Abstract
Our study focuses on the relationship between inflammatory biomarkers and hypertension among sedentary adults in the United States, using data from the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2018. We categorized 24,614 participants into two groups based on their daily sedentary time: 9607 individuals in the sedentary group (≥7 h) and 15,007 in the non‐sedentary group (<7 h). We found that the sedentary group had a significantly higher prevalence of hypertension than the non‐sedentary group. Using weighted multiple logistic regression and smoothing curves, we assessed the correlation between inflammatory biomarkers and hypertension among the sedentary adults. The odds ratios for hypertension were 1.92 for the monocyte to high‐density lipoprotein ratio (MHR), 1.15 for the systemic inflammation response index (SIRI), and 1.19 for the natural logarithm of the systemic immune‐inflammation index (lnSII), all showing nonlinear associations. Furthermore, a significant positive correlation was found between sedentary time and inflammatory biomarkers (MHR, SIRI, and lnSII). Our findings suggest that prolonged sedentary behavior in the US significantly increases hypertension risk, likely due to marked increases in inflammation markers.
Keywords: hypertension, monocyte to high‐density lipoprotein ratio, sedentary behavior, systemic immune‐inflammation index, systemic inflammation response index
1. INTRODUCTION
The Global Burden of Disease studies highlight the importance of managing abnormal blood pressure, a leading global risk factor for mortality and morbidity. 1 Hypertension and related complications, such as ischemic heart disease and stroke, accounts for approximately 9.4 million deaths annually worldwide. 2 Similarly, in the United States, hypertension not only affects millions but also imposes a significant economic burden. 3 Despite adherence to the American Heart Association's anti‐hypertensive medication guidelines, early intervention remains critical for reducing cardiovascular disease risks. 4
However, traditional research on chronic diseases often overlooks behavioral risks. Sedentary behavior, defined as activities such as sitting, lying down, or reclining that do not exceed 1.5 METs, 5 has emerged as a significant public health concern. Studies show that adults spend approximately two‐thirds of their waking hours in such low‐activity states. 6 This prevalent lifestyle pattern not only impairs immune function but is also intricately linked to various chronic disease risk factors, such as hypertension. Sedentary behavior contributes to endothelial dysfunction, 7 , 8 insulin resistance, 9 , 10 altered lipid metabolism, 11 and, critically, amplified low‐grade inflammation, as evidenced by elevated levels of cytokines like IL‐1, IL‐6, and TNF‐α. 12 , 13 These factors cumulatively heighten the risk of developing hypertension.
Recent studies have investigated the association between novel inflammatory biomarkers—monocyte to high‐density lipoprotein ratio (MHR), systemic inflammation response index (SIRI), and systemic immune‐inflammation index (SII)—and hypertension. For example, MHR has been shown to predict non‐dipping hypertension. 14 Research in Chinese populations has indicated positive correlations between hypertension and systemic immune inflammation. 15 Elevated levels of the SIRI 16 and SII 17 in hypertensive individuals were linked to increased all‐cause and cardiovascular mortality. Furthermore, a higher SII has been correlated with significant morning blood pressure spikes. 18 Although these markers have been extensively studied in the general population, their relationship with hypertension among sedentary individuals requires further investigation.
Considering the research gaps in sedentary behavior and hypertension, our study specifically targets populations with extensive sedentary habits, particularly those exceeding 7 h of daily sedentary time (ST). We hypothesize that such lifestyles exacerbate systemic immune inflammation, which in turn increases the risk of hypertension. This 7‐h threshold is associated with an elevated risk of cardiovascular disease, as suggested by epidemiological evidence. 6 , 19
2. METHODS
2.1. Study population
We analyzed records from the National Health and Nutrition Examination Survey (NHANES) database covering the period from 2009 to 2018. This survey assesses nutrition and health, and its protocol was approved by the NCHS Ethics Review Board. All participants provided written informed consent. Since the data were obtained from the official NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm), no further ethical approval was necessary.
Out of the initial 49,693 participants, exclusions were as follows: (1) individuals younger than 20 years old; (2) those without MHR data or identified as outliers; (3) those without ST data or recording less than 30 minutes; (4) those missing physician's documented hypertension diagnosis or blood pressure readings. Consequently, 24,614 participants qualified for the study: 9607 with ST ≥ 7 h/day and 15,007 with ST < 7 h/day. The recruitment process is illustrated in Figure 1.
FIGURE 1.
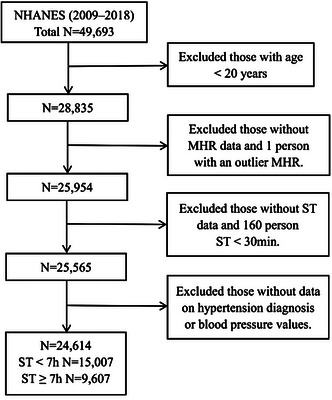
Flow diagram of the participants.
2.2. Study variables
Hypertension diagnosis was confirmed if any of the following criteria were met: average systolic blood pressure ≥140 mmHg, average diastolic blood pressure ≥90 mmHg, a physician's diagnosis, or the use of antihypertensive medication.
Inflammatory markers were determined using the following formulas: (1) MHR = monocyte count/HDL‐C; (2) SIRI = neutrophil count × monocyte count/lymphocyte count; (3) SII = platelet count × neutrophil count/lymphocyte count. Units are 109 cells/μL for cell counts and mmol/L for HDL‐C. Each marker was divided into quartiles for analysis, with the lowest quartile designated as the reference category. SII values underwent logarithmic transformation (lnSII) to correct for right skewness.
ST and physical activity were quantified using the Global Physical Activity Questionnaire. Participants recorded their typical daily sitting or reclining time during waking hours. ST was classified as <7 h/day or ≥7 h/day. Physical activity was measured in metabolic equivalents (METs) per week, with individuals achieving ≥ 600 METs/week considered “active”. 20
2.3. Covariate assessment
To accurately identify the factors influencing our study outcomes, we considered a range of covariates from the NHANES website (www.cdc.gov/nchs/nhanes/), which provides a detailed description of the data collection process. These covariates include: (1) demographics data: age, gender, race, education level, and family income to poverty rate (PIR). (2) examination, laboratory, dietary and questionnaire data: body mass index (BMI), fasting blood glucose (FBG), energy intake, drinking, smoking, disease status, and the use of anti‐hypertensive drugs.
BMI was calculated as weight (kg)/height (m2), and categorized as normal weight (<25.0 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2).
2.4. Statistical analyses
Data analyses were conducted using R software version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and EmpowerStats (X&Y Solutions, Inc, Boston, MA, USA). Continuous variables were presented as means ± standard deviations (M ± SD), and categorical variables as frequencies (percentages). Baseline characteristics were compared using analysis of variance (ANOVA) for continuous variables and chi‐square tests for categorical variables. Weighted univariate and multivariate logistic regression were performed to assess the associations between inflammatory markers and hypertension among sedentary individuals. Odds ratios (OR) and 95% confidence intervals (CI) were used as effect measures. Additionally, the relationship between ST and inflammatory biomarkers was explored using weighted multiple linear regression. In both multiple logistic and linear regression analyses, three progressively adjusted models were utilized. Smooth curve fitting used to address the nonlinear problem. All statistical tests were two‐tailed, with P‐values < .05 indicating statistical significance.
3. RESULTS
3.1. Baseline clinical characteristics
Table 1 presents the baseline characteristics of participants, categorized by ST and hypertension status. The data suggests that prolonged sitting duration is associated with increased BMI, elevated inflammation markers, increased risk of chronic diseases, extensive history of medication use, and reduced levels of physical activity. Additionally, hypertensive individuals generally appear to be older and have lower PIR. They also tend to have higher BMI, fasting blood glucose, and inflammation markers than those without hypertension. We also found that approximately 67.1% of hypertensive patients use anti‐hypertensive medications.
TABLE 1.
Baseline characteristics of participants in NHANES 2009−2018.
| Non‐HP (n = 65,034) | HP (n = 63,966) | ST < 7 h (n= 79,392) | ST ≥ 7 h (n = 49,608) | |
|---|---|---|---|---|
| PIR | 2.7 ± 1.6 | 2.5 ± 1.5** | 2.4 ± 1.5 | 2.8 ± 1.6** |
| SBP (mmHg) | 116.9 ± 11.0 | 136.0 ± 19.9** | 126.8 ± 18.8 | 127.8 ± 18.4** |
| DBP (mmHg) | 69.9 ± 9.4 | 72.8 ± 14.3** | 71.4 ± 12.1 | 71.3 ± 12.3 # |
| BMI (kg/m2) | 28.3 ± 6.3 | 30.9 ± 7.2** | 29.2 ± 6.5 | 30.3 ± 7.5** |
| FBG (mmol/L) | 6.08 ± 1.24 | 6.44 ± 1.65** | 6.26 ± 1.46 | 6.29 ± 1.49 # |
| ST (h) | 5.8 ± 3.4 | 6.2 ± 3.4** | – | – |
| MHR | 0.4 ± 0.2 | 0.5 ± 0.3** | 0.4 ± 0.2 | 0.5 ± 0.3** |
| SII (*109/L) | 499.4 ± 294.5 | 543.5 ± 460.9** | 515.5 ± 412.0 | 530.5 ± 342.2** |
| lnSII | 6.08 ± 0.51 | 6.13 ± 0.58** | 6.09 ± 0.54 | 6.12 ± 0.55** |
| SIRI (*109/L) | 1.1 ± 0.8 | 1.4 ± 1.1** | 1.2 ± 0.9 | 1.3 ± 1.0** |
| Gender | ** | ** | ||
| Women | 7302 (52.0%) | 5342 (50.5%) | 7247 (51.6%) | 5362 (50.7%) |
| Men | 6737 (48.0%) | 5233 (49.5%) | 6792 (48.4%) | 5213 (49.3%) |
| Race | ** | ** | ||
| Mexican American | 2303 (16.4%) | 1301 (12.3%) | 2686 (17.9%) | 845 (8.8%) |
| Other Hispanic | 1572 (11.2%) | 1026 (9.7%) | 1831 (12.2%) | 740 (7.7%) |
| Non‐Hispanic White | 5756 (41.0%) | 4346 (41.1%) | 5613 (37.4%) | 4506 (46.9%) |
| Non‐Hispanic black | 2176 (15.5%) | 2718 (25.7%) | 2911 (19.4%) | 2152 (22.4%) |
| Other race | 2232 (15.9%) | 1184 (11.2%) | 1966 (13.1%) | 1364 (14.2%) |
| Education level | ** | ** | ||
| <High school | 3215 (22.9%) | 2834 (26.8%) | 4382 (29.2%) | 1710 (17.8%) |
| ≥High school | 10824 (77.1%) | 7741 (73.2%) | 10625 (70.8) | 7897 (82.2%) |
| Smoking (n, %) | ** | ** | ||
| No | 8129 (57.9%) | 5383 (50.9%) | 8254 (55.0%) | 5140 (53.5%) |
| Yes | 5910 (42.1%) | 5192 (49.1%) | 6753 (45.0%) | 4467 (46.5%) |
| Drinking | ** | ** | ||
| No | 3622 (25.8%) | 3395 (32.1%) | 4547 (30.3%) | 2565 (26.7%) |
| Yes | 10417 (74.2%) | 7180 (67.9%) | 10460 (69.7) | 7042 (73.3%) |
| Physical activity | ** | ** | ||
| Active (n, %) | 12060 (85.9%) | 8999 (85.1%) | 13071 (87.1) | 7974 (83.0%) |
| Inactive (n, %)) | 1979 (14.1%) | 1576 (14.9%) | 1936 (12.9%) | 1633 (17.0%) |
| Diabetes (n, %) | ** | ** | ||
| No | 12396 (88.3%) | 7180 (67.9%) | 11841 (78.9) | 7407 (77.1%) |
| Yes | 1643 (11.7%) | 3395 (32.1%) | 3166 (21.1%) | 2200 (22.9%) |
| CHD (n, %) | ** | ** | ||
| No | 13604 (96.9%) | 9306 (88.0%) | 14002 (93.3) | 8762 (91.2%) |
| Yes | 435 (3.1%) | 1269 (12.0%) | 1005 (6.7%) | 845 (8.8%) |
| Hypertension | ** | |||
| No | – | – | 7774 (51.8%) | 4621 (48.1%) |
| Yes | – | – | 7233 (48.2%) | 4986 (51.9%) |
| Anti‐hypertensive drugs | ** | ** | ||
| Yes | – | 67.1% | 31.3% | 36.4% |
| No | – | 32.9% | 68.7% | 63.6% |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; MHR, monocyte to‐high‐density lipoprotein ratio; PIR, poverty to income ratio; SBP, systolic blood pressure (mmHg); SII, systematic immune‐inflammation index; SIRI, systemic inflammation response index; ST, sedentary time.
Continuous variables (mean ± standard deviation) were compared using weighted analysis of variance (ANOVA); Categorical variables (n (%)) were evaluated using weighted Chi‐square test.
# No significant; * P < .05; **P < .001.
3.2. Hypertension prevalence of different inflammatory biomarkers and ST
Figure 2 illustrates the variations in hypertension prevalence across different levels of ST and inflammatory parameters. Individuals engaging in prolonged sedentary behavior (over 7 h per day) and in the higher quartiles of inflammatory biomarkers have a significantly increased hypertension prevalence. In conclusion, both sedentary lifestyles and high levels of inflammatory markers increase hypertension risk, which underscores the importance of analyzing lifestyle and biological factors.
FIGURE 2.
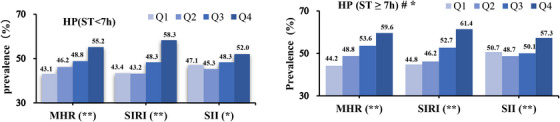
The prevalence of hypertension was compared across different sedentary time intervals and quartiles of inflammatory parameters using the Chi‐square test. # The prevalence of hypertension is higher among populations with ST ≥ 7 h/day compared to those with <7 h/day. The two‐tailed condition was defined as statistical significance. * P < .05, ** P < .001.
3.3. Influencing factors and confounders in hypertension risk
Table 2 employs univariate and multivariate logistic regression analyses to identify factors strongly associated with hypertension. Identified factors include demographics (age, gender, PIR), lifestyle choices (smoking, drinking), inflammatory markers (MHR, lnSII, SIRI), metabolic indicators (BMI, fasting blood glucose), and histories of medical conditions and medication. Moreover, the association between inflammatory markers and hypertension is more pronounced in sedentary individuals, as detailed in Table S1.
TABLE 2.
Univariate and multivariate logistic regression analysis of predictors for hypertension.
| Variable |
ST ≥ 7 h Univariate OR (95% CI) P‐value |
ST ≥ 7 h Multivariate OR (95% CI) P‐value |
|---|---|---|
| Age (years) | 1.062 (1.061, 1.064)** | 1.063 (1.059, 1.067)** |
| Gender—Women | 1.132 (1.093, 1.173)** | 1.058 (1.032, 1.157)** |
| PIR | 0.868 (0.858, 0.878)** | 0.942 (0.911, 0.975)** |
| BMI (kg/m2) | 1.062 (1.059, 1.065)** | 1.063 (1.055, 1.071)** |
| FBG (mmol/L) | 1.201 (1.156, 1.248)** | 0.976 (0.940, 1.013) # |
| MHR | 2.931 (2.709, 3.171)** | 1.917 (1.521, 2.416)** |
| lnSII (*109/L) | 1.207 (1.169, 1.247)** | 1.193 (1.107, 1.309)** |
| SIRI (*109/L) | 1.368 (1.339, 1.398)** | 1.145 (1.072, 1.201)** |
| Race | 0.963(0.961, 0.966)** | 0.963 (0.961, 0.966)** |
| Mexican American | 1 | 1 |
| Other Hispanic | 1.144 (1.049, 1.249)* | 1.283 (1.107, 1.635)** |
| Non‐Hispanic White | 1.160 (1.087, 1.238)** | 1.103 (0.914, 1.330) # |
| Non‐Hispanic black | 1.889 (1.760, 2.028)** | 2.268 (1.858, 2.770)** |
| Other Race | 0.691 (0.640, 0.746)** | 1.296 (1.147, 1.616)** |
| Education level | ||
| <High school | 1 | 1 |
| ≥High school | 0.619 (0.591, 0.649)** | 1.029 (0.891, 1.189) # |
| Smoking (n, %) | ||
| No | 1 | 1 |
| Yes | 1.470 (1.418, 1.523)** | 1.138 (1.053, 1.268)** |
| Drinking | ||
| No | 1 | 1 |
| Yes | 0.752 (0.723, 0.783)** | 1.065 (0.942, 1.202) # |
| Physical activity | ||
| Active (n, %) | 1 | 1 |
| Inactive (n, %) | 1.021 (0.985, 1.098) # | 1.050 (0.963, 1.156) # |
| Diabetes (n, %) | ||
| No | 1 | 1 |
| Yes | 3.671 (3.502, 3.848)** | 1.719 (1.487, 1.989)** |
| CHD (n, %) | ||
| No | 1 | 1 |
| Yes | 3.631 (3.372, 3.909)** | 1.454 (1.187, 1.780)** |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; MHR, monocyte‐to‐high‐density lipoprotein ratio; PIR, poverty to income ratio; SII, systematic immune‐inflammation index; SIRI, systemic inflammation response index; ST, sedentary time.
Covariates of multivariate logistic regression analysis: age, gender, PIR, BMI, fasting blood glucose, MHR, lnSII, SIRI, race, education level, smoking, drinking, physical activity, diabetes, CHD and anti‐hypertensive drugs.
# No significant; * P < .05; **P < .001.
3.4. Correlation of inflammation with hypertension among sedentary population
Table 3 demonstrates a significant positive correlation between inflammatory markers and hypertension among sedentary individuals, as revealed by three multivariate logistic regression models. In the fully adjusted model, MHR, SIRI, and lnSII are significantly associated with hypertension risk, with odds ratios of 1.92 (95% CI: 1.52–2.42), 1.15 (95% CI: 1.07–1.20), and 1.19 (95% CI: 1.11–1.31), respectively. Furthermore, Table S2 provides gender stratified analysis results, showing gender differences in the quartiles of inflammatory markers related to hypertension risk. MHR shows a stronger correlation with an increased risk of hypertension among women, especially in the highest quartile, with a 1.69‐fold increase in risk. Similarly, SIRI is more closely linked to an increased risk of hypertension in men, showing a 1.52‐fold increase.
TABLE 3.
Associations between the inflammatory indicators and HP in sedentary population.
| Variable |
Model I OR (95% CI) P‐value |
Model II OR (95% CI) P‐value |
Model III OR (95% CI) P‐value |
|---|---|---|---|
| MHR | 2.50 (2.38, 2.62)** | 3.80 (3.59, 4.03)** | 1.92 (1.52, 2.42)** |
| Q1 | 1 | 1 | 1 |
| Q2 | 1.16 (1.13, 1.20)** | 1.29 (1.24, 1.33)** | 1.08 (1.04, 1.20)* |
| Q3 | 1.34 (1.29, 1.38)** | 1.56 (1.50, 1.61)** | 1.16 (1.09, 1.32)** |
| Q4 | 1.73 (1.67, 1.78)** | 2.25 (2.17, 2.34)** | 1.45 (1.22, 1.66)** |
| SIRI | 1.35 (1.34, 1.37)** | 1.26 (1.24, 1.28)** | 1.15 (1.07, 1.20)** |
| Q1 | 1 | 1 | 1 |
| Q2 | 1.01 (0.98, 1.05) # | 1.15 (1.11, 1.19)** | 1.08 (1.03, 1.19)* |
| Q3 | 1.28 (1.24, 1.32)** | 1.39 (1.34, 1.44)** | 1.23 (1.14, 1.40)** |
| Q4 | 1.89 (1.83, 1.95)** | 1.77 (1.71, 1.84)** | 1.36 (1.17, 1.58)** |
| lnSII | 1.17 (1.15, 1.20)** | 1.32 (1.29, 1.35)** | 1.19 (1.11, 1.31)** |
| Q1 | 1 | 1 | 1 |
| Q2 | 0.93 (0.90, 0.96)** | 1.10 (1.07, 1.14)** | 1.05 (0.96, 1.18) # |
| Q3 | 1.02 (0.99, 1.05) # | 1.24 (1.20, 1.28)** | 1.06 (1.01, 1.12)* |
| Q4 | 1.26 (1.23, 1.30)** | 1.49 (1.43, 1.54)** | 1.30 (1.14, 1.49)** |
Abbreviations: MHR, monocyte‐to‐high‐density lipoprotein ratio; SII, systematic immune‐inflammation index; SIRI, systematic inflammation response index. Inflammatory indicators include continuous variable and quartile variable.
Three multivariate logistic regression models: Model I: no covariates were adjusted. Model II: Model I + age, gender and race were adjusted. Model III: Model II + educational level, PIR, BMI, fasting blood glucose, energy, smoking, drinking, physical activity, diabetes, coronary heart disease and anti‐hypertensive drugs were adjusted.
# No significant; * P < .05; **P < .001.
3.5. Correlation of sedentary behavior with inflammation
Table 4 examines the relationship between the duration of sedentary behavior and inflammatory indicators. After adjusting the models, a positive association was found: greater total ST was linked to increases in MHR, lnSII, and SIRI. Individuals engaging in less than 7 h sedentary time per day demonstrated smaller effect sizes compared to those with 7 or more hours. The statistical significance of these associations is confirmed by confidence intervals that do not cross zero and p‐values below .05, with several below .001, indicating that sedentary behavior markedly increases levels of systemic inflammation. Additionally, we conducted a gender‐based stratification analysis in Table S3. The results indicated that sedentary time remained positively correlated with inflammatory markers.
TABLE 4.
Associations between the sedentary time and inflammatory indicators.
| Model I β (95% CI) P‐value | Model II β (95% CI) P‐value | Model III β (95% CI) P‐value | |
|---|---|---|---|
| MHR | |||
| Total sedentary time | 0.080 (0.063, 0.097)** | 0.077 (0.060, 0.093)** | 0.063 (0.045, 0.081)** |
| ST < 7h | 0.096 (0.060, 0.110)** | 0.093 (0.051, 0.106)** | 0.062 (0.018,0.077)* |
| ST ≥ 7h | 0.112 (0.068, 0.131)** | 0.098 (0.059, 0.113)** | 0.078 (0.032, 0.086)* |
| lnSII | |||
| Total sedentary time | 0.082 (0.067, 0.093)** | 0.100 (0.063, 0.188)** | 0.068 (0.028, 0.084)** |
| ST < 7h | 0.051 (0.046, 0.070)** | 0.094 (0.083, 0.130)** | 0.055 (−0.036, 0.069) # |
| ST ≥ 7h | 0.061 (0.023, 0.082)* | 0.061 (0.031, 0.082)** | 0.086 (0.078, 0.117)* |
| SIRI | |||
| Total sedentary time | 0.070 (0.057, 0.092)** | 0.055 (0.037, 0.067)** | 0.053 (0.024, 0.063)** |
| ST < 7h | 0.071 (0.044, 0.083)** | 0.042 (0.030, 0.061)** | 0.033 (0.017, 0.051)** |
| ST ≥ 7h | 0.032 (0.019, 0.048)* | 0.034 (0.016, 0.048)** | 0.025 (0.013, 0.034)* |
Abbreviations: MHR, monocyte‐to‐high‐density lipoprotein ratio; SII, systematic immune‐inflammation index; SIRI, systematic inflammation response index; β, regression coefficient.
Three multiple linear regression models were used to determine the standard β coefficient. Model I: no covariates were adjusted. Model II: Model I + age, gender and race were adjusted. Model III: Model II + educational level, PIR, BMI, energy, proteins, carbohydrates, fats, smoking, drinking, physical activity, hypertension, diabetes and coronary heart disease.
# No significant; * P < .05; ** P < .001.
3.6. Subgroup analysis of hypertension correlation with inflammatory markers
Figure S1 analyzes various subgroups. Gender analysis indicates a stronger correlation between MHR and hypertension in females than in males, with an odds ratio of 2.50 (95% CI 1.67−3.75). The interaction by age group was not significant regarding the association between inflammation and hypertension risk, indicated by a p‐value above .05. Inflammatory biomarkers indicate a higher hypertension risk in obese individuals compared to those overweight. Notably, our data suggest that individuals with a normal BMI may have an elevated risk compared to those who are overweight. Non‐smokers exhibit a higher hypertension risk associated with these inflammatory biomarkers than smokers. Engagement in physical activity appears to mitigate this associated risk.
3.7. Nonlinear correlation of inflammatory markers and hypertension risk
Figure 3 further explores the nonlinear relationships between inflammatory markers and hypertension. It shows that MHR levels are initially associated with increasing hypertension risk, which peaks at a threshold of 0.75. Beyond this point, the risk declines temporarily before rising again after the 0.95 threshold. Higher levels of SIRI are positively correlated with increased hypertension risk, as demonstrated in Figure 3C. Figure 3E reveals a U‐shaped curve for lnSII, indicating heightened risks at both lower and higher levels. Charts 3b, 3d, and 3f confirm a positive correlation, showing an upward trend in hypertension prevalence with increasing quartiles of MHR, SIRI, and lnSII.
FIGURE 3.
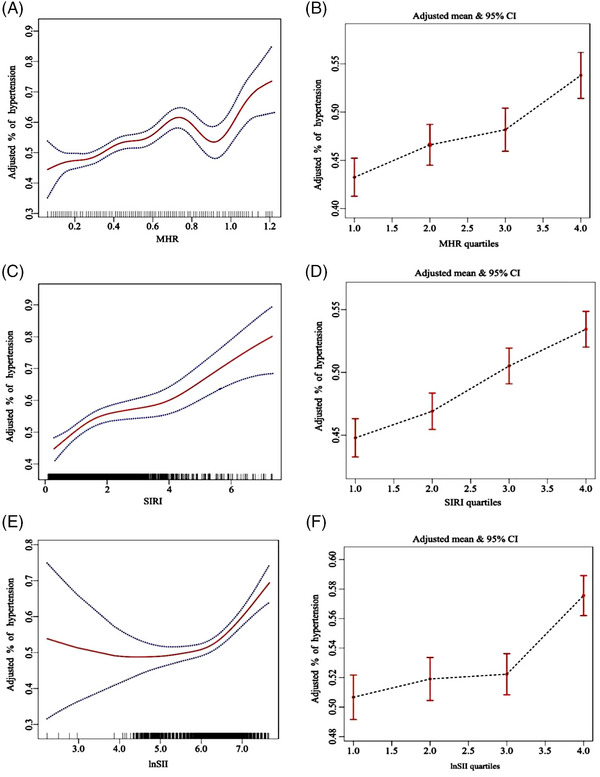
The relationship between hypertension and inflammatory markers (MHR, SIRI, lnSII) by using weighted generalized additive model and smooth curve fitting, after adjusting age, gender, race, poverty income ratio, education level, drinking, smoking, physical activity, BMI, fasting blood glucose, energy, diabetes, coronary heart disease, and anti‐hypertensive drugs.
4. DISCUSSION
The key findings of our study are as follows: (1) The relationship between inflammatory markers and hypertension is more pronounced in sedentary individuals (ST ≥ 7 h/d) within the study population; (2) After controlling for confounding factors, MHR, SIRI, and lnSII all exhibit positive correlations with hypertension. Additionally, significant positive correlations persist between ST and inflammatory markers in adjusted models; (3) Subgroup analyses indicate potential differences in the health impacts of sedentary behavior across genders, BMI, race, and physical activity.
In this cross‐sectional study, we investigated the relationship between new inflammatory biomarkers and hypertension in individuals with prolonged sedentary behavior (ST ≥ 7 h/d). While previous research has primarily focused on the association between sedentary duration and cardiovascular diseases, 21 , 22 as well as the link between inflammation markers and cardiovascular diseases, 23 , 24 there has been limited analysis regarding the specific connection between inflammatory markers and hypertension in sedentary populations. 25 , 26 Our findings indicate a stronger association between these inflammatory markers and hypertension in sedentary individuals. Previous studies, including those utilizing NHANES data 27 and cohort studies in China 15 have reported positive correlations between inflammatory markers (such as MHR, adjusted OR of 1.07 and SII, RR value of 1.003) and hypertension risk. However, in our fully adjusted model, the estimated OR for MHR with hypertension was 1.92 (95% CI: 1.52–2.42), and for lnSII it was 1.19 (95% CI: 1.11–1.31), emphasizing the potential influence of sedentary behavior on inflammation levels and hypertension risk. We also found that longer ST significantly increases the levels of inflammatory markers, potentially explaining the heightened hypertension risk in sedentary populations. This finding is consistent with existing research that suggests sedentary behavior contributes to chronic immune cell overactivation and low‐grade inflammation, 28 potentially exacerbating hypertension and its complications.
Contrary to Zhou's study, 27 we observed a curvilinear positive correlation between MHR and hypertension, which fluctuates rather than increases linearly. The relationship between higher levels of SIRI and increased hypertension risk is curvilinear, consistent with previous studies and indicating a progressively greater risk as SIRI levels rise. 29 Additionally, the U‐shaped curve linking lnSII levels with hypertension risk suggests that both low and high levels increase risk. This pattern likely provides a more accurate representation of immune signaling in disease and its progression, as noted in previous studies on hypertension prognosis. 17 Our findings suggest that this complex relationship more accurately reflects the actual conditions within the studied population, indicating potential pathological mechanisms at various inflammation marker levels. Insights gained from these findings could significantly impact the development of more precise prevention and intervention strategies for hypertension.
Our subgroup analysis showed significant gender differences in the relationship between inflammatory markers and hypertension risk. For example, sedentary women had the highest risk of hypertension from the MHR marker (OR = 2.50), aligning with similar gender disparities seen with MHR in coronary heart disease. 30 Analysis of 105,239 participants indicated that prolonged sitting elevates metabolic syndrome risk more in women, 28 suggesting gender‐specific physiological differences. The obese population exhibited the highest risk of hypertension, as shown by inflammatory markers (MHR's OR = 2.8, SIRI's OR = 1.53, lnSII's OR = 1.22), aligning with previous studies linking higher BMI to increased hypertension risk. 31 , 32 Interestingly, our data indicate that individuals with normal BMI face a higher risk than those overweight, echoing previous research, 33 suggesting normal BMI may also be a risk factor. Additionally, our findings demonstrate that physical activity significantly reduces hypertension risk—by 66% for MHR, 25% for SIRI, and 43% for lnSII—supporting research on the anti‐inflammatory benefits of exercise. 34 These results suggest promoting physical activity as a key strategy to prevent hypertension, especially in populations with high inflammation.
Our study is strengthened by utilizing a nationally representative sample and selecting cellular inflammatory factors, diverging from traditional markers used in prior research. This novel approach provides deeper insights into the links between hypertension, sedentary behavior, and cellular inflammation. However, the cross‐sectional design and reliance on self‐reported sedentary behavior limit our ability to determine causality and may introduce bias. Future studies should use longitudinal designs and objective measures of physical activity to confirm our results and control for confounding factors.
5. CONCLUSIONS
Our research suggests that a sedentary period of seven hours may represent a critical threshold, as inflammatory levels and hypertension risk may increase beyond this duration. Future prospective studies are needed to establish causality and explore sedentary behavior and inflammation in the prevention and treatment of hypertension.
AUTHOR CONTRIBUTIONS
Shuo Sha and Huan‐Zhen Chen: Conceived and designed the experiments; conducted the experiments; analyzed and interpreted the data and wrote the paper. Xing‐Peng Bu and Ai‐Wen Wang: Contributed materials; analysis tools; valuable suggestions and data.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Sha S, Bu X‐P, Wang A‐W, Chen H‐Z. Association between inflammatory biomarkers and hypertension among sedentary adults in US: NHANES 2009–2018. J Clin Hypertens. 2024;26:945–954. 10.1111/jch.14851
Shuo Sha is the first author and Xing‐Peng Bu and Ai‐Wen Wang share second authorship.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in National Health and Nutrition Examination Surveys (NHANES) at https://www.cdc.gov/nchs/nhanes/index.htm
REFERENCES
- 1. Chew NWS, Ng CH, Tan DJH, et al. The global burden of metabolic disease: data from 2000 to 2019. Cell Metab. 2023;35(3):414‐428. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2019 Ageing Collaborators . Global, regional, and national burden of diseases and injuries for adults 70 years and older: systematic analysis for the Global Burden of Disease 2019 Study. BMJ. 2022;376:068208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ouyang F, Cheng X, Zhou W, He J, Xiao S. Increased mortality trends in patients with chronic non‐communicable diseases and comorbid hypertension in the United States, 2000–2019. Front Public Health. 2022;10:753861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasan RS, Song RJ, Xanthakis V, et al. Hypertension‐mediated organ damage: prevalence, correlates, and prognosis in the community. Hypertension. 2022;79(3):505‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raffin J, de Souto Barreto P, Le Traon AP, Vellas B, Aubertin‐Leheudre M, Rolland Y. Sedentary behavior and the biological hallmarks of aging. Ageing Res Rev. 2023;83:101807. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Q, Chen C, Zhang J, Ye Y, Fan X. Sedentary behavior and health outcomes in patients with heart failure: a systematic review and meta‐analysis. Heart Fail Rev. 2022;27(4):1017‐1028. [DOI] [PubMed] [Google Scholar]
- 7. Hong J, Park E, Lee J, Lee Y, Rooney BV, Park Y. Exercise training mitigates ER stress and UCP2 deficiency‐associated coronary vascular dysfunction in atherosclerosis. Sci Rep. 2021;11(1):15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daniele A, Lucas SJE, Rendeiro C. Detrimental effects of physical inactivity on peripheral and brain vasculature in humans: insights into mechanisms, long‐term health consequences and protective strategies. Front Physiol. 2022;13:998380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farrahi V, Rostami M, Dumuid D, et al. Joint profiles of sedentary time and physical activity in adults and their associations with cardiometabolic health. Med Sci Sports Exerc. 2022;54(12):2118‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee S, Gulseth HL, Langleite TM, et al. Branched‐chain amino acid metabolism, insulin sensitivity and liver fat response to exercise training in sedentary dysglycaemic and normoglycaemic men. Diabetologia. 2021;64(2):410‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinto AJ, Bergouignan A, Dempsey PC, et al. Physiology of sedentary behavior. Physiol Rev. 2023;103(4):2561‐2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huston P. A sedentary and unhealthy lifestyle fuels chronic disease progression by changing interstitial cell behaviour: a network analysis. Front Physiol. 2022;13:904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vandercappellen EJ, Koster A, Savelberg HHCM, et al. Sedentary behaviour and physical activity are associated with biomarkers of endothelial dysfunction and low‐grade inflammation‐relevance for (pre)diabetes: the Maastricht Study. Diabetologia. 2022;65(5):777‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Ding Y, Jiang W. Neutrophil and monocyte ratios to high‐density lipoprotein cholesterol as biomarkers in non‐dipping hypertension. Clin Exp Hypertens. 2023;45(1):2210785. [DOI] [PubMed] [Google Scholar]
- 15. Ma LL, Xiao HB, Zhang J, et al. Association between systemic immune inflammatory/inflammatory response index and hypertension: a cohort study of functional community. Nutr Metab Cardiovasc Dis. 2023. Published online October 4. S0939‐4753(23)00386‐1. [DOI] [PubMed] [Google Scholar]
- 16. Cheang I, Zhu X, Lu X, et al. Associations of inflammation with risk of cardiovascular and all‐cause mortality in adults with hypertension: an inflammatory prognostic scoring system. J Inflamm Res. 2022;15:6125‐6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao Y, Li P, Zhang Y, et al. Association of systemic immune inflammatory index with all‐cause and cause‐specific mortality in hypertensive individuals: results from NHANES. Front Immunol. 2023;14:1087345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saylik F, Sarıkaya R. Can systemic immune‐inflammation index detect the presence of exxaggerated morning blood pressure surge in newly diagnosed treatment‐naive hypertensive patients? Clin Exp Hypertens. 2021;43(8):772‐779. [DOI] [PubMed] [Google Scholar]
- 19. Pandey A, Salahuddin U, Garg S, et al. Continuous dose‐response association between sedentary time and risk for cardiovascular disease: a meta‐analysis. JAMA Cardiol. 2016;1(5):575‐583. [DOI] [PubMed] [Google Scholar]
- 20. Cao Z, Xu C, Zhang P, Wang Y. Associations of sedentary time and physical activity with adverse health conditions: outcome‐wide analyses using isotemporal substitution model. EClinicalMedicine. 2022;48:101424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yerramalla MS, Van Hees VT, Chen M, Fayosse A, Chastin SFM, Sabia S. Objectively measured total sedentary time and pattern of sedentary accumulation in older adults: associations with incident cardiovascular disease and all‐cause mortality. The Journals of Gerontology: Series A. 2022;77(4):842‐850. Lipsitz LA, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duran AT, Friel CP, Serafini MA, Ensari I, Cheung YK, Diaz KM. Breaking up prolonged sitting to improve cardiometabolic risk: dose‐response analysis of a randomized crossover trial. Med Sci Sports Exerc. 2023;55(5):847‐855. [DOI] [PubMed] [Google Scholar]
- 23. Jayedi A, Rahimi K, Bautista LE, Nazarzadeh M, Zargar MS, Shab‐Bidar S. Inflammation markers and risk of developing hypertension: a meta‐analysis of cohort studies. Heart. 2019;105(9):686‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kälsch AI, Scharnagl H, Kleber ME, et al. Long‐ and short‐term association of low‐grade systemic inflammation with cardiovascular mortality in the LURIC study. Clin Res Cardiol. 2020;109(3):358‐373. [DOI] [PubMed] [Google Scholar]
- 25. Demirtaş B, Aydin T. Monocyte‐to‐high‐density lipoprotein ratio: an independent predictor of reverse dipper pattern in hypertensive patients. Eur Rev Med Pharmacol Sci. 2023;27(19):9012‐9021. [DOI] [PubMed] [Google Scholar]
- 26. Shi Y, Zhou W. Threshold effect of systemic immune inflammatory index on hypertension in American adults (NHANES 2017–2020). J Hypertens. 2023;41(12):2107‐2112. [DOI] [PubMed] [Google Scholar]
- 27. Zhou Y, Dan H, Bai L, et al. Continuous positive linear association between the monocyte to high‐density lipoprotein cholesterol ratio and hypertension: a cross‐sectional study. Int J Hypertens. 2022;2022:1‐9. Ong KL, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J, Zhang H, Yang L, et al. Sedentary time and the risk of metabolic syndrome: a systematic review and dose‐response meta‐analysis. Obes Rev. 2022;23(12):13510. [DOI] [PubMed] [Google Scholar]
- 29. Zhao S, Dong S, Qin Y, Wang Y, Zhang B, Liu A. Inflammation index SIRI is associated with increased all‐cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. 2022;9:1066219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan S, Sha S, Wang D, Li S, Jia Y. Association between monocyte to high‐density lipoprotein ratio and coronary heart disease in US adults in the National Health and Nutrition Examination Surveys 2009–2018. Coronary Artery Disease. 2023;34(2):111‐118. [DOI] [PubMed] [Google Scholar]
- 31. Wang Q, Song X, Du S, et al. Multiple trajectories of body mass index and waist circumference and their associations with hypertension and blood pressure in chinese adults from 1991 to 2018: a prospective study. Nutrients. 2023;15(3):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson P, Logan I, Tomson C, Sheerin N, Ellam T. Obesity, sex, race, and early onset hypertension. Hypertension. 2020;76(3):859‐865. [DOI] [PubMed] [Google Scholar]
- 33. Foti K, Hardy ST, Chang AR, Selvin E, Coresh J, Muntner P. BMI and blood pressure control among United States adults with hypertension. J Hypertens. 2022;40(4):741‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El Assar M, Álvarez‐Bustos A, Sosa P, Angulo J, Rodríguez‐Mañas L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. 2022;23(15):8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are openly available in National Health and Nutrition Examination Surveys (NHANES) at https://www.cdc.gov/nchs/nhanes/index.htm


