Abstract
Since the 1970s, artificial intelligence (AI) has played an increasingly pivotal role in the medical field, enhancing the efficiency of disease diagnosis and treatment. Amidst an aging population and the proliferation of chronic disease, the prevalence of complex surgeries for high-risk multimorbid patients and hard-to-heal wounds has escalated. Healthcare professionals face the challenge of delivering safe and effective care to all patients concurrently. Inadequate management of skin wounds exacerbates the risk of infection and complications, which can obstruct the healing process and diminish patients’ quality of life. AI shows substantial promise in revolutionizing wound care and management, thus enhancing the treatment of hospitalized patients and enabling healthcare workers to allocate their time more effectively. This review details the advancements in applying AI for skin wound assessment and the prediction of healing timelines. It emphasizes the use of diverse algorithms to automate and streamline the measurement, classification, and identification of chronic wound healing stages, and to predict wound healing times. Moreover, the review addresses existing limitations and explores future directions.
Keywords: Skin wound healing, artificial intelligence, skin wound measurement, skin wound classification, burn degree assessment, chronic wound prediction
Introduction
Artificial intelligence (AI) encompasses computer algorithms designed to mimic human cognitive function [1,2]. Since the 1970s, AI technologies such as machine learning (ML), neural networks, semantic recognition, and image analysis have become integral to the medical field, substantially enhancing the diagnosis and treatment of diseases [3]. In the 21st century, fueled by advancements in deep learning (DL), ML algorithms, hardware, and data storage capabilities, AI has undergone a significant evolution, offering profound support in clinical settings [4].
In clinical practice, skin wounds are frequently encountered [5], necessitating that clinicians base their treatment decisions and assessments of wound healing progress on various factors, including wound size, classification, and tissue composition [6]. These assessments largely depend on the subjective visual evaluations of physicians and clinical staff [7,8], highlighting an opportunity to integrate emerging technologies [9]. Inaccurate evaluation can result in serious consequences such as improper dressing selection, overlooked non-healing wounds, and delayed specialist referrals [10]. With the increasing number of surgeries involving high-risk and multimorbid patients, effective perioperative wound management becomes critical [11]. AI is not only valuable for surgical wounds but also offers significant benefits for assessing healing times in chronic and burn wounds [9,12]. Inadequate skin wound management heightens the risk of surgical site infections and other complications [11]. Additionally, patients with chronic wounds often require intricate care due to comorbidities that complicate the healing process [13].
Numerous articles have explored the use of imaging techniques for skin wounds, yet there remains a notable gap in the systematic summarization of innovative AI methodologies and their applications in this domain [14]. The forefront of recent advancements in AI - particularly over the last three years - has been dominated by machine learning (ML) and deep learning (DL) [15]. AI facilitates the rapid analysis of vast arrays of wound images, working in tandem with intelligent algorithms and extensive databases to accurately identify, classify, and predict wound tissue characteristics. Importantly, AI has the ability to improve accuracy through ongoing learning. Despite the availability of seemingly ample medical data sets and sophisticated algorithms for many years, there is still a significant lack of algorithms that meaningfully affect clinical care [16]. This review aims to provide a comprehensive overview of the current applications of AI in skin wound assessment and the prediction of healing times, setting the stage for future developments.
Skin wound assessment
Monitoring changes in wound surface area over several weeks is a critical metric for evaluating the effectiveness of therapeutic interventions [17]. Traditional methods of measuring wounds using a scalpel to gauge width and length are often imprecise, potentially resulting in less than optimal treatments and outcomes [18]. Artificial intelligence significantly enhances the precision of measuring wound dimensions, topology, edge positioning, and the percentage of different tissue types. This advancement markedly improves the accuracy of wound closure assessments in clinical settings. For instance, the Automatic Skin Ulcer Region Assessment framework developed by Daniel et al. efficiently segmented wounds and measured their sizes with a low error rate of 14% through a semi-automatic method [19]. Additionally, Zhao et al. created a vision-laser scanner, utilizing an artificial neural network, to reconstruct wounds’ three-dimensional edges and topologies [20]. Jones et al. employed a Convolutional Neural Network (CNN) to determine epidermal and dermal thickness and the percentage of re-epithelialization [21], while Ramachandram et al. introduced a deep learning approach for objective tissue identification and measurement [22].
Traditionally, collecting and manually observing regular wound images to determine study metrics has been a time-consuming and laborious process. Furthermore, defining wound margins is often subjective and can vary among experts [23]. To overcome these challenges, Carrión et al. developed a deep learning (DL)-based image analysis pipeline capable of processing non-uniform wound images. This system extracts critical data such as key wound locations, performs image cropping, and calculates metrics related to the size of the wound periphery over time [23]. This pipeline facilitated a high-throughput assessment and accurate tracking of wound size. In their research, it provided essential details like wound closure percentages and dimensions for further analysis. The system proved effective with minimal human intervention and could accurately estimate wound sizes even when up to 50% of the reference images were absent.
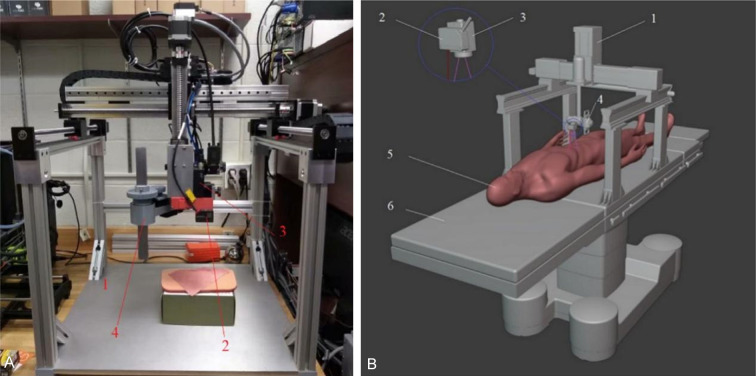
To optimize the area selected for automated analysis, Wang et al. deployed support vector machines (SVM) to define wound boundaries precisely [24]. These boundaries were further refined through the application of the conditional random field method. Estimations of wound extent rely on optical theory, comparing images across color channels, and using a fuzzy spectral clustering segmentation algorithm to delineate the wound area [25]. These methods, however, are confined to two-dimensional imagery. To capture the three-dimensional architecture of wounds, Edward et al. used a vision-laser scanner to generate a 3D point cloud of the wound’s edges and topology [20]. Integrating this scanner with gantry robots, as depicted in Figure 1, enhances accuracy and improves patient outcomesby reducing human error, lowering infection rates, and accelerating healing. Additionally, a custom-designed supplementary laser LED was employed to provide an extra measurement point, leveraging an artificial neural network to decrease scanning time [20]. In collaboration with RSI, Andreas Körber and his team connected digital photography with optical raster through a digital scanner and picture processing software (DigiSkin), achieving precise three-dimensional imaging of chronic wounds [26]. Moreover, Wang et al. developed an integrated system that combines visual features with deep learning algorithms for wound segmentation and area estimation, using a newly developed convolutional encoder-decoder network - a variant of ConvNet [18]. This system is not only computationally efficient and reliable but also includes capabilities for detecting wound infections and predicting healing outcome. The specific applications and benefits of these methods are detailed in Table 1.
Figure 1.
Gantry robotic wound closure system. A. Set configuration; B. Visionary sketch of setting in operating room. 1: gantry robot, 2: laser range sensor, 3: 2D camera, 4: fixture placement device, 5: patient body, 6: surgeon bed. Source: Zhao YM, Currie EH, Kavoussi L, Rabbany SY. Laser scanner for 3D reconstruction of a wound’s edge and topology. International Journal of Computer Assisted Radiology and Surgery 2021; 16: 1761-1773 [20].
Table 1.
Comparison of AI methods regarding wound measurement
| Wound Measurement Method | Principle | Application | Merits | Limitations |
|---|---|---|---|---|
| Automatic image analysis pipeline [23] | Computer vision, object detection algorithms (YOLO) | Automated measurement of wound size and automatic assessment of average wound closure percentage. High fidelity results on unseen data with minimal human intervention | Automated and enables high fidelity results | Not good at dealing with some of the challenges like occlusion and blur. |
| Quantitative measurements are not exactly aligned | ||||
| Fuzzy spectral clustering [25] | Gray scale based fuzzy similarity measure, spectral clustering segmentation algorithm | Accurate depiction of the wound area and automatic calculation of the contrast between wound and non-wound areas | Effective depiction of wound areas in non-uniformly illuminated images | The wounds that are near to heal or the images having very low (i.e. nearly zero) contrast between healed wound area and healthy skin are not accurately segmented. |
| The method is not completely automatic. | ||||
| Vision laser scanner [20] | Use laser ranging scanning to generate 3D point cloud, artificial neural network estimation method | Accurate 3D reconstruction of wound margins and topology | Simultaneous generation of 3D point clouds of wound skin and its edges | The scanner can only deal with small size wound (~3-inch length) |
| Integrated system [18] | Convolutional encoder-decoder networks (a variant of ConvNet), Hough transformation, computer vision tasks | Wound segmentation in an end-to-end different manner and estimation of wound surface area by transformation of pixel length to actual length | High computational efficiency, validity and reliability as a multifunctional, integrated and unified framework system |
The use of AI technology varies across different types of wounds. Commonly treated clinical skin wounds include lower extremity venous ulcers, diabetic foot ulcers, pressure ulcers, burn wounds, and surgically infected wounds. Integrating AI with computer vision and imaging technologies enables non-contact measurements, enhancing the regular monitoring of ulcer wounds [27]. This allows patients to send images of their wounds from their homes, reducing the need for frequent hospital visits. Moreover, leveraging data from electronic medical records combined with machine learning (ML) algorithms has proven highly effective in predicting the development of pressure ulcers [28]. AI systems can customize predictions based on unique patient data from medical records. In the context of burn wounds, AI-driven systems for assessing burn depth have demonstrated significant clinical value [29]. Additionally, Egberts et al. employed a neural network to predict the healing trajectory of burn wounds, successfully simulating skin contraction over periods longer than one year [30]. For surgical site infections (SSIs), ML algorithms trained with comprehensive health data, including detailed wound status descriptors, have effectively predicted SSI risks [31].
The integration of various optical wound assessment tools and multi-modal imaging devices has significantly improved the stability and precision of wound evaluations. These technological advancements provide detailed measurements of wound area and volume, and also yield insights into the tissue composition within the wound bed [32]. The ongoing development of intelligent information evaluation systems has greatly improved the ease and systematic management of digital imagery and wound-related data. This progress has paved the way for sophisticated intelligent monitoring systems, which play a crucial role in enhancing healing rates and expediting patient recovery [33].
Skin wound classification
Histologic classification of skin wounds
The variety of tissue types within a wound serves as a crucial indicator of its healing progress [22]. Precise analysis of wound tissue characteristics, such as the area and the percentage of granulation tissue (PGT), is essential for enhancing wound care and recovery [34]. Traditional histologic analysis, commonly used for disease diagnosis, requires extensive and time-consuming tissue preparation [35]. To address this, Howell et al. developed an AI-based tool for both qualitative and quantitative wound assessment. They benchmarked this tool against human expert evaluations, establishing a reliable AI framework to measure wound area and PGT [36]. Further exploring tissue pathology, Maknuna et al. introduced a rapid method for characterizing scar lesions in H&E-stained tissues [23]. They utilized both supervised and unsupervised learning approaches to teach the computer to identify patterns and extract insights from unclassified data [37,38]. Their use of the K-means algorithm enabled detailed analysis of features like collagen density and its directional variance, confirming a substantial 50% difference between normal and scar tissue. This method proves effective for delineating scar tissue’s pathologic attributes and aiding in the formulation of targeted treatment strategies [37]. Additionally, AI medical devices have enhanced the precision of remote wound assessment and classification [39].
Beyond the measurement tools previously mentioned, several researchers have explored the application of Convolutional Neural Networks (CNN) in wound assessment. Utilizing deep learning techniques, they have effectively identified and segmented unique features within image regions. Since its adoption in 2012, CNNs have been extensively applied across various biomedical fields, showcasing its proficiency in classifying and segmenting large volumes of image data swiftly and accurately [40,41]. Specifically for skin wounds, segmentation involves precisely outlining the wound area in the image and isolating the necessary components for analysis. To enhance the quantitative analysis of skin wound histology, Jones et al. developed a CNN capable of automatically calculating parameters such as wound depth, wound width, as well as the thicknesses of epidermal and dermal layers, and the percentage of re-epithelialization [21]. This CNN proved its efficacy by accurately segmenting entire sections of H&E-stained wounds on a pixel-wise level in under 30 seconds using a standard desktop computer. These technological advances set the stage for more detailed quantification of histologic features in wound imagery.
Burn degree assessment
Precise assessment of burn severity is critical for effective wound care and treatment [42]. An erroneous evaluation can result in delayed wound management, adversely affecting future treatment outcomes [43]. In contemporary medical practices, artificial intelligence (AI) is utilized to assess burn severity by estimating the total body surface area affected, depth of burns, and extent of scarring [44]. Additionally, Spatial Frequency-Domain Imaging (SFDI) technology, which leverages the relationship between histologic observations and tissue property changes, has proven to be an invaluable tool. This technology can predict the severity of burns within a 24-hour period by analyzing images captured at various wavelengths and frequencies [45-47]. The use of Support Vector Machine (SVM) classifiers further enhances the precision of these predictions [48].
Concurrently, Cirillo et al. showcased the effectiveness of AI in determining burn depth [49]. Using semantic segmentation of images from polarized high-performance light cameras, their AI model proficiently identified four distinct levels of burn severity [50]: superficial (I), superficial to intermediate (II), medium to deep (III), and deep to full thickness (IV), achieving remarkable accuracy rates of up to 92% [49]. Constructing such a model requires extensive learning and training, supported by a substantial training dataset. Nevertheless, the prospects for further refining the algorithm through the acquisition of more images are both viable and promising for future advancements [49,51].
Skin wound prediction
Chronic wound prediction
Chronic wounds pose a significant global challenge, defined by localized skin and tissue injuries with a compromised physiologic healing response [25,52]. The Wound Healing Association describes chronic wounds as a failure to restore the normal structure and function of damaged tissue in a regular and timely manner [53]. Typically, the healing process for chronic wounds extends beyond four weeks, significantly impairing affected individuals’ quality of life and well-being, and contributing to elevated mortality rates [54]. Therefore, precise assessment, prediction, and management of chronic wounds are crucial for reducing the healthcare system’s lburden and enhancing the speed and quality of patient recovery [55].
In clinical practice, common lower extremity wounds such as arterial, diabetic, pressure, and venous ulcers [56] pose a high risk to older adults, who are more susceptible due to various age-related changes [57]. These changes include an increased prevalence of chronic conditions like cardiovascular disease and diabetes, along with impaired mobility, incontinence, low weight, poor nutritional status, and cognitive impairment [58].
Age-related intrinsic alterations in skin wound healing - such as modified inflammatory responses, reduced levels of supportive extracellular matrix (ECM) and growth factors, delayed epithelialization, and diminished angiogenic activity - contribute to slower wound closure rates in older adults [59]. With the aging population, the incidence of such wounds has significantly increased, intensifying the demand on limited healthcare resources [60]. To address this, researchers have developed an AI-powered wearable sensor linked with advanced wound dressing bandages. This system uses a deep artificial neural network (ANN) algorithm for monitoring chronic wounds and identifying their healing stages [61]. This near-field sensing technology provides critical data for treatment decisions and assesses the effectiveness of wound care medications. Within the realm of telemedicine, Chakraborty et al. have introduced a model that uses Linear Discriminant Analysis to classify tissue types, achieving a tissue prediction accuracy of 91.45% [62]. This approach enables remote diagnosis of chronic wound healing statuses, aiding clinicians in making more informed decisions based on quantitative tissue composition data. Moreover, advancements in statistical computing have propelled the development of several promising machine learning techniques [63]. Jung et al. utilized modern ML methods to create a predictive model for delayed wound healing, training it with collected wound data to enable early and precise predictions of delayed healing outcomes [64].
Chronic wounds represent a significant global health challenge, where accurate diagnosis and effective treatment are crucial for facilitating healing and averting further complications [65]. In the healthcare sector, AI is increasingly utilized to analyze medical data predictively, adapting seamlessly to new information [66]. Although electronic medical records are extensively used for documenting wounds, managing and tracking every aspect of patient care for those with chronic wounds is still a complex task [67]. Nonetheless, leveraging big data analytics and machine learning offers substantial promise in reducing treatment costs, decreasing the time needed for simulations, and enhancing the overall quality of care [68].
Wound healing time prediction
The ability to predict wound healing time holds significant clinical value, enabling physicians to swiftly tailor treatment plans to individual needs [69]. Through accurate predictions, doctors can decide whether a patient requires multiple debridements or if early closure is feasible, as well as determine the best timing for closing traumatic wounds. This predictive capability not only reduces the duration to wound closure but also minimizes the risk of wound complications and failures [70].
Assessing the thickness of the epidermis and scabs is critical for understanding the skin wound healing process, as it provides key insights into the normalcy of the re-epithelialization process [71-73]. Optical Coherence Tomography (OCT), a real-time, non-invasive imaging technique, enables the cross-sectional evaluation of tissue microstructures. Integrating OCT with AI algorithms allows for the automated measurement of the thickness of epithelial tissues and scabs, thereby facilitating predictions about wound healing times [74]. Predicting the healing of amputation wounds, however, remains a complex challenge due to factors such as severe ischemia and the absence of reliable assessment tools. To address this, Squiers et al. implemented a novel imaging system to capture multispectral images of the lower extremities [75]. Analyzing these images in conjunction with patient clinical risk factors through machine learning algorithms has enhanced the accuracy of predictions regarding amputation wound healing. Additionally, this approach potentially reduces the necessity for reoperations and the occurrence of delayed healing.
In 2020, Chinese researchers developed a CNN-based artificial model to recognize burn depth, which was effectively used to predict the healing time of burn wounds. This model accurately estimated the wound healing timeline by analyzing the depth of the burn [76].
Wound healing is a complex and dynamic process, making the accurate prediction of healing times a persistent challenge for clinicians. However, with the growing accessibility of vast data sets and enhanced computing capabilities, AI-based models are poised to play a crucial role in the prediction and assessment of wound healing timelines [77].
Summary and outlook
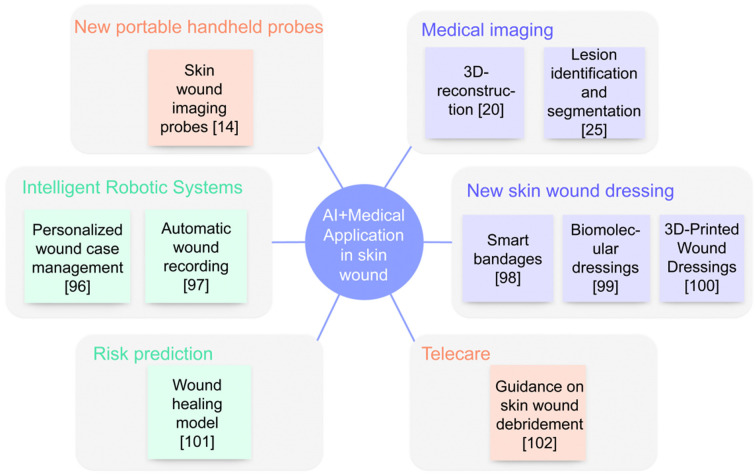
Currently, AI is evolving at an unprecedented pace, particularly in the medical sector where it significantly enhances rapid image interpretation, diagnosis, risk prediction, and adjuvant treatment [78,79]. Specific examples of AI applications in skin wound management are illustrated in Figure 2. Over the last decade, the swift advancement of computer processing technology has facilitated the deeper integration of AI systems across various medical imaging technologies such as X-ray, ultrasound, computed tomography, and magnetic resonance imaging [77]. Machine learning (ML) and deep learning (DL) have been instrumental in analyzing medical images from these technologies, demonstrating high accuracy and reliability [80]. AI’s capabilities extend across a broad spectrum of functions, including assisting in diagnosis, selecting therapies, predicting risks, stratifying diseases, reducing medical errors, and enhancing productivity [81]. In a notable study, Aaron Jones et al. implemented a quasi-experimental design across four settings within the Australian Health Service. They gathered data from standard and intervention groups, revealing that 101 out of 132 wounds showed improvement during the intervention, with a mean wound size reduction of 53.99%. This research underscores the practicality and effectiveness of AI in wound management [82].
Figure 2.
AI+ Medical application scenarios in skin wounds. Scenarios for the use of AI in skin wounds, including a variety of novel imaging techniques, risk prediction systems incorporating algorithms, intelligent robotics, and the accompanying promise of telemedicine and personalized medicine.
Despite the advancements, challenges remain from the tedious and time-consuming processes involved in wound image collection, classification, and interpretation, compounded by the lack of robust and efficient data analysis systems [83]. A primary barrier is data availability; hospitals often hesitate to share data due to privacy concerns [84], while machine learning (ML) requires extensive datasets for effective training, often difficult to secure [85]. Advancements in algorithms and the broader adoption of cloud computing may mitigate these issues [86], and stricter data privacy regulations could also provide support [87]. Another significant challenge is the clinical implementation of AI, with limited empirical evidence on its impact on patient outcome [88]. Moreover, AI interventions should expedite, not hinder, medical processes, including the necessary training for healthcare providers [89]. Ethical concerns also persist, as poor decisions in healthcare can lead to severe repercussions, and accountability remains a critical issue [90]. The legal complexities associated with applying traditional tort liability to AI technologies due to their opaque and unpredictable nature call for innovative legal standards and models, such as AI personhood or joint liability, to establish a fair and predictable framework for AI-related medical malpractice [91].
The future of AI in skin wound management looks promising, particularly with the advent of Explainable Artificial Intelligence (XAI) based on deep learning (DL) in medical image analysis. XAI is evolving as a vital tool that enhances AI’s ability to offer novel insight into data, thereby enriching the resource base with new discovery elements [92]. As DL-based methods become more widespread, the demand for explainability increases, especially in critical areas such as medical image analysis, which plays a crucial role in skin wound assessment [93]. Beyond diverse imaging techniques and AI-integrated systems, AI-powered remote consultation systems using smartphones and tablets for data gathering and connectivity are gaining traction [94]. For instance, recent advancements in AI technologies have improved the remote monitoring of diabetic foot ulcers by mobile applications [95]. Digital solutions for the remote diagnosis and monitoring of wounds in community settings have rapidly evolved. The COVID-19 pandemic has further spurred the research and development of these innovative technologies. Applying ML algorithms in diagnosing and managing chronic wounds presents a viable strategy to enhance the care of hospitalized patients while optimizing the efficiency of healthcare professionals [12]. With extensive and diverse predictors and data sets, ML becomes an invaluable tool for stratifying risk among patients with a predisposition to chronic wounds [63]. The move towards personalized telemedicine is shaping up to deliver optimal patient outcomes, and with the development of intelligent robotic systems, the dawn of AI-driven personalized telemedicine appears imminent [1].
AI has dramatically transformed the field of wound care, revolutionizing the assessment, measurement, classification, and prediction of wounds. At present, AI applications in skin wounds mainly concentrate on two areas: wound image analysis and data integration [96]. However, the development of AI-based systems to a level suitable for clinical use, ensuring the delivery of high-quality wound care, is still underway [79]. By setting stringent standards for wound data collection and creating more user-friendly and efficient recording systems, AI is poised to significantly enhance wound care practice [97-102]. This will provide patients with a more comprehensive and higher-quality care experience.
Disclosure of conflict of interest
None.
References
- 1.Wu YZ, Chen XC, Yi D. Advances and perspective of artificial intelligence in clinical area. J Army Med Univ. 2022;44:89–102. [Google Scholar]
- 2.Patil RS, Kulkarni SB, Gaikwad VL. Artificial intelligence in pharmaceutical regulatory affairs. Drug Discov Today. 2023;28:103700. doi: 10.1016/j.drudis.2023.103700. [DOI] [PubMed] [Google Scholar]
- 3.Stefanelli M. The socio-organizational age of artificial intelligence in medicine. Artif Intell Med. 2001;23:25–47. doi: 10.1016/s0933-3657(01)00074-4. [DOI] [PubMed] [Google Scholar]
- 4.Liu PR, Huo TT, Lu L, Zhang JY, Liu SX, Xie M, Ye ZW. Current status and prospect of artificial intelligence application in medicine. Chin Med J. 2021;101:3677–3683. [Google Scholar]
- 5.Subramaniam T, Fauzi MB, Lokanathan Y, Law JX. The role of calcium in wound healing. Int J Mol Sci. 2021;22:6486. doi: 10.3390/ijms22126486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masson-Meyers DS, Andrade TAM, Caetano GF, Guimaraes FR, Leite MN, Leite SN, Frade MAC. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101:21–37. doi: 10.1111/iep.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg. 2006;117(Suppl):193S–209S. doi: 10.1097/01.prs.0000225459.93750.29. discussion 210S-211S. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Renick P, Senkowsky J, Nair A, Tang L. Diagnostics for wound infections. Adv Wound Care (New Rochelle) 2021;10:317–327. doi: 10.1089/wound.2019.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen CK, Ghatak S, Gnyawali SC, Roy S, Gordillo GM. Cutaneous imaging technologies in acute burn and chronic wound care. Plast Reconstr Surg. 2016;138(Suppl):119S–128S. doi: 10.1097/PRS.0000000000002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Anisuzzaman DM, Williamson V, Dhar MK, Rostami B, Niezgoda J, Gopalakrishnan S, Yu Z. Fully automatic wound segmentation with deep convolutional neural networks. Sci Rep. 2020;10:21897. doi: 10.1038/s41598-020-78799-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie BM, Walker RM, McInnes E, Moore Z, Eskes AM, O’Connor T, Harbeck E, White C, Scott IA, Vermeulen H, Chaboyer W. Preoperative and postoperative recommendations to surgical wound care interventions: a systematic meta-review of Cochrane reviews. Int J Nurs Stud. 2020;102:103486. doi: 10.1016/j.ijnurstu.2019.103486. [DOI] [PubMed] [Google Scholar]
- 12.Dabas M, Schwartz D, Beeckman D, Gefen A. Application of artificial intelligence methodologies to chronic wound care and management: a scoping review. Adv Wound Care (New Rochelle) 2023;12:205–240. doi: 10.1089/wound.2021.0144. [DOI] [PubMed] [Google Scholar]
- 13.Howell RS, Kohan LS, Woods JS, Criscitelli T, Gillette BM, Donovan V, Gorenstein S. Wound care center of excellence: a process for continuous monitoring and improvement of wound care quality. Adv Skin Wound Care. 2018;31:204–213. doi: 10.1097/01.ASW.0000531354.39232.70. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Mohamedi AH, Senkowsky J, Nair A, Tang L. Imaging in chronic wound diagnostics. Adv Wound Care (New Rochelle) 2020;9:245–263. doi: 10.1089/wound.2019.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomura A, Noguchi M, Kometani M, Furukawa K, Yoneda T. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21:61. doi: 10.1007/s11892-021-01423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deo RC. Machine learning in medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foltynski P, Ladyzynski P, Ciechanowska A, Migalska-Musial K, Judzewicz G, Sabalinska S. Wound area measurement with digital planimetry: improved accuracy and precision with calibration based on 2 rulers. PLoS One. 2015;10:e0134622. doi: 10.1371/journal.pone.0134622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Yan X, Smith M, Kochhar K, Rubin M, Warren SM, Wrobel J, Lee H. A unified framework for automatic wound segmentation and analysis with deep convolutional neural networks. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:2415–2418. doi: 10.1109/EMBC.2015.7318881. [DOI] [PubMed] [Google Scholar]
- 19.Chino DYT, Scabora LC, Cazzolato MT, Jorge AES, Traina-Jr C, Traina AJM. Segmenting skin ulcers and measuring the wound area using deep convolutional networks. Comput Methods Programs Biomed. 2020;191:105376. doi: 10.1016/j.cmpb.2020.105376. [DOI] [PubMed] [Google Scholar]
- 20.Zhao YM, Currie EH, Kavoussi L, Rabbany SY. Laser scanner for 3D reconstruction of a wound’s edge and topology. Int J Comput Assist Radiol Surg. 2021;16:1761–1773. doi: 10.1007/s11548-021-02459-1. [DOI] [PubMed] [Google Scholar]
- 21.Jones JD, Quinn KP. Automated quantitative analysis of wound histology using deep-learning neural networks. J Invest Dermatol. 2021;141:1367–1370. doi: 10.1016/j.jid.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandram D, Ramirez-GarciaLuna JL, Fraser RDJ, Martínez-Jiménez MA, Arriaga-Caballero JE, Allport J. Fully automated wound tissue segmentation using deep learning on mobile devices: cohort study. JMIR Mhealth Uhealth. 2022;10:e36977. doi: 10.2196/36977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrión H, Jafari M, Bagood MD, Yang HY, Isseroff RR, Gomez M. Automatic wound detection and size estimation using deep learning algorithms. PLoS Comput Biol. 2022;18:e1009852. doi: 10.1371/journal.pcbi.1009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Pedersen PC, Agu E, Strong DM, Tulu B. Area determination of diabetic foot ulcer images using a cascaded two-stage SVM-based classification. IEEE Trans Biomed Eng. 2017;64:2098–2109. doi: 10.1109/TBME.2016.2632522. [DOI] [PubMed] [Google Scholar]
- 25.Manohar Dhane D, Maity M, Mungle T, Bar C, Achar A, Kolekar M, Chakraborty C. Fuzzy spectral clustering for automated delineation of chronic wound region using digital images. Comput Biol Med. 2017;89:551–560. doi: 10.1016/j.compbiomed.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Körber A, Rietkötter J, Grabbe S, Dissemond J. Three-dimensional documentation of wound healing: first results of a new objective method for measurement. J Dtsch Dermatol Ges. 2006;4:848–854. doi: 10.1111/j.1610-0387.2006.06113.x. [DOI] [PubMed] [Google Scholar]
- 27.Kairys A, Pauliukiene R, Raudonis V, Ceponis J. Towards home-based diabetic foot ulcer monitoring: a systematic review. Sensors (Basel) 2023;23:3618. doi: 10.3390/s23073618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alderden J, Pepper GA, Wilson A, Whitney JD, Richardson S, Butcher R, Jo Y, Cummins MR. Predicting pressure injury in critical care patients: a machine-learning model. Am J Crit Care. 2018;27:461–468. doi: 10.4037/ajcc2018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirillo MD, Mirdell R, Sjöberg F, Pham TD. Time-independent prediction of burn depth using deep convolutional neural networks. J Burn Care Res. 2019;40:857–863. doi: 10.1093/jbcr/irz103. [DOI] [PubMed] [Google Scholar]
- 30.Egberts G, Schaaphok M, Vermolen F, Zuijlen PV. A Bayesian finite-element trained machine learning approach for predicting post-burn contraction. Neural Comput Appl. 2022;34:8635–8642. doi: 10.1007/s00521-021-06772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ke C, Jin Y, Evans H, Lober B, Qian X, Liu J, Huang S. Prognostics of surgical site infections using dynamic health data. J Biomed Inform. 2017;65:22–33. doi: 10.1016/j.jbi.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Paul DW, Ghassemi P, Ramella-Roman JC, Prindeze NJ, Moffatt LT, Alkhalil A, Shupp JW. Noninvasive imaging technologies for cutaneous wound assessment: a review. Wound Repair Regen. 2015;23:149–162. doi: 10.1111/wrr.12262. [DOI] [PubMed] [Google Scholar]
- 33.Lucas Y, Niri R, Treuillet S, Douzi H, Castaneda B. Wound size imaging: ready for smart assessment and monitoring. Adv Wound Care (New Rochelle) 2021;10:641–661. doi: 10.1089/wound.2018.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flanagan M. The characteristics and formation of granulation tissue. J Wound Care. 1998;7:508–510. doi: 10.12968/jowc.1998.7.10.508. [DOI] [PubMed] [Google Scholar]
- 35.Rivenson Y, Wang H, Wei Z, de Haan K, Zhang Y, Wu Y, Günaydın H, Zuckerman JE, Chong T, Sisk AE, Westbrook LM, Wallace WD, Ozcan A. Virtual histological staining of unlabelled tissue-autofluorescence images via deep learning. Nat Biomed Eng. 2019;3:466–477. doi: 10.1038/s41551-019-0362-y. [DOI] [PubMed] [Google Scholar]
- 36.Howell RS, Liu HH, Khan AA, Woods JS, Lin LJ, Saxena M, Saxena H, Castellano M, Petrone P, Slone E, Chiu ES, Gillette BM, Gorenstein SA. Development of a method for clinical evaluation of artificial intelligence-based digital wound assessment tools. JAMA Netw Open. 2021;4:e217234. doi: 10.1001/jamanetworkopen.2021.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maknuna L, Kim H, Lee Y, Choi Y, Kim H, Yi M, Kang HW. Automated structural analysis and quantitative characterization of scar tissue using machine learning. Diagnostics (Basel) 2022;12:534. doi: 10.3390/diagnostics12020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidhuber J. Deep learning in neural networks: an overview. Neural Netw. 2015;61:85–117. doi: 10.1016/j.neunet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Zoppo G, Marrone F, Pittarello M, Farina M, Uberti A, Demarchi D, Secco J, Corinto F, Ricci E. AI technology for remote clinical assessment and monitoring. J Wound Care. 2020;29:692–706. doi: 10.12968/jowc.2020.29.12.692. [DOI] [PubMed] [Google Scholar]
- 40.Sarvamangala DR, Kulkarni RV. Convolutional neural networks in medical image understanding: a survey. Evol Intell. 2022;15:1–22. doi: 10.1007/s12065-020-00540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita R, Nishio M, Do RKG, Togashi K. Convolutional neural networks: an overview and application in radiology. Insights Imaging. 2018;9:611–629. doi: 10.1007/s13244-018-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai F, Zhang D, Su K, Xin N. Burn images segmentation based on burn-GAN. J Burn Care Res. 2021;42:755–762. doi: 10.1093/jbcr/iraa208. [DOI] [PubMed] [Google Scholar]
- 43.Yoshino Y, Ohtsuka M, Kawaguchi M, Sakai K, Hashimoto A, Hayashi M, Madokoro N, Asano Y, Abe M, Ishii T, Isei T, Ito T, Inoue Y, Imafuku S, Irisawa R, Ohtsuka M, Ogawa F, Kadono T, Kawakami T, Kukino R, Kono T, Kodera M, Takahara M, Tanioka M, Nakanishi T, Nakamura Y, Hasegawa M, Fujimoto M, Fujiwara H, Maekawa T, Matsuo K, Yamasaki O, Le Pavoux A, Tachibana T, Ihn H Wound/Burn Guidelines Committee. The wound/burn guidelines - 6: guidelines for the management of burns. J Dermatol. 2016;43:989–1010. doi: 10.1111/1346-8138.13288. [DOI] [PubMed] [Google Scholar]
- 44.Huang S, Dang J, Sheckter CC, Yenikomshian HA, Gillenwater J. A systematic review of machine learning and automation in burn wound evaluation: a promising but developing frontier. Burns. 2021;47:1691–1704. doi: 10.1016/j.burns.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Ponticorvo A, Rowland R, Baldado M, Burmeister DM, Christy RJ, Bernal NP, Durkin AJ. Evaluating clinical observation versus spatial frequency domain imaging (SFDI), laser speckle imaging (LSI) and thermal imaging for the assessment of burn depth. Burns. 2019;45:450–460. doi: 10.1016/j.burns.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponticorvo A, Burmeister DM, Yang B, Choi B, Christy RJ, Durkin AJ. Quantitative assessment of graded burn wounds in a porcine model using spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI) Biomed Opt Express. 2014;5:3467–3481. doi: 10.1364/BOE.5.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen JQ, Crouzet C, Mai T, Riola K, Uchitel D, Liaw LH, Bernal N, Ponticorvo A, Choi B, Durkin AJ. Spatial frequency domain imaging of burn wounds in a preclinical model of graded burn severity. J Biomed Opt. 2013;18:66010. doi: 10.1117/1.JBO.18.6.066010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowland R, Ponticorvo A, Baldado M, Kennedy GT, Burmeister DM, Christy RJ, Bernal NP, Durkin AJ. Burn wound classification model using spatial frequency-domain imaging and machine learning. J Biomed Opt. 2019;24:1–9. doi: 10.1117/1.JBO.24.5.056007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cirillo MD, Mirdell R, Sjöberg F, Pham TD. Improving burn depth assessment for pediatric scalds by AI based on semantic segmentation of polarized light photography images. Burns. 2021;47:1586–1593. doi: 10.1016/j.burns.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Hettiaratchy S, Papini R. Initial management of a major burn: II--assessment and resuscitation. BMJ. 2004;329:101–103. doi: 10.1136/bmj.329.7457.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson RH, Rowland R, Kennedy GT, Campbell C, Joe VC, Chin TL, Burmeister DM, Christy RJ, Durkin AJ. Review of machine learning for optical imaging of burn wound severity assessment. J Biomed Opt. 2024;29:020901. doi: 10.1117/1.JBO.29.2.020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130:489–493. [PubMed] [Google Scholar]
- 53.Kirsner RS. The Wound Healing Society chronic wound ulcer healing guidelines update of the 2006 guidelines--blending old with new. Wound Repair Regen. 2016;24:110–111. doi: 10.1111/wrr.12393. [DOI] [PubMed] [Google Scholar]
- 54.van Koppen CJ, Hartmann RW. Advances in the treatment of chronic wounds: a patent review. Expert Opin Ther Pat. 2015;25:931–937. doi: 10.1517/13543776.2015.1045879. [DOI] [PubMed] [Google Scholar]
- 55.Kolimi P, Narala S, Nyavanandi D, Youssef AAA, Dudhipala N. Innovative treatment strategies to accelerate wound healing: trajectory and recent advancements. Cells. 2022;11:2439. doi: 10.3390/cells11152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowers S, Franco E. Chronic wounds: evaluation and management. Am Fam Physician. 2020;101:159–166. [PubMed] [Google Scholar]
- 57.Alam W, Hasson J, Reed M. Clinical approach to chronic wound management in older adults. J Am Geriatr Soc. 2021;69:2327–2334. doi: 10.1111/jgs.17177. [DOI] [PubMed] [Google Scholar]
- 58.Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, DiPietro L, Falanga V, Fife C, Gardner S, Grice E, Harmon J, Hazzard WR, High KP, Houghton P, Jacobson N, Kirsner RS, Kovacs EJ, Margolis D, McFarland Horne F, Reed MJ, Sullivan DH, Thom S, Tomic-Canic M, Walston J, Whitney J, Williams J, Zieman S, Schmader K. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen. 2015;23:1–13. doi: 10.1111/wrr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol. 2016;74:607–25. doi: 10.1016/j.jaad.2015.08.070. quiz 625-6. [DOI] [PubMed] [Google Scholar]
- 61.Kalasin S, Sangnuang P, Surareungchai W. Intelligent wearable sensors interconnected with advanced wound dressing bandages for contactless chronic skin monitoring: artificial intelligence for predicting tissue regeneration. Anal Chem. 2022;94:6842–6852. doi: 10.1021/acs.analchem.2c00782. [DOI] [PubMed] [Google Scholar]
- 62.Chakraborty C, Gupta B, Ghosh SK, Das DK, Chakraborty C. Telemedicine supported chronic wound tissue prediction using classification approaches. J Med Syst. 2016;40:68. doi: 10.1007/s10916-015-0424-y. [DOI] [PubMed] [Google Scholar]
- 63.Veličković VM, Spelman T, Clark M, Probst S, Armstrong DG, Steyerberg E. Individualized risk prediction for improved chronic wound management. Adv Wound Care (New Rochelle) 2023;12:387–398. doi: 10.1089/wound.2022.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung K, Covington S, Sen CK, Januszyk M, Kirsner RS, Gurtner GC, Shah NH. Rapid identification of slow healing wounds. Wound Repair Regen. 2016;24:181–188. doi: 10.1111/wrr.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haalboom M. Chronic wounds: innovations in diagnostics and therapeutics. Curr Med Chem. 2018;25:5772–5781. doi: 10.2174/0929867324666170710120556. [DOI] [PubMed] [Google Scholar]
- 66.Rajesh E, Basheer S, Dhanaraj RK, Yadav S, Kadry S, Khan MA, Kim YJ, Cha JH. Machine learning for online automatic prediction of common disease attributes using never-ending image learner. Diagnostics (Basel) 2022;13:95. doi: 10.3390/diagnostics13010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woods JS, Saxena M, Nagamine T, Howell RS, Criscitelli T, Gorenstein S, M Gillette B. The future of data-driven wound care. AORN J. 2018;107:455–463. doi: 10.1002/aorn.12102. [DOI] [PubMed] [Google Scholar]
- 68.Khan S, Khan HU, Nazir S. Systematic analysis of healthcare big data analytics for efficient care and disease diagnosing. Sci Rep. 2022;12:22377. doi: 10.1038/s41598-022-26090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Plast Reconstr Surg. 2006;117(Suppl):239S–244S. doi: 10.1097/01.prs.0000222891.74489.33. [DOI] [PubMed] [Google Scholar]
- 70.Lisboa FA, Dente CJ, Schobel SA, Khatri V, Potter BK, Kirk AD, Elster EA. Utilizing precision medicine to estimate timing for surgical closure of traumatic extremity wounds. Ann Surg. 2019;270:535–543. doi: 10.1097/SLA.0000000000003470. [DOI] [PubMed] [Google Scholar]
- 71.Xu P, Wu Y, Zhou L, Yang Z, Zhang X, Hu X, Yang J, Wang M, Wang B, Luo G, He W, Cheng B. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma. 2020;8:tkaa028. doi: 10.1093/burnst/tkaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011;19:441–453. doi: 10.1016/j.fsc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–365. doi: 10.1016/j.addr.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Ji Y, Yang S, Zhou K, Rocliffe HR, Pellicoro A, Cash JL, Wang R, Li C, Huang Z. Deep-learning approach for automated thickness measurement of epithelial tissue and scab using optical coherence tomography. J Biomed Opt. 2022;27:015002. doi: 10.1117/1.JBO.27.1.015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Squiers JJ, Thatcher JE, Bastawros DS, Applewhite AJ, Baxter RD, Yi F, Quan P, Yu S, DiMaio JM, Gable DR. Machine learning analysis of multispectral imaging and clinical risk factors to predict amputation wound healing. J Vasc Surg. 2022;75:279–285. doi: 10.1016/j.jvs.2021.06.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Ke Z, He Z, Chen X, Zhang Y, Xie P, Li T, Zhou J, Li F, Yang C, Zhang P, Huang C, Kai L. Real-time burn depth assessment using artificial networks: a large-scale, multicentre study. Burns. 2020;46:1829–1838. doi: 10.1016/j.burns.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Anisuzzaman DM, Wang C, Rostami B, Gopalakrishnan S, Niezgoda J, Yu Z. Image-based artificial intelligence in wound assessment: a systematic review. Adv Wound Care (New Rochelle) 2022;11:687–709. doi: 10.1089/wound.2021.0091. [DOI] [PubMed] [Google Scholar]
- 78.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 79.Rippon MG, Fleming L, Chen T, Rogers AA, Ousey K. Artificial intelligence in wound care: diagnosis, assessment and treatment of hard-to-heal wounds: a narrative review. J Wound Care. 2024;33:229–242. doi: 10.12968/jowc.2024.33.4.229. [DOI] [PubMed] [Google Scholar]
- 80.Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37:505–515. doi: 10.1148/rg.2017160130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barakat-Johnson M, Jones A, Burger M, Leong T, Frotjold A, Randall S, Fethney J, Coyer F. Reshaping wound care: evaluation of an artificial intelligence app to improve wound assessment and management. Stud Health Technol Inform. 2024;310:941–945. doi: 10.3233/SHTI231103. [DOI] [PubMed] [Google Scholar]
- 83.Hassan M, Awan FM, Naz A, deAndrés-Galiana EJ, Alvarez O, Cernea A, Fernández-Brillet L, Fernández-Martínez JL, Kloczkowski A. Innovations in genomics and big data analytics for personalized medicine and health care: a review. Int J Mol Sci. 2022;23:4645. doi: 10.3390/ijms23094645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waithira N, Mutinda B, Cheah PY. Data management and sharing policy: the first step towards promoting data sharing. BMC Med. 2019;17:80. doi: 10.1186/s12916-019-1315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, Wang Y, Dong Q, Shen H, Wang Y. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2:230–243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez K, Fodeh SJ, Allam A, Brandt CA, Krauthammer M. Reducing annotation burden through multimodal learning. Front Big Data. 2020;3:19. doi: 10.3389/fdata.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pesapane F, Volonté C, Codari M, Sardanelli F. Artificial intelligence as a medical device in radiology: ethical and regulatory issues in Europe and the United States. Insights Imaging. 2018;9:745–753. doi: 10.1007/s13244-018-0645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aung YYM, Wong DCS, Ting DSW. The promise of artificial intelligence: a review of the opportunities and challenges of artificial intelligence in healthcare. Br Med Bull. 2021;139:4–15. doi: 10.1093/bmb/ldab016. [DOI] [PubMed] [Google Scholar]
- 89.Loftus TJ, Tighe PJ, Filiberto AC, Efron PA, Brakenridge SC, Mohr AM, Rashidi P, Upchurch GR Jr, Bihorac A. Artificial intelligence and surgical decision-making. JAMA Surg. 2020;155:148–158. doi: 10.1001/jamasurg.2019.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esmaeilzadeh P. Challenges and strategies for wide-scale artificial intelligence (AI) deployment in healthcare practices: a perspective for healthcare organizations. Artif Intell Med. 2024;151:102861. doi: 10.1016/j.artmed.2024.102861. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan HR, Schweikart SJ. Are current tort liability doctrines adequate for addressing injury caused by AI? AMA J Ethics. 2019;21:E160–166. doi: 10.1001/amajethics.2019.160. [DOI] [PubMed] [Google Scholar]
- 92.Santorsola M, Lescai F. The promise of explainable deep learning for omics data analysis: adding new discovery tools to AI. N Biotechnol. 2023;77:1–11. doi: 10.1016/j.nbt.2023.06.002. [DOI] [PubMed] [Google Scholar]
- 93.van der Velden BHM, Kuijf HJ, Gilhuijs KGA, Viergever MA. Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med Image Anal. 2022;79:102470. doi: 10.1016/j.media.2022.102470. [DOI] [PubMed] [Google Scholar]
- 94.Karako K, Song P, Chen Y, Tang W. Realizing 5G- and AI-based doctor-to-doctor remote diagnosis: opportunities, challenges, and prospects. Biosci Trends. 2020;14:314–317. doi: 10.5582/bst.2020.03364. [DOI] [PubMed] [Google Scholar]
- 95.Pappachan JM, Cassidy B, Fernandez CJ, Chandrabalan V, Yap MH. The role of artificial intelligence technology in the care of diabetic foot ulcers: the past, the present, and the future. World J Diabetes. 2022;13:1131–1139. doi: 10.4239/wjd.v13.i12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xing MY, Li MX, Li J, Li B. Advances in the application of artificial intelligence in the field of chronic wound care. Adv Clin Med. 2022;12:11013–11018. [Google Scholar]
- 97.Filko D, Marijanović D, Nyarko EK. Automatic robot-driven 3d reconstruction system for chronic wounds. Sensors (Basel) 2021;21:8308. doi: 10.3390/s21248308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Derakhshandeh H, Kashaf SS, Aghabaglou F, Ghanavati IO, Tamayol A. Smart bandages: the future of wound care. Trends Biotechnol. 2018;36:1259–1274. doi: 10.1016/j.tibtech.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Felgueiras HP, Tavares TD, Amorim MTP. Biodegradable, spun nanocomposite polymeric fibrous dressings loaded with bioactive biomolecules for an effective wound healing: a review. IOP Conf Ser Mater Sci Eng. 2019;634:012033. [Google Scholar]
- 100.Alizadehgiashi M, Nemr CR, Chekini M, Pinto Ramos D, Mittal N, Ahmed SU, Khuu N, Kelley SO, Kumacheva E. Multifunctional 3D-printed wound dressings. ACS Nano. 2021;15:12375–12387. doi: 10.1021/acsnano.1c04499. [DOI] [PubMed] [Google Scholar]
- 101.Cho SK, Mattke S, Gordon H, Sheridan M, Ennis W. Development of a model to predict healing of chronic wounds within 12 weeks. Adv Wound Care (New Rochelle) 2020;9:516–524. doi: 10.1089/wound.2019.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Armstrong DG, Rowe VL, D’Huyvetter K, Sherman RA. Telehealth-guided home-based maggot debridement therapy for chronic complex wounds: peri- and post-pandemic potential. Int Wound J. 2020;17:1490–1495. doi: 10.1111/iwj.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]