluoroquinolone) S24 (MH "Tetracyclines+") S23 (MH "Cephalosporins+") S22 (MH "Penicillins+") S21 (MH "Antibiotics+") S20 S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 S19 TI deep infection* or deep sepsis or infected collection or AB deep infection* or deep sepsis or infected collection S18 TI wound* N5 dehisc* or AB wound* N5 dehisc* S17 TI surg* N5 dehisc* or AB surg* N5 dehisc* S16 TI surg* N5 incision* or AB surg* N5 incision* S15 TI surg* N5 site* or AB surg* N5 site* S14 TI surg* N5 wound* or AB surg* N5 wound* S13 TI surg* N5 infection* or AB surg* N5 infection* S12 (MH "Surgical Wound Dehiscence") S11 (MH "Soft Tissue Infections") S10 (MH "Sepsis+") S9 (MH "Wound Infection+") S8 S5 or S6 or S7 S7 TI ( methicillin‐resistan* or meticillin‐resistan* or MRSA ) OR AB ( methicillin‐resistan* or meticillin‐resistan* or MRSA ) S6 (MH "Methicillin‐Resistant Staphylococcus Aureus") S5 S1 and S4 S4 S2 or S3 S3 (MH "Staphylococcus Aureus+") S2 (MH "Staphylococcal Infections+") S1 (MH "Methicillin Resistance")
Data and analyses
Comparison 1. Linezolid versus vancomycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication of MRSA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Available‐case analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Intention‐to‐treat (best‐best scenario) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Intention‐to‐treat (best‐worst scenario) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Intention‐to‐treat (worst‐best scenario) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Intention‐to‐treat (worst‐worst scenario) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Weigelt 2004.
| Methods | RCT | |
| Participants | Country: USA
Number randomised: 65
Post‐randomisation drop‐outs: 6 (9.2%)
Revised sample size: 59
Average age: not stated
Male:female ratio: not stated
Inclusion criteria: hospitalised patients, 18 years or older, with clinical signs and symptoms of a suspected MRSA SSI (local erythema of the skin and soft tissues with systemic signs or symptoms of infection)
Exclusion criteria: people 1. with known Gram‐negative infections, osteomyelitis, endocarditis, meningitis, septic arthritis, necrotizing fasciitis, or gas gangrene 2. concurrently receiving another investigational medication 3. with an infected device that was not being removed 4. with medical co‐morbidities that would require long hospitalisations 5. with hypersensitivity to linezolid or vancomycin |
|
| Interventions | Participants were randomly assigned to 2 groups: Group 1 (n = 30): linezolid (600 mg either intravenously or orally every 12 h for 7‐14 days) Group 2 (n = 29): vancomycin (1 g intravenously every 12 h for 7‐14 days) | |
| Outcomes | Eradication of MRSA | |
| Notes | Definition of eradication of MRSA: culture negative for MRSA 1 week after the end of treatment Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were post‐randomisation drop‐outs but the conclusions did not alter when we by imputed the data using different scenarios |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes that will generally be assessed were not reported |
MRSA = methicillin‐resistant Staphylococcus aureus RCT = randomised controlled trial SSI = surgical site infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jacqueline 2011 | Performed in animals |
| Lucasti 2008 | No separate data for participants who underwent surgery |
| Philpott 2003 | MRSA episodes were treated with additional antibiotics and did not involve an RCT of a choice of antibiotic treatment for MRSA |
| Van Laethem 1988 | No separate data for participants who underwent surgery |
Abbreviations
MRSA = methicillin‐resistant Staphylococcus aureus RCT = randomised controlled trial
Contributions of authors
Kurinchi Gurusamy: conceived, designed and co‐ordinated the review; extracted data; checked the quality of data extraction; undertook quality assessment; analysed or interpreted data; checked quality assessment; performed part of data analysis or interpretation; performed statistical analysis; checked the quality of statistical analysis and completed the first draft of the review. Performed translation and part of writing or editing of the review; made an intellectual contribution to the review and approved the final review prior to submission. Wrote to trial authors, experts, and companies; secured funding for the review and acts as guarantor of the review. Rahul Koti: extracted data; undertook quality assessment; made an intellectual contribution to the review and approved the final review prior to submission. Clare Toon: extracted data and approved the final review prior to submission. Peter Wilson: made an intellectual contribution to the review, advised on and approved the final review prior to submission. Brian Davidson: conceived, designed, and made an intellectual contribution to the review; secured funding for the review; advised on and approved the final review prior to submission.
Contributions of editorial base
Nicky Cullum: edited the protocol; advised on methodology, interpretation and review content. Joan Webster, Editor: approved the final review prior to submission. Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content; edited the review. Ruth Foxlee: designed the search strategy and edited the search methods section. Rachel Richardson: edited the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
National Institute for Health Research, the health research wing of the UK Government Department of Health funds K Gurusamy to complete this review.
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
Declarations of interest
KS Gurusamy and BR Davidson are funded by a joint funding scheme between Department of Health and Wellcome Trust on a completely unrelated project. R Koti and C Toon ‐ none known. APR Wilson is a consultant microbiologist in the NHS advising on antibiotic use and advises some private hospitals on infection control. He is a member of a clinical trial drug safety monitoring board for a monoclonal antibody. He has been an expert witness in infection related cases. He has a number of non‐commercial grants for research in the area of transmission of infection. APR Wilson was part funded by the UCLH/UCL Comprehensive Biomedical Centre with funding from the Department of Health's NIHR Biomedical Research Centres.
This project was funded by the National Institute for Health Research (NIHR).
Disclaimer
Department of Health disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS (National Health Service), or the Department of Health.
New
References
References to studies included in this review
Weigelt 2004 {published data only}
- Weigelt J, Kaafarani HM, Itani KM, Swanson RN. Linezolid eradicates MRSA better than vancomycin from surgical‐site infections. American Journal of Surgery 2004;188(6):760‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Jacqueline 2011 {published data only}
- Jacqueline C, Amador G, Desessard C, Mabecque V, Miegeville AF, Caillon J, et al. Assessment of linezolid, vancomycin, and rifampicin as monotherapy in comparison with combination of linezolid/rifampicin and vancomycin/rifampicin in the treatment of methicillin‐resistant Staphylococcus aureus acute osteomyelitis. Clinical Microbiology and Infection 2011;17(Supplement S4):S428. [Google Scholar]
Lucasti 2008 {published data only}
- Lucasti C, Jasovich A, Umeh O, Jiang J, Kaniga K, Friedland I. Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra‐abdominal infection: a phase III, prospective, multicenter, randomized, double‐blind, noninferiority study. Clinical Therapeutics 2008;30(5):868‐83. [DOI] [PubMed] [Google Scholar]
Philpott 2003 {published data only}
- Philpott‐Howard J, Burroughs A, Fisher N, Hastings M, Kibbler C, Mutimer D, et al. Piperacillin‐tazobactam versus ciprofloxacin plus amoxicillin in the treatment of infective episodes after liver transplantation. Journal of Antimicrobial Chemotherapy 2003;52:993‐1000. [DOI] [PubMed] [Google Scholar]
Van Laethem 1988 {published data only}
- Laethem Y, Hermans P, Wit S, Goosens H, Clumeck N. Teicoplanin compared with vancomycin in methicillin‐resistant Staphylococcus aureus infections: preliminary results. Journal of Antimicrobial Chemotherapy 1988;21(Supplement A):81‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Barber 1961
- Barber M. Methicillin‐resistant staphylococci. Journal of Clinical Pathology 1961 Jul;14:385‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chemaly 2010
- Chemaly RF, Hachem RY, Husni RN, Bahna B, Rjaili GA, Waked A, et al. Characteristics and outcomes of methicillin‐resistant Staphylococcus aureus surgical‐site infections in patients with cancer: a case‐control study. Annals of Surgical Oncology 2010;17(6):1499‐506. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Donlan 2002
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews 2002;15(2):167‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
ECDC 2009a
- ECDC. European Centre for Disease Prevention and Control. Annual epidemiological report on communicable diseases in Europe. http://www.ecdc.europa.eu/en/publications/Publications/0910_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf 2009 (accessed on 17 June 2011). [PubMed]
ECDC 2009b
- ECDC. European Centre for Disease Prevention and Control. The bacterial challenge: Time to react. A call to narrow the gap between multi‐drug resistant bacteria in the EU and the development of new anti‐bacterial agents. http://www.ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf 2009 (accessed on 17 June 2011).
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraser 2010
- Fraser S, Brady R, Graham C, Paterson‐Brown S, Gibb A. Methicillin‐resistant Staphylococcus aureus in surgical patients: identification of high‐risk populations for the development of targeted screening programmes. Annals of the Royal College of Surgeons of England 2010;92(4):311‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Gurusamy 2013a
- Gurusamy KS, Koti R, Toon C, Wilson P, Davidson BR. Antibiotic therapy for the treatment of methicillin‐resistant Staphylococcus aureus (MRSA) in non‐surgical wounds. Cochrane Database of Systematic Reviews In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2013b
- Gurusamy KS, Koti R, Wilson P, Davidson BR. Antibiotic prophylaxis for the prevention of methicillin‐resistant Staphylococcus aureus (MRSA) related complications in surgical patients. Cochrane Database of Systematic Reviews In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbarth 2008
- Harbarth S, Huttner B, Gervaz P, Fankhauser C, Chraiti MN, Schrenzel J, et al. Risk factors for methicillin‐resistant Staphylococcus aureus surgical site infection. Infection Control and Hospital Epidemiology 2008;29(9):890‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ and Altman DG on behalf of the Cochrane Statistical Methods Group (Editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horan 1992
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infection Control and Hospital Epidemiology 1992;13(10):606‐8. [PubMed] [Google Scholar]
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Jevons 1961
- Jevons PM. “Celbenin” ‐ resistant staphylococci. British Medical Journal 1961;1(5219):124‐5. [PMC free article] [PubMed] [Google Scholar]
Kallen 2010
- Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, et al. Health care‐associated invasive MRSA infections, 2005‐2008. JAMA 2010;304(6):641‐8. [DOI] [PubMed] [Google Scholar]
Kang 2012
- Kang CI, Song JH, Ko KS, Chung DR, Peck KR. Clinical features and outcomes of Staphylococcus aureus infections in non‐neutropenic cancer patients. Supportive Care in Cancer 2012;20(3):483‐8. [DOI] [PubMed] [Google Scholar]
Kaye 2008
- Kaye KS, Anderson DJ, Choi Y, Link K, Thacker P, Sexton DJ. The deadly toll of invasive methicillin‐resistant Staphylococcus aureus infection in community hospitals. Clinical Infectious Diseases 2008;46(10):1568‐77. [DOI] [PubMed] [Google Scholar]
Keel 2011
- Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2011;55(7):3393‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Klimek 1976
- Klimek JJ, Marsik FJ, Bartlett RC, Weir B, Shea P, Quintiliani R. Clinical, epidemiologic and bacteriologic observations of an outbreak of methicillin‐resistant Staphylococcus aureus at a large community hospital. American Journal of Medicine 1976;61(3):340‐5. [DOI] [PubMed] [Google Scholar]
Knox 1961
- Knox R, Smith JT. The nature of penicillin resistance in staphylococci. Lancet 1961;2(7201):520‐2. [DOI] [PubMed] [Google Scholar]
Kuehnert 2005
- Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin‐resistant–Staphylococcus aureus hospitalizations, United States. Emerging Infectious Diseases 2005;11(6):868‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamagni 2011
- Lamagni TL, Potz N, Powell D, Pebody R, Wilson J, Duckworth G. Mortality in patients with meticillin‐resistant Staphylococcus aureus bacteraemia, England 2004‐2005. Journal of Hospital Infection 2011;77(1):16‐20. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lewis 2011
- Lewis T, Chaudhry R, Nightingale P, Lambert P, Das I. Methicillin‐resistant Staphylococcus aureus bacteremia: epidemiology, outcome, and laboratory characteristics in a tertiary referral center in the UK. International Journal of Infectious Diseases 2011;15(2):e131‐5. [DOI] [PubMed] [Google Scholar]
Liu 2011
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children: executive summary. Clinical Infectious Diseases 2011;52(3):285‐92. [DOI] [PubMed] [Google Scholar]
Loeb 2003
- Loeb MB, Main C, Eady A, Walkers‐Dilks C. Antimicrobial drugs for treating methicillin‐resistant Staphylococcus aureus colonization. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Melzer 2013
- Melzer M, Welch C. Outcomes in UK patients with hospital‐acquired bacteraemia and the risk of catheter‐associated urinary tract infections. Postgraduate Medical Journal 2013;Mar 21:Epub ahead of print. [DOI: 10.1136/postgradmedj-2012-131393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
O'Toole 1970
- O'Toole RD, Drew WL, Dahlgren BJ, Beaty HN. An outbreak of methicillin‐resistant Staphylococcus aureus infection. Observations in hospital and nursing home. JAMA 1970;213(2):257‐63. [PubMed] [Google Scholar]
OED 2011
- OED. Oxford English Dictionary. The definitive record of the English language. http://www.oed.com/ 2011 (accessed on 7th April, 2011).
Otter 2011
- Otter JA, French GL. Community‐associated meticillin‐resistant Staphylococcus aureus strains as a cause of healthcare‐associated infection. Journal of Hospital Infection 2011;79(3):189‐93. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Reddy 2007
- Reddy SL, Grayson AD, Smith G, Warwick R, Chalmers JA. Methicillin resistant Staphylococcus aureus infections following cardiac surgery: incidence, impact and identifying adverse outcome traits. European Journal of Cardio‐Thoracic Surgery 2007;32(1):113‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ridgeway 2005
- Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. Journal of Bone and Joint Surgery. British Volume 2005;87(6):844‐50. [DOI] [PubMed] [Google Scholar]
Sanchez 2013
- Sanchez CJ Jr, Mende K, Beckius ML, Akers KS, Romano DR, Wenke JC, et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infectious Diseases 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanjay 2010
- Sanjay P, Fawzi A, Kulli C, Polignano FM, Tait IS. Impact of methicillin‐resistant Staphylococcus Aureus (MRSA) infection on patient outcome after pancreatoduodenectomy (PD)‐‐a cause for concern?. Pancreas 2010;39(8):1211‐4. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann H, Brożek J, Oxman A, editors. Grade handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The Grade Working Group, 2009. Available from http://www.Cc‐ims.Net/gradepro.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH (editors) on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shukla 2009
- Shukla S, Nixon M, Acharya M, Korim MT, Pandey R. Incidence of MRSA surgical‐site infection in MRSA carriers in an orthopaedic trauma unit. Journal of Bone and Joint Surgery. British Volume 2009;91(2):225‐8. [DOI] [PubMed] [Google Scholar]
SIGN 2011
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random 2011.
Skov 2005
- Skov R, Kolmos HJ, Peltonen R, Vuopio‐Varkila J, Hardardottir H, Gudlaugsson O, et al. MRSA infections increasing in the Nordic countries. Eurosurveillance 2005;10(31):http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2765. [Google Scholar]
Vinh 2009
- Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. Journal of Infection 2009;59(Supplement 1):S59‐74. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang JT, Wang JL, Fang CT, Chie WC, Lai MS, Lauderdale TL, et al. Risk factors for mortality of nosocomial methicillin‐resistant Staphylococcus aureus (MRSA) bloodstream infection: with investigation of the potential role of community‐associated MRSA strains. Journal of Infection 2010;61(6):449‐57. [DOI] [PubMed] [Google Scholar]
Watanabe 2008
- Watanabe A, Kohnoe S, Shimabukuro R, Yamanaka T, Iso Y, Baba H, et al. Risk factors associated with surgical site infection in upper and lower gastrointestinal surgery. Surgery Today 2008;38(5):404‐12. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wulf 2008
- Wulf M, Voss A. MRSA in livestock animals‐an epidemic waiting to happen?. Clinical Microbiology and Infection 2008;14(6):519‐21. [DOI] [PubMed] [Google Scholar]


